Improved Workflow for Analysis of Vascular Myocyte Time-Series and Line-Scan Ca2+ Imaging Datasets
Abstract
1. Introduction
2. Results
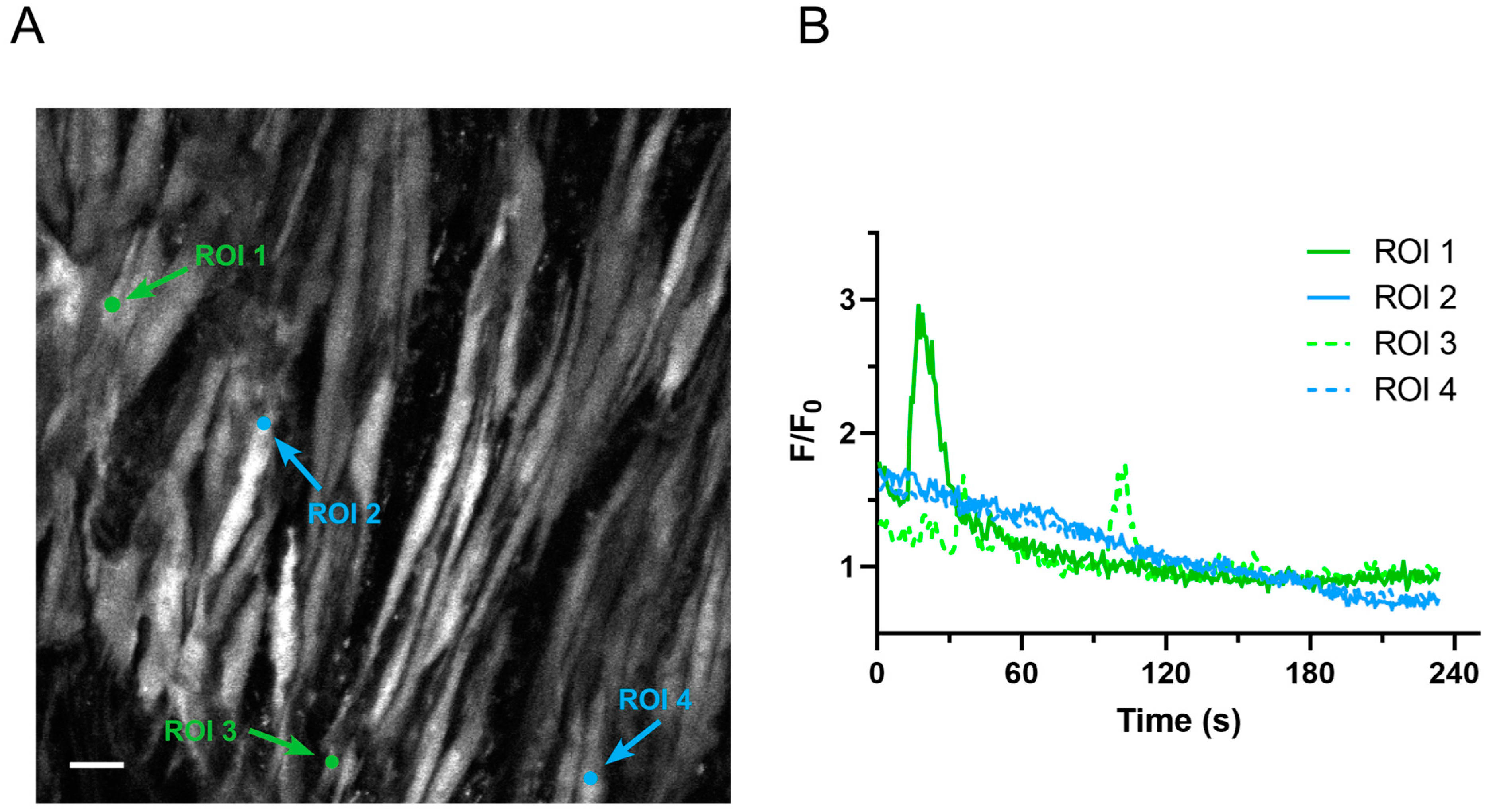
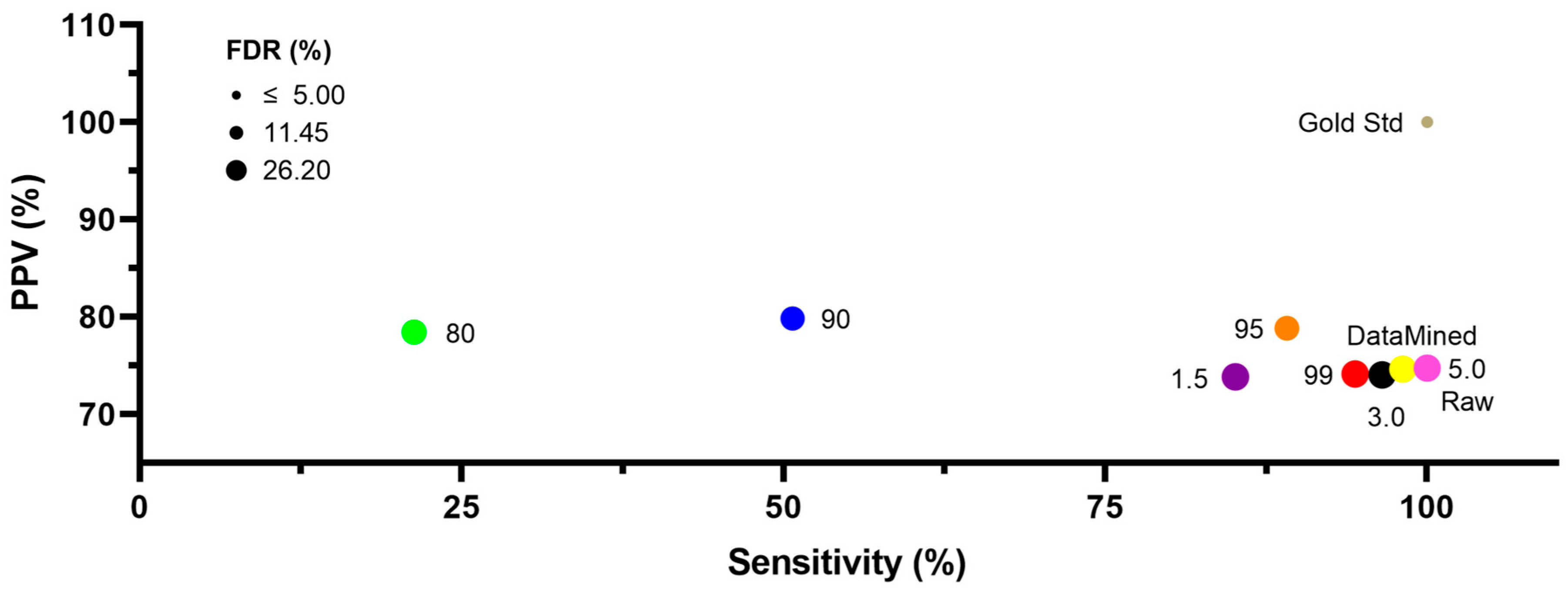
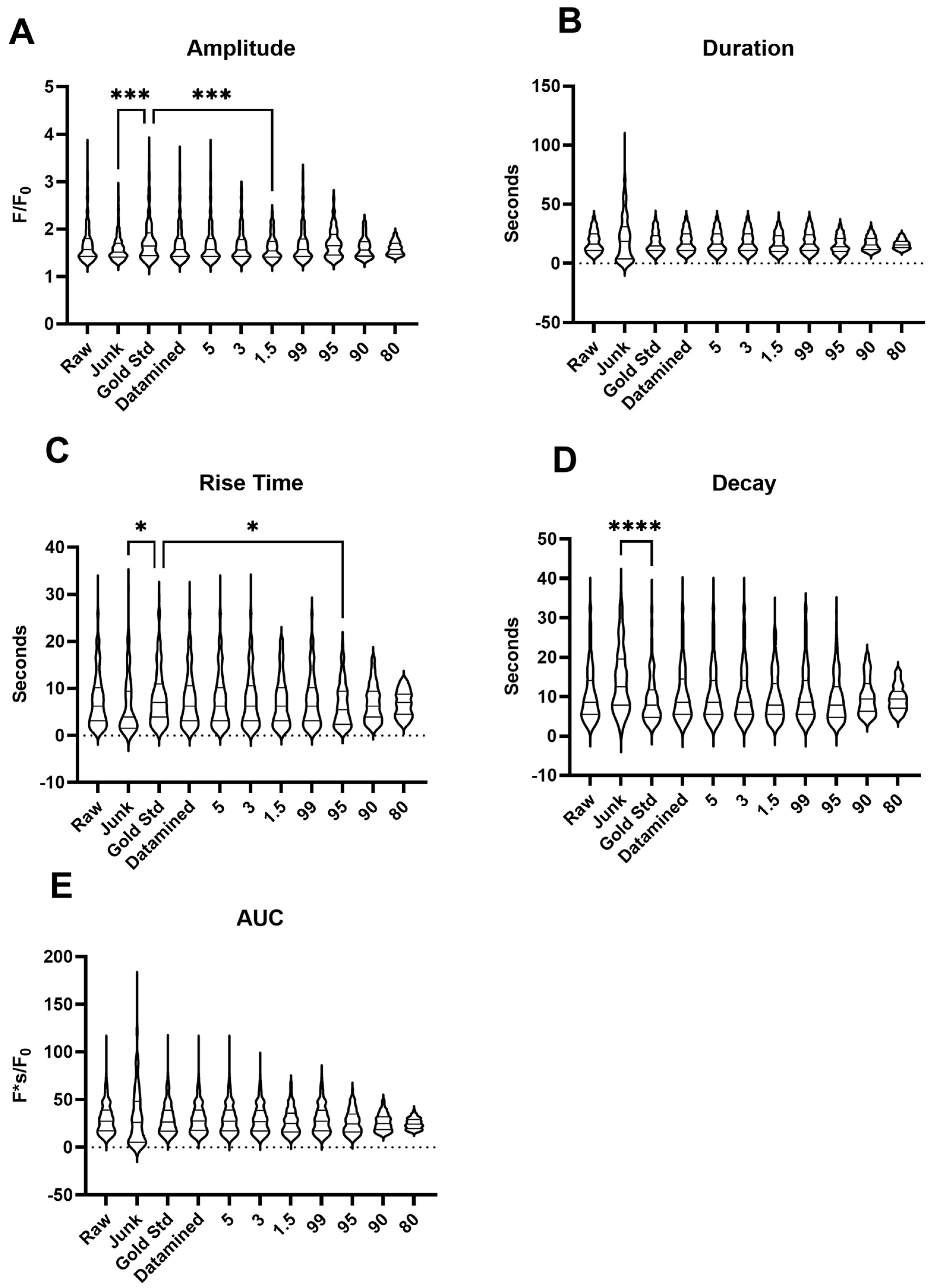
2.1. Ca2+ Oscillations
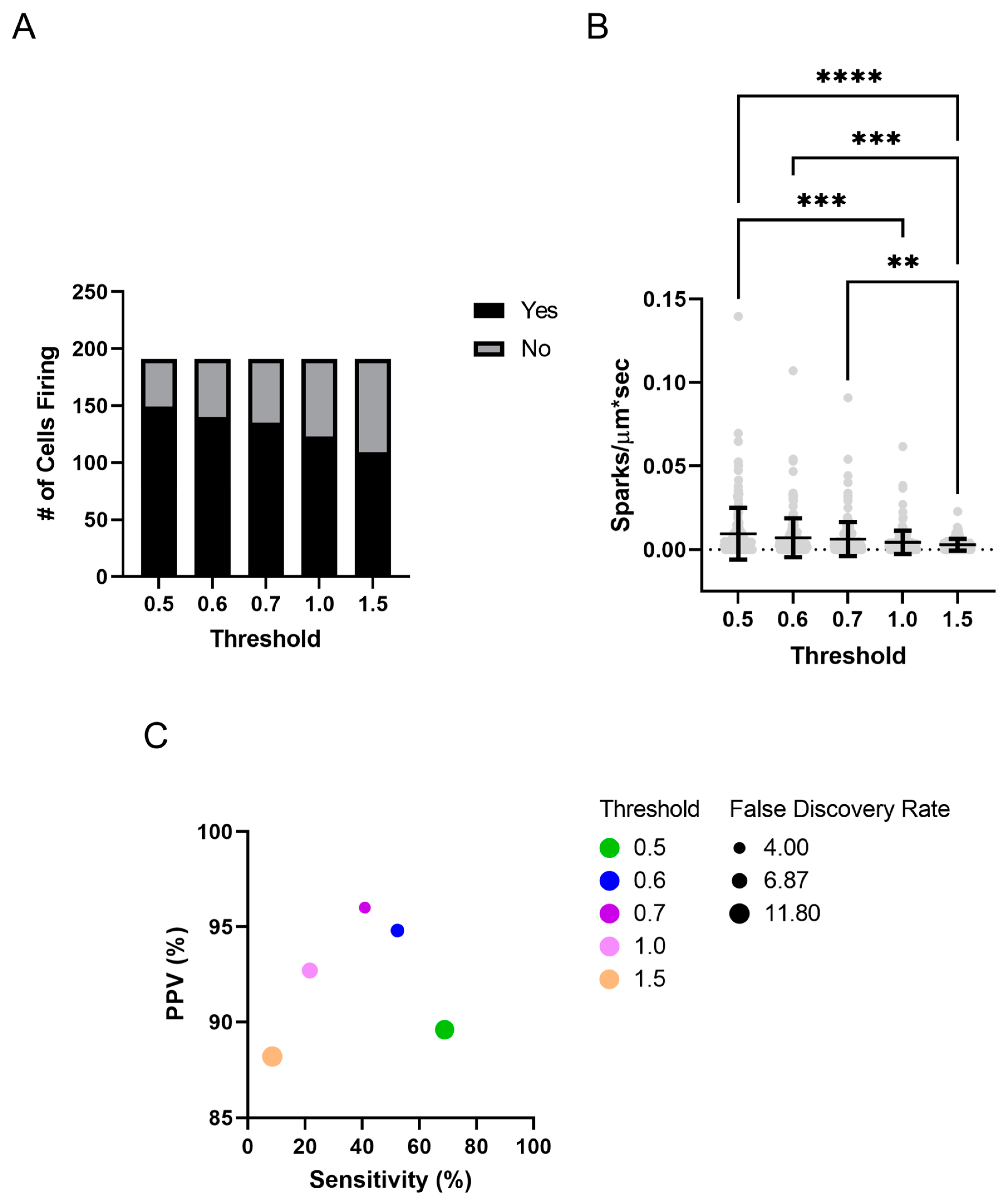
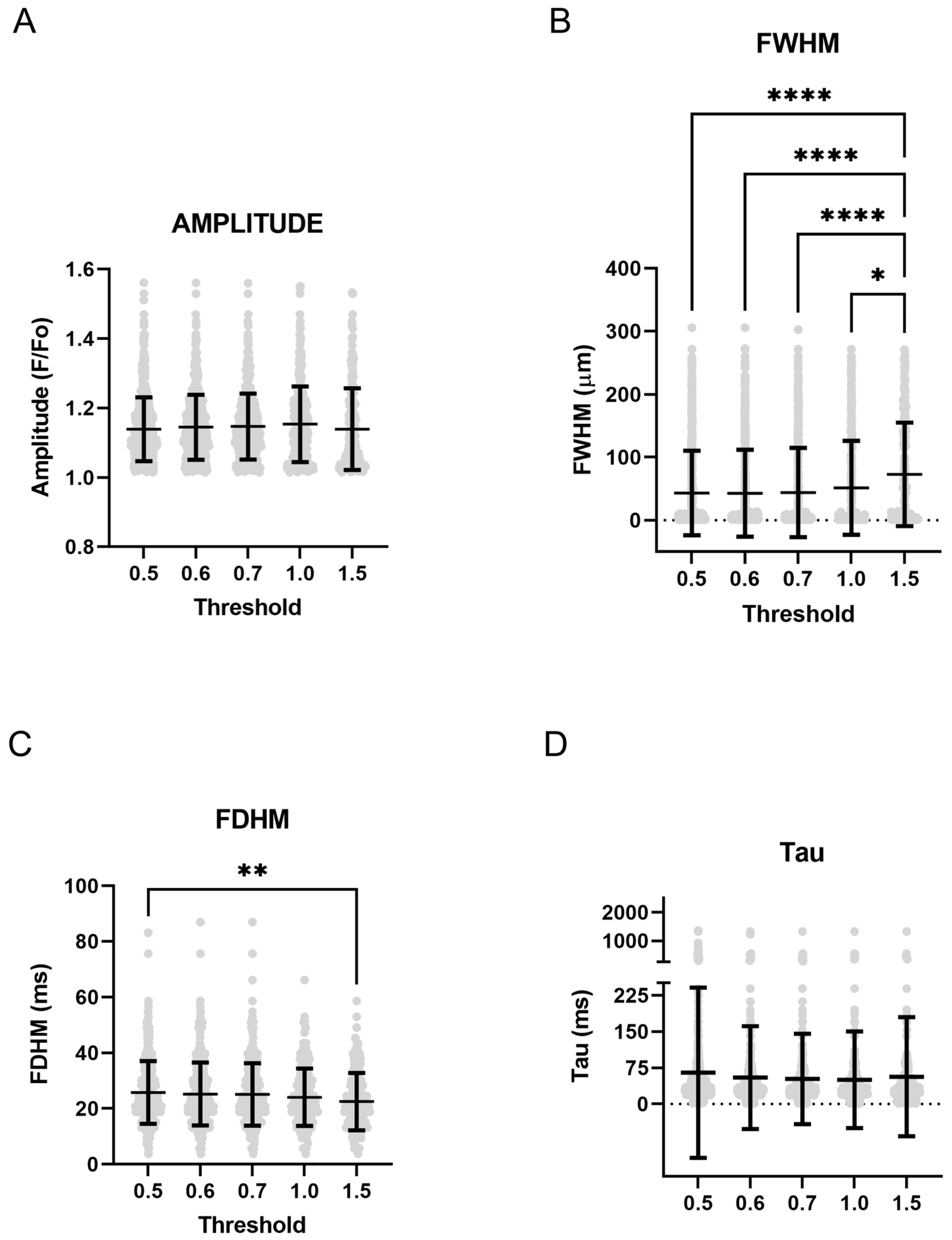
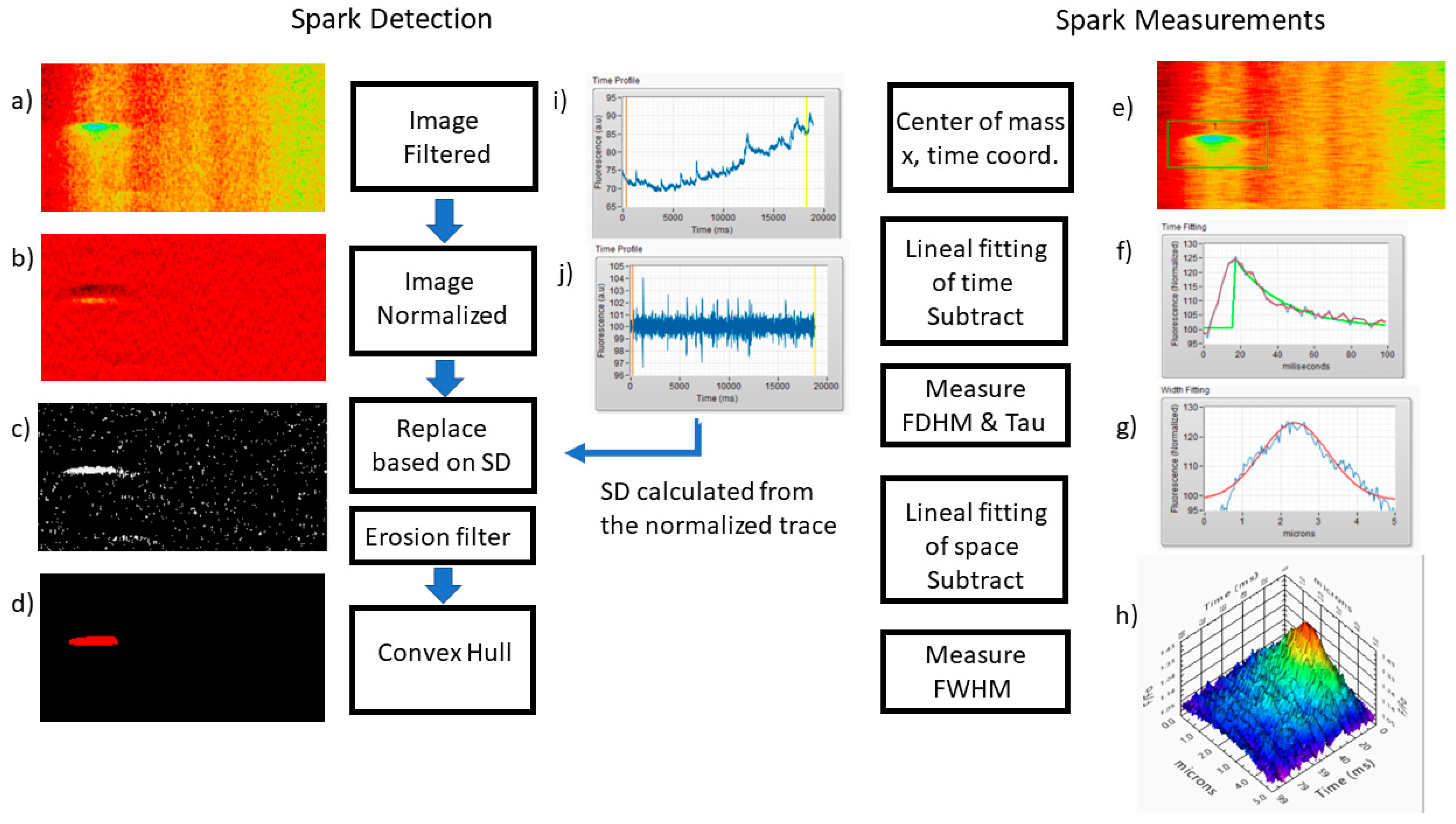
2.2. Ca2+ Sparks
3. Discussion
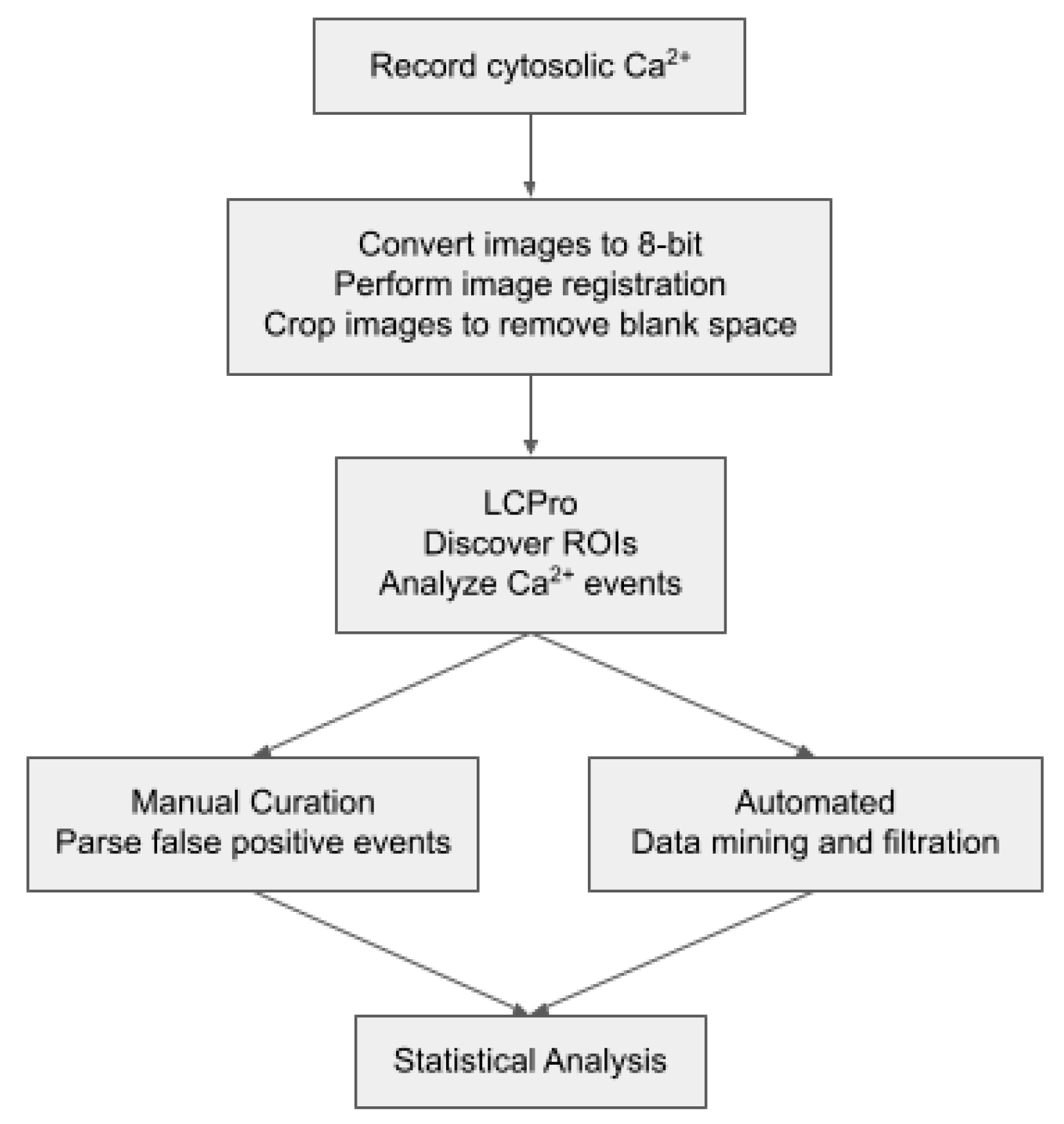
4. Materials and Methods
4.1. Confocal Microscopy Studies
4.2. Ca2+ Oscillatory Signal Analysis
4.3. Line Scan Analysis
4.4. Chemicals Reagents and Drugs
4.5. Statistical Methods and Sampling
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williams, D.A.; Fogarty, K.E.; Tsien, R.Y.; Fay, F.S. Calcium Gradients in Single Smooth Muscle Cells Revealed by the Digital Imaging Microscope Using Fura-2. Nature 1985, 318, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.Y.; Rink, T.J.; Poenie, M. Measurement of Cytosolic Free Ca2+ in Individual Small Cells Using Fluorescence Microscopy with Dual Excitation Wavelengths. Cell Calcium 1985, 6, 145–157. [Google Scholar] [CrossRef]
- Minta, A.; Kao, J.P.; Tsien, R.Y. Fluorescent Indicators for Cytosolic Calcium Based on Rhodamine and Fluorescein Chromophores. J. Biol. Chem. 1989, 264, 8171–8178. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.E.; Jin, C.; Reed, J.C.; Tsien, R.Y. Bcl-2-Mediated Alterations in Endoplasmic Reticulum Ca2+ Analyzed with an Improved Genetically Encoded Fluorescent Sensor. Proc. Natl. Acad. Sci. USA 2004, 101, 17404–17409. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.; Lopez, V.; Anti, N.A.O.; Alvarez, R.; Kafeero, I.; Welsh, D.G.; Romero, M.; Kaushal, S.; Johnson, C.M.; Bosviel, R.; et al. Gestational Long-Term Hypoxia Induces Metabolomic Reprogramming and Phenotypic Transformations in Fetal Sheep Pulmonary Arteries. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 320, L770–L784. [Google Scholar] [CrossRef]
- Blum-Johnston, C.; Thorpe, R.B.; Wee, C.; Opsahl, R.; Romero, M.; Murray, S.; Brunelle, A.; Blood, Q.; Wilson, R.; Blood, A.B.; et al. Long-Term Hypoxia Uncouples Ca2+ and ENOS in Bradykinin-Mediated Pulmonary Arterial Relaxation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R870–R882. [Google Scholar] [CrossRef]
- Shen, C.P.; Romero, M.; Brunelle, A.; Wolfe, C.; Dobyns, A.; Francis, M.; Taylor, M.S.; Puglisi, J.L.; Longo, L.D.; Zhang, L.; et al. Long-Term High-Altitude Hypoxia Influences Pulmonary Arterial L-Type Calcium Channel-Mediated Ca2+ Signals and Contraction in Fetal and Adult Sheep. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R433–R446. [Google Scholar] [CrossRef]
- Wilson, S.M.; Pappone, P.A. P2 Receptor Modulation of Voltage-Gated Potassium Currents in Brown Adipocytes. J. Gen. Physiol. 1999, 113, 125–138. [Google Scholar] [CrossRef]
- del Corsso, C.; Ostrovskaya, O.; McAllister, C.E.; Murray, K.; Hatton, W.J.; Gurney, A.M.; Spencer, N.J.; Wilson, S.M. Effects of Aging on Ca2+ Signaling in Murine Mesenteric Arterial Smooth Muscle Cells. Mech. Ageing Dev. 2006, 127, 315–323. [Google Scholar] [CrossRef]
