Apoptosis Related Human Wharton’s Jelly-Derived Stem Cells Differentiation into Osteoblasts, Chondrocytes, Adipocytes and Neural-like Cells—Complete Transcriptomic Assays
Abstract
1. Introduction
2. Results
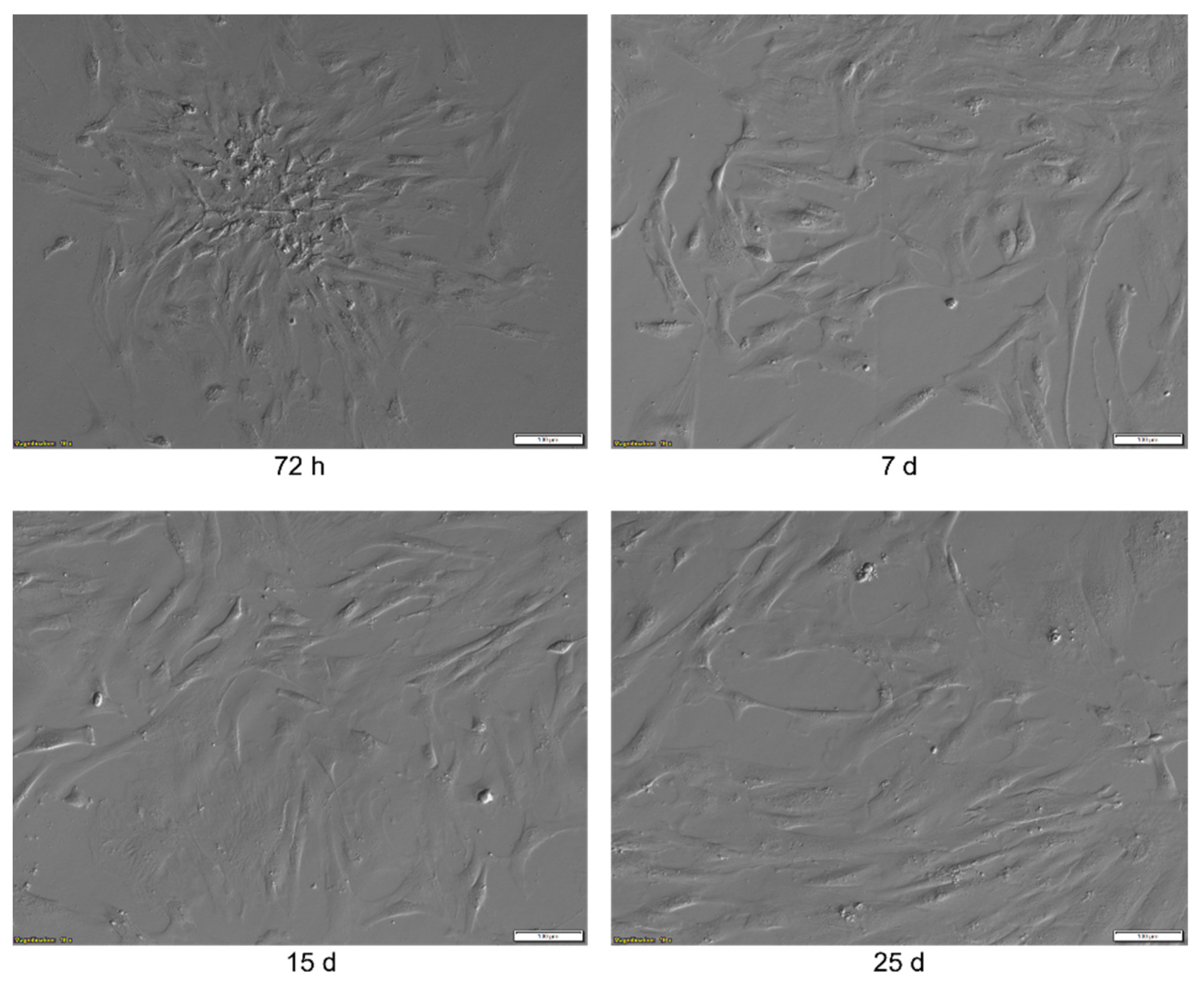
2.1. Morphological Analysis
2.2. Flow Cytometry Analysis
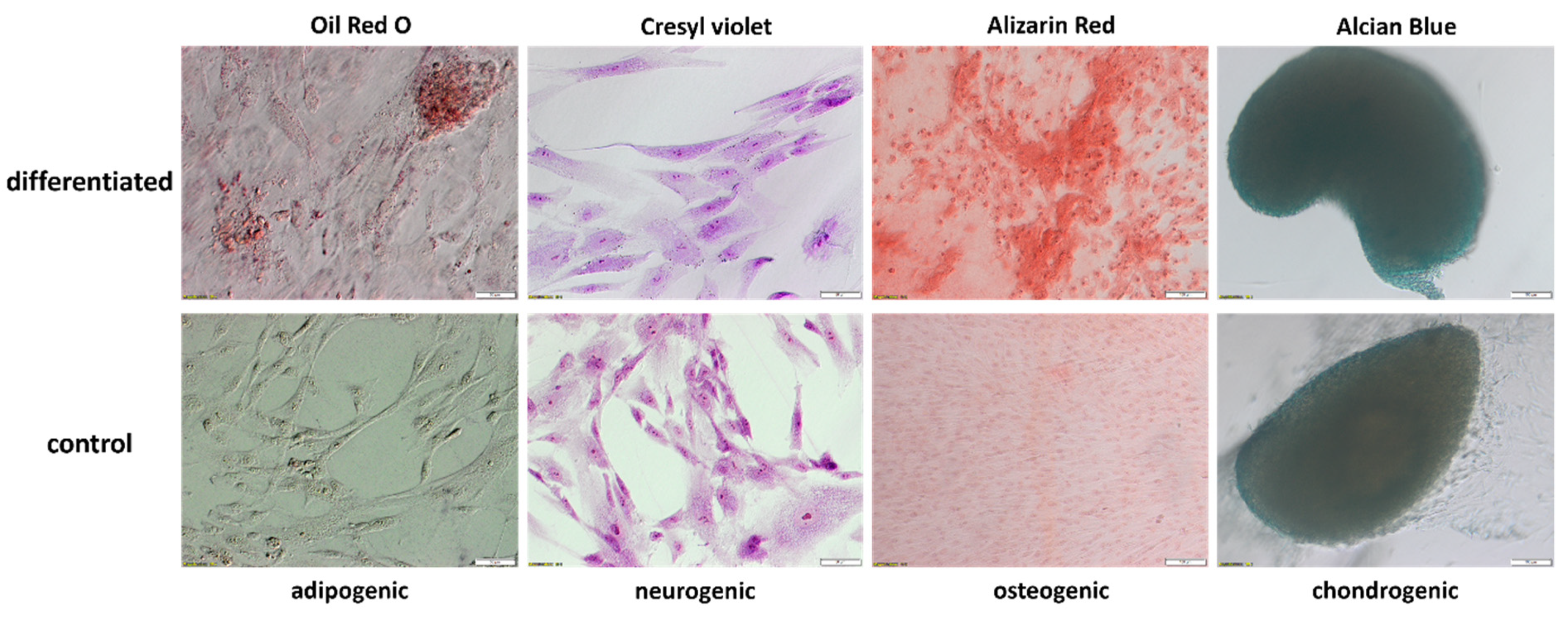
2.3. Evaluation of WJ-MSCs Differentiation
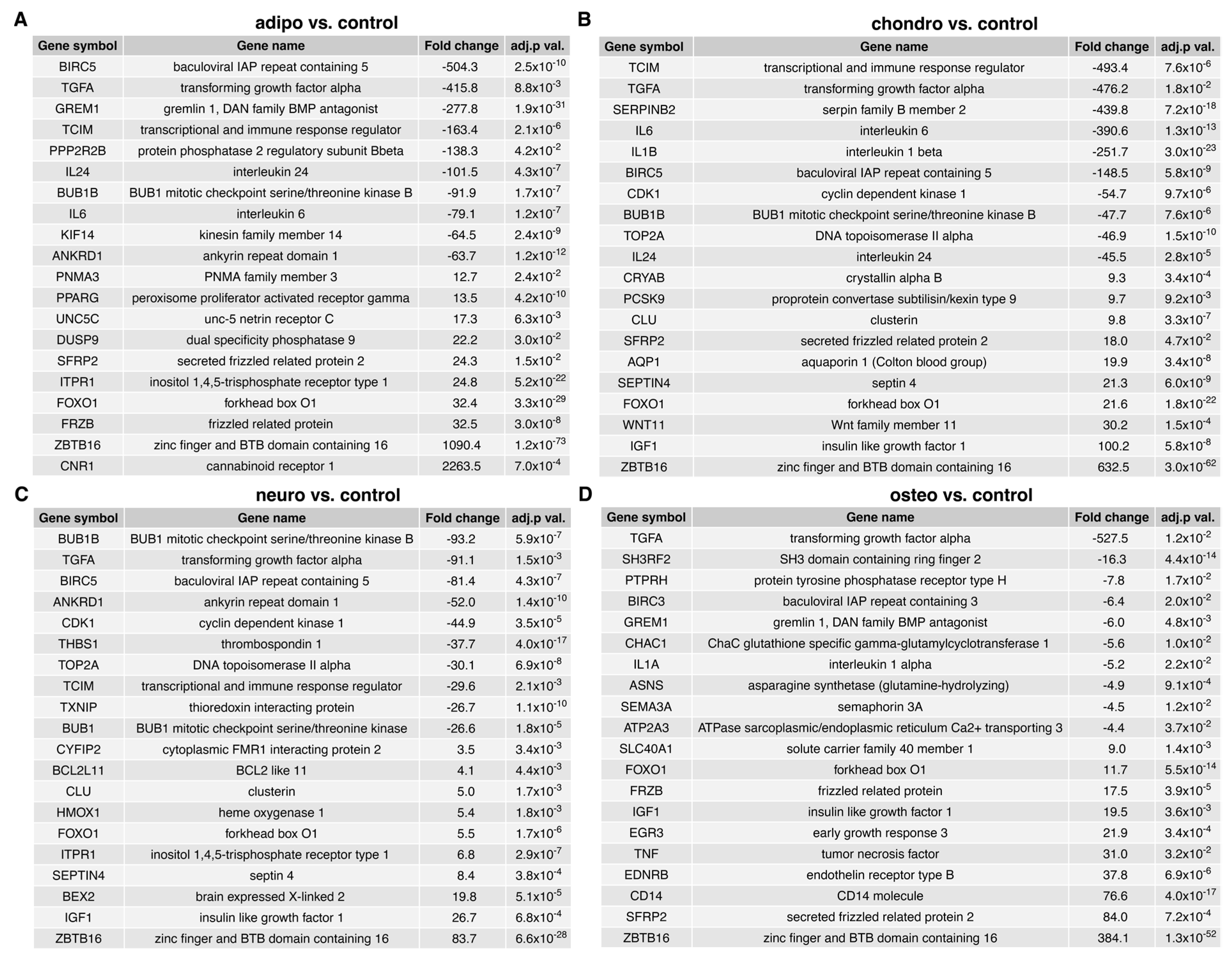
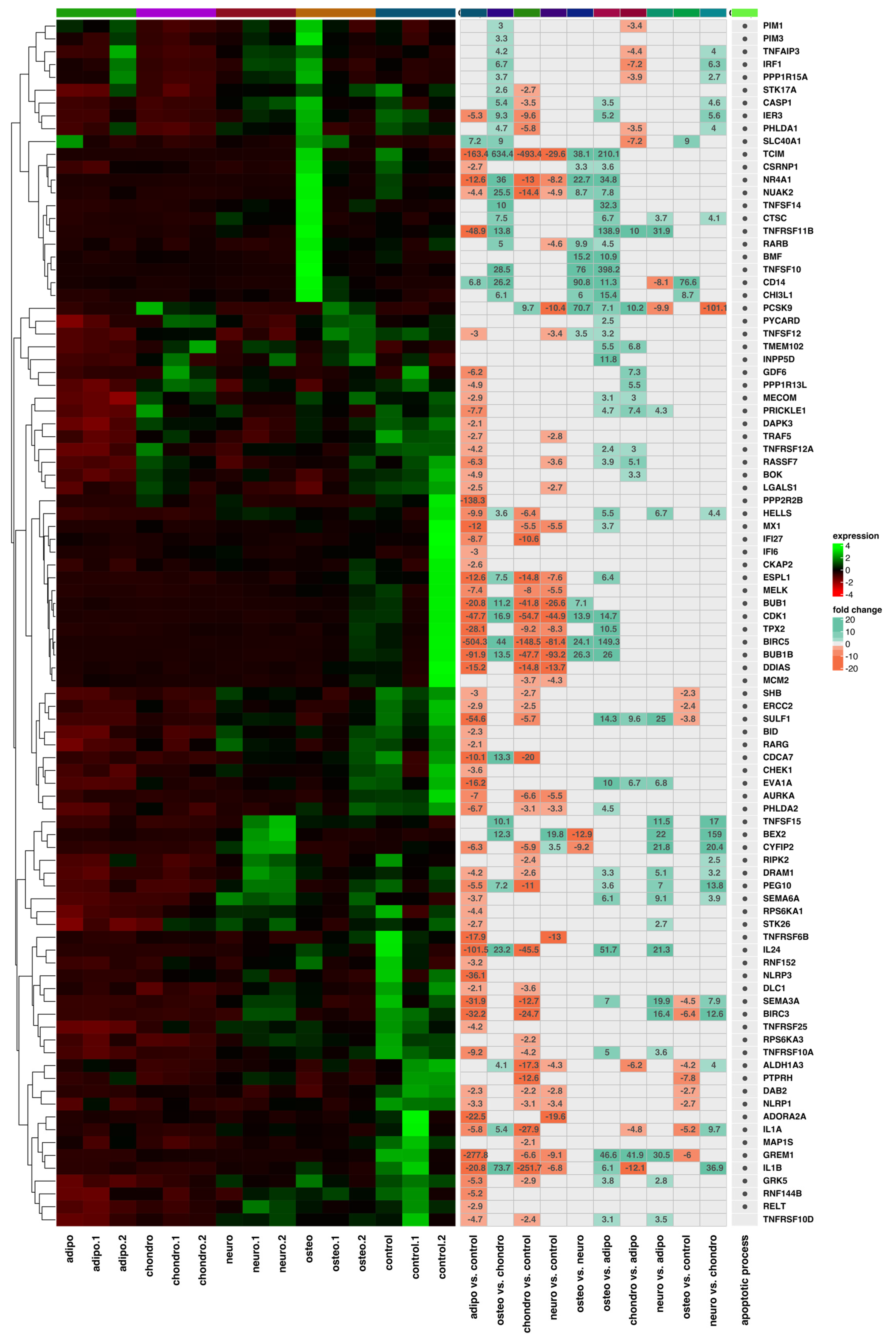
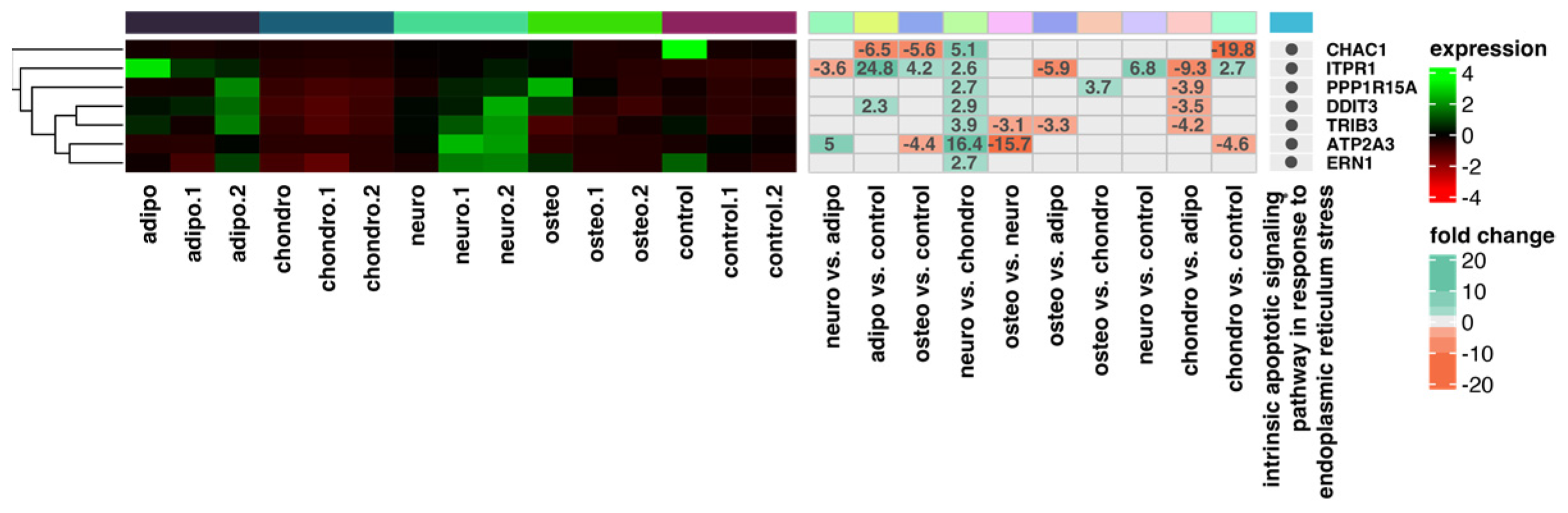
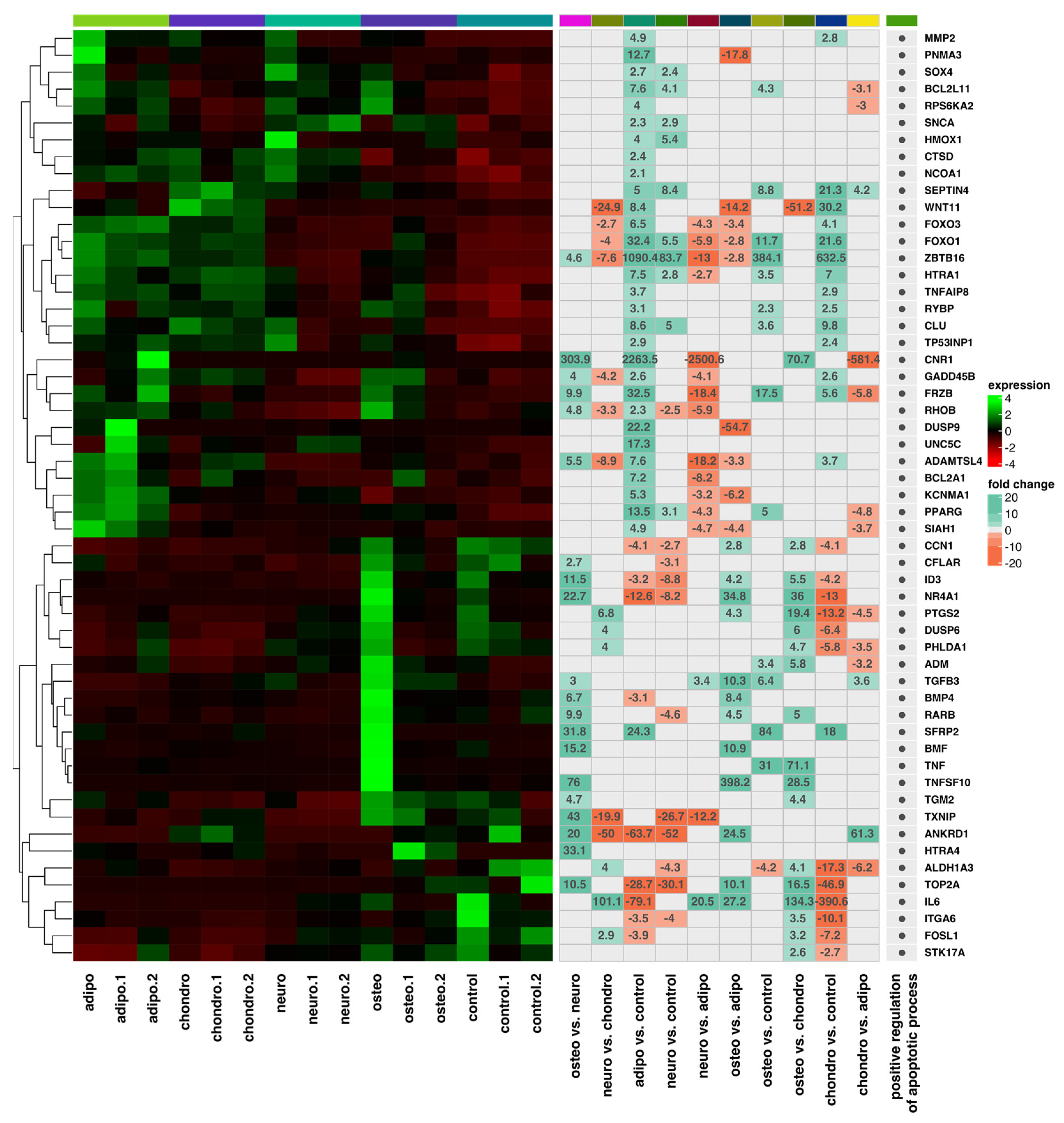
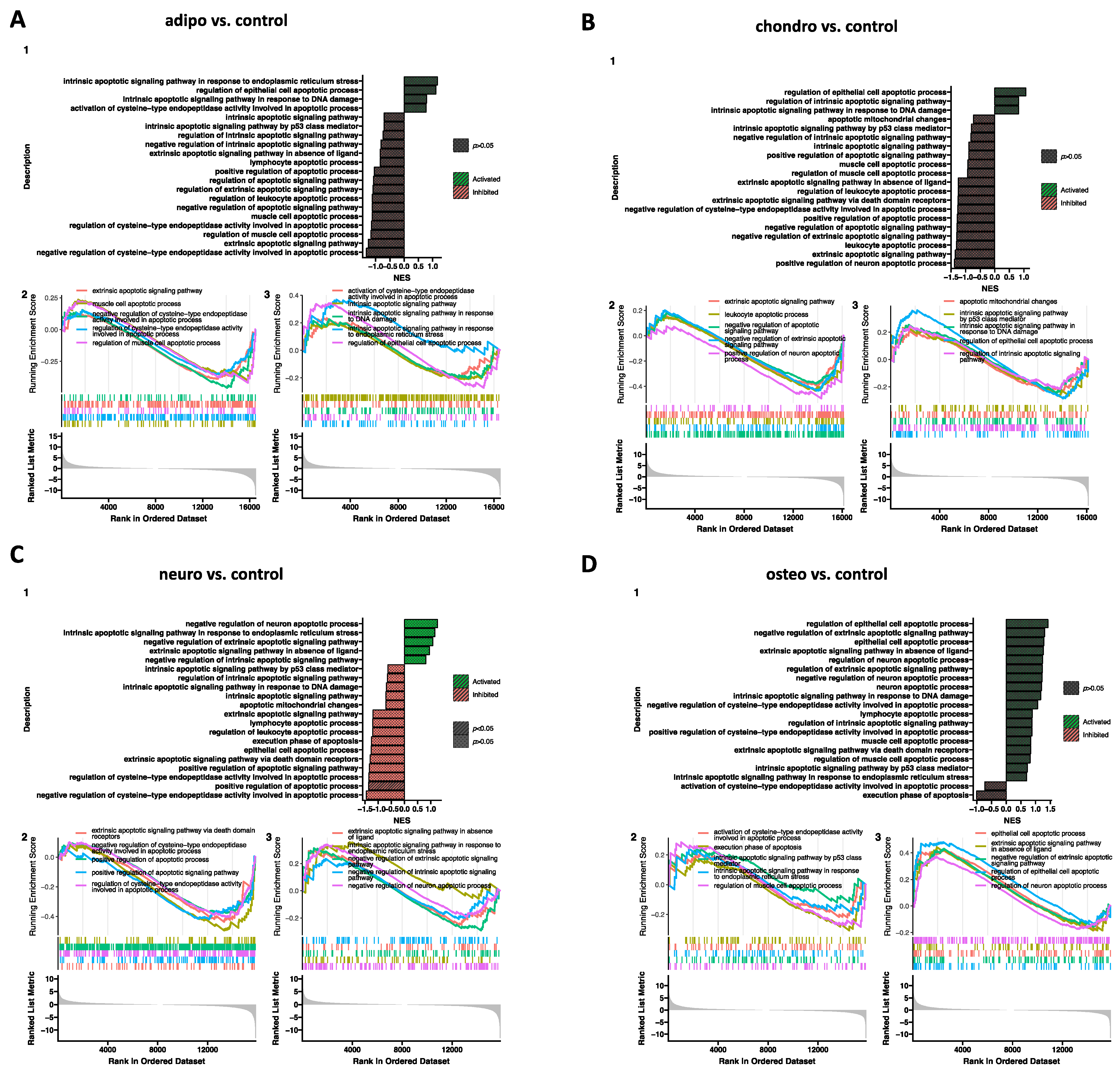
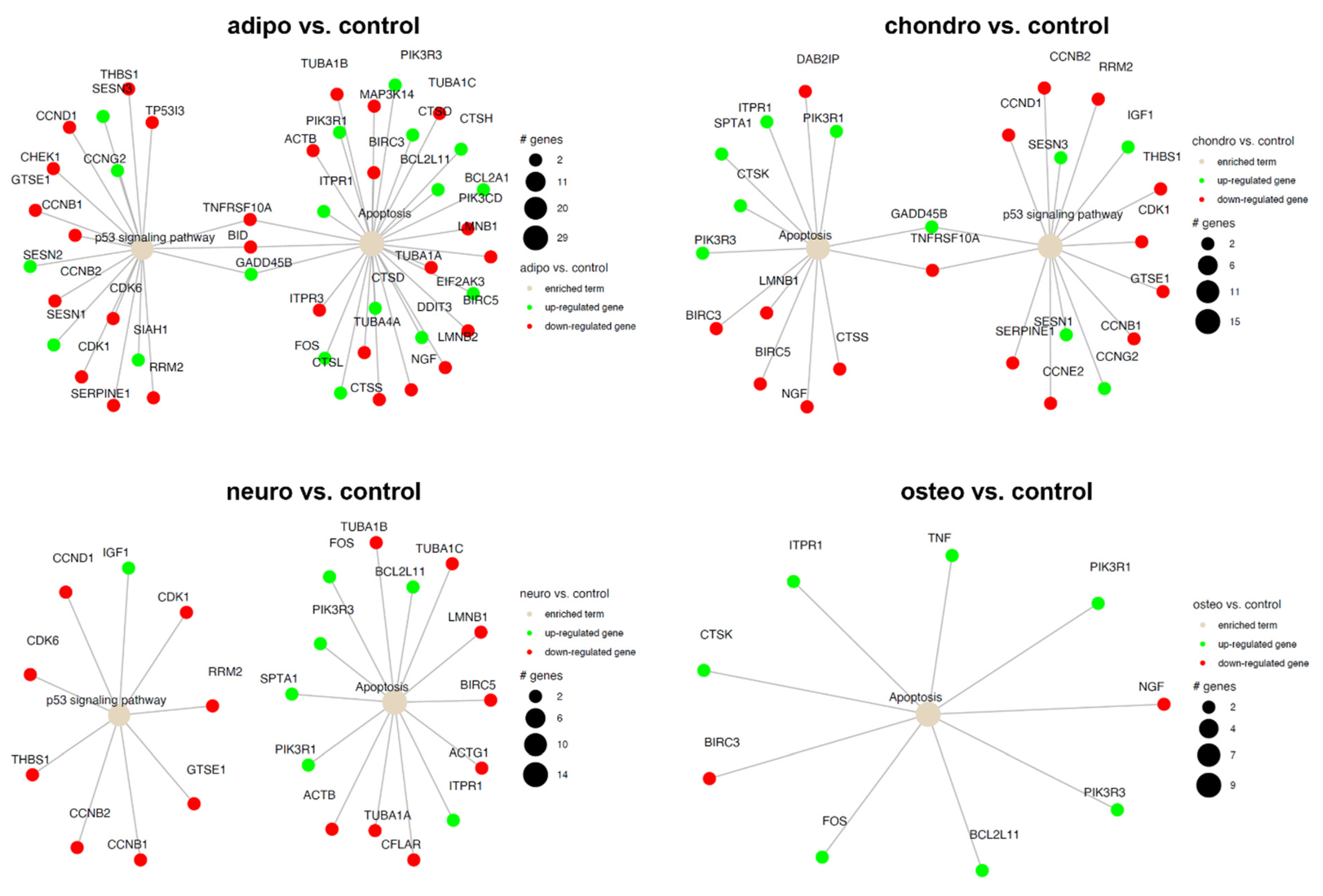
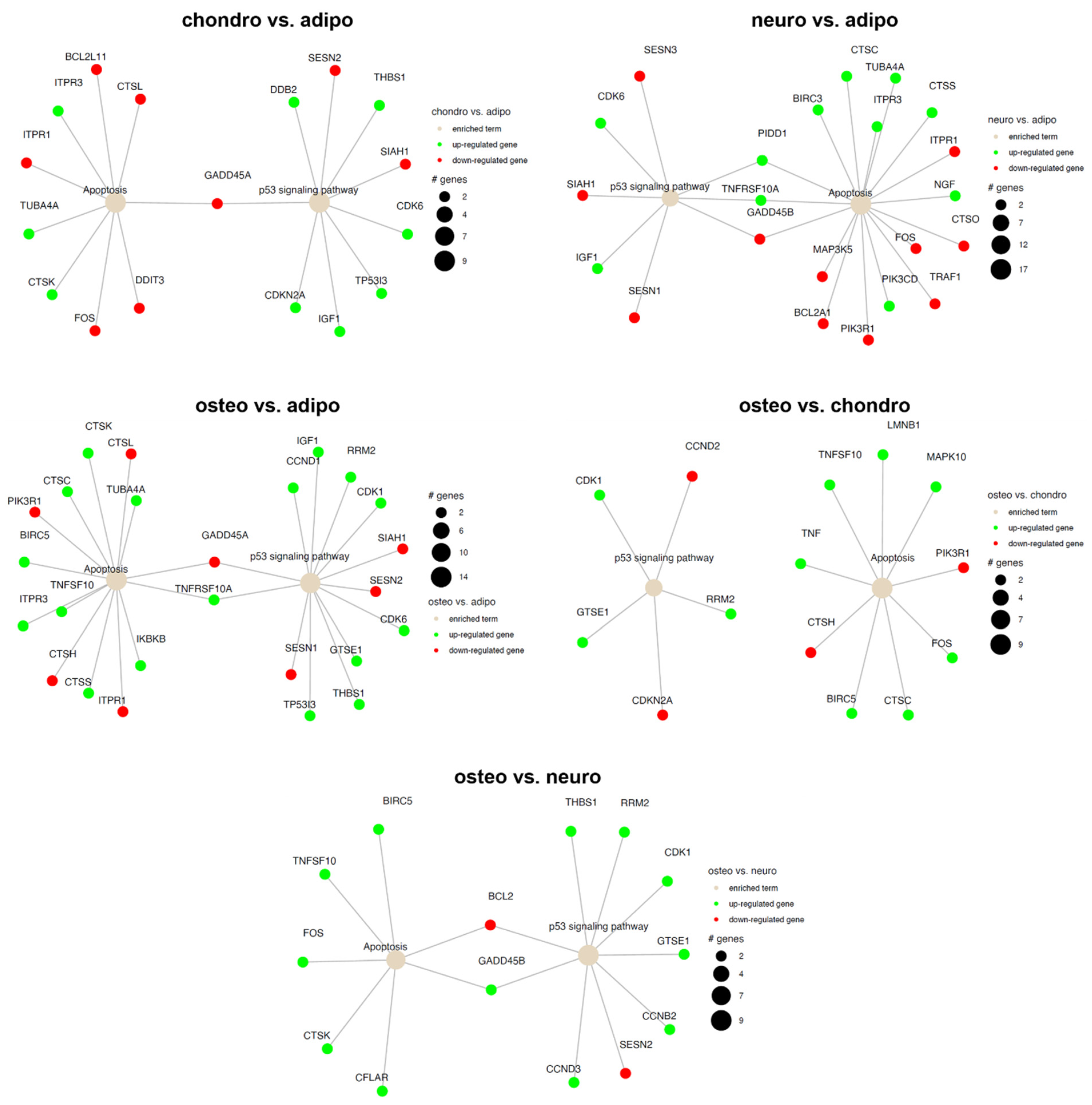
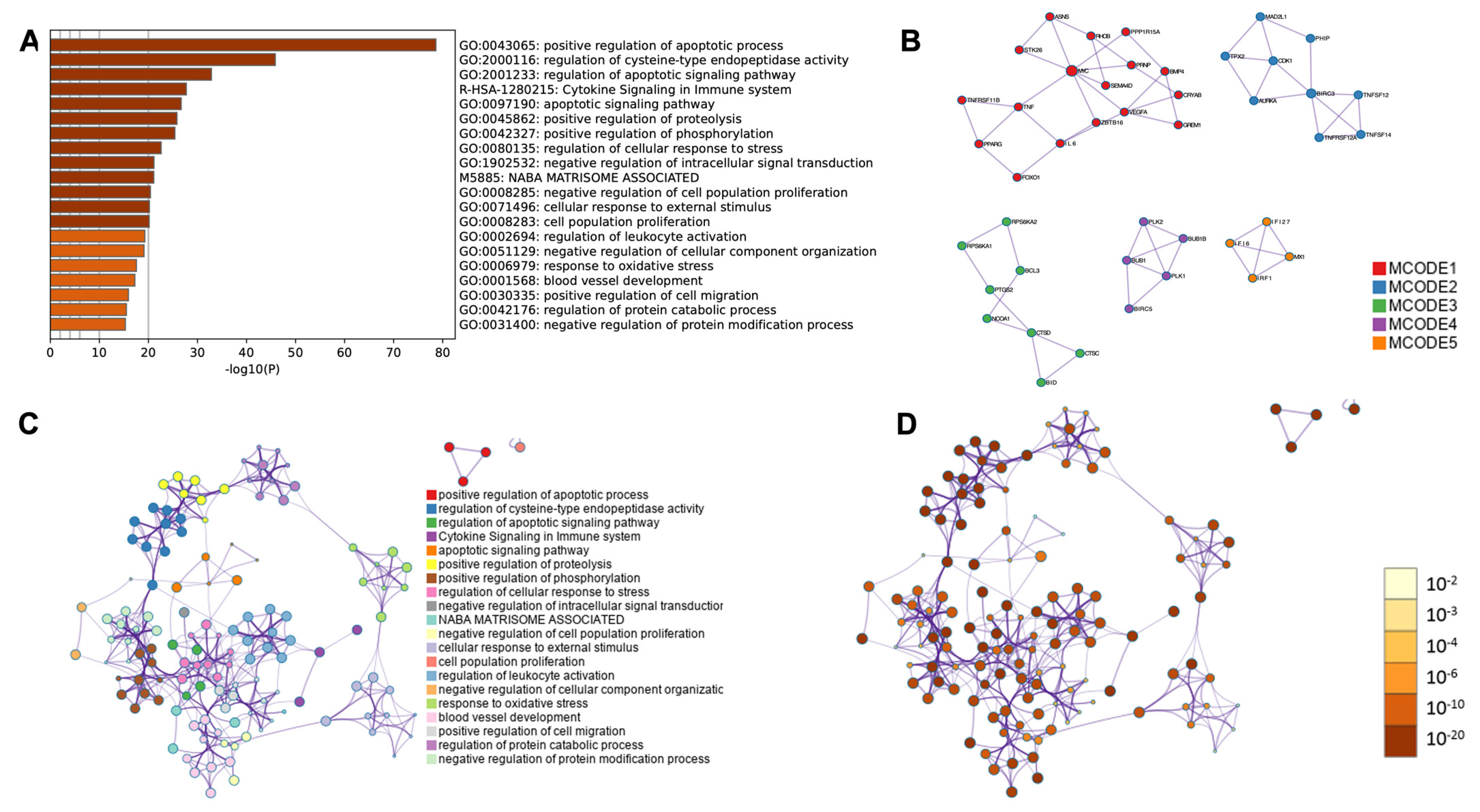
2.4. RNA-Seq Analysis
3. Discussion
4. Materials and Methods
4.1. Material Collection
4.2. Wharton’s Jelly-Derived Mesenchymal Stem Cells Isolation
4.3. In Vitro Cell Culture
4.4. Flow Cytometry Analysis
4.5. Multilineage Differentiation
4.5.1. Osteogenic Differentiation
4.5.2. Neurogenic Differentiation
4.5.3. Chondrogenic Differentiation
4.5.4. Adipogenic Differentiation
4.6. RNA Isolation
4.7. RNA-Seq
4.8. Bioinformatical and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarrige, M.; Frank, E.; Herardot, E.; Martineau, S.; Darle, A.; Benabides, M.; Domingues, S.; Chose, O.; Habeler, W.; Lorant, J.; et al. The future of regenerative medicine: Cell therapy using pluripotent stem cells and acellular therapies based on extracellular vesicles. Cells 2021, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Doğan, A. Embryonic stem cells in development and regenerative medicine. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2018; Volume 1079, pp. 1–15. [Google Scholar]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Young, H.E.; Black, A.C. Adult Stem Cells. Anat. Rec.-Part A Discov. Mol. Cell. Evol. Biol. 2004, 276, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Pochon, C.; Notarantonio, A.B.; Laroye, C.; Reppel, L.; Bensoussan, D.; Bertrand, A.; Rubio, M.T.; D’Aveni, M. Wharton’s jelly-derived stromal cells and their cell therapy applications in allogeneic haematopoietic stem cell transplantation. J. Cell. Mol. Med. 2022, 26, 1339–1350. [Google Scholar] [CrossRef]
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016, 2016, 6901286. [Google Scholar] [CrossRef]
- Conconi, M.T.; Liddo, R.D.; Tommasini, M.; Calore, C.; Parnigotto, P.P. Phenotype and Differentiation Potential of Stromal Populations Obtained from Various Zones of Human Umbilical Cord: An Overview. Open Tissue Eng. Regen. Med. J. 2011, 4, 6–20. [Google Scholar] [CrossRef]
- Can, A.; Karahuseyinoglu, S. Concise Review: Human Umbilical Cord Stroma with Regard to the Source of Fetus-Derived Stem Cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef]
- Bongso, A.; Fong, C.Y. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev. Rep. 2013, 9, 226–240. [Google Scholar] [CrossRef]
- Lyons, F.G.; Mattei, T.A. Sources, Identification, and Clinical Implications of Heterogeneity in Human Umbilical Cord Stem Cells. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1169, pp. 243–256. [Google Scholar]
- Sobolewski, K.; Bańkowski, E.; Chyczewski, L.; Jaworski, S. Collagen and glycosaminoglycans of wharton’s jelly. Neonatology 1997, 71, 11–21. [Google Scholar] [CrossRef]
