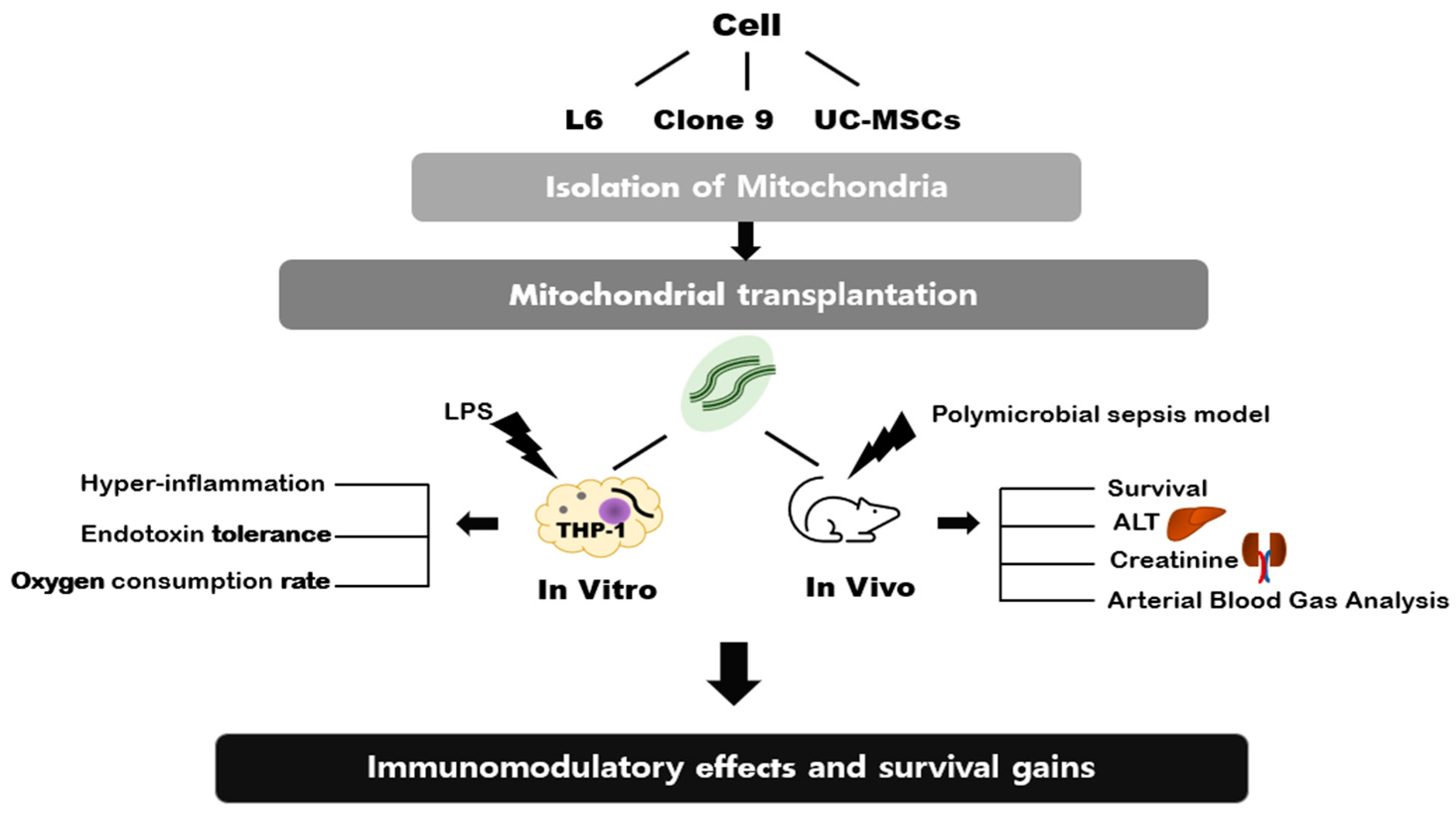
The Effects of Mitochondrial Transplantation on Sepsis Depend on the Type of Cell from Which They Are Isolated
Abstract
1. Introduction
2. Results
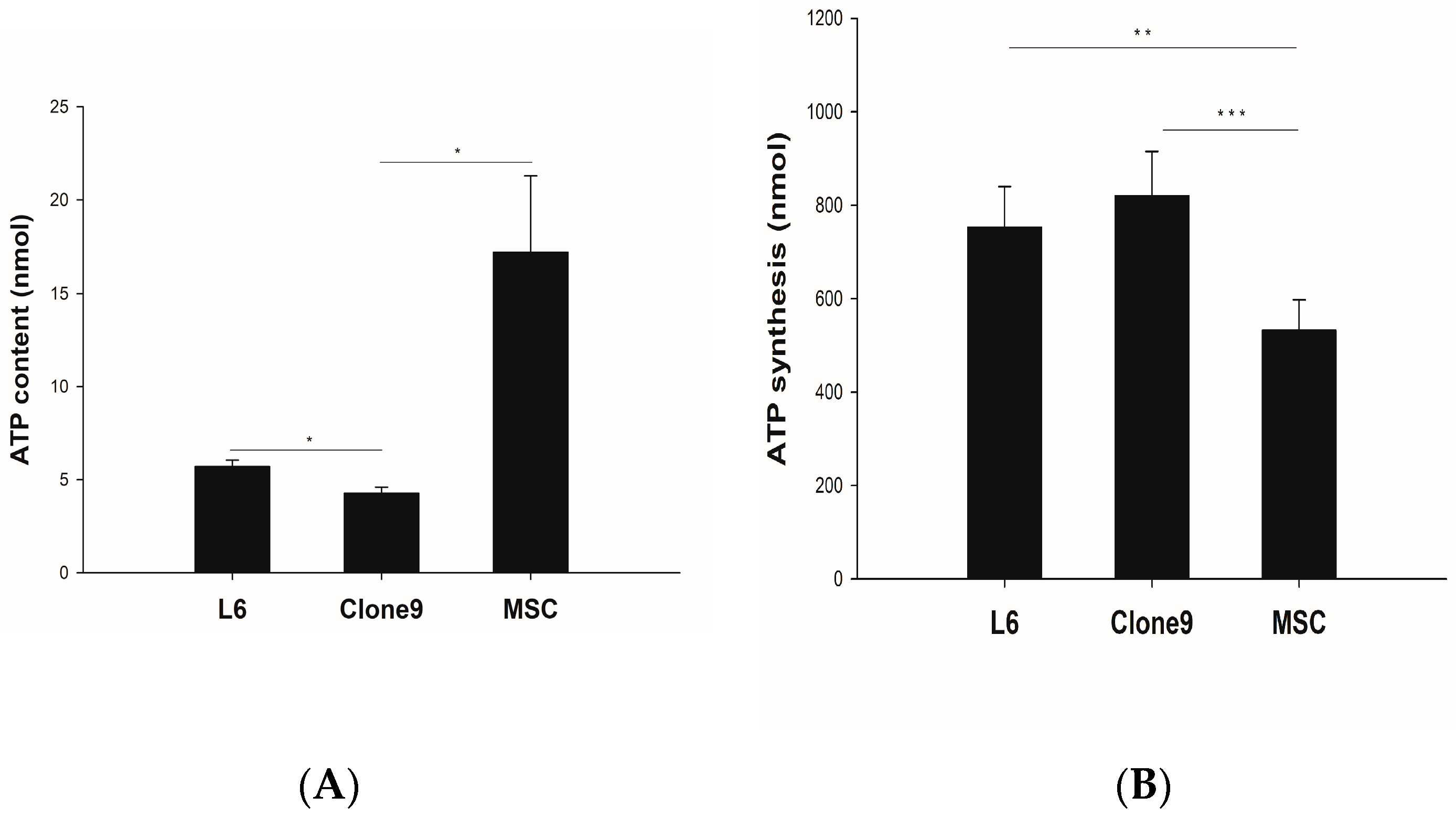
2.1. ATP Content and Synthesis
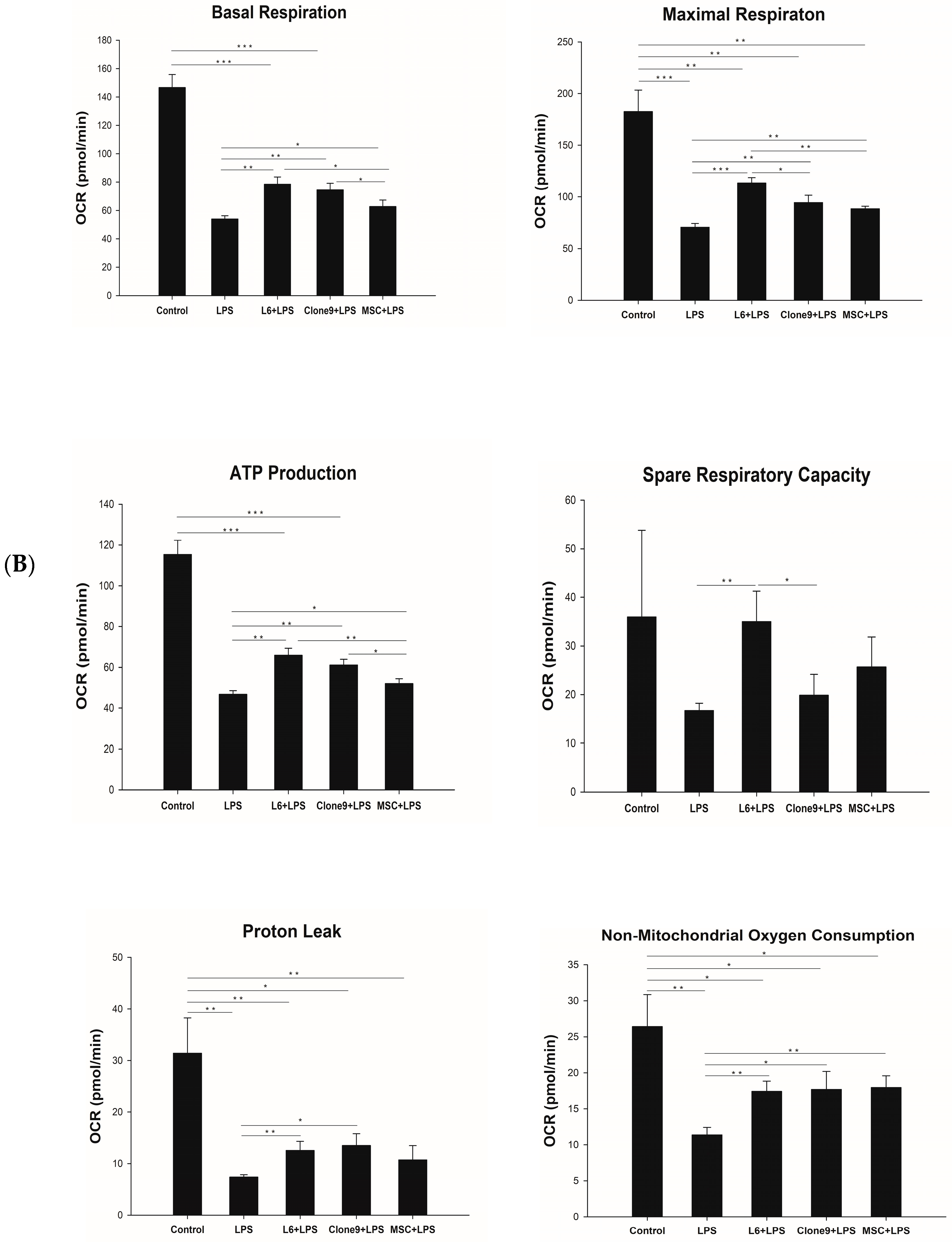
2.2. The Effect of Mitochondrial Transplantation on Mitochondrial Functions: In Vitro LPS Stimulation of THP-1
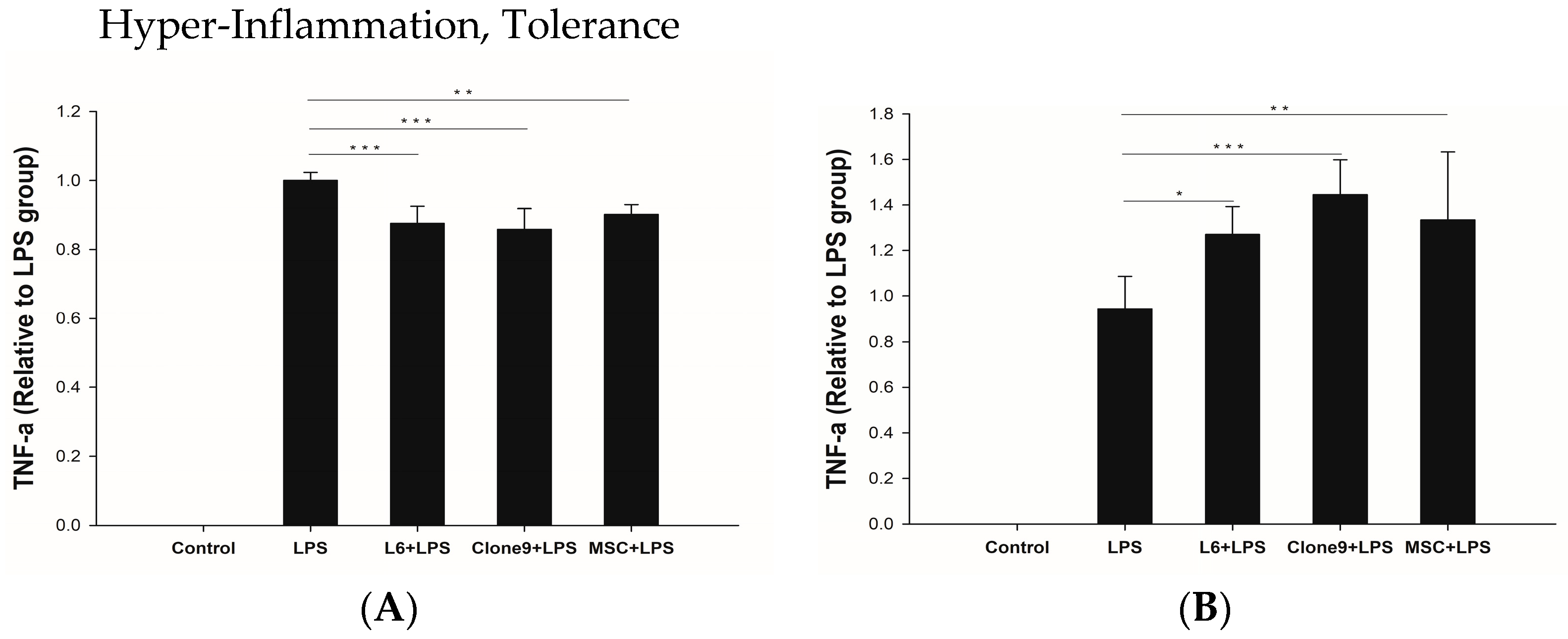
2.3. The Immune Modulation Effects of Mitochondrial Transplantation: In Vitro LPS Stimulation of THP-1
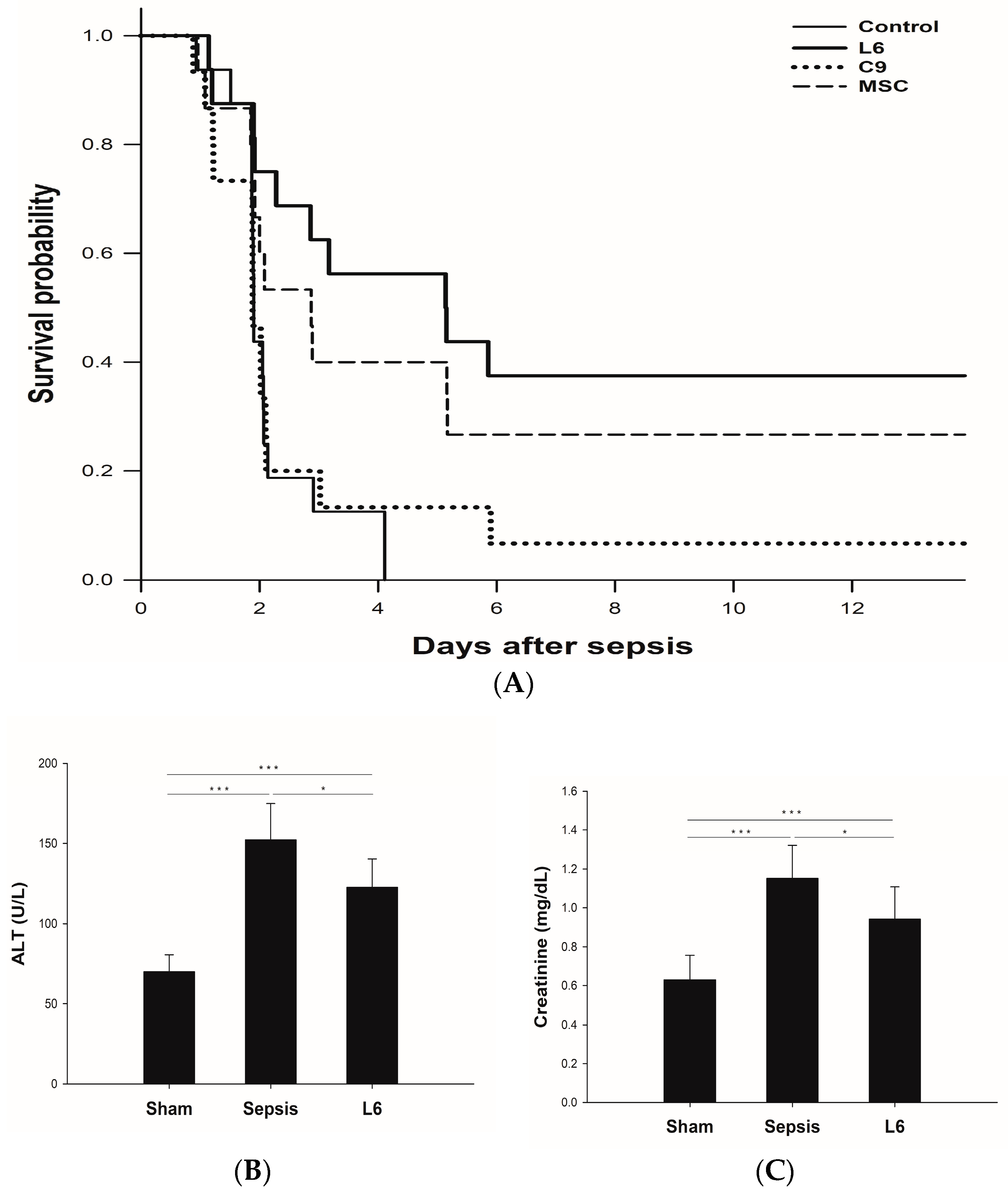
2.4. Survival Study According to the Injection of Mitochondria on Sepsis
2.5. Plasma Chemistry Assay: ALT and Creatinine
2.6. Arterial Blood Gas Analysis
3. Discussion
4. Materials and Methods
4.1. Mitochondria Isolation from Different Cell Types and Mitochondrial Transplantation
4.2. ATP Assay
4.3. Stimulation of THP-1 with LPS
4.4. Measurement of Oxygen Consumption Rate
4.5. In Vivo Rat Sepsis Model
4.6. Quantification of Cytokines
