Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach
Abstract
1. Introduction
2. Results
3. Methods and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Putungan, D.B.; Lin, S.-H.; Wei, C.-M.; Kuo, J.-L. Li Adsorption, Hydrogen Storage and Dissociation Using Monolayer MoS2: An Ab Initio Random Structure Searching Approach. Phys. Chem. Chem. Phys. 2015, 17, 11367–11374. [Google Scholar] [CrossRef]
- Rangarajan, S.; Mavrikakis, M. On the Preferred Active Sites of Promoted MoS2 for Hydrodesulfurization with Minimal Organonitrogen Inhibition. ACS Catal. 2017, 7, 501–509. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High-Performance Chemical Sensing Using Schottky-Contacted Chemical Vapor Deposition Grown Monolayer MoS2 Transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef]
- Donarelli, M.; Prezioso, S.; Perrozzi, F.; Bisti, F.; Nardone, M.; Giancaterini, L.; Cantalini, C.; Ottaviano, L. Response to NO2 and Other Gases of Resistive Chemically Exfoliated MoS2-Based Gas Sensors. Sens. Actuators B Chem. 2015, 207, 602–613. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.-H.; Park, S.-G.; Kwon, J.-D.; Lee, Y.-J.; Lee, K.-H.; Lee, B.H.; et al. Chemical Sensing of 2D Graphene/MoS2 Heterostructure Device. ACS Appl. Mater. Interfaces 2015, 7, 16775–16780. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Kim, A.R.; Park, Y.; Yoon, J.; Lee, Y.-J.; Lee, S.; Yoo, T.J.; Kang, C.G.; Lee, B.H.; Ko, H.C.; et al. Bifunctional Sensing Characteristics of Chemical Vapor Deposition Synthesized Atomic-Layered MoS2. ACS Appl. Mater. Interfaces 2015, 7, 2952–2959. [Google Scholar] [CrossRef]
- Vrubel, H.; Merki, D.; Hu, X. Hydrogen Evolution Catalyzed by MoS3 and MoS2 Particles. Energy Environ. Sci. 2012, 5, 6136. [Google Scholar] [CrossRef]
