Synthesis and Biological Properties of Pyranocoumarin Derivatives as Potent Anti-Inflammatory Agents
Abstract
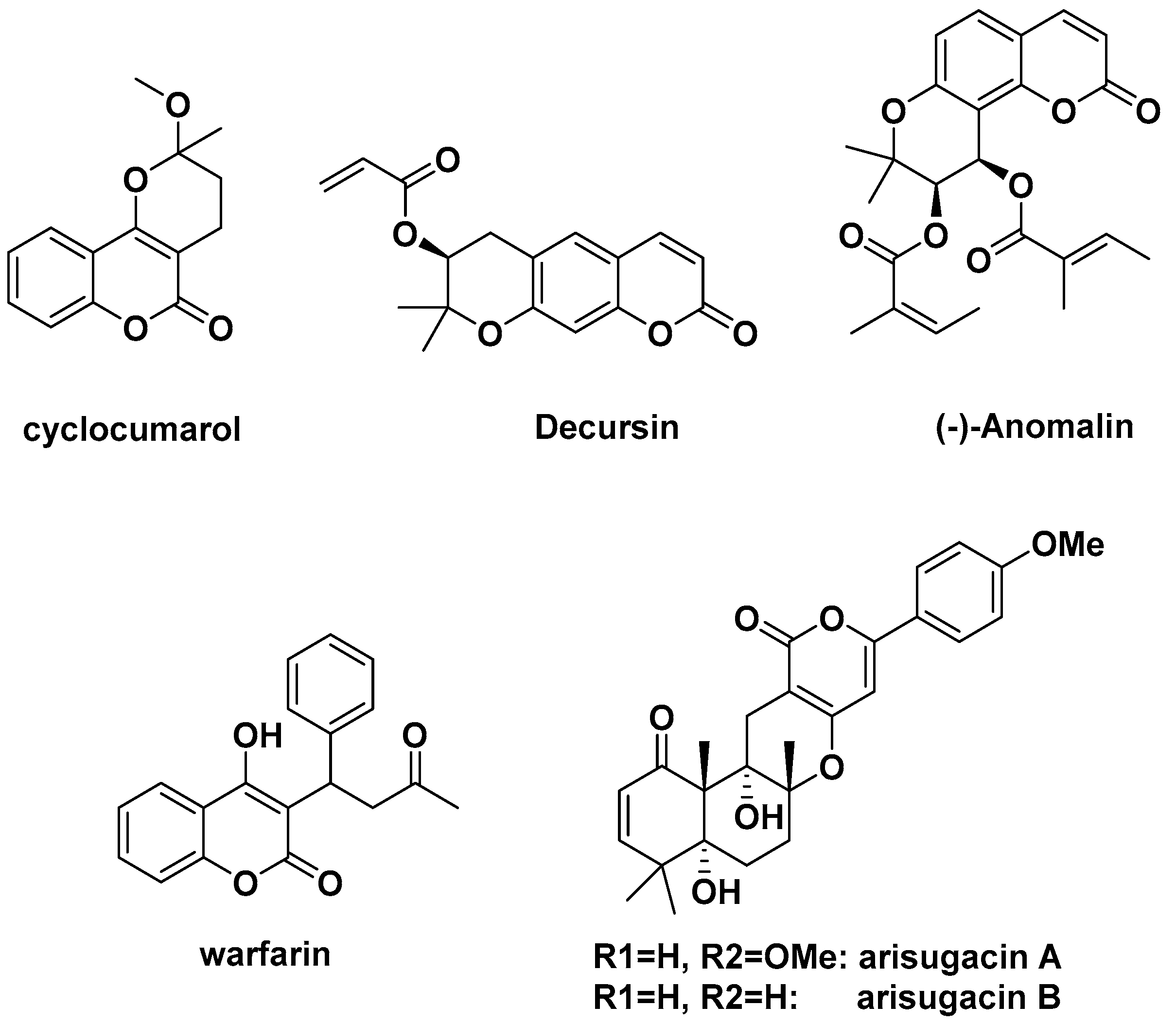
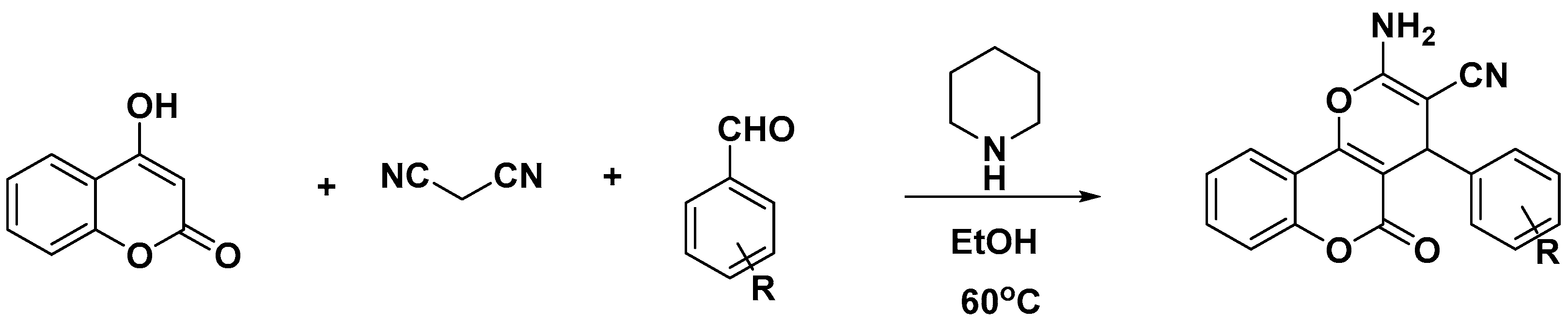
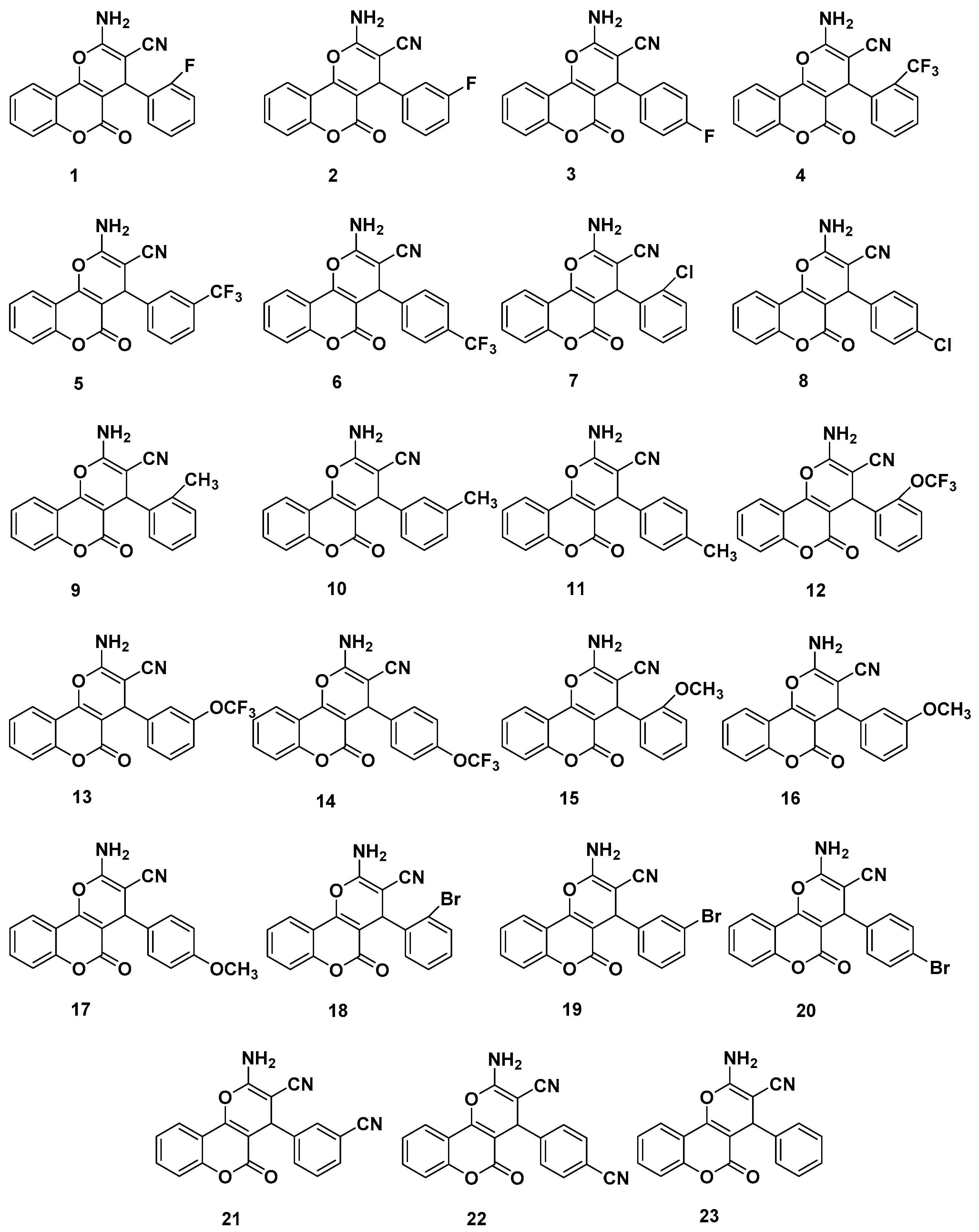
1. Introduction
2. Results
2.1. Effects of Coumarin Derivatives on the Viability of RAW264.7 Cells
2.2. Effects of Coumarin Derivatives on Nitric Oxide Production in LPS-Stimulated RAW264.7 Cells
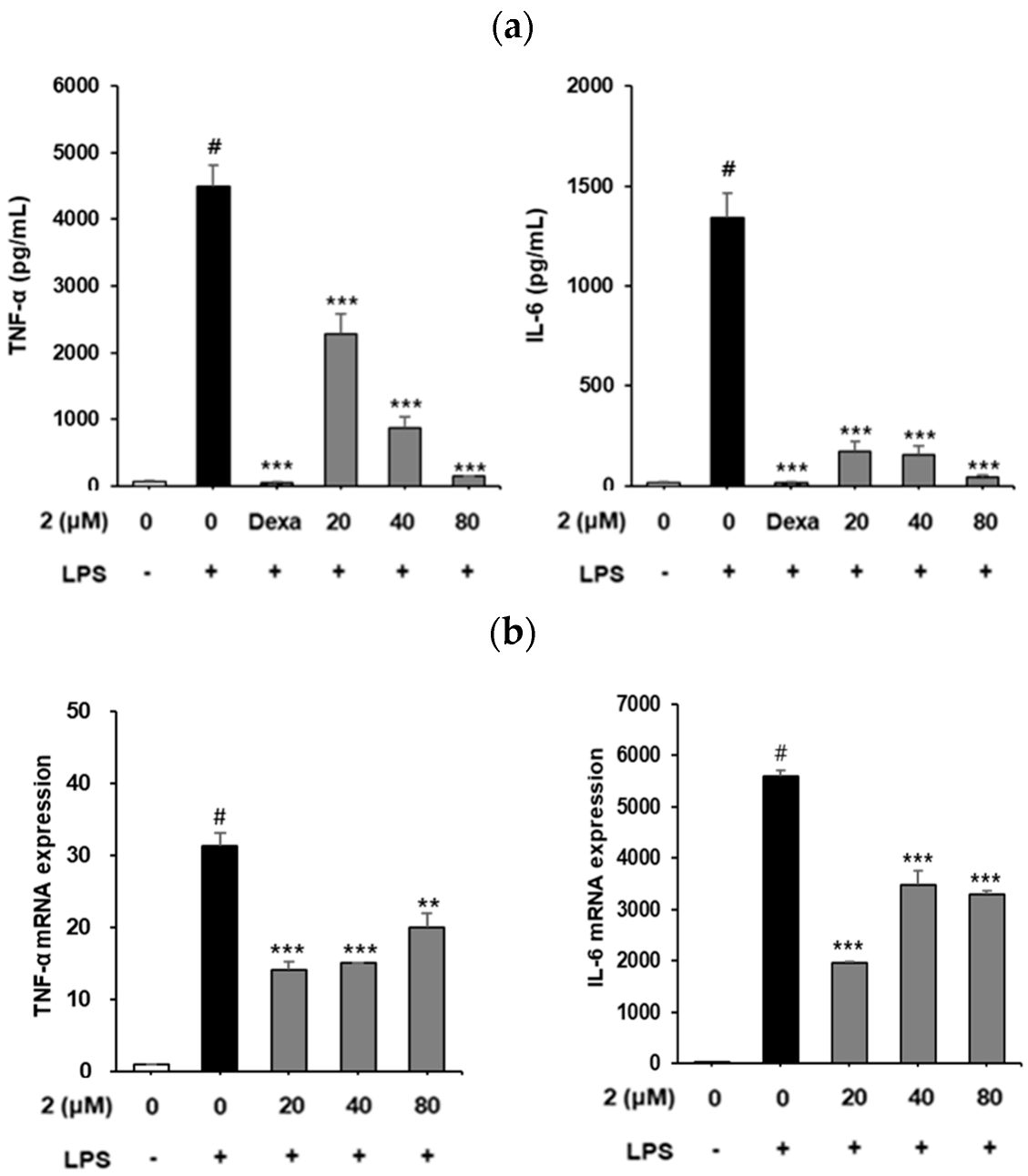
2.3. Analysis of the Effect of Coumarin Derivative 2 on IL-6 and TNF-α Production and mRNA Expression in RAW264.7 Cells
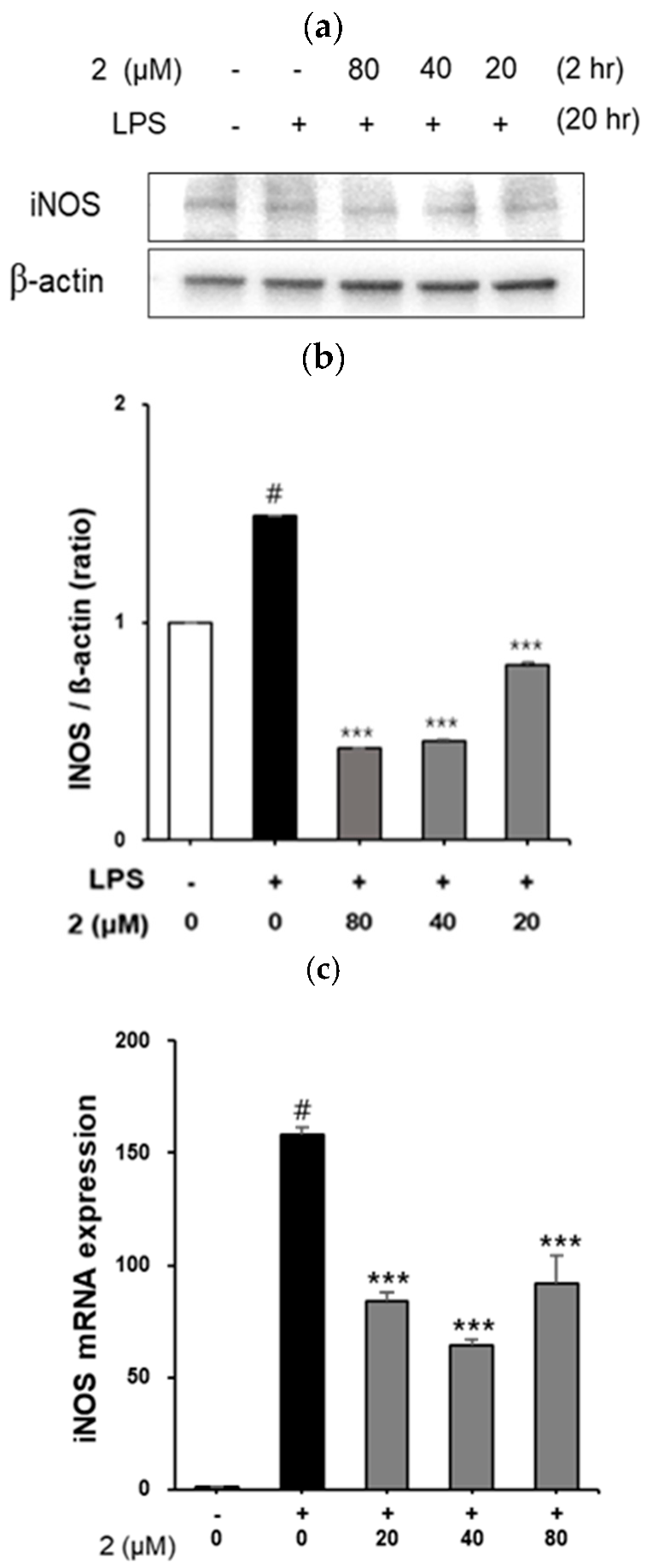
2.4. Effect of Coumarin Derivative 2 on iNOS Expression in LPS-Induced RAW264.7 Cells
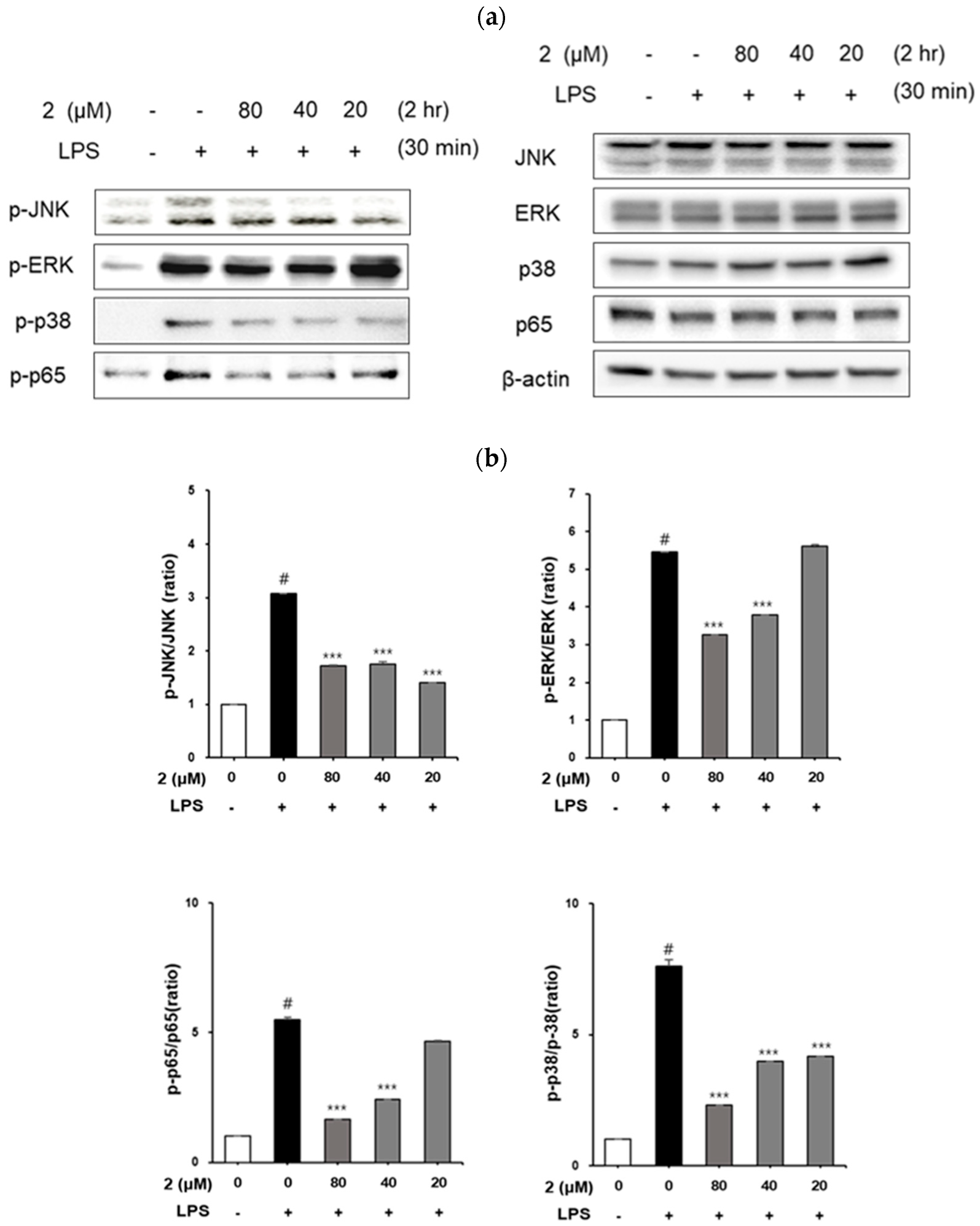
2.5. Analysis of the Effect of Coumarin Derivative 2 on MAPKs and NF-kB p65 Phosphorylation in LPS-Induced RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Determination of NO
4.5. Determination of TNF-α and IL-6
4.6. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.7. Preparation of Cell Lysate and Immunoblotting
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Curini, M.; Cravotto, G.; Epifano, F.; Giannone, G. Chemistry and biological activity of natural and synthetic prenyloxycoumarins. Curr. Med. Chem. 2006, 13, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Melliou, E.; Magiatis, P.; Mitaku, S.; Skaltsounis, A.L.; Chinou, E.; Chinou, I. Natural and synthetic 2,2-dimethylpyranocoumarins with antibacterial activity. J. Nat. Prod. 2005, 68, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K.; Singh, S.; Khajuria, A.; Guru, S.K.; Joshi, P.; Meena, S.; Nadkarni, J.R.; Singh, A.; Bharate, S.S.; Bhushan, S.; et al. Pyrano-isochromanones as IL-6 inhibitors: Synthesis, in vitro and in vivo antiarthritic activity. J. Med. Chem. 2014, 57, 7085–7097. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pham, P.T.; Skripnikova, E.V.; Zheng, S.; Lovings, L.J.; Wang, Y.; Goyal, N.; Bellow, S.M.; Mensah, L.M.; Chatters, A.J.; et al. A ligand-based drug design. Discovery of 4-trifluoromethyl-7,8-pyranocoumarin as a selective inhibitor of human cytochrome P450 1A2. J. Med. Chem. 2015, 58, 6481–6493. [Google Scholar] [CrossRef] [PubMed]