- Goyal, R.; Creel, K.D.; Chavis, E.; Smith, G.D.; Longo, L.D.; Wilson, S.M. Maturation of Intracellular Calcium Homeostasis in Sheep Pulmonary Arterial Smooth Muscle Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L905–L914. [Google Scholar] [CrossRef]
- Janiak, R.; Wilson, S.M.; Montague, S.; Hume, J.R. Heterogeneity of Calcium Stores and Elementary Release Events in Canine Pulmonary Arterial Smooth Muscle Cells. Am. J. Physiol. Cell Physiol. 2001, 280, C22–C33. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Song, R.; Romero, M.; Dasgupta, C.; Min, J.; Hatcher, D.; Xiao, D.; Blood, A.; Wilson, S.M.; Zhang, L. Gestational Hypoxia Inhibits Pregnancy-Induced Upregulation of Ca2+ Sparks and Spontaneous Transient Outward Currents in Uterine Arteries Via Heightened Endoplasmic Reticulum/Oxidative Stress. Hypertension 2020, 76, 930–942. [Google Scholar] [CrossRef]
- Harraz, O.F.; Brett, S.E.; Zechariah, A.; Romero, M.; Puglisi, J.L.; Wilson, S.M.; Welsh, D.G. Genetic Ablation of CaV3.2 Channels Enhances the Arterial Myogenic Response by Modulating the RyR-BKCa Axis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1843–1851. [Google Scholar] [CrossRef]
- Reid, C.; Romero, M.; Chang, S.B.; Osman, N.; Puglisi, J.L.; Wilson, C.G.; Blood, A.B.; Zhang, L.; Wilson, S.M. Long-Term Hypoxia Negatively Influences Ca2+ Signaling in Basilar Arterial Myocytes of Fetal and Adult Sheep. Front. Physiol. 2021, 12, 760176. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.; Qian, X.; Charbel, C.; Ledoux, J.; Parker, J.C.; Taylor, M.S. Automated Region of Interest Analysis of Dynamic Ca2+ Signals in Image Sequences. Am. J. Physiol. Cell Physiol. 2012, 303, C236–C243. [Google Scholar] [CrossRef] [PubMed]
- Blum-Johnston, C.; Thorpe, R.B.; Wee, C.; Romero, M.; Brunelle, A.; Blood, Q.; Wilson, R.; Blood, A.B.; Francis, M.; Taylor, M.S.; et al. Developmental Acceleration of Bradykinin-Dependent Relaxation by Prenatal Chronic Hypoxia Impedes Normal Development after Birth. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L271–L286. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and Current Challenges in Calcium Signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Puri, B.K. Calcium Signaling and Gene Expression. Adv. Exp. Med. Biol. 2020, 1131, 537–545. [Google Scholar]
- Clapham, D.E. Calcium Signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Kaifosh, P.; Zaremba, J.D.; Danielson, N.B.; Losonczy, A. SIMA: Python Software for Analysis of Dynamic Fluorescence Imaging Data. Front. Neuroinform. 2014, 8, 80. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Wu, J.; Zhang, Y.; Zhao, Z.; Lin, X.; Qiao, H.; Xie, H.; Wang, H.; Fang, L.; et al. Reinforcing Neuron Extraction and Spike Inference in Calcium Imaging Using Deep Self-Supervised Denoising. Nat. Methods 2021, 18, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Sità, L.; Brondi, M.; Lagomarsino de Leon Roig, P.; Curreli, S.; Panniello, M.; Vecchia, D.; Fellin, T. A Deep-Learning Approach for Online Cell Identification and Trace Extraction in Functional Two-Photon Calcium Imaging. Nat. Commun. 