Hif1α/Dhrs3a Pathway Participates in Lipid Droplet Accumulation via Retinol and Ppar-γ in Fish Hepatocytes
Abstract
1. Introduction
2. Results
2.1. LD Accumulation Is Accompanied by Increased Dhrs3 Expression
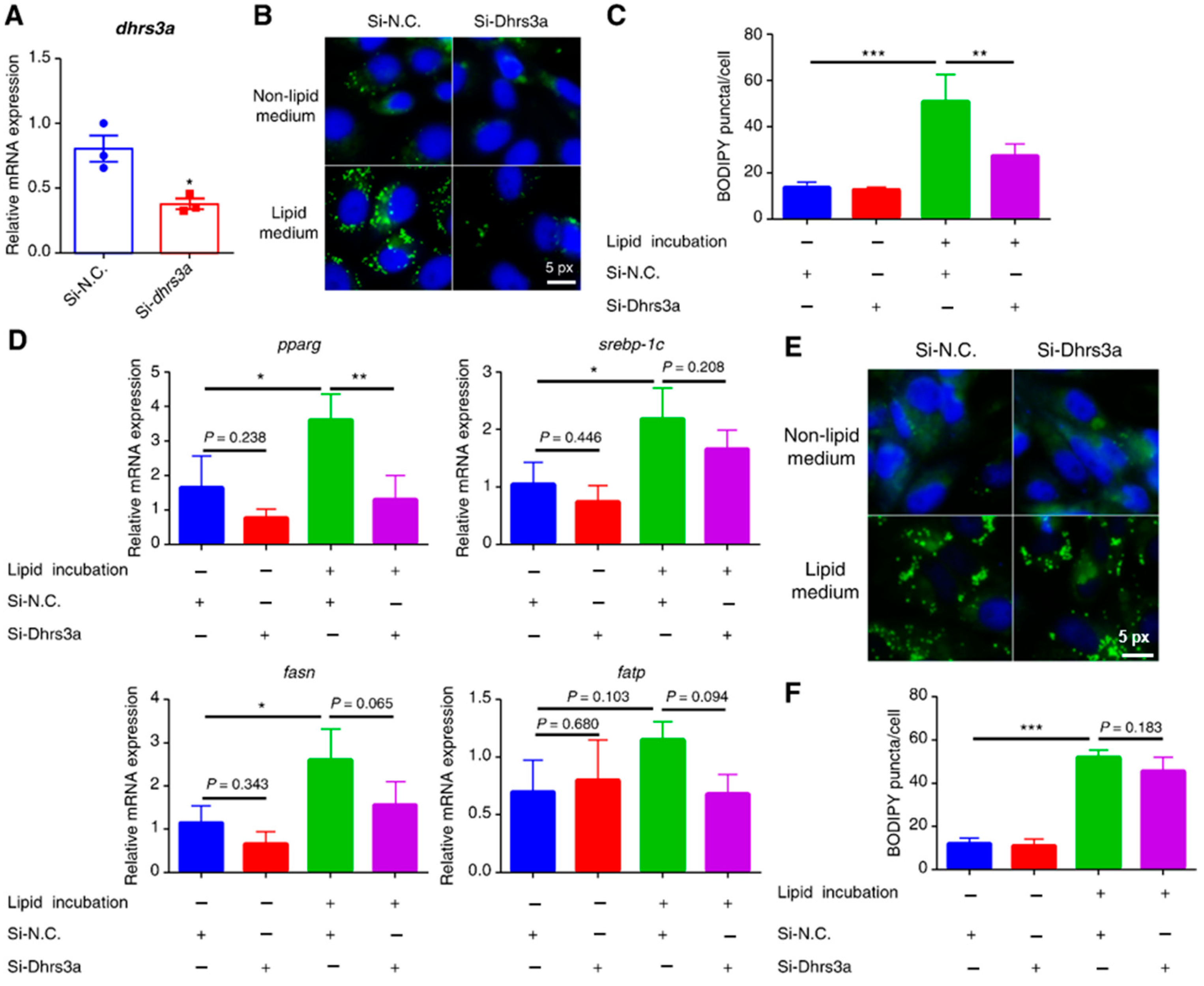
2.2. Dhrs3a Knockdown Delays LD Accumulation in Cells in a Lipid-Rich Medium
2.3. Exogenous Retinyl Acetate Maintains LD Accumulation
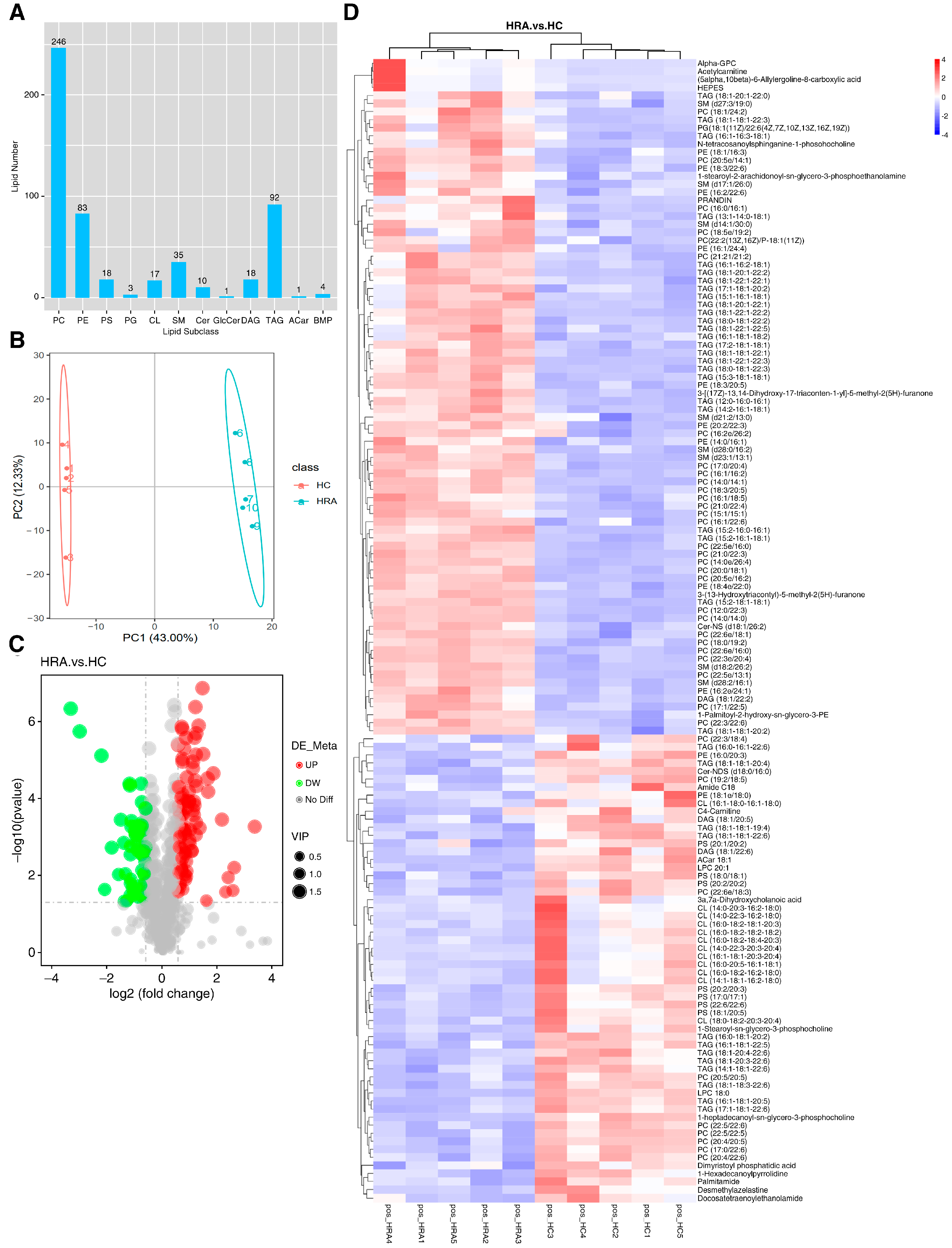
2.4. Exogenous Retinyl Acetate Alters the Lipidome of Zebrafish
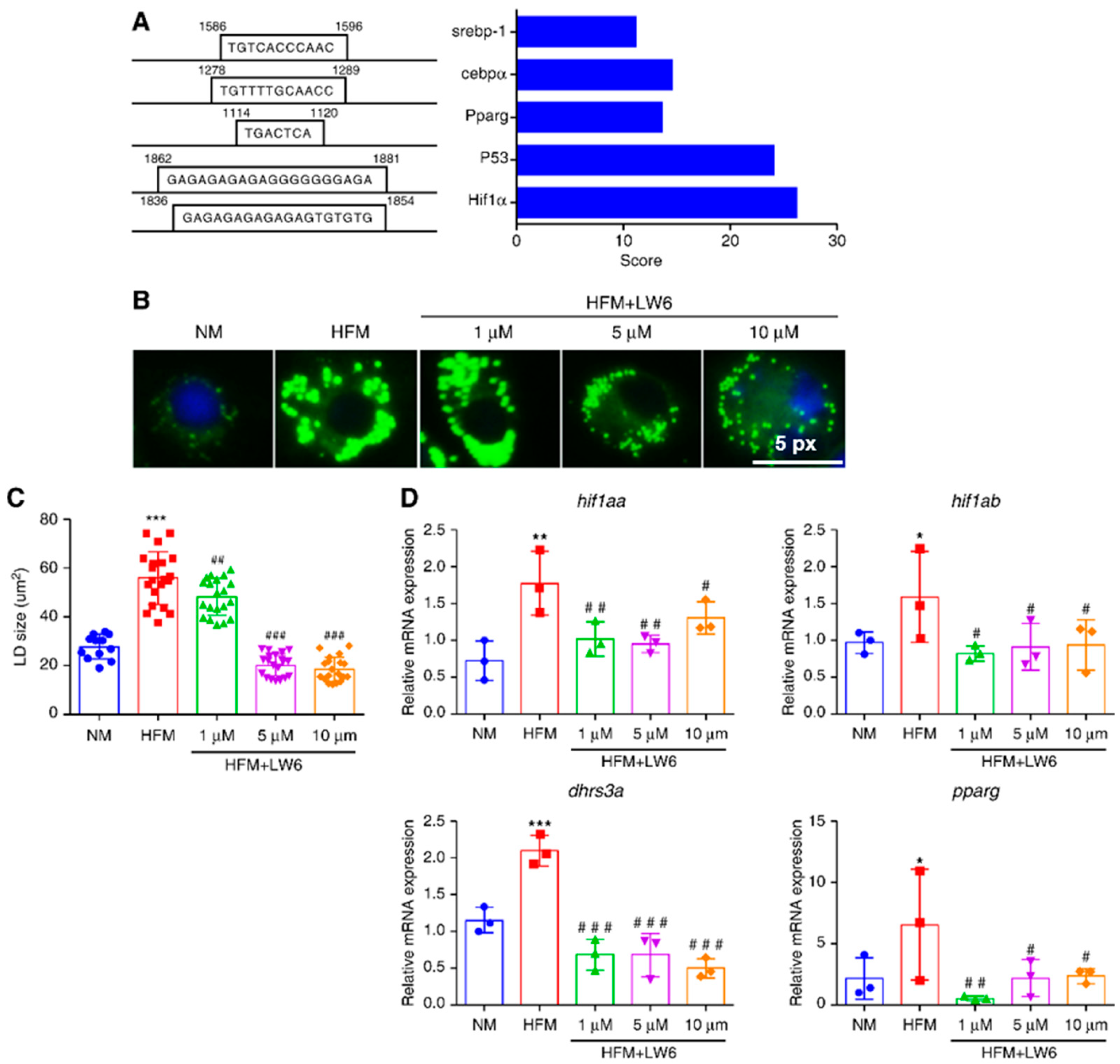
2.5. Hif1α Regulates dhrs3a and LD Accumulation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. RNA Interference and Treatment with an Inhibitor
4.3. LD Staining
4.4. TG and Retinol Content Assay
4.5. Immunofluorescence Staining
4.6. Transcriptome Analysis
4.7. qRT-PCR
4.8. Lipidomic Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, S. Chapter 3 Lipid droplets as organelles. In International Review of Cell and Molecular Biology; Galluzzi, L., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 337, pp. 83–110. [Google Scholar]
- Yang, L.; Ding, Y.; Chen, Y.; Zhang, S.; Huo, C.; Wang, Y.; Yu, J.; Zhang, P.; Na, H.; Zhang, H.; et al. The proteomics of lipid droplets: Structure, dynamics, and functions of the organelle conserved from bacteria to humans. J. Lipid Res. 2012, 53, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Kory, N.; Farese, R.V., Jr.; Walther, T.C. Targeting fat: Mechanisms of protein localization to lipid droplets. Trends Cell Biol. 2016, 26, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Henne, W.M.; Reese, M.L.; Goodman, J.M. The assembly of lipid droplets and their roles in challenged cells. EMBO J. 2018, 37, e98947. [Google Scholar] [CrossRef]