- Khan, M.A.; Leuenberger, M.N. Room-Temperature Superparamagnetism Due to Giant Magnetic Anisotropy in MoS Defected Single-Layer MoS2. J. Phys. Condens. Matter 2018, 30, 155802. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.J.; Jeong, B.G.; Kwon, C.; Cha, Y.; Choi, S.H.; Kim, K.K.; Jeong, M.S. Electrical Role of Sulfur Vacancies in MoS2: Transient Current Approach. Appl. Surf. Sci. 2023, 613, 155900. [Google Scholar] [CrossRef]
- González, C.; Biel, B.; Dappe, Y.J. Theoretical Characterisation of Point Defects on a MoS 2 Monolayer by Scanning Tunnelling Microscopy. Nanotechnology 2016, 27, 105702. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Rawal, T.B.; Rahman, T.S. Single-Layer MoS2 with Sulfur Vacancies: Structure and Catalytic Application. J. Phys. Chem. C 2014, 118, 5346–5351. [Google Scholar] [CrossRef]
- An, Y.; Fan, X.; Liu, H.; Luo, Z. Improved Catalytic Performance of Monolayer Nano-Triangles WS2 and MoS2 on HER by 3d Metals Doping. Comput. Mater. Sci. 2019, 159, 333–340. [Google Scholar] [CrossRef]
- Yin, M.Y.; Wang, X.C.; Mi, W.B.; Yang, B.H. First Principles Prediction on the Interfaces of Fe/MoS2, Co/MoS2 and Fe3O4/MoS2. Comput. Mater. Sci. 2015, 99, 326–335. [Google Scholar] [CrossRef]
- Nan, H.; Wang, Z.; Wang, W.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.; Tan, P.; Miao, F.; Wang, X.; et al. Strong Photoluminescence Enhancement of MoS2 through Defect Engineering and Oxygen Bonding. ACS Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef]
- Miralrio, A.; Rangel Cortes, E.; Castro, M. Electronic Properties and Enhanced Reactivity of MoS2 Monolayers with Substitutional Gold Atoms Embedded into Sulfur Vacancies. Appl. Surf. Sci. 2018, 455, 758–770. [Google Scholar] [CrossRef]
