The Role of EMT-Related lncRNAs in Ovarian Cancer
Abstract
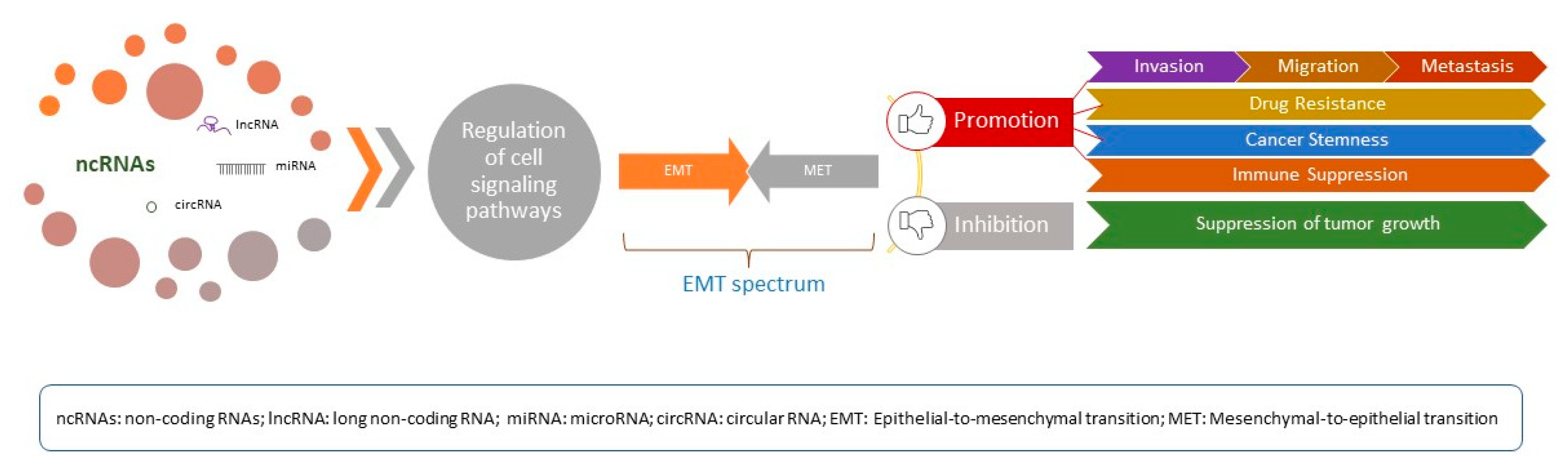
1. Introduction
2. Brief Overview of EMT in Oncology
3. EMT in Ovarian Cancer
4. Regulation of EMT by Long Non-Coding RNAs in Ovarian Cancer: Molecular Mechanisms
5. EMT-Related lncRNAs: Current Evidence
5.1. EMT-Related lncRNAs in Cancer Diagnosis
5.2. EMT-Related lncRNAs in Cancer Prognosis
5.3. EMT-Related lncRNAs as Therapeutic Targets in Cancer
6. LncRNAs as Biomarkers and Therapeutic Targets: Future Perspectives and Challenges
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.; Zuñiga, F.; Elfeky, O.; Guanzon, D.; Lai, A.; Rice, G.E.; Perrin, L.; Hooper, J.; Salomon, C. The potential role of miRNAs and exosomes in chemotherapy in ovarian cancer. Endocr. Relat. Cancer 2018, 25, R663–R685. [Google Scholar] [CrossRef]
- D’Oria, O.; D’Auge, T.G.; Baiocco, E.; Vincenzoni, C.; Mancini, E.; Bruno, V.; Chiofalo, B.; Mancari, R.; Vizza, R.; Cutillo, G.; et al. The role of preoperative frailty assessment in patients affected by gynecological cancer: A narrative review. Ital. J. Gynaecol. Obstet. 2022, 34, 76–83. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef]
- Pal, A.; Barrett, T.F.; Paolini, R.; Parikh, A.; Puram, S.V. Partial EMT in head and neck cancer biology: A spectrum instead of a switch. Oncogene 2021, 40, 5049–5065. [Google Scholar] [CrossRef]
- Coffey, A.J.; Kokocinski, F.; Calafato, M.S.; Scott, C.E.; Palta, P.; Drury, E.; Joyce, C.J.; Leproust, E.M.; Harrow, J.; Hunt, S.; et al. The GENCODE exome: Sequencing the complete human exome. Eur. J. Hum. Genet. 2011, 19, 827–831. [Google Scholar] [CrossRef]
- Mei, Y.; Khan, H.; Shishikura, M.; Ishiyama, S.; Khan, A.; Orita, H.; Brock, M.V. pfeRNAs-A Novel Class of Small Non-coding RNAs With Real Translational Potential. J. Surg. Res. 2023, 284, 237–244. [Google Scholar] [CrossRef]
- Gugnoni, M.; Ciarrocchi, A. Long Noncoding RNA and Epithelial Mesenchymal Transition in Cancer. Int. J. Mol. Sci. 2019, 20, 1924. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in Diagnosis, Prediction, and Oncogenesis of Ovarian Cancer. Biochim. Biophys. Acta Rev. Cancer. 2021, 1875, 188503. [Google Scholar] [CrossRef]
- Kamal, R.; Hamed, S.; Mansour, S.; Mounir, Y.; Sallam, S.A. Ovarian Cancer Screening-Ultrasound; Impact on Ovarian Cancer Mortality. Br. J. Radiol. 2018, 91, 20170571. [Google Scholar] [CrossRef] [PubMed]
- Mor, G.; Visintin, I.; Lai, Y.; Zhao, H.; Schwartz, P.; Rutherford, T.; Yue, L.; Bray-Ward, P.; Ward, D.C. Serum Protein Markers for Early Detection of Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 7677–7682. [Google Scholar] [CrossRef] [PubMed]
- Greenburg, G.; Hay, E.D. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J. Cell Biol. 1982, 95, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Gibbons, D.L.; Kurie, J.M. The role of epithelial-mesenchymal transition programming in invasion and metastasis: A clinical perspective. Cancer Manag. Res. 2013, 5, 187–195. [Google Scholar] [CrossRef]
- Chanda, A.; Sarkar, A.; Bonni, S. The SUMO System and TGFβ Signaling Interplay in Regulation of Epithelial-Mesenchymal Transition: Implications for Cancer Progression. Cancers 2018, 10, 264. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Derynck, R.; Muthusamy, B.P.; Saeteurn, K.Y. Signaling pathway cooperation in TGF-β-induced epithelial-mesenchymal transition. Curr. Opin. Cell Biol. 2014, 31, 56–66. [Google Scholar] [CrossRef]
- Skrypek, N.; Goossens, S.; De Smedt, E.; Vandamme, N.; Berx, G. Epithelial-to-mesenchymal transition: Epigenetic reprogramming driving cellular plasticity. Trends Genet. 2017, 33, 943–959. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.A.; Kraut, N.; Beug, H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 2005, 17, 548–558. [Google Scholar] [CrossRef]
- Grünert, S.; Jechlinger, M.; Beug, H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat. Rev. Mol. Cell Biol. 2003, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.I.; Castillo, M.D.; Litzinger, M.; Hamilton, D.H.; Palena, C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 2011, 71, 5296–5306. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, N.J.; Sasser, A.K.; Axel, A.E.; Vesuna, F.; Raman, V.; Ramirez, N. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene 2009, 28, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Deng, J.; Rychahou, P.G.; Qiu, S.; Evers, B.M.; Zhou, B.P. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell 2009, 15, 416–428. [Google Scholar] [CrossRef]
- Mak, M.P.; Tong, P.; Diao, L.; Cardnell, R.J.; Gibbons, D.L.; William, W.N. A Patient-derived, pan-cancer EMt signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin. Cancer Res. 2016, 22, 609–620. [Google Scholar] [CrossRef]
- Singh, S.; Chakrabarti, R. Consequences of EMT-Driven Changes in the Immune Microenvironment of Breast Cancer and Therapeutic Response of Cancer Cells. J. Clin. Med. 2019, 8, 642. [Google Scholar] [CrossRef]
- Ma, H.Y.; Liu, X.Z.; Liang, C.M. Inflammatory Microenvironment Contributes to Epithelial-Mesenchymal Transition in Gastric Cancer. World J. Gastroenterol. 2016, 22, 6619–6628. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Caserta, C.A.; Rumio, C.; Marcucci, F. The Vicious Cross-Talk between Tumor Cells with an EMT Phenotype and Cells of the Immune System. Cells 2019, 8, 460. [Google Scholar] [CrossRef] [PubMed]
- Napoletano, C.; Bellati, F.; Landi, R.; Pauselli, S.; Marchetti, C.; Visconti, V.; Sale, P.; Liberati, M.; Rughetti, A.; Frati, L.; et al. Ovarian cancer cytoreduction induces changes in T cell population subsets reducing immunosuppression. J. Cell. Mol. Med. 2010, 14, 2748–2759. [Google Scholar] [CrossRef] [PubMed]
- Gasparri, M.L.; Attar, R.; Palaia, I.; Perniola, G.; Marchetti, C.; Di Donato, V.; Farooqi, A.A.; Papadia, A.; Panici, P.B. Tumor infiltrating lymphocytes in ovarian cancer. Asian Pac. J. Cancer Prev. 2015, 16, 3635–3638. [Google Scholar] [CrossRef]
- Wu, C.; Cipollone, J.; Maines-Bandiera, S.; Tan, C.; Karsan, A.; Auersperg, N.; Roskelley, C.D. The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation 2008, 76, 193–205. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian Cancer Development and Metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Shield, K.; Ackland, M.L.; Ahmed, N.; Rice, G.E. Multicellular Spheroids in Ovarian Cancer Metastases:Biology and Pathology. Gynecol. Oncol. 2009, 113, 143–148. [Google Scholar] [CrossRef]
- Klymenko, Y.; Kim, O.; Stack, M.S. Complex Determinants of Epithelial: Mesenchymal Phenotypic Plasticity in Ovarian Cancer. Cancers 2017, 9, 104. [Google Scholar] [CrossRef]
- Gil, O.D.; Lee, C.; Ariztia, E.V.; Wang, F.Q.; Smith, P.J.; Hope, J.M.; Fishman, D.A. Lysophosphatidic acid (LPA) promotes E-cadherin ectodomain shedding and OVCA429 cell invasion in an uPA-dependent manner. Gynecol. Oncol. 2008, 108, 361–369. [Google Scholar] [CrossRef]
- Ray, U.; Roy, S.S.; Chowdhury, S.R. Lysophosphatidic Acid Promotes Epithelial to Mesenchymal Transition in Ovarian Cancer Cells by Repressing SIRT1. Cell. Physiol. Biochem. 2017, 41, 795–805. [Google Scholar] [CrossRef]
