A Bioactive Compound-Loaded Zinc-Aminoclay Encapsulated, Pickering Emulsion System for Treating Acne-Inducing Microbes
Abstract
1. Introduction
2. Results and Discussion