- Miralrio, A.; Rangel, E.; Castro, M. Activation of MoS2 Monolayers by Substitutional Copper and Silver Atoms Embedded in Sulfur Vacancies: A Theoretical Study. Appl. Surf. Sci. 2019, 481, 611–624. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, M.S.; Srivastava, A.; Khan, M.S.; Husain, M. Si-Doped MoS2 Sheet as Phosgene Gas Sensor: A First Principles Study. AIP Conf. Proc. 2019, 2115, 030438. [Google Scholar] [CrossRef]
- Zhao, P.; Zheng, J.; Guo, P.; Jiang, Z.; Cao, L.; Wan, Y. Electronic and Magnetic Properties of Re-Doped Single-Layer MoS2: A DFT Study. Comput. Mater. Sci. 2017, 128, 287–293. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Qiu, Y.; Zhu, J.; Zhang, Y.; Hu, G. A DFT Study of Transition Metal (Fe, Co, Ni, Cu, Ag, Au, Rh, Pd, Pt and Ir)-Embedded Monolayer MoS2 for Gas Adsorption. Comput. Mater. Sci. 2017, 138, 255–266. [Google Scholar] [CrossRef]
- Tong, P.; Sheng, Y.; Hou, R.; Iqbal, M.; Chen, L.; Li, J. Recent Progress on Coatings of Biomedical Magnesium Alloy. Smart Mater. Med. 2022, 3, 104–116. [Google Scholar] [CrossRef]
- Tuckett, R. Greenhouse Gases and the Emerging Climate Emergency. In Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 19–45. ISBN 978-0-12-821575-3. [Google Scholar]
- Sachs, J.D. From Millennium Development Goals to Sustainable Development Goals. Lancet 2012, 379, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, C.Y.; Liu, L.L.; Zhou, B.; Zhang, Q.K.; Chen, Z.Q.; Tang, Z. Adsorption of Gas Molecules on Cu Impurities Embedded Monolayer MoS2: A First- Principles Study. Appl. Surf. Sci. 2016, 382, 280–287. [Google Scholar] [CrossRef]
- Wang, Z.; Mi, B. Environmental Applications of 2D Molybdenum Disulfide (MoS 2 ) Nanosheets. Environ. Sci. Technol. 2017, 51, 8229–8244. [Google Scholar] [CrossRef]
