Abstract
Epithelial ovarian cancer (EOC) is one of the deadliest gynecological cancers worldwide, mainly because of its initially asymptomatic nature and consequently late diagnosis. Long non-coding RNAs (lncRNA) are non-coding transcripts of more than 200 nucleotides, whose deregulation is involved in pathologies such as EOC, and are therefore envisaged as future biomarkers. We present a meta-analysis of available gene expression profiling (microarray and RNA sequencing) studies from EOC patients to identify lncRNA genes with diagnostic and prognostic value. In this meta-analysis, we include 46 independent cohorts, along with available expression profiling data from EOC cell lines. Differential expression analyses were conducted to identify those lncRNAs that are deregulated in (i) EOC versus healthy ovary tissue, (ii) unfavorable versus more favorable prognosis, (iii) metastatic versus primary tumors, (iv) chemoresistant versus chemosensitive EOC, and (v) correlation to specific histological subtypes of EOC. From the results of this meta-analysis, we established a panel of lncRNAs that are highly correlated with EOC. The panel includes several lncRNAs that are already known and even functionally characterized in EOC, but also lncRNAs that have not been previously correlated with this cancer, and which are discussed in relation to their putative role in EOC and their potential use as clinically relevant tools.
1. Introduction
Epithelial ovarian cancer (EOC) is the second most common cause of death due to gynecological cancers, with approximately 314,000 new cases and 205,000 deaths worldwide in 2020, and with increasing trends predicted [1]. EOC patients are usually diagnosed at an advanced stage of the disease due to the initially asymptomatic character of the tumor, leading to a dramatic five-year overall survival rate below 40% [2]. An early diagnosis correlates with a better prognosis but, unfortunately, an efficient, approved, and easy protocol based on biomarkers is not available for EOC [3]. Long non-coding RNAs (lncRNAs) are transcripts longer than 200 nucleotides that regulate gene expression at different levels, taking part in physiological and pathological processes, including EOC [4]. More than 150 lncRNAs have been studied and related to EOC so far [5,6,7]. Transcriptome-wide approaches, such as expression microarrays and RNA deep sequencing, produce a large amount of information that is shared with the scientific community thanks to online repositories, such as the Gene Expression Omnibus (GEO) or the European Nucleotide Archive [8]. There are many gene expression profiling studies in the context of EOC, which also provide clinical information about patients and, depending on the cohort size, are able to capture the patient-to-patient variability, thereby allowing the validation and discovery of biological markers and the advance of precision medicine [9]. Several transcriptomic meta-analyses have been previously published analyzing these data in EOC; however, they either used a small number of studies, those available at the moment [10,11,12,13], or were focused on protein-coding genes and neglected lncRNAs [14,15,16,17]. The present work is the first lncRNA meta-analysis in EOC comprising a high and significant number of studies—specifically, 46 independent cohorts—and the first to integrate information from expression microarrays and bulk RNA sequencing. The objective of this work is to reanalyze and compare all of the EOC-patient-derived microarray and RNA sequencing studies available to date, in order to find lncRNAs that highly correlate to clinical aspects and that might have clinical application in the management of EOC patients.
2. Results
In this meta-analysis, we analyzed publicly available independent transcriptomic datasets from EOC-patient-derived samples, which contain lncRNA expression data as well as associated clinical data from patients included in the studies. They are representative of the actual clinical knowledge on EOC and integrate the wide variability between individuals and cohorts.
In our search for gene expression profiling data in ovarian cancer, we initially found a total of 63 studies, of which 51 and 12 accounted for microarray and bulk RNA-Seq technologies, respectively. In terms of the type of analyzed sample, 48 studies used tissue, 2 blood, 2 blood serum, 1 urine, and 1 saliva, whereas 9 worked with cell lines (2 of them including their derived exosomes). All of the studies were considered epithelial ovarian cancer cases, although four of them contained additional data from stromal cells from the tumor that were not used in this meta-analysis. Seventeen studies restrained the cancer samples to one specific subtype, such as high-grade serous or clear cell; another six compared several subtypes within the same study, while the remaining thirty-eight studies did not specify the EOC subtype. Those samples named in the original studies as “serous”, “high-grade serous”, or “low-grade serous” were jointly considered. Out of the 51 studies with cancer patients (excluding cell line studies) who were naïve to treatment at the time of sample extraction, 22 of them did not include samples from healthy tissue for comparison, but 7 of these 22 studies included either cancer cells from ascites and/or peritoneal metastatic samples matched to the primary tumor. There were 11 studies with prognostic information, including survival and/or progression over time. Twelve studies only considered miRNAs, due to the selected microarray platform. The full list of initially identified studies, including the cohort size of each study, can be found in the Supplementary Materials (Table S1).
Our objective was to identify lncRNAs (antisense, sense, intronic, intergenic, divergent, overlapping, and non-overlapping) with clinical value related to epithelial ovarian cancer. Thus, we discarded 17 studies from the 63 initially identified according to the criteria specified in Section 4, thereby excluding studies with information only from miRNAs and/or carried out only with cell lines. Due to the heterogeneity of clinical information available in each study, we performed different rounds of analyses focusing on different studies, which included information of relevance in diagnosis or prognosis, correlation to metastasis, chemotherapy resistance, and histological EOC subtypes. Therefore, in the following sections, we use the same criteria to present the results of the different analyses performed.
2.1. Analysis of lncRNAs with Putative Diagnostic Value in EOC
In this first round of our analysis, we sought to identify differentially expressed lncRNAs—either upregulated or downregulated—in EOC compared with healthy samples. For this category, we considered 24 studies that used both cancer and control samples; however, EGAD00001000877 data are not publicly available, and the RNA-Seq libraries from GSE192410 were prepared from circular RNA only; therefore, these data were discarded. We analyzed the remaining 22 studies, whose metadata and results for the differential expression analyses are available in the Supplementary Materials (Table S2). The RNA analyzed in these studies was derived from tissue samples, except for GSE29220, whose RNA was derived from saliva samples. Only one study, GSE137238, presented matched cancer and normal ovarian tissue from the same woman, whereas in the rest, the case and control samples came from different women.
A summary from these 22 analyses is shown in Figure 1, in which the number of up- and downregulated lncRNAs in EOC is depicted, as well as the sum of the other gene biotypes (protein-coding genes, pseudogenes, micro-RNA genes, T-cell receptor genes, immunoglobulin genes, small nuclear RNA genes, small nucleolar RNA genes, small Cajal body-specific RNA genes, ribosomal RNA genes, and ribozyme genes). The study generating the highest number of deregulated targets was GSE190688, with 6934 up- and 5325 downregulated, of which 1691 and 1497 were lncRNAs, respectively. For GSE29220, statistically significant deregulation was not identified.
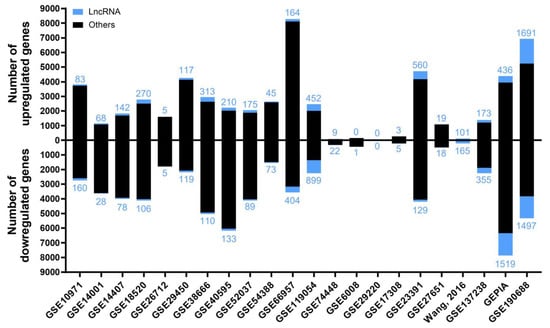
Figure 1.
The number of deregulated genes in each selected study in the “Diagnostic” analysis. The bar graph shows the absolute frequency of upregulated (upper part) and downregulated (lower part) genes, depicting the fraction corresponding to lncRNA genes in blue at the ends of the bars.
After obtaining the list of differentially expressed genes for each study, we carried out multiple pairwise comparisons to determine which lncRNAs were deregulated across the different cohorts with the same trend (up or down). A total of 271 lncRNAs with contradictory trends among different studies (that is, upregulated in some studies but downregulated in others) were excluded, but they are listed in the Supplementary Materials (Table S3). There were 247 upregulated and 243 downregulated lncRNAs that were statistically significant in at least three of the analyzed studies (listed in Table 1 and Table 2, respectively); the majority of which (200 and 209, respectively) were not previously related to EOC (Supplementary Materials, Table S4). The most frequently upregulated lncRNAs were RNF157-AS1 and BBOX1-AS1, both in 12 different studies, whereas MAGI2-AS3 was the most frequently downregulated lncRNA, in 15 different studies. The lncRNAs that showed significant upregulation and that were more frequently found in different cohorts, but that had not been previously related to EOC diagnosis, were ENSG00000187951, MIR205HG, ZNF232-AS1, ENSG00000285756, LINC01297, TFAP2A-AS1, LINC01977, and LINC01770, as shown in Figure 2a. The lncRNAs that were more frequently downregulated in different cohorts but that had not been previously related to EOC diagnosis were PGM5-AS1, ENSG00000267058, EPM2A-DT, NR2F1-AS1, KLF3-AS1, GLIDR, ERVK13-3, and CLN8-AS1, as shown in Figure 2b. There were several lncRNAs that we considered to be contradictory from the results that we found in the meta-analyses (Supplementary Materials, Table S3) and that were related to EOC in the literature, such as PART1 [18] or XIST [19]. This fact does not invalidate the experimental evidence collected about their role in EOC, although it downplays their importance as diagnostic biomarkers for the disease, since different trends are observed in different cohorts.

Table 1.
Upregulated lncRNAs in ovarian cancer tissue in at least three different transcriptomic studies from the “Diagnostic” analysis. For genes in bold, the position of the non-cancerous cell line OELE matches the first quartile when sorting the cell lines in ascending normalized counts.

Table 2.
Downregulated lncRNAs in ovarian cancer tissue in at least three different transcriptomic studies from the “Diagnostic” analysis. For genes in bold, the position of the non-cancerous cell line OELE matches the fourth quartile when sorting the cell lines in ascending normalized counts.
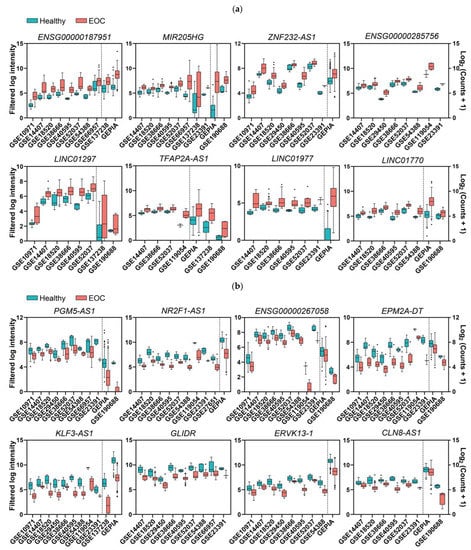
Figure 2.
Expression levels in EOC and normal ovary tissues of the top deregulated lncRNAs that were discovered in the EOC meta-analysis. (a,b) Panels correspond to upregulated and downregulated genes, respectively. The middle line in each boxplot represents the median value, and the black dots are the outlier values. Comparisons from the dotted line to the left are from microarray studies, and comparisons from the dotted line to the right are from RNA-Seq studies, except for GLIDR and ENSG00000285756, for which all comparisons are from microarrays. ZNF232-AS1, ATP2A1-AS1, LINC01977, TFAP2A-AS1, and NR2F1-AS1 are not represented in this figure, because only fold changes and p-values are available in this study [20]. All comparisons are p ≤ 0.01.
Once we had obtained these two lists, we checked whether these lncRNAs were also dysregulated in 57 epithelial ovarian cancer cell lines when compared with OELE—a non-cancerous human ovarian epithelium cell line—as a criterion for further validation. We obtained raw read counts from RNA sequencing of each of these cell lines from the Cancer Cell Line Encyclopedia (CCLE), rounded them to the closest integer, and normalized them using DESeq2. After that, we sorted the 58 cell lines in ascending normalized read counts for each gene, annotating the position and quartile in which OELE was present. We considered that deregulation in patients was consistent with the cell line data when OELE occupied a position within the first quartile (lower expression) for upregulated genes or the fourth quartile (higher expression) for downregulated genes. There were 42 upregulated and 57 downregulated lncRNAs that fulfilled these criteria (in bold in Table 1 and Table 2, and reported, together with position and quartile information, in the Supplementary Materials (Table S4)).
2.2. Analysis of lncRNAs with Putative Prognostic Value in EOC
This second analysis consisted of differentially expressed lncRNAs showing a significant correlation with favorable or unfavorable prognosis—meaning increased or decreased, respectively, overall survival (OS) and/or disease-free survival (DFS) periods after diagnosis or time without relapse after being treated or surgically debulked. In this category, we selected 11 studies containing information about death and relapse events over time. The metadata information of each study and the results from our meta-analysis can be found in the Supplementary Materials (Table S5). The numbers of genes resulting from the Cox proportional hazards model affecting OS and DFS for each study are depicted in Figure 3.
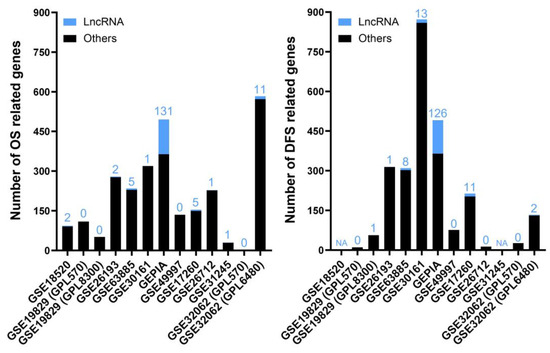
Figure 3.
The numbers of genes affecting OS (left) and DFS (right) in each selected study for the “Prognostic” analysis. The bar graph shows the absolute frequency of genes; the portion in blue at the ends of the bars is the fraction corresponding to lncRNA genes. NA (not applicable) means that there is no patient information about DFS for those studies, whereas 0 means that there are no statistically significant genes.
These analyses yielded 123 and 32 lncRNAs positively correlated with longer or shorter OS periods, respectively, as listed in Table 3. We also found 125 lncRNAs that were positively correlated with longer DFS periods and 34 lncRNAs with shorter DFS periods, as presented in Table 4. The references from the genes that were previously associated with EOC in the literature can be found in the Supplementary Materials (Table S6). When comparing the resulting gene lists from each analysis pairwise, only six lncRNAs (in bold in Table 3 and Table 4) were confirmed in two different studies: RNF157-AS1, AQP5-AS1, CRNDE, and ZFAS1 in OS studies, and MALAT1 and SNHG8 in disease-free survival studies. However, SNHG8 showed opposed trends in the two studies; therefore, we excluded it from the final lists of this category (but it is included in the Supplementary Materials (Table S3)). Figure 4 shows the survival periods of three selected lncRNAs.

Table 3.
LncRNA genes positively correlated with longer (left) or shorter (right) OS periods in EOC patients identified in the studies from the “Prognosis” analysis. LncRNAs in bold are present in two different studies.

Table 4.
LncRNA genes positively correlated with longer (left) or shorter (right) DFS periods in EOC patients identified in the studies from the “Prognosis” analysis. LncRNAs in bold are present in two different studies.
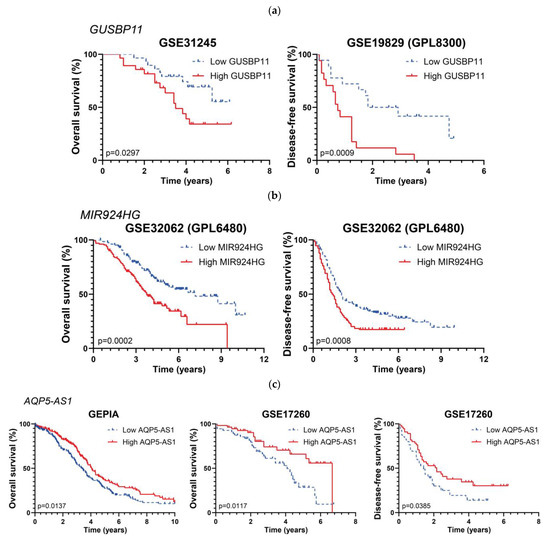
Figure 4.
Kaplan–Meier curves for three lncRNAs affecting survival periods in EOC patients: (a) GUSBP11, (b), MIR924HG, (c), and AQP5-AS1. Graph titles indicate each corresponding study.
2.3. Analysis of lncRNAs Deregulated in EOC Metastasis
The third analysis comprised seven different studies (summarized in the Supplementary Materials (Table S7)) containing transcriptomic information derived from the primary tumor, cancer cells from the ascitic fluid, and/or peritoneal solid metastasis samples, matched for each patient.
We analyzed the seven studies individually to look for differentially expressed lncRNA genes related to the metastatic process by comparing (i) peritoneal solid metastasis versus cancer cells from the ascitic fluid, (ii) peritoneal solid metastasis versus primary tumor, and (iii) cancer cells from the ascitic fluid versus primary tumor, using the variable “patient” as a blocking factor. A summary of the differentially expressed genes for each comparison and study is shown in Figure 5. The two studies that yielded the highest numbers of differentially expressed genes were GSE133296 and GSE137237.
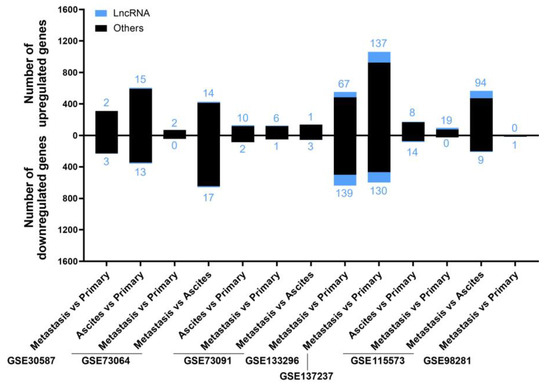
Figure 5.
The number of deregulated genes in each comparison within the selected studies for the “Metastatic” analysis. The bar graph shows the absolute frequency of upregulated (upper part) and downregulated (lower part) genes, depicting the fraction corresponding to lncRNA genes in blue at the ends of the bars.
After carrying out the individual analyses, we found 287 upregulated and 287 downregulated lncRNAs, of which 30 and 8, respectively, were found in two or three different studies carried out with patients, or in one study carried out with patients and differentially expressed in EOC cell lines from a metastatic origin when comparing them with those originating from primary tumors (Table 5 and Table 6). Additionally, we identified 26 lncRNAs that were upregulated or downregulated in cancer cells from ascites but did not change their levels between primary tumor and solid peritoneal metastasis, which we named “switch” (Table 7). The full lists, including references from genes previously associated with EOC, can be found in the Supplementary Materials (Table S8). Those lncRNAs whose expression was contradictory between different analyzed studies were excluded from Table S8 but included in Table S3.

Table 5.
Upregulated lncRNAs in metastasis in two or three patient transcriptomic studies, or in one patient transcriptomic study and also in metastatic versus primary tumor cell line groups from CCLE. Genes in bold coincide with significant differentially expressed (DE) lncRNAs between the primary and metastatic cell line groups using DESeq2, and underlined genes coincide with those that were significant in the receiver operating curve analyses.

Table 6.
Downregulated lncRNAs in one patient transcriptomic study and also in metastatic versus primary tumor cell line groups from CCLE. Genes in bold coincide with significant DE lncRNAs between the primary and metastatic cell line groups using DESeq2, and underlined genes coincide with those that were significant in the receiver operating curve analyses.

Table 7.
“Switch” lncRNAs in metastasis patient transcriptomic studies.