- Ha, J.H.; Ward, J.D.; Radhakrishnan, R.; Jayaraman, M.; Song, Y.S.; Dhanasekaran, D.N. Lysophosphatidic acid stimulates epithelial to mesenchymal transition marker Slug/Snail2 in ovarian cancer cells via Gαi2, Src, and HIF1α signaling nexus. Oncotarget 2016, 7, 37664–37679. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Burkhalter, R.; Symowicz, J.; Chaffin, K.; Ellerbroek, S.; Stack, M.S. Lysophosphatidic Acid Disrupts Junctional Integrity and Epithelial Cohesion in Ovarian Cancer Cells. J. Oncol. 2012, 2012, 501492. [Google Scholar] [CrossRef] [PubMed]
- Burkhalter, R.J.; Westfall, S.D.; Liu, Y.; Stack, M.S. Lysophosphatidic Acid Initiates Epithelial to Mesenchymal Transition and Induces β-Catenin-mediated Transcription in Epithelial Ovarian Carcinoma. J. Biol. Chem. 2015, 290, 22143–22154. [Google Scholar] [CrossRef] [PubMed]
- Fishman, D.A.; Liu, Y.; Ellerbroek, S.M.; Stack, M.S. Lysophosphatidic Acid Promotes Matrix Metalloproteinase (MMP) Activation and MMP-Dependent Invasion in Ovarian Cancer Cells. Cancer Res. 2001, 61, 3194–3199. [Google Scholar]
- Vergara, D.; Merlot, B.; Lucot, J.; Collinet, P.; Vinatier, D.; Fournier, I.; Salzet, M. Epithelial–mesenchymal Transition in Ovarian Cancer. Cancer Lett. 2010, 291, 59–66. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, Y.; Steed, H.; Davidge, S.; Fu, Y. TGF_ and EGF Synergistically Induce a More Invasive Phenotype of Epithelial Ovarian Cancer Cells. Biochem. Biophys. Res. Commun. 2010, 401, 376–381. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, X.; Feng, J.; Deng, L.L.; Liu, Y.; Li, B.; Zhu, M.; Lu, C.; Zhou, L. MT2-MMP Induces Proteolysis and Leads to EMT in Carcinomas. Oncotarget 2016, 7, 48193–48205. [Google Scholar] [CrossRef]
- Dahl, K.D.C.; Symowicz, J.; Ning, Y.; Gutierrez, E.; Fishman, D.A.; Adley, B.P.; Stack, M.S.; Hudson, L.G. Matrix Metalloproteinase 9 is a Mediator of Epidermal Growth Factor-Dependent E-Cadherin Loss in Ovarian Carcinoma Cells. Cancer Res. 2008, 68, 4606–4613. [Google Scholar] [CrossRef]
- Covington, M.D.; Burghardt, R.C.; Parrish, A.R. Ischemia-Induced Cleavage of Cadherins in NRK Cells Requires MT1-MMP (MMP-14). Am. J. Physiol. Renal Physiol. 2006, 290, F43–F51. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Kamata, N.; Fujimoto, R.; Tsutsumi, S.; Tomonari, M.; Taki, M.; Hosokawa, H.; Nagayama, M. Increased Invasion and Matrix Metalloproteinase-2 Expression by Snail-Induced Mesenchymal Transition in Squamous Cell Carcinomas. Int. J. Oncol. 2003, 22, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Tsunoda, T.; Seiki, M.; Nakamura, Y.; Furukawa, Y. Identification of Membrane-Type Matrix Metalloproteinase-1 as a Target of the [Beta]-Catenin/Tcf4 Complex in Human Colorectal Cancers. Oncogene 2002, 21, 5861. [Google Scholar] [CrossRef]
- Asem, M.S.; Buechler, S.; Wates, R.B.; Miller, D.L.; Stack, M.S. Wnt5a Signaling in Cancer. Cancers 2016, 8, 79. [Google Scholar] [CrossRef]
- Yin, J.; Zeng, F.; Wu, N.; Kang, K.; Yang, Z.; Yang, H. Interleukin-8 Promotes Human Ovarian Cancer Cell Migration by Epithelial–mesenchymal Transition Induction in Vitro. Clin. Transl. Oncol. 2015, 17, 365–370. [Google Scholar] [CrossRef] [PubMed]
- So, K.A.; Min, K.J.; Hong, J.H.; Lee, J. Interleukin-6 Expression by Interactions between Gynecologic Cancer Cells and Human Mesenchymal Stem Cells Promotes Epithelial-Mesenchymal Transition. Int. J. Oncol. 2015, 47, 1451–1459. [Google Scholar] [CrossRef]
- Fang, X.; Yu, S.; Bast, R.C.; Liu, S.; Xu, H.J.; Hu, S.X.; LaPushin, R.; Claret, F.X.; Aggarwal, B.B.; Lu, Y.; et al. Mechanisms for Lysophosphatidic Acid-Induced Cytokine Production in Ovarian Cancer Cells. J. Biol. Chem. 2004, 279, 9653–9661. [Google Scholar] [CrossRef]
- Tamura, G.; Yin, J.; Wang, S.; Fleisher, A.S.; Zou, T.; Abraham, J.M.; Kong, D.; Smolinski, K.N.; Wilson, K.T.; James, S.P.; et al. E-Cadherin Gene Promoter Hypermethylation in Primary Human Gastric Carcinomas. J. Natl. Cancer Inst. 2000, 92, 569–573. [Google Scholar] [CrossRef]
- Fukagawa, A.; Ishii, H.; Miyazawa, K.; Saitoh, M. δEF1 Associates with DNMT1 and Maintains DNA Methylation of the E-cadherin Promoter in Breast Cancer Cells. Cancer Med. 2015, 4, 125–135. [Google Scholar] [CrossRef]
- Bücker, L.; Lehmann, U. CDH1 (E-cadherin) Gene Methylation in Human Breast Cancer: Critical Appraisal of a Long and Twisted Story. Cancers 2022, 14, 4377. [Google Scholar] [CrossRef]
- Adhikary, A.; Chakraborty, S.; Mazumdar, M.; Ghosh, S.; Mukherjee, S.; Manna, A.; Mohanty, S.; Nakka, K.K.; Joshi, S.; De, A.; et al. Inhibition of Epithelial to Mesenchymal Transition by E-Cadherin Up-Regulation Via Repression of Slug Transcription and Inhibition of E-Cadherin Degradation: Dual Role of Scaffold/Matrix Attachment Region-Binding Protein 1 (SMAR1) in Breast Cancer Cells. J. Biol. Chem. 2014, 289, 25431–25444. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, H.; Vieth, E.; Lee, J.; Segar, M.; Liu, Y.; Nephew, K.P.; Matei, D. TGF-B Induces Global Changes in DNA Methylation during the Epithelial-to-Mesenchymal Transition in Ovarian Cancer Cells. Epigenetics 2014, 9, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhang, C.; Hassan, S.; Biswas, M.H.; Balaji, K.C. Protein Kinase D1 Suppresses Epithelial-to-Mesenchymal Transition through Phosphorylation of Snail. Cancer Res. 2010, 70, 7810–7819. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, H.S.; Kim, N.H.; Ji, S.; Cha, S.Y.; Kang, J.G.; Ota, I.; Shimada, K.; Konishi, N.; Nam, H.W.; et al. Snail1 is Stabilized by O-GlcNAc Modification in Hyperglycaemic Condition. EMBO J. 2010, 29, 3787–3796. [Google Scholar] [CrossRef]
- He, M.; Zhou, Z.; Shah, A.A.; Hong, Y.; Chen, Q.; Wan, Y. New Insights into Posttranslational Modifications of Hippo Pathway in Carcinogenesis and Therapeutics. Cell Div. 2016, 11, 4. [Google Scholar] [CrossRef]
- Long, J.; Zuo, D.; Park, M. Pc2-mediated sumoylation of Smad-interacting protein 1 attenuates transcriptional repression of E-cadherin. J. Biol. Chem. 2005, 280, 35477–35489. [Google Scholar] [CrossRef]
- Xiong, T.; Wang, Y.; Zhang, Y.; Yuan, J.; Zhu, C.; Jiang, W. lncRNA AC005224.4/miR-140-3p/SNAI2 regulating axis facilitates the invasion and metastasis of ovarian cancer through epithelial-mesenchymal transition. Chin. Med. J. 2023, 136, 1098–1110. [Google Scholar] [CrossRef]
- Eoh, K.J.; Lee, D.W.; Nam, E.J.; Kim, J.I.; Moon, H.; Kim, S.W.; Kim, Y.T. HOXA-AS3 induces tumor progression through the epithelial-mesenchymal transition pathway in epithelial ovarian cancer. Oncol. Rep. 2023, 49, 64. [Google Scholar] [CrossRef]
- Dong, L.; Wang, H.; Gao, Y.; Wang, S.; Wang, W. Long non-coding RNA PVT1 promotes the proliferation, migration and EMT process of ovarian cancer cells by regulating CTGF. Oncol. Lett. 2022, 25, 71. [Google Scholar] [CrossRef]
- Chen, Y.; Du, H.; Bao, L.; Liu, W. LncRNA PVT1 promotes ovarian cancer progression by silencing miR-214. Cancer Biol. Med. 2018, 15, 238–250. [Google Scholar] [CrossRef]