2022, 13, 1529. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, A.; Friedrich, J.; Gunn, P.; Kalfon, J.; Brown, B.L.; Koay, S.A.; Taxidis, J.; Najafi, F.; Gauthier, J.L.; Zhou, P.; et al. CaImAn an Open Source Tool for Scalable Calcium Imaging Data Analysis. Elife 2019, 8, e38173. [Google Scholar] [CrossRef] [PubMed]
- Knighten, J.M.; Aziz, T.; Pleshinger, D.J.; Annamdevula, N.; Rich, T.C.; Taylor, M.S.; Andrews, J.F.; Macarilla, C.T.; Francis, C.M. Algorithm for Biological Second Messenger Analysis with Dynamic Regions of Interest. PLoS ONE 2023, 18, e0284394. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, G.; Ascoli, G.A.; Zhou, J.; Liu, L. Neuron Tracing from Light Microscopy Images: Automation, Deep Learning and Bench Testing. Bioinformatics 2022, 38, 5329–5339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, G.; Han, X.; Wu, J.; Li, Z.; Li, X.; Xiao, G.; Xie, H.; Fang, L.; Dai, Q. Rapid Detection of Neurons in Widefield Calcium Imaging Datasets after Training with Synthetic Data. Nat. Methods 2023, 20, 747–754. [Google Scholar] [CrossRef]
- Alzoubi, I.; Bao, G.; Zhang, R.; Loh, C.; Zheng, Y.; Cherepanoff, S.; Gracie, G.; Lee, M.; Kuligowski, M.; Alexander, K.L.; et al. An Open-Source AI Framework for the Analysis of Single Cells in Whole-Slide Images with a Note on CD276 in Glioblastoma. Cancers 2022, 14, 3441. [Google Scholar] [CrossRef]