- Meyer, F.A.; Laver-Rudich, Z.; Tanenbaum, R. Evidence for a mechanical coupling of glycoprotein microfibrils with collagen fibrils in Wharton’s jelly. BBA-Gen. Subj. 1983, 755, 376–387. [Google Scholar] [CrossRef]
- Takechi, K.; Kuwabara, Y.; Mizuno, M. Ultrastructural and immunohistochemical studies of Wharton’s jelly umbilical cord cells. Placenta 1993, 14, 235–245. [Google Scholar] [CrossRef]
- Nanaev, A.K.; Kohnen, G.; Milovanov, A.P.; Domogatsky, S.P.; Kaufmann, P. Stromal differentiation and architecture of the human umbilical cord. Placenta 1997, 18, 53–64. [Google Scholar] [CrossRef]
- Damsgaard, T.M.E.; Nielsen, B.W.; Sørensen, F.B.; Henriques, U.; Schiøtz, P.O. Estimation of the total number of mast cells in the human umbilical cord: A methodological study. Apmis 1992, 100, 845–850. [Google Scholar] [CrossRef]
- Wang, X.Y.; Lan, Y.; He, W.Y.; Zhang, L.; Yao, H.Y.; Hou, C.M.; Tong, Y.; Liu, Y.L.; Yang, G.; Liu, X.D.; et al. Identification of mesenchymal stem cells in aorta-gonad-mesonephros and yolk sac of human embryos. Blood 2008, 111, 2436–2443. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Subramanian, A.; Fong, C.Y.; Biswas, A.; Bongso, A. Comparative characterization of cells from the various compartments of the human umbilical cord shows that the Wharton’s jelly compartment provides the best source of clinically utilizable mesenchymal stem cells. PLoS ONE 2015, 10, e0127992. [Google Scholar] [CrossRef]
- Cabrera-Pérez, R.; Monguió-Tortajada, M.; Gámez-Valero, A.; Rojas-Márquez, R.; Borràs, F.E.; Roura, S.; Vives, J. Osteogenic commitment of Wharton’s jelly mesenchymal stromal cells: Mechanisms and implications for bioprocess development and clinical application. Stem Cell Res. Ther. 2019, 10, 356. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.S.; Yazid, M.D.; Sainik, N.Q.A.V.; Razali, R.A.; Saim, A.B.; Idrus, R.B.H. Osteogenic induction of Wharton’s jelly-derived mesenchymal stem cell for bone regeneration: A systematic review. Stem Cells Int. 2018, 2018, 2406462. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Teixeira, M.V.T.; Carias, R.B.V.; Granjeiro, J.M.; Borojevic, R. Gene expression and protein secretion during human mesenchymal cell differentiation into adipogenic cells. BMC Cell Biol. 2014, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Amable, P.R.; Teixeira, M.V.T.; Carias, R.B.V.; Granjeiro, J.M.; Borojevic, R. Protein synthesis and secretion in human mesenchymal cells derived from bone marrow, adipose tissue and Wharton’s jelly. Stem Cell Res. Ther. 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Reppel, L.; Margossian, T.; Yaghi, L.; Moreau, P.; Mercier, N.; Leger, L.; Hupont, S.; Stoltz, J.-F.; Bensoussan, D.; Huselstein, C. Hypoxic Culture Conditions for Mesenchymal Stromal/Stem Cells from Wharton’s Jelly: A Critical Parameter to Consider in a Therapeutic Context. Curr. Stem Cell Res. Ther. 2014, 9, 306–318. [Google Scholar] [CrossRef]
- Reppel, L.; Schiavi, J.; Charif, N.; Leger, L.; Yu, H.; Pinzano, A.; Henrionnet, C.; Stoltz, J.F.; Bensoussan, D.; Huselstein, C. Chondrogenic induction of mesenchymal stromal/stem cells from Wharton’s jelly embedded in alginate hydrogel and without added growth factor: An alternative stem cell source for cartilage tissue engineering. Stem Cell Res. Ther. 2015, 6, 260. [Google Scholar] [CrossRef]
