Dysfunctional Mitochondria in the Cardiac Fibers of a Williams–Beuren Syndrome Mouse Model
Abstract
1. Introduction
2. Results
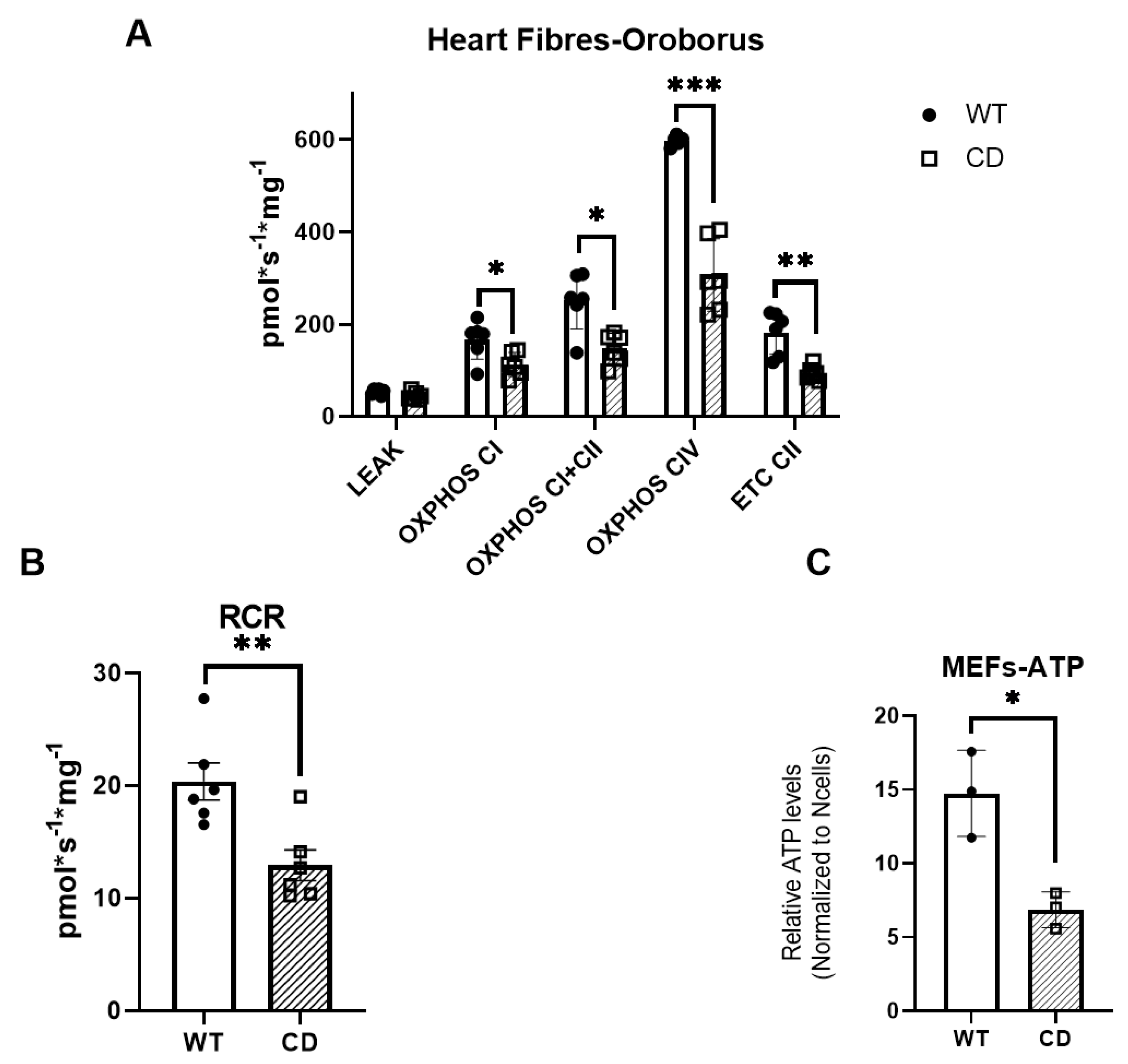
2.1. The Cardiac Fibers of CD Mice Exhibit Deficient Oxygen Consumption
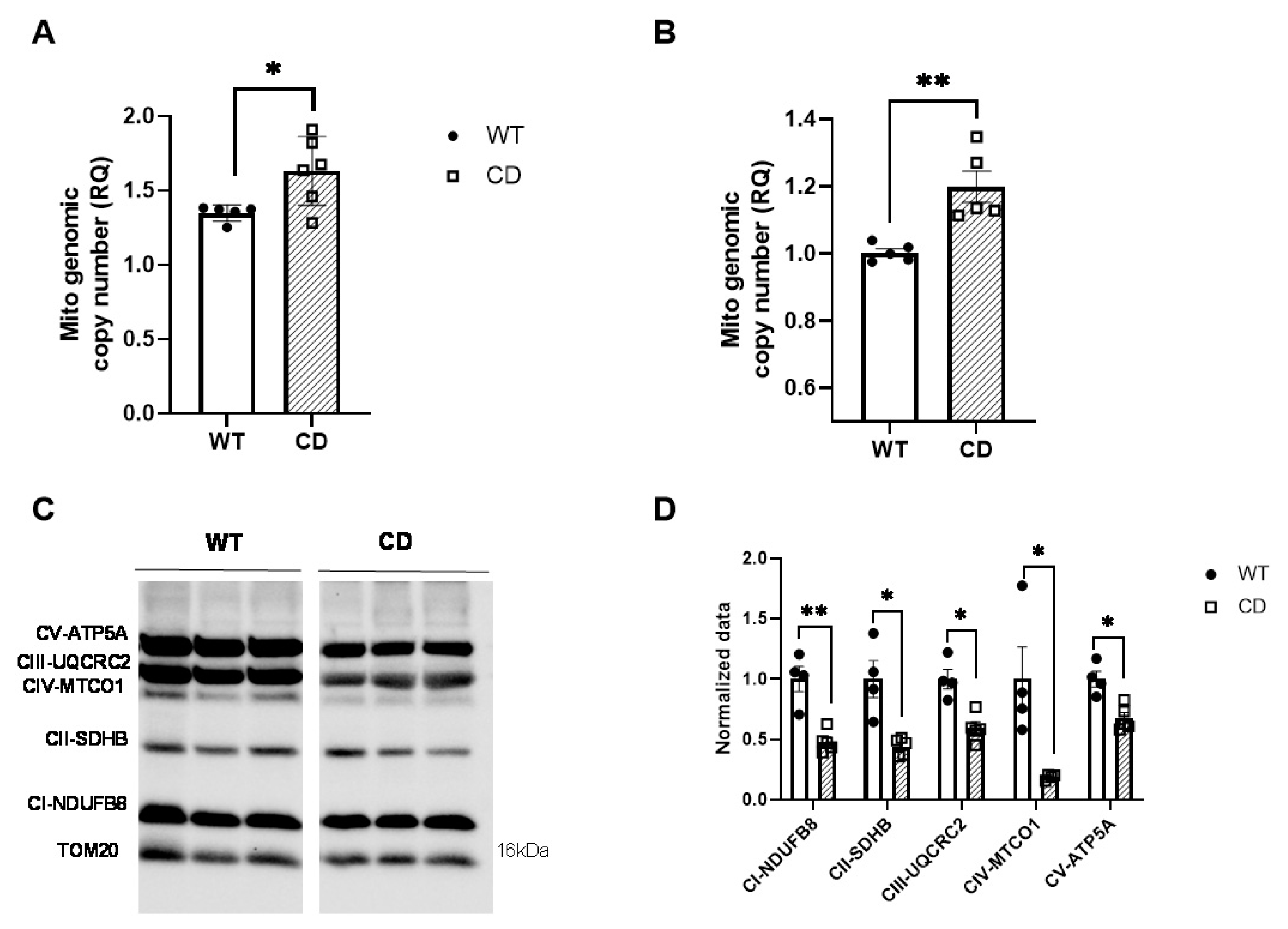
2.2. CD Cardiac Tissue Show Decreased Levels of the OXPHOS Complex
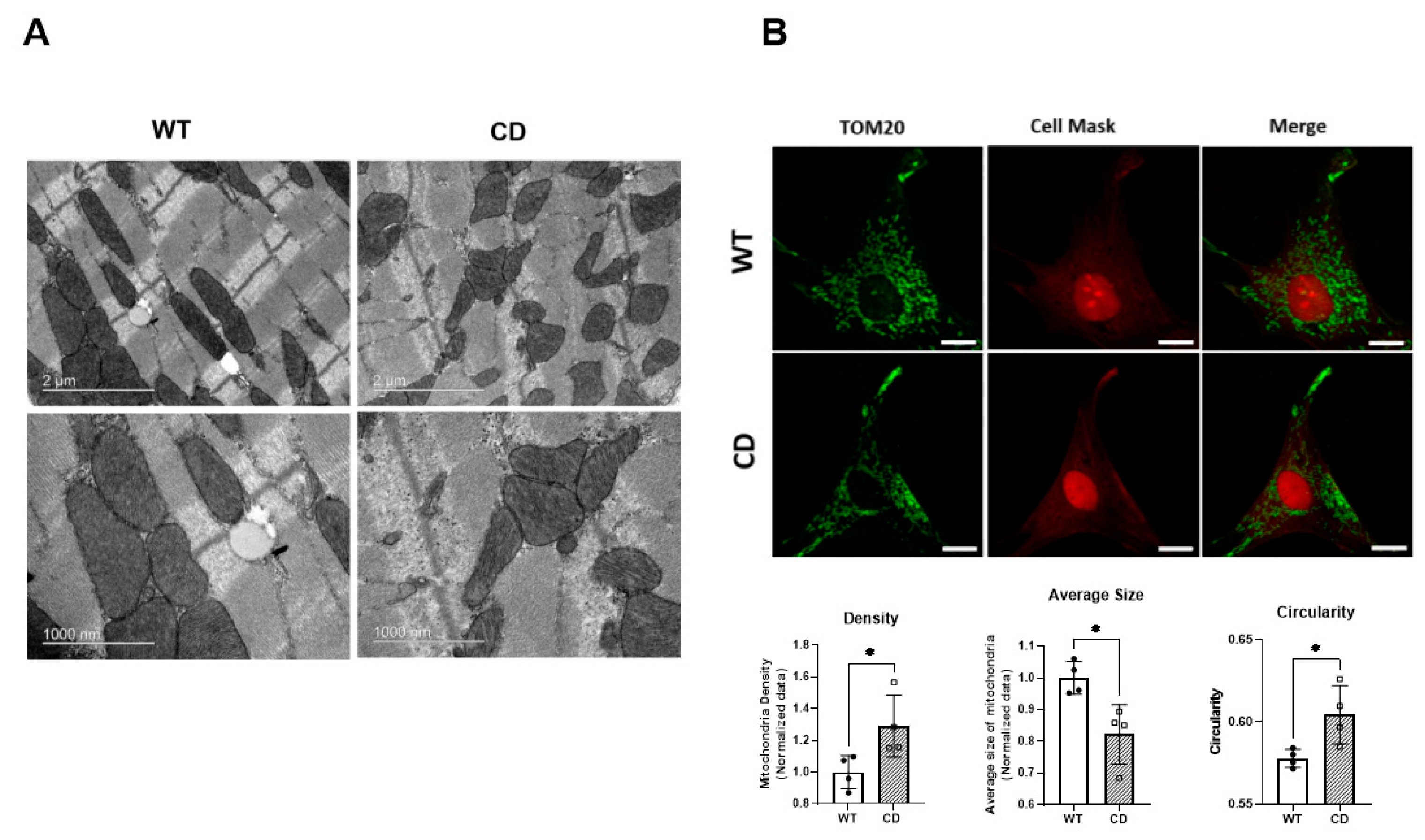
2.3. Altered Mitochondrial Morphology in CD Cardiac Fibers
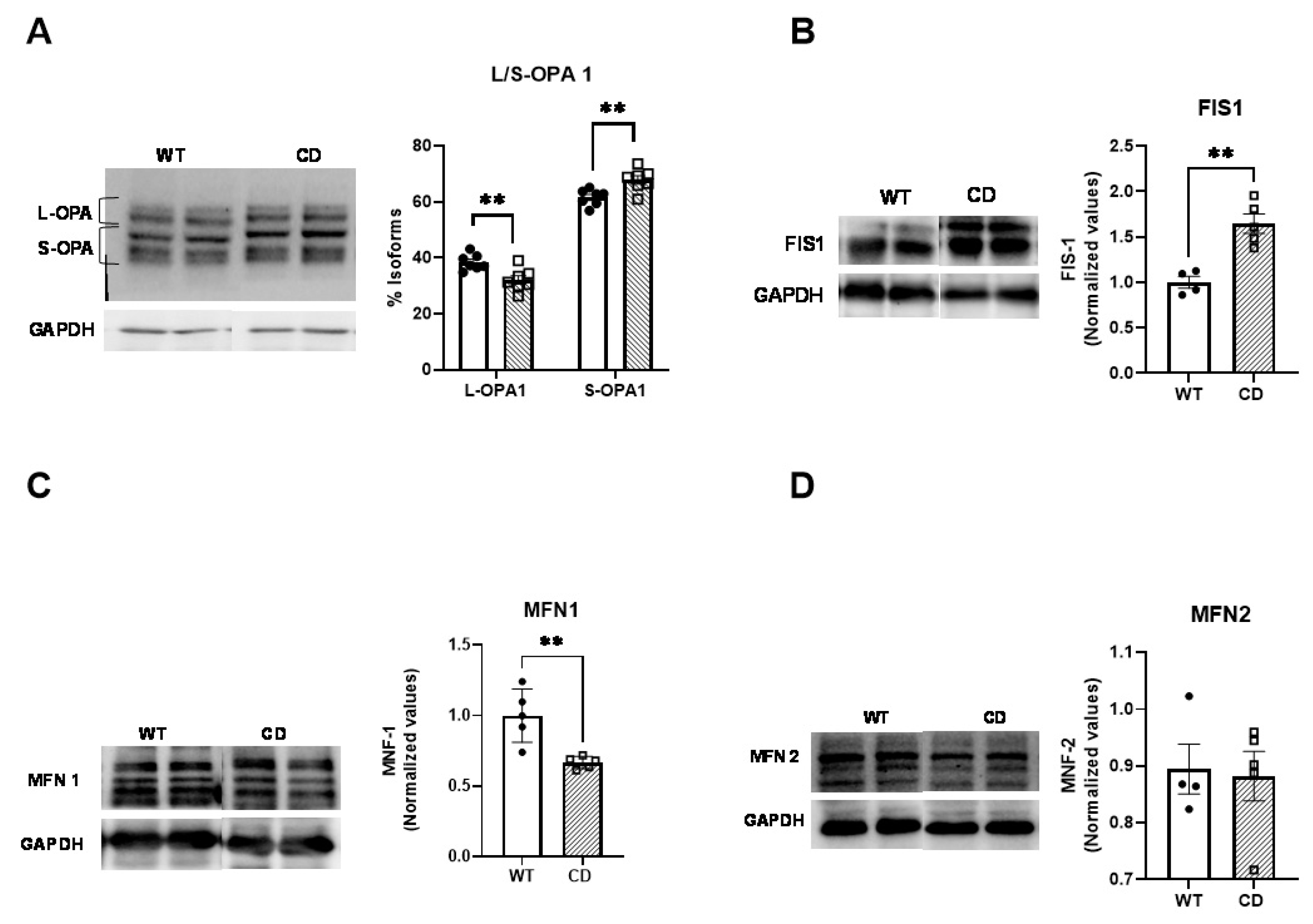
2.4. Altered Mitochondrial Dynamics in CD Cardiac Fibers
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance
4.2. Cell Lines and Culture Conditions
4.3. Assessment of Mitochondrial Function Using OROBOROS
4.4. ATP Measurements
4.5. Western Blotting
4.6. qPCR Experiments
4.7. Analysis of Mitochondrial Morphology
4.8. Transmission Electron Microscopy (TEM)
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strømme, P.; Bjømstad, P.G.; Ramstad, K. Prevalence Estimation of Williams Syndrome. J. Child Neurol. 2002, 17, 269–271. [Google Scholar] [CrossRef]
- Bayés, M.; Magano, L.F.; Rivera, N.; Flores, R.; Pérez Jurado, L.A. Mutational Mechanisms of Williams-Beuren Syndrome Deletions. Am. J. Hum. Genet. 2003, 73, 131–151. [Google Scholar] [CrossRef]
- Kozel, B.A.; Barak, B.; Kim, C.A.; Mervis, C.B.; Osborne, L.R.; Porter, M.; Pober, B.R. Williams Syndrome. Nat. Rev. Dis. Prim. 2021, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.T. Cardiovascular Disease in Williams Syndrome. Curr. Opin. Pediatr. 2018, 30, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Wessel, A.; Gravenhorst, V.; Buchhorn, R.; Gosch, A.; Partsch, C.-J.; Pankau, R. Risk of Sudden Death in the Williams-Beuren Syndrome. Am. J. Med. Genet. A 2004, 127A, 234–237. [Google Scholar] [CrossRef]
