Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer
Abstract
1. Introduction
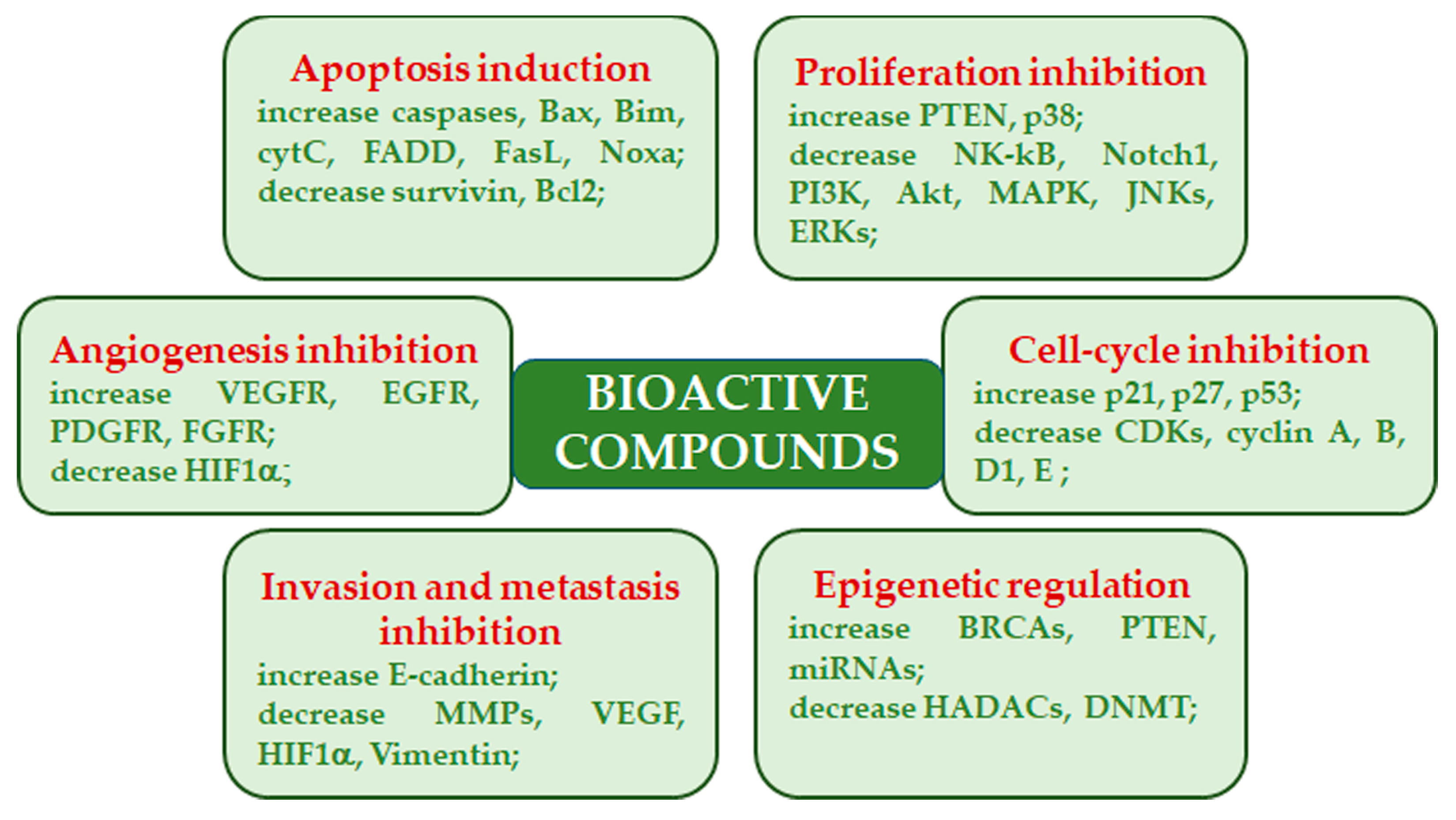
2. Bioactive Compounds in Medicinal Mushrooms and Their Mechanisms of Action
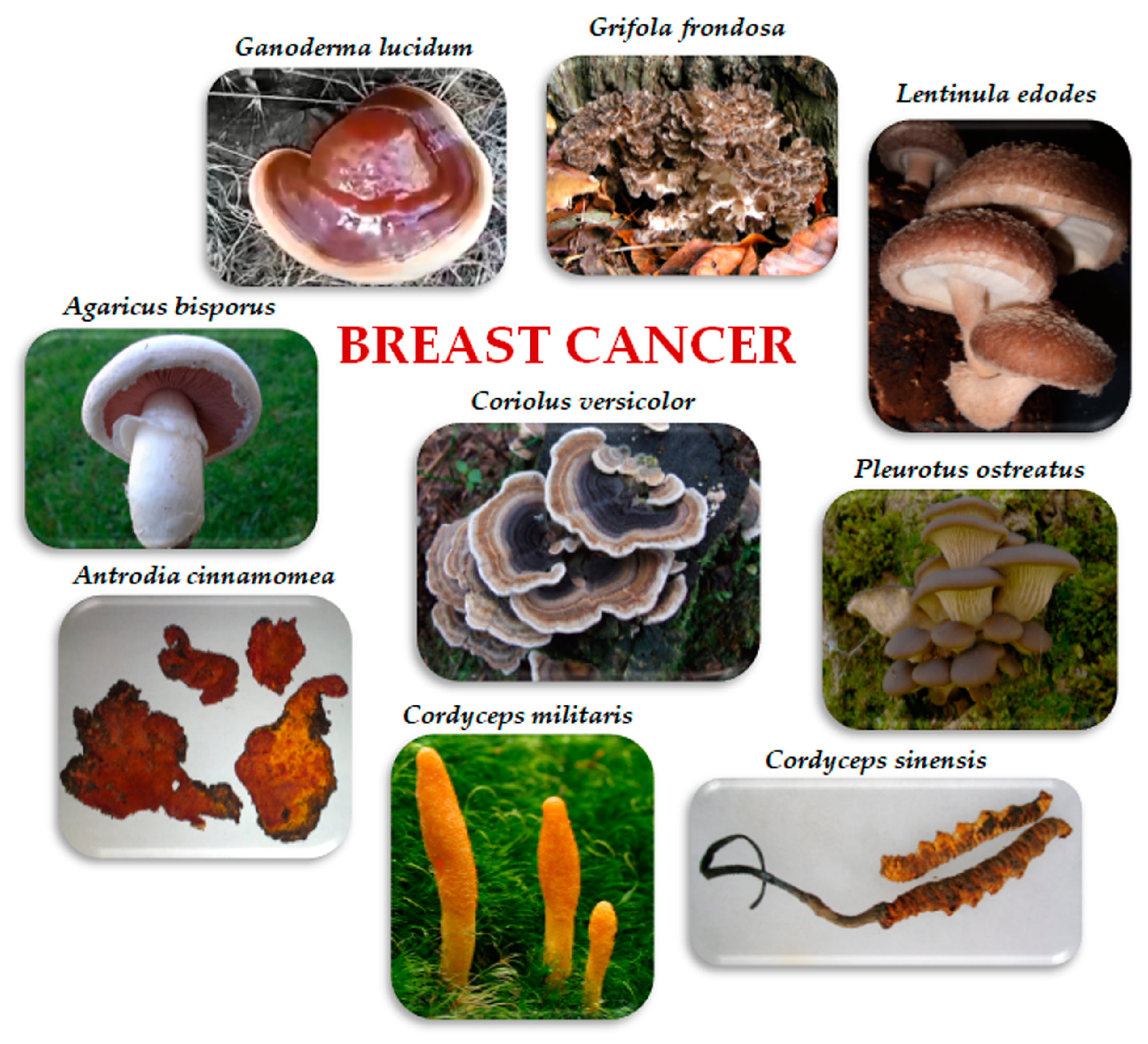
3. Edible/Medicinal Mushrooms in Breast Cancer
3.1. Agaricus bisporus (J.E. Lange) Imbach
3.2. Antrodia cinnamomea T.T. Chang and W.N. Chou
3.3. Cordyceps sinensis (Berk.) Sacc. and Cordyceps militaris (L.) Fr.
3.4. Coriolus versicolor (L.) Quél.
3.5. Ganoderma lucidum (Curtis) P. Karst. (Reishi)
3.6. Grifola frondosa (Dicks.) Gray (Maitake)
3.7. Lentinula edodes (Berk.) Pegler (Shitake)
3.8. Pleurotus ostreatus (Jacq.) P. Kumm.
| Species | Main Bioactive Constituents | Mechanisms |
|---|---|---|
| Agaricus bisporum | Polysaccharides (ABP-1 and ABP-2 fractions), in particular, β-glucans (β-(1→6)-d-glucan, B16), lectins, amino acids, unsaturated fatty acids (linoleic and linolenic acids), vitamin B, vitamin C, sterols, phenolic and indole compounds, ergosterol, flavonoids, ergocalciferol, ergosterol | Inhibition of cell proliferation, suppression of tumor growth in nude mice xenografts; induction of macrophages polarization towards M1 phenotype and production of Il-6, IL-1 β, TNF-α, CoX-2; induction of nitric oxide, activation of NF-κB and cell growth inhibition, probably due to the activity on macrophages; inhibition of proteins synthesis; lectins induce cytotoxicity, apoptosis, and immune system modulation [33,47,48,49,50,51] |
| Antrodia cinnamomea | Polysaccharides, terpenoids (ergostane, lanostane), lignans, glycoproteins, benzene derivatives, ubiquinone derivatives, maleic and succinil acids derivatives, Anticin A, Antrocin C, Antcin K, antcin C, antcin B | Induction of apoptosis, suppression of mRNA expression of S-phase kinase-associated protein 2 (skp2); decrease of urokinase plasminogen activator (uPA) activity, uPA receptor (uPAR), vascular endothelial growth factor (VEGF), and MMP-9 and MMP-2; inhibition of TGF-β1-induced migration arrest epithelial to mesenchymal transition (EMT); suppression of the ERK1/2, p38, and JNK1/2 phosphorylation; inhibition of Akt/mTOR and NF-κB pathways; apoptosis induction, cell cycle arrest, antimetastatic effect, dysfunction of mitochondrial caspase-3/-9 activation, cytochrome c release, degradation of PARP, and Bcl2/Bax dysregulation; HDAC inhibition, autophagy induction (LC3-II, p62, and FOX1 increase) [31,53,57,58,59,60,61,62,126]; proliferation inhibition related to the arrest of cells at the G1 phase and induction of autophagy; stress of the endoplasmic reticulum; reduction of tumor size [62] |
| Cordyceps sinensis and Cordyceps militaris | Cordycepin (3-deoxyadenosine), ergosterol, mannitol, modifies nucleosides | Induction of apoptosis by promoting expression and translocation of Bax to mitochondria and decreasing Bcl2 levels by releasing cytochrome C, activating p53, caspase-9, caspase 3, caspase-8; inhibition of cell growth, migration, and invasion, through reduction of the EMT (TWIST1, SLUG, SNAIL1, ZEB reduction, N-cadherin downregulation, E-cadherin upregulation); inhibition of migration; antiproliferative activity through induction of apoptotic cell death; LDH release, PARP increase, ROS production, inhibition of AKT activation and PI3K/Akt; increased level of Cu/Zn superoxide dismutase in cancer cells; induction of autophagy, DNA damage, and targeting of cancer stem cells [58,63,65]; decrease in tumor weight and size; reduction of the number of metastasis; increase survival; increased expression levels of cleaved PARP, cleaved caspase-3, cleaved caspase-8, and Bax [63,66,67] |
| Coriolus versicolor | Protein-bound polysaccharides (polysaccharide peptide, PSP, and glycoprotein PSK, Krestin), terpenes, proteins, peptides, amino acids, purpurins | Suppression of cell proliferation through apoptotic cell death induction, upregulation of p53, and downregulation of Bcl-2; NK cell activation, p53, and Bcl-2 downregulation; inhibition of migration (MMP9 activity and protein levels downregulation); cytotoxicity via necroptosis activated through the TNF-α/TNFR1 pathway stimulation [77,78,79]; suppression of cancer cell proliferation, reduction of tumor weight and antimetastatic effect, simultaneously protecting bones against breast cancer-induced osteolysis; migration and invasion inhibition; immunomodulatory (increase IL-2, 6, 12 TNF-α, INF-γ, histamine, prostaglandin E) and antimigratory effects [80,81,82,126] |
| Ganoderma lucidum | Polysaccharides (α-1,3, β-1,3 and β-1,6-D-glucans, ganoderan), triterpenes, ganoderic acids, ganodermic acid, ganodermic alcohols, lucidones, lucinedic acid, ergosterol, 5,6-dehydroergosterol, ergosterol peroxide, and palmitic acid | Inhibitory effect against Akt phosphorylation on Ser473 and downregulation of Akt expression, inhibition of NF-κB, also related to estrogen receptors, cyclin D1, and subsequently cdk4 [126]; suppression of adhesion, migration, and invasion of cancer cells, down-regulation of oncogene c-myc expression and secretion of uPA and inhibition of MMP2 and MMP9 [92,98]; apoptosis induction through downregulation of cyclin F, Bcl-2, Bcl-xL and upregulation of Bax and caspase-9 levels [91,94,95]; G1 phase arrest, apoptosis induction via caspase 3/7 activation, and PARP cleavage [89]; inhibition of tumor growth and migration via inhibition of Wnt/β-catenin signaling; suppression of cancer cell growth through apoptosis induction via mitochondria-mediated pathway; effects on protein expression of E-cadherin, mammalian target of rapamycin (mTOR), human eukaryotic translation initiation factor 4G (eIF4G), and p70 ribosomal protein S6 kinase (p70S6K) and activity of extracellular regulated kinase (ERK 1/2), reduction in tumor size and weight; downregulation of immune checkpoints; effects on cancer stem cells [91,99,100,106,126,127]; reduction in incidence of mammary tumors [96] |
