Unique Peptides of Cathelicidin-1 in the Early Detection of Mastitis—In Silico Analysis
Abstract
1. Introduction
1.1. Role of Cathelicidin-1 in Mastitis in Sheep
1.2. Unique Peptides and Core Unique Peptides
1.3. Hypothesis and Objectives of the Present Study
2. Results
2.1. Reference Protein Data
2.2. Core Unique Peptides of Cathelicidin-1
2.3. Composite Core Unique Peptides of Cathelicidin-1
2.4. Unique Peptides of Cathelicidin-1 in Tryptic Digest Peptides
2.5. Position of Core Unique Peptides of Cathelicidin-1 within its Three-Dimensional Structure
3. Discussion
3.1. Preamble-Cathelicidins in the Diagnosis of Mastitis in Sheep
3.2. Potential Usefulness of Unique Peptides
3.3. Application of the Methodology of Unique Peptides for Detection of Cathelicidin-1
3.4. Detection of Unique Peptides in Tryptic Digest Peptides
4. Materials and Methods
4.1. Reference Protein Data
4.2. Detection of Unique Peptides and Core Unique Peptides of Cathelicidin-1
4.3. Detection of Composite Core Unique Peptides of Cathelicidin-1
4.4. Detection of Unique Peptides of Cathelicidin-1 from Tryptic Digest Peptides
4.5. Position of Core Unique Peptides of Cathelicidin-1 within its Three-Dimensional Structure
4.6. Data Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority. Scientific opinion on the welfare risks related to the farming of sheep for wool, meat and milk production. EFSA J. 2014, 12, 3933–4060. [Google Scholar]
- Gelasakis, A.Ι.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.C.; Fthenakis, G.C. Mastitis in sheep—The last 10 years and the future of research. Vet. Microbiol. 2015, 181, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.C.; Jones, J.E.T. The effect of inoculation of coagulase-negative staphylococci into the ovine mammary gland. J. Comp. Pathol. 1990, 102, 211–219. [Google Scholar] [CrossRef]
- Katsafadou, A.I.; Politis, A.P.; Mavrogianni, V.S.; Barbagianni, M.S.; Vasileiou, N.G.C.; Fthenakis, G.C.; Fragkou, I.A. Mammary defences and immunity against mastitis in sheep. Animals 2019, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Pisanu, S.; Marogna, G.; Cubeddu, T.; Pagnozzi, D.; Cacciotto, C.; Campesi, F.; Schianchi, G.; Rocca, S.; Uzzau, S. Production and release of antimicrobial and immune defense proteins by mammary epithelial cells following Streptococcus uberis infection of sheep. Infect. Immun. 2013, 81, 3182–3197. [Google Scholar] [CrossRef]