- Pober, B.R. Williams-Beuren Syndrome. N. Engl. J. Med. 2010, 362, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, M.; Antonell, A.; Magano, L.F.; Muñoz, F.J.; Flores, R.; Bayés, M.; Jurado, L.A.P. Hemizygosity at the NCF1 Gene in Patients with Williams-Beuren Syndrome Decreases Their Risk of Hypertension. Am. J. Hum. Genet. 2006, 78, 533–542. [Google Scholar] [CrossRef]
- Kozel, B.A.; Danback, J.R.; Waxler, J.L.; Knutsen, R.H.; de Las Fuentes, L.; Reusz, G.S.; Kis, E.; Bhatt, A.B.; Pober, B.R. Williams Syndrome Predisposes to Vascular Stiffness Modified by Antihypertensive Use and Copy Number Changes in NCF1. Hypertension 2014, 63, 74–79. [Google Scholar] [CrossRef]
- Campuzano, V.; Segura-Puimedon, M.; Terrado, V.; Sánchez-Rodríguez, C.; Coustets, M.; Menacho-Márquez, M.; Nevado, J.; Bustelo, X.R.X.R.; Francke, U.; Pérez-Jurado, L.A.L.A. Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome. PLoS Genet. 2012, 8, e1002458. [Google Scholar] [CrossRef]
- Segura-Puimedon, M.; Sahún, I.; Velot, E.; Dubus, P.; Borralleras, C.; Rodrigues, A.J.A.J.; Valero, M.C.; Valverde, O.; Sousa, N.; Herault, Y.; et al. Heterozygous Deletion of the Williams-Beuren Syndrome Critical Interval in Mice Recapitulates Most Features of the Human Disorder. Hum. Mol. Genet. 2014, 23, 6481–6494. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Soumaka, E.; Rapti, K.; Mavroidis, M.; Tsangaris, G.; Maris, A.; Weisleder, N.; Capetanaki, Y. Alterations in the Heart Mitochondrial Proteome in a Desmin Null Heart Failure Model. J. Mol. Cell. Cardiol. 