- Jackson, C.L. Lipid droplet biogenesis. Curr. Opin. Cell Biol. 2019, 59, 88–96. [Google Scholar] [CrossRef]
- Lundová, T.; Zemanová, L.; Malčeková, B.; Skarka, A.; Štambergová, H.; Havránková, J.; Šafr, M.; Wsól, V. Molecular and biochemical characterisation of human short-chain dehydrogenase/reductase member 3 (DHRS3). Chem. Biol. Interact. 2015, 234, 178–187. [Google Scholar] [CrossRef]
- Haeseleer, F.; Huang, J.; Lebioda, L.; Saari, J.C.; Palczewski, K. Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J. Biol. Chem. 1998, 273, 21790–21799. [Google Scholar] [CrossRef]
- Cerignoli, F.; Guo, X.; Cardinali, B.; Rinaldi, C.; Casaletto, J.; Frati, L.; Screpanti, I.; Gudas, L.J.; Gulino, A.; Thiele, C.J.; et al. retSDR1, a short-chain retinol dehydrogenase/reductase, is retinoic acid-inducible and frequently deleted in human neuroblastoma cell lines. Cancer Res. 2002, 62, 1196–1204. [Google Scholar]
- Feng, L.; Hernandez, R.E.; Waxman, J.S.; Yelon, D.; Moens, C.B. Dhrs3a regulates retinoic acid biosynthesis through a feedback inhibition mechanism. Dev. Biol. 2010, 338, 1–14. [Google Scholar] [CrossRef]
- Pataki, C.I.; Rodrigues, J.; Zhang, L.; Qian, J.; Efron, B.; Hastie, T.; Elias, J.E.; Levitt, M.; Kopito, R.R. Proteomic analysis of monolayer-integrated proteins on lipid droplets identifies amphipathic interfacial α-helical membrane anchors. Proc. Natl. Acad. Sci. USA 2018, 115, E8172–E8180. [Google Scholar] [CrossRef]