- Sharma, A.; Anu; Khan, M.S.; Husain, M.; Khan, M.S.; Srivastava, A. Sensing of CO and NO on Cu-Doped MoS2 Monolayer-Based Single Electron Transistor: A First Principles Study. IEEE Sens. J. 2018, 18, 2853–2860. [Google Scholar] [CrossRef]
- Wu, S.; Liu, H.; Qu, M.; Du, A.; Fan, J.; Sun, Q. B/C-Doped MoS2 as Promising Materials for Nitrogen Removal from Natural Gas: A First-Principles Computational Study. Comput. Mater. Sci. 2022, 214, 111735. [Google Scholar] [CrossRef]
- Sharma, A.; Srivastava, A.; Husain, M.; Khan, M.S. Computational Investigations of Cu-Embedded MoS2 Sheet for CO Oxidation Catalysis. J. Mater. Sci. 2018, 53, 9578–9588. [Google Scholar] [CrossRef]
- Reddy, B.K.S.; Borse, P.H. Review—Recent Material Advances and Their Mechanistic Approaches for Room Temperature Chemiresistive Gas Sensors. J. Electrochem. Soc. 2021, 168, 057521. [Google Scholar] [CrossRef]
- Ullah, F.; Ibrahim, K.; Mistry, K.; Samad, A.; Shahin, A.; Sanderson, J.; Musselman, K. WS2 and WS2-ZnO Chemiresistive Gas Sensors: The Role of Analyte Charge Asymmetry and Molecular Size. ACS Sens. 2023, 8, 1630–1638. [Google Scholar] [CrossRef]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent Advances in the Science and Technology of Ultra Low Sulfur Diesel (ULSD) Production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Rana, M.S.; Sámano, V.; Ancheyta, J.; Diaz, J.A.I. A Review of Recent Advances on Process Technologies for Upgrading of Heavy Oils and Residua. Fuel 2007, 86, 1216–1231. [Google Scholar] [CrossRef]
- Walton, A.S.; Lauritsen, J.V.; Topsøe, H.; Besenbacher, F. MoS2 Nanoparticle Morphologies in Hydrodesulfurization Catalysis Studied by Scanning Tunneling Microscopy. J. Catal. 2013, 308, 306–318. [Google Scholar] [CrossRef]