After obtaining these lists, we tested again whether we could correlate these results with cell lines using CCLE expression data, since there are 32 and 25 epithelial ovarian cancer cell lines that are derived from primary tumors and metastases, respectively. We first looked for differentially expressed genes between the primary and metastatic cell line groups using DESeq2 and obtained three upregulated and one downregulated (p-value < 0.05 and |log2 fold-change| > 0.585) lncRNAs that were present in the upregulated and downregulated lists obtained from the analyses of the patient studies. The second approach was to carry out receiver operating characteristic (ROC) curve analyses to see if the genes outlined from the patient analyses also showed differences in the comparison between the metastatic and primary tumor cell line groups. We obtained five upregulated and seven downregulated lncRNAs (area under the curve > 0.5 and p-value < 0.05) contained in the lists obtained from the analyses of the metastatic patient studies. The results for both DESeq2 and ROC for the metastasis category can be found in the Supplementary Materials (Table S8). In terms of the influence of lncRNAs in EOC metastasis, upregulation of LINC02544, LINC01235, HECW2-AS1, and MIR31HG in relation to this process was derived from this meta-analysis (Figure 6), and since it has not been previously found in EOC, this deserves special mention.
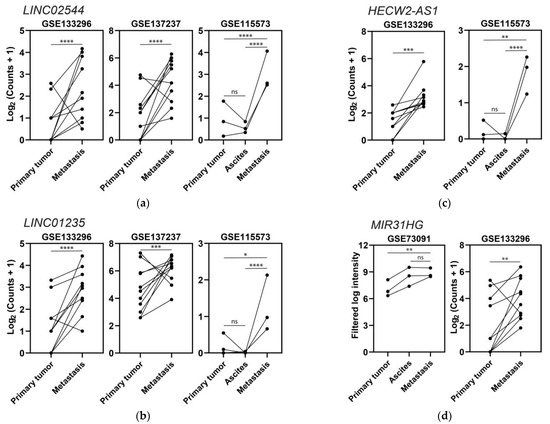
Figure 6.
Expression levels of four lncRNAs upregulated in metastases: (a) LINC02544, (b) LINC01235, (c) HECW2-AS1, and (d) MIR31HG. Dots represent tissue samples, and lines join the samples from each patient; * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, ns p > 0.05.
2.4. Looking for Outstanding lncRNAs Related in Common to Diagnosis, Prognosis, and Metastasis in EOC
At this point of the analysis, we decided to overlap the final lists for the first three categories to highlight the most interesting lncRNAs in terms of clinical value. We generated two different intersections, as represented in Figure 7: one comparing the upregulated lncRNAs, which represent the ones that would be putative oncogenes, and another one comparing the downregulated lncRNAs, which represent the ones that could be considered tumor suppressors.
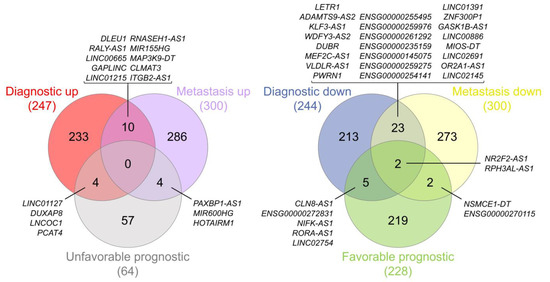
Figure 7.
Venn diagrams representing the intersections between the final lists of the diagnostic, prognostic, and metastasis analyses—specifically, potential oncogenes (left) and potential tumor suppressors (right). Diagrams were generated using interactivenn.net.
We only obtained results in the intersection of the three lists for the potential tumor suppressor comparison, whereas in the other comparison the intersections found were only pairwise. The lncRNAs considered to be probable tumor suppressors in EOC were NR2F2-AS1 and RPH3AL-AS1, and their data from the diagnostic, metastasis, and prognostic analyses are represented in Figure 8 (see also Figure S1).
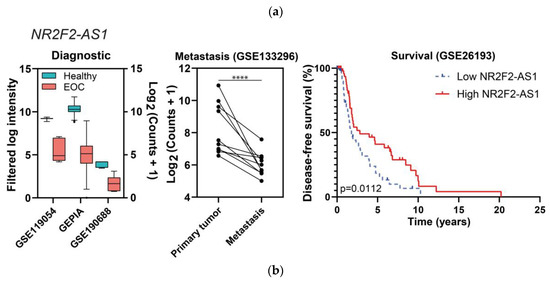
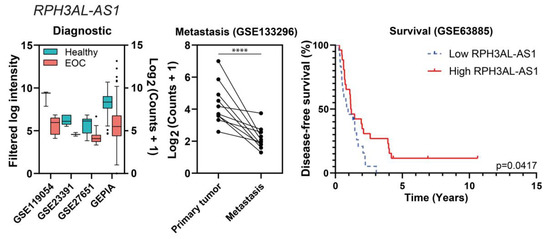
Figure 8.
“Diagnostic” and “Metastasis” differential expression results, and survival plots of the potential tumor suppressors NR2F2-AS1 (a) and RPH3AL-AS1 (b). In the left panels, all of the comparisons are p ≤ 0.01. In the middle panels, dots represent tissue samples and lines join the three samples from each patient. In the case of RPH3AL-AS1, one study from the diagnostic category is not represented because raw information from [20] is not available, only fold changes and p-values; **** p ≤ 0.0001.
NR2F2-AS1 and RPH3AL-AS1 were both downregulated in EOC in comparison with healthy tissues in four independent studies (NR2F2-AS1 was also downregulated in EOC cell lines) and in one study of metastatic tumors, correlating with a favorable prognosis (specifically, with improved disease-free survival), as shown in Figure 8.
2.5. Analysis of lncRNAs with Putative Influence in Resistance to Chemotherapy
Next, we aimed to identify lncRNAs related to standard chemotherapy resistance (jointly considering cisplatin or carboplatin and/or a taxane or cyclophosphamide) by comparing expression levels from partial or non-responder and responder patients. In this case, only three studies (all microarrays) had information regarding the response to treatment, and they are listed together with the results from the individual analyses in the Supplementary Materials (Table S9). We could only find six upregulated and nine downregulated lncRNAs, as shown in Figure 9. As expected, due to the low number of deregulated genes in this category, multiple pairwise comparisons did not produce any overlap between studies. The references from previous associations between these genes and EOC can be found in the Supplementary Materials (Table S10).
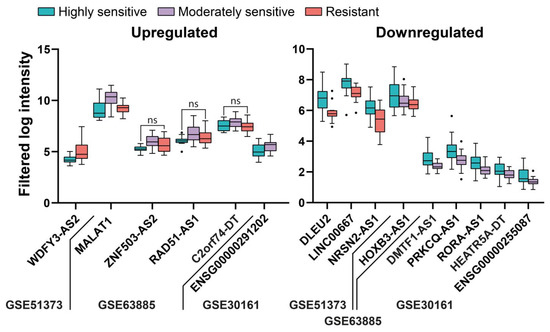
Figure 9.
Deregulated lncRNAs in tissue from patients according to the sensitivity to standard chemotherapy. All of the comparisons are p < 0.05 except for those indicated with ns, indicating p > 0.05.
Although there have been few transcriptomic studies considering the influence of lncRNAs in resistance to chemotherapy in EOC, our meta-analysis confirmed the influence of two lncRNAs previously associated with resistance to chemotherapy in EOC—WDFY3-AS2 [21], and MALAT1 [22]—as well as revealing the influence of other lncRNAs that have been associated with EOC but not with chemoresistance until now, i.e., LINC00667 [12], NRSN2-AS1 [23], and RAD51-AS1 [24]. Others had not been associated with EOC but had been associated with other cancers, i.e., DLEU2 with endometrial [25] and prostate cancers [26], LINC00667 with breast cancer [27], and PRKCQ-AS1 with multiple myeloma [28].
2.6. Analysis of lncRNAs with a Putative Specific Value Associated with Histological EOC Subtypes
Epithelial ovarian cancer can be further classified into five subtypes according to histological structure, mutations in certain tumor suppressors or proto-oncogenes, chemosensitivity, spreading behavior, and patient prognosis [29]. These five subtypes and their relative frequencies are high-grade serous (HGSOC, 70%), low-grade serous (LGSOC, >5%), endometrioid (ENOC, 10%), clear cell (CCOC, 10%), and mucinous (MOC, 3%) [29]. As there were six studies with the subtype information available (Supplementary Materials, Table S11), in the last analysis, we looked for lncRNAs whose expression was specific for each EOC subtype.
The results of each analysis for the subtype category are contained in the Supplementary Materials (Table S11) and summarized in Figure 10. Regarding HGSOC and LGSOC, we did not consider LGSOC independently, because there was only information about this subtype in two studies (GSE14001 and GSE26751), in which there were HGSOC and healthy individuals and no other subtypes to compare with. We did not find any ambiguous lncRNAs within any of the subtypes across different studies. Those lncRNAs that were present in two or three studies of HGSOC or CCOC are listed in Table 8 and Table 9, respectively. The full list of deregulated lncRNAs according to each subtype can be found in the Supplementary Materials (Table S12).
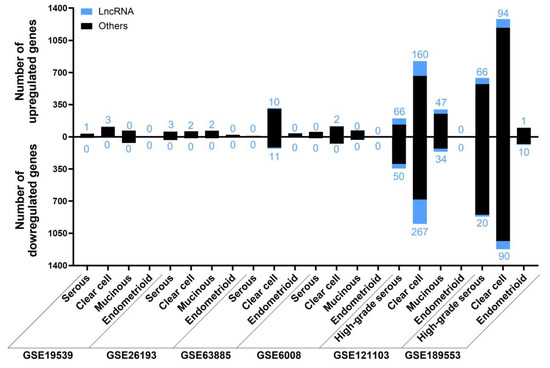
Figure 10.
The number of deregulated genes in each selected study for the “Subtype” analysis. The bar graph shows the absolute frequency of upregulated (upper part) and downregulated (lower part) genes, depicting the fraction corresponding to lncRNA genes in blue at the ends of the bars.

Table 8.
Differentially expressed lncRNAs in high-grade serous ovarian cancer patients identified in two or three (*) studies.

Table 9.
Differentially expressed lncRNAs in clear cell serous ovarian cancer patients identified in two, three (*) or four (in bold) studies.
We performed two different multiple pairwise comparisons (up- and downregulated lncRNAs, separately) between the lists drawn for each subtype to identify true subtype-specific lncRNAs and exclude those present in more than one subtype. The results of these overlaps are shown in Figure 11. We found an a priori unexpected specificity between lncRNAs and histological subtypes of EOC. Surprisingly, only 1 out of the 418 upregulated and 3 out of the 467 downregulated lncRNAs in any subtype were deregulated in the same way in two different subtypes (included in the Supplementary Materials (Table S3)), meaning that the remaining lncRNAs are potentially subtype-specific.
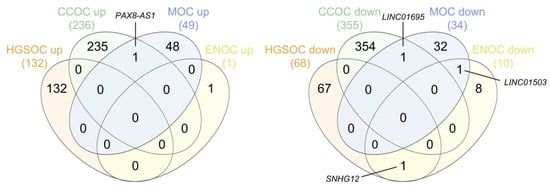
Figure 11.
Venn diagrams representing the intersections between the final lists of the upregulated (left) and downregulated (right) subtype-specific lncRNAs. HGSOC, high-grade serous ovarian carcinoma; CCOC, clear cell ovarian carcinoma; MOC, mucinous ovarian carcinoma; ENOC, endometrioid ovarian carcinoma. Diagrams were generated using interactivenn.net.
3. Discussion
The lack of early detection methods for EOC and its consequent late diagnosis result in dramatic high mortality. We aimed to update the significance of putative lncRNA-based biomarkers in EOC in light of available studies combining gene expression and clinical data.
As the first objective of our analysis, we sought to identify differentially expressed lncRNAs (either upregulated or downregulated) in EOC compared with healthy samples that could be identified as significant candidates for future experimental analysis and translation to clinical practice as diagnostic biomarkers. Data from 22 different cohorts of patients were included in this analysis. Indeed, lncRNA expression in these cohorts had been previously analyzed in some cases, but not all. The reanalysis of all of these available data revealed worthwhile information because it allowed us to bring to light available, but not evident, information. We found 247 upregulated and 243 downregulated lncRNAs (listed in Table 1 and Table 2, respectively), 200 and 209 of which were not previously related to EOC, respectively. The most frequently upregulated lncRNAs were RNF157-AS1 and BBOX1-AS1, both in 12 different studies, whereas MAGI2-AS3 was the most frequently downregulated lncRNA, in 15 different studies. Validation of these novel EOC-related lncRNAs is supported by three pieces of evidence: First, the meta-analysis, applied to protein-coding genes across the same studies, rendered a group of genes that are well-known EOC markers as significantly deregulated, including CP [30], CD24 [31], and INAVA [14]—upregulated in 18, 17, and 16 studies, respectively—as well as AOX1 [14] and PDGFD [32], downregulated in 16 and 13 studies, respectively. Second, our analysis also found other lncRNAs previously related to EOC (cited in the Supplementary Materials (Table S4)). These include, among others, RNF157-AS1 [33] and UCA1 [34], which interact with proteins to regulate transcription; BBOX1-AS1 [35], DUXAP8 [36], LINC01503 [37], HAGLR (as HOXD-AS1) [38], LINC00665 [39], and HAGLROS [40], which act as competing endogenous RNAs (ceRNAs) with miRNAs, affecting gene expression at the post-transcriptional level; and MAGI2-AS2 [41], HAND2-AS1 [42], ZNF300P1 [43], and HYMAI [44], whose downregulation in EOC is explained by hypermethylation of the promoter. The third piece of evidence for the validation of our meta-analysis comes from the fact that 99 deregulated lncRNAs in EOC patients were also deregulated in EOC cell lines (Supplementary Materials, Table S4). The lncRNAs that showed significant upregulation, and which were more frequently found in different cohorts, but that had not been previously related to EOC diagnosis, were ENSG00000187951, MIR205HG, ZNF232-AS1, ENSG00000285756, LINC01297, TFAP2A-AS1, LINC01977, and LINC01770, as shown in Figure 2a. Some of them have been previously related to other cancer types. MIR205HG is the host gene for the microRNA miR-205 but, although originating from the same primary transcript through alternative splicing, its lncRNA and miRNA are functionally independent [45,46,47]. MIR205HG promotes lung squamous cell carcinoma [48], osteosarcoma [49], melanoma [50], cervical cancer [51,52], head and neck squamous carcinoma [46], and esophageal squamous carcinoma [53]; however, in esophageal adenocarcinoma, it is downregulated and hinders HNRNPA0 mRNA translation by interacting with LIN28A [54] and affecting the Hedgehog pathway [55]. MIR205HG also acts in physiological processes, such as in embryogenesis by regulating the transcription of Pit1, Zbtb20, prolactin, and growth hormone in the anterior pituitary in mouse models [47]; or in cell fate by preventing luminal differentiation of human prostate basal cells through the interferon pathway by forming a DNA:RNA triplex in the Alu regulatory elements in the proximal promoter of target genes [45,56]. LINC01770 is upregulated in endometrial cancer in comparison with endometrial dysplasia tissues [57]. LINC01977 is upregulated and correlated with poor prognosis in lung adenocarcinoma [58] and breast cancer [59]; in lung adenocarcinoma, TGF-β derived from infiltrated tumor-associated macrophages (TAM2) activates SMAD3, which binds LINC01977 to induce its nuclear transport, where it upregulates transcription by simultaneously binding the promoter and super-enhancer, facilitating the interaction between SMAD3 and CBP/P300 to activate ZEB1 transcription [58]. In breast cancer, LINC01977 expression is also correlated with chemoresistance to doxorubicin by targeting the miR-212-3p/GOLM1 axis [59]. LINC01297 is upregulated in estrogen receptor (ER)-positive breast cancer, in comparison with ER-negative breast cancer [60] and lung adenocarcinoma [61]; there is also a positive correlation between LINC01297 and the expression of its nearby gene LINC01296, which acts as an oncogene in bladder cancer [62]. Conversely, ENSG00000285756 and TFAP2A-AS1, which were EOC-upregulated lncRNAs in our analysis, are downregulated in other cancer types. ENSG00000285756 is downregulated in cervical cancer [63], and TFAP2A-AS1 is downregulated and correlated with a good prognosis in breast cancer, acting in vitro as a tumor suppressor by sponging miR-933 to modulate SMAD2 mRNA stability [64]; TFAP2A-AS1 is also transcriptionally activated by KLF15 and inhibits the proliferation and migration of gastric cancer cells by sponging miR-3657 to regulate NISCH mRNA stability [65]. The lncRNAs that were more frequently downregulated in different cohorts, but that had not been previously related to EOC diagnosis, were PGM5-AS1, ENSG00000267058, EPM2A-DT, NR2F1-AS1, KLF3-AS1, GLIDR, ERVK13-3, and CLN8-AS1, as shown in Figure 2b. PGM5-AS1 (ENSG00000224958) is downregulated in both EOC patients and EOC cell lines; it is also downregulated and negatively correlated with oxaliplatin resistance in colorectal cancer [66] but, on the other hand, it is upregulated and sponges miR-140-5p to prevent FBN1 mRNA degradation in osteosarcoma [67]. KLF3-AS1 is downregulated in esophageal squamous cell carcinoma stem cells, promoting cell migration and invasion by being unable to sponge miR-185-5p, which induces KLF3 mRNA degradation, thereby preventing transcriptional repression of SOX2 and OCT4 by KLF3 [68], and acts as a competing endogenous tumor suppressor RNA in gastric cancer [69] and osteosarcoma [70]. Conversely, several lncRNAs that are downregulated in EOC are upregulated and stimulate cancer progression in other tissues. NR2F1-AS1 is upregulated in non-small cell lung [71], thyroid [72], pancreatic [73], and hepatocellular [74] cancers; in pancreatic cancer, it is induced by hypoxia, its expression is positively correlated with the expression of its sense gene NR2F1, and they both trigger the AKT and mTOR pathways, promoting proliferation, migration, and invasion [75]. NR2F1-AS1 is also upregulated in dormant breast cancer stem-like cells and increases tumor dissemination by recruiting PTBP1 to the mRNA of its sense gene (NR2F1), promoting its translation so that NR2F1 represses ΔNp63 transcription [76]. GLIDR is upregulated and promotes glioma [77], lung [78], and prostate [79] cancers by acting as competing endogenous RNAs for miRNAs. ERVK13-3 is upregulated in osteosarcoma [80].
Our second objective was to find differentially expressed lncRNAs showing a significant correlation with favorable or unfavorable prognosis. From our initial selection of studies from 46 different cohorts, only 11 contained information about death and relapse events over time. The analysis of these data rendered a limited amount of lncRNAs whose differential expression could be related to prognosis. Among them, high expression of GUSBP11 and MIR924HG (underlined in Table 3 and Table 4) was positively correlated with shorter OS and DFS, meaning a negative prognosis. In accordance with our data for EOC, high expression levels of GUSBP11 are predicted to bind miR-22-3p to avoid CCN2A mRNA degradation, correlating to poor overall survival in hepatocellular carcinoma patients [81]. Conversely, GUSBP11 expression correlates with better prognosis in head and neck squamous cell carcinoma [82], bladder cancer [83], papillary renal cell carcinoma [84], and pancreatic adenocarcinoma [85]. Regarding MIR924HG, there is no information about it in the literature. However, miR-924, which is hosted in the MIR924HG locus, acts as a tumor suppressor in non-small-cell lung carcinoma [86] and hepatocellular carcinoma [87]. In our analysis, AQP5-AS1 was associated with a more favorable prognosis due to its correlation with longer survival periods in different studies. There is no information regarding the antisense lncRNA AQP5-AS1 in the literature, but the sense transcript encodes the protein AQP5, which is overexpressed in OC tissues [88] and promotes proliferation and migration in OC [89]; however, contradictory data can also be found, since high AQP5 expression is correlated with a better prognosis in OC patients [90].