- Deisenroth, C.; Itahana, Y.; Tollini, L.; Jin, A.; Zhang, Y. p53-inducible DHRS3 is an endoplasmic reticulum protein associated with lipid droplet accumulation. J. Biol. Chem. 2011, 286, 28343–28356. [Google Scholar] [CrossRef]
- Mejhert, N.; Kuruvilla, L.; Gabriel, K.R.; Elliott, S.D.; Guie, M.A.; Wang, H.; Lai, Z.W.; Lane, E.A.; Christiano, R.; Danial, N.N.; et al. Partitioning of MLX-family transcription factors to lipid droplets regulates metabolic gene expression. Mol. Cell 2020, 77, 1251–1264.e9. [Google Scholar] [CrossRef]
- Semenza, G.L. Expression of hypoxia-inducible factor 1: Mechanisms and consequences. Biochem. Pharmacol. 2000, 59, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Wu, H.; Cordasic, N.; Oefner, P.; Dietel, B.; Thiele, C.; Weidemann, A.; Eckardt, K.U.; Warnecke, C. Hypoxia-inducible protein 2 Hig2/Hilpda mediates neutral lipid accumulation in macrophages and contributes to atherosclerosis in apolipoprotein E–deficient mice. FASEB J. 2017, 31, 4971–4984. [Google Scholar] [CrossRef]
- Wang, J.; Han, S.L.; Li, L.Y.; Lu, D.L.; Mchele Limbu, S.M.; Li, D.L.; Zhang, M.L.; Du, Z.Y. Lipophagy is essential for lipid metabolism in fish. Sci. Bull. 2018, 63, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ji, H.; Li, X.X.; Shi, X.C.; Du, Z.Y.; Chen, L.Q. Lipolytic enzymes involving lipolysis in Teleost: Synteny, structure, tissue distribution, and expression in grass carp (Ctenopharyngodon idella). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 198, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yang, Z.; Shi, X.C.; Ji, H.; Du, Z.Y.; Chen, L.Q. G0S2a1 (G0/G1 switch gene 2a1) is downregulated by TNF-α in grass carp (Ctenopharyngodon idellus) hepatocytes through PPARα inhibition. Gene 2018, 641, 1–7. [Google Scholar] [CrossRef]