4.7. Serum Alanine Aminotransferase (ALT) and Creatinine Concentration in Plasma
4.8. Arterial Blood Gas Analysis
4.9. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; Del Nido, P.J.; McCully, J.D. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H966–H982. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Liang, M.-Z.; Chen, L. Current progress of mitochondrial transplantation that promotes neuronal regeneration. Transl. Neurodegener. 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, B.; Yavari, R.; Badalzadeh, R.; Mahmoodpoor, A. An Overview on Mitochondrial-Based Therapies in Sepsis-Related Myocardial Dysfunction: Mitochondrial Transplantation as a Promising Approach. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 3277274. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Lee, M.J.; Chung, T.N.; Lee, H.A.R.; Lee, J.H.; Choi, S.Y.; Park, Y.J.; Kim, C.H.; Jin, I.; Kim, S.H. The immune modulatory effects of mitochondrial transplantation on cecal slurry model in rat. Crit. Care 2021, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Roushandeh, A.M.; Kuwahara, Y.; Roudkenar, M.H. Mitochondrial transplantation as a potential and novel master key for treatment of various incurable diseases. Cytotechnology 2019, 71, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; Villena-Gutierrez, R. Cardiac Mitochondrial Transplantation: The Force Awakens; American College of Cardiology Foundation: Washington, DC, USA, 2021; pp. 1089–1092. [Google Scholar]
- Yuan, X.; Logan, T.M.; Ma, T. Metabolism in human mesenchymal stromal cells: A missing link between hMSC biomanufacturing and therapy? Front. Immunol. 2019, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Yao, Y.; Zhao, T.; Chen, Y.-Y.; Shen, Y.-L.; Wang, L.-L.; Zhu, Y. Stem cell-derived mitochondria transplantation: A novel strategy and the challenges for the treatment of tissue injury. Stem Cell Res. Ther. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Yang, B.; Zhou, L.; Ren, F.; Duan, Z.P.; Ma, Y.J. Promotion of mitochondrial energy metabolism during hepatocyte apoptosis in a rat model of acute liver failure. Mol. Med. Rep. 2015, 12, 5035–5041. [Google Scholar] [CrossRef] [PubMed]