- Mitchell, K.E.; Weiss, M.L.; Mitchell, B.M.; Martin, P.; Davis, D.; Morales, L.; Helwig, B.; Beerenstrauch, M.; Abou-Easa, K.; Hildreth, T.; et al. Matrix Cells from Wharton’s Jelly Form Neurons and Glia. Stem Cells 2003, 21, 50–60. [Google Scholar] [CrossRef]
- Fu, Y.S.; Shih, Y.T.; Cheng, Y.C.; Min, M.Y. Transformation of human umbilical mesenchymal cells into neurons in vitro. J. Biomed. Sci. 2004, 11, 652–660. [Google Scholar] [CrossRef]
- Liang, J.; Wu, S.; Zhao, H.; Li, S.L.; Liu, Z.X.; Wu, J.; Zhou, L. Human umbilical cord mesenchymal stem cells derived from Wharton’s jelly differentiate into cholinergic-like neurons in vitro. Neurosci. Lett. 2013, 532, 59–63. [Google Scholar] [CrossRef]
- Alizadeh, R.; Bagher, Z.; Kamrava, S.K.; Falah, M.; Ghasemi Hamidabadi, H.; Eskandarian Boroujeni, M.; Mohammadi, F.; Khodaverdi, S.; Zare-Sadeghi, A.; Olya, A.; et al. Differentiation of human mesenchymal stem cells (MSC) to dopaminergic neurons: A comparison between Wharton’s Jelly and olfactory mucosa as sources of MSCs. J. Chem. Neuroanat. 2019, 96, 126–133. [Google Scholar] [CrossRef]
- Wang, H.-S.; Hung, S.-C.; Peng, S.-T.; Huang, C.-C.; Wei, H.-M.; Guo, Y.-J.; Fu, Y.-S.; Lai, M.-C.; Chen, C.-C. Mesenchymal Stem Cells in the Wharton’s Jelly of the Human Umbilical Cord. Stem Cells 2004, 22, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Conconi, M.T.; Burra, P.; Di Liddo, R.; Calore, C.; Turetta, M.; Bellini, S.; Bo, P.; Nussdorfer, G.G.; Parnigotto, P.P. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int. J. Mol. Med. 2006, 18, 1089–1096. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Lie, P.C.; Wei, X. Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton’s jelly into hepatocyte-like cells. Cytotherapy 2009, 11, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Bharti, D.; Shivakumar, S.B.; Park, J.K.; Ullah, I.; Subbarao, R.B.; Park, J.S.; Lee, S.L.; Park, B.W.; Rho, G.J. Comparative analysis of human Wharton’s jelly mesenchymal stem cells derived from different parts of the same umbilical cord. Cell Tissue Res. 2018, 372, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Prasajak, P.; Leeanansaksiri, W. Developing a new two-step protocol to generate functional hepatocytes from wharton’s jelly-derived mesenchymal stem cells under hypoxic condition. Stem Cells Int. 2013, 2013, 762196. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, J.; Cui, H.P.; Wang, X.M.; Rong, H.; Shao, B.; Cui, H. Wharton’s jelly mesenchymal stem cells differentiate into retinal progenitor cells. Neural Regen. Res. 2013, 8, 1783–1792. [Google Scholar] [CrossRef]
- Huang, P.; Lin, L.M.; Wu, X.Y.; Tang, Q.L.; Feng, X.Y.; Lin, G.Y.; Lin, X.; Wang, H.W.; Huang, T.H.; Ma, L. Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into germ-like cells in vitro. J. Cell. Biochem. 2010, 109, 747–754. [Google Scholar] [CrossRef]
- Chao, K.C.; Chao, K.F.; Fu, Y.S.; Liu, S.H. Islet-like clusters derived from mesenchymal stem cells in Wharton’s jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS ONE 2008, 3, e1451. [Google Scholar] [CrossRef]