- Liu, P.; Ji, H.; Li, C.; Chen, L.-Q.; Du, Z.-Y. Morphology, mitochondrial development and adipogenic-related genes expression during adipocytes differentiation in grass carp (Ctenopharyngodon idellus). Sci. Bull. 2015, 60, 1241–1251. [Google Scholar] [CrossRef]
- Liu, P.; Ji, H.; Li, C.; Tian, J.; Wang, Y.; Yu, P. Ontogenetic development of adipose tissue in grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 2015, 41, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Du, Y.; Yu, E.; Lei, C.; Xia, Y.; Jiang, P.; Li, H.; Zhang, K.; Li, Z.; Gong, W.; et al. Prostaglandin 2α promotes autophagy and mitochondrial energy production in fish hepatocytes. Cells 2022, 11, 1870. [Google Scholar] [CrossRef]
- Goo, Y.H.; Son, S.H.; Paul, A. Lipid droplet-associated hydrolase promotes lipid droplet fusion and enhances ATGL degradation and triglyceride accumulation. Sci. Rep. 2017, 7, 2743. [Google Scholar] [CrossRef] [PubMed]
- Moessinger, C.; Kuerschner, L.; Spandl, J.; Shevchenko, A.; Thiele, C. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J. Biol. Chem. 2011, 286, 21330–21339. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Napoli, J.L. The retinol dehydrogenase Rdh10 localizes to lipid droplets during acyl ester biosynthesis. J. Biol. Chem. 2013, 288, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, R.; Chen, Q.; Ross, A.C. DHRS3, a retinal reductase, is differentially regulated by retinoic acid and lipopolysaccharide-induced inflammation in THP-1 cells and rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G578–G588. [Google Scholar] [CrossRef]