- Treffers, C.; Chen, L.; Anderson, R.C.; Yu, P.L. Isolation and characterisation of antimicrobial peptides from deer neutrophils. Int. J. Antimicrob. Agents 2005, 26, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Pisanu, S.; Ghisaura, S.; Pagnozzi, D.; Marogna, G.; Tanca, A.; Biosa, G.; Cacciotto, C.; Alberti, A.; Pittau, M.; et al. Proteomics and pathway analyses of the milk fat globule in sheep naturally infected by Mycoplasma agalactiae provide indications of the in vivo response of the mammary epithelium to bacterial infection. Infect. Immun. 2011, 79, 3833–3845. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Tedde, V.; Dore, S.; Pisanu, S.; Puggioni, G.M.; Roggio, A.M.; Pagnozzi, D.; Lollai, S.; Cannas, E.A.; Uzzau, S. Evaluation of milk cathelicidin for detection of dairy sheep mastitis. J. Dairy Sci. 2016, 99, 6446–6456. [Google Scholar] [CrossRef]
- Addis, M.F.; Tedde, V.; Puggioni, G.M.; Pisanu, S.; Casula, A.; Locatelli, C.; Rota, N.; Bronzo, V.; Moroni, P.; Uzzau, S. Evaluation of milk cathelicidin for detection of bovine mastitis. J. Dairy Sci. 2016, 99, 8250–8258. [Google Scholar] [CrossRef] [PubMed]
- Katsafadou, A.I.; Tsangaris, G.T.; Vasileiou, N.G.C.; Ioannidi, K.S.; Anagnostopoulos, A.K.; Billinis, C.; Fragkou, I.A.; Papadopoulos, E.; Mavrogianni, V.S.; Michael, C.K.; et al. Detection of cathelicidin-1 in the milk as an early indicator of mastitis in ewes. Pathogens 2019, 8, 270. [Google Scholar] [CrossRef]
- Fragkou, I.A.; Boscos, C.M.; Fthenakis, G.C. Diagnosis of clinical or subclinical mastitis in ewes. Small Rumin. Res. 2014, 118, 86–92. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, Y.H. Whole-cell protein identification using the concept of unique peptides. Genom. Proteom. Bioinform. 2010, 8, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Alexandridou, A.; Tsangaris, G.T.; Vougas, K.; Nikita, K.; Spyrou, G. UniMaP: Finding unique mass and peptide signatures in the human proteome. Bioinformatics 2009, 25, 3035–3037. [Google Scholar] [CrossRef]
- Kontopodis, E.; Pierros, V.; Stravopodis, D.J.; Tsangaris, G.T. Prediction of SARS-CoV-2 Omicron variant immunogenicity, immune escape and pathogenicity, through the analysis of spike protein-specific core unique peptides. Vaccines 2022, 10, 357. [Google Scholar] [CrossRef]