The third objective was the identification of differentially expressed lncRNAs associated with metastasis in EOC. Seven studies included samples that allowed comparisons of (i) peritoneal solid metastasis vs. cancer cells from the ascitic fluid, (ii) peritoneal solid metastasis vs. primary tumor, and (iii) cancer cells from the ascitic fluid vs. primary tumor. Upregulation of LINC02544, LINC01235, HECW2-AS1, and MIR31HG in relation to this process was found from this meta-analysis and, since it has not been previously found in EOC, deserves special mention. Previous data about the influence of these lncRNAs in invasion or metastasis and unfavorable prognosis can be found in other cancers and reinforce our findings. LINC02544 is overexpressed in lung squamous cell carcinoma patients with lymph node metastasis, and it promotes proliferation, migration, and invasion in vitro by sponging miR-138-5p—a potential target of E2F3 [91]; increased expression of LINC02544 has also been related to in vitro invasion and unfavorable prognosis in breast cancer [92]. LINC01235 is upregulated in gastric cancer patients versus healthy individuals and metastatic versus non-metastatic gastric cancer patients, promoting migration, invasion, and EMT, as well as negatively affecting prognosis [93,94]. MIR31HG has been associated with unfavorable prognosis in gastric cancer [95], lung adenocarcinoma [96], head and neck squamous cell carcinoma [97,98], colorectal cancer [99], and non-small cell lung carcinoma [100].
The intersection of differentially expressed lncRNAs obtained in the three previous analyses (diagnosis, prognosis, and metastasis) highlighted the importance of two lncRNAs (NR2F2-AS1 and RPH3AL-AS1) in EOC. A priori, we did not expect a large overlap between lncRNAs useful in diagnosis and prognosis, since lncRNAs discovered in each category may be regulating different phases and functions of oncogenesis. A greater overlap exists between metastasis and prognosis, since the functional relationship between metastasis and prognosis is very straightforward, and the presence of metastasis is usually associated with shorter survival times. NR2F2-AS1 is upregulated in several malignancies, such as non-small cell lung cancer, clear cell renal cell carcinoma, and prostate, cervical, nasopharynx, and esophageal cancers, being considered an oncogene [101], contrary to what we observed in EOC. Its gene product NR2F2 or COUP-TFII is highly expressed in the ovarian stroma and is negligible in the ovarian epithelium, although NR2F2 is downregulated at the mRNA level in OC tissues. NR2F2 expression increases in the epithelial component and is associated with shorter periods before recurrence [102]. NR2F2 acts as an oncogene in other cancers, such as renal, prostate, or breast cancers [103]. RPH3AL-AS1 is located within the cytoband 17p13.3, and its downregulation in EOC could be due to the high deletion frequency of this chromosomal region in OC patients [104,105].
Next, as a fourth objective, we aimed to identify lncRNAs related to standard chemotherapy resistance. Among the studies considered in this meta-analysis, there were only three studies containing information that allowed us to study this correlation. Although the results from our meta-analysis confirm the influence of two lncRNAs previously associated with resistance to chemotherapy in EOC (WDFY3-AS2 [21] and MALAT1 [22]) and some new lncRNAs that were also detected, more studies are needed to validate these results.
Epithelial ovarian cancer can be further classified into five subtypes according to its histological structure: high-grade serous, low-grade serous, endometrioid, clear cell, and mucinous [29]. We found in our meta-analysis that the differential expression of lncRNAs is highly specific for the different subtypes, which can be used for diagnostic purposes.
One of the most interesting characteristics of lncRNAs is that they can be detected in tumor-derived small extracellular vesicles, as well as free molecules or protein-associated complexes circulating in the blood. The fact that circulating levels of some lncRNAs in serum or plasma samples can correlate to those found in tumor tissue increases their importance as biomarkers in liquid biopsy for EOC, with ongoing clinical trials [5]. There are examples of some lncRNAs identified in our meta-analysis that have been previously found in liquid biopsies. HAND2-AS1—which is downregulated in EOC tumors, as confirmed in 13 studies (Table 2)—had been also detected in blood plasma from triple-negative breast cancer patients [106]. The expression of SP2-AS1, which was downregulated in eight studies (Table 2), was previously detected in blood and associated with the risk of endometriosis and ENOC [107]. UCA1, which was upregulated in 10 EOC studies (Table 2), also showed increased levels in serum-derived exosomes from cisplatin-resistant OC patients [108] and plasma from colorectal cancer patients [109]. PGM5-AS1, downregulated in tumor tissues from 12 EOC studies (Table 2), was also downregulated in plasma from colorectal cancer patients [109]. The lncRNA ESRG, upregulated in eight EOC studies (Table 2), was detected in exosomes present in effusion supernatants from HGSOC [110]. GUSBP11, which was correlated with a poor prognosis in EOC (Table 3 and Table 4, and Figure 4), was upregulated in plasma from gastric cancer patients in comparison with healthy individuals [111].
Finally, it is also important to remark that EOC is intrinsically associated with late diagnosis and, therefore, most of the studied samples included in this meta-analysis come from advanced stages of the disease, which implies that early-stage samples are underrepresented. Studies with larger cohort sizes and increased representation of samples from the initial stages of EOC, along with the use of sensitive, normalized, universally standardized, and reproducible techniques to detect circulating lncRNAs, are still needed to make possible the translation of these findings into the gynecological clinical setting.
4. Materials and Methods
4.1. Selection of Suitable Gene Expression Datasets for Meta-Analysis
Microarray- and bulk RNA-Seq-based gene expression profiling studies for ovarian cancer were identified in PubMed and the Gene Expression Omnibus (GEO—https://www.ncbi.nlm.nih.gov/geo/ accessed on 15 January 2023) [112]. The search terms included “ovarian cancer” AND (“microarray” OR “RNA-Seq”) AND “patients”. Eligible studies and datasets had to fulfill the following requirements: (i) include case and control human studies, (ii) perform transcriptomic analyses, and (iii) have available raw and/or processed microarray or RNA-Seq data. Studies were not considered if they were (i) letters, abstracts, and human case reports, i.e., not full and original research studies; (ii) studies based only on cell lines as a model of study; (iii) RT-qPCR-based studies only; (iv) studies neglecting ncRNAs—specifically lncRNAs; or (v) only focused on stromal or germinal ovarian cancer.
4.2. Data Extraction and Processing
Microarray intensity files (CEL or text), probe information tables, and RNA-Seq read count tables, along with the experimental metadata included in the series matrix files, were downloaded from the GEO Accession Display for each selected dataset, whereas FASTQ files were downloaded from the European Nucleotide Archive (ENA) browser. It is worth noting that we only considered genes with Ensembl IDs, as their GENCODE annotation is manually supervised and, therefore, updated and reliable. Because of this, some lncRNAs with only NCBI Gene IDs were not considered, although they were perhaps still interesting for EOC; thus, we may have underestimated our results for the sake of a more confident annotation. The version of the annotation was Ensembl 108 (GRCh38.p13).
4.2.1. Microarray
Microarray data were processed using BRB-ArrayTools (version 4.6.2), developed by Dr. Richard Simon (Biometric Research Program, National Cancer of Institute, BeThesda, Rockville, MA, USA) and the BRB-ArrayTools Development Team. Affymetrix CEL files were imported with the Data Import Wizard option, using the JustRMA normalization method and standard Affymetrix probe set IDs. The text files were imported using the General Format Importer option, adjusting the red and green thresholds to 10 and 100, respectively, and the background intensity and spot flag information were considered when available. Regardless of the importing option, the experiment descriptor files created from the corresponding series matrix files were also imported, and in all cases the option “Average the replicate spots within an array” was selected. Affymetrix Human Genome U133 Plus “1.0” and 2.0 arrays were annotated with chip-specific Bioconductor packages, whereas the rest were annotated using information included in the intensity file or the probe information table, both resulting in EntrezId, UniGene ID, and/or GenBank Nucleotide Accession Number lists. These IDs were converted to Ensembl Gene IDs using bioDBnet [113], unified in one list, and annotated by merging with the human genome information (hg38, GRCh38.p13) using RStudio (v4.1.0)’s merge function from the data.table package.
4.2.2. RNA-Seq
RNA-Seq data were processed using RStudio (version 2022.12.0 Build 353), R (version 4.1.0), Bioconductor (version 3.14), and DESeq2 (version 1.34). For GSE190688, GSE98281, and GSE115573 studies, read count files were not available; hence, after concatenating the GSE115573 files belonging to each sample, we mapped the three of them and quantified the reads using Kallisto [114] against human (hg38) cDNA and ncRNA transcriptomes obtained from Ensembl FTP (January 2023). After merging both cDNA and ncRNA abundance files, they were imported into RStudio using tximport and implemented into DESeq2 using the function DESeqDataSetFromTximport. For the rest of the cases, read counts for each Ensembl Gene ID and sample were imported to R in a single file, including the experimental design. Gene filtering and normalization were carried out using DESeq2. In the case of GSE189553, read counts were rounded to the closest integer so that they could be analyzed by DESeq2.
When using datasets from Gene Expression Profiling Interactive Analysis [115], gene expression profiling was compared between ovarian cancer patients from the Cancer Genome Atlas (TCGA) and normal ovarian tissues from Genotype-Tissue Expression (GTEx). Specifically, in the “FUNCTIONS”, “Expression Analysis”, and “Differential Genes” tab, the “OV” dataset, which corresponds to ovarian adenocarcinoma, was selected. Moreover, from GEPIA2, the top differential genes affecting prognostic variables were downloaded. Specifically, in the “FUNCTIONS”, “Survival Analysis”, and “Most Differential Survival Genes” tab, the “OV” dataset was again selected. Additionally, GTEx normal ovary read counts were downloaded from https://gtexportal.org/home/datasets (accessed on 15 January 2023), and TCGA ovarian adenocarcinoma read counts and raw survival information were downloaded from UCSC Xena (https://xenabrowser.net/datapages/ (accessed on 15 January 2023)).
Cell line RNA-Seq read count data generated by RNA-Seq by Expectation-Maximization (RSEM), along with metadata related to the histological subtype and metastatic or non-metastatic origin of the cell lines, were downloaded from the Cancer Cell Line Encyclopedia [116]. We filtered the information from 57 epithelial ovarian cancer cell lines and 1 immortalized, non-cancerous ovarian epithelium cell line (OELE). There are 74 cell lines derived from the ovaries, but COLO704, HEY, OVMIU, DOV13, OC315, JHOS3, OVCA420, and OVCA433 do not have transcriptomic information available, and OVCAR5, HSKTC, SNU840, KGN, PA1, COV434, BIN67, and SCCOHT1 were not considered in our meta-analysis because they are not models of epithelial ovarian cancer. RSEM counts were rounded to the closest integer and then normalized using DESeq2.
4.3. Data Analysis
Four different types of comparisons were carried out to identify differentially expressed lncRNA genes in EOC: (I) “Diagnostic category”—cancer samples versus normal samples; (II) “Metastasis category”—peritoneal metastasis versus primary tumor, peritoneal metastasis versus cancer cells from ascites or effusion, or cancer cells from ascites or effusion versus primary tumor; (III) “Drug resistance category”—resistant versus sensitive or partial responders versus complete responders; and (IV) “Subtype category”—samples with one histological subtype versus the remaining ones. An additional comparison was carried out between metastasis-derived cell lines and primary tumor-derived cell lines using data from cell line transcriptomes.
4.3.1. Differential Expression in Microarray
To identify differentially expressed genes between ovarian cancer patients and women free of the disease, class comparisons between groups of arrays were carried out using the function “Class comparison”. Two-sample t-tests were used, setting the significance threshold of univariate tests as 0.01, assuming (when possible) a random variance model, and blocking by patient or sample (when matched samples were available).
4.3.2. Differential Expression in RNA-Seq
Differential expression was run with DESeq2 and, finally, annotated to the human genome hg38, as previously described for microarray data. The false discovery rate (FDR) cutoff was set as 0.05 and |Log2FC| as 0.585, blocking by patient or sample when matched samples were available. In the case of GEPIA2, the differential gene expression was calculated using the LIMMA method, with the |Log2FC| cutoff set as 0.585 and the q-value cutoff set as 0.01, selecting both overexpressed and underexpressed genes.
4.3.3. Differential Survival in Microarray
Overall survival (OS) and disease-free survival (DFS) gene lists were generated using BRB-ArrayTools “Survival analysis” and “Genes affecting survival”. This option carries out Cox proportional hazards model (Wald statistic) univariate tests, and the p-value was set to 0.01.
4.3.4. Differential Survival in RNA-Seq (GEPIA2)
OS and DFS gene lists were generated based on gene expression by applying the Log-rank test (Mantel-Cox test). The OV TCGA cohort threshold between the “high” and “low” groups was set to the median, the confidence interval to 95%, and the p-value to 0.01.
4.4. Pairwise lncRNA Analysis
Significantly dysregulated lncRNAs with Ensembl IDs from microarray or RNA-Seq studies were selected and separated into “upregulated” or “downregulated” lists, respectively, for each individual study according to their fold change in the previously described comparisons.
All possible pairwise comparisons between studies of each category were carried out within the “upregulated” and “downregulated” lists, separately, using the Excel match function between Ensembl Gene IDs. As an exclusion criterion for all of the categories, genes that had contradictory trends in different studies were considered to be ambiguous and were discarded. For the “Diagnostic category”, only lncRNA genes that were differentially expressed in at least three independent studies were selected. For the “Survival category”, lncRNAs were separated into those positively correlated with favorable patient prognosis or unfavorable prognosis. Finally, selected lncRNA genes for each category were further compared pairwise with the remaining categories, again using the Excel match function (version 2305).
4.5. ROC Analysis
Receiver operating characteristic (ROC) curve analysis was performed using easyROC (version 1.3.1.) (http://www.biosoft.hacettepe.edu.tr/easyROC/ (accessed on 15 January 2023)) [117].
4.6. Figures and Venn Diagrams
Figures were drown with graphpad prism version 8.0.2.Venn diagrams were generated using InteractiVenn (http://www.interactivenn.net/# (accessed on 15 January 2023) [118]).
4.7. Nomenclature
Novel transcripts that do not have yet a gene symbol are identified by their Ensembl Gene ID.
5. Conclusions
The study of lncRNAs in cancer is an emerging field, and according to GENCODE Release 43 [119] the number of known lncRNA genes in the human genome so far is 19,928, which is slightly higher than the 19,393 protein-coding genes. Despite this large number, only a small proportion of lncRNAs have been associated with EOC, and with this study we have increased this number, with 1631 new lncRNA genes. This effort has produced valuable information to take into account in future projects, since specific lncRNAs are associated with EOC for diagnosis (ENSG00000187951, MIR205HG, ZNF232-AS1, ENSG00000285756, LINC01297, TFAP2A-AS1, LINC01977, LINC01770, which are upregulated; and PGM5-AS1, ENSG00000267058, EPM2A-DT, NR2F1-AS1, KLF3-AS1, GLIDR, ERVK13-3, CLN8-AS1, which are downregulated), prognosis (GUSBP11, MIR924HG, unfavorable; and AQP5-AS1, favorable), and metastasis (LINC02544, LINC01235, HECW2-AS1, and MIR31HG). Furthermore, the differential expression of lncRNAs is highly specific to the different subtypes, which can be used for diagnosis purposes.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms241310798/s1. Complete results of the meta-analysis and references of previous studies [120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261,262,263,264,265,266,267,268,269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286,287,288,289,290,291,292,293,294,295,296,297,298,299,300,301,302,303,304,305,306,307,308,309,310,311].