| Grifola Frondosa | β-glucans and α-glucan (D-fraction, X-fraction, Grifolan, MZ-fraction, and MT-α-glucan), proteins, carbohydrates, ergocalciferol, minerals | Apoptosis induction through the release of CytC from mitochondria, alterations in genes involved in cell proliferation and invasion; upregulation of E-cadherin protein levels, promotion of cell adhesion, downregulation of cell motility and MMP2 and MMP9; decrease in β-catenin levels; modulation of Bax/Bcl2 ratio, affecting the pro-survival pathways related to PI3K/Akt and ERK [58,109]; immunomodulatory effects on macrophages, NK and T cells; decrease in metastasis; inhibition of carcinogenesis, angiogenesis, and cancer invasiveness; prolonged survival [58,109] |
| Lentinula edodes | β-glucans (lentinan), phenolic compounds, ergothioneine, sterols (ergosterol), eritadenine, peptides (lenthionine) | Induction of apoptosis associated with mitochondrial membrane potential decrease and decreased cdk4 and cyclin D1 resulting in cell cycle arrest; increased p21, p53, and Bax levels; inhibition of migration, autophagy induction [115,116,117,126]; reduction in tumor growth through suppression of cell proliferation and apoptosis promotion; inhibition of multiple pathways (PI3K-Akt-mTOR, ERK, p53) [118] |
| Pleurotus ostreatus | α-glucans, β-glucans, lentanin, lipopolisaccharides, resveratrol, concavallin A, mevinolin, ergosterol | Cell growth inhibition related to cell cycle arrest at the G0/G1 phase, upregulation of the p21, p53, p27, and p19 genes and downregulation of E2f transcription factor 1, PCNA, CDK4, CDK6, and transcription factor DP-1; induction of oxidative stress and apoptotic cell death due to the upregulation of p53 and Bax, downregulation of Bcl2, and increase in caspase 3/7 activity; increased cytotoxic activity of natural killer cells; inhibition of angiogenesis and metastasis by the inhibition of MMP2 and MMP9 expression; downregulation of VEGF [58,121,124]; decrease in tumor volume and increased body weight; decrease in tumor incidence, volume, and metastasis [121,124,125] |
4. Human Studies
4.1. Studies on Dietary Consumption of Edible Mushrooms and Breast Cancer Risk
4.2. Effect of Fungal Extracts on Breast Cancer: Clinical Studies and Meta-Analyses
5. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Porter, P. “Westernizing” Women’s Risks? Breast Cancer in Lower-Income Countries. N. Engl. J. Med. 2008, 358, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Agostinetto, E.; Gligorov, J.; Piccart, M. Systemic Therapy for Early-Stage Breast Cancer: Learning from the Past to Build the Future. Nat. Rev. Clin. Oncol. 2022, 19, 763–774. [Google Scholar] [CrossRef]
- Wu, H.-C.; Do, C.; Andrulis, I.L.; John, E.M.; Daly, M.B.; Buys, S.S.; Chung, W.K.; Knight, J.A.; Bradbury, A.R.; Keegan, T.H.M.; et al. Breast Cancer Family History and Allele-Specific DNA Methylation in the Legacy Girls Study. Epigenetics 2018, 13, 240–250. [Google Scholar] [CrossRef]
- Shiovitz, S.; Korde, L.A. Genetics of Breast Cancer: A Topic in Evolution. Ann. Oncol. 2015, 26, 1291–1299. [Google Scholar] [CrossRef]
- Shahbandi, A.; Nguyen, H.D.; Jackson, J.G. TP53 Mutations and Outcomes in Breast Cancer: Reading beyond the Headlines. Trends Cancer 2020, 6, 98–110. [Google Scholar] [CrossRef]
- Corso, G.; Intra, M.; Trentin, C.; Veronesi, P.; Galimberti, V. CDH1 Germline Mutations and Hereditary Lobular Breast Cancer. Fam. Cancer 2016, 15, 215–219. [Google Scholar] [CrossRef]
- Kechagioglou, P.; Papi, R.M.; Provatopoulou, X.; Kalogera, E.; Papadimitriou, E.; Grigoropoulos, P.; Nonni, A.; Zografos, G.; Kyriakidis, D.A.; Gounaris, A. Tumor Suppressor PTEN in Breast Cancer: Heterozygosity, Mutations and Protein Expression. Anticancer Res. 2014, 34, 1387–1400. [Google Scholar]
- Chen, J.; Lindblom, A. Germline Mutation Screening of the STK11/LKB1 Gene in Familial Breast Cancer with LOH on 19p. Clin. Genet. 2000, 57, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Lesueur, F.; Nguyen-Dumont, T.; Pertesi, M.; Odefrey, F.; Hammet, F.; Neuhausen, S.L.; John, E.M.; Andrulis, I.L.; Terry, M.B.; et al. Rare Mutations in XRCC2 Increase the Risk of Breast Cancer. Am. J. Hum. Genet. 2012, 90, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Rachdaoui, N.; Sarkar, D.K. Effects of Alcohol on the Endocrine System. Endocrinol. Metab. Clin. N. Am. 2013, 42, 593–615. [Google Scholar] [CrossRef]
- Dandamudi, A.; Tommie, J.; Nommsen-Rivers, L.; Couch, S. Dietary Patterns and Breast Cancer Risk: A Systematic Review. Anticancer Res. 2018, 38, 3209–3222. [Google Scholar] [CrossRef]
- Kolb, R.; Zhang, W. Obesity and Breast Cancer: A Case of Inflamed Adipose Tissue. Cancers 2020, 12, 1686. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, A.; Barati-Boldaji, R.; Soltani, S.; Mohammadipoor, N.; Esmaeilinezhad, Z.; Clark, C.C.T.; Babajafari, S.; Akbarzadeh, M. Intake of Various Food Groups and Risk of Breast Cancer: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Adv. Nutr. 2021, 12, 809–849. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M. Diet and Risk of Breast Cancer. Wspolczesna Onkol. 2016, 20, 13–19. [Google Scholar] [CrossRef]
- Miglietta, F.; Bottosso, M.; Griguolo, G.; Dieci, M.V.; Guarneri, V. Major Advancements in Metastatic Breast Cancer Treatment: When Expanding Options Means Prolonging Survival. ESMO Open 2022, 7, 100409. [Google Scholar] [CrossRef]
- Van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef]
- Berretta, M.; Dal Lago, L.; Tinazzi, M.; Ronchi, A.; La Rocca, G.; Montella, L.; Di Francia, R.; Facchini, B.A.; Bignucolo, A.; Montopoli, M. Evaluation of Concomitant Use of Anticancer Drugs and Herbal Products: From Interactions to Synergic Activity. Cancers 2022, 14, 5203. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.-J.J.; Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V.; Harnett, J.E. Interactions between Natural Products and Cancer Treatments: Underlying Mechanisms and Clinical Importance. Cancer Chemother. Pharmacol. 2023, 91, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-Glucans from Grifola Frondosa and Ganoderma Lucidum in Breast Cancer: An Example of Complementary and Integrative Medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The Chemistry, Pharmacology and Therapeutic Potential of the Edible Mushroom Dictyophora indusiata (Vent Ex. Pers.) Fischer (Synn. Phallus indusiatus). Biomedicines 2019, 7, 98. [Google Scholar] [CrossRef]