- Pierros, V.; Kontopodis, E.; Stravopodis, D.J.; Tsangaris, G.T. Unique peptide signatures of SARS-CοV-2 virus against human proteome reveal variants’ immune escape and infectiveness. Heliyon 2022, 8, e09222. [Google Scholar] [CrossRef] [PubMed]
- UniProt. P54230 CTHL1_SHEEP. 2023. Available online: https://www.uniprot.org/uniprotkb/P54230/entry (accessed on 18 April 2023).
- UniProt. P22226 CTHL1_BOVIN. 2023. Available online: https://www.uniprot.org/uniprotkb/P22226/entry (accessed on 18 April 2023).
- AlphaFold Protein Structure Database. Cathelicidin-1 AlphaFold Structure Prediction. 2023. Available online: https://alphafold.ebi.ac.uk/entry/P54230?fbclid=IwAR0bPMzS62Q0j8hB-8Wr9n8f99ww29M8pkf0L_taYWprz8KrzG59GkaDSLU (accessed on 24 May 2023).
- AlphaFold Protein Structure Database. Cathelicidin-1 AlphaFold Structure Prediction. 2023. Available online: https://alphafold.ebi.ac.uk/entry/P22226?fbclid=IwAR31Qa_xJkxfjAI0sCZ9_Phar4DZv12w8WcqIJT3Fte3g4C4iFWfxYNF1sk (accessed on 24 May 2023).
- Swiss-Model. P54230 (CTHL1_SHEEP). 2023. Available online: https://swissmodel.expasy.org/repository/uniprot/P54230?csm=1690638C791B1736 (accessed on 19 April 2023).
- Swiss-Model. P22226 (CTHL1_BOVIN). 2023. Available online: https://swissmodel.expasy.org/repository/uniprot/P22226?csm=008CD7DC6CB91BF9 (accessed on 19 April 2023).
- Vasileiou, N.G.C.; Fthenakis, G.C.; Mavrogianni, V.S. Comparison of the efficacy of intramammary or injectable antibiotic administration against staphylococcal mastitis in ewes. Pathogens 2022, 11, 1164. [Google Scholar] [CrossRef]
- Lianou, D.T.; Petinaki, E.; Cripps, P.J.; Gougoulis, D.A.; Michael, C.K.; Tsilipounidaki, K.; Skoulakis, A.; Katsafadou, A.I.; Vasileiou, N.G.C.; Giannoulis, T.; et al. Antibiotic resistance of staphylococci from bulk-tank milk of sheep flocks: Prevalence, patterns, association with biofilm formation, effects on milk quality, and risk factors. Biology 2021, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Lianou, D.T.; Fthenakis, G.C. Use of antibiotics against bacterial infections on dairy sheep and goat farms: Patterns of usage and associations with health management and human resources. Antibiotics 2022, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.; Barbagianni, M.; Katsafadou, A.; Lianou, D.; Michael, C.; Vasileiou, N.; Mavrogianni, V. Update on diagnosis of mastitis in sheep. Reprod. Domest. Anim. 2022, 57, 27. [Google Scholar]