- Wang, W.; Yu, S.; Li, W.; Hu, H.; Zou, G. Silencing of lncRNA SNHG17 inhibits the tumorigenesis of epithelial ovarian cancer through regulation of miR-485-5p/AKT1 axis. Biochem. Biophys. Res. Commun. 2022, 637, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Zhang, X.; Zhao, X. Long non-coding RNA OIP5-AS1 suppresses microRNA-92a to augment proliferation and metastasis of ovarian cancer cells through upregulating ITGA6. J. Ovarian Res. 2022, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, J.; Liu, D.; Liu, L. OIP5-AS1/miR-137/ZNF217 Axis Promotes Malignant Behaviors in Epithelial Ovarian Cancer. Cancer Manag. Res. 2020, 12, 6707–6717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Luo, B.H.; Wu, Q.H.; Li, Q.L.; Yang, K.D. LncRNA HCG18 upregulates TRAF4/TRAF5 to facilitate proliferation, migration and EMT of epithelial ovarian cancer by targeting miR-29a/b. Mol. Med. 2022, 28, 2. [Google Scholar] [CrossRef]
- Liu, P.; Huang, H.; Qi, X.; Bian, C.; Cheng, M.; Liu, L.; Xue, L.; Zhao, X.; Yi, T.; Quan, Y. Hypoxia-Induced LncRNA-MIR210HG Promotes Cancer Progression by Inhibiting HIF-1α Degradation in Ovarian Cancer. Front. Oncol. 2021, 11, 701488. [Google Scholar] [CrossRef]
- Jacobs Catane, L.; Moshel, O.; Smith, Y.; Davidson, B.; Reich, R. Splice-Variant Knock-Out of TGFβ Receptors Perturbates the Proteome of Ovarian Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 12647. [Google Scholar] [CrossRef]
- Mao, T.L.; Fan, M.H.; Dlamini, N.; Liu, C.L. LncRNA MALAT1 Facilitates Ovarian Cancer Progression through Promoting Chemoresistance and Invasiveness in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 10201. [Google Scholar] [CrossRef]
- Gordon, M.A.; Babbs, B.; Cochrane, D.R.; Bitler, B.G.; Richer, J.K. The long non-coding RNA MALAT1 promotes ovarian cancer progression by regulating RBFOX2-mediated alternative splicing. Mol. Carcinog. 2019, 58, 196–205. [Google Scholar] [CrossRef]
- Jin, Y.; Feng, S.J.; Qiu, S.; Shao, N.; Zheng, J.H. LncRNA MALAT1 promotes proliferation and metastasis in epithelial ovarian cancer via the PI3K-AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3176–3184. [Google Scholar]
- Xue, Y.; Wang, P.; Jiang, F.; Yu, J.; Ding, H.; Zhang, Z.; Pei, H.; Li, B. A Newly Identified lncBCAS1-4_1 Associated with Vitamin D Signaling and EMT in Ovarian Cancer Cells. Front. Oncol. 2021, 11, 691500. [Google Scholar] [CrossRef]
- Kim, L.K.; Park, S.A.; Yang, Y.; Kim, Y.T.; Heo, T.H.; Kim, H.J. LncRNA SRA mediates cell migration, invasion, and progression of ovarian cancer via NOTCH signaling and epithelial-mesenchymal transition. Biosci. Rep. 2021, 41, BSR20210565. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, G.; Yang, F.; He, L.; Xie, X.; Li, L.; Yang, L.; Ma, Y.; Zhang, Q.; Chen, J.; et al. Elevated LINC00909 Promotes Tumor Progression of Ovarian Cancer via Regulating the miR-23b-3p/MRC2 Axis. Oxidative Med. Cell. Longev. 2021, 2021, 5574130. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, C.; Xu, Y.; Yang, L.; Chen, Y.; Zhu, Y. The LINC00922 aggravates ovarian cancer progression via sponging miR-361-3p. J. Ovarian Res. 2021, 14, 77. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, W. H3K27ac-induced lncRNA PAXIP1-AS1 promotes cell proliferation, migration, EMT and apoptosis in ovarian cancer by targeting miR-6744-5p/PCBP2 axis. J. Ovarian Res. 2021, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tan, S.; Bai, X.; Ma, S.; Chen, X. Long non-coding RNA LINC01215 promotes epithelial-mesenchymal transition and lymph node metastasis in epithelial ovarian cancer through RUNX3 promoter methylation. Transl. Oncol. 2021, 14, 101135. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ren, C.; Wang, B.; Xue, J.; Li, F.; Liu, J.; Yang, L. LncRNA MAFG-AS1 promotes the malignant phenotype of ovarian cancer by upregulating NFKB1-dependent IGF1. Cancer Gene Ther. 2022, 29, 277–291. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lin, F.; Sun, H.; Lin, Y.; Wang, Z.; Wang, X. The lnc-CTSLP8 upregulates CTSL1 as a competitive endogenous RNA and promotes ovarian cancer metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 151. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Yang, L.; Zhang, J. Long Non-coding RNA LINC01969 Promotes Ovarian Cancer by Regulating the miR-144-5p/LARP1 Axis as a Competing Endogenous RNA. Front. Cell Dev. Biol. 2021, 8, 625730. [Google Scholar] [CrossRef]
- Park, S.A.; Kim, L.K.; Kim, Y.T.; Heo, T.H.; Kim, H.J. Long Noncoding RNA E2F4as Promotes Progression and Predicts Patient Prognosis in Human Ovarian Cancer. Cancers 2020, 12, 3626. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, P.; Sun, H.; Liu, Y. LINC01094/miR-577 axis regulates the progression of ovarian cancer. J. Ovarian Res. 2020, 13, 122. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.B.; Liu, Y.M.; Li, F.X.; Zhang, L.P.; Liao, Z.M. LncRNA HOTTIP promotes the proliferation and invasion of ovarian cancer cells by activating the MEK/ERK pathway. Mol. Med. Rep. 2020, 22, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Cao, X.; Luo, Y.; Zhang, G.; Zhang, D. A Positive Feedback Loop of lncRNA DSCR8/miR-98-5p/STAT3/HIF-1α Plays a Role in the Progression of Ovarian Cancer. Front. Oncol. 2020, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhang, L.; Yang, H.; Luo, K.; Qing, C. Long non-coding RNA NEAT1 promotes ovarian cancer cell invasion and migration by interacting with miR-1321 and regulating tight junction protein 3 expression. Mol. Med. Rep. 2020, 22, 3429–3439. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wu, Z.; Rao, D.; Zhuo, B.; Chen, Q. Long non-coding RNA LINC00858 aggravates the oncogenic phenotypes of ovarian cancer cells through miR-134-5p/RAD18 signaling. Arch. Gynecol. Obstet. 2020, 302, 1243–1254. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Lu, T.; Liu, X.; Yin, W.; Zhang, H. LncRNA SNHG8 induces ovarian carcinoma cells cellular process and stemness through Wnt/β-catenin pathway. Cancer Biomark. 2020, 28, 459–471. [Google Scholar] [CrossRef]
- Wang, L.; He, M.; Fu, L.; Jin, Y. Role of lncRNAHCP5/microRNA-525-5p/PRC1 crosstalk in the malignant behaviors of ovarian cancer cells. Exp. Cell Res. 2020, 394, 112129. [Google Scholar] [CrossRef]
- Xu, H.; Zheng, J.F.; Hou, C.Z.; Li, Y.; Liu, P.S. Up-regulation of long intergenic noncoding RNA 01296 in ovarian cancer impacts invasion, apoptosis and cell cycle distribution via regulating EMT. Cell. Signal. 2019, 62, 109341. [Google Scholar] [CrossRef]
- Xu, H.; Mao, H.L.; Zhao, X.R.; Li, Y.; Liu, P.S. MiR-29c-3p, a target miRNA of LINC01296, accelerates tumor malignancy: Therapeutic potential of a LINC01296/miR-29c-3p axis in ovarian cancer. J. Ovarian Res. 2020, 13, 31. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, A.; Song, T.; Gao, F.; Sun, H.; Kong, X. lncRNA MIAT Regulates Cell Growth, Migration, and Invasion Through Sponging miR-150-5p in Ovarian Cancer. Cancer Biother. Radiopharm. 2020, 35, 650–660. [Google Scholar] [CrossRef]