- Jiang, J.; Sliva, D. Novel Medicinal Mushroom Blend Suppresses Growth and Invasiveness of Human Breast Cancer Cells. Int. J. Oncol. 2010, 37, 1529–1536. [Google Scholar] [CrossRef]
- Joseph, T.P.; Chanda, W.; Padhiar, A.A.; Batool, S.; LiQun, S.; Zhong, M.T.; Huang, M. A Preclinical Evaluation of the Antitumor Activities of Edible and Medicinal Mushrooms: A Molecular Insight. Integr. Cancer Ther. 2018, 17, 200–209. [Google Scholar] [CrossRef]
- Dowaraka-Persad, B.; Neergheen, V.S. Mushroom-Derived Compounds as Metabolic Modulators in Cancer. Molecules 2023, 28, 1441. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Medicinal Mushrooms: Clinical Perspective and Challenges. Drug Discov. Today 2022, 27, 636–651. [Google Scholar] [CrossRef]
- Panda, S.K.; Sahoo, G.; Swain, S.S.; Luyten, W. Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery. Pharmaceuticals 2022, 15, 176. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal Mushroom Science: Current Perspectives, Advances, Evidences, and Challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Popović, V.; Živković, J.; Davidović, S.; Stevanović, M.; Stojković, D. Mycotherapy of Cancer: An Update on Cytotoxic and Antitumor Activities of Mushrooms, Bioactive Principles and Molecular Mechanisms of Their Action. Curr. Top. Med. Chem. 2013, 13, 2791–2806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Chawla, P.; Kaur, S.; Najda, A.; Fogarasi, M.; Fogarasi, S. Bioactives from Mushroom: Health Attributes and Food Industry Applications. Materials 2021, 14, 7640. [Google Scholar] [CrossRef]
- Pathak, M.P.; Pathak, K.; Saikia, R.; Gogoi, U.; Ahmad, M.Z.; Patowary, P.; Das, A. Immunomodulatory Effect of Mushrooms and Their Bioactive Compounds in Cancer: A Comprehensive Review. Biomed. Pharmacother. 2022, 149, 112901. [Google Scholar] [CrossRef]
- Elkhateeb, W.A. What Medicinal Mushroom Can Do? Chem. Res. J. 2020, 5, 106–118. [Google Scholar]
- Demain, A.L.; Vaishnav, P. Natural Products for Cancer Chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Figueiredo, L.; Régis, W.C.B. Medicinal Mushrooms in Adjuvant Cancer Therapies: An Approach to Anticancer Effects and Presumed Mechanisms of Action. Nutrire 2017, 42, 28. [Google Scholar] [CrossRef]
- Hyder, M.S.; Dutta, S.D. Mushroom-Derived Polysaccharides as Antitumor and Anticancer Agent: A Concise Review. Biocatal. Agric. Biotechnol. 2021, 35, 102085. [Google Scholar] [CrossRef]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet and Risk of Breast, Endometrial and Ovarian Cancer: UK Women’s Cohort Study. Br. J. Nutr. 2019, 122, 564–574. [Google Scholar] [CrossRef]
- Ba, D.M.; Ssentongo, P.; Beelman, R.B.; Muscat, J.; Gao, X.; Richie, J.P. Higher Mushroom Consumption Is Associated with Lower Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, L.; Chen, W.; Zhu, B.; Shen, N.; Ke, J.; Lou, J.; Song, R.; Zhong, R.; Miao, X. Dietary Mushroom Intake May Reduce the Risk of Breast Cancer: Evidence from a Meta-Analysis of Observational Studies. PLoS ONE 2014, 9, e93437. [Google Scholar] [CrossRef] [PubMed]
- Koyyalamudi, S.R.; Jeong, S.-C.; Song, C.-H.; Cho, K.Y.; Pang, G. Vitamin D2 Formation and Bioavailability from Agaricus Bisporus Button Mushrooms Treated with Ultraviolet Irradiation. J. Agric. Food Chem. 2009, 57, 3351–3355. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Muszyńska, B.; Kała, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and Biological Properties of Agaricus Bisporus Fruiting Bodies—A Review. Polish J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef]
- Adams, L.S.; Phung, S.; Wu, X.; Ki, L.; Chen, S. White Button Mushroom (Agaricus Bisporus) Exhibits Antiproliferative and Proapoptotic Properties and Inhibits Prostate Tumor Growth in Athymic Mice. Nutr. Cancer 2008, 60, 744–756. [Google Scholar] [CrossRef]
- Chen, S.; Oh, S.-R.; Phung, S.; Hur, G.; Ye, J.J.; Kwok, S.L.; Shrode, G.E.; Belury, M.; Adams, L.S.; Williams, D. Anti-Aromatase Activity of Phytochemicals in White Button Mushrooms (Agaricus Bisporus). Cancer Res. 2006, 66, 12026–12034. [Google Scholar] [CrossRef]
- Rutckeviski, R.; Corso, C.R.; Román-Ochoa, Y.; Cipriani, T.R.; Centa, A.; Smiderle, F.R. Agaricus Bisporus β-(1 → 6)-D-Glucan Induces M1 Phenotype on Macrophages and Increases Sensitivity to Doxorubicin of Triple Negative Breast Cancer Cells. Carbohydr. Polym. 2022, 278, 118917. [Google Scholar] [CrossRef]
- Jeong, S.C.; Koyyalamudi, S.R.; Jeong, Y.T.; Song, C.H.; Pang, G. Macrophage Immunomodulating and Antitumor Activities of Polysaccharides Isolated from Agaricus Bisporus White Button Mushrooms. J. Med. Food 2012, 15, 58–65. [Google Scholar] [CrossRef]
- Novaes, M.R.C.G.; Valadares, F.; Reis, M.C.; Gonçalves, D.R.; Menezes, M.C. The Effects of Dietary Supplementation with Agaricales Mushrooms and Other Medicinal Fungi on Breast Cancer: Evidence-Based Medicine. Clinics 2011, 66, 2133–2139. [Google Scholar] [CrossRef]
- Rachmawati, H.; Sundari, S.; Nabila, N.; Tandrasasmita, O.M.; Amalia, R.; Siahaan, T.J.; Tjandrawinata, R.R.; Ismaya, W.T. Orf239342 from the Mushroom Agaricus Bisporus Is a Mannose Binding Protein. Biochem. Biophys. Res. Commun. 2019, 515, 99–103. [Google Scholar] [CrossRef]
- Poiroux, G.; Barre, A.; van Damme, E.J.M.; Benoist, H.; Rougé, P. Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy. Int. J. Mol. Sci. 2017, 18, 1232. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Liu, Y.-C.; El-Shazly, M.; Wu, T.-Y.; Chang, J.-G.; Wu, Y.-C. Antrodia Cinnamomea, a Treasured Medicinal Mushroom, Induces Growth Arrest in Breast Cancer Cells, T47D Cells: New Mechanisms Emerge. Int. J. Mol. Sci. 2019, 20, 833. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Guan, Y.-Y.; Hu, P.-F.; Chen, L.; Xu, G.-R.; Liu, L.; Cheung, P.C.K. Production of Bioactive Metabolites by Submerged Fermentation of the Medicinal Mushroom Antrodia Cinnamomea: Recent Advances and Future Development. Crit. Rev. Biotechnol. 2019, 39, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia Cinnamomea—An Updated Minireview of Its Bioactive Components and Biological Activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef]