2005, 38, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Elsnicova, B.; Hornikova, D.; Tibenska, V.; Kolar, D.; Tlapakova, T.; Schmid, B.; Mallek, M.; Eggers, B.; Schlötzer-Schrehardt, U.; Peeva, V.; et al. Desmin Knock-Out Cardiomyopathy: A Heart on the Verge of Metabolic Crisis. Int. J. Mol. Sci. 2022, 23, 12020. [Google Scholar] [CrossRef]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial Diseases. Nat. Rev. Dis. Prim. 2016, 2, 16080. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Trincado, C.; García-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial Dynamics, Mitophagy and Cardiovascular Disease. J. Physiol. 2016, 594, 509–525. [Google Scholar] [CrossRef]
- Brand, M.D.; Nicholls, D.G. Assessing Mitochondrial Dysfunction in Cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef]
- Chen, H.; Chomyn, A.; Chan, D.C. Disruption of Fusion Results in Mitochondrial Heterogeneity and Dysfunction. J. Biol. Chem. 2005, 280, 26185–26192. [Google Scholar] [CrossRef] [PubMed]
- Zorzano, A.; Liesa, M.; Sebastián, D.; Segalés, J.; Palacín, M. Mitochondrial Fusion Proteins: Dual Regulators of Morphology and Metabolism. Semin. Cell Dev. Biol. 2010, 21, 566–574. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Mitochondrial Dynamics—Fusion, Fission, Movement, and Mitophagy—In Neurodegenerative Diseases. Hum. Mol. Genet. 2009, 18, R169–R176. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Chen, H.; Vermulst, M.; Wang, Y.E.; Chomyn, A.; Prolla, T.A.; McCaffery, J.M.; Chan, D.C. Mitochondrial Fusion Is Required for MtDNA Stability in Skeletal Muscle and Tolerance of MtDNA Mutations. Cell 2010, 141, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Sarasija, S.; Norman, K.R. A γ-Secretase Independent Role for Presenilin in Calcium Homeostasis Impacts Mitochondrial Function and Morphology in Caenorhabditis Elegans. Genetics 2015, 201, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Tebbenkamp, A.T.N.; Varela, L.; Choi, J.; Paredes, M.I.; Giani, A.M.; Song, J.E.; Sestan-Pesa, M.; Franjic, D.; Sousa, A.M.M.; Liu, Z.W.; et al. The 7q11.23 Protein DNAJC30 Interacts with ATP Synthase and Links Mitochondria to Brain Development. Cell 2018, 175, 1088–1104.e23. [Google Scholar] [CrossRef]
- Baxter, M.; Voronkov, M.; Poolman, T.; Galli, G.; Pinali, C.; Goosey, L.; Knight, A.; Krakowiak, K.; Maidstone, R.; Iqbal, M.; et al. Cardiac Mitochondrial Function Depends on BUD23 Mediated Ribosome Programming. Elife 2020, 9, e50705. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Romero, A.; Galera-López, L.; Ortiz-Romero, P.; Llorente-Ovejero, A.; de Los Reyes-Ramírez, L.; Bengoetxea de Tena, I.; Garcia-Elias, A.; Mas-Stachurska, A.; Reixachs-Solé, M.; Pastor, A.; et al. Cannabinoid Signaling Modulation through JZL184 Restores Key Phenotypes of a Mouse Model for Williams-Beuren Syndrome. Elife 2022, 11, e50705. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, N.; Ortiz-Romero, P.; Rodriguez-Rovira, I.; Pérez-Jurado, L.A.; Egea, G.; Campuzano, V. The Combined Treatment of Curcumin with Verapamil Ameliorates the Cardiovascular Pathology in a Williams-Beuren Syndrome Mouse Model. Int. J. Mol. Sci. 2023, 24, 3261. [Google Scholar] [CrossRef]