- Xu, C.; Zhu, L.X.; Sun, D.M.; Yao, H.; Han, D.X. Regulatory mechanism of lncRNA NORAD on proliferation and invasion of ovarian cancer cells through miR-199a-3p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1672–1681. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.J.; An, Q. LINC00963 Promotes Ovarian Cancer Proliferation, Migration and EMT via the miR-378g/CHI3L1 Axis. Cancer Manag. Res. 2020, 12, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhang, X.; Zhao, Y.; Qian, H.; Wang, H.; He, C.; Liu, X.; Guo, T.; Lin, M.; Yu, H.; et al. Role of lncRNA-ATB in ovarian cancer and its mechanisms of action. Exp. Ther. Med. 2020, 19, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.; Deng, X.; Luo, M.; Wang, X.; Hu, H.; Liu, C.; Zhong, M. Long noncoding RNA H19 promotes transforming growth factor-β-induced epithelial-mesenchymal transition by acting as a competing endogenous RNA of miR-370-3p in ovarian cancer cells. Onco Targets Ther. 2018, 11, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ding, Y.; Yang, X. Overexpression of Long Noncoding RNA H19 Downregulates miR-140-5p and Activates PI3K/AKT Signaling Pathway to Promote Invasion, Migration and Epithelial-Mesenchymal Transition of Ovarian Cancer Cells. Biomed. Res. Int. 2021, 2021, 6619730. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yu, M.; Yang, R.; Zhang, L.; Zhang, L.; Zhu, D.; Luo, H.; Hong, Y.; Yu, T.; Sun, J.; et al. A PTAL-miR-101-FN1 Axis Promotes EMT and Invasion-Metastasis in Serous Ovarian Cancer. Mol. Ther. Oncolyt. 2019, 16, 53–62. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Wu, L.; Pei, M. Interaction between LncRNA-ROR and miR-145 contributes to epithelial-mesenchymal transition of ovarian cancer cells. Gen. Physiol. Biophys. 2019, 38, 461–471. [Google Scholar] [CrossRef]
- Lou, Y.; Jiang, H.; Cui, Z.; Wang, L.; Wang, X.; Tian, T. Linc-ROR induces epithelial-to-mesenchymal transition in ovarian cancer by increasing Wnt/β-catenin signaling. Oncotarget 2017, 8, 69983–69994. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, X.J.; Tang, X.Y.; Zheng, T.T.; Zhang, X.Y.; Hua, K.Q. Long noncoding RNA TC0101441 induces epithelial-mesenchymal transition in epithelial ovarian cancer metastasis by downregulating KiSS1. Int. J. Cancer 2020, 146, 2588–2598. [Google Scholar] [CrossRef]
- Yan, H.; Li, H.; Silva, M.A.; Guan, Y.; Yang, L.; Zhu, L.; Zhang, Z.; Li, G.; Ren, C. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J. Exp. Clin. Cancer Res. 2019, 38, 356. [Google Scholar] [CrossRef]
- Zhuang, X.H.; Liu, Y.; Li, J.L. Overexpression of long noncoding RNA HOXB-AS3 indicates an unfavorable prognosis and promotes tumorigenesis in epithelial ovarian cancer via Wnt/β-catenin signaling pathway. Biosci. Rep. 2019, 39, BSR20190906. [Google Scholar] [CrossRef]
- Dong, S.; Wang, R.; Wang, H.; Ding, Q.; Zhou, X.; Wang, J.; Zhang, K.; Long, Y.; Lu, S.; Hong, T.; et al. HOXD-AS1 promotes the epithelial to mesenchymal transition of ovarian cancer cells by regulating miR-186-5p and PIK3R3. J. Exp. Clin. Cancer Res. 2019, 38, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dun, Y.; Zhou, S.; Huang, X.H. LncRNA HOXD-AS1 promotes epithelial ovarian cancer cells proliferation and invasion by targeting miR-133a-3p and activating Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2017, 96, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Li, N.; Cui, Y.L. The lncRNA CCAT1 upregulates TGFβR1 via sponging miR-490-3p to promote TGFβ1-induced EMT of ovarian cancer cells. Cancer Cell Int. 2018, 18, 145. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, H.; Ren, F.; Jia, Y.; Zhang, R. Long non-coding RNA CCAT1 promotes metastasis and poor prognosis in epithelial ovarian cancer. Exp. Cell Res. 2017, 359, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Silva, M.A.; Li, H.; Zhu, L.; Li, P.; Li, X.; Wang, X.; Gao, J.; Wang, P.; Zhang, Z. Long noncoding RNA DQ786243 interacts with miR-506 and promotes progression of ovarian cancer through targeting cAMP responsive element binding protein 1. J. Cell. Biochem. 2018, 119, 9764–9780. [Google Scholar] [CrossRef]
- Liang, H.; Yu, T.; Han, Y.; Jiang, H.; Wang, C.; You, T.; Zhao, X.; Shan, H.; Yang, R.; Yang, L.; et al. LncRNA PTAR promotes EMT and invasion-metastasis in serous ovarian cancer by competitively binding miR-101-3p to regulate ZEB1 expression. Mol. Cancer 2021, 17, 119, Erratum in Mol. Cancer 2021, 20, 64. [Google Scholar] [CrossRef]
- Shu, C.; Yan, D.; Mo, Y.; Gu, J.; Shah, N.; He, J. Long noncoding RNA lncARSR promotes epithelial ovarian cancer cell proliferation and invasion by association with HuR and miR-200 family. Am. J. Cancer Res. 2018, 8, 981–992. [Google Scholar]
- Liang, H.; Zhao, X.; Wang, C.; Sun, J.; Chen, Y.; Wang, G.; Fang, L.; Yang, R.; Yu, M.; Gu, Y.; et al. Systematic analyses reveal long non-coding RNA (PTAF)-mediated promotion of EMT and invasion-metastasis in serous ovarian cancer. Mol. Cancer 2018, 17, 96. [Google Scholar] [CrossRef]
- Wang, B.; Liu, M.; Zhuang, R.; Jiang, J.; Gao, J.; Wang, H.; Chen, H.; Zhang, Z.; Kuang, Y.; Li, P. Long non-coding RNA CCAT2 promotes epithelial-mesenchymal transition involving Wnt/β-catenin pathway in epithelial ovarian carcinoma cells. Oncol. Lett. 2018, 15, 3369–3375. [Google Scholar] [CrossRef]
- Mitra, R.; Chen, X.; Greenawalt, E.J.; Maulik, U.; Jiang, W.; Zhao, Z.; Eischen, C.M. Decoding critical long non-coding RNA in ovarian cancer epithelial-to-mesenchymal transition. Nat. Commun. 2017, 8, 1604. [Google Scholar] [CrossRef]
- Yim, G.W.; Kim, H.J.; Kim, L.K.; Kim, S.W.; Kim, S.; Nam, E.J.; Kim, Y.T. Long Non-coding RNA HOXA11 Antisense Promotes Cell Proliferation and Invasion and Predicts Patient Prognosis in Serous Ovarian Cancer. Cancer Res. Treat. 2017, 49, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.J.; Lin, Y.Y.; Ye, L.C.; Ding, J.X.; Feng, W.W.; Jin, H.Y.; Zhang, Y.; Li, Q.; Hua, K.Q. Overexpression of long non-coding RNA HOTAIR predicts poor patient prognosis and promotes tumor metastasis in epithelial ovarian cancer. Gynecol. Oncol. 2014, 134, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tang, X.; Zheng, T.; Qiu, J.; Hua, K. Long non-coding RNA AOC4P suppresses epithelial ovarian cancer metastasis by regulating epithelial-mesenchymal transition. J. Ovarian Res. 2020, 13, 45. [Google Scholar] [CrossRef]
- Tian, J.; Yang, L.; Wang, Z.; Yan, H. MIR503HG impeded ovarian cancer progression by interacting with SPI1 and preventing TMEFF1 transcription. Aging 2022, 14, 5390–5405. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, H.; Chen, J. Linc00261 Inhibited High-Grade Serous Ovarian Cancer Progression through miR-552-ATG10-EMT Axis. Comput. Math. Methods Med. 2022, 2022, 9450353. [Google Scholar] [CrossRef]
- Lv, W.; Jia, Y.; Wang, J.; Duan, Y.; Wang, X.; Liu, T.; Hao, S.; Liu, L. Long non-coding RNA SNHG10 upregulates BIN1 to suppress the tumorigenesis and epithelial-mesenchymal transition of epithelial ovarian cancer via sponging miR-200a-3p. Cell Death Discov. 2022, 8, 60. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Sun, J.; Hu, C.; Ge, X.; Li, R. LncRNA HCG11 represses ovarian cancer cell growth via AKT signaling pathway. J. Obstet. Gynaecol. Res. 2022, 48, 796–805. [Google Scholar] [CrossRef]
- Yang, J.; Peng, S.; Zhang, K. LncRNA RP11-499E18.1 Inhibits Proliferation, Migration, and Epithelial-Mesenchymal Transition Process of Ovarian Cancer Cells by Dissociating PAK2-SOX2 Interaction. Front. Cell Dev. Biol. 2021, 9, 697831. [Google Scholar] [CrossRef]
- Hao, T.; Huang, S.; Han, F. LINC-PINT suppresses tumour cell proliferation, migration and invasion through targeting miR-374a-5p in ovarian cancer. Cell Biochem. Funct. 2020, 38, 1089–1099. [Google Scholar] [CrossRef]