- Wu, L.F.; Wang, N.N.; Liu, Y.S.; Wei, X. Differentiation of wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng.-Part A 2009, 15, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.H.; Zhou, B.; Lu, S.H.; Feng, B.; Yang, S.G.; Du, W.T.; Gu, D.S.; Han, Z.C.; Liu, Y.L. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J. Cell. Biochem. 2007, 100, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Gao, J.; Jiang, Y.; Sun, B.; Lu, W.; Su, M.; Xu, Y.; Yang, X.; Zhang, Y. Differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells into endometrial cells. Stem Cell Res. Ther. 2017, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Potier, E.; Ferreira, E.; Meunier, A.; Sedel, L.; Logeart-Avramoglou, D.; Petite, H. Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng. 2007, 13, 1325–1331. [Google Scholar] [CrossRef]
- Stelcer, E.; Komarowska, H.; Jopek, K.; Żok, A.; Iżycki, D.; Malińska, A.; Szczepaniak, B.; Komekbai, Z.; Karczewski, M.; Wierzbicki, T.; et al. Biological response of adrenal carcinoma and melanoma cells to mitotane treatment. Oncol. Lett. 2022, 23, 120. [Google Scholar] [CrossRef]
- Budna, J.; Chachuła, A.; Kaźmierczak, D.; Rybska, M.; Ciesiółka, S.; Bryja, A.; Kranc, W.; Borys, S.; Zok, A.; Bukowska, D.; et al. Morphogenesis-related gene-expression profile in porcine oocytes before and after in vitro maturation. Zygote 2017, 25, 331–340. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57. [Google Scholar] [CrossRef]
- Golkar-Narenji, A.; Antosik, P.; Nolin, S.; Rucinski, M.; Jopek, K.; Zok, A.; Sobolewski, J.; Jankowski, M.; Zdun, M.; Bukowska, D.; et al. Gene Ontology Groups and Signaling Pathways Regulating the Process of Avian Satellite Cell Differentiation. Genes 2022, 13, 242. [Google Scholar] [CrossRef]
- Jankowski, M.; Dompe, C.; Sibiak, R.; Wąsiatycz, G.; Mozdziak, P.; Jaśkowski, J.M.; Antosik, P.; Kempisty, B.; Dyszkiewicz-Konwińska, M. In Vitro Cultures of Adipose-Derived Stem Cells: An Overview of Methods, Molecular Analyses, and Clinical Applications. Cells 2020, 9, 1783. [Google Scholar] [CrossRef]
- Binder, B.Y.K.; Genetos, D.C.; Leach, J.K. Lysophosphatidic Acid Protects Human Mesenchymal Stromal Cells from Differentiation-Dependent Vulnerability to Apoptosis. Tissue Eng. Part A 2014, 20, 1156. [Google Scholar] [CrossRef] [PubMed]
- Pesarini, J.R.; de Oliveira, E.J.T.; Pessatto, L.R.; Rabacow, A.P.M.; Camassola, M.; dos Santos, B.P.; de Barros, M.E.; Cantero, W.d.B.; Antoniolli-Silva, A.C.M.B.; Oliveira, R.J. Calcitriol combined with calcium chloride causes apoptosis in undifferentiated adipose tissue-derived human mesenchymal stem cells, but this effect decreases during adipogenic differentiation. Biomed. Pharmacother. 2018, 108, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Lo Furno, D.; Graziano, A.C.E.; Caggia, S.; Perrotta, R.E.; Tarico, M.S.; Giuffrida, R.; Cardile, V. Decrease of apoptosis markers during adipogenic differentiation of mesenchymal stem cells from human adipose tissue. Apoptosis 2013, 18, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Oliver, L.; Hue, E.; Séry, Q.; Lafargue, A.; Pecqueur, C.; Paris, F.; Vallette, F.M. Differentiation-related response to DNA breaks in human mesenchymal stem cells. Stem Cells 2013, 31, 800–807. [Google Scholar] [CrossRef]