- Vasileiou, N.G.C.; Cripps, P.J.; Ioannidi, K.S.; Chatzopoulos, D.C.; Gougoulis, D.A.; Sarrou, S.; Orfanou, D.C.; Politis, A.P.; Gonzalez-Valerio, T.C.; Argyros, S.; et al. Extensive countrywide field investigation of subclinical mastitis in sheep in Greece. J. Dairy Sci. 2018, 101, 7297–7310. [Google Scholar] [CrossRef] [PubMed]
- Minns, D.; Smith, K.J.; Alessandrini, V.; Hardisty, G.; Melrose, L.; Jackson-Jones, L.; MacDonald, A.S.; Davidson, D.J.; Gwyer Findlay, E. The neutrophil antimicrobial peptide cathelicidin promotes Th17 differentiation. Nat. Commun. 2021, 12, 1285. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, T.; Cacciotto, C.; Pisanu, S.; Tedde, V.; Alberti, A.; Pittau, M.; Dore, S.; Cannas, A.; Uzzau, S.; Rocca, S.; et al. Cathelicidin production and release by mammary epithelial cells during infectious mastitis. Vet. Immunol. Immunopathol. 2017, 189, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Smolenski, G.A.; Wieliczko, R.J.; Pryor, S.M.; Broadhurst, M.K.; Wheeler, T.T.; Haigh, B.J. The abundance of milk cathelicidin proteins during bovine mastitis. Vet. Immunol. Immunopathol. 2011, 143, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Katsafadou, A.I.; Tsangaris, G.T.; Anagnostopoulos, A.K.; Billinis, C.; Barbagianni, M.S.; Vasileiou, N.G.C.; Spanos, S.A.; Mavrogianni, V.S.; Fthenakis, G.C. Differential quantitative proteomics study of experimental Mannheimia haemolytica mastitis in sheep. J. Proteom. 2019, 205, 103393. [Google Scholar] [CrossRef]
- Wang, P.; Wilson, S. Mass spectrometry-based protein identification by integrating de novo sequencing with database searching. Bioinformatics 2013, 14, 24. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Y.; Xu, W.; Dong, L.; Wang, Y.; Gao, B.; Li, G.; Zhang, W. Apolipoprotein A1-Unique Peptide as a diagnostic biomarker for acute ischemic stroke. Int. J. Mol. Sci. 2016, 17, 458. [Google Scholar] [CrossRef]