- Li, W.; Ma, S.; Bai, X.; Pan, W.; Ai, L.; Tan, W. Long noncoding RNA WDFY3-AS2 suppresses tumor progression by acting as a competing endogenous RNA of microRNA-18a in ovarian cancer. J. Cell. Physiol. 2020, 235, 1141–1154. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Xie, H.; Yuan, J.; Jiang, X.Y.; Yong, J.H.; Zeng, D.; Dou, Y.Y.; Xiao, S.S. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol. Ther. 2019, 20, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Jin, C.; Li, H.; Qin, Q.; Li, L. LncRNA ADAMTS9-AS2 regulates ovarian cancer progression by targeting miR-182-5p/FOXF2 signaling pathway. Int. J. Biol. Macromol. 2018, 120, 1705–1713. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.X.; Sun, G.; Shangguan, M.Y.; Gui, Z.; Bao, Y.; Li, Y.F.; Jia, Z.H. Novel role of lncRNA CHRF in cisplatin resistance of ovarian cancer is mediated by miR-10b induced EMT and STAT3 signaling. Sci. Rep. 2020, 10, 14768. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; He, J.; Sun, H.; Jin, Z. Long non-coding RNA H19 mediates ovarian cancer cell cisplatin-resistance and migration during EMT. Int. J. Clin. Exp. Pathol. 2019, 12, 2506–2515. [Google Scholar] [PubMed]
- Naghsh-Nilchi, A.; Ebrahimi Ghahnavieh, L.; Dehghanian, F. Construction of miRNA-lncRNA-mRNA co-expression network affecting EMT-mediated cisplatin resistance in ovarian cancer. J. Cell. Mol. Med. 2022, 26, 4530–4547. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Y.; Cheng, H.; Tian, J.; Yang, S. Roles of a TMPO-AS1/microRNA-200c/TMEFF2 ceRNA network in the malignant behaviors and 5-FU resistance of ovarian cancer cells. Exp. Mol. Pathol. 2020, 115, 104481. [Google Scholar] [CrossRef]
- Weber, D.G.; Johnen, G.; Casjens, S.; Bryk, O.; Pesch, B.; Jöckel, K.H.; Kollmeier, J.; Brüning, T. Evaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancer. BMC Res. Notes 2013, 6, 518. [Google Scholar] [CrossRef]
- Smolle, M.A.; Bauernhofer, T.; Pummer, K.; Calin, G.A.; Pichler, M. Current insights into long non-coding RNAs (LncRNAs) in prostate cancer. Int. J. Mol. Sci. 2017, 18, 473. [Google Scholar] [CrossRef]
- Ren, S.; Wang, F.; Shen, J.; Sun, Y.; Xu, W.; Lu, J.; Wei, M.; Xu, C.; Wu, C.; Zhang, Z.; et al. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur. J. Cancer 2013, 49, 2949–2959. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, Z.; Hu, M.; Luo, X.; Cui, Z. Diagnostic efficacy of long noncoding RNA MALAT-1 in human cancers: A meta-analysis study. Oncotarget 2017, 8, 102291–102300. [Google Scholar] [CrossRef]
- Hu, X.; Bao, J.; Wang, Z.; Zhang, Z.; Gu, P.; Tao, F.; Cui, D.; Jiang, W. The plasma lncRNA acting as fingerprint in non-small-cell lung cancer. Tumour Biol. 2016, 37, 3497–3504. [Google Scholar] [CrossRef] [PubMed]
- Senousy, M.A.; El-Abd, A.M.; Abdel-Malek, R.R.; Rizk, S.M. Circulating long non-coding RNAs HOTAIR, Linc-p21, GAS5 and XIST expression profiles in diffuse large B-cell lymphoma: Association with R-CHOP responsiveness. Sci. Rep. 2021, 11, 2095. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.K.; Pastori, C.; Penas, C.; Komotar, R.J.; Ivan, M.E.; Wahlestedt, C.; Ayad, N.G. Serum long noncoding RNA HOTAIR as a novel diagnostic and prognostic biomarker in glioblastoma multiforme. Mol. Cancer 2018, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, X.; Zheng, Z.; Ma, X.; Hu, X.; Wu, D.; Wang, M. Serum HOTAIR as a novel diagnostic biomarker for esophageal squamous cell carcinoma. Mol. Cancer 2017, 16, 75. [Google Scholar] [CrossRef]
- Zhang, K.; Luo, Z.; Zhang, Y.; Zhang, L.; Wu, L.; Liu, L.; Yang, J.; Song, X.; Liu, J. Circulating lncRNA H19 in plasma as a novel biomarker for breast cancer. Cancer Biomark. 2016, 17, 187–194. [Google Scholar] [CrossRef]
- Hashad, D.; Elbanna, A.; Ibrahim, A.; Khedr, G. Evaluation of the Role of Circulating Long Non-Coding RNA H19 as a Promising Novel Biomarker in Plasma of Patients with Gastric Cancer. J. Clin. Lab. Anal. 2016, 30, 1100–1105. [Google Scholar] [CrossRef]
- Lan, X.; Sun, W.; Dong, W.; Wang, Z.; Zhang, T.; He, L.; Zhang, H. Downregulation of long noncoding RNA H19 contributes to the proliferation and migration of papillary thyroid carcinoma. Gene 2018, 646, 98–105. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, Y.; Zhang, X.; Yang, Y.; Zheng, X.; Li, X.; Liu, Y.; Zhang, Y. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer. Mol. Cancer 2018, 17, 68. [Google Scholar] [CrossRef]
- Zhao, W.; Song, M.; Zhang, J.; Kuerban, M.; Wang, H. Combined identification of long non-coding RNA CCAT1 and HOTAIR in serum as an effective screening for colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 14131–14140. [Google Scholar]
- Cao, X.; Yao, J.; Jia, M.; Shen, X.; Zhang, J.; Ju, S. Serum CCAT2 as a biomarker for adjuvant diagnosis and prognostic prediction of cervical cancer. J. Ovarian Res. 2022, 15, 20. [Google Scholar] [CrossRef]
- Qiu, J.J.; Lin, X.J.; Tang, X.Y.; Zheng, T.T.; Lin, Y.Y.; Hua, K.Q. Exosomal metastasis-associated lung adenocarcinoma transcript 1 promotes angiogenesis and predicts poor prognosis in epithelial ovarian cancer. Int. J. Biol. Sci. 2018, 14, 1960–1973. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.T.; Shi, D.B.; Wang, Y.W.; Li, X.X.; Xu, Y.; Tripathi, P.; Gu, W.L.; Cai, G.X.; Cai, S.J. High expression of lncRNA MALAT1 suggests a biomarker of poor prognosis in colorectal cancer. Int. J. Clin. Exp. Pathol. 2014, 7, 3174–3181. [Google Scholar]
- Lai, M.C.; Yang, Z.; Zhou, L.; Zhu, Q.Q.; Xie, H.Y.; Zhang, F.; Wu, L.M.; Chen, L.M.; Zheng, S.S. Long non-coding RNA MALAT-1 overexpression predicts tumor recurrence of hepatocellular carcinoma after liver transplantation. Med. Oncol. 2012, 29, 1810–1816. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Spieker, T.; Koschmieder, S.; Schäffers, S.; Humberg, J.; Jungen, D.; Bulk, E.; Hascher, A.; Wittmer, D.; Marra, A.; et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. Thorac. Oncol. 2011, 6, 1984–1992, Erratum in J. Thorac. Oncol. 2012, 7, 1206. [Google Scholar] [CrossRef]
- Wang, Z.; Katsaros, D.; Biglia, N.; Shen, Y.; Fu, Y.; Loo, L.W.M.; Jia, W.; Obata, Y.; Yu, H. High expression of long non-coding RNA MALAT1 in breast cancer is associated with poor relapse-free survival. Breast Cancer Res. Treat. 2018, 171, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Gao, G.; Bazhabayi, M.; Zhang, K.; Liu, F.; Xiao, X. MALAT1 and BACH1 are prognostic biomarkers for triple-negative breast cancer. J. Cancer Res. Ther. 2019, 15, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhao, Z.Y.; Wu, R.; Zhang, Y.; Zhang, Z.Y. Prognostic value of long noncoding RNAs in gastric cancer: A meta-analysis. Onco Targets Ther. 2018, 11, 4877–4891. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Z.; Yang, H.; Lin, Y. Increased MALAT1 expression predicts poor prognosis in esophageal cancer patients. Biomed. Pharmacother. 2016, 83, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, P.; Mao, G.; Deng, J.; Peng, G.; Ning, X.; Yang, H.; Sun, H. Long non-coding RNA MALAT1 as a valuable biomarker for prognosis in osteosarcoma: A systematic review and meta-analysis. Int. J. Surg. 2019, 72, 206–213. [Google Scholar] [CrossRef]