- Ming Liu, T.; Martina, M.; Hutmacher, D.W.; Hoi Po Hui, J.; Hin Lee, E.; Lim, B.; Hoi Hui, J.P. Identification of Common Pathways Mediating Differentiation of Bone Marrow- and Adipose Tissue-Derived Human Mesenchymal Stem Cells into Three Mesenchymal Lineages. Stem Cells 2007, 25, 750–760. [Google Scholar] [CrossRef]
- Onizuka, S.; Iwata, T.; Park, S.J.; Nakai, K.; Yamato, M.; Okano, T.; Izumi, Y. ZBTB16 as a Downstream Target Gene of Osterix Regulates Osteoblastogenesis of Human Multipotent Mesenchymal Stromal Cells. J. Cell. Biochem. 2016, 117, 2423. [Google Scholar] [CrossRef]
- Marofi, F.; Vahedi, G.; Solali, S.; Alivand, M.; Salarinasab, S.; Zadi Heydarabad, M.; Farshdousti Hagh, M. Gene expression of TWIST1 and ZBTB16 is regulated by methylation modifications during the osteoblastic differentiation of mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 6230–6243. [Google Scholar] [CrossRef]
- Liu, T.M.; Guo, X.M.; Tan, H.S.; Hui, J.H.; Lim, B.; Lee, E.H. Zinc-finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum. 2011, 63, 2711–2720. [Google Scholar] [CrossRef]
- Al-Ali, M.M.; Khan, A.A.; Fayyad, A.M.; Abdallah, S.H.; Khattak, M.N.K. Transcriptomic profiling of the telomerase transformed Mesenchymal stromal cells derived adipocytes in response to rosiglitazone. BMC Genom. Data 2022, 23, 17. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dessels, C.; Durandt, C.; Pepper, M.S. Genome-wide analysis of gene expression during adipogenesis in human adipose-derived stromal cells reveals novel patterns of gene expression during adipocyte differentiation. Stem Cell Res. 2016, 16, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Sobieszczuk, D.F.; Poliakov, A.; Xu, Q.; Wilkinson, D.G. A feedback loop mediated by degradation of an inhibitor is required to initiate neuronal differentiation. Genes Dev. 2010, 24, 206. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Z.; Sun, Y.E.; Liu, Y.; Wu, Z.; Ma, B.; Cheng, L. Neuroprotective Effects of Human Umbilical Cord-Derived Mesenchymal Stem Cells From Different Donors on Spinal Cord Injury in Mice. Front. Cell. Neurosci. 2021, 15, 768711. [Google Scholar] [CrossRef]
- Ludikhuize, M.C.; Rodríguez Colman, M.J. Metabolic Regulation of Stem Cells and Differentiation: A Forkhead Box O Transcription Factor Perspective. Antioxid. Redox Signal. 2021, 34, 1004–1024. [Google Scholar] [CrossRef]
- Kurakazu, I.; Akasaki, Y.; Hayashida, M.; Tsushima, H.; Goto, N.; Sueishi, T.; Toya, M.; Kuwahara, M.; Okazaki, K.; Duffy, T.; et al. FOXO1 transcription factor regulates chondrogenic differentiation through transforming growth factor β1 signaling. J. Biol. Chem. 2019, 294, 17555. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hu, B.; Xie, L.Q.; Jiang, T.J.; Xia, Z.Y.; Peng, H. Scara3 regulates bone marrow mesenchymal stem cell fate switch between osteoblasts and adipocytes by promoting Foxo1. Cell Prolif. 2021, 54, e13095. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, M.F.; Flowers, S.; Bhattacharya, R.; Faibish, D.; Behl, Y.; Kotton, D.N.; Gerstenfeld, L.; Moran, E.; Graves, D.T. FOXO1 Modulates Osteoblast Differentiation. Bone 2011, 48, 1043. [Google Scholar] [CrossRef]