- Mckee, T.C.; Covington, C.D.; Fuller, R.W.; Bokesch, H.R.; Young, S.; Cardellina, J.H.; Kadushin, M.R.; Soejarto, D.D.; Stevens, P.F.; Cragg, G.M.B.; et al. Pyranocoumarins from tropical species of the genus Calophyllum: A chemotaxonomic study of extracts in the National Cancer Institute collection. J. Nat. Prod. 1998, 61, 1252–1256. [Google Scholar] [CrossRef]
- Conti, C.; Proietti Monaco, L.P.; Desideri, N. Design, synthesis and in vitro evaluation of novel chroman-4-one, chroman, and 2H-chromene derivatives as human rhinovirus capsid-binding inhibitors. Bioorg. Med. Chem. 2011, 19, 7357–7364. [Google Scholar] [CrossRef]
- Khandy, M.T.; Sofronova, A.K.; Gorpenchenko, T.Y.; Chirikova, N.K. Plant pyranocoumarins: Description, biosynthesis, application. Plants 2022, 11, 3135. [Google Scholar] [CrossRef]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.A.; Mahmoud, A.H.; Ibrahim, B.M.M.; Bari, A.; Villinger, A. Synthesis and evaluation of new coumarin derivatives as antioxidant, antimicrobial, and anti-inflammatory agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef]
- Rayar, A.M.; Lagarde, N.; Martin, F.; Blanchard, F.; Liagre, B.; Ferroud, C.; Zagury, J.F.; Montes, M.; Sylla-Iyarreta Veitía, M.S.-I. New selective cyclooxygenase-2 inhibitors from cyclocoumarol: Synthesis, characterization, biological evaluation and molecular modeling. Eur. J. Med. Chem. 2018, 146, 577–587. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, C.; Wang, Z.; Lee, H.J.; Hu, H.; Malewicz, B.; Lee, H.J.; Lee, J.H.; Baek, N.I.; Jeong, J.H.; et al. A novel class of pyranocoumarin anti-androgen receptor signaling compounds. Mol. Cancer Ther. 2007, 6, 907–917. [Google Scholar] [CrossRef]