- Ostano, P.; Mello-Grand, M.; Sesia, D.; Gregnanin, I.; Peraldo-Neia, C.; Guana, F.; Jachetti, E.; Farsetti, A.; Chiorino, G. Gene Expression Signature Predictive of Neuroendocrine Transformation in Prostate Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 1078. [Google Scholar] [CrossRef]
- Sun, X.; Du, P.; Yuan, W.; Du, Z.; Yu, M.; Yu, X.; Hu, T. Long non-coding RNA HOTAIR regulates cyclin J via inhibition of microRNA-205 expression in bladder cancer. Cell Death Dis. 2015, 6, e1907. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Shiroki, T.; Nakagawa, T.; Yokoyama, M.; Tamai, K.; Yamanami, H.; Fujiya, T.; Sato, I.; Yamaguchi, K.; Tanaka, N.; et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS ONE 2013, 8, e77070. [Google Scholar] [CrossRef] [PubMed]
- Kogo, R.; Shimamura, T.; Mimori, K.; Kawahara, K.; Imoto, S.; Sudo, T.; Tanaka, F.; Shibata, K.; Suzuki, A.; Komune, S.; et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011, 71, 6320–6326, Erratum in Cancer Res. 2012, 72, 1039. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhou, X.; Chang, H.; Li, H.; Liu, F.; Ma, C.; Lu, J. CCAT1 promotes hepatocellular carcinoma cell proliferation and invasion. Int. J. Clin. Exp. Pathol. 2015, 8, 5427–5434. [Google Scholar] [PubMed]
- Lin, H.; Cheng, W.; Yan, H.; Zhang, X. Overexpression of the long noncoding RNA CCAT1 promotes metastasis via epithelial-tomesenchymal transition in lung adenocarcinoma. Oncol. Lett. 2018, 16, 1809–1814. [Google Scholar] [CrossRef]
- Jiang, X.M.; Li, Z.L.; Li, J.L.; Zheng, W.Y.; Li, X.H.; Cui, Y.F.; Sun, D.J. LncRNA CCAT1 as the unfavorable prognostic biomarker for cholangiocarcinoma. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1242–1247. [Google Scholar]
- Zhang, T.J.; Zhou, J.D.; Zhang, W.; Lin, J.; Ma, J.C.; Wen, X.M.; Yuan, Q.; Li, X.X.; Xu, Z.J.; Qian, J. H19 overexpression promotes leukemogenesis and predicts unfavorable prognosis in acute myeloid leukemia. Clin. Epigenet. 2018, 10, 47. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Ling, H.; Ivan, C.; Pichler, M.; Matsushita, D.; Goblirsch, M.; Stiegelbauer, V.; Shigeyasu, K.; Zhang, X.; Chen, M.; et al. H19 Noncoding RNA, an Independent Prognostic Factor, Regulates Essential Rb-E2F and CDK8-β-Catenin Signaling in Colorectal Cancer. EBioMedicine 2016, 13, 113–124. [Google Scholar] [CrossRef]
- Shi, G.; Li, H.; Gao, F.; Tan, Q. lncRNA H19 predicts poor prognosis in patients with melanoma and regulates cell growth, invasion, migration and epithelial-mesenchymal transition in melanoma cells. Onco Targets Ther. 2018, 11, 3583–3595. [Google Scholar] [CrossRef]
- Quagliata, L.; Matter, M.S.; Piscuoglio, S.; Arabi, L.; Ruiz, C.; Procino, A.; Kovac, M.; Moretti, F.; Makowska, Z.; Boldanova, T.; et al. Long noncoding RNA HOTTIP/HOXA13 expression is associated with disease progression and predicts outcome in hepatocellular carcinoma patients. Hepatology 2014, 59, 911–923. [Google Scholar] [CrossRef]
- Ye, H.; Liu., K.; Qian., K. Overexpression of long noncoding RNA HOTTIP promotes tumor invasion and predicts poor prognosis in gastric cancer. Onco Targets Ther. 2016, 9, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qian, J.; Xiang, Y.; Chen, Y.; Qu, J. The prognostic value of long noncoding RNA HOTTIP on clinical outcomes in breast cancer. Oncotarget 2017, 8, 6833–6844. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.K.; Xiao, Y.; Wan, X.B.; Zhao, Y.Z.; Li, J.; Li, Y.; Han, G.S.; Chen, X.B.; Zou, Q.Y.; Wang, G.C.; et al. Association of long non-coding RNA HOTTIP with progression and prognosis in colorectal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11458–11463. [Google Scholar] [PubMed]
- Li, F.; Cao, L.; Hang, D.; Wang, F.; Wang, Q. Long non-coding RNA HOTTIP is up-regulated and associated with poor prognosis in patients with osteosarcoma. Int. J. Clin. Exp. Pathol. 2015, 8, 11414–11420. [Google Scholar] [PubMed]
- Hanna, N.; Ohana, P.; Konikoff, F.M.; Leichtmann, G.; Hubert, A.; Appelbaum, L.; Kopelman, Y.; Czerniak, A.; Hochberg, A. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012, 19, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Lavie, O.; Edelman, D.; Levy, T.; Fishman, A.; Hubert, A.; Segev, Y.; Raveh, E.; Gilon, M.; Hochberg, A. A phase 1/2a, dose-escalation, safety, pharmacokinetic, and preliminary efficacy study of intraperitoneal administration of BC-819 (H19-DTA) in subjects with recurrent ovarian/peritoneal cancer. Arch. Gynecol. Obstet. 2017, 295, 751–761. [Google Scholar] [CrossRef]
- Smaldone, M.C.; Davies, B.J. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr. Opin. Mol. Ther. 2010, 12, 607–616. [Google Scholar]
- Ozcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–109. [Google Scholar] [CrossRef]
- Renganathan, A.; Felley-Bosco, E. Long noncoding RNAs in cancer and therapeutic potential. Adv. Exp. Med. Biol. 2017, 1008, 199–222. [Google Scholar] [CrossRef]
- Gong, N.; Teng, X.; Li, J.; Liang, X.J. Antisense oligonucleotide-conjugated nanostructure-targeting lncRNA MALAT1 inhibits cancer metastasis. ACS Appl. Mater. Interfaces 2019, 11, 37–42. [Google Scholar] [CrossRef]
- Tinzl, M.; Marberger, M.; Horvath, S.; Chypre, C. DD3PCA3 RNA analysis in urine—A new perspective for detecting prostate cancer. Eur. Urol. 2004, 46, 182–187. [Google Scholar] [CrossRef]
- Isin, M.; Ozgur, E.; Cetin, G.; Erten, N.; Aktan, M.; Gezer, U.; Dalay, N. Investigation of circulating lncRNAs in B-cell neoplasms. Clin. Chim. Acta 2014, 431, 255–259. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, K.; Wang, Z.; Wang, Y.; Liu, J.; Lin, L.; Shao, Y.; Gao, L.; Yin, H.; Cui, C.; et al. DNA in serum extracellular vesicles is stable under different storage conditions. BMC Cancer 2016, 16, 753. [Google Scholar] [CrossRef]
- Zhang, H.G.; Grizzle, W.E. Exosomes: A novel pathway of local and distant intercellular communication that facilitates the growth and metastasis of neoplastic lesions. Am. J. Pathol. 2014, 184, 28–41. [Google Scholar] [CrossRef]
- Ono, H.; Motoi, N.; Nagano, H.; Miyauchi, E.; Ushijima, M.; Matsuura, M.; Okumura, S.; Nishio, M.; Hirose, T.; Inase, N.; et al. Long noncoding RNA HOTAIR is relevant to cellular proliferation, invasiveness, and clinical relapse in small-cell lung cancer. Cancer Med. 2014, 3, 632–642. [Google Scholar] [CrossRef]
- Qi, P.; Zhou, X.Y.; Du, X. Circulating long non-coding RNAs in cancer: Current status and future perspectives. Mol. Cancer 2016, 15, 39. [Google Scholar] [CrossRef]
- Shi, J.; Li, X.; Zhang, F.; Zhang, C.; Guan, Q.; Cao, X.; Zhu, W.; Zhang, X.; Cheng, Y.; Ou, K.; et al. Circulating lncRNAs associated with occurrence of colorectal cancer progression. Am. J. Cancer Res. 2015, 5, 2258–2265. [Google Scholar] [PubMed]
- Kunej, T.; Obsteter, J.; Pogacar, Z.; Horvat, S.; Calin, G.A. The decalog of long non-coding RNA involvement in cancer diagnosis and monitoring. Crit. Rev. Clin. Lab. Sci. 2014, 51, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Solanas, M.; Moral, R.; Garcia, G.; Grau, L.; Vela, E.; Escrich, R.; Costa, I.; Escrich, E. Differential expression of H19 and vitamin D3 upregulated protein 1 as a mechanism of the modulatory effects of high virgin olive oil and high corn oil diets on experimental mammary tumours. Eur. J. Cancer Prev. 2009, 1, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Faraldi, M.; Gomarasca, M.; Sansoni, V.; Perego, S.; Banfi, G.; Lombardi, G. Normalization strategies differently affect circulating miRNA profile associated with the training status. Sci. Rep. 2019, 9, 1584. [Google Scholar] [CrossRef]