- Gladden, J.D.; Zelickson, B.R.; Wei, C.-C.; Ulasova, E.; Zheng, J.; Ahmed, M.I.; Chen, Y.; Bamman, M.; Ballinger, S.; Darley-Usmar, V.; et al. Novel Insights into Interactions between Mitochondria and Xanthine Oxidase in Acute Cardiac Volume Overload. Free Radic. Biol. Med. 2011, 51, 1975–1984. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.A.; Cicalese, S.; Preston, K.J.; Kawai, T.; Okuno, K.; Choi, E.T.; Kasahara, S.; Uchida, H.A.; Otaka, N.; Scalia, R.; et al. Targeting Mitochondrial Fission as a Potential Therapeutic for Abdominal Aortic Aneurysm. Cardiovasc. Res. 2021, 117, 971–982. [Google Scholar] [CrossRef]
- Ortiz-Romero, P.; González-Simón, A.; Egea, G.; Pérez-Jurado, L.A.; Campuzano, V. Co-Treatment With Verapamil and Curcumin Attenuates the Behavioral Alterations Observed in Williams-Beuren Syndrome Mice by Regulation of MAPK Pathway and Microglia Overexpression. Front. Pharmacol. 2021, 12, 670785. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Romero, P.; Borralleras, C.; Bosch-Morató, M.; Guivernau, B.; Albericio, G.; Muñoz, F.J.F.J.; Pérez-Jurado, L.A.; Campuzano, V. Epigallocatechin-3-Gallate Improves Cardiac Hypertrophy and Short-Term Memory Deficits in a Williams-Beuren Syndrome Mouse Model. PLoS ONE 2018, 13, e0194476. [Google Scholar] [CrossRef]
- Cha, S.G.; Song, M.K.; Lee, S.Y.; Kim, G.B.; Kwak, J.G.; Kim, W.H.; Bae, E.J. Long-Term Cardiovascular Outcome of Williams Syndrome. Congenit. Heart Dis. 2019, 14, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Del Pasqua, A.; Rinelli, G.; Toscano, A.; Iacobelli, R.; Digilio, C.; Marino, B.; Saffirio, C.; Mondillo, S.; Pasquini, L.; Sanders, S.P.; et al. New Findings Concerning Cardiovascular Manifestations Emerging from Long-Term Follow-up of 150 Patients with the Williams-Beuren-Beuren