- Onder, A.; Trendafilova, A. A review on anomalin: A natural bioactive pyranocoumarin from the past to the future. Chem. Biodivers. 2022, 19, e202200167. [Google Scholar] [CrossRef]
- Kuno, F.; Otoguro, K.; Shiomi, K.; Iwai, Y.; Omura, S. Arisugacins A and B, Novel and selective acetylcholinesterase inhibitors from Penicillium sp. FO-4259. I. Screening, taxonomy, fermentation, isolation and biological activity. J. Antibiot. 1996, 49, 742–747. [Google Scholar] [CrossRef]
- Omura, S.; Kuno, F.; Otoguro, K.; Sunazuka, T.; Shiomi, K.; Masuma, R.; Iwai, Y. Arisugacin, a novel and selective inhibitor of acetylcholinesterase from Penicillium sp. FO-4259. J. Antibiot. 1995, 48, 745–746. [Google Scholar] [CrossRef]
- Otoguro, K.; Kuno, F.; Omura, S. Arisugacins, selective acetylcholinesterase inhibitors of microbial origin. Pharmacol. Ther. 1997, 76, 45–54. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the inflammatory response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef]
- Zedler, S.; Faist, E. The impact of endogenous triggers on trauma-associated inflammation. Curr. Opin. Crit. Care 2006, 12, 595–601. [Google Scholar] [CrossRef]
- Isomäki, P.; Punnonen, J.J. Pro- and anti-inflammatory cytokines in rheumatoid arthritis. Ann. Med. 1997, 29, 499–507. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Ju, D.; Sun, S.C. NF-kB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, e17023. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Li, Y.; Wang, Z.; Xiao, M.; Yin, P.; Lu, Y.; Qian, X.; Xu, Y.; Liu, J. 7b, A novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted-inhibiting TAK1 following down-regulation of ERK1/2- and p38 MAPK-mediated activation of NF-κB in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2013, 17, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef]