- Karlsson, R.; Thorsell, A.; Gomila, M.; Salvà-Serra, F.; Jakobsson, H.E.; Gonzales-Siles, L.; Jaén-Luchoro, D.; Skovbjerg, S.; Fuchs, J.; Karlsson, A.; et al. Discovery of species-unique peptide biomarkers of bacterial pathogens by tandem mass spectrometry-based proteotyping. Mol. Cell. Proteom. 2020, 19, 518–528. [Google Scholar] [CrossRef]
- Kesarwani, V.; Gupta, R.; Raju Vetukuri, R.R.; Kushwaha, S.K.; Gandhi, S. Identification of unique peptides for SARS-CoV-2 diagnostics and vaccine development by an in silico proteomics Approach. Front. Immunol. 2021, 12, 725240. [Google Scholar] [CrossRef]
- Zanetti, M.; Gennaro, R.; Romeo, D. Cathelicidins: A novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995, 374, 1–5. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Alberts, B.; Heald, R.; Johnson, A.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P.; Wilson, J.; Hunt, T. Molecular Biology of the Cell, 7th ed.; W. W. Norton & Company: New York, NY, USA, 2022; p. 1552. [Google Scholar]
- Yakimov, A.P.; Afanaseva, A.S.; Khodorkovskiy, M.A.; Petukhov, M.G. Design of stable α-helical peptides and thermostable proteins in biotechnology and biomedicine. Acta Nat. 2016, 8, 70–81. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.; Lee, H.; Lee, J.O. Application of antihelix antibodies in protein structure determination. Proc. Natl. Acad. Sci. USA 2019, 116, 17786–17791. [Google Scholar] [CrossRef] [PubMed]
- Koide, S. Repurposing off-the-shelf antihelix antibodies for enabling structural biology. Proc. Natl. Acad. Sci. USA 2019, 116, 17611–17613. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.B.; McConney, A.A.; Gallo, M.; Woods, A.L.; Senn, G.J.; Hamelin, D. An investigation of the effectiveness of concept mapping as an instructional tool. Sci. Educ. 1993, 77, 95–111. [Google Scholar] [CrossRef]
- Shehu, A.; Kavraki, L.E. Modeling structures and motions of loops in protein molecules. Entropy 2012, 14, 252–290. [Google Scholar] [CrossRef]
- Papaleo, E.; Saladino, G.; Lambrughi, M.; Lindorff-Larsen, K.; Gervasio, F.L.; Nussinov, R. The role of protein loops and linkers in conformational dynamics and allostery. Chem. Rev. 2016, 116, 6391–6423. [Google Scholar] [CrossRef]
- Richardson, J.S.; Richardson, D.C. Natural beta-sheet proteins use negative design to avoid edge-to-edge aggregation. Proc. Natl. Acad. Sci. USA 2002, 99, 2754–2759. [Google Scholar] [CrossRef]