Author Contributions
Conceptualization, M.S.-M., M.L.-M. and M.E.C.; data curation, M.S.-M.; formal analysis, M.S.-M.; funding acquisition, M.L.-M., Á.V.-V. and M.E.C.; methodology, L.L.-C. and Á.V.-V.; project administration, M.L.-M.; resources, A.B.-A., E.R.-B. and M.Q.-V.; supervision, M.L.-M. and M.E.C.; writing—original draft, M.S.-M. and M.E.C.; writing—review and editing, M.S.-M., M.L.-M., L.L.-C., Á.V.-V., A.B.-A., E.R.-B., M.Q.-V. and M.E.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Plan Estatal I + D + i, Instituto de Salud Carlos III (ISCIII, Spain grant no. PI18/01417) and the Ministerio de Ciencia e Innovación (grant no. PID2021-124564OB-I00), and co-funded by the Fondo Europeo de Desarrollo Regional-FEDER (The European Regional Development Fund-ERDF) “A way of Making Europe” and by the Xunta de Galicia (Consolidación Grupos Referencia Competitiva grant no. ED431C 2020-08). M.S.-M. acknowledges the Ministry of Science, Innovation, and Universities of Spain for funding through an FPU fellowship.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of Ovarian Cancer: A Review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Ueland, F. A Perspective on Ovarian Cancer Biomarkers: Past, Present and Yet-To-Come. Diagnostics 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, A.; Chen, L. LncRNAs in Ovarian Cancer. Clin. Chim. Acta 2019, 490, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Salamini-Montemurri, M.; Lamas-Maceiras, M.; Barreiro-Alonso, A.; Vizoso-Vázquez, Á.; Rodríguez-Belmonte, E.; Quindós-Varela, M.; Esperanza Cerdán, M. The Challenges and Opportunities of LncRNAs in Ovarian Cancer Research and Clinical Use. Cancers 2020, 12, 1020. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Doxtater, K.; Keramatnia, F.; Zacheaus, C.; Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Role of LncRNAs in Ovarian Cancer: Defining New Biomarkers for Therapeutic Purposes. Drug Discov. Today 2018, 23, 1635–1643. [Google Scholar] [CrossRef]
- Worku, T.; Bhattarai, D.; Ayers, D.; Wang, K.; Wang, C.; Rehman, Z.; Talpur, H.S.; Yang, L. Long Non-Coding RNAs: The New Horizon of Gene Regulation in Ovarian Cancer. Cell. Physiol. Biochem. 2017, 44, 948–966. [Google Scholar] [CrossRef]
- Negi, A.; Shukla, A.; Jaiswar, A.; Shrinet, J.; Jasrotia, R.S. Applications and Challenges of Microarray and RNA-Sequencing. In Bioinformatics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 91–103. [Google Scholar]
- Pleasance, E.; Bohm, A.; Williamson, L.M.; Nelson, J.M.T.; Shen, Y.; Bonakdar, M.; Titmuss, E.; Csizmok, V.; Wee, K.; Hosseinzadeh, S.; et al. Whole-Genome and Transcriptome Analysis Enhances Precision Cancer Treatment Options. Ann. Oncol. 2022, 33, 939–949. [Google Scholar] [CrossRef]
- Ma, S.-Y.; Wei, P.; Qu, F. KCNMA1-AS1 Attenuates Apoptosis of Epithelial Ovarian Cancer Cells and Serves as a Risk Factor for Poor Prognosis of Epithelial Ovarian Cancer. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4629–4641. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, W. Long Non-coding RNA LINC01627 Is a Prognostic Risk Factor for Epithelial Ovarian Cancer. Oncol. Lett. 2019, 18, 2861–2868. [Google Scholar] [CrossRef]
- Chen, Y.; Bi, F.; An, Y.; Yang, Q. Identification of Pathological Grade and Prognosis-associated LncRNA for Ovarian Cancer. J. Cell. Biochem. 2019, 120, 14444–14454. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, Y.; Zheng, H.; Ding, Y.; Wang, X. An Integrated Analysis Reveals the Oncogenic Function of LncRNA LINC00511 in Human Ovarian Cancer. Cancer Med. 2019, 8, 3026–3035. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Zhang, Z.; Zou, J.; Li, J.; Wei, R.; Guo, Q.; Zhu, X.; Chu, C.; Fu, X.; et al. Meta-Analysis Based Gene Expression Profiling Reveals Functional Genes in Ovarian Cancer. Biosci. Rep. 2020, 40, BSR20202911. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Z.; Liang, B.; Chen, S.; Zhang, X.; Tong, X.; Lou, W.; Le, L.; Tang, X.; Fu, F. Identification of Core Genes in Ovarian Cancer by an Integrative Meta-Analysis. J. Ovarian Res. 2018, 11, 94. [Google Scholar] [CrossRef]
- Dong, H.; Hong, S.; Xu, X.; Xiao, Y.; Jin, L.; Xiong, M. Meta-Analysis and Network Analysis of Five Ovarian Cancer Gene Expression Dataset. In Proceedings of the 2010 Third International Joint Conference on Computational Science and Optimization, Huangshan, China, 28–31 May 2010; pp. 242–246. [Google Scholar]
- Fridley, B.L.; Dai, J.; Raghavan, R.; Li, Q.; Winham, S.J.; Hou, X.; Weroha, S.J.; Wang, C.; Kalli, K.R.; Cunningham, J.M.; et al. Transcriptomic Characterization of Endometrioid, Clear Cell, and High-Grade Serous Epithelial Ovarian Carcinoma. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lei, Y.; Li, S.; Li, F.; Lei, J. LncRNA PART1 Stimulates the Development of Ovarian Cancer by Up-Regulating RACGAP1 and RRM2. Reprod. Sci. 2022, 29, 2224–2235. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, H.; Zhou, J.; Wang, J.; Xu, Y.; Zhang, H.; Gu, Y.; Fu, F.; Shen, Y.; Zhang, G.; et al. Inhibition of Long Non-Coding RNA XIST Upregulates MicroRNA-149-3p to Repress Ovarian Cancer Cell Progression. Cell Death Dis. 2021, 12, 145. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Z.; Dai, C.; Cao, J.; Liu, X.; Xu, J.; Lv, M.; Gu, Y.; Zhang, J.; Hua, X.; et al. LncRNAs Expression Profiling in Normal Ovary, Benign Ovarian Cyst and Malignant Epithelial Ovarian Cancer. Sci. Rep. 2016, 6, 38983. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Xia, L.; Zhang, M. LncRNA WDFY3-AS2 Promotes Cisplatin Resistance and the Cancer Stem Cell in Ovarian Cancer by Regulating Hsa-MiR-139-5p/SDC4 Axis. Cancer Cell Int. 2021, 21, 284. [Google Scholar] [CrossRef]
- Mao, T.-L.; Fan, M.-H.; Dlamini, N.; Liu, C.-L. LncRNA MALAT1 Facilitates Ovarian Cancer Progression through Promoting Chemoresistance and Invasiveness in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 10201. [Google Scholar] [CrossRef]
- Chen, Q.; Xie, J.; Yang, Y. Long Non-Coding RNA NRSN2-AS1 Facilitates Tumorigenesis and Progression of Ovarian Cancer via MiR-744-5p/PRKX Axis. Biol. Reprod. 2022, 106, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, G.; Qiu, J.; Zhang, N.; Ding, J.; Hua, K. E2F1-Regulated Long Non-Coding RNA RAD51-AS1 Promotes Cell Cycle Progression, Inhibits Apoptosis and Predicts Poor Prognosis in Epithelial Ovarian Cancer. Sci. Rep. 2017, 7, 4469. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Xiong, Y.; Konno, Y.; Ihira, K.; Kobayashi, N.; Yue, J.; Watari, H. Long Non-Coding RNA DLEU2 Drives EMT and Glycolysis in Endometrial Cancer through HK2 by Competitively Binding with MiR-455 and by Modulating the EZH2/MiR-181a Pathway. J. Exp. Clin. Cancer Res. 2021, 40, 216. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, H.; Yang, L.; Zhan, M.; Shi, Y.; Zhang, C.; Gao, D.; Gu, M.; Chen, Y.; Wang, Z. E2F Transcription Factor 2-Activated DLEU2 Contributes to Prostate Tumorigenesis by Upregulating Serum and Glucocorticoid-Induced Protein Kinase 1. Cell Death Dis. 2022, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zhang, Y.; Yang, X.; Li, X.; Zheng, Y.; Liu, Y.; Zhang, X. N6-Methyladenine- Induced LINC00667 Promoted Breast Cancer Progression through M6A/KIAA1429 Positive Feedback Loop. Bioengineered 2022, 13, 13462–13473. [Google Scholar] [CrossRef]
- Malek, E.; Kim, B.; Driscoll, J. Identification of Long Non-Coding RNAs Deregulated in Multiple Myeloma Cells Resistant to Proteasome Inhibitors. Genes 2016, 7, 84. [Google Scholar] [CrossRef]
- Gilks, C.B.; Prat, J. Ovarian Carcinoma Pathology and Genetics: Recent Advances. Hum. Pathol. 2009, 40, 1213–1223. [Google Scholar] [CrossRef]
- Lee, C.M.; Lo, H.-W.; Shao, R.-P.; Wang, S.-C.; Xia, W.; Gershenson, D.M.; Hung, M.-C. Selective Activation of Ceruloplasmin Promoter in Ovarian Tumors. Cancer Res. 2004, 64, 1788–1793. [Google Scholar] [CrossRef]
- Tarhriz, V.; Bandehpour, M.; Dastmalchi, S.; Ouladsahebmadarek, E.; Zarredar, H.; Eyvazi, S. Overview of CD24 as a New Molecular Marker in Ovarian Cancer. J. Cell. Physiol. 2019, 234, 2134–2142. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, M.; Cai, Y.; Rong, Z.; Wang, C.; Xu, Z.; Xu, H.; Song, W.; Hou, Y.; Lou, G. Platelet-derived Growth Factor-D Expression Mediates the Effect of Differentiated Degree on Prognosis in Epithelial Ovarian Cancer. J. Cell. Biochem. 2019, 120, 6920–6925. [Google Scholar] [CrossRef]
- Xu, P.; Xu, S.; Pan, H.; Dai, C.; Xu, Y.; Wang, L.; Cong, Y.; Zhang, H.; Cao, J.; Ge, L.; et al. Differential Effects of the LncRNA RNF157-AS1 on Epithelial Ovarian Cancer Cells through Suppression of DIRAS3- and ULK1-Mediated Autophagy. Cell Death Dis. 2023, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Spindler, T.J.; de Souza Fonseca, M.A.; Corona, R.I.; Seo, J.H.; Dezem, F.S.; Li, L.; Lee, J.M.; Long, H.W.; Sellers, T.A.; et al. Super-Enhancer-Associated LncRNA UCA1 Interacts Directly with AMOT to Activate YAP Target Genes in Epithelial Ovarian Cancer. iScience 2019, 17, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, R.; Yang, Y.; Jiang, J. LncRNA BBOX1-AS1 Aggravates the Development of Ovarian Cancer by Sequestering MiR-361-3p to Augment PODXL Expression. Reprod. Sci. 2021, 28, 736–744. [Google Scholar] [CrossRef]
- Li, L.-M.; Hao, S.-J.; Ni, M.; Jin, S.; Tian, Y.-Q. DUXAP8 Promotes the Proliferation and Migration of Ovarian Cancer Cells via Down-Regulating MicroRNA-29a-3p Expression. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhai, Y.; Chen, Y. GATA1-Induced Upregulation of LINC01503 Promotes Carboplatin Resistance in Ovarian Carcinoma by Upregulating PD-L1 via Sponging MiR-766-5p. J. Ovarian Res. 2021, 14, 108. [Google Scholar] [CrossRef]
- Zhang, Y.; Dun, Y.; Zhou, S.; Huang, X.-H. LncRNA HOXD-AS1 Promotes Epithelial Ovarian Cancer Cells Proliferation and Invasion by Targeting MiR-133a-3p and Activating Wnt/β-Catenin Signaling Pathway. Biomed. Pharmacother. 2017, 96, 1216–1221. [Google Scholar] [CrossRef]
- Xu, D.; Song, Q.; Liu, Y.; Chen, W.; LU, L.; Xu, M.; Fang, X.; Zhao, W.; Zhou, H. LINC00665 Promotes Ovarian Cancer Progression through Regulating the MiRNA-34a-5p/E2F3 Axis. J. Cancer 2021, 12, 1755–1763. [Google Scholar] [CrossRef]
- Zhu, L.; Mei, M. Interference of Long Non-coding RNA HAGLROS Inhibits the Proliferation and Promotes the Apoptosis of Ovarian Cancer Cells by Targeting MiR-26b-5p. Exp. Ther. Med. 2021, 22, 879. [Google Scholar] [CrossRef]
- Gokulnath, P.; de Cristofaro, T.; Manipur, I.; Di Palma, T.; Soriano, A.A.; Guarracino, M.R.; Zannini, M. Long Non-Coding RNA MAGI2-AS3 Is a New Player with a Tumor Suppressive Role in High Grade Serous Ovarian Carcinoma. Cancers 2019, 11, 2008. [Google Scholar] [CrossRef]
- Gokulnath, P.; de Cristofaro, T.; Manipur, I.; Di Palma, T.; Soriano, A.A.; Guarracino, M.R.; Zannini, M. Long Non-Coding RNA HAND2-AS1 Acts as a Tumor Suppressor in High-Grade Serous Ovarian Carcinoma. Int. J. Mol. Sci. 2020, 21, 4059. [Google Scholar] [CrossRef]
- Gloss, B.; Moran-Jones, K.; Lin, V.; Gonzalez, M.; Scurry, J.; Hacker, N.F.; Sutherland, R.L.; Clark, S.J.; Samimi, G. ZNF300P1 Encodes a LincRNA That Regulates Cell Polarity and Is Epigenetically Silenced in Type II Epithelial Ovarian Cancer. Mol. Cancer 2014, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Kamikihara, T.; Arima, T.; Kato, K.; Matsuda, T.; Kato, H.; Douchi, T.; Nagata, Y.; Nakao, M.; Wake, N. Epigenetic Silencing of the Imprinted GeneZAC by DNA Methylation Is an Early Event in the Progression of Human Ovarian Cancer. Int. J. Cancer 2005, 115, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Profumo, V.; Forte, B.; Percio, S.; Rotundo, F.; Doldi, V.; Ferrari, E.; Fenderico, N.; Dugo, M.; Romagnoli, D.; Benelli, M.; et al. LEADeR Role of MiR-205 Host Gene as Long Noncoding RNA in Prostate Basal Cell Differentiation. Nat. Commun. 2019, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Di Agostino, S.; Valenti, F.; Sacconi, A.; Fontemaggi, G.; Pallocca, M.; Pulito, C.; Ganci, F.; Muti, P.; Strano, S.; Blandino, G. Long Non-Coding MIR205HG Depletes Hsa-MiR-590-3p Leading to Unrestrained Proliferation in Head and Neck Squamous Cell Carcinoma. Theranostics 2018, 8, 1850–1868. [Google Scholar] [CrossRef]
- Du, Q.; Hoover, A.R.; Dozmorov, I.; Raj, P.; Khan, S.; Molina, E.; Chang, T.-C.; de la Morena, M.T.; Cleaver, O.B.; Mendell, J.T.; et al. MIR205HG Is a Long Noncoding RNA That Regulates Growth Hormone and Prolactin Production in the Anterior Pituitary. Dev. Cell 2019, 49, 618–631.e5. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Zhang, R.; Li, C.; Xiong, J.; Wei, Y. MIR205HG Acts as a CeRNA to Expedite Cell Proliferation and Progression in Lung Squamous Cell Carcinoma via Targeting MiR-299-3p/MAP3K2 Axis. BMC Pulm. Med. 2020, 20, 163. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Long, X.; Pu, Q. MIR205 Host Gene (MIR205HG) Drives Osteosarcoma Metastasis via Regulating the MicroRNA 2114-3p (MiR-2114-3p)/Twist Family BHLH Transcription Factor 2 (TWIST2) Axis. Bioengineered 2021, 12, 1576–1586. [Google Scholar] [CrossRef]
- Guo, J.; Gan, Q.; Gan, C.; Zhang, X.; Ma, X.; Dong, M. LncRNA MIR205HG Regulates Melanomagenesis via the MiR-299-3p/VEGFA Axis. Aging 2021, 13, 5297–5311. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Y.; Zheng, L. Analysis of Differentially Expressed Long Non-coding RNAs Revealed a Pro-tumor Role of MIR205HG in Cervical Cancer. Mol. Med. Rep. 2021, 25, 42. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Huang, H. Long Non-Coding RNA MIR205HG Function as a CeRNA to Accelerate Tumor Growth and Progression via Sponging MiR-122–5p in Cervical Cancer. Biochem. Biophys. Res. Commun. 2019, 514, 78–85. [Google Scholar] [CrossRef]
- Hongle, L.; Jia, J.; Yang, L.; Chu, J.; Sheng, J.; Wang, C.; Meng, W.; Jia, Z.; Yin, H.; Wan, J.; et al. LncRNA MIR205HG Drives Esophageal Squamous Cell Carcinoma Progression by Regulating MiR-214/SOX4 Axis. OncoTargets Ther. 2020, 13, 13097–13109. [Google Scholar]
- Dong, X.; Chen, X.; Lu, D.; Diao, D.; Liu, X.; Mai, S.; Feng, S.; Xiong, G. LncRNA MiR205HG Hinders HNRNPA0 Translation: Anti-oncogenic Effects in Esophageal Carcinoma. Mol. Oncol. 2022, 16, 795–812. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Tieu, A.H.; Cheng, Y.; Ma, K.; Akshintala, V.S.; Simsek, C.; Prasath, V.; Shin, E.J.; Ngamruengphong, S.; Khashab, M.A.; et al. Novel Long Noncoding RNA MiR205HG Functions as an Esophageal Tumor-Suppressive Hedgehog Inhibitor. Cancers 2021, 13, 1707. [Google Scholar] [CrossRef] [PubMed]
- Bezzecchi, E.; Pagani, G.; Forte, B.; Percio, S.; Zaffaroni, N.; Dolfini, D.; Gandellini, P. MIR205HG/LEADR Long Noncoding RNA Binds to Primed Proximal Regulatory Regions in Prostate Basal Cells Through a Triplex- and Alu-Mediated Mechanism. Front. Cell Dev. Biol. 2022, 10, 1293. [Google Scholar] [CrossRef]
- Hao, C.; Lin, S.; Liu, P.; Liang, W.; Li, Z.; Li, Y. Potential Serum Metabolites and Long-chain Noncoding RNA Biomarkers for Endometrial Cancer Tissue. J. Obstet. Gynaecol. Res. 2023, 49, 725–743. [Google Scholar] [CrossRef]
- Zhang, T.; Xia, W.; Song, X.; Mao, Q.; Huang, X.; Chen, B.; Liang, Y.; Wang, H.; Chen, Y.; Yu, X.; et al. Super-Enhancer Hijacking LINC01977 Promotes Malignancy of Early-Stage Lung Adenocarcinoma Addicted to the Canonical TGF-β/SMAD3 Pathway. J. Hematol. Oncol. 2022, 15, 114. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Wang, X.; Liang, Y.; Luo, D.; Han, D.; Li, C.; Chen, T.; Zhang, H.; Liu, Y.; et al. LINC01977 Promotes Breast Cancer Progression and Chemoresistance to Doxorubicin by Targeting MiR-212-3p/GOLM1 Axis. Front. Oncol. 2021, 11, 657094. [Google Scholar] [CrossRef]
- Van Grembergen, O.; Bizet, M.; de Bony, E.J.; Calonne, E.; Putmans, P.; Brohée, S.; Olsen, C.; Guo, M.; Bontempi, G.; Sotiriou, C.; et al. Portraying Breast Cancers with Long Noncoding RNAs. Sci. Adv. 2016, 2, e1600220. [Google Scholar] [CrossRef]