Syndrome. Cardiol. Young 2009, 19, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Troia, A.; Knutsen, R.H.; Halabi, C.M.; Malide, D.; Yu, Z.X.; Wardlaw-Pickett, A.; Kronquist, E.K.; Tsang, K.M.; Kovacs, A.; Mecham, R.P.; et al. Inhibition of NOX1 Mitigates Blood Pressure Increases in Elastin Insufficiency. Function 2021, 2, zqab015. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.; Hofhaus, G.; Schröder, R.R.; Kühlbrandt, W. Dimer Ribbons of ATP Synthase Shape the Inner Mitochondrial Membrane. EMBO J. 2008, 27, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.C.; Di Benedetto, G.; Scorrano, L. During Autophagy Mitochondria Elongate, Are Spared from Degradation and Sustain Cell Viability. Nat. Cell Biol. 2011, 13, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Nakamura, K.; Iijima, M.; Sesaki, H. Mitochondrial Dynamics in Neurodegeneration. Trends Cell Biol. 2013, 23, 64–71. [Google Scholar] [CrossRef]
- Morciano, G.; Boncompagni, C.; Ramaccini, D.; Pedriali, G.; Bouhamida, E.; Tremoli, E.; Giorgi, C.; Pinton, P. Comprehensive Analysis of Mitochondrial Dynamics Alterations in Heart Diseases. Int. J. Mol. Sci. 2023, 24, 3414. [Google Scholar] [CrossRef]
- Aung, L.H.H.; Jumbo, J.C.C.; Wang, Y.; Li, P. Therapeutic Potential and Recent Advances on Targeting Mitochondrial Dynamics in Cardiac Hypertrophy: A Concise Review. Mol. Ther. Nucleic Acids 2021, 25, 416–443. [Google Scholar] [CrossRef]
- Givvimani, S.; Munjal, C.; Tyagi, N.; Sen, U.; Metreveli, N.; Tyagi, S.C. Mitochondrial Division/Mitophagy Inhibitor (Mdivi) Ameliorates Pressure Overload Induced Heart Failure. PLoS ONE 2012, 7, e32388. [Google Scholar] [CrossRef]