- Cheng, P.N.; Pham, J.D.; Nowick, J.S. The supramolecular chemistry of β-sheets. J. Am. Chem. Soc. 2013, 135, 5477–5492. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Gatto, G.J., Jr.; Stryer, L. Biochemistry, 9th ed.; Freeman W.H.: New York, NY, USA, 2019; p. 1296. [Google Scholar]
- Di Vona, M.L.; Rossolini, G.M.; Sette, M. A Strategy based on loop analysis to develop peptide epitopes: Application to SARS-CoV-2 spike protein. Front. Mol. Biosci. 2021, 8, 658687. [Google Scholar] [CrossRef]
- Mavrogianni, V.S.; Menzies, P.I.; Fragkou, I.A.; Fthenakis, G.C. Principles of mastitis treatment in sheep and goats. Vet. Clin. North Am. Food Anim. Pract. 2011, 11, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, U.; Albaghdadi, H.; Luo, Y.; Arbabi, M.; Desvaux, C.; Veres, T.; Stanimirovic, D.; Abulrob, A. Molecular imaging of glioblastoma multiforme using anti-insulin-like growth factor-binding protein-7 single-domain antibodies. Br. J. Cancer 2010, 103, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Rakovich, T.Y.; Mahfoud, O.K.; Mohamed, B.M.; Prina-Mello, A.; Crosbie-Staunton, K.; Van Den Broeck, T.; De Kimpe, L.; Sukhanova, A.; Baty, D.; Rakovich, A.; et al. Highly sensitive single domain antibodyquantum dot conjugates for detection of HER2 biomarker in lung and breast cancer cells. ACS Nano 2014, 8, 5682–5695. [Google Scholar] [CrossRef]
- Zheng, F.; Put, S.; Bouwens, L.; Lahoutte, T.; Matthys, P.; Muyldermans, S.; De Baetselier, P.; Devoogdt, N.; Raes, G.; Schoonooghe, S. Molecular imaging with macrophage CRIg-targeting nanobodies for early and preclinical diagnosis in a mouse model of rheumatoid arthritis. J. Nucl. Med. 2014, 55, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, E.J.; Mariuzza, R.A. Molecular recognition in antibody-antigen complexes. Adv. Protein Chem. 2002, 61, 119–160. [Google Scholar]
- Mian, I.S.; Bradwell, A.R.; Olson, A.J. Structure, function and properties of antibody binding sites. J. Mol. Biol. 1991, 217, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Ofran, Y.; Schlessinger, A.; Rost, B. Automated identification of complementarity determining regions (CDRs) reveals peculiar characteristics of CDRs and B cell epitopes. J. Immunol. 2008, 181, 6230–6235. [Google Scholar] [CrossRef]