- McCully, J.D.; Levitsky, S.; Del Nido, P.J.; Cowan, D.B. Mitochondrial transplantation for therapeutic use. Clin. Transl. Med. 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Wallace, D.C.; Burelle, Y. The rise of mitochondria in medicine. Mitochondrion 2016, 30, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Russell, O.M.; Gorman, G.S.; Lightowlers, R.N.; Turnbull, D.M. Mitochondrial diseases: Hope for the future. Cell 2020, 181, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.; Russell, O.M. Mitochondrial transplantation—A possible therapeutic for mitochondrial dysfunction? Mitochondrial transfer is a potential cure for many diseases but proof of efficacy and safety is still lacking. EMBO Rep. 2020, 21, e50964. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Takegawa, R.; Shoaib, M.; Aoki, T.; Choudhary, R.C.; Kuschner, C.E.; Nishikimi, M.; Miyara, S.J.; Rolston, D.M.; Guevara, S. Mitochondrial transplantation therapy for ischemia reperfusion injury: A systematic review of animal and human studies. J. Transl. Med. 2021, 19, 214. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ma, Y.; Liu, Y.; Wan, Q. Measurement of mitochondrial respiration in adherent cells by Seahorse XF96 Cell Mito Stress Test. STAR Protoc. 2021, 2, 100245. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, K.; Jo, Y.H.; Lee, J.H.; Hwang, J.E. Dose-dependent mortality and organ injury in a cecal slurry peritonitis model. J. Surg. Res. 2016, 206, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Lee, M.J.; Bae, J.; Lee, J.H.; Lee, H.A.R.; Mun, S.; Kim, Y.-s.; Yune, C.J.; Chung, T.N.; Kim, K. Effects of glucocorticoid therapy on sepsis depend both on the dose of steroids and on the severity and phase of the animal sepsis model. Life 2022, 12, 421. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-S.; Lee, H.A.R.; Lee, M.J.; Park, Y.J.; Mun, S.; Yune, C.J.; Chung, T.N.; Bae, J.; Kim, M.J.; Choi, Y.-S.; et al. The Effects of Mitochondrial Transplantation on Sepsis Depend on the Type of Cell from Which They Are Isolated. Int. J. Mol. Sci. 2023, 24, 10113. https://doi.org/10.3390/ijms241210113