- Albiter, M.A.; Huirache-Acuña, R.; Paraguay-Delgado, F.; Rico, J.L.; Alonso-Nuñez, G. Synthesis of MoS2 Nanorods and Their Catalytic Test in the HDS of Dibenzothiophene. Nanotechnology 2006, 17, 3473–3481. [Google Scholar] [CrossRef] [PubMed]
- Muralikrishna, S.; Manjunath, K.; Samrat, D.; Reddy, V.; Ramakrishnappa, T.; Nagaraju, D.H. Hydrothermal Synthesis of 2D MoS2 Nanosheets for Electrocatalytic Hydrogen Evolution Reaction. RSC Adv. 2015, 5, 89389–89396. [Google Scholar] [CrossRef]
- Samy, O.; Zeng, S.; Birowosuto, M.D.; El Moutaouakil, A. A Review on MoS2 Properties, Synthesis, Sensing Applications and Challenges. Crystals 2021, 11, 355. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, X.; Tang, J.; Cui, H.; Li, Y. Noble Metal (Pt or Au)-Doped Monolayer MoS2 as a Promising Adsorbent and Gas-Sensing Material to SO2, SOF2 and SO2F2: A DFT Study. Appl. Phys. A 2018, 124, 194. [Google Scholar] [CrossRef]
- Halwidl, D.; Mayr-Schmölzer, W.; Setvin, M.; Fobes, D.; Peng, J.; Mao, Z.; Schmid, M.; Mittendorfer, F.; Redinger, J.; Diebold, U. A Full Monolayer of Superoxide: Oxygen Activation on the Unmodified Ca3Ru2O7 (001) Surface. J. Mater. Chem. A 2018, 6, 5703–5713. [Google Scholar] [CrossRef]
- Chen, Z.W.; Yan, J.M.; Zheng, W.T.; Jiang, Q. Cu4 Cluster Doped Monolayer MoS2 for CO Oxidation. Sci. Rep. 2015, 5, 11230. [Google Scholar] [CrossRef]
- Gadzhiev, O.B.; Ignatov, S.K.; Gangopadhyay, S.; Masunov, A.E.; Petrov, A.I. Mechanism of Nitric Oxide Oxidation Reaction (2NO + O2 → 2NO2) Revisited. J. Chem. Theory Comput. 2011, 7, 2021–2024. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Dou, X.; Zhang, Q.; Shah, S.N.A.; Khan, M.; Uchiyama, K.; Lin, J.-M. MoS2-Quantum Dot Triggered Reactive Oxygen Species Generation and Depletion: Responsible for Enhanced Chemiluminescence. Chem. Sci. 2019, 10, 497–500. [Google Scholar] [CrossRef]