- Li, H.-X.; Wang, J.-J.; Lu, C.-L.; Gao, Y.-J.; Gao, L.; Yang, Z.-Q. Review of Bioactivity, Isolation, and Identification of Active Compounds from Antrodia Cinnamomea. Bioengineering 2022, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Hsieh, H.-W.; Lin, C.-C.; Wang, S.-Y. Antcin-A Modulates Epithelial-to-Mesenchymal Transition and Inhibits Migratory and Invasive Potentials of Human Breast Cancer Cells via P53-Mediated MiR-200c Activation. Planta Med. 2019, 85, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Ng, T.B.; Chan, H.H.L.; Liu, Q.; Man, G.C.W.; Zhang, C.Z.; Guan, S.; Ng, C.C.W.; Fang, E.F.; Wang, H.; et al. Mushroom Extracts and Compounds with Suppressive Action on Breast Cancer: Evidence from Studies Using Cultured Cancer Cells, Tumor-Bearing Animals, and Clinical Trials. Appl. Microbiol. Biotechnol. 2020, 104, 4675–4703. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Chueh, P.-J.; Mau, J.-L.; Wang, S.-Y. Antrodin C Inhibits Epithelial-to-Mesenchymal Transition and Metastasis of Breast Cancer Cells via Suppression of Smad2/3 and β-Catenin Signaling Pathways. PLoS ONE 2015, 10, e117111. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Kuo, Y.-H.; Tsai, C.-T.; Huang, Y.-T.; Chen, S.-C.; Chang, H.-W.; Lin, E.; Lin, W.-H.; Hseu, Y.-C. Anti-Metastatic Activities of Antrodia Camphorata against Human Breast Cancer Cells Mediated through Suppression of the MAPK Signaling Pathway. Food Chem. Toxicol. 2011, 49, 290–298. [Google Scholar] [CrossRef]
- Yang, H.-L.; Chen, C.-S.; Chang, W.-H.; Lu, F.-J.; Lai, Y.-C.; Chen, C.-C.; Hseu, T.-H.; Kuo, C.-T.; Hseu, Y.-C. Growth Inhibition and Induction of Apoptosis in MCF-7 Breast Cancer Cells by Antrodia Camphorata. Cancer Lett. 2006, 231, 215–227. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Lin, Y.-Y.; Yang, Y.-H.; Lin, C.-L.; Kuan, F.-C.; Lu, C.-N.; Chang, G.-H.; Tsai, M.-S.; Hsu, C.-M.; Yeh, R.-A.; et al. Antrodia Cinnamomea Extract Inhibits the Proliferation of Tamoxifen-Resistant Breast Cancer Cells through Apoptosis and Skp2/MicroRNAs Pathway. BMC Complement. Altern. Med. 2018, 18, 152. [Google Scholar] [CrossRef]
- Wei, C.; Khan, M.A.; Du, J.; Cheng, J.; Tania, M.; Leung, E.L.-H.; Fu, J. Cordycepin Inhibits Triple-Negative Breast Cancer Cell Migration and Invasion by Regulating EMT-TFs SLUG, TWIST1, SNAIL1, and ZEB1. Front. Oncol. 2022, 12, 898583. [Google Scholar] [CrossRef] [PubMed]
- Shashidhar, M.G.; Giridhar, P.; Udaya Sankar, K.; Manohar, B. Bioactive Principles from Cordyceps Sinensis: A Potent Food Supplement—A Review. J. Funct. Foods 2013, 5, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, W.-Y.; Jung, K.; Kwon, Y.S.; Kim, D.; Hwang, G.S.; Kim, C.-E.; Lee, S.; Kang, K.S. The Inhibitory Effect of Cordycepin on the Proliferation of MCF-7 Breast Cancer Cells, and Its Mechanism: An Investigation Using Network Pharmacology-Based Analysis. Biomolecules 2019, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; De Luca, F.; Di Iorio, C.; Ratto, D.; Siciliani, S.; Ferrari, B.; Cobelli, F.; Borsci, G.; Priori, E.C.; Chinosi, S.; et al. Novel Medicinal Mushroom Blend as a Promising Supplement in Integrative Oncology: A Multi-Tiered Study Using 4t1 Triple-Negative Mouse Breast Cancer Model. Int. J. Mol. Sci. 2020, 21, 3479. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, Y.; Teng, M.; Zhang, S.; Yin, M.; Lu, J.; Liu, Y.; Lee, R.J.; Wang, D.; Teng, L. Cordyceps Militaris Induces Tumor Cell Death via the Caspase-Dependent Mitochondrial Pathway in HepG2 and MCF-7 Cells. Mol. Med. Rep. 2016, 13, 5132–5140. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.-R.; Ahmed, M.; Park, H.-J. Cordyceps Militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms. Microorganisms 2022, 10, 405. [Google Scholar] [CrossRef]
- Tima, S.; Tapingkae, T.; To-Anun, C.; Noireung, P.; Intaparn, P.; Chaiyana, W.; Sirithunyalug, J.; Panyajai, P.; Viriyaadhammaa, N.; Nirachonkul, W.; et al. Antileukaemic Cell Proliferation and Cytotoxic Activity of Edible Golden Cordyceps (Cordyceps Militaris) Extracts. Evid.-Based Complement. Altern. Med. 2022, 2022, 5347718. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, M.; Jiang, Y.; Liu, Y.; Luo, H.; Hao, C.; Zeng, P.; Zhang, L. Preclinical and Clinical Studies of Coriolus Versicolor Polysaccharopeptide as an Immunotherapeutic in China. Discov. Med. 2017, 23, 207–219. [Google Scholar]
- Wang, Z.; Dong, B.; Feng, Z.; Yu, S.; Bao, Y. A Study on Immunomodulatory Mechanism of Polysaccharopeptide Mediated by TLR4 Signaling Pathway. BMC Immunol. 2015, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory Properties of Coriolus Versicolor: The Role of Polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Chang, Y.; Zhang, L. Coriolus Versicolor Polysaccharopeptide as an Immunotherapeutic in China; Springer: Berlin/Heidelberg, Germany, 2019; Volume 163, ISBN 9780128177402. [Google Scholar]
- Lu, H.; Yang, Y.; Gad, E.; Wenner, C.A.; Chang, A.; Larson, E.R.; Dang, Y.; Martzen, M.; Standish, L.J.; Disis, M.L. Polysaccharide Krestin Is a Novel TLR2 Agonist That Mediates Inhibition of Tumor Growth via Stimulation of CD8 T Cells and NK Cells. Clin. Cancer Res. 2011, 17, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Ooi, V.E.C.; Liu, F. Immunomodulation and Anti-Cancer Activity of Polysaccharide-Protein Complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.; Kennedy, D.A.; Ishii, M.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, D. Polysaccharide K and Coriolus Versicolor Extracts for Lung Cancer: A Systematic Review. Integr. Cancer Ther. 2015, 14, 201–211. [Google Scholar] [CrossRef]
- Habtemariam, S. Trametes Versicolor (Synn. Coriolus Versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Kim, C.-F.; Leung, K.-N.; Fung, K.-P.; Tse, T.-F.; Chan, H.; Lau, C.B.-S. Differential Anti-Tumor Activity of Coriolus Versicolor (Yunzhi) Extract through P53- and/or Bcl-2-Dependent Apoptotic Pathway in Human Breast Cancer Cells. Cancer Biol. Ther. 2005, 4, 638–644. [Google Scholar] [CrossRef]