- Hu, J.; Wang, T.; Chen, Q. Competitive Endogenous RNA Network Identifies Four Long Non-Coding RNA Signature as a Candidate Prognostic Biomarker for Lung Adenocarcinoma. Transl. Cancer Res. 2019, 8, 1046–1064. [Google Scholar] [CrossRef]
- Seitz, A.K.; Christensen, L.L.; Christensen, E.; Faarkrog, K.; Ostenfeld, M.S.; Hedegaard, J.; Nordentoft, I.; Nielsen, M.M.; Palmfeldt, J.; Thomson, M.; et al. Profiling of Long Non-Coding RNAs Identifies LINC00958 and LINC01296 as Candidate Oncogenes in Bladder Cancer. Sci. Rep. 2017, 7, 395. [Google Scholar] [CrossRef]
- Li, L.; Peng, Q.; Gong, M.; Ling, L.; Xu, Y.; Liu, Q. Using LncRNA Sequencing to Reveal a Putative LncRNA-MRNA Correlation Network and the Potential Role of PCBP1-AS1 in the Pathogenesis of Cervical Cancer. Front. Oncol. 2021, 11, 634732. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Guo, H.; Tang, J. Long Non-Coding RNA TFAP2A-AS1 Inhibits Cell Proliferation and Invasion in Breast Cancer via MiR-933/SMAD2. Med. Sci. Monit. 2019, 25, 1242–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, L.; Wu, J.; You, J.; Hong, Q.; Ye, F. Transcription Factor KLF15 Inhibits the Proliferation and Migration of Gastric Cancer Cells via Regulating the TFAP2A-AS1/NISCH Axis. Biol. Direct 2021, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Hui, B.; Lu, C.; Wang, J.; Xu, Y.; Yang, Y.; Ji, H.; Li, X.; Xu, L.; Wang, J.; Tang, W.; et al. Engineered Exosomes for Co-delivery of PGM5-AS1 and Oxaliplatin to Reverse Drug Resistance in Colon Cancer. J. Cell. Physiol. 2022, 237, 911–933. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P.; Gao, H.; Wang, X.; Yan, M. Long Non-coding RNA PGM5-AS1 Promotes Epithelial-mesenchymal Transition, Invasion and Metastasis of Osteosarcoma Cells by Impairing MiR-140-5p-mediated FBN1 Inhibition. Mol. Oncol. 2020, 14, 2660–2677. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Deng, M.; Xue, N.-N.; Li, T.-X.; Guo, Y.-X.; Gao, L.; Zhao, D.; Fan, R.-T. LncRNA KLF3-AS1 Suppresses Cell Migration and Invasion in ESCC by Impairing MiR-185-5p-Targeted KLF3 Inhibition. Mol. Ther.-Nucleic Acids 2020, 20, 231–241. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, K.; Xia, Y.; Liang, L.; Zhu, X. Long Noncoding RNA KLF3-AS1 Acts as an Endogenous RNA of MiR-223 to Attenuate Gastric Cancer Progression and Chemoresistance. Front. Oncol. 2021, 11, 704339. [Google Scholar] [CrossRef]
- Chen, C.; Liu, L. Silencing of LncRNA KLF3-AS1 Represses Cell Growth in Osteosarcoma via MiR-338-3p/MEF2C Axis. J. Clin. Lab. Anal. 2022, 36, e24698. [Google Scholar] [CrossRef]
- Jin, L.; Chen, C.; Huang, L.; Sun, Q.; Bu, L. Long Noncoding RNA NR2F1-AS1 Stimulates the Tumorigenic Behavior of Non-Small Cell Lung Cancer Cells by Sponging MiR-363-3p to Increase SOX4. Open Med. 2021, 17, 87–95. [Google Scholar] [CrossRef]
- Guo, F.; Fu, Q.; Wang, Y.; Sui, G. Long Non-coding RNA NR2F1-AS1 Promoted Proliferation and Migration yet Suppressed Apoptosis of Thyroid Cancer Cells through Regulating MiRNA-338-3p/CCND1 Axis. J. Cell. Mol. Med. 2019, 23, 5907–5919. [Google Scholar] [CrossRef]
- Luo, D.; Liu, Y.; Li, Z.; Zhu, H.; Yu, X. NR2F1-AS1 Promotes Pancreatic Ductal Adenocarcinoma Progression Through Competing Endogenous RNA Regulatory Network Constructed by Sponging MiRNA-146a-5p/MiRNA-877-5p. Front. Cell Dev. Biol. 2021, 9, 736980. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, J.; Ding, C.; Jin, X.; Jia, Z.; Peng, J. LncRNA NR2F1-AS1 Regulates Hepatocellular Carcinoma Oxaliplatin Resistance by Targeting ABCC1 via MiR-363. J. Cell. Mol. Med. 2018, 22, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Cai, K.; Zheng, D.; Zhu, C.; Li, L.; Wang, F.; He, Z.; Yu, C.; Sun, C. Hypoxia-Induced Long Noncoding RNA NR2F1-AS1 Maintains Pancreatic Cancer Proliferation, Migration, and Invasion by Activating the NR2F1/AKT/MTOR Axis. Cell Death Dis. 2022, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Wu, Q.; Fang, H.; Wang, Y.; Xiao, Y.; Cong, M.; Wang, T.; He, Y.; Ma, C.; et al. Long Non-Coding RNA NR2F1-AS1 Induces Breast Cancer Lung Metastatic Dormancy by Regulating NR2F1 and ΔNp63. Nat. Commun. 2021, 12, 5232. [Google Scholar] [CrossRef]
- Yu, Q.; Li, X.; Feng, T. GLIDR Promotes the Progression of Glioma by Regulating the MiR-4677-3p/MAGI2 Axis. Exp. Cell Res. 2021, 406, 112726. [Google Scholar] [CrossRef] [PubMed]
- Tai, G.; Fu, H.; Bai, H.; Liu, H.; Li, L.; Song, T. Long Non-Coding RNA GLIDR Accelerates the Tumorigenesis of Lung Adenocarcinoma by MiR-1270/TCF12 Axis. Cell Cycle 2021, 20, 1653–1662. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Cao, Q.; Li, H.; Zhao, Z.; Wei, B.; Yuan, H.; Chen, Z.; Yang, S. GLIDR Promotes the Aggressiveness Progression of Prostate Cancer Cells by Sponging MiR-128–3p. Pathol.-Res. Pract. 2023, 242, 154343. [Google Scholar] [CrossRef]
- Xie, H.; Dai, L.; Ye, B.; Chen, R.; Wang, B.; Zhang, N.; Miao, H.; Liang, W. Long Non-Coding RNA ERVK13-1 Aggravates Osteosarcoma through the Involvement of MicroRNA-873-5p/KLF5 Axis. Acta Biochim. Pol. 2022, 69, 703–710. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, Z.; Wang, X.; Zhang, Q.; Lyu, L.; Tang, B. The Predictive Competing Endogenous RNA Regulatory Networks and Potential Prognostic and Immunological Roles of Cyclin A2 in Pan-Cancer Analysis. Front. Mol. Biosci. 2022, 9, 809509. [Google Scholar] [CrossRef]
- Cao, W.; Liu, J.; Liu, Z.; Wang, X.; Han, Z.-G.; Ji, T.; Chen, W.; Zou, X. A Three-LncRNA Signature Derived from the Atlas of NcRNA in Cancer (TANRIC) Database Predicts the Survival of Patients with Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2017, 65, 94–101. [Google Scholar] [CrossRef]
- Cai, J.; Xie, H.; Yan, Y.; Huang, Z.; Tang, P.; Cao, X.; Wang, Z.; Yang, C.; Wen, J.; Tan, M.; et al. A Novel Cuproptosis-Related LncRNA Signature Predicts Prognosis and Therapeutic Response in Bladder Cancer. Front. Genet. 2023, 13, 1082691. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Liao, X.; Zhang, Y.; Xu, B.; Song, Y.; Bian, G.; Fu, X. Anti-Tumor Role of CAMK2B in Remodeling the Stromal Microenvironment and Inhibiting Proliferation in Papillary Renal Cell Carcinoma. Front. Oncol. 2022, 12, 29. [Google Scholar] [CrossRef]
- Lyu, H.; Zhang, J.; Wei, Q.; Huang, Y.; Zhang, R.; Xiao, S.; Guo, D.; Chen, X.-Z.; Zhou, C.; Tang, J. Identification of Wnt/β-Catenin- and Autophagy-Related LncRNA Signature for Predicting Immune Efficacy in Pancreatic Adenocarcinoma. Biology 2023, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, X.; Yang, B.; Xia, Z.; Chen, Q. MiR-924 as a Tumor Suppressor Inhibits Non-Small Cell Lung Cancer by Inhibiting RHBDD1/Wnt/β-Catenin Signaling Pathway. Cancer Cell Int. 2020, 20, 491. [Google Scholar] [CrossRef]
- Fan, H.; Lv, P.; Mu, T.; Zhao, X.; Liu, Y.; Feng, Y.; Lv, J.; Liu, M.; Tang, H. LncRNA N335586/MiR-924/CKMT1A Axis Contributes to Cell Migration and Invasion in Hepatocellular Carcinoma Cells. Cancer Lett. 2018, 429, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-H.; Shi, Y.-F.; Cheng, Q.; Deng, L. Expression and Localization of Aquaporin-5 in the Epithelial Ovarian Tumors. Gynecol. Oncol. 2006, 100, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Zhu, Y.; Zhang, X.; Chen, X.; Zheng, W.; Yang, J. Down-Regulated Aquaporin 5 Inhibits Proliferation and Migration of Human Epithelial Ovarian Cancer 3AO Cells. J. Ovarian Res. 2014, 7, 78. [Google Scholar] [CrossRef]
- Chetry, M.; Li, S.; Liu, H.; Hu, X.; Zhu, X. Prognostic Values of Aquaporins MRNA Expression in Human Ovarian Cancer. Biosci. Rep. 2018, 38, BSR20180108. [Google Scholar] [CrossRef]
- Wei, W.; Xu, T.; Zhang, Y.; Huang, Y.; Wang, X. Upregulation of Long Noncoding RNA Linc02544 and Its Association with Overall Survival Rate and the Influence on Cell Proliferation and Migration in Lung Squamous Cell Carcinoma. Discov. Oncol. 2022, 13, 41. [Google Scholar] [CrossRef]
- Guo, Z.-H.; Yao, L.-T.; Guo, A.-Y. Clinical and Biological Impact of LINC02544 Expression in Breast Cancer after Neoadjuvant Chemotherapy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 10573–10585. [Google Scholar]
- Zhang, C.; Liang, Y.; Zhang, C.-D.; Pei, J.-P.; Wu, K.-Z.; Li, Y.-Z.; Dai, D.-Q. The Novel Role and Function of LINC01235 in Metastasis of Gastric Cancer Cells by Inducing Epithelial-Mesenchymal Transition. Genomics 2021, 113, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-E.; Xing, Y.; Ran, B.-L.; Zhang, C.; Pan, S.-W.; An, W.; Chen, Q.-C.; Xu, H.-M. LINC01235-TWIST2 Feedback Loop Facilitates Epithelial–Mesenchymal Transition in Gastric Cancer by Inhibiting THBS2. Aging 2020, 12, 25060–25075. [Google Scholar] [CrossRef]
- Tu, C.; Ren, X.; He, J.; Li, S.; Qi, L.; Duan, Z.; Wang, W.; Li, Z. The Predictive Value of LncRNA MIR31HG Expression on Clinical Outcomes in Patients with Solid Malignant Tumors. Cancer Cell Int. 2020, 20, 115. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Hu, D.; Yang, P.; Li, Y.; Bashir, S.; Nai, A.; Ma, F.; Jia, G.; Xu, M. A Novel Cuproptosis-Related Prognostic LncRNA Signature and LncRNA MIR31HG/MiR-193a-3p/TNFRSF21 Regulatory Axis in Lung Adenocarcinoma. Front. Oncol. 2022, 12, 927706. [Google Scholar] [CrossRef]
- Wang, R.; Ma, Z.; Feng, L.; Yang, Y.; Tan, C.; Shi, Q.; Lian, M.; He, S.; Ma, H.; Fang, J. LncRNA MIR31HG Targets HIF1A and P21 to Facilitate Head and Neck Cancer Cell Proliferation and Tumorigenesis by Promoting Cell-Cycle Progression. Mol. Cancer 2018, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-W.; Hung, W.-W.; Chou, C.-H.; Tu, H.-F.; Chang, S.-R.; Liu, Y.-C.; Liu, C.-J.; Lin, S.-C. LncRNA MIR31HG Drives Oncogenicity by Inhibiting the Limb-Bud and Heart Development Gene (LBH) during Oral Carcinoma. Int. J. Mol. Sci. 2021, 22, 8383. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, B.; Cao, J.; Zhao, L.; Wang, G. MIR31HG Expression Predicts Poor Prognosis and Promotes Colorectal Cancer Progression. Cancer Manag. Res. 2022, 14, 1973–1986. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, X.; Wang, X.; Li, J. MIR31HG Promotes Cell Proliferation and Invasion by Activating the Wnt/Β-catenin Signaling Pathway in Non-small Cell Lung Cancer. Oncol. Lett. 2018, 17, 221–229. [Google Scholar] [CrossRef]
- Ghorbanzadeh, S.; Poor-Ghassem, N.; Afsa, M.; Nikbakht, M.; Malekzadeh, K. Long Non-Coding RNA NR2F2-AS1: Its Expanding Oncogenic Roles in Tumor Progression. Hum. Cell 2022, 35, 1355–1363. [Google Scholar] [CrossRef]
- Hawkins, S.M.; Loomans, H.A.; Wan, Y.-W.; Ghosh-Choudhury, T.; Coffey, D.; Xiao, W.; Liu, Z.; Sangi-Haghpeykar, H.; Anderson, M.L. Expression and Functional Pathway Analysis of Nuclear Receptor NR2F2 in Ovarian Cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1152–E1162. [Google Scholar] [CrossRef]
- Zheng, J.; Qin, W.; Jiao, D.; Ren, J.; Wei, M.; Shi, S.; Xi, W.; Wang, H.; Yang, A.-G.; Huan, Y.; et al. Knockdown of COUP-TFII Inhibits Cell Proliferation and Induces Apoptosis through Upregulating BRCA1 in Renal Cell Carcinoma Cells. Int. J. Cancer 2016, 139, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Nomura, N.; Mori, H.; Wake, N. Poor Correlation with Loss of Heterozygosity on Chromosome 17p and P53 Mutations in Ovarian Cancers. Gynecol. Oncol. 1996, 63, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.J.; Ziegler, M.R.; Radford, D.M.; Fair, K.L.; Steinbrueck, T.; Xynos, F.P.; Donis-Keller, H. Allelic Deletion on Chromosome 17p13.3 in Early Ovarian Cancer. Cancer Res. 1996, 56, 606–611. [Google Scholar] [PubMed]
- Wei, M.; Liu, L.; Wang, Z. Long Non-coding RNA Heart and Neural Crest Derivatives Expressed 2-antisense RNA 1 Overexpression Inhibits the Proliferation of Cancer Cells by Reducing RUNX2 Expression in Triple-negative Breast Cancer. Oncol. Lett. 2019, 18, 6775–6780. [Google Scholar] [CrossRef]
- Mortlock, S.; Corona, R.I.; Kho, P.F.; Pharoah, P.; Seo, J.-H.; Freedman, M.L.; Gayther, S.A.; Siedhoff, M.T.; Rogers, P.A.W.; Leuchter, R.; et al. A Multi-Level Investigation of the Genetic Relationship between Endometriosis and Ovarian Cancer Histotypes. Cell Rep. Med. 2022, 3, 100542. [Google Scholar] [CrossRef]
- Li, Z.; Niu, H.; Qin, Q.; Yang, S.; Wang, Q.; Yu, C.; Wei, Z.; Jin, Z.; Wang, X.; Yang, A.; et al. LncRNA UCA1 Mediates Resistance to Cisplatin by Regulating the MiR-143/FOSL2-Signaling Pathway in Ovarian Cancer. Mol. Ther.-Nucleic Acids 2019, 17, 92–101. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Pan, D.; Xin, Z.; Bu, F.; Zhang, Y.; Tian, Q.; Feng, X. Circulating LncRNA UCA1 and LncRNA PGM5-AS1 Act as Potential Diagnostic Biomarkers for Early-Stage Colorectal Cancer. Biosci. Rep. 2021, 41, BSR20211115. [Google Scholar] [CrossRef]
- Filippov-Levy, N.; Cohen-Schussheim, H.; Tropé, C.G.; Hetland Falkenthal, T.E.; Smith, Y.; Davidson, B.; Reich, R. Expression and Clinical Role of Long Non-Coding RNA in High-Grade Serous Carcinoma. Gynecol. Oncol. 2018, 148, 559–566. [Google Scholar] [CrossRef]
- Zheng, R.; Liang, J.; Lu, J.; Li, S.; Zhang, G.; Wang, X.; Liu, M.; Wang, W.; Chu, H.; Tao, G.; et al. Genome-Wide Long Non-Coding RNAs Identified a Panel of Novel Plasma Biomarkers for Gastric Cancer Diagnosis. Gastric Cancer 2019, 22, 731–741. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2012, 41, D991–D995. [Google Scholar] [CrossRef]
- Mudunuri, U.; Che, A.; Yi, M.; Stephens, R.M. BioDBnet: The Biological Database Network. Bioinformatics 2009, 25, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-Optimal Probabilistic RNA-Seq Quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An Enhanced Web Server for Large-Scale Expression Profiling and Interactive Analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef] [PubMed]
- Ghandi, M.; Huang, F.W.; Jané-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R.; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-Generation Characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef]
- Goksuluk, D.; Korkmaz, S.; Zararsiz, G.; Karaagaoglu, A.E. EasyROC: An Interactive Web-Tool for ROC Curve Analysis Using R Language Environment. R J. 2016, 8, 213. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Frankish, A.; Diekhans, M.; Jungreis, I.; Lagarde, J.; Loveland, J.E.; Mudge, J.M.; Sisu, C.; Wright, J.C.; Armstrong, J.; Barnes, I.; et al. GENCODE 2021. Nucleic Acids Res. 2021, 49, D916–D923. [Google Scholar] [CrossRef]
- Tone, A.A.; Begley, H.; Sharma, M.; Murphy, J.; Rosen, B.; Brown, T.J.; Shaw, P.A. Gene Expression Profiles of Luteal Phase Fallopian Tube Epithelium from BRCA Mutation Carriers Resemble High-Grade Serous Carcinoma. Clin. Cancer Res. 2008, 14, 4067–4078. [Google Scholar] [CrossRef]
- Tung, C.S.; Mok, S.C.; Tsang, Y.T.M.; Zu, Z.; Song, H.; Liu, J.; Deavers, M.T.; Malpica, A.; Wolf, J.K.; Lu, K.H.; et al. PAX2 Expression in Low Malignant Potential Ovarian Tumors and Low-Grade Ovarian Serous Carcinomas. Mod. Pathol. 2009, 22, 1243–1250. [Google Scholar] [CrossRef]
- Bowen, N.J.; Walker, L.D.; Matyunina, L.V.; Logani, S.; Totten, K.A.; Benigno, B.B.; McDonald, J.F. Gene Expression Profiling Supports the Hypothesis That Human Ovarian Surface Epithelia Are Multipotent and Capable of Serving as Ovarian Cancer Initiating Cells. BMC Med. Genom. 2009, 2, 71. [Google Scholar] [CrossRef]
- Mok, S.C.; Bonome, T.; Vathipadiekal, V.; Bell, A.; Johnson, M.E.; Wong, K.; Park, D.-C.; Hao, K.; Yip, D.K.P.; Donninger, H.; et al. A Gene Signature Predictive for Outcome in Advanced Ovarian Cancer Identifies a Survival Factor: Microfibril-Associated Glycoprotein 2. Cancer Cell 2009, 16, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Bonome, T.; Levine, D.A.; Shih, J.; Randonovich, M.; Pise-Masison, C.A.; Bogomolniy, F.; Ozbun, L.; Brady, J.; Barrett, J.C.; Boyd, J.; et al. A Gene Signature Predicting for Survival in Suboptimally Debulked Patients with Ovarian Cancer. Cancer Res. 2008, 68, 5478–5486. [Google Scholar] [CrossRef] [PubMed]