- Prasad, L.; Sitharama Iyengar, S. Wavelet Analysis with Applications to Image Processing; CRC Press: Boca Raton, FL, USA, 2020; ISBN 9781000714166. [Google Scholar]
- Meyer, F.; Beucher, S. Morphological Segmentation. J. Vis. Commun. Image Represent. 1990, 1, 21–46. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, M.; Wu, G.; Yao, C.; Zhang, J. Recent Advances in Morphological Cell Image Analysis. Comput. Math. Methods Med. 2012, 2012, 101536. [Google Scholar] [CrossRef]
- Tada, M.; Takeuchi, A.; Hashizume, M.; Kitamura, K.; Kano, M. A Highly Sensitive Fluorescent Indicator Dye for Calcium Imaging of Neural Activity in Vitro and in Vivo. Eur. J. Neurosci. 2014, 39, 1720–1728. [Google Scholar] [CrossRef]
- Akerboom, J.; Carreras Calderón, N.; Tian, L.; Wabnig, S.; Prigge, M.; Tolö, J.; Gordus, A.; Orger, M.B.; Severi, K.E.; Macklin, J.J.; et al. Genetically Encoded Calcium Indicators for Multi-Color Neural Activity Imaging and Combination with Optogenetics. Front. Mol. Neurosci. 2013, 6, 2. [Google Scholar] [CrossRef]
- Lock, J.T.; Parker, I.; Smith, I.F. A Comparison of Fluorescent Ca2+ Indicators for Imaging Local Ca2+ Signals in Cultured Cells. Cell Calcium 2015, 58, 638–648. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Poenie, M.; Tsien, R.Y. A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J. Biol. Chem. 1985, 260, 3440–3450. [Google Scholar] [CrossRef]
- Hyrc, K.L.; Minta, A.; Escamilla, P.R.; Chan, P.P.L.; Meshik, X.A.; Goldberg, M.P. Synthesis and Properties of Asante Calcium Red--a Novel Family of Long Excitation Wavelength Calcium Indicators. Cell Calcium 2013, 54, 320–333. [Google Scholar] [CrossRef]