- Aoyagi, H.; Iino, Y.; Takeo, T.; Horii, Y.; Morishita, Y.; Horiuchi, R. Effects of OK-432 (Picibanil) on the Estrogen Receptors of MCF-7 Cells and Potentiation of Antiproliferative Effects of Tamoxifen in Combination with OK-432. Oncology 1997, 54, 414–423. [Google Scholar] [CrossRef]
- Luo, K.-W.; Yue, G.G.-L.; Ko, C.-H.; Lee, J.K.-M.; Gao, S.; Li, L.-F.; Li, G.; Fung, K.-P.; Leung, P.-C.; Lau, C.B.-S. In Vivo and in Vitro Anti-Tumor and Anti-Metastasis Effects of Coriolus Versicolor Aqueous Extract on Mouse Mammary 4T1 Carcinoma. Phytomedicine 2014, 21, 1078–1087. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Extract from the Coriolus Versicolor Fungus as an Anti-Inflammatory Agent with Cytotoxic Properties against Endothelial Cells and Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9063. [Google Scholar] [CrossRef]
- Pawlikowska, M.; Jadrzejewski, T.; Broayna, A.A.; Wrotek, S. Protein-Bound Polysaccharides from Coriolus Versicolor Induce RIPK1/RIPK3/MLKL-Mediated Necroptosis in ER-Positive Breast Cancer and Amelanotic Melanoma Cells. Cell. Physiol. Biochem. 2020, 54, 591–604. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, S.-H.; Dai, Y.-C. Species Clarification of the Prize Medicinal Ganoderma Mushroom Lingzhi. Fungal Divers. 2012, 56, 49–62. [Google Scholar] [CrossRef]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary Metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Hennicke, F.; Cheikh-Ali, Z.; Liebisch, T.; MacIá-Vicente, J.G.; Bode, H.B.; Piepenbring, M. Distinguishing Commercially Grown Ganoderma Lucidum from Ganoderma Lingzhi from Europe and East Asia on the Basis of Morphology, Molecular Phylogeny, and Triterpenic Acid Profiles. Phytochemistry 2016, 127, 29–37. [Google Scholar] [CrossRef]
- Ryvarden, L. Studies in Neotropical Polypores 2: A Preliminary Key to Neotropical Species of Ganoderma with a Laccate Pileus. Mycologia 2000, 92, 180–191. [Google Scholar] [CrossRef]
- Xie, Y.-Z.; Yang, F.; Tan, W.; Li, X.; Jiao, C.; Huang, R.; Yang, B.B. The Anti-Cancer Components of Ganoderma Lucidum Possesses Cardiovascular Protective Effect by Regulating Circular RNA Expression. Oncoscience 2016, 3, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, A.; Jedinak, A.; Nguyen, H.; Terry, C.; Baldridge, L.A.; Jiang, J.; Sliva, D. Triterpenes from Ganoderma Lucidum Induce Autophagy in Colon Cancer through the Inhibition of P38 Mitogen-Activated Kinase (P38 MAPK). Nutr. Cancer 2010, 62, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Montemayor, M.M.; Ling, T.; Suárez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negrón, C.L.; Lacourt-Ventura, M.Y.; Valentín-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of Biologically Active Ganoderma Lucidum Compounds and Synthesis of Improved Derivatives That Confer Anti-Cancer Activities in Vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Lilburn, C. Medicinal Mushrooms (Specifically Ganoderma Lucidum or Reishi) as an Adjuvant Treatment in Breast Cancer. Aust. J. Herb. Naturop. Med. 2022, 34, 18–23. [Google Scholar] [CrossRef]
- Suarez-Arroyo, I.J.; Rosario-Acevedo, R.; Aguilar-Perez, A.; Clemente, P.L.; Cubano, L.A.; Serrano, J.; Schneider, R.J.; Martínez-Montemayor, M.M. Anti-Tumor Effects of Ganoderma Lucidum (Reishi) in Inflammatory Breast Cancer in In Vivo and In Vitro Models. PLoS ONE 2013, 8, e57431. [Google Scholar] [CrossRef]
- Barbieri, A.; Quagliariello, V.; Del Vecchio, V.; Falco, M.; Luciano, A.; Amruthraj, N.J.; Nasti, G.; Ottaiano, A.; Berretta, M.; Iaffaioli, R.V.; et al. Anticancer and Anti-Inflammatory Properties of Ganoderma Lucidum Extract Effects on Melanoma and Triple-Negative Breast Cancer Treatment. Nutrients 2017, 9, 210. [Google Scholar] [CrossRef]
- Adamec, J.; Jannasch, A.; Dudhgaonkar, S.; Jedinak, A.; Sedlak, M.; Sliva, D. Development of a New Method for Improved Identification and Relative Quantification of Unknown Metabolites in Complex Samples: Determination of a Triterpenoid Metabolic Fingerprint for the in Situ Characterization of Ganoderma Bioactive Compounds. J. Sep. Sci. 2009, 32, 4052–4058. [Google Scholar] [CrossRef]
- Jiang, J.; Grieb, B.; Thyagarajan, A.; Sliva, D. Ganoderic Acids Suppress Growth and Invasive Behavior of Breast Cancer Cells by Modulating AP-1 and NF-ΚB Signaling. Int. J. Mol. Med. 2008, 21, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.-Y.; Sartippour, M.R.; Brooks, M.N.; Zhang, Q.; Hardy, M.; Go, V.L.; Li, F.P.; Heber, D. Ganoderma Lucidum Spore Extract Inhibits Endothelial and Breast Cancer Cells in Vitro. Oncol. Rep. 2004, 12, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Smina, T.P.; Nitha, B.; Devasagayam, T.P.A.; Janardhanan, K.K. Ganoderma Lucidum Total Triterpenes Induce Apoptosis in MCF-7 Cells and Attenuate DMBA Induced Mammary and Skin Carcinomas in Experimental Animals. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2017, 813, 45–51. [Google Scholar] [CrossRef]
- Acevedo-Díaz, A.; Ortiz-Soto, G.; Suárez-Arroyo, I.J.; Zayas-Santiago, A.; Montemayor, M.M.M. Ganoderma Lucidum Extract Reduces the Motility of Breast Cancer Cells Mediated by the RAC–Lamellipodin Axis. Nutrients 2019, 11, 1116. [Google Scholar] [CrossRef]
- Loganathan, J.; Jiang, J.; Smith, A.; Jedinak, A.; Thyagarajan-Sahu, A.; Sandusky, G.E.; Nakshatri, H.; Sliva, D. The Mushroom Ganoderma Lucidum Suppresses Breast-to-Lung Cancer Metastasis through the Inhibition of pro-Invasive Genes. Int. J. Oncol. 2014, 45, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Arroyo, I.J.; Rios-Fuller, T.J.; Feliz-Mosquea, Y.R.; Lacourt-Ventura, M.; Leal-Alviarez, D.J.; Maldonado-Martinez, G.; Cubano, L.A.; Martínez-Montemayor, M.M. Ganoderma Lucidum Combined with the EGFR Tyrosine Kinase Inhibitor, Erlotinib Synergize to Reduce Inflammatory Breast Cancer Progression. J. Cancer 2016, 7, 500–511. [Google Scholar] [CrossRef]