- Teixeira, C.C.; Liu, Y.; Thant, L.M.; Pang, J.; Palmer, G.; Alikhani, M. Foxo1, a Novel Regulator of Osteoblast Differentiation and Skeletogenesis. J. Biol. Chem. 2010, 285, 31055. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Tian, M.; Huang, Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J. Mol. Endocrinol. 2019, 62, R239–R253. [Google Scholar] [CrossRef] [PubMed]
- Nakae, J.; Kitamura, T.; Kitamura, Y.; Biggs, W.H.; Arden, K.C.; Accili, D. The forkhead transcription factor Fox01 regulates adipocyte differentiation. Dev. Cell 2003, 4, 119–129. [Google Scholar] [CrossRef]
- Domínguez-Castro, M.; Domínguez-Galicia, A.; Pérez-Pérez, O.; Hernández-Pineda, J.; Mancilla-Herrera, I.; Bazán-Tejeda, M.L.; Rodríguez-Cruz, L.; González-Torres, M.C.; Montoya-Estrada, A.; Reyes-Muñoz, E.; et al. Hyperglycemia affects neuronal differentiation and Nestin, FOXO1, and LMO3 mRNA expression of human Wharton’s jelly mesenchymal stem cells of children from diabetic mothers. Biochem. Biophys. Res. Commun. 2022, 637, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lotan, R.; Rotem, A.; Gonen, H.; Finberg, J.P.M.; Kemeny, S.; Steller, H.; Ciechanover, A.; Larisch, S. Regulation of the proapoptotic ARTS protein by ubiquitin-mediated degradation. J. Biol. Chem. 2005, 280, 25802–25810. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, Y.; Rotem, A.; Lotan, R.; Steller, H.; Larisch, S. The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J. 2004, 23, 1627. [Google Scholar] [CrossRef]
- Qi, Z.; Guo, W.; Zheng, S.; Fu, C.; Ma, Y.; Pan, S.; Liu, Q.; Yang, X. Enhancement of neural stem cell survival, proliferation and differentiation by IGF-1 delivery in graphene oxide-incorporated PLGA electrospun nanofibrous mats. RSC Adv. 2019, 9, 8315. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, Y.; Chen, X.; Yuan, J.; Liu, X.; Chen, Y.; Zhao, Y.; Liu, P.; Li, Y. Effects of IGF-1 on neural differentiation of human umbilical cord derived mesenchymal stem cells. Life Sci. 2016, 151, 93–101. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, B.; Zhao, J.; Pan, W.; Xu, J.; Chen, S. IGF-I induces adipose derived mesenchymal cell chondrogenic differentiation in vitro and enhances chondrogenesis in vivo. Vitr. Cell. Dev. Biol. Anim. 2016, 52, 356–364. [Google Scholar] [CrossRef]
- Feng, J.; Meng, Z. Insulin growth factor-1 promotes the proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells through the Wnt/β-catenin pathway. Exp. Ther. Med. 2021, 22, 891. [Google Scholar] [CrossRef]
- Kinoshita, A.; Ohyama, K.; Tanimura, S.; Matsuda, K.; Kishino, T.; Negishi, Y.; Asahina, N.; Shiraishi, H.; Hosoki, K.; Tomiwa, K.; et al. Itpr1 regulates the formation of anterior eye segment tissues derived from neural crest cells. Development 2021, 148, dev188755. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Wang, Y.; He, L.; Xu, D.; Yan, E.; Guo, J.; Ma, C.; Zhang, P.; Yin, J. Lack of adipocyte IP3R1 reduces diet-induced obesity and greatly improves whole-body glucose homeostasis. Cell Death Discov. 2023, 9, 87. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Liu, M.; Zhou, Z.; Li, Q.; Huang, T.; Yue, Y.; Tian, Y. Effect and Related Mechanism of Platelet-Rich Plasma on the Osteogenic Differentiation of Human Adipose-Derived Stem Cells. BioMed Res. Int. 2022, 2022, 1256002. [Google Scholar] [CrossRef]