- Gezer, U.; Özgür, E.; Cetinkaya, M.; Isin, M.; Dalay, N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell. Biol. Int. 2014, 38, 1076–1079. [Google Scholar] [CrossRef] [PubMed]

| lncRNA | Expression | Result | Expression of EMT Markers/Genes | Potential Application | Subtype of OC | Ref. |
|---|---|---|---|---|---|---|
| AC005224.4 | ↑ | EMT promotion in vitro | AC005224.4 overexpression resulted in decreased E-cadherin expression and increased expressions of N-cadherin, Snail, and vimentin, whereas its knockdown led to the opposite effects | Therapeutic target | - | [67] |
| HOXA-AS3 | ↑ | EMT promotion in vitro | HOXA-AS3 knockdown resulted in E-cadherin upregulation and β-catenin, AKT, and vimentin downregulation | Prognostic marker and therapeutic target | EOC | [68] |
| PVT1 | ↑ | EMT promotion in vitro | PVT1 knockdown suppressed CTGF and vimentin expression but led to E-cadherin overexpression | Therapeutic target | - | [69] |
| EMT promotion | PVT1 knockdown resulted in (i) the downregulation of vimentin, β-catenin, transcription factors, Snail, and Slug and (ii) E-cadherin upregulation | Diagnostic marker and therapeutic target | EOC | [70] | ||
| lncRNA SNHG17 | ↑ | EMT promotion | SNHG17 knockdown upregulated E-cadherin level and reduced the levels of N-cadherin and vimentin | Therapeutic target | EOC | [71] |
| OIP5-AS1 | ↑ | EMT promotion | OIP5-AS1 inhibition increased E-cadherin and decreased vimentin expression | Therapeutic target | - | [72] |
| OIP5-AS1 knockdown increased E-cadherin levels and decreased N-cadherin, vimentin, Slug, and Twist levels | Therapeutic target | EOC | [73] | |||
| HCG18 | ↑ | EMT promotion | HCG18 knockdown increased E-cadherin levels, decreased MMP2, MMP9, and vimentin, and downregulated ZEB1, Slug, TWIST1, and Snail | Therapeutic target | EOC | [74] |
| MIR210HG | ↑ (under hypoxia conditions) | EMT promotion | MIR210HG knockdown increased E-cadherin and decreased N-cadherin expression under hypoxic conditions | Therapeutic target | - | [75] |
| MALAT1 | ↑ | EMT promotion | Upregulation of MALAT 1 resulted in increased levels of SNAIL protein | Therapeutic target | - | [76] |
| Diagnostic marker/therapeutic target | EOC | [77] | ||||
| MALAT1-regulated RBFOX2 plays a pro-tumorigenic role in OC, and one of its potential targets, KIF1B, may also affect tumorigenicity | - | EOC | [78] | |||
| - | Therapeutic target | EOC | [79] | |||
| lncBCAS1-4_1 | ↑ | EMT promotion | lncBCAS1-4_1 upregulation led to significant increase of N-cadherin and vimentin as well as EMT-related transcriptional factor (ZEB1) | Therapeutic target | - | [80] |
| SRA | ↑ | EMT promotion | SRA knockdown increased the levels of E-cadherin and decreased the expression of β-catenin, N-cadherin, Snail, and vimentin; SRA overexpression triggered the opposite effects | Predictive biomarker | - | [81] |
| LINC00909 | ↑ | EMT promotion | Ectopic LINC00909 expression decreased E-cadherin and increased N-cadherin/vimentin levels; LINC00909 knockdown partially reversed the process of EMT | Diagnostic biomarker | - | [82] |
| LINC00922 | ↑ | EMT promotion | LINC00922 knockdown led to E-cadherin overexpression and inhibits the expression of vimentin | Therapeutic target | - | [83] |
| PAXIP1-AS1 | ↑ | EMT promotion | PAXIP1-AS1 knockdown led to decreased levels of MMP2, MMP9, and N-cadherin and increased E-cadherin expression | - | - | [84] |
| LINC01215 | ↑ | EMT promotion | LINC01215 knockdown led to decreased expressions of MMP-2, MMP-9, and vimentin and increased the expression of E-cadherin | Therapeutic target | EOC | [85] |
| MAFG-AS1 | ↑ | EMT promotion | MAFG-AS1 led to increased E-cadherin expression and reduced N-cadherin, MMP-2, MMP-9, and vimentin expression | Therapeutic target | - | [86] |
| CTSLP8 | ↑ | EMT promotion | CTSLP8 knockdown increased E-cadherin expression and decreased N-cadherin expression. In addition, overexpression of CTSLP8 upregulated, while CTSLP8 knockout suppressed the expression of ZEB1 and Snail | Therapeutic target | - | [87] |
| LINC01969 | ↑ | EMT promotion | LINC01969 knockdown increased E-cadherin expression but decreased Snail and vimentin expression levels | Prognostic biomarker | - | [88] |
| E2F4as | ↑ | EMT promotion | E2F4as knockdown resulted in increased expression of E-cadherin and decreased expression of N-cadherin, β-catenin, vimentin, Wnt-5β, Snail, and claudin-1 expression | Diagnostic, prognostic biomarker and therapeutic target | - | [89] |
| LINC01094 | ↑ | EMT promotion | LINC01094 knockdown upregulated E-cadherin expression and downregulated vimentin expression | Therapeutic target | - | [90] |
| HOTTIP | ↑ | EMT promotion | HOTTIP knockdown led to elevated E-cadherin and reduced N-cadherin, vimentin, and Snail expression | Therapeutic target | - | [91] |
| DSCR8 | ↑ | EMT promotion | DSCR8 overexpression decreased E-cadherin expression and increased N-cadherin and vimentin expression | Therapeutic target | - | [92] |
| NEAT1 | ↑ | EMT promotion | NEAT1 knockdown led to E-cadherin upregulation and N-cadherin and vimentin downregulation | Therapeutic target | - | [93] |
| LINC00858 | ↑ | EMT promotion | LINC00858 knockdown led to E-cadherin activation and N-cadherin, Slug, and Twist protein inhibition | - | - | [94] |
| SNHG8 | ↑ | EMT promotion | SNHG8 knockdown led to increased expression of E-cadherin and decreased levels of N-cadherin and Snail | - | - | [95] |
| HCP5 | ↑ | EMT promotion | HCP5 silencing led to E-cadherin upregulation and vimentin downregulation | Therapeutic potential | - | [96] |
| LINC01296 | ↑ | EMT promotion | LINC01296 knock down led to increased E-cadherin expression and decreased expression of N-cadherin and vimentin | Diagnostic and prognostic biomarker and therapeutic target | - | [97] |
| [98] | ||||||
| MIAT | ↑ | EMT promotion | MIAT knockdown led to increased expression of E-cadherin and decreased expression of N-cadherin, Snail, and ZEB1 | Prognostic biomarker/therapeutic target | - | [99] |
| NORAD | ↑ | EMT promotion | NORAD knockdown led to increased levels of E-cadherin and decreased N-cadherin and vimentin levels, whereas overexpression of NORAD, triggered opposite effects | Therapeutic target | - | [100] |
| LINC00963 | ↑ | EMT promotion | LINC00963 knockdown increased E-cadherin and decreased vimentin levels | Therapeutic target | - | [101] |
| ATB | ↑ | EMT promotion | LncRNA-ATB knockdown decreased the expression of p-STAT3 and vimentin and increased E-cadherin expression | Therapeutic target | - | [102] |
| H19 | ↑ | EMT promotion | H19 knockdown increased E-cadherin levels and decreased the levels of Snail and vimentin, whereas overexpression of H19 had the opposite effect | Therapeutic target | - | [103] |
| H19 | ↑ | EMT promotion | H19 overexpression led to increased E-cadherin and vimentin and decreased N-cadherin | Therapeutic target | - | [104] |
| AC004988.1 (PTAL) | ↑ in mesenchymal subtype samples | EMT promotion | PTAL silencing resulted in E-cadherin and ZO-1 upregulation and N-cadherin, vimentin, and Slug downregulation | Therapeutic target | serous OC | [105] |