- Martínez-Montemayor, M.M.; Acevedo, R.R.; Otero-Franqui, E.; Cubano, L.A.; Dharmawardhane, S.F. Ganoderma Lucidum (Reishi) Inhibits Cancer Cell Growth and Expression of Key Molecules in Inflammatory Breast Cancer. Nutr. Cancer 2011, 63, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-Y.; Hua, K.-F.; Lin, C.-C.; Lin, C.-H.; Hsu, J.; Wong, C.-H. Extract of Reishi Polysaccharides Induces Cytokine Expression via TLR4-Modulated Protein Kinase Signaling Pathways. J. Immunol. 2004, 173, 5989–5999. [Google Scholar] [CrossRef]
- Liao, S.-F.; Liang, C.-H.; Ho, M.-Y.; Hsu, T.-L.; Tsai, T.-I.; Hsieh, Y.S.-Y.; Tsai, C.-M.; Li, S.-T.; Cheng, Y.-Y.; Tsao, S.-M.; et al. Immunization of Fucose-Containing Polysaccharides from Reishi Mushroom Induces Antibodies to Tumor-Associated Globo H-Series Epitopes. Proc. Natl. Acad. Sci. USA 2013, 110, 13809–13814. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.-M.; Hsu, H.-Y. Fucose-Containing Fraction of Ling-Zhi Enhances Lipid Rafts-Dependent Ubiquitination of TGFβ Receptor Degradation and Attenuates Breast Cancer Tumorigenesis. Sci. Rep. 2016, 6, 36563. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, J.S.; Kim, Z.H.; Huang, R.B.; Chae, Y.L.; Wang, R.S. Khz-Cp (Crude Polysaccharide Extract Obtained from the Fusion of Ganoderma Lucidum and Polyporus Umbellatus Mycelia) Induces Apoptosis by Increasing Intracellular Calcium Levels and Activating P38 and NADPH Oxidase-Dependent Generation of Reactive Oxygen. BMC Complement. Altern. Med. 2014, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, J.S.; Kim, Z.H.; Huang, R.B.; Chae, Y.L.; Wang, R.S. Induction of Apoptosis in MCF-7 Human Breast Cancer Cells by Khz (Fusion of Ganoderma Lucidum and Polyporus Umbellatus Mycelium). Mol. Med. Rep. 2016, 13, 1243–1249. [Google Scholar] [CrossRef]
- Su, J.; Su, L.; Li, D.; Shuai, O.; Zhang, Y.; Liang, H.; Jiao, C.; Xu, Z.; Lai, Y.; Xie, Y. Antitumor Activity of Extract From the Sporoderm-Breaking Spore of Ganoderma Lucidum: Restoration on Exhausted Cytotoxic T Cell With Gut Microbiota Remodeling. Front. Immunol. 2018, 9, 1765. [Google Scholar] [CrossRef]
- Deepalakshmi, K.; Mirunalini, S. Modulatory Effect of Ganoderma Lucidum on Expression of Xenobiotic Enzymes, Oxidant-Antioxidant and Hormonal Status in 7,12-Dimethylbenz(a)Anthracene- Induced Mammary Carcinoma in Rats. Pharmacogn. Mag. 2013, 9, 167–175. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Siu, K.-C.; Geng, P. Bioactive Ingredients and Medicinal Values of Grifola Frondosa (Maitake). Foods 2021, 10, 95. [Google Scholar] [CrossRef]
- Alonso, E.N.; Ferronato, M.J.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Guevara, J.A.; Facchinetti, M.M.; Curino, A.C. Antitumoral and Antimetastatic Activity of Maitake D-Fraction in Triple-Negative Breast Cancer Cells. Oncotarget 2018, 9, 23396–23412. [Google Scholar] [CrossRef]
- Lu, H.; Lou, H.; Hu, J.; Liu, Z.; Chen, Q. Macrofungi: A Review of Cultivation Strategies, Bioactivity, and Application of Mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef]
- Subramaniam, S.; Jiao, S.; Zhang, Z.; Jing, P. Impact of Post-Harvest Processing or Thermal Dehydration on Physiochemical, Nutritional and Sensory Quality of Shiitake Mushrooms. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2560–2595. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World; Wiley: Hoboken, NJ, USA, 2017; ISBN 9781119149446. [Google Scholar]
- Morales, D.; Smiderle, F.R.; Piris, A.J.; Soler-Rivas, C.; Prodanov, M. Production of a β-D-Glucan-Rich Extract from Shiitake Mushrooms (Lentinula Edodes) by an Extraction/Microfiltration/Reverse Osmosis (Nanofiltration) Process. Innov. Food Sci. Emerg. Technol. 2019, 51, 80–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Wang, X.; Zhang, L.; Cheung, P.C.K. Advances in Lentinan: Isolation, Structure, Chain Conformation and Bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Din, S.R.U.; Zhong, M.; Nisar, M.A.; Saleem, M.Z.; Hussain, A.; Khinsar, K.H.; Alam, S.; Ayub, G.; Kanwal, S.; Li, X.; et al. Latcripin-7A, Derivative of Lentinula Edodes C91–3, Reduces Migration and Induces Apoptosis, Autophagy, and Cell Cycle Arrest at G1 Phase in Breast Cancer Cells. Appl. Microbiol. Biotechnol. 2020, 104, 10165–10179. [Google Scholar] [CrossRef] [PubMed]
- Israilides, C.; Kletsas, D.; Arapoglou, D.; Philippoussis, A.; Pratsinis, H.; Ebringerová, A.; Hříbalová, V.; Harding, S.E. In Vitro Cytostatic and Immunomodulatory Properties of the Medicinal Mushroom Lentinula edodes. Phytomedicine 2008, 15, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Li, Q.; Yu, S.; Zhang, J.; He, L.; Ronis, M.J.J.; Badger, T.M. Inhibition of Growth and Induction of Apoptosis in Human Cancer Cell Lines by an Ethyl Acetate Fraction from Shiitake Mushrooms. J. Altern. Complement. Med. 2006, 12, 125–132. [Google Scholar] [CrossRef]
- Xu, H.; Zou, S.; Xu, X. The β-Glucan from Lentinus Edodes Suppresses Cell Proliferation and Promotes Apoptosis in Estrogen Receptor Positive Breast Cancers. Oncotarget 2017, 8, 86693–86709. [Google Scholar] [CrossRef]
- Chang, S.T.; Buswell, J.A. Mushroom Nutriceuticals. World J. Microbiol. Biotechnol. 1996, 12, 473–476. [Google Scholar] [CrossRef]
- Okhuoya, J.A. Edible Mushrooms: As Functional Foods and Nutriceuticals. Trop. J. Nat. Prod. Res. 2017, 1, 186–187. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Singh, M.P. Promising Anticancer Activity of Polysaccharides and Other Macromolecules Derived from Oyster Mushroom (Pleurotus Sp.): An Updated Review. Int. J. Biol. Macromol. 2021, 182, 1628–1637. [Google Scholar] [CrossRef]
- Mishra, V.; Tomar, S.; Yadav, P.; Vishwakarma, S.; Singh, M.P. Elemental Analysis, Phytochemical Screening and Evaluation of Antioxidant, Antibacterial and Anticancer Activity of Pleurotus Ostreatus through In Vitro and In Silico Approaches. Metabolites 2022, 12, 821. [Google Scholar] [CrossRef]
- Jedinak, A.; Sliva, D. Pleurotus Ostreatus Inhibits Proliferation of Human Breast and Colon Cancer Cells through P53-Dependent as Well as P53-Independent Pathway. Int. J. Oncol. 2008, 33, 1307–1313. [Google Scholar] [CrossRef]