- Torres, G.; Morales, P.E.; García-Miguel, M.; Norambuena-Soto, I.; Cartes-Saavedra, B.; Vidal-Peña, G.; Moncada-Ruff, D.; Sanhueza-Olivares, F.; San Martín, A.; Chiong, M. Glucagon-like Peptide-1 Inhibits Vascular Smooth Muscle Cell Dedifferentiation through Mitochondrial Dynamics Regulation. Biochem. Pharmacol. 2016, 104, 52–61. [Google Scholar] [CrossRef]
- Ding, M.; Feng, N.; Tang, D.; Feng, J.; Li, Z.; Jia, M.; Liu, Z.; Gu, X.; Wang, Y.; Fu, F.; et al. Melatonin Prevents Drp1-Mediated Mitochondrial Fission in Diabetic Hearts through SIRT1-PGC1α Pathway. J. Pineal Res. 2018, 65, e12491. [Google Scholar] [CrossRef]
- Hull, T.D.; Boddu, R.; Guo, L.; Tisher, C.C.; Traylor, A.M.; Patel, B.; Joseph, R.; Prabhu, S.D.; Suliman, H.B.; Piantadosi, C.A.; et al. Heme Oxygenase-1 Regulates Mitochondrial Quality Control in the Heart. JCI Insight 2016, 1, e85817. [Google Scholar] [CrossRef] [PubMed]
- Borralleras, C.; Sahun, I.; Pérez-Jurado, L.A.; Campuzano, V. Intracisternal Gtf2i Gene Therapy Ameliorates Deficits in Cognition and Synaptic Plasticity of a Mouse Model of Williams-Beuren Syndrome. Mol. Ther. 2015, 23, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Pesta, D.; Gnaiger, E. High-Resolution Respirometry: OXPHOS Protocols for Human Cells and Permeabilized Fibers from Small Biopsies of Human Muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, M.; Puigdellívol, M.; Alberch, J.; Ginés, S. Cdk5-Mediated Mitochondrial Fission: A Key Player in Dopaminergic Toxicity in Huntington’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 2145–2160. [Google Scholar] [CrossRef]