- Mann, J.R.; Backlund, M.G.; DuBois, R.N. Mechanisms of disease: Inflammatory mediators and cancer prevention. Nat. Clin. Pract. Oncol. 2005, 2, 202–210. [Google Scholar] [CrossRef]
- Hwangbo, H.; Jeung, J.S.; Kim, M.Y.; Ji, S.Y.; Yoon, S.H.; Kim, T.H.; Kim, S.O.; Choi, Y.H. A study on antioxidant and anti-inflammatory effects based on analysis of functional components of Cornus officinalis Siebold & Zucc. J. Life Sci. 2021, 31, 287–297. [Google Scholar]
- Celec, P. Nuclear factor kappa B–molecular biomedicine: The next generation. Biomed. Pharmacother. 2004, 58, 365–371. [Google Scholar] [CrossRef]
- Garg, A.; Aggarwal, B.B. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia 2002, 16, 1053–1068. [Google Scholar] [CrossRef]
- Athman, R.; Philpott, D. Innate immunity via toll-like receptors and Nod proteins. Curr. Opin. Microbiol. 2004, 7, 25–32. [Google Scholar] [CrossRef]
- Beinke, S.; Ley, S.C. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem. J. 2004, 382, 393–409. [Google Scholar] [CrossRef]
- Iwalewa, E.O.; McGaw, L.J.; Naidoo, V.; Eloff, J.N. Inflammation: The foundation of diseases and disorders. A review of phytomedicines of South African origin used to treat pain and inflammatory conditions. Afr. J. Biotechnol. 2007, 6, 2868–2885. [Google Scholar] [CrossRef]
- Hoffmann, C. COX-2 in brain and spinal cord implications for therapeutic use. Curr. Med. Chem. 2000, 7, 1113–1120. [Google Scholar] [CrossRef]
- Yun, H.Y.; Dawson, V.L.; Dawson, T.M. Neurobiology of nitric oxide. Crit. Rev. Neurobiol. 1996, 10, 291–316. [Google Scholar] [CrossRef]
- Du, C.; Bhatia, M.; Tang, S.C.W.; Zhang, M.; Steiner, T. Mediators of inflammation: Inflammation in cancer, chronic diseases, and wound healing. Mediat. Inflam. 2015, 2015, 570653. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Seo, W.G.; Pae, H.O.; Oh, G.S.; Chai, K.Y.; Yun, Y.G.; Kwon, T.O.; Chung, H.T. Inhibitory effect of ethyl acetate fraction from Cudrania tricuspidata on the expression of nitric oxide synthase gene in RAW264.7 macrophages stimulated with interferon-γ and lipopolysaccharide. Gen. Pharmacol. 2000, 35, 21–28. [Google Scholar] [CrossRef]