- Giang, M.; Papamatheakis, D.G.; Nguyen, D.; Paez, R.; Blum Johnston, C.; Kim, J.; Brunnell, A.; Blood, Q.; Goyal, R.; Longo, L.D.; et al. Muscarinic Receptor Activation Affects Pulmonary Artery Contractility in Sheep: The Impact of Maturation and Chronic Hypoxia on Endothelium-Dependent and Endothelium-Independent Function. High Alt. Med. Biol. 2016, 17, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Hadley, S.R.; Blood, Q.; Rubalcava, M.; Waskel, E.; Lumbard, B.; Le, P.; Longo, L.D.; Buchholz, J.N.; Wilson, S.M. Maternal High-Altitude Hypoxia and Suppression of Ryanodine Receptor-Mediated Ca2+ Sparks in Fetal Sheep Pulmonary Arterial Myocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, L799–L813. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Q.; Dasgupta, C.; Song, R.; Romero, M.; Wilson, S.M.; Zhang, L. MicroRNA-210 Mediates Hypoxia-Induced Repression of Spontaneous Transient Outward Currents in Sheep Uterine Arteries During Gestation. Hypertension 2021, 77, 1412–1427. [Google Scholar] [CrossRef] [PubMed]
- National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research, and Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Guide for the Care and Use of Laboratory Animals: Eighth Edition; National Academies Press, 2011. Available online: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf (accessed on 1 June 2023).

| LC Pro | |
| Strengths | Limitations |