- Lenhard, J.M. PPAR gamma/RXR as a molecular target for diabetes. Recept. Channels 2001, 7, 249–258. [Google Scholar]
- Ivashov, V.A.; Grillitsch, K.; Koefeler, H.; Leitner, E.; Baeumlisberger, D.; Karas, M.; Daum, G. Lipidome and proteome of lipid droplets from the methylotrophic yeast pichia pastoris. Biochim. Biophys. Acta 2013, 1831, 282–290. [Google Scholar] [CrossRef]
- Penno, A.; Hackenbroich, G.; Thiele, C. Phospholipids and lipid droplets. Biochim. Biophys. Acta 2013, 1831, 589–594. [Google Scholar] [CrossRef]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; De Benedictis, V.; Ruggiero, F.M.; Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta 2014, 1837, 408–417. [Google Scholar] [CrossRef]
- Billings, S.E.; Pierzchalski, K.; Butler Tjaden, N.E.B.; Pang, X.Y.; Trainor, P.A.; Kane, M.A.; Moise, A.R. The retinaldehyde reductase DHRS3 is essential for preventing the formation of excess retinoic acid during embryonic development. FASEB J. 2013, 27, 4877–4889. [Google Scholar] [CrossRef]
- Mylonis, I.; Simos, G.; Paraskeva, E. Hypoxia-inducible factors and the regulation of lipid metabolism. Cells 2019, 8, 214. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xu, H.; Wei, Y.; Liang, M. Effects of acute hypoxia on nutrient metabolism and physiological function in turbot, Scophthalmus maximus. Fish Physiol. Biochem. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Sun, J.L.; Gu, Y.; Yao, F.C.; Liang, Y.S.; Liu, Y.F.; Zhang, K.X.; Song, F.B.; Zhou, L.; Wang, Z.W.; et al. Hypoxia alters glucose and lipid metabolisms in golden pompano (Trachinotus blochii). Aquaculture 2023, 562, 738747. [Google Scholar] [CrossRef]