- Li, Q.; Hu, B.; Yang, Q.; Cai, X.; Nie, M.; Jin, Y.; Zhou, L.; Xu, Y.; Pan, Q.; Fang, L. Interaction Mechanism between Multi-Layered MoS2 and H2O2 for Self-Generation of Reactive Oxygen Species. Environ. Res. 2020, 191, 110227. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A Modular and Open-Source Software Project for Quantum Simulations of Materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Petkov, V.; Billinge, S.J.L.; Larson, P.; Mahanti, S.D.; Vogt, T.; Rangan, K.K.; Kanatzidis, M.G. Structure of Nanocrystalline Materials Using Atomic Pair Distribution Function Analysis: Study of LiMoS2. Phys. Rev. B 2002, 65, 092105. [Google Scholar] [CrossRef]
- Koós, A.A.; Vancsó, P.; Magda, G.Z.; Osváth, Z.; Kertész, K.; Dobrik, G.; Hwang, C.; Tapasztó, L.; Biró, L.P. STM Study of the MoS2 Flakes Grown on Graphite: A Model System for Atomically Clean 2D Heterostructure Interfaces. Carbon 2016, 105, 408–415. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A Fast and Robust Algorithm for Bader Decomposition of Charge Density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
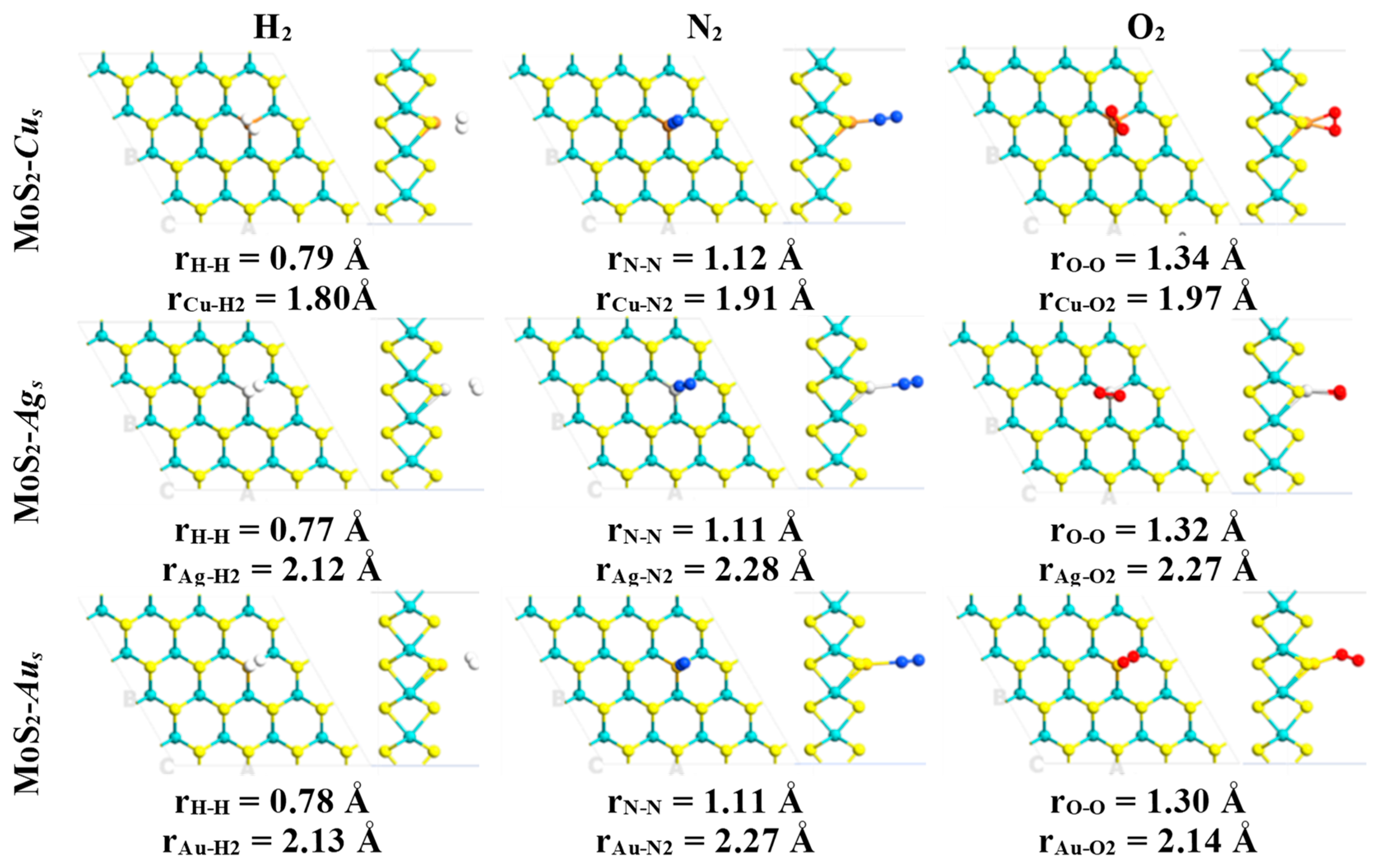
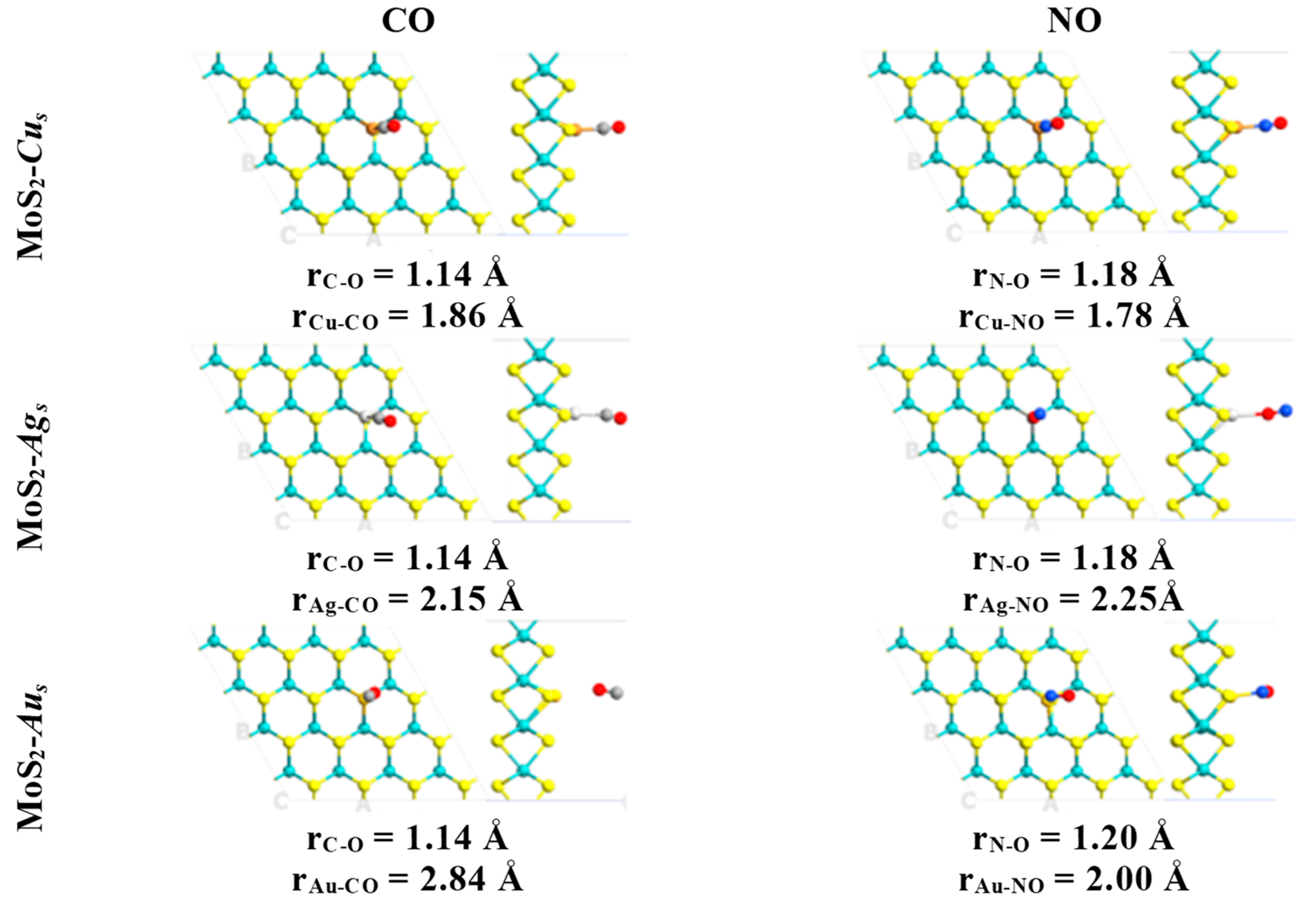
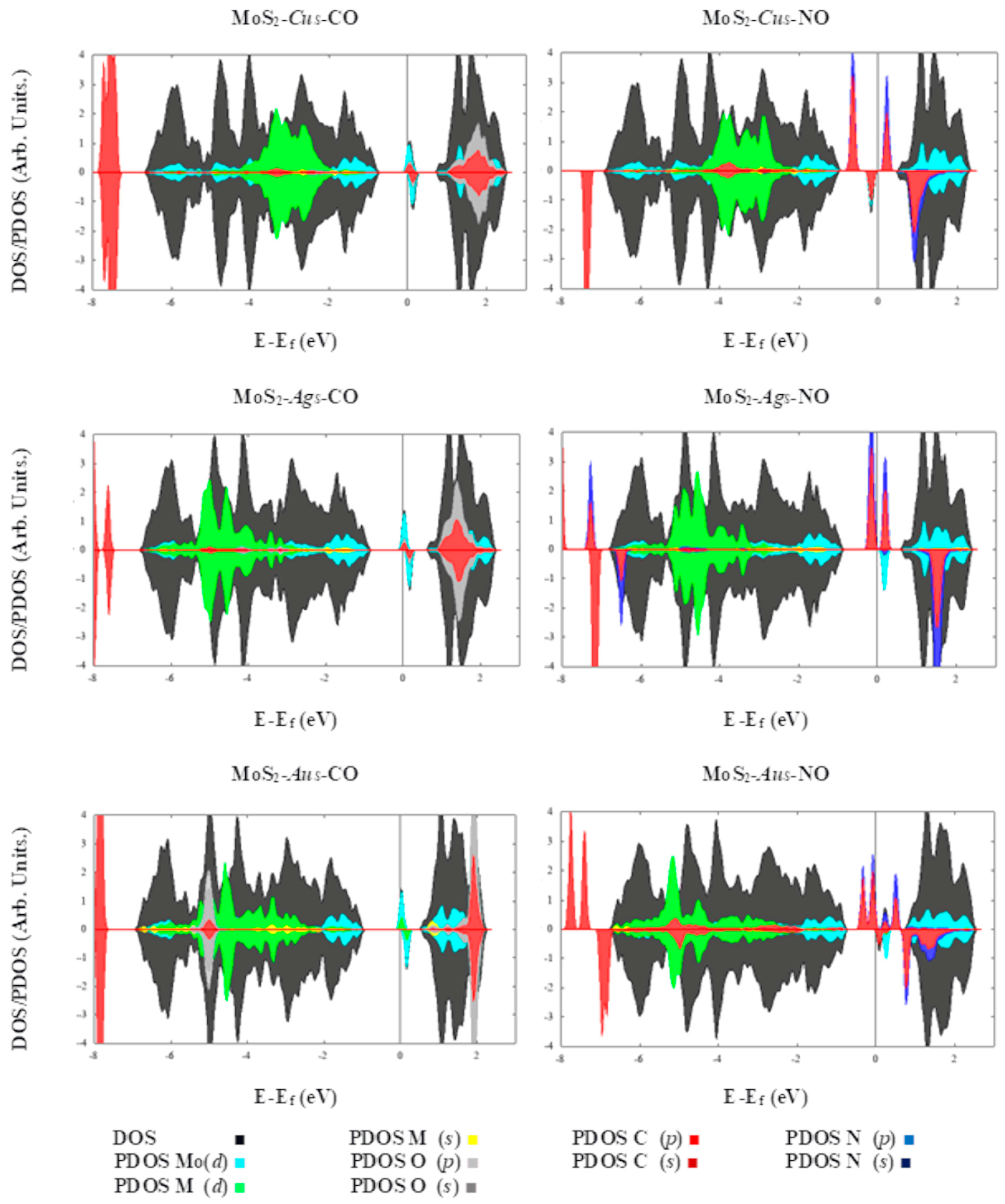
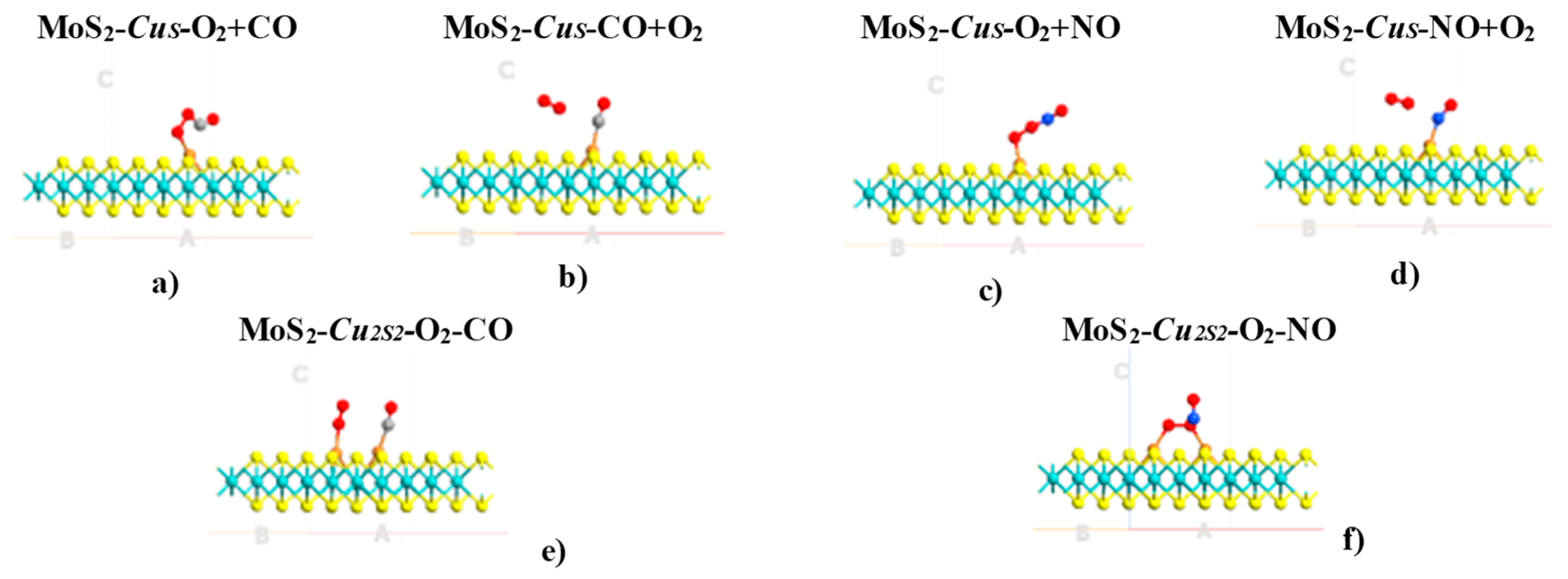
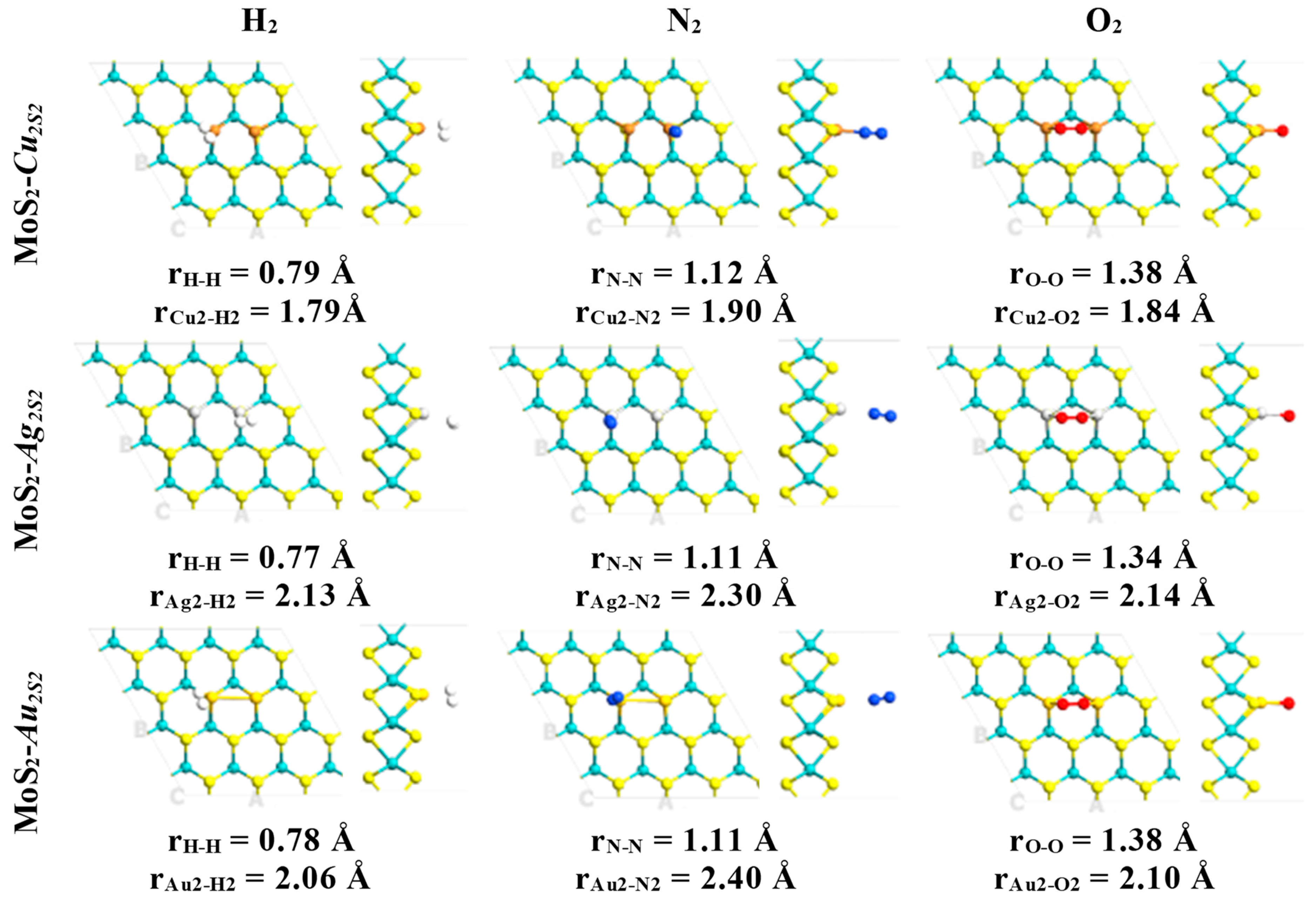
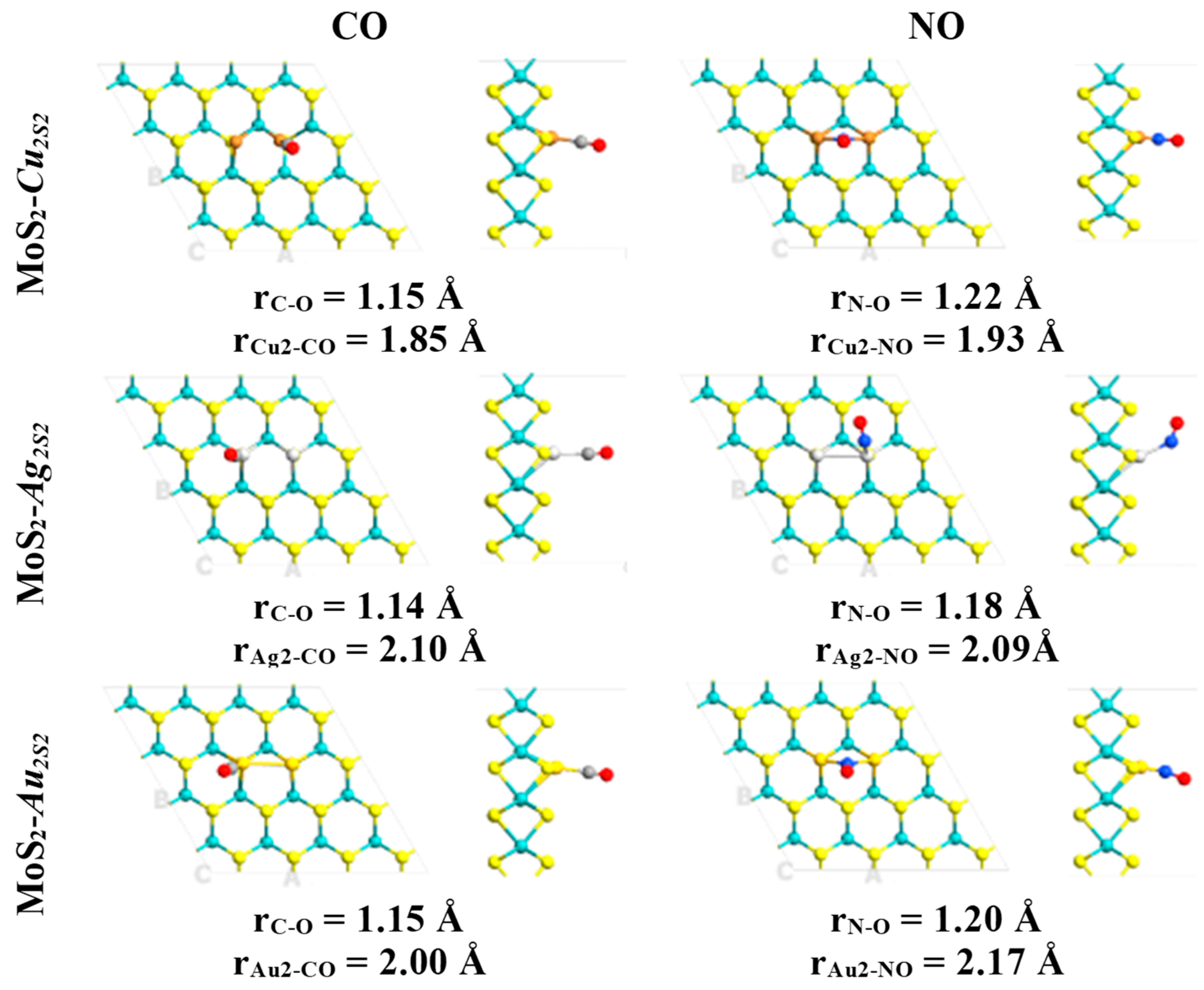
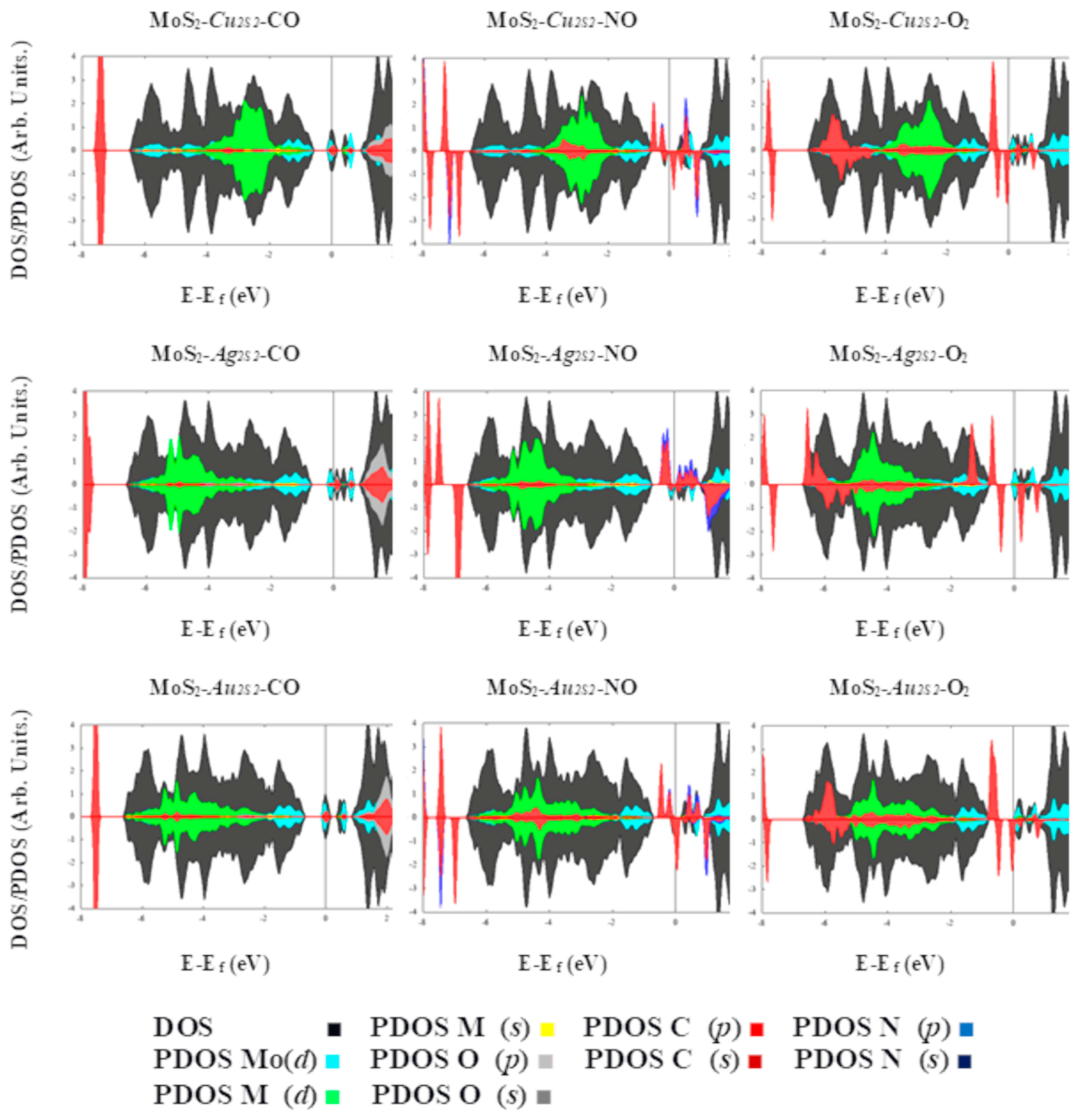
| System | Eads (kcal·mol−1)/eV | Total Magnetization (μB) | Magnetization on AB Molecule (μB) | Total Charge on AB Molecule (e) | Adsorption Mode |
|---|---|---|---|---|---|
| MoS2-Cus-H2 | 9.35/0.45 | 0.67 | 0.00 (0.00) | 0.02 | Cu-H2 |