- Stany, M.P.; Vathipadiekal, V.; Ozbun, L.; Stone, R.L.; Mok, S.C.; Xue, H.; Kagami, T.; Wang, Y.; McAlpine, J.N.; Bowtell, D.; et al. Identification of Novel Therapeutic Targets in Microdissected Clear Cell Ovarian Cancers. PLoS ONE 2011, 6, e21121. [Google Scholar] [CrossRef] [PubMed]
- Lili, L.N.; Matyunina, L.V.; Walker, L.D.; Benigno, B.B.; McDonald, J.F. Molecular Profiling Predicts the Existence of Two Functionally Distinct Classes of Ovarian Cancer Stroma. Biomed Res. Int. 2013, 2013, 846387. [Google Scholar] [CrossRef] [PubMed]
- Yeung, T.-L.; Leung, C.S.; Wong, K.-K.; Samimi, G.; Thompson, M.S.; Liu, J.; Zaid, T.M.; Ghosh, S.; Birrer, M.J.; Mok, S.C. TGF-β Modulates Ovarian Cancer Invasion by Upregulating CAF-Derived Versican in the Tumor Microenvironment. Cancer Res. 2013, 73, 5016–5028. [Google Scholar] [CrossRef]
- Hill, C.G.; Matyunina, L.V.; Walker, D.; Benigno, B.B.; McDonald, J.F. Transcriptional Override: A Regulatory Network Model of Indirect Responses to Modulations in MicroRNA Expression. BMC Syst. Biol. 2014, 8, 36. [Google Scholar] [CrossRef]
- Yeung, T.-L.; Leung, C.S.; Wong, K.-K.; Gutierrez-Hartmann, A.; Kwong, J.; Gershenson, D.M.; Mok, S.C. ELF3 Is a Negative Regulator of Epithelial-Mesenchymal Transition in Ovarian Cancer Cells. Oncotarget 2017, 8, 16951–16963. [Google Scholar] [CrossRef]
- Dong, S.; Wang, R.; Wang, H.; Ding, Q.; Zhou, X.; Wang, J.; Zhang, K.; Long, Y.; Lu, S.; Hong, T.; et al. HOXD-AS1 Promotes the Epithelial to Mesenchymal Transition of Ovarian Cancer Cells by Regulating MiR-186-5p and PIK3R3. J. Exp. Clin. Cancer Res. 2019, 38, 110. [Google Scholar] [CrossRef]
- Mitra, S.; Tiwari, K.; Podicheti, R.; Pandhiri, T.; Rusch, D.B.; Bonetto, A.; Zhang, C.; Mitra, A.K. Transcriptome Profiling Reveals Matrisome Alteration as a Key Feature of Ovarian Cancer Progression. Cancers 2019, 11, 1513. [Google Scholar] [CrossRef]
- Shahab, S.W.; Matyunina, L.V.; Mezencev, R.; Walker, L.D.; Bowen, N.J.; Benigno, B.B.; McDonald, J.F. Evidence for the Complexity of MicroRNA-Mediated Regulation in Ovarian Cancer: A Systems Approach. PLoS ONE 2011, 6, e22508. [Google Scholar] [CrossRef]
- King, E.R.; Tung, C.S.; Tsang, Y.T.M.; Zu, Z.; Lok, G.T.M.; Deavers, M.T.; Malpica, A.; Wolf, J.K.; Lu, K.H.; Birrer, M.J.; et al. The Anterior Gradient Homolog 3 (AGR3) Gene Is Associated With Differentiation and Survival in Ovarian Cancer. Am. J. Surg. Pathol. 2011, 35, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Lee, J.; Haemmerle, M.; Ling, H.; Previs, R.A.; Pradeep, S.; Wu, S.Y.; Ivan, C.; Ferracin, M.; Dennison, J.B.; et al. Long Noncoding RNA Ceruloplasmin Promotes Cancer Growth by Altering Glycolysis. Cell Rep. 2015, 13, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Hendrix-Lucas, N.; Kuick, R.; Zhai, Y.; Schwartz, D.R.; Akyol, A.; Hanash, S.; Misek, D.E.; Katabuchi, H.; Williams, B.O.; et al. Mouse Model of Human Ovarian Endometrioid Adenocarcinoma Based on Somatic Defects in the Wnt/β-Catenin and PI3K/Pten Signaling Pathways. Cancer Cell 2007, 11, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Kim, J.H.; Zhou, H.; Kim, B.W.; Wong, D.T. Salivary Transcriptomic Biomarkers for Detection of Ovarian Cancer: For Serous Papillary Adenocarcinoma. J. Mol. Med. 2012, 90, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Merritt, M.A.; Parsons, P.G.; Newton, T.R.; Martyn, A.C.; Webb, P.M.; Green, A.C.; Papadimos, D.J.; Boyle, G.M. Expression Profiling Identifies Genes Involved in Neoplastic Transformation of Serous Ovarian Cancer. BMC Cancer 2009, 9, 378. [Google Scholar] [CrossRef]
- Dou, Z.; Qiu, C.; Zhang, X.; Yao, S.; Zhao, C.; Wang, Z.; Chu, R.; Chen, J.; Chen, Z.; Li, R.; et al. HJURP Promotes Malignant Progression and Mediates Sensitivity to Cisplatin and WEE1-Inhibitor in Serous Ovarian Cancer. Int. J. Biol. Sci. 2022, 18, 1188–1210. [Google Scholar] [CrossRef]
- Zhao, J.; Song, X.; Xu, T.; Yang, Q.; Liu, J.; Jiang, B.; Wu, J. Identification of Potential Prognostic Competing Triplets in High-Grade Serous Ovarian Cancer. Front. Genet. 2021, 11, 1586. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, C. LncRNA DLX6-AS1 Aggravates the Development of Ovarian Cancer via Modulating FHL2 by Sponging MiR-195-5p. Cancer Cell Int. 2020, 20, 370. [Google Scholar] [CrossRef]
- Xie, M.; Fu, Q.; Wang, P.; Cui, Y. STAT1-Induced Upregulation LncRNA LINC00958 Accelerates the Epithelial Ovarian Cancer Tumorigenesis by Regulating Wnt/β-Catenin Signaling. Dis. Mrk. 2021, 2021, 1405045. [Google Scholar] [CrossRef]
- Cremaschi, P.; Carriero, R.; Astrologo, S.; Colì, C.; Lisa, A.; Parolo, S.; Bione, S. An Association Rule Mining Approach to Discover LncRNAs Expression Patterns in Cancer Datasets. Biomed Res. Int. 2015, 2015, 146250. [Google Scholar] [CrossRef]
- Jing, L.; Gong, M.; Lu, X.; Jiang, Y.; Li, H.; Cheng, W. LINC01127 Promotes the Development of Ovarian Tumors by Regulating the Cell Cycle. Am. J. Transl. Res. 2019, 11, 406–417. [Google Scholar] [PubMed]
- Wang, W.; Song, F.; Feng, X.; Chu, X.; Dai, H.; Tian, J.; Fang, X.; Song, F.; Liu, B.; Li, L.; et al. Functional Interrogation of Enhancer Connectome Prioritizes Candidate Target Genes at Ovarian Cancer Susceptibility Loci. Front. Genet. 2021, 12, 646179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, T.; Ji, J.; Zhao, F.; Li, C.; Han, X. RHPN1-AS1 Promotes Cell Proliferation and Migration via MiR-665/Akt3 in Ovarian Cancer. Cancer Gene Ther. 2021, 28, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Zhou, J.-Q.; Liao, Y.-S. Autophagy-Related Long Non-Coding RNA Signature for Ovarian Cancer. J. Int. Med. Res. 2020, 48, 030006052097076. [Google Scholar] [CrossRef]
- Chang, H.; Li, B.; Zhang, X.; Meng, X. NCK1-AS1 Promotes NCK1 Expression to Facilitate Tumorigenesis and Chemo-Resistance in Ovarian Cancer. Biochem. Biophys. Res. Commun. 2020, 522, 292–299. [Google Scholar] [CrossRef]
- Wang, L.-L.; Sun, K.-X.; Wu, D.-D.; Xiu, Y.-L.; Chen, X.; Chen, S.; Zong, Z.-H.; Sang, X.-B.; Liu, Y.; Zhao, Y. DLEU1 Contributes to Ovarian Carcinoma Tumourigenesis and Development by Interacting with MiR-490-3p and Altering CDK1 Expression. J. Cell. Mol. Med. 2017, 21, 3055–3065. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; Kong, D.; Hu, L.; Liu, D.; Wu, J. Long Noncoding RNA CASC9 Promotes LIN7A Expression via MiR-758-3p to Facilitate the Malignancy of Ovarian Cancer. J. Cell. Physiol. 2019, 234, 10800–10808. [Google Scholar] [CrossRef]
- Manichaikul, A.; Peres, L.C.; Wang, X.; Barnard, M.E.; Chyn, D.; Sheng, X.; Du, Z.; Tyrer, J.; Dennis, J.; Schwartz, A.G.; et al. Identification of Novel Epithelial Ovarian Cancer Loci in Women of African Ancestry. Int. J. Cancer 2020, 146, 2987–2998. [Google Scholar] [CrossRef]
- Xue, H.; Wu, Z.; Rao, D.; Zhuo, B.; Chen, Q. Long Non-Coding RNA LINC00858 Aggravates the Oncogenic Phenotypes of Ovarian Cancer Cells through MiR-134-5p/RAD18 Signaling. Arch. Gynecol. Obstet. 2020, 302, 1243–1254. [Google Scholar] [CrossRef]
- Ding, Y.; Tan, X.; Abasi, A.; Dai, Y.; Wu, R.; Zhang, T.; Li, K.; Yan, M.; Huang, X. LncRNA TRPM2-AS Promotes Ovarian Cancer Progression and Cisplatin Resistance by Sponging MiR-138-5p to Release SDC3 MRNA. Aging 2021, 13, 6832–6848. [Google Scholar] [CrossRef]
- Feng, S.; Yin, H.; Zhang, K.; Shan, M.; Ji, X.; Luo, S.; Shen, Y. Integrated Clinical Characteristics and Omics Analysis Identifies a Ferroptosis and Iron-Metabolism-Related LncRNA Signature for Predicting Prognosis and Therapeutic Responses in Ovarian Cancer. J. Ovarian Res. 2022, 15, 10. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.R.; Lv, Y.H.; Yao, H.M.; Zhang, H.Y.; Zhou, Y.; Liu, S.E. LncRNA PCAT6 Promotes Occurrence and Development of Ovarian Cancer by Inhibiting PTEN. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8230–8238. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhai, J.; Fu, Y. LncRNA CDKN2B-AS1 Promotes the Progression of Ovarian Cancer by MiR-143-3p/SMAD3 Axis and Predicts a Poor Prognosis. Neoplasma 2020, 67, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, H.; Liu, G.; Tang, R.; Ding, Y.; Xu, P.; Wang, H.; Miao, J.; Gu, X.; Han, S. LBX2-AS1 Promotes Ovarian Cancer Progression by Facilitating E2F2 Gene Expression via MiR-455-5p and MiR-491-5p Sponging. J. Cell. Mol. Med. 2021, 25, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tan, S.; Bai, X.; Ma, S.; Chen, X. Long Non-Coding RNA LINC01215 Promotes Epithelial-Mesenchymal Transition and Lymph Node Metastasis in Epithelial Ovarian Cancer through RUNX3 Promoter Methylation. Transl. Oncol. 2021, 14, 101135. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lin, Y.-B.; Li, L.; Liu, J. LncRNA TLR8-AS1 Promotes Metastasis and Chemoresistance of Ovarian Cancer through Enhancing TLR8 MRNA Stability. Biochem. Biophys. Res. Commun. 2020, 526, 857–864. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Wang, Z.; Guo, W.; Yang, D. MYC-binding LncRNA EPIC1 Promotes AKT-mTORC1 Signaling and Rapamycin Resistance in Breast and Ovarian Cancer. Mol. Carcinog. 2020, 59, 1188–1198. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhang, Y.; Luo, C.; Zhu, T.; Zhang, R.; Yao, R. LINC01342 Promotes the Progression of Ovarian Cancer by Absorbing MicroRNA-30c-2-3p to Upregulate HIF3A. J. Cell. Physiol. 2020, 235, 3939–3949. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, S.; Lv, Z.X.; Zhao, X.J. Promoting Action of Long Non-Coding RNA Small Nucleolar RNA Host Gene 4 in Ovarian Cancer. Acta Biochim. Pol. 2023, 70, 59–68. [Google Scholar] [CrossRef]
- Chu, Z.-P.; Dai, J.; Jia, L.-G.; Li, J.; Zhang, Z.-Y.; Yan, P. Increased Expression of Long Noncoding RNA HMMR-AS1 in Epithelial Ovarian Cancer: An Independent Prognostic Factor. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8145–8150. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, H.; Huang, Q.; Wu, J.; Zhang, M. A Prognostic Model Based on Immune-Related Long Noncoding RNAs for Patients with Epithelial Ovarian Cancer. J. Ovarian Res. 2022, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Tian, Y.; Wang, R.; Zhao, C.; Kong, Y.; Tian, R.; Wang, W.; Ma, L. FOXP4-AS1 Is a Favorable Prognostic-Related Enhancer RNA in Ovarian Cancer. Biosci. Rep. 2021, 41, BSR20204008. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gong, M.; Zhao, M.; Wang, X.; Cheng, W.; Xia, Y. LncRNAs KB-1836B5, LINC00566 and FAM27L Are Associated with the Survival Time of Patients with Ovarian Cancer. Oncol. Lett. 2018, 16, 3735–3745. [Google Scholar] [CrossRef] [PubMed]
- Jie, Y.; Ye, L.; Chen, H.; Yu, X.; Cai, L.; He, W.; Fu, Y. ELFN1-AS1 Accelerates Cell Proliferation, Invasion and Migration via Regulating MiR-497-3p/CLDN4 Axis in Ovarian Cancer. Bioengineered 2020, 11, 872–882. [Google Scholar] [CrossRef]
- Wen, A.; Luo, L.; Du, C.; Luo, X. Long Non-coding RNA MiR155HG Silencing Restrains Ovarian Cancer Progression by Targeting the MicroRNA-155-5p/Tyrosinase-related Protein 1 Axis. Exp. Ther. Med. 2021, 22, 1237. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhan, X. Identification of Clinical Trait–Related LncRNA and MRNA Biomarkers with Weighted Gene Co-Expression Network Analysis as Useful Tool for Personalized Medicine in Ovarian Cancer. EPMA J. 2019, 10, 273–290. [Google Scholar] [CrossRef]
- Zhou, M.; Sun, Y.; Sun, Y.; Xu, W.; Zhang, Z.; Zhao, H.; Zhong, Z.; Sun, J. Comprehensive Analysis of LncRNA Expression Profiles Reveals a Novel LncRNA Signature to Discriminate Nonequivalent Outcomes in Patients with Ovarian Cancer. Oncotarget 2016, 7, 32433–32448. [Google Scholar] [CrossRef]
- Tao, F.; Tian, X.; Lu, M.; Zhang, Z. A Novel LncRNA, Lnc-OC1, Promotes Ovarian Cancer Cell Proliferation and Migration by Sponging MiR-34a and MiR-34c. J. Genet. Genom. 2018, 45, 137–145. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Yang, J.; Wang, Z.; Zhang, Z.; Peng, J.; Wang, Y.; Hong, L. A Novel Tumor Mutational Burden-Based Risk Model Predicts Prognosis and Correlates with Immune Infiltration in Ovarian Cancer. Front. Immunol. 2022, 13, 943389. [Google Scholar] [CrossRef]
- Li, S.; Shen, S.; Ge, W.; Cen, Y.; Zhang, S.; Cheng, X.; Wang, X.; Xie, X.; Lu, W. Long Non-Coding RNA SLC25A21-AS1 Inhibits the Development of Epithelial Ovarian Cancer by Specifically Inducing PTBP3 Degradation. Biomark. Res. 2023, 11, 12. [Google Scholar] [CrossRef]
- Xu, H.; Wang, X.; Zhang, Y.; Zheng, W.; Zhang, H. GATA6-AS1 Inhibits Ovarian Cancer Cell Proliferation and Migratory and Invasive Abilities by Sponging MiR-19a-5p and Upregulating TET2. Oncol. Lett. 2021, 22, 718. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wu, G.; Yang, F.; He, L.; Xie, X.; Li, L.; Yang, L.; Ma, Y.; Zhang, Q.; Chen, J.; et al. Elevated LINC00909 Promotes Tumor Progression of Ovarian Cancer via Regulating the MiR-23b-3p/MRC2 Axis. Oxid. Med. Cell. Longev. 2021, 2021, 5574130. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Yu, L.; Zhu, B.; Gan, H.Y.; Guo, B.Q. LncRNA GIHCG Promotes Development of Ovarian Cancer by Regulating MicroRNA-429. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8127–8134. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Lu, T.; Liu, X.; Yin, W.; Zhang, H. LncRNA SNHG8 Induces Ovarian Carcinoma Cells Cellular Process and Stemness through Wnt/β-Catenin Pathway. Cancer Biomark. 2020, 28, 459–471. [Google Scholar] [CrossRef]
- Dai, C.; Xu, P.; Liu, S.; Xu, S.; Xu, J.; Fu, Z.; Cao, J.; Lv, M.; Zhou, J.; Liu, G.; et al. Long Noncoding RNA ZEB1-AS1 Affects Paclitaxel and Cisplatin Resistance by Regulating MMP19 in Epithelial Ovarian Cancer Cells. Arch. Gynecol. Obstet. 2021, 303, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.; Wang, W.; Wu, L.; Qi, C.; Yan, W.; Lu, W.; Tian, J.; Shang, A. LINC00936/MicroRNA-221-3p Regulates Tumor Progression in Ovarian Cancer by Interacting with LAMA3. Recent Pat. Anticancer. Drug Discov. 2023, 18, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.-X.; Zhang, C.-Y.; Bai, N.; Feng, C.; Zhang, Z.-M.; Wang, L.; Gao, Z.-Z. LncRNA CRNDE Promotes Cell Proliferation, Migration and Invasion of Ovarian Cancer via MiR-423-5p/FSCN1 Axis. Mol. Cell. Biochem. 2022, 477, 1477–1488. [Google Scholar] [CrossRef]
- Wang, A.; Jin, C.; Li, H.; Qin, Q.; Li, L. LncRNA ADAMTS9-AS2 Regulates Ovarian Cancer Progression by Targeting MiR-182-5p/FOXF2 Signaling Pathway. Int. J. Biol. Macromol. 2018, 120, 1705–1713. [Google Scholar] [CrossRef]
- Aichen, Z.; Kun, W.; Xiaochun, S.; Lingling, T. LncRNA FGD5-AS1 Promotes the Malignant Phenotypes of Ovarian Cancer Cells via Targeting MiR-142-5p. Apoptosis 2021, 26, 348–360. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, J.; Zhang, H.; Cao, B.; Xu, G.; Zhang, Z.; Tong, J. Four Prognosis-Associated LncRNAs Serve as Biomarkers in Ovarian Cancer. Front. Genet. 2021, 12, 672674. [Google Scholar] [CrossRef]
- Han, S.; Li, D.; Xiao, M. LncRNA ZFAS1 Serves as a Prognostic Biomarker to Predict the Survival of Patients with Ovarian Cancer. Exp. Ther. Med. 2019, 18, 4673–4681. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yuan, D.; Zhu, D.; Xu, T.; Huang, A.; Jiang, L.; Liu, C.; Qian, H.; Bu, X. LncRNA-MSC-AS1 Inhibits the Ovarian Cancer Progression by Targeting MiR-425-5p. J. Ovarian Res. 2021, 14, 109. [Google Scholar] [CrossRef]
- Han, Z.; Li, D.; Yang, Y.; Zhang, H. LINC-DUBR Suppresses Malignant Progression of Ovarian Cancer by Downregulating MiR-107 to Induce SMAC Expression. J. Healthc. Eng. 2022, 2022, 4535655. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Shen, L.; Lin, Q.; Dong, C.; Maswela, B.; Illahi, G.S.; Wu, X. SNHG5 Enhances Paclitaxel Sensitivity of Ovarian Cancer Cells through Sponging MiR-23a. Biomed. Pharmacother. 2020, 123, 109711. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Reid, B.M.; Sellers, T.A.; Karreth, F.A. Abstract B34: LINC00886, a Risk Locus-Associated Long Noncoding RNA, Promotes Ovarian Cancer Progression. Clin. Cancer Res. 2020, 26, B34. [Google Scholar] [CrossRef]