- Liu, S.M.; Yang, T.C.; Ming, T.W.; Gaun, T.K.W.; Zhou, T.; Wang, S.; Ye, B.G. Isosteroid alkaloids with different chemical structures from Fritillariae cirrhosae bulbus alleviate LPS-induced inflammatory response in RAW264.7 cells by MAPK signaling pathway. Int. Immunopharmacol. 2020, 78, 106047. [Google Scholar] [CrossRef]
- Boumpas, D.T.; Chrousos, G.P.; Wilder, R.L.; Cupps, T.R.; Balow, J.E. 30 glucocorticoid therapy for immune-mediated diseases: Basic and clinical correlates. Ann. Intern. Med. 1993, 119, 1198–1208. [Google Scholar] [CrossRef]
- Shin, M.S.; Kim, S.B.; Lee, J.; Choi, H.S.; Park, J.; Park, J.Y.; Lee, S.; Hwang, G.S.; Koo, B.A.; Kang, K.S. Beneficial effect of herbal formulation KM1608 on inflammatory bowl diseases: A preliminary experimental study. Molecules 2018, 23, 2068. [Google Scholar] [CrossRef]
- Shin, M.S.; Park, J.Y.; Lee, J.; Yoo, H.H.; Hahm, D.H.; Lee, S.C.; Lee, S.; Hwang, G.S.; Jung, K.; Kang, K.S. Anti-inflammatory effects and corresponding mechanisms of cirsimaritin extracted from Cirsium japonicum var. maackii Maxim. Bioorg. Med. Chem. Lett. 2017, 27, 3076–3080. [Google Scholar] [CrossRef]
- Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 2007, 449, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, S.J.; Hwang, J.W.; Kim, E.H.; Park, P.J.; Jeong, J.H. Anti-inflammatory effects of extracts from Ligustrum ovalifolium H. Leaves on RAW264.7 macrophages. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1205–1210. [Google Scholar] [CrossRef]
- Sarkar, D.; Fisher, P.B. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006, 236, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Nyati, K.K.; Masuda, K.; Zaman, M.M.U.; Dubey, P.K.; Millrine, D.; Chalise, J.P.; Higa, M.; Li, S.; Standley, D.M.; Saito, K.; et al. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017, 45, 2687–2703. [Google Scholar] [CrossRef]