| MoS2-Cus-O2 | 24.88/1.07 | 0.95 | 0.34 (0.34) | −0.50 | Cu-O2 |
| MoS2-Cus-N2 | 15.21/0.66 | 0.46 | 0.00 (0.02) | −0.09 | Cu-N-N |
| MoS2-Cus-CO | 28.54/1.24 | 0.43 | 0.01 (0.02) | −0.10 | Cu-C-O |
| MoS2-Cus-NO | 33.18/1.44 | 2.07 | 0.47 (0.70) | −0.28 | Cu-N-O |
| MoS2-Ags-H2 | 6.12/0.27 | 0.89 | 0.00 (0.00) | 0.05 | Ag-H2 |
| MoS2-Ags-O2 | 13.16/0.57 | 0.94 | 0.62 (0.64) | −0.42 | Ag-O2 |
| MoS2-Ags-N2 | 7.67/0.33 | 0.82 | 0.00 (0.01) | −0.02 | Ag-N-N |
| MoS2-Ags-CO | 17.85/0.77 | 0.69 | 0.01 (0.02) | −0.01 | Ag-C-O |
| MoS2-Ags-NO | 7.95/0.35 | 1.75 | 0.35 (0.88) | −0.19 | Ag-O-N |
| MoS2-Aus-H2 | 5.20/0.23 | 0.57 | 0.00 (0.00) | 0.06 | Au-H2 |
| MoS2-Aus-O2 | 11.74/0.51 | 0.86 | 0.42 (0.61) | −0.40 | Au-O-O |
| MoS2-Aus-N2 | 6.69/0.29 | 0.26 | 0.00 (0.01) | −0.02 | Au-N-N |
| MoS2-Aus-CO | 2.95/0.13 | 0.66 | 0.00 (0.00) | 0.00 | Au-O-C |
| MoS2-Aus-NO | 2.48/0.11 | 1.53 | 0.44 (0.58) | −0.25 | Au-N-O |
| System | Eads (kcal·mol−1)/eV | Total Magnetization (μB) | Magnetization on AB Molecule (μB) | Total Charge on AB Molecule (e) | Adsorption Mode |
|---|---|---|---|---|---|
| MoS2-Cu2S2-H2 | 9.09/0.395 | 0.03 | 0.00 (0.00) | 0.03 | Cu-H2 |
| MoS2-Cu2S2-O2 | 38.04/1.65 | 0.26 | 0.21 (0.21) | −0.85 | Cu-O-O-Cu |
| MoS2-Cu2S2-N2 | 15.24/0.66 | 0.04 | 0.00 (0.01) | −0.12 | Cu-N-N |
| MoS2-Cu2S2-CO | 30.21/1.31 | 0.03 | 0.00 (0.00) | −0.12 | Cu-C-O |
| MoS2-Cu2S2-NO | 38.89/1.69 | 0.94 | 0.45 (0.45) | −0.51 | Cu-N-O |
| MoS2-Ag2S2-H2 | 5.306/0.23 | 0.02 | 0.00 (0.00) | 0.04 | Ag-H2 |
| MoS2-Ag2S2-O2 | 22.41/0.97 | 0.94 | 0.48 (0.48) | −0.60 | Ag-O-O-Ag |
| MoS2-Ag2S2-N2 | 7.35/0.32 | 0.02 | 0.00 (0.00) | −0.02 | Ag-N-N |
| MoS2-Ag2S2-CO | 18.55/0.80 | 0.02 | 0.00 (0.00) | −0.03 | Ag-C-O |
| MoS2-Ag2S2-NO | 21.41/0.93 | 1.15 | 0.41 (0.66) | −0.25 | Ag-N-O |