- Li, W.; Ma, S.; Bai, X.; Pan, W.; Ai, L.; Tan, W. Long Noncoding RNA WDFY3-AS2 Suppresses Tumor Progression by Acting as a Competing Endogenous RNA of MicroRNA-18a in Ovarian Cancer. J. Cell. Physiol. 2020, 235, 1141–1154. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Zhang, A.; Wang, Q.; Ge, J.; Li, Q.; Xiao, J. Long Non-Coding RNA SDCBP2-AS1 Delays the Progression of Ovarian Cancer via MicroRNA-100-5p-Targeted EPDR1. World J. Surg. Oncol. 2021, 19, 199. [Google Scholar] [CrossRef]
- Feng, H.; Gu, Z.-Y.; Li, Q.; Liu, Q.-H.; Yang, X.-Y.; Zhang, J.-J. Identification of Significant Genes with Poor Prognosis in Ovarian Cancer via Bioinformatical Analysis. J. Ovarian Res. 2019, 12, 35. [Google Scholar] [CrossRef]
- Cai, L.; Hu, X.; Ye, L.; Bai, P.; Jie, Y.; Shu, K. Long Non-Coding RNA ADAMTS9-AS1 Attenuates Ferroptosis by Targeting MicroRNA-587/Solute Carrier Family 7 Member 11 Axis in Epithelial Ovarian Cancer. Bioengineered 2022, 13, 8226–8239. [Google Scholar] [CrossRef]
- Yang, M.; Zhai, Z.; Guo, S.; Li, X.; Zhu, Y.; Wang, Y. Long Non-Coding RNA FLJ33360 Participates in Ovarian Cancer Progression by Sponging MiR-30b-3p. OncoTargets. Ther. 2019, 12, 4469–4480. [Google Scholar] [CrossRef]
- Lei, J.; He, Z.; Wang, J.; Hu, M.; Zhou, P.; Lian, C.; Hua, L.; Wu, S.; Zhou, J. Identification of MEG8/MiR-378d/SOBP Axis as a Novel Regulatory Network and Associated with Immune Infiltrates in Ovarian Carcinoma by Integrated Bioinformatics Analysis. Cancer Med. 2021, 10, 2924–2939. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wang, F.; Gao, C.; Cao, Y.; Wang, J. Development and Verification of an Autophagy-Related LncRNA Signature to Predict Clinical Outcomes and Therapeutic Responses in Ovarian Cancer. Front. Med. 2021, 8, 666973. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, H.; Xi, M.; Lu, X. Long Noncoding RNA C17orf91 Is a Potential Prognostic Marker and Functions as an Oncogene in Ovarian Cancer. J. Ovarian Res. 2016, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Gao, H.; Li, X.; Zhu, Y.; Peng, S.; Yu, J.; Zhan, G.; Wang, J.; Liu, N.; Guo, X. LncRNA TPT1-AS1 Promotes Tumorigenesis and Metastasis in Epithelial Ovarian Cancer by Inducing TPT1 Expression. Cancer Sci. 2019, 110, 1587–1598. [Google Scholar] [CrossRef]
- Xu, R.; Peng, H.; Yang, N.; Liu, Z.; Lu, W. Nuclear LncRNA CERNA1 Enhances the Cisplatin-Induced Cell Apoptosis and Overcomes Chemoresistance via Epigenetic Activation of BCL2L10 in Ovarian Cancer. Genes Dis. 2023, 10, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Spentzos, D.; Karlan, B.Y.; Taniguchi, T.; Fountzilas, E.; Francoeur, N.; Levine, D.A.; Cannistra, S.A. Gene Expression Profile of BRCA Ness That Correlates With Responsiveness to Chemotherapy and With Outcome in Patients With Epithelial Ovarian Cancer. J. Clin. Oncol. 2010, 28, 3555–3561. [Google Scholar] [CrossRef]
- Mateescu, B.; Batista, L.; Cardon, M.; Gruosso, T.; de Feraudy, Y.; Mariani, O.; Nicolas, A.; Meyniel, J.-P.; Cottu, P.; Sastre-Garau, X.; et al. MiR-141 and MiR-200a Act on Ovarian Tumorigenesis by Controlling Oxidative Stress Response. Nat. Med. 2011, 17, 1627–1635. [Google Scholar] [CrossRef]
- Lisowska, K.M.; Olbryt, M.; Student, S.; Kujawa, K.A.; Cortez, A.J.; Simek, K.; Dansonka-Mieszkowska, A.; Rzepecka, I.K.; Tudrej, P.; Kupryjańczyk, J. Unsupervised Analysis Reveals Two Molecular Subgroups of Serous Ovarian Cancer with Distinct Gene Expression Profiles and Survival. J. Cancer Res. Clin. Oncol. 2016, 142, 1239–1252. [Google Scholar] [CrossRef]
- Ferriss, J.S.; Kim, Y.; Duska, L.; Birrer, M.; Levine, D.A.; Moskaluk, C.; Theodorescu, D.; Lee, J.K. Multi-Gene Expression Predictors of Single Drug Responses to Adjuvant Chemotherapy in Ovarian Carcinoma: Predicting Platinum Resistance. PLoS ONE 2012, 7, e30550. [Google Scholar] [CrossRef]
- Pils, D.; Hager, G.; Tong, D.; Aust, S.; Heinze, G.; Kohl, M.; Schuster, E.; Wolf, A.; Sehouli, J.; Braicu, I.; et al. Validating the Impact of a Molecular Subtype in Ovarian Cancer on Outcomes: A Study of the OVCAD Consortium. Cancer Sci. 2012, 103, 1334–1341. [Google Scholar] [CrossRef]
- Yoshihara, K.; Tajima, A.; Yahata, T.; Kodama, S.; Fujiwara, H.; Suzuki, M.; Onishi, Y.; Hatae, M.; Sueyoshi, K.; Fujiwara, H.; et al. Gene Expression Profile for Predicting Survival in Advanced-Stage Serous Ovarian Cancer Across Two Independent Datasets. PLoS ONE 2010, 5, e9615. [Google Scholar] [CrossRef] [PubMed]
- Spentzos, D.; Levine, D.A.; Kolia, S.; Otu, H.; Boyd, J.; Libermann, T.A.; Cannistra, S.A. Unique Gene Expression Profile Based on Pathologic Response in Epithelial Ovarian Cancer. J. Clin. Oncol. 2005, 23, 7911–7918. [Google Scholar] [CrossRef]
- Yoshihara, K.; Tsunoda, T.; Shigemizu, D.; Fujiwara, H.; Hatae, M.; Fujiwara, H.; Masuzaki, H.; Katabuchi, H.; Kawakami, Y.; Okamoto, A.; et al. High-Risk Ovarian Cancer Based on 126-Gene Expression Signature Is Uniquely Characterized by Downregulation of Antigen Presentation Pathway. Clin. Cancer Res. 2012, 18, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Shen, J.; He, Q.; Xu, S. Identification of a Novel Immune-Related LncRNA CTD-2288O8.1 Regulating Cisplatin Resistance in Ovarian Cancer Based on Integrated Analysis. Front. Genet. 2022, 13, 814291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, Z.; Yan, Y. Role of a Pyroptosis-Related LncRNA Signature in Risk Stratification and Immunotherapy of Ovarian Cancer. Front. Med. 2022, 8, 2842. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Q.; Zhu, Y.; Huo, X.; Bao, J.; Su, M. Derivation, Comprehensive Analysis, and Assay Validation of a Pyroptosis-Related LncRNA Prognostic Signature in Patients With Ovarian Cancer. Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Feng, M.; Huang, X. M6A-Related LncRNA Signature Is Involved in Immunosuppression and Predicts the Patient Prognosis of the Age-Associated Ovarian Cancer. J. Immunol. Res. 2022, 2022, 3258400. [Google Scholar] [CrossRef]
- Liang, H.; Bai, Y.; Wang, H.; Yang, X. Identification of LncRNA Prognostic Markers for Ovarian Cancer by Integration of Co-Expression and CeRNA Network. Front. Genet. 2021, 11, 566497. [Google Scholar] [CrossRef]
- Guo, Q.; Cheng, Y.; Liang, T.; He, Y.; Ren, C.; Sun, L.; Zhang, G. Comprehensive Analysis of LncRNA-MRNA Co-Expression Patterns Identifies Immune-Associated LncRNA Biomarkers in Ovarian Cancer Malignant Progression. Sci. Rep. 2016, 5, 17683. [Google Scholar] [CrossRef]
- Lin, N.; Lin, J.; Tanaka, Y.; Sun, P.; Zhou, X. Identification and Validation of a Five-LncRNA Signature for Predicting Survival with Targeted Drug Candidates in Ovarian Cancer. Bioengineered 2021, 12, 3263–3274. [Google Scholar] [CrossRef]
- Wang, L.; He, M.; Fu, L.; Jin, Y. Role of LncRNAHCP5/MicroRNA-525–5p/PRC1 Crosstalk in the Malignant Behaviors of Ovarian Cancer Cells. Exp. Cell Res. 2020, 394, 112129. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Tan, J. N6-Methyladenosine-Related LncRNAs Is a Potential Marker for Predicting Prognosis and Immunotherapy in Ovarian Cancer. Hereditas 2022, 159, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, H.; Zhang, M.; Xing, L.; Yang, C.; Xia, B.; Lou, G. Integrated Analysis of a Competing Endogenous RNA Network Reveals an 11-LncRNA Prognostic Signature in Ovarian Cancer. Aging 2020, 12, 25153–25171. [Google Scholar] [CrossRef]
- Newtson, A.; Reyes, H.; Devor, E.J.; Goodheart, M.J.; Bosquet, J.G. Identification of Novel Fusion Transcripts in High Grade Serous Ovarian Cancer. Int. J. Mol. Sci. 2021, 22, 4791. [Google Scholar] [CrossRef] [PubMed]
- Hyter, S.; Hirst, J.; Pathak, H.; Pessetto, Z.Y.; Koestler, D.C.; Raghavan, R.; Pei, D.; Godwin, A.K. Developing a Genetic Signature to Predict Drug Response in Ovarian Cancer. Oncotarget 2018, 9, 14828–14848. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Jia, Y.; Wang, J.; Duan, Y.; Wang, X.; Liu, T.; Hao, S.; Liu, L. Long Non-Coding RNA SNHG10 Upregulates BIN1 to Suppress the Tumorigenesis and Epithelial–Mesenchymal Transition of Epithelial Ovarian Cancer via Sponging MiR-200a-3p. Cell Death Discov. 2022, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Xia, Y. LncRNA HLA-F-AS1 Attenuates the Ovarian Cancer Development by Targeting MiR-21-3p/PEG3 Axis. Anticancer. Drugs 2022, 33, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hou, Y.; Li, A.; Li, Z.; Wang, W.; Xie, H.; Rong, Z.; Lou, G.; Li, K. Identification of a Six-LncRNA Signature Associated with Recurrence of Ovarian Cancer. Sci. Rep. 2017, 7, 752. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.-G.; Zhang, K.-Q. LINC00452 Promotes Ovarian Carcinogenesis through Increasing ROCK1 by Sponging MiR-501-3p and Suppressing Ubiquitin-Mediated Degradation. Aging 2020, 12, 21129–21146. [Google Scholar] [CrossRef]
- Fu, Y.; Biglia, N.; Wang, Z.; Shen, Y.; Risch, H.A.; Lu, L.; Canuto, E.M.; Jia, W.; Katsaros, D.; Yu, H. Long Non-Coding RNAs, ASAP1-IT1, FAM215A, and LINC00472, in Epithelial Ovarian Cancer. Gynecol. Oncol. 2016, 143, 642–649. [Google Scholar] [CrossRef]
- Gong, M.; Luo, C.; Meng, H.; Li, S.; Nie, S.; Jiang, Y.; Wan, Y.; Li, H.; Cheng, W. Upregulated LINC00565 Accelerates Ovarian Cancer Progression By Targeting GAS6. Onco. Targets. Ther. 2019, 12, 10011–10022. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Huang, S.; Han, F. LINC-PINT Suppresses Tumour Cell Proliferation, Migration and Invasion through Targeting MiR-374a-5p in Ovarian Cancer. Cell Biochem. Funct. 2020, 38, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, D.-Z.; Zhai, Y.-N.; Xue, K.; Xu, F.; Gu, X.-Y.; Wang, S.-M. Microarray Expression Profiling of Long Non-Coding RNAs in Epithelial Ovarian Cancer. Oncol. Lett. 2017, 14, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.; Zhang, L.; Li, H.; Duan, H.; Wang, D.; Zhao, X.; Xie, Y. The Enhancer RNA ADCY10P1 Is Associated with the Progression of Ovarian Cancer. J. Ovarian Res. 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Wang, F.; Wang, L. Hypoxia-Related LncRNA Prognostic Model of Ovarian Cancer Based on Big Data Analysis. J. Oncol. 2023, 2023, 6037121. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhan, X. Anti-Parasite Drug Ivermectin Can Suppress Ovarian Cancer by Regulating LncRNA-EIF4A3-MRNA Axes. EPMA J. 2020, 11, 289–309. [Google Scholar] [CrossRef]
- Zhang, W.; Fei, J.; Yu, S.; Shen, J.; Zhu, X.; Sadhukhan, A.; Lu, W.; Zhou, J. LINC01088 Inhibits Tumorigenesis of Ovarian Epithelial Cells by Targeting MiR-24-1-5p. Sci. Rep. 2018, 8, 2876. [Google Scholar] [CrossRef]
- Shao, S.; Tian, J.; Zhang, H.; Wang, S. LncRNA Myocardial Infarction-Associated Transcript Promotes Cell Proliferation and Inhibits Cell Apoptosis by Targeting MiR-330-5p in Epithelial Ovarian Cancer Cells. Arch. Med. Sci. 2018, 14, 1263–1270. [Google Scholar] [CrossRef]
- Liu, S.; Lai, W.; Shi, Y.; Liu, N.; Ouyang, L.; Zhang, Z.; Chen, L.; Wang, X.; Qian, B.; Xiao, D.; et al. Annotation and Cluster Analysis of Long Noncoding RNA Linked to Male Sex and Estrogen in Cancers. NPJ Precis. Oncol. 2020, 4, 5. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, H.; Fu, Y. Effects of Autophagy-Related Genes on the Prognosis and Immune Microenvironment of Ovarian Cancer. Biomed Res. Int. 2022, 2022, 6609195. [Google Scholar] [CrossRef]
- Luan, A.; Hou, L.; Zhang, F. Silencing of SBF2-AS1 Inhibits Cell Growth and Invasion by Sponging MicroRNA-338-3p in Serous Ovarian Carcinoma. Kaohsiung J. Med. Sci. 2022, 38, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gao, L.; Zhang, M.; Ning, N.; Wang, Y.; Wu, D.; Li, X. Identification and Analysis of An Epigenetically Regulated Five-LncRNA Signature Associated With Outcome and Chemotherapy Response in Ovarian Cancer. Front. Cell Dev. Biol. 2021, 9, 644940. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, A.S.; Fischer, A.; Miller, D.H.; Vang, S.; MacLaughlan, S.; Wu, H.-T.; Yu, J.; Steinhoff, M.; Collins, C.; Smith, P.J.S.; et al. Expression Profiling of Primary and Metastatic Ovarian Tumors Reveals Differences Indicative of Aggressive Disease. PLoS ONE 2014, 9, e94476. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Yang, Z.; Xu, S.; Li, X.; Yang, X.; Jin, P.; Liu, Y.; Zhou, X.; Zhang, T.; Gong, C.; et al. Heterotypic CAF-Tumor Spheroids Promote Early Peritoneal Metastasis of Ovarian Cancer. J. Exp. Med. 2019, 216, 688–703. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Taylor-Harding, B.; Raz, Y.; Haro, M.; Recouvreux, M.S.; Taylan, E.; Lester, J.; Millstein, J.; Walts, A.E.; Karlan, B.Y.; et al. Are Epithelial Ovarian Cancers of the Mesenchymal Subtype Actually Intraperitoneal Metastases to the Ovary? Front. Cell Dev. Biol. 2020, 8, 647. [Google Scholar] [CrossRef]
- Sallinen, H.; Janhonen, S.; Pölönen, P.; Niskanen, H.; Liu, O.H.; Kivelä, A.; Hartikainen, J.M.; Anttila, M.; Heinäniemi, M.; Ylä-Herttuala, S.; et al. Comparative Transcriptome Analysis of Matched Primary and Distant Metastatic Ovarian Carcinoma. BMC Cancer 2019, 19, 1121. [Google Scholar] [CrossRef]
- Wang, L.; Ren, C.; Xu, Y.; Yang, L.; Chen, Y.; Zhu, Y. The LINC00922 Aggravates Ovarian Cancer Progression via Sponging MiR-361-3p. J. Ovarian Res. 2021, 14, 77. [Google Scholar] [CrossRef]
- Eoh, K.; Lee, D.; Nam, E.; Kim, J.; Moon, H.; Kim, S.; Kim, Y. HOXA-AS3 Induces Tumor Progression through the Epithelial-mesenchymal Transition Pathway in Epithelial Ovarian Cancer. Oncol. Rep. 2023, 49, 64. [Google Scholar] [CrossRef]
- Yao, N.; Sun, J.-Q.; Yu, L.; Ma, L.; Guo, B.-Q. LINC00968 Accelerates the Progression of Epithelial Ovarian Cancer via Mediating the Cell Cycle Progression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4642–4649. [Google Scholar]
- Liu, M.; Shen, C.; Wang, C. Long Noncoding RNA LINC01133 Confers Tumor-Suppressive Functions in Ovarian Cancer by Regulating Leucine-Rich Repeat Kinase 2 as an MiR-205 Sponge. Am. J. Pathol. 2019, 189, 2323–2339. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Wang, Y.; Liu, L. An Integration Analysis of MRNAs and MiRNAs Microarray Data to Identify Key Regulators for Ovarian Endometriosis Based on Competing Endogenous RNAs. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, J.; Zhu, L.; Zhou, Y.; Tong, J. Comprehensive Analyses of Glycolysis-Related LncRNAs for Ovarian Cancer Patients. J. Ovarian Res. 2021, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Guan, N.; Zheng, H.; Wu, X.; Xie, L.; Tong, X. SP1-Regulated Non-Coding RNA SNHG22 Promotes Ovarian Cancer Growth and Glycolysis. Cancer Manag. Res. 2021, 13, 7299–7309. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, Y.; Yin, W.; Qian, S. Development and Verification of a 7-LncRNA Prognostic Model Based on Tumor Immunity for Patients with Ovarian Cancer. J. Ovarian Res. 2023, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, H. Identification and Validation of a Seven M6A-Related LncRNAs Signature Predicting Prognosis of Ovarian Cancer. BMC Cancer 2022, 22, 633. [Google Scholar] [CrossRef]
- Li, J.; Feng, L.; Tian, C.; Tang, Y.L.; Tang, Y.; Hu, F.Q. Long Noncoding RNA-JPX Predicts the Poor Prognosis of Ovarian Cancer Patients and Promotes Tumor Cell Proliferation, Invasion and Migration by the PI3K/Akt/MTOR Signaling Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8135–8144. [Google Scholar] [CrossRef]
- Lin, H.; Xu, X.; Chen, K.; Fu, Z.; Wang, S.; Chen, Y.; Zhang, H.; Niu, Y.; Chen, H.; Yu, H.; et al. LncRNA CASC15, MiR-23b Cluster and SMAD3 Form a Novel Positive Feedback Loop to Promote Epithelial-Mesenchymal Transition and Metastasis in Ovarian Cancer. Int. J. Biol. Sci. 2022, 18, 1989–2002. [Google Scholar] [CrossRef]