- Sidibeh, C.O.; Pereira, M.J.; Lau Börjesson, J.; Kamble, P.G.; Skrtic, S.; Katsogiannos, P.; Sundbom, M.; Svensson, M.K.; Eriksson, J.W. Role of cannabinoid receptor 1 in human adipose tissue for lipolysis regulation and insulin resistance. Endocrine 2017, 55, 839. [Google Scholar] [CrossRef]
- Katagiri, W.; Osugi, M.; Kawai, T.; Hibi, H. Secreted Frizzled-Related Protein Promotes Bone Regeneration by Human Bone Marrow-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2015, 16, 23250. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Huang, X.; Rodrigues, E.D.; Leijten, J.C.H.; Verrips, T.; El Khattabi, M.; Karperien, M.; Post, J.N. Endogenous DKK1 and FRZB Regulate Chondrogenesis and Hypertrophy in Three-Dimensional Cultures of Human Chondrocytes and Human Mesenchymal Stem Cells. Stem Cells Dev. 2016, 25, 1808. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Jung, E.; Hwang, W.; Kim, Y.S.; Park, D. Isorhamnetin-induced anti-adipogenesis is mediated by stabilization of beta-catenin protein. Life Sci. 2010, 86, 416–423. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, E.; Yang, M.; Lu, L. Overexpression of Wnt11 promotes chondrogenic differentiation of bone marrow-derived mesenchymal stem cells in synergism with TGF-β. Mol. Cell. Biochem. 2014, 390, 123–131. [Google Scholar] [CrossRef]
- Naderi, A.; Liu, J.; Bennett, I.C. BEX2 regulates mitochondrial apoptosis and G1 cell cycle in breast cancer. Int. J. Cancer 2010, 126, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Meng, Q.; Xu, X.; Zhi, T.; Shi, Q.; Wang, Y.; Yu, R. Bex2 regulates cell proliferation and apoptosis in malignant glioma cells via the c-Jun NH2-terminal kinase pathway. Biochem. Biophys. Res. Commun. 2012, 427, 574–580. [Google Scholar] [CrossRef]
- Alvarez, E.; Zhou, W.; Witta, S.E.; Freed, C.R. Characterization of the Bex gene family in humans, mice, and rats. Gene 2005, 357, 18–28. [Google Scholar] [CrossRef]
- Mashhadikhan, M.; Kheiri, H.; Dehghanifard, A. DNA methylation and gene expression of sFRP2, sFRP4, Dkk 1, and Wif1 during osteoblastic differentiation of bone marrow derived mesenchymal stem cells. J. Oral Biosci. 2020, 62, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cao, Y.; Yu, G.; Wang, J.; Lin, X.; Ge, L.; Du, J.; Wang, L.; Diao, S.; Lian, X.; et al. SFRP2 enhances the osteogenic differentiation of apical papilla stem cells by antagonizing the canonical WNT pathway. Cell. Mol. Biol. Lett. 2017, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Pomduk, K.; Kheolamai, P.; U-Pratya, Y.; Wattanapanitch, M.; Klincumhom, N.; Issaragrisil, S. Enhanced human mesenchymal stem cell survival under oxidative stress by overexpression of secreted frizzled-related protein 2 gene. Ann. Hematol. 2015, 94, 319–327. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, N.; Xu, X.; Xu, Y.; Li, S.; Zhang, J.; Yang, P. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011, 44, 420. [Google Scholar] [CrossRef] [PubMed]
- Marupanthorn, K.; Tantrawatpan, C.; Tantikanlayaporn, D.; Kheolamai, P.; Manochantr, S. The Effects of TNF-α on Osteogenic Differentiation of Umbilical Cord Derived Mesenchymal Stem Cells. J. Med. Assoc. Thai. 2015, 98 (Suppl. 3), S34–S40. [Google Scholar]