|
|
| Recommendation | |
| |
| Sparklab 5.8 | |
| Strengths | Limitations |
|
|
| Recommendation | |
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boskind, M.; Nelapudi, N.; Williamson, G.; Mendez, B.; Juarez, R.; Zhang, L.; Blood, A.B.; Wilson, C.G.; Puglisi, J.L.; Wilson, S.M. Improved Workflow for Analysis of Vascular Myocyte Time-Series and Line-Scan Ca2+ Imaging Datasets. Int. J. Mol. Sci. 2023, 24, 9729. https://doi.org/10.3390/ijms24119729
Boskind M, Nelapudi N, Williamson G, Mendez B, Juarez R, Zhang L, Blood AB, Wilson CG, Puglisi JL, Wilson SM. Improved Workflow for Analysis of Vascular Myocyte Time-Series and Line-Scan Ca2+ Imaging Datasets. International Journal of Molecular Sciences. 2023; 24(11):9729. https://doi.org/10.3390/ijms24119729
Chicago/Turabian StyleBoskind, Madison, Nikitha Nelapudi, Grace Williamson, Bobby Mendez, Rucha Juarez, Lubo Zhang, Arlin B. Blood, Christopher G. Wilson, Jose Luis Puglisi, and Sean M. Wilson. 2023. "Improved Workflow for Analysis of Vascular Myocyte Time-Series and Line-Scan Ca2+ Imaging Datasets" International Journal of Molecular Sciences 24, no. 11: 9729. https://doi.org/10.3390/ijms24119729
APA StyleBoskind, M., Nelapudi, N., Williamson, G., Mendez, B., Juarez, R., Zhang, L., Blood, A. B., Wilson, C. G., Puglisi, J. L., & Wilson, S. M. (2023). Improved Workflow for Analysis of Vascular Myocyte Time-Series and Line-Scan Ca2+ Imaging Datasets. International Journal of Molecular Sciences, 24(11), 9729. https://doi.org/10.3390/ijms24119729