- Krishnamoorthy, D.; Sankaran, M. Modulatory Effect of Pleurotus Ostreatus on Oxidant/Antioxidant Status in 7, 12-Dimethylbenz (a) Anthracene Induced Mammary Carcinoma in Experimental Rats—A Dose-Response Study. J. Cancer Res. Ther. 2016, 12, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.D.; Islam, M.D. Pleurotus Highking Mushroom Induces Apoptosis by Altering the Balance of Proapoptotic and Antiapoptotic Genes in Breast Cancer Cells and Inhibits Tumor Sphere Formation. Medicina 2019, 55, 716. [Google Scholar] [CrossRef]
- Nowakowski, P.; Markiewicz-Żukowska, R.; Bielecka, J.; Mielcarek, K.; Grabia, M.; Socha, K. Treasures from the Forest: Evaluation of Mushroom Extracts as Anti-Cancer Agents. Biomed. Pharmacother. 2021, 143, 112106. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuller, T.J.; Ortiz-Soto, G.; Lacourt-Ventura, M.; Maldonado-Martinez, G.; Cubano, L.A.; Schneider, R.J.; Martinez-Montemayor, M.M. Ganoderma Lucidum Extract (GLE) Impairs Breast Cancer Stem Cells by Targeting the STAT3 Pathway. Oncotarget 2018, 9, 35907–35921. [Google Scholar] [CrossRef]
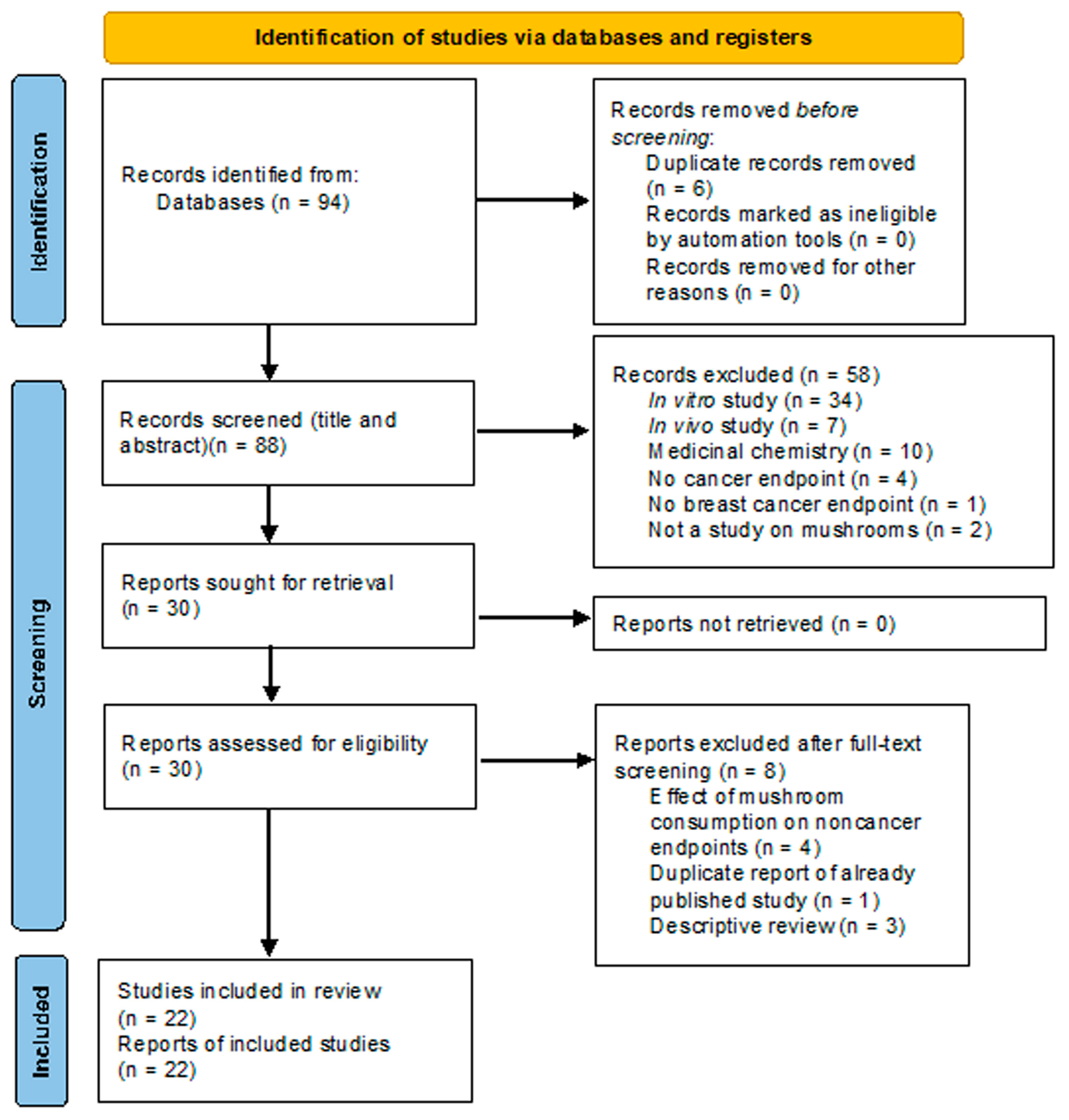
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zhang, S.; Sugawara, Y.; Chen, S.; Beelman, R.B.; Tsuduki, T.; Tomata, Y.; Matsuyama, S.; Tsuji, I. Mushroom Consumption and Incident Risk of Prostate Cancer in Japan: A Pooled Analysis of the Miyagi Cohort Study and the Ohsaki Cohort Study. Int. J. Cancer 2020, 146, 2712–2720. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, W.K.; Kim, M.K.; Lee, S.S.; Choi, B.Y. Dietary Factors and Gastric Cancer in Korea: A Case-Control Study. Int. J. Cancer 2002, 97, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Jian, H.; Xing, X.; Holman, C.D.J. Dietary Intakes of Mushrooms and Green Tea Combine to Reduce the Risk of Breast Cancer in Chinese Women. Int. J. Cancer 2009, 124, 1404–1408. [Google Scholar] [CrossRef]
- Seo, A.H.; Kim, K.; Nam, S.-J.; Kong, G.; Mi, K.K. A Case-Control Study on the Dietary Intake of Mushrooms and Breast Cancer Risk among Korean Women. Int. J. Cancer 2008, 122, 919–923. [Google Scholar] [CrossRef]
- Shin, A.; Kim, J.; Lim, S.-Y.; Kim, G.; Sung, M.-K.; Lee, E.-S.; Ro, J. Dietary Mushroom Intake and the Risk of Breast Cancer Based on Hormone Receptor Status. Nutr. Cancer 2010, 62, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Yang, M.; Keum, N.; Giovannucci, E.L.; Sun, Q.; Chavarro, J.E. Mushroom Consumption and Risk of Total and Site-Specific Cancer in Two Large U.S. Prospective Cohorts. Cancer Prev. Res. 2019, 12, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Valadares, F.; Novaes, M.R.C.G.; Cañete, R. Effect of Agaricus Sylvaticus Supplementation on Nutritional Status and Adverse Events of Chemotherapy of Breast Cancer: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Indian J. Pharmacol. 2013, 45, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-Y.; Hung, Y.-C.; Chen, Y.-H.; Chen, Y.-H.; Huang, Y.-C.; Kao, C.-W.; Su, Y.-L.; Chiu, H.-H.E.; Rau, K.-M. A Preliminary Randomised Controlled Study of Short-Term Antrodia Cinnamomea Treatment Combined with Chemotherapy for Patients with Advanced Cancer. BMC Complement. Altern. Med. 2016, 16, 322. [Google Scholar] [CrossRef]
- Torkelson, C.J.; Sweet, E.; Martzen, M.R.; Sasagawa, M.; Wenner, C.A.; Gay, J.; Putiri, A.; Standish, L.J. Phase 1 Clinical Trial of Trametes Versicolor in Women with Breast Cancer. ISRN Oncol. 2012, 2012, 251632. [Google Scholar] [CrossRef]
- Wong, C.-K.; Bao, Y.-X.; Wong, E.L.-Y.; Leung, P.-C.; Fung, K.P.; Lam, C.W.K. Immunomodulatory Activities of Yunzhi and Danshen in Post-Treatment Breast Cancer Patients. Am. J. Chin. Med. 2005, 33, 381–395. [Google Scholar] [CrossRef]
- Jin, X.; Ruiz Beguerie, J.; Sze, D.M.-Y.; Chan, G.C.F. Ganoderma Lucidum (Reishi Mushroom) for Cancer Treatment. Cochrane Database Syst. Rev. 2016, 2016, CD007731. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, J.; Tang, D.; Zhang, Q. Dynamic Biomarkers Indicate the Immunological Benefits Provided by Ganoderma Spore Powder in Post-Operative Breast and Lung Cancer Patients. Clin. Transl. Oncol. 2021, 23, 1481–1490. [Google Scholar] [CrossRef]
- Ali, N.A.M.; Saeed, H.A.; Othman, R.T. Immunostimulatory and Anti-Inflammatory Effect of Ganoderma lucidum on Breast Cancer Patients. Asian Pacific J. Cancer Biol. 2018, 3, 51–57. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Zhao, L.; Huang, X.; Wang, J.; Kang, X. Spore Powder of Ganoderma Lucidum Improves Cancer-Related Fatigue in Breast Cancer Patients Undergoing Endocrine Therapy: A Pilot Clinical Trial. Evid.-Based Complement. Altern. Med. 2012, 2012, 809614. [Google Scholar] [CrossRef]
- Bao, P.-P.; Lu, W.; Cui, Y.; Zheng, Y.; Gu, K.; Chen, Z.; Zheng, W.; Shu, X.O. Ginseng and Ganoderma Lucidum Use after Breast Cancer Diagnosis and Quality of Life: A Report from the Shanghai Breast Cancer Survival Study. PLoS ONE 2012, 7, e39343. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Lin, H.; Seidman, A.; Fornier, M.; D’Andrea, G.; Wesa, K.; Yeung, S.; Cunningham-Rundles, S.; Vickers, A.J.; Cassileth, B. A Phase I/II Trial of a Polysaccharide Extract from Grifola Frondosa (Maitake Mushroom) in Breast Cancer Patients: Immunological Effects. J. Cancer Res. Clin. Oncol. 2009, 135, 1215–1221. [Google Scholar] [CrossRef]
- Nagashima, Y.; Sanpei, N.; Yamamoto, S.; Yoshino, S.; Tangoku, A.; Oka, M. Evaluation of Host Immunity and Side Effects in Breast Cancer Patients Treated with Adjuvant Chemotherapy (FEC Therapy). Gan Kagaku Ryoho Cancer Chemother. 2005, 32, 1550–1552. [Google Scholar]
- Yamaguchi, Y.; Miyahara, E.; Hihara, J. Efficacy and Safety of Orally Administered Lentinula Edodes Mycelia Extract for Patients Undergoing Cancer Chemotherapy: A Pilot Study. Am. J. Chin. Med. 2011, 39, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Takimoto, Y.; Suzuki, R.; Arai, T.; Uebaba, K.; Nakai, M.; Strong, J.M.; Tokuda, H. Efficacy of Oral Administration of Lentinula Eododes Mycelia Extract for Breast Cancer Patients Undergoing Postoperative Hormone Therapy. Asian Pacific J. Cancer Prev. 2013, 14, 3469–3472. [Google Scholar] [CrossRef] [PubMed]