- Yu, C.M.; Peng, H.P.; Chen, I.C.; Lee, Y.C.; Chen, J.B.; Tsai, K.C.; Chen, C.T.; Chang, J.H.; Yang, E.W.; Hsu, P.C.; et al. Rationalization and design of the complementarity determining region sequences in an antibody-antigen recognition interface. PLoS ONE 2012, 7, e33340. [Google Scholar] [CrossRef]
- Laskay, U.A.; Lobas, A.A.; Srzentić, K.; Gorshkov, M.V.; Tsybin, Y.O. Proteome digestion specificity analysis for the rtional design of extended bottom-up and middle-down proteomics experiments. J. Proteome Res. 2013, 12, 5558–5569. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, 439–444. [Google Scholar] [CrossRef]
- Mariani, V.; Biasini, M.; Barbato, A.; Schwede, T. lDDT: A local superposition-free score for comparing protein structures and models using distance difference tests. Bioinformatics 2013, 29, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef] [PubMed]


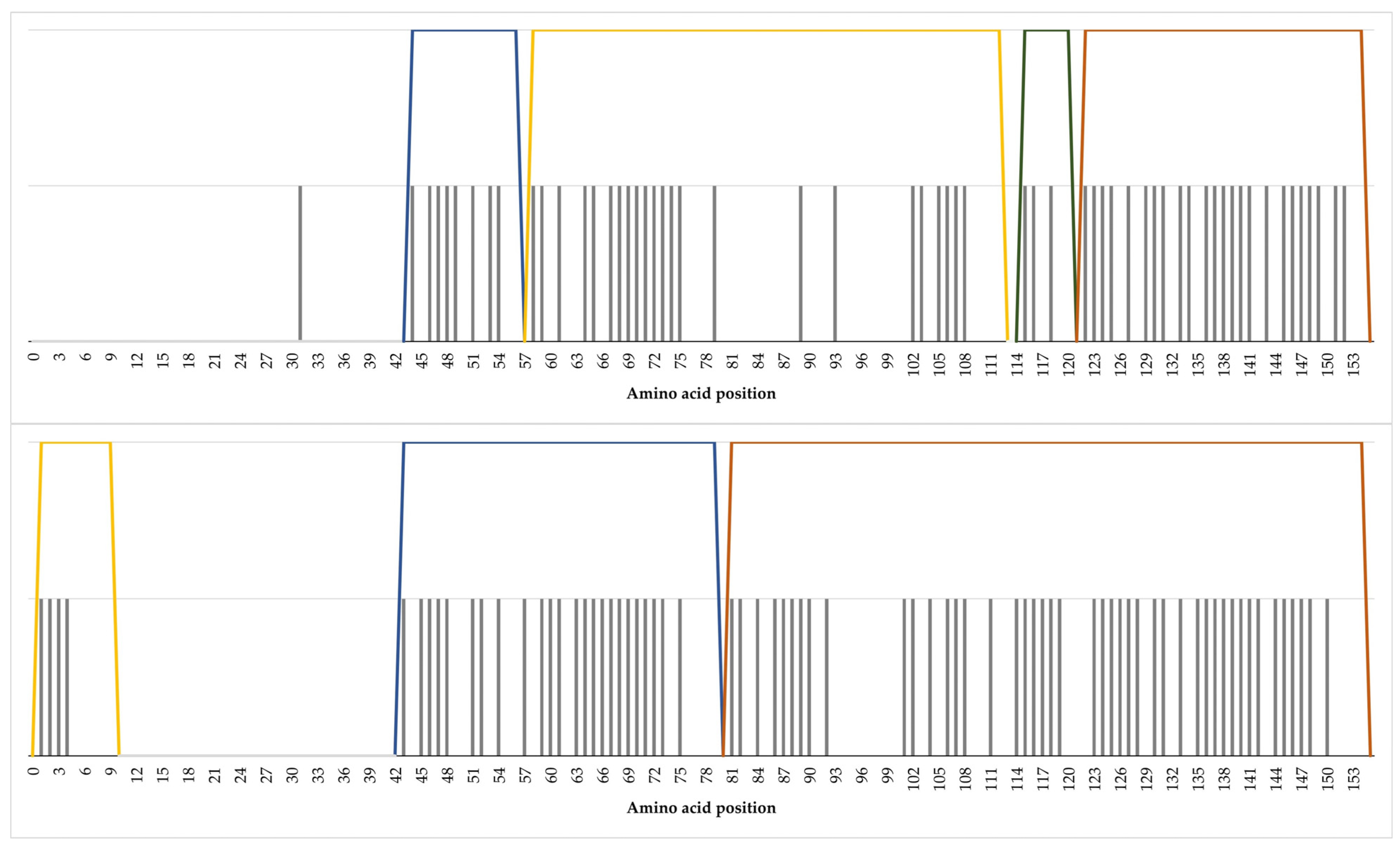
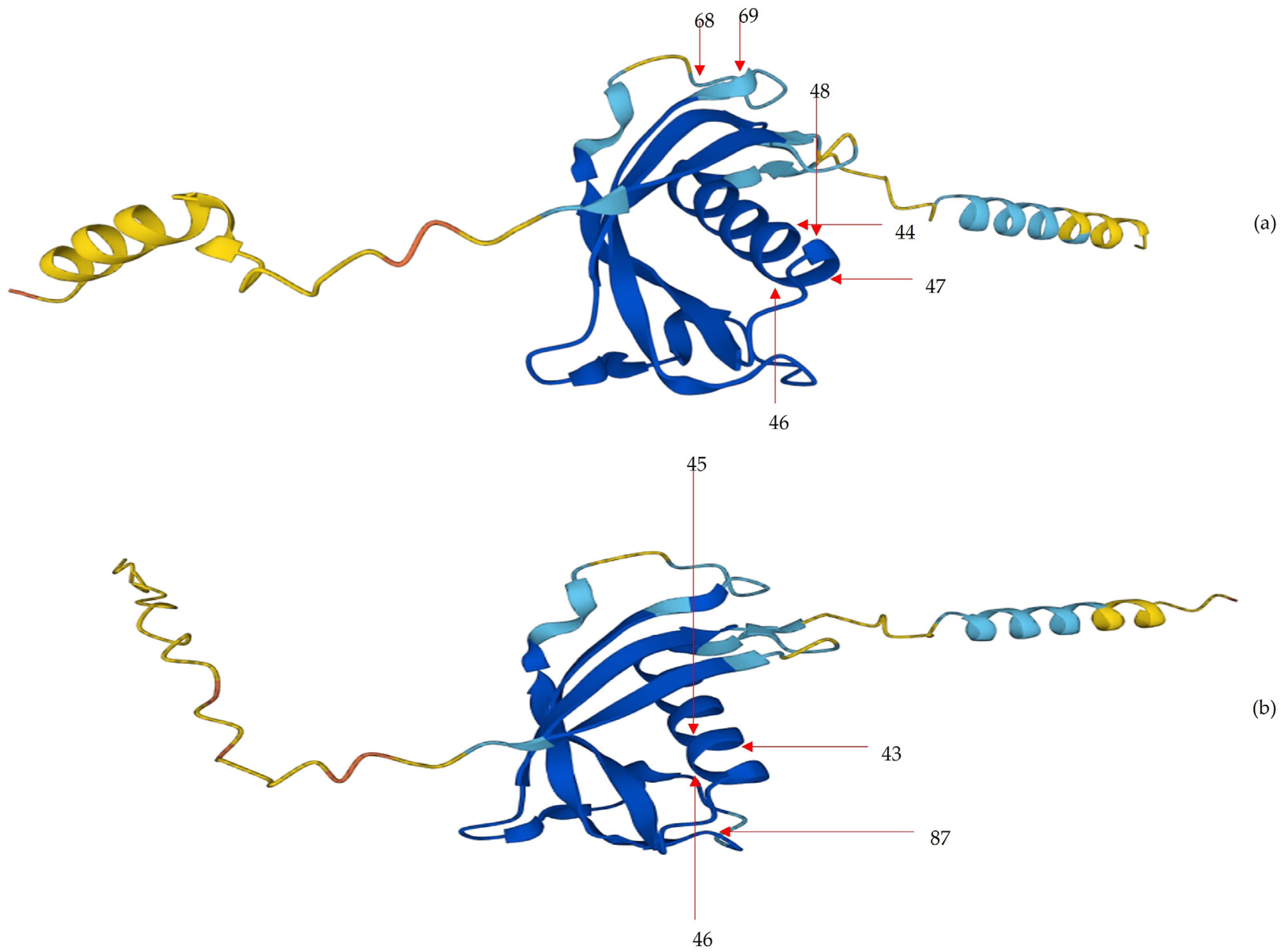
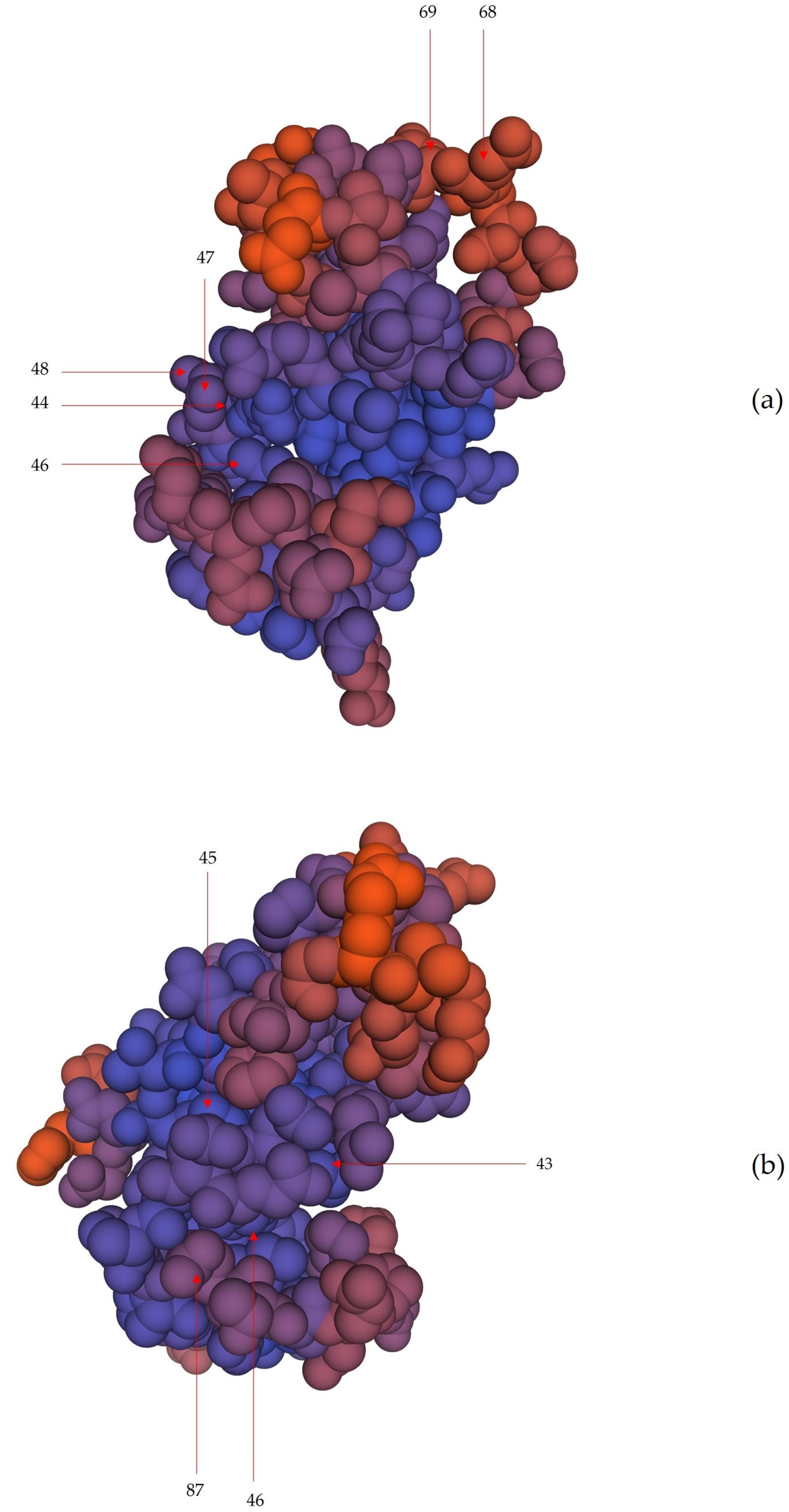
| Length of CUPs 1 | CUPs in Cathelicidin-1 of Sheep Origin (n) | CUPs in Cathelicidin-1 of Cattle Origin (n) |
|---|---|---|
| 4 | 26 | 0 |
| 5 | 29 | 32 |
| 6 | 0 | 35 |
| 7 | 2 | 5 |
| 8 | 0 | 0 |
| 9 | 0 | 0 |
| 10 | 0 | 0 |
| 11 | 1 | 0 |
| 12 | 2 | 0 |
| 13 | 0 | 1 |
| Median | 5 2 | 6 2 |
| Position 1 | Sequence of Core Unique Peptides | Length 2 |
|---|---|---|
| 59–63 | ELDQP | 5 |
| 75–79 | RVSFR | 5 |
| 107–111 | CEGTV | 5 |
| 116–120 | VRGNF | 5 |
| Position 1 | Cathelicidin-1 of Sheep Origin | Cathelicidin-1 of Cattle Origin | ||||
|---|---|---|---|---|---|---|
| Sequence of Composite Core Unique Peptides | Length 2 | No. of CUPs 3 | Sequence of Composite Core Unique Peptides | Length | No. of CUPs | |
| 1–9 | METPRASLS | 9 | 4 | |||
| 43–79 | DQLNEQSSEPNIYRLLE LDQPPQDDEDPDSPKR VSFR | 37 | 24 | |||
| 44–57 | QLNEQSSEPNIYRL | 14 | 8 | |||
| 58–112 | LELDQPPQDDEDPDSP KRVSFRVKETVCPRTT QQPPEQCDFKENGLLK RCEGTVT | 55 | 23 | |||
| 81–154 | KETVCSRTTQQPPEQC DFKENGLLKRCEGTVT LDQVRGNFDITCNNH QSIRITKQPWAPPQAA RLCRIVVIRVC | 74 | 45 | |||
| 115–121 | QVRGNFD | 7 | 3 | |||
| 122–155 | ITCNNHQSIRITKQP WAPPQAARICRII FLRVCR | 34 | 24 | |||
| Position 1 | Sequence of Unique Tryptic Digest Peptides | Length 2 | No. of CUPs 3 Included in Sheep Cathelicidin-1 | No. of CUPs Included in Cattle Cathelicidin-1 |