| MoS2-Au2S2-H2 | 5.102/0.22 | 0.03 | 0.01 (0.01) | 0.06 | Au-H2 |
| MoS2-Au2S2-O2 | 22.078/0.96 | 0.28 | 0.25 (0.25) | −0.71 | Au-O-O-Au |
| MoS2-Au2S2-N2 | 5.903/0.26 | 0.04 | 0.00 (0.00) | −0.02 | Au-N-N |
| MoS2-Au2S2-CO | 24.82/1.08 | 0.03 | 0.00 (0.00) | −0.05 | Au-C-O |
| MoS2-Au2S2-NO | 25.65/1.11 | 0.87 | 0.33 (0.36) | −0.37 | Au-N-O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutierrez-Rodriguez, J.; Castro, M.; Nieto-Jalil, J.M.; Medina, D.I.; Montes de Oca, S.; García-González, J.A.; Rangel-Cortes, E.; Miralrio, A. Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach. Int. J. Mol. Sci. 2023, 24, 10284. https://doi.org/10.3390/ijms241210284
Gutierrez-Rodriguez J, Castro M, Nieto-Jalil JM, Medina DI, Montes de Oca S, García-González JA, Rangel-Cortes E, Miralrio A. Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach. International Journal of Molecular Sciences. 2023; 24(12):10284. https://doi.org/10.3390/ijms241210284
Chicago/Turabian StyleGutierrez-Rodriguez, Josue, Miguel Castro, Jose Manuel Nieto-Jalil, Dora Iliana Medina, Saul Montes de Oca, José Andrés García-González, Eduardo Rangel-Cortes, and Alan Miralrio. 2023. "Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach" International Journal of Molecular Sciences 24, no. 12: 10284. https://doi.org/10.3390/ijms241210284
APA StyleGutierrez-Rodriguez, J., Castro, M., Nieto-Jalil, J. M., Medina, D. I., Montes de Oca, S., García-González, J. A., Rangel-Cortes, E., & Miralrio, A. (2023). Substitutional Coinage Metals as Promising Defects for Adsorption and Detection of Gases on MoS2 Monolayers: A Computational Approach. International Journal of Molecular Sciences, 24(12), 10284. https://doi.org/10.3390/ijms241210284