| ROR | ↑ | EMT promotion | ROR knockdown led to increased E-cadherin expression and decreased N-cadherin and vimentin expression | Therapeutic target | - | [106] |
| ROR | ↑ | EMT promotion | ROR silencing led to higher E-cadherin expression and lower vimentin, β-catenin, and c-myc levels | Therapeutic target | HGSOC | [107] |
| TC0101441 | ↑ | EMT promotion | TC0101441 knockdown increased the expression of E-cadherin but decreased the expression of N-cadherin and Snail | Prognostic marker and Therapeutic target | EOC | [108] |
| FLVCR1-AS1 | ↑ | EMT promotion | FLVCR1-AS1 knockdown upregulated E-cadherin expression and downregulated vimentin and Snail expression | Therapeutic target | serous OC | [109] |
| HOXB-AS3 | ↑ | EMT promotion | HOXB-AS3 knockdown led to elevation of E-cadherin levels and repressed N-cadherin and vimentin levels | Prognostic biomarker and Therapeutic target | EOC | [110] |
| HOXD-AS1 | ↑ | EMT promotion | HOXD-AS1 downregulation, upregulated E-cadherin expression and downregulated vimentin expression | Therapeutic target | EOC | [111,112] |
| CCAT1 | ↑ | EMT promotion | CCAT1 knockdown enhanced E-cadherin and claudin expression and reduced vimentin, N-cadherin, and MMP9 expression | Prognostic biomarker and therapeutic target | - | [113] |
| CCAT1 downregulation promoted E-cadherin expression and reduced vimentin and N-cadherin expression, while CCAT1 upregulation had the opposite results | Diagnostic and prognostic biomarker and therapeutic target | EOC | [114] | |||
| DQ786243 | ↑ | EMT promotion | HOXA-AS3 knockdown resulted in E-cadherin upregulation and β-catenin, AKT, and vimentin downregulation in OC cell lines | Therapeutic target | - | [115] |
| PTAR (see erratum) | ↑ | EMT promotion | PTAR downregulation led to increased E-cadherin expression and reduced fibronectin1, ZEB1, and vimentin expression | Therapeutic target | serous OC | [116] |
| ARSR | ↑ | EMT promotion | Overexpression of lncARSR reduced E-cadherin and ZO-1 and increased N-cadherin and vimentin | Therapeutic target | EOC | [117] |
| PTAF | ↑ | EMT promotion | PTAF knockdown led to increased E-cadherin expression and decreased SNAI2 expression | Therapeutic target | serous OC | [118] |
| CCAT2 | ↑ | EMT promotion | CCAT2 knockdown led to upregulation of E-cadherin and downregulation of N-cadherin, SNAI, and Twist | Therapeutic target | EOC | [119] |
| DNM3OS, MEG3, and MIAT | ↑ | EMT promotion | DNM3OS knockdown led to elevated of E-cadherin and reduced levels of N-cadherin, Snail, and Slug | Therapeutic target | HGSOC | [120] |
| HOXA11 | ↑ | EMT promotion | HOXA11 knockdown led to E-cadherin upregulation and N-cadherin, β-catenin, and vimentin downregulation. Moreover, the expression of Twist and Snail were also downregulated | Prognostic biomarker and Therapeutic target | serous OC | [121] |
| HOTAIR | ↑ | EMT promotion | HOTAIR knockdown resulted in increased expression of E-cadherin and decreased vimentin and Snail expression | Prognostic biomarker and Therapeutic target | EOC | [122] |
| lncRNA | Expression | Result | Expression of EMT Markers/Genes | Potential Application | Subtype of OC | Ref. |
|---|---|---|---|---|---|---|
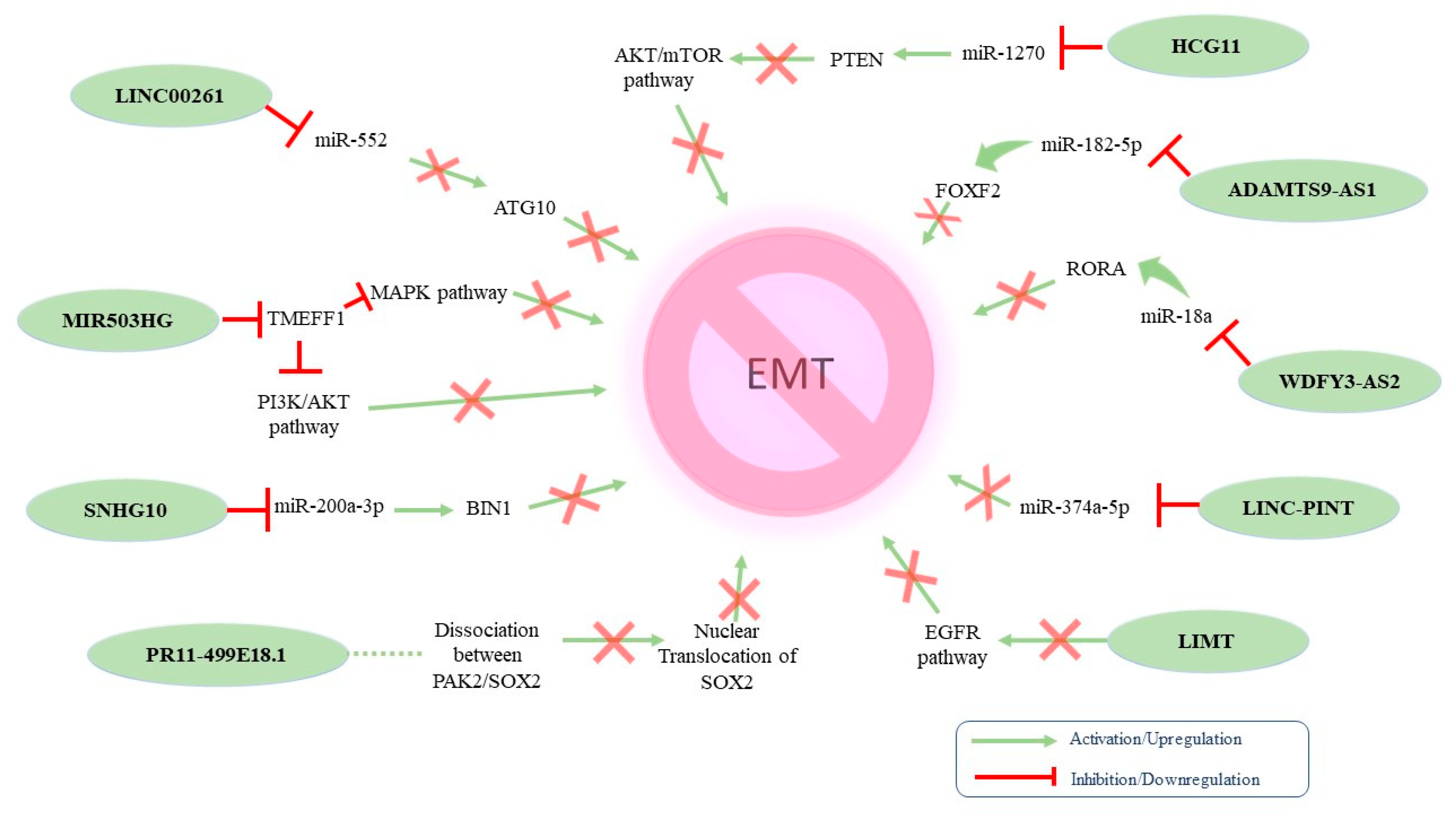
| MIR503HG | ↓ | EMT inhibition | MIR503HG knockdown suppressed E-cadherin expression and increased N-cadherin and vimentin expression | Therapeutic target | - | [124] |
| Linc00261 | ↓ | EMT inhibition | Linc00261 knockdown led to E-cadherin downregulation and increased expression of Slug, Twist1, and N-cadherin, whereas the opposite was observed following Linc00261 overexpression | Therapeutic target | HGSOC | [125] |
| SNHG10 | ↓ | EMT inhibition | SNHG10 overexpression led to increased E-cadherin levels and decreased N-cadherin, vimentin, and Snail levels | Predictive biomarker and therapeutic target | EOC | [126] |
| HCG11 | ↓ | EMT inhibition | Ectopic expression of HCG11 increased E-cadherin levels and reduced N-cadherin expression | Therapeutic target | - | [127] |
| RP11-499E18.1 | ↓ | EMT inhibition | RP11-499E18.1 overexpression led to increased E-cadherin and decreased vimentin expression. RP11-499E18.1 knockdown exerted the opposite effects | Diagnostic marker | - | [128] |
| LINC-PINT | ↓ | EMT inhibition | LINC-PINT silencing was associated with decreased levels of E-cadherin and high N-cadherin and vimentin levels | Target for OC treatment | - | [129] |
| AOC4P | ↓ | EMT inhibition | MMP9 and COL1A2 genes were upregulated in two AOC4P-siRNA-transfected cell lines | Target for anti-metastatic strategies | EOC | [123] |
| WDFY3-AS2 | ↓ | EMT inhibition | WDFY3 overexpression increased E-cadherin levels and decreased N-cadherin and vimentin levels | Therapeutic potential | - | [130] |
| LIMT | ↓ | EMT inhibition | Co-culturing of OC cells with M2-like TAMs (which suppress LIMT) downregulated the expression of E-cadherin while N-cadherin and vimentin were upregulated | Diagnostic, prognostic and therapeutic potential | EOC | [131] |
| ADAMTS9-AS2 | ↓ | EMT inhibition | ADAMTS9-AS2 overexpression induced E-cadherin expression and decreased vimentin expression while ADAMTS9-AS2 inhibition resulted in the opposite effects | Therapeutic target | - | [132] |
| lncRNA | Expression | Result | Expression of EMT Markers/Genes | Potential Application | Subtype of OC | Ref. |
|---|---|---|---|---|---|---|
| HCP5 | ↑ | EMT phenotype in CR cells | - | Prognostic value | - | [135] |
| CHRF | ↑ | EMT promotion in CR cells | CHRF downregulation led to decreased levels of E-cadherin and increased vimentin levels in CR cells | Therapeutic target for sensitizing CR cells | - | [133] |
| TMPO-AS1 | ↑ | EMT promotion | TMPO-AS1 silencing led to increased levels of E-cadherin and decreased levels of vimentin | Therapeutic potential | - | [136] |
| H19 | ↑ | EMT promotion | Twist, Slug, and Snail were dramatically upregulated and E-cadherin decreased in CR cells | Therapeutic target for sensitizing CR cells | - | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampropoulou, D.I.; Papadimitriou, M.; Papadimitriou, C.; Filippou, D.; Kourlaba, G.; Aravantinos, G.; Gazouli, M. The Role of EMT-Related lncRNAs in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 10079. https://doi.org/10.3390/ijms241210079
Lampropoulou DI, Papadimitriou M, Papadimitriou C, Filippou D, Kourlaba G, Aravantinos G, Gazouli M. The Role of EMT-Related lncRNAs in Ovarian Cancer. International Journal of Molecular Sciences. 2023; 24(12):10079. https://doi.org/10.3390/ijms241210079
Chicago/Turabian StyleLampropoulou, Dimitra Ioanna, Marios Papadimitriou, Christos Papadimitriou, Dimitrios Filippou, Georgia Kourlaba, Gerasimos Aravantinos, and Maria Gazouli. 2023. "The Role of EMT-Related lncRNAs in Ovarian Cancer" International Journal of Molecular Sciences 24, no. 12: 10079. https://doi.org/10.3390/ijms241210079
APA StyleLampropoulou, D. I., Papadimitriou, M., Papadimitriou, C., Filippou, D., Kourlaba, G., Aravantinos, G., & Gazouli, M. (2023). The Role of EMT-Related lncRNAs in Ovarian Cancer. International Journal of Molecular Sciences, 24(12), 10079. https://doi.org/10.3390/ijms241210079