- Valente, A.J.; Maddalena, L.A.; Robb, E.L.; Moradi, F.; Stuart, J.A. A Simple ImageJ Macro Tool for Analyzing Mitochondrial Network Morphology in Mammalian Cell Culture. Acta Histochem. 2017, 119, 315–326. [Google Scholar] [CrossRef]
- Meneses-Salas, E.; García-Melero, A.; Kanerva, K.; Blanco-Muñoz, P.; Morales-Paytuvi, F.; Bonjoch, J.; Casas, J.; Egert, A.; Beevi, S.S.; Jose, J.; et al. Annexin A6 Modulates TBC1D15/Rab7/StARD3 Axis to Control Endosomal Cholesterol Export in NPC1 Cells. Cell. Mol. Life Sci. 2020, 77, 2839–2857. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, N.; Tobías-Baraja, E.; Gonzalez, A.; Garrabou, G.; Egea, G.; Campuzano, V. Dysfunctional Mitochondria in the Cardiac Fibers of a Williams–Beuren Syndrome Mouse Model. Int. J. Mol. Sci. 2023, 24, 10071. https://doi.org/10.3390/ijms241210071
Abdalla N, Tobías-Baraja E, Gonzalez A, Garrabou G, Egea G, Campuzano V. Dysfunctional Mitochondria in the Cardiac Fibers of a Williams–Beuren Syndrome Mouse Model. International Journal of Molecular Sciences. 2023; 24(12):10071. https://doi.org/10.3390/ijms241210071
Chicago/Turabian StyleAbdalla, Noura, Ester Tobías-Baraja, Alejandro Gonzalez, Gloria Garrabou, Gustavo Egea, and Victoria Campuzano. 2023. "Dysfunctional Mitochondria in the Cardiac Fibers of a Williams–Beuren Syndrome Mouse Model" International Journal of Molecular Sciences 24, no. 12: 10071. https://doi.org/10.3390/ijms241210071
APA StyleAbdalla, N., Tobías-Baraja, E., Gonzalez, A., Garrabou, G., Egea, G., & Campuzano, V. (2023). Dysfunctional Mitochondria in the Cardiac Fibers of a Williams–Beuren Syndrome Mouse Model. International Journal of Molecular Sciences, 24(12), 10071. https://doi.org/10.3390/ijms241210071