- Chan, E.D.; Riches, D.W. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am. J. Physiol. Cell Physiol. 2001, 280, C441–C450. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Wang, H.; Yang, Z.; Li, J.; Suo, H. Prevent effects of Lactobacillus fermentum HY01 on dextran sulfate sodium-induced colitis in mice. Nutrients 2017, 9, 545. [Google Scholar] [CrossRef]
- Dendorfer, U. Molecular biology of cytokines. Artif. Organs 1996, 20, 437–444. [Google Scholar] [CrossRef]
- Liden, J.; Rafter, I.; Truss, M.; Gustafsson, J.A.; Okret, S. Glucocorticoid effects on NF-κB binding in the transcription of the ICAM-1 gene. Biochem. Biophys. Res. Commun. 2000, 273, 1008–1014. [Google Scholar] [CrossRef]
- Pruett, S.B.; Fan, R.; Zheng, Q. Characterization of glucocorticoid receptor translocation, cytoplasmic IκB, nuclear NF-κB, and activation of NF-κB in T lymphocytes exposed to stress-inducible concentrations of corticosterone in vivo. Int. Immunopharmacol. 2003, 3, 1–16. [Google Scholar] [CrossRef]
| Control | LPS | Dexamethasone | ||||
|---|---|---|---|---|---|---|
| Average (%) | SD | Average (%) | SD | Average (%) | SD | |
| 100.0 | 0.7 | 97.4 | 1.9 | 102.5 | 1.8 | |
| Coumarin Derivative | 20 μM | 40 μM | 80 μM | |||
| Average (%) | SD | Average (%) | SD | Average (%) | SD | |
| 1 | 90.43 | 2.65 | 96.02 | 2.22 | 109.35 | 2.98 |
| 2 | 106.05 | 7.64 | 97.13 | 0.00 | 100.77 | 14.46 |
| 3 | 95.37 | 5.31 | 100.88 | 1.08 | 110.15 | 5.85 |
| 4 | 79.89 | 5.74 | 91.19 | 2.55 | 88.66 | 0.92 |
| 5 | 86.79 | 1.52 | 109.69 | 0.32 | 113.21 | 0.65 |
| 6 | 98.66 | 14.52 | 110.92 | 8.34 | 110.61 | 2.82 |
| 7 | 105.59 | 1.68 | 121.03 | 1.52 | 124.32 | 7.37 |
| 8 | 103.10 | 5.96 | 106.55 | 0.32 | 93.49 | 3.20 |
| 9 | 118.45 | 0.85 | 111.03 | 0.91 | 112.10 | 1.09 |
| 10 | 110.82 | 0.00 | 113.30 | 3.28 | 104.16 | 2.12 |
| 11 | 113.13 | 2.55 | 108.24 | 4.01 | 122.49 | 3.88 |