- Tian, J.-J.; Jin, Y.-Q.; Yu, E.-M.; Sun, J.-H.; Xia, Y.; Zhang, K.; Li, Z.; Gong, W.; Wang, G.; Xie, J. Farnesoid X receptor is an effective target for modulating lipid accumulation in grass carp, Ctenopharyngodon idella. Aquaculture 2021, 534, 736248. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Du, Y.; Wang, B.; Ji, M.; Li, H.; Xia, Y.; Zhang, K.; Li, Z.; Xie, W.; Gong, W.; et al. Hif1α/Dhrs3a Pathway Participates in Lipid Droplet Accumulation via Retinol and Ppar-γ in Fish Hepatocytes. Int. J. Mol. Sci. 2023, 24, 10236. https://doi.org/10.3390/ijms241210236
Tian J, Du Y, Wang B, Ji M, Li H, Xia Y, Zhang K, Li Z, Xie W, Gong W, et al. Hif1α/Dhrs3a Pathway Participates in Lipid Droplet Accumulation via Retinol and Ppar-γ in Fish Hepatocytes. International Journal of Molecular Sciences. 2023; 24(12):10236. https://doi.org/10.3390/ijms241210236
Chicago/Turabian StyleTian, Jingjing, Yihui Du, Binbin Wang, Mengmeng Ji, Hongyan Li, Yun Xia, Kai Zhang, Zhifei Li, Wenping Xie, Wangbao Gong, and et al. 2023. "Hif1α/Dhrs3a Pathway Participates in Lipid Droplet Accumulation via Retinol and Ppar-γ in Fish Hepatocytes" International Journal of Molecular Sciences 24, no. 12: 10236. https://doi.org/10.3390/ijms241210236
APA StyleTian, J., Du, Y., Wang, B., Ji, M., Li, H., Xia, Y., Zhang, K., Li, Z., Xie, W., Gong, W., Yu, E., Wang, G., & Xie, J. (2023). Hif1α/Dhrs3a Pathway Participates in Lipid Droplet Accumulation via Retinol and Ppar-γ in Fish Hepatocytes. International Journal of Molecular Sciences, 24(12), 10236. https://doi.org/10.3390/ijms241210236