Kim Y-S, Lee HAR, Lee MJ, Park YJ, Mun S, Yune CJ, Chung TN, Bae J, Kim MJ, Choi Y-S, et al. The Effects of Mitochondrial Transplantation on Sepsis Depend on the Type of Cell from Which They Are Isolated. International Journal of Molecular Sciences. 2023; 24(12):10113. https://doi.org/10.3390/ijms241210113
Chicago/Turabian StyleKim, Yun-Seok, Han A Reum Lee, Min Ji Lee, Ye Jin Park, Sehwan Mun, Chang June Yune, Tae Nyoung Chung, Jinkun Bae, Mi Jin Kim, Yong-Soo Choi, and et al. 2023. "The Effects of Mitochondrial Transplantation on Sepsis Depend on the Type of Cell from Which They Are Isolated" International Journal of Molecular Sciences 24, no. 12: 10113. https://doi.org/10.3390/ijms241210113
APA StyleKim, Y.-S., Lee, H. A. R., Lee, M. J., Park, Y. J., Mun, S., Yune, C. J., Chung, T. N., Bae, J., Kim, M. J., Choi, Y.-S., & Kim, K. (2023). The Effects of Mitochondrial Transplantation on Sepsis Depend on the Type of Cell from Which They Are Isolated. International Journal of Molecular Sciences, 24(12), 10113. https://doi.org/10.3390/ijms241210113