- Wang, L.; Ye, T.Y.; Wu, H.; Chen, S.Y.; Weng, J.R.; Xi, X.W. LINC00702 Accelerates the Progression of Ovarian Cancer through Interacting with EZH2 to Inhibit the Transcription of KLF2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 201–208. [Google Scholar] [CrossRef]
- Tian, J.; Yang, L.; Wang, Z.; Yan, H. MIR503HG Impeded Ovarian Cancer Progression by Interacting with SPI1 and Preventing TMEFF1 Transcription. Aging 2022, 14, 5390–5405. [Google Scholar] [CrossRef]
- Pan, X.; Guo, Z.; Chen, Y.; Zheng, S.; Peng, M.; Yang, Y.; Wang, Z. STAT3-Induced LncRNA SNHG17 Exerts Oncogenic Effects on Ovarian Cancer through Regulating CDK6. Mol. Ther.-Nucleic Acids 2020, 22, 38–49. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, Y.; Gou, R.; Nie, X.; Li, X.; Liu, J.; Lin, B. Identification Three LncRNA Prognostic Signature of Ovarian Cancer Based on Genome-Wide Copy Number Variation. Biomed. Pharmacother. 2020, 124, 109810. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, G. DNM3OS Facilitates Ovarian Cancer Progression by Regulating MiR-193a-3p/MAP3K3 Axis. Yonsei Med. J. 2021, 62, 535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ji, G.; Le, X.; Wang, C.; Xu, L.; Feng, M.; Zhang, Y.; Yang, H.; Xuan, Y.; Yang, Y.; et al. Long Noncoding RNA LINC00092 Acts in Cancer-Associated Fibroblasts to Drive Glycolysis and Progression of Ovarian Cancer. Cancer Res. 2017, 77, 1369–1382. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, B.; Zhan, X. Comprehensive Analysis of Tumor Microenvironment Identified Prognostic Immune-Related Gene Signature in Ovarian Cancer. Front. Genet. 2021, 12, 616073. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Shi, X.-Y.; Li, Z.-L.; Li, M.; Zhang, M.-M.; Yan, S.-J.; Wei, Z.-L. Downregulation of LINC01508 Contributes to Cisplatin Resistance in Ovarian Cancer via the Regulation of the Hippo-YAP Pathway. J. Gynecol. Oncol. 2021, 32, e77. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Xiao, X.; Chen, J. Silencing of the Long Noncoding RNA LINC01132 Alleviates the Oncogenicity of Epithelial Ovarian Cancer by Regulating the MicroRNA-431-5p/SOX9 Axis. Int. J. Mol. Med. 2021, 48, 151. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Huang, K.; Xiong, X.; Shi, Y.; Wang, X.; Pan, X.; Cong, Y.; Sun, Y.; Ge, L.; et al. Long Noncoding RNA RFPL1S-202 Inhibits Ovarian Cancer Progression by Downregulating the IFN-β/STAT1 Signaling. Exp. Cell Res. 2023, 422, 113438. [Google Scholar] [CrossRef]
- Kaneuchi, M.; Sasaki, M.; Tanaka, Y.; Shiina, H.; Yamada, H.; Yamamoto, R.; Sakuragi, N.; Enokida, H.; Verma, M.; Dahiya, R. WT1 and WT1-AS Genes Are Inactivated by Promoter Methylation in Ovarian Clear Cell Adenocarcinoma. Cancer 2005, 104, 1924–1930. [Google Scholar] [CrossRef]
- Liu, B.; Yan, L.; Chi, Y.; Sun, Y.; Yang, X. Long Non-Coding RNA AFAP1-AS1 Facilitates Ovarian Cancer Progression by Regulating the MiR-107/PDK4 Axis. J. Ovarian Res. 2021, 14, 60. [Google Scholar] [CrossRef]
- Taniguchi-Ponciano, K.; Huerta-Padilla, V.; Baeza-Xochihua, V.; Ponce-Navarrete, G.; Salcedo, E.; Gomez-Apo, E.; Chavez-Macias, L.; Aviles-Duran, J.; Ruiz-Sanchez, H.; Valdivia, A.; et al. Revisiting the Genomic and Transcriptomic Landscapes from Female Malignancies Could Provide Molecular Markers and Targets for Precision Medicine. Arch. Med. Res. 2019, 50, 428–436. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Y.; Sun, J.; Hu, C.; Ge, X.; Li, R. LncRNA HCG11 Represses Ovarian Cancer Cell Growth via AKT Signaling Pathway. J. Obstet. Gynaecol. Res. 2022, 48, 796–805. [Google Scholar] [CrossRef]
- Xue, Z.; Zhu, X.; Teng, Y. Long Non-coding RNA CASC2 Inhibits Progression and Predicts Favorable Prognosis in Epithelial Ovarian Cancer. Mol. Med. Rep. 2018, 18, 5173–5181. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhan, X.; Zhan, X. The LncRNA SNHG3 Regulates Energy Metabolism of Ovarian Cancer by an Analysis of Mitochondrial Proteomes. Gynecol. Oncol. 2018, 150, 343–354. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Pang, W.; Zhang, H.; Zeng, Z.; Wang, H. LncRNA LUCAT1 Promotes Proliferation of Ovarian Cancer Cells by Regulating MiR-199a-5p Expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1682–1687. [Google Scholar] [PubMed]
- Fang, Y.-N.; Huang, Z.-L.; Li, H.; Tan, W.-B.; Zhang, Q.-G.; Wang, L.; Wu, J.-L. LINC01116 Promotes the Progression of Epithelial Ovarian Cancer via Regulating Cell Apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5127–5133. [Google Scholar] [PubMed]
- Guo, R.; Qin, Y. LEMD1-AS1 Suppresses Ovarian Cancer Progression Through Regulating MiR-183-5p/TP53 Axis. Onco. Targets. Ther. 2020, 13, 7387–7398. [Google Scholar] [CrossRef]
- Ruan, Z.; Zhao, D. Long Intergenic Noncoding RNA LINC00284 Knockdown Reduces Angiogenesis in Ovarian Cancer Cells via Up-regulation of MEST through NF-κB1. FASEB J. 2019, 33, 12047–12059. [Google Scholar] [CrossRef]
- Wu, X.; Qiu, L.; Feng, H.; Zhang, H.; Yu, H.; Du, Y.; Wu, H.; Zhu, S.; Ruan, Y.; Jiang, H. KHDRBS3 Promotes Paclitaxel Resistance and Induces Glycolysis through Modulated MIR17HG/CLDN6 Signaling in Epithelial Ovarian Cancer. Life Sci. 2022, 293, 120328. [Google Scholar] [CrossRef]
- Yang, H.; Han, C. Abstract 2139: A Seven LncRNA Based Risk Score System for Predicting the Recurrence and Prognosis of Ovarian Cancer Patients. Cancer Res. 2020, 80, 2139. [Google Scholar] [CrossRef]
- Geng, L.; Wang, Z.; Tian, Y. Down-Regulation of ZNF252P-AS1 Alleviates Ovarian Cancer Progression by Binding MiR-324-3p to Downregulate LY6K. J. Ovarian Res. 2022, 15, 1. [Google Scholar] [CrossRef]
- Wang, K.; Mei, S.; Cai, M.; Zhai, D.; Zhang, D.; Yu, J.; Ni, Z.; Yu, C. Ferroptosis-Related Long Noncoding RNAs as Prognostic Biomarkers for Ovarian Cancer. Front. Oncol. 2022, 12, 888699. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Huang, H.; Qi, X.; Bian, C.; Cheng, M.; Liu, L.; Xue, L.; Zhao, X.; Yi, T.; Quan, Y. Hypoxia-Induced LncRNA-MIR210HG Promotes Cancer Progression By Inhibiting HIF-1α Degradation in Ovarian Cancer. Front. Oncol. 2021, 11, 4971. [Google Scholar] [CrossRef] [PubMed]
- Buttarelli, M.; De Donato, M.; Raspaglio, G.; Babini, G.; Ciucci, A.; Martinelli, E.; Baccaro, P.; Pasciuto, T.; Fagotti, A.; Scambia, G.; et al. Clinical Value of LncRNA MEG3 in High-Grade Serous Ovarian Cancer. Cancers 2020, 12, 966. [Google Scholar] [CrossRef]
- Abildgaard, C.; do Canto, L.M.; Rainho, C.A.; Marchi, F.A.; Calanca, N.; Waldstrøm, M.; Steffensen, K.D.; Rogatto, S.R. The Long Non-Coding RNA SNHG12 as a Mediator of Carboplatin Resistance in Ovarian Cancer via Epigenetic Mechanisms. Cancers 2022, 14, 1664. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jin, H.; Duan, X.; Liu, H.; Zhao, X.; Fan, S.; Wang, Y.; Yao, T. LncRNA PSMA3-AS1 Promotes Cell Proliferation, Migration, and Invasion in Ovarian Cancer by Activating the PI3K/Akt Pathway via the MiR-378a-3p/GALNT3 Axis. Environ. Toxicol. 2021, 36, 2562–2577. [Google Scholar] [CrossRef]
- Koti, M.; Gooding, R.J.; Nuin, P.; Haslehurst, A.; Crane, C.; Weberpals, J.; Childs, T.; Bryson, P.; Dharsee, M.; Evans, K.; et al. Identification of the IGF1/PI3K/NF ΚB/ERK Gene Signalling Networks Associated with Chemotherapy Resistance and Treatment Response in High-Grade Serous Epithelial Ovarian Cancer. BMC Cancer 2013, 13, 549. [Google Scholar] [CrossRef]
- Xu, S.; Jia, G.; Zhang, H.; Wang, L.; Cong, Y.; Lv, M.; Xu, J.; Ruan, H.; Jia, X.; Xu, P.; et al. LncRNA HOXB-AS3 Promotes Growth, Invasion and Migration of Epithelial Ovarian Cancer by Altering Glycolysis. Life Sci. 2021, 264, 118636. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Williams, L.H.; Boyle, S.E.; Bearfoot, J.L.; Sridhar, A.; Speed, T.P.; Gorringe, K.L.; Campbell, I.G. Identification of Candidate Growth Promoting Genes in Ovarian Cancer through Integrated Copy Number and Expression Analysis. PLoS ONE 2010, 5, e9983. [Google Scholar] [CrossRef]
- Akasu-Nagayoshi, Y.; Hayashi, T.; Kawabata, A.; Shimizu, N.; Yamada, A.; Yokota, N.; Nakato, R.; Shirahige, K.; Okamoto, A.; Akiyama, T. PHOSPHATE Exporter XPR1/SLC53A1 Is Required for the Tumorigenicity of Epithelial Ovarian Cancer. Cancer Sci. 2022, 113, 2034–2043. [Google Scholar] [CrossRef]
- Zhao, Q.; Fan, C. A Novel Risk Score System for Assessment of Ovarian Cancer Based on Co-Expression Network Analysis and Expression Level of Five LncRNAs. BMC Med. Genet. 2019, 20, 103. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, Z.; Huang, Y.; Wang, K.; Xie, Y.; Yang, N. LncRNA GAS5 Inhibits the Proliferation and Invasion of Ovarian Clear Cell Carcinoma via the MiR-31-5p/ARID1A Axis. Kaohsiung J. Med. Sci. 2021, 37, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, Q.; Huang, X. Abnormal 5-Methylcytosine LncRNA Methylome Is Involved in Human High-Grade Serous Ovarian Cancer. Am. J. Transl. Res. 2021, 13, 13625–13639. [Google Scholar] [PubMed]
- Duan, M.; Fang, M.; Wang, C.; Wang, H.; Li, M. LncRNA EMX2OS Induces Proliferation, Invasion and Sphere Formation of Ovarian Cancer Cells via Regulating the MiR-654-3p/AKT3/PD-L1 Axis. Cancer Manag. Res. 2020, 12, 2141–2154. [Google Scholar] [CrossRef]
- Chen, X.; Wu, W.; Cao, X.; Zhao, X.; Li, W.; Deng, C.; Huang, Z. LncRNA Mortal Obligate RNA Transcript Was Downregulated in Ovarian Carcinoma and Inhibits Cancer Cell Proliferation by Downregulating MiRNA-21. J. Cell. Biochem. 2019, 120, 11949–11954. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, Y.; Li, X.; Wang, D. Ovarian Cancer-specific Dysregulated Genes with Prognostic Significance: ScRNA-Seq with Bulk RNA-Seq Data and Experimental Validation. Ann. N. Y. Acad. Sci. 2022, 1512, 154–173. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Nie, X.; Guo, Q.; Liu, Q.; Qi, Y.; Liu, J.; Lin, B. Construction of Novel MRNA-MiRNA-LncRNA Regulatory Networks Associated with Prognosis of Ovarian Cancer. J. Cancer 2020, 11, 7057–7072. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Feng, L.; Yin, P.; Song, S.; Wu, F.; Yan, P.; Liang, Z. RGS5 Decreases the Proliferation of Human Ovarian Carcinoma-derived Primary Endothelial Cells through the MAPK/ERK Signaling Pathway in Hypoxia. Oncol. Rep. 2018, 41, 165–177. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Zhao, X.; Zhang, P. Long Non-coding RNA FOXD2-AS1 Promotes Proliferation, Migration and Invasion of Ovarian Cancer Cells via Regulating the Expression of MiR-4492. Exp. Ther. Med. 2021, 21, 307. [Google Scholar] [CrossRef]
- Liu, Q.-Y.; Jiang, X.-X.; Tian, H.-N.; Guo, H.-L.; Guo, H.; Guo, Y. Long Non-Coding RNA OIP5-AS1 Plays an Oncogenic Role in Ovarian Cancer through Targeting MiR-324-3p/NFIB Axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7266–7275. [Google Scholar]
- Zheng, Q.; Zhang, J.; Liu, Y.; Dong, W.; Dai, X.; Du, X.; Gu, D. LINC01119 Encapsulated by Cancer-Associated Adipocytes-Derived Exosomes Promotes M2 Polarization of Macrophages to Induce Immune Escape in Ovarian Cancer in a 3D Co-Culture Cell-Based Model. Clin. Transl. Oncol. 2023. [Google Scholar] [CrossRef]
- Naghsh-Nilchi, A.; Ebrahimi Ghahnavieh, L.; Dehghanian, F. Construction of MiRNA-lncRNA-mRNA Co-expression Network Affecting EMT- Mediated Cisplatin Resistance in Ovarian Cancer. J. Cell. Mol. Med. 2022, 26, 4530–4547. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.-J.; An, Q. LINC00963 Promotes Ovarian Cancer Proliferation, Migration and EMT via the MiR-378g/CHI3L1 Axis. Cancer Manag. Res. 2020, 12, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Burdennyy, A.M.; Filippova, E.A.; Ivanova, N.A.; Lukina, S.S.; Pronina, I.V.; Loginov, V.I.; Fridman, M.V.; Kazubskaya, T.P.; Utkin, D.O.; Braga, E.A.; et al. Hypermethylation of Genes in New Long Noncoding RNA in Ovarian Tumors and Metastases: A Dual Effect. Bull. Exp. Biol. Med. 2021, 171, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Kerslake, R.; Sisu, C.; Panfilov, S.; Hall, M.; Khan, N.; Jeyaneethi, J.; Randeva, H.; Kyrou, I.; Karteris, E. Differential Regulation of Genes by the Glucogenic Hormone Asprosin in Ovarian Cancer. J. Clin. Med. 2022, 11, 5942. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Jiang, Y.; Yang, J. Long Noncoding RNA LINC01554 as a Novel Biomarker for Diagnosis and Prognosis Prediction of Epithelial Ovarian Cancer. Dis. Mrk. 2021, 2021, 1244612. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Hao, Y.; Rao, B.; Zhang, Z. A Ferroptosis-Related LncRNA Signature Predicts Prognosis in Ovarian Cancer Patients. Transl. Cancer Res. 2021, 10, 4802–4816. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, B.; Liu, M.; Qiao, H.; Zhang, S.; Qiu, J.; Ying, X. Long Non-Coding RNA SNHG25 Promotes Epithelial Ovarian Cancer Progression by up-Regulating COMP. J. Cancer 2021, 12, 1660–1668. [Google Scholar] [CrossRef]
- Chen, H.; Tian, X.; Luan, Y.; Lu, H. Downregulated Long Noncoding RNA DGCR5 Acts as a New Promising Biomarker for the Diagnosis and Prognosis of Ovarian Cancer. Technol. Cancer Res. Treat. 2019, 18, 153303381989680. [Google Scholar] [CrossRef]
- Arab, K.; Park, Y.J.; Lindroth, A.M.; Schäfer, A.; Oakes, C.; Weichenhan, D.; Lukanova, A.; Lundin, E.; Risch, A.; Meister, M.; et al. Long Noncoding RNA TARID Directs Demethylation and Activation of the Tumor Suppressor TCF21 via GADD45A. Mol. Cell 2014, 55, 604–614. [Google Scholar] [CrossRef]
- Gao, C.; Zhao, D.; Zhao, Q.; Dong, D.; Mu, L.; Zhao, X.; Guo, M.; Xu, A.; Fang, L.; Liu, Q.; et al. Microarray Profiling and Co-Expression Network Analysis of LncRNAs and MRNAs in Ovarian Cancer. Cell Death Discov. 2019, 5, 93. [Google Scholar] [CrossRef]
- Cardillo, N.; Russo, D.; Newtson, A.; Reyes, H.; Lyons, Y.; Devor, E.; Bender, D.; Goodheart, M.J.; Gonzalez-Bosquet, J. Identification of Novel LncRNAs in Ovarian Cancer and Their Impact on Overall Survival. Int. J. Mol. Sci. 2021, 22, 1079. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; You, J.; Han, Y.; Liu, Y.; Huang, M.; Lu, X.; Chen, J.; Zheng, Y. LINC00184 Promotes Ovarian Cancer Cells Proliferation and Cisplatin Resistance by Elevating CNTN1 Expression via Sponging MiR-1305. Onco. Targets. Ther. 2021, 14, 2711–2726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Luo, B.-H.; Wu, Q.-H.; Li, Q.-L.; Yang, K.-D. LncRNA HCG18 Upregulates TRAF4/TRAF5 to Facilitate Proliferation, Migration and EMT of Epithelial Ovarian Cancer by Targeting MiR-29a/B. Mol. Med. 2022, 28, 2. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, Y.-J. LncRNA SNHG20 Promotes Migration and Invasion of Ovarian Cancer via Modulating the MicroRNA-148a/ROCK1 Axis. J. Ovarian Res. 2021, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Su, H.; Tan, Y. UNC5B-AS1 Promoted Ovarian Cancer Progression by Regulating the H3K27me on NDRG2 via EZH2. Cell Biol. Int. 2020, 44, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fang, L.; Hu, D.; Chen, S.; Shen, S.; Chen, K.; Mu, J.; Li, J.; Zhang, H.; Yong-lin, L.; et al. Necroptosis-Associated Long Noncoding RNAs Can Predict Prognosis and Differentiate between Cold and Hot Tumors in Ovarian Cancer. Front. Oncol. 2022, 12, 967207. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, J.; Xu, Y. Long Non-coding RNA NNT-AS1 Contributes to Cell Proliferation, Metastasis and Apoptosis in Human Ovarian Cancer. Oncol. Lett. 2018, 15, 9264–9270. [Google Scholar] [CrossRef]
- Qi, B.; Liu, S.; Liu, D.; Yao, H.; Yan, R. Comprehensive Analysis of CRIP1 in Patients with Ovarian Cancer, Including CeRNA Network, Immune-Infiltration Pattern, and Clinical Benefit. Dis. Mrk. 2022, 2022, 2687867. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, Y.; Guo, Q.; Zhu, J.; Cao, L.; Guo, X.; Xu, F.; Weng, W.; Ju, X.; Wu, X. Long Non-coding RNA SNHG6 Promotes Cell Proliferation and Migration through Sponging MiR-4465 in Ovarian Clear Cell Carcinoma. J. Cell. Mol. Med. 2019, 23, 5025–5036. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).