- Heidenreich, S.; Schmidt, M.; August, C.; Cullen, P.; Rademaekers, A.; Pauels, H. Regulation of human monocyte apoptosis by the CD14 molecule. J. Immunol. 1997, 159, 3178–3188. [Google Scholar] [CrossRef]
- Li, K.; Dan, Z.; Hu, X.; Ouzhu, M.; Ciren, Y.; Wang, Z.; Wang, J.; Yang, X.; Ze, Y. CD14 overexpression upregulates TNF-α-mediated inflammatory responses and suppresses the malignancy of gastric carcinoma cells. Mol. Cell. Biochem. 2013, 376, 137. [Google Scholar] [CrossRef]
- Lee, M.-S.; Wang, J.; Yuan, H.; Jiao, H.; Tsai, T.-L.; Squire, M.W.; Li, W.-J. Endothelin-1 differentially directs lineage specification of adipose- and bone marrow–derived mesenchymal stem cells. FASEB J. 2019, 33, 996. [Google Scholar] [CrossRef]
- Rucinski, M.; Zok, A.; Guidolin, D.; de Caro, R.; Malendowicz, L.K. Expression of precerebellins in cultured rat calvaria osteoblast-like cells. Int. J. Mol. Med. 2008, 22, 553–558. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Fresno, C.; Fernández, E.A. RDAVIDWebService: A versatile R interface to DAVID. Bioinformatics 2013, 29, 2810–2811. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Cohen, R. Weighted false discovery rate controlling procedures for clinical trials. Biostatistics 2017, 18, 91–104. [Google Scholar] [CrossRef]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef]
- Goesmann, A.; Haubrock, M.; Meyer, F.; Kalinowski, J.; Giegerich, R. PathFinder: Reconstruction and dynamic visualization of metabolic pathways. Bioinformatics 2002, 18, 124–129. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefańska, K.; Nemcova, L.; Blatkiewicz, M.; Pieńkowski, W.; Ruciński, M.; Zabel, M.; Mozdziak, P.; Podhorska-Okołów, M.; Dzięgiel, P.; Kempisty, B. Apoptosis Related Human Wharton’s Jelly-Derived Stem Cells Differentiation into Osteoblasts, Chondrocytes, Adipocytes and Neural-like Cells—Complete Transcriptomic Assays. Int. J. Mol. Sci. 2023, 24, 10023. https://doi.org/10.3390/ijms241210023
Stefańska K, Nemcova L, Blatkiewicz M, Pieńkowski W, Ruciński M, Zabel M, Mozdziak P, Podhorska-Okołów M, Dzięgiel P, Kempisty B. Apoptosis Related Human Wharton’s Jelly-Derived Stem Cells Differentiation into Osteoblasts, Chondrocytes, Adipocytes and Neural-like Cells—Complete Transcriptomic Assays. International Journal of Molecular Sciences. 2023; 24(12):10023. https://doi.org/10.3390/ijms241210023
Chicago/Turabian StyleStefańska, Katarzyna, Lucie Nemcova, Małgorzata Blatkiewicz, Wojciech Pieńkowski, Marcin Ruciński, Maciej Zabel, Paul Mozdziak, Marzenna Podhorska-Okołów, Piotr Dzięgiel, and Bartosz Kempisty. 2023. "Apoptosis Related Human Wharton’s Jelly-Derived Stem Cells Differentiation into Osteoblasts, Chondrocytes, Adipocytes and Neural-like Cells—Complete Transcriptomic Assays" International Journal of Molecular Sciences 24, no. 12: 10023. https://doi.org/10.3390/ijms241210023
APA StyleStefańska, K., Nemcova, L., Blatkiewicz, M., Pieńkowski, W., Ruciński, M., Zabel, M., Mozdziak, P., Podhorska-Okołów, M., Dzięgiel, P., & Kempisty, B. (2023). Apoptosis Related Human Wharton’s Jelly-Derived Stem Cells Differentiation into Osteoblasts, Chondrocytes, Adipocytes and Neural-like Cells—Complete Transcriptomic Assays. International Journal of Molecular Sciences, 24(12), 10023. https://doi.org/10.3390/ijms241210023