| 12 | 99.18 | 0.79 | 101.85 | 1.64 | 105.92 | 1.21 |
| 13 | 113.78 | 6.25 | 117.64 | 1.03 | 112.23 | 3.82 |
| 14 | 109.53 | 10.80 | 95.75 | 1.64 | 101.67 | 3.10 |
| 15 | 108.58 | 1.46 | 111.67 | 4.98 | 113.69 | 1.88 |
| 16 | 106.95 | 3.88 | 101.80 | 0.49 | 96.27 | 3.46 |
| 17 | 122.42 | 6.34 | 121.99 | 3.55 | 136.60 | 0.07 |
| 18 | 129.27 | 2.39 | 129.70 | 0.27 | 137.99 | 3.27 |
| 19 | 117.31 | 2.39 | 119.48 | 2.45 | 119.72 | 0.20 |
| 20 | 114.22 | 6.34 | 123.92 | 5.86 | 135.00 | 11.73 |
| 21 | 130.57 | 0.68 | 122.08 | 1.09 | 102.27 | 1.02 |
| 22 | 120.20 | 3.48 | 121.65 | 0.34 | 113.60 | 1.50 |
| 23 | 119.14 | 0.34 | 117.94 | 0.55 | 116.68 | 0.68 |
| Control | LPS | Dexamethasone | ||||
|---|---|---|---|---|---|---|
| Average (μM) | SD | Average (μM) | SD | Average (μM) | SD | |
| 3.1 | 0.2 | 25.9 | 0.5 | 6.5 # | 0.8 | |
| 3.1 | 0.2 | 25.9 | 3.1 | 0.2 | 25.9 | |
| Coumarin Derivative | 20 μM | 40 μM | 80 μM | |||
| Average (μM) | SD | Average (μM) | SD | Average (μM) | SD | |
| 1 | 29.50 | 1.34 | 29.82 | 0.45 | 28.71 | 2.02 |
| 2 | 22.53 | 1.79 | 15.24 *** | 0.45 | 10.17 *** | 0.45 |
| 3 | 27.60 | 1.34 | 28.86 | 1.34 | 22.84 | 0.45 |
| 4 | 15.71 | 0.22 | 17.45 *** | 2.24 | 18.09 *** | 2.24 |
| 5 | 30.77 | 3.14 | 28.71 | 0.67 | 25.38 | 0.45 |
| 6 | 30.92 | 0.67 | 26.49 | 0.67 | 16.82 *** | 0.45 |
| 7 | 32.83 | 2.46 | 29.66 | 1.12 | 27.60 | 1.34 |
| 8 | 32.19 | 3.81 | 24.11 | 0.00 | 10.64 *** | 0.67 |
| 9 | 23.27 | 0.59 | 24.10 | 2.17 | 21.32 | 0.98 |
| 10 | 24.24 | 0.00 | 20.34 ** | 0.00 | 9.21 *** | 0.79 |
| 11 | 24.80 | 0.79 | 26.47 | 0.00 | 26.19 | 0.00 |
| 12 | 16.58 | 0.20 | 17.28 *** | 0.79 | 17.98 *** | 2.17 |
| 13 | 27.17 | 1.77 | 26.33 | 0.59 | 24.94 | 0.20 |
| 14 | 27.72 | 0.98 | 24.52 | 0.79 | 16.31 *** | 0.20 |
| 15 | 26.33 | 0.59 | 22.71 | 1.38 | 19.37 ** | 0.59 |
| 16 | 27.58 | 1.97 | 26.33 | 0.59 | 16.17 *** | 0.39 |
| 17 | 28.68 | 0.91 | 26.11 | 0.45 | 20.16 ** | 0.68 |