- Eliza, W.L.Y.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus Versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. Recent Patents Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Zhong, Z.; Chen, L.; Wang, Y. Ganoderma Lucidum Polysaccharides: Immunomodulation and Potential Anti-Tumor Activities. Am. J. Chin. Med. 2011, 39, 15–27. [Google Scholar] [CrossRef]
- Zhong, L.; Yan, P.; Lam, W.C.; Yao, L.; Bian, Z. Coriolus Versicolor and Ganoderma Lucidum Related Natural Products as an Adjunct Therapy for Cancers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2019, 10, 703. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, K.; Zheng, Y.; Zheng, W.; Lu, W.; Shu, X.O. The Use of Complementary and Alternative Medicine among Chinese Women with Breast Cancer. J. Altern. Complement. Med. 2008, 14, 1049–1055. [Google Scholar] [CrossRef]
- Kosaka, A.; Wani, T.; Hattori, Y.; Yamashita, A. Effect of Lentinan on the Adrenalectomized Rats and Human Patients with Breast Cancer. Jpn. J. Cancer Chemother. 1982, 9, 1474–1481. [Google Scholar]
- Panda, M.K.; Paul, M.; Singdevsachan, S.K.; Tayung, K.; Das, S.K.; Thatoi, H. Promising Anti-Cancer Therapeutics from Mushrooms: Current Findings and Future Perceptions. Curr. Pharm. Biotechnol. 2021, 22, 1164–1191. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE Guidelines: 3. Rating the Quality of Evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Arshadi, N.; Nouri, H.; Moghimi, H. Increasing the Production of the Bioactive Compounds in Medicinal Mushrooms: An Omics Perspective. Microb. Cell Fact. 2023, 22, 11. [Google Scholar] [CrossRef] [PubMed]

| Species | Trial Design, Trial Identifier (When Available), Number of Patients (n) | Symptomatic Effects, Adverse Effects | Immune Cell Effects (vs. Controls) | Cytokine Level Effects (vs. Controls) | Other Effects | Reference |
|---|---|---|---|---|---|---|
| Agaricus sylvaticus | Randomized, placebo-controlled, double-blind trial, n = 46 | ↓ GI adverse effects and anorexia vs. placebo | // | // | // | [135] |
| Antrodia cinnamomea | Randomized, double-blind, placebo-controlled trial, clinicaltrials.gov identifier: NCT01287286, n = 37 | ↑ GI adverse effects vs. placebo | // | // | Overall survival, disease control rate, quality of life, adverse events: no differences between arms; platelet counts ↓ in AC arm; quality of sleep ↑ in AC arm | [136] |
| Coriolus versicolor | Phase I, dose-escalation trial, clinicaltrials.gov identifier: NCT00680667, n = 11 | Severe anxiety (adverse effect) | ↑ CD8+ T cells, ↑ CD19+ B cells; CD16+/56+ NK cell counts unchanged but activity ↑ | // | // | [137] |
| Cohort study, identifier not available, n = 82 | // | ↑ CD8+, ↑ CD4+, ↑ B-cells, ↑ T-helper/T suppressor cells ratio | ↓ sIL-2R | // | [138] | |
| Ganoderma lucidum | Meta-analysis, n = 153 | // | ↑ CD3+, ↑ CD4+, ↑ CD8+ cells; ↑ NK cell activity | // | // | [139] |
| Randomized, double-blind trial, identifier not available, n = 69 (breast cancer) | No serious adverse effects | ↑ CD3+, ↑ CD4+, ↑ CD3+/HLADR-cells; ↓ CD4+, ↓ CD25+, ↓ Treg (CD4+/CD25+), ↓ CD3+/HLADR+ cells | ↑ IL-12, ↓ IL-10 | // | [140] | |
| Prospective observational study, identifier not available, n = 40 | // | // | ↑ IFN-γ, ↓ IL-8, ↓ TNF-α | // | [141] | |
| Randomized controlled study, identifier not available, n = 48 | Improvement of fatigue, anxiety, depression, QoL vs. controls; social functioning unchanged | // | ↓ IL-6, ↓ TNF-α | // | [142] | |
| Population observational study, identifier not available, n = 4149 | Improvement of QoL and physical and psychological well-being | // | // | // | [143] | |
| Grifola frondosa | Phase I/II trial, identifier not available, n = 34 | No serious adverse effects | ↑ CD4+/CD25+ T cells, ↑ CD3+/CD25+ T cells, ↑ CD45RA+/CD4+ cells, ↑ CD45RO+/CD8+ cells | ↑ IL-10, ↑ IL-2 and, ↑ IFN-γ, ↑ TNF-α | // | [144] |
| Lentinula edodes | Case series, add-on to chemotherapy, identifier not available, n = 10 | // | Addition of LE to adjuvant chemotherapy caused sustained NK activity and prevented leukocyte drop | // | // | [145] |
| Case series; add-on to chemotherapy, identifier not available, n = 3 | Improvement of QoL | ↑ NK cell activity | ↓ immunosuppressive acidic protein | // | [146] | |
| Case series, add-on to post-operative hormone therapy, identifier not available, n = 20 | Improvement of QoL | // | ↑ IFNγ, ↑ IFNγ/L-10 | // | [147] |
| Species | Type of Studies | Strength of Recommendation | ||
|---|---|---|---|---|
| In Vitro | In Vivo | Clinical Studies | ||
| Agaricus bisporum | *** | ** | * | ** |
| Antrodia cinnamomea | *** | *** | * | ** |
| Cordyceps sinensis | ** | * | * | * |
| Cordyceps militaris | ** | * | - | * |
| Coriolus versicolor | *** | *** | *** | *** |
| Ganoderma lucidum | *** | *** | *** | *** |
| Grifola Frondosa | *** | ** | *** | *** |
| Lentinula edodes | *** | ** | *** | *** |
| Pleurotus ostreatus | *** | ** | - | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gariboldi, M.B.; Marras, E.; Ferrario, N.; Vivona, V.; Prini, P.; Vignati, F.; Perletti, G. Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer. Int. J. Mol. Sci. 2023, 24, 10120. https://doi.org/10.3390/ijms241210120
Gariboldi MB, Marras E, Ferrario N, Vivona V, Prini P, Vignati F, Perletti G. Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer. International Journal of Molecular Sciences. 2023; 24(12):10120. https://doi.org/10.3390/ijms241210120
Chicago/Turabian StyleGariboldi, Marzia Bruna, Emanuela Marras, Nicole Ferrario, Veronica Vivona, Pamela Prini, Francesca Vignati, and Gianpaolo Perletti. 2023. "Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer" International Journal of Molecular Sciences 24, no. 12: 10120. https://doi.org/10.3390/ijms241210120
APA StyleGariboldi, M. B., Marras, E., Ferrario, N., Vivona, V., Prini, P., Vignati, F., & Perletti, G. (2023). Anti-Cancer Potential of Edible/Medicinal Mushrooms in Breast Cancer. International Journal of Molecular Sciences, 24(12), 10120. https://doi.org/10.3390/ijms241210120