|---|---|---|---|---|
| 41–56 | AVDQLNEQSSEPNIYR | 16 | 7 | 6 |
| 57–74 | LLELDQPPQDDEDPDSPK | 18 | 9 | 11 |
| 88–99 | TTQQPPEQCDFK | 12 | 1 | 3 |
| 107–117 | CEGTVTLDQVR | 11 | 2 | 3 |
| 118–131 | GNFDITCNNHQSIR | 14 | 6 | 7 |
| 135–144 | QPWAPPQAAR | 10 | 5 | 5 |
| Position 1 | Sequence of CUPs 2 | Length 3 | Motifs of Protein Secondary Structure |
|---|---|---|---|
| Cathelicidin-1 of Sheep Origin | |||
| 44–48 | QLNEQ | 5 | 44–48: α-helix |
| 46–49 | NEQS | 4 | 46–48: α-helix, 49: loop |
| 47–51 | EQSSE | 5 | 47–48: α-helix, 49–51: loop |
| 48–52 | QSSEP | 5 | 48: α-helix, 49–52: loop |
| 68–71 | EDPD | 4 | 68–71: loop |
| 69–72 | DPDS | 4 | 69–72: loop |
| Cathelicidin-1 of Cattle Origin | |||
| 43–48 | DQLNEQ | 6 | 43–48: α-helix |
| 45–49 | LNEQS | 5 | 45–48: α-helix, 49: loop |
| 46–51 | NEQSSE | 6 | 46–48: α-helix, 49–51: loop |
| 87–91 | RTTQQ | 5 | 87–91: loop |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourganou, M.V.; Kontopodis, E.; Tsangaris, G.T.; Pierros, V.; Vasileiou, N.G.C.; Mavrogianni, V.S.; Fthenakis, G.C.; Katsafadou, A.I. Unique Peptides of Cathelicidin-1 in the Early Detection of Mastitis—In Silico Analysis. Int. J. Mol. Sci. 2023, 24, 10160. https://doi.org/10.3390/ijms241210160
Bourganou MV, Kontopodis E, Tsangaris GT, Pierros V, Vasileiou NGC, Mavrogianni VS, Fthenakis GC, Katsafadou AI. Unique Peptides of Cathelicidin-1 in the Early Detection of Mastitis—In Silico Analysis. International Journal of Molecular Sciences. 2023; 24(12):10160. https://doi.org/10.3390/ijms241210160
Chicago/Turabian StyleBourganou, Maria V., Evangelos Kontopodis, George Th. Tsangaris, Vasileios Pierros, Natalia G. C. Vasileiou, Vasia S. Mavrogianni, George C. Fthenakis, and Angeliki I. Katsafadou. 2023. "Unique Peptides of Cathelicidin-1 in the Early Detection of Mastitis—In Silico Analysis" International Journal of Molecular Sciences 24, no. 12: 10160. https://doi.org/10.3390/ijms241210160
APA StyleBourganou, M. V., Kontopodis, E., Tsangaris, G. T., Pierros, V., Vasileiou, N. G. C., Mavrogianni, V. S., Fthenakis, G. C., & Katsafadou, A. I. (2023). Unique Peptides of Cathelicidin-1 in the Early Detection of Mastitis—In Silico Analysis. International Journal of Molecular Sciences, 24(12), 10160. https://doi.org/10.3390/ijms241210160