| 18 | 28.03 | 0.91 | 27.87 | 0.23 | 25.95 | 2.50 |
| 19 | 27.07 | 1.36 | 17.76 | 1.36 | 10.05 *** | 0.45 |
| 20 | 31.89 | 1.82 | 30.12 | 0.23 | 19.52 ** | 1.59 |
| 21 | 28.68 | 1.82 | 22.73 | 2.95 | 11.97 *** | 0.45 |
| 22 | 31.41 | 1.14 | 28.84 | 0.23 | 22.89 | 0.45 |
| 23 | 30.77 | 0.68 | 29.80 | 0.23 | 27.39 | 0.45 |
| Antibodies | Source | Catalog Number |
|---|---|---|
| JNK | Cell Signaling Technology | #9252 |
| ERK1/2 | Cell Signaling Technology | #9102 |
| p38 | Cell Signaling Technology | #9212 |
| p65 | Cell Signaling Technology | #4764 |
| p-JNK | Cell Signaling Technology | #9251 |
| p-ERK1/2 | Cell Signaling Technology | #9101 |
| p-p38 | Cell Signaling Technology | #4631 |
| p-p65 | Cell Signaling Technology | #3033 |
| iNOS | Cell Signaling Technology | #13120 |
| β-actin | Cell Signaling Technology | #4967 |
| Anti-rabbit IgG | Cell Signaling Technology | #7074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, S.J.; Lee, H.; Shin, M.-S.; Lee, J.W. Synthesis and Biological Properties of Pyranocoumarin Derivatives as Potent Anti-Inflammatory Agents. Int. J. Mol. Sci. 2023, 24, 10026. https://doi.org/10.3390/ijms241210026
Min SJ, Lee H, Shin M-S, Lee JW. Synthesis and Biological Properties of Pyranocoumarin Derivatives as Potent Anti-Inflammatory Agents. International Journal of Molecular Sciences. 2023; 24(12):10026. https://doi.org/10.3390/ijms241210026
Chicago/Turabian StyleMin, Su Ji, Heesu Lee, Myoung-Sook Shin, and Jae Wook Lee. 2023. "Synthesis and Biological Properties of Pyranocoumarin Derivatives as Potent Anti-Inflammatory Agents" International Journal of Molecular Sciences 24, no. 12: 10026. https://doi.org/10.3390/ijms241210026
APA StyleMin, S. J., Lee, H., Shin, M.-S., & Lee, J. W. (2023). Synthesis and Biological Properties of Pyranocoumarin Derivatives as Potent Anti-Inflammatory Agents. International Journal of Molecular Sciences, 24(12), 10026. https://doi.org/10.3390/ijms241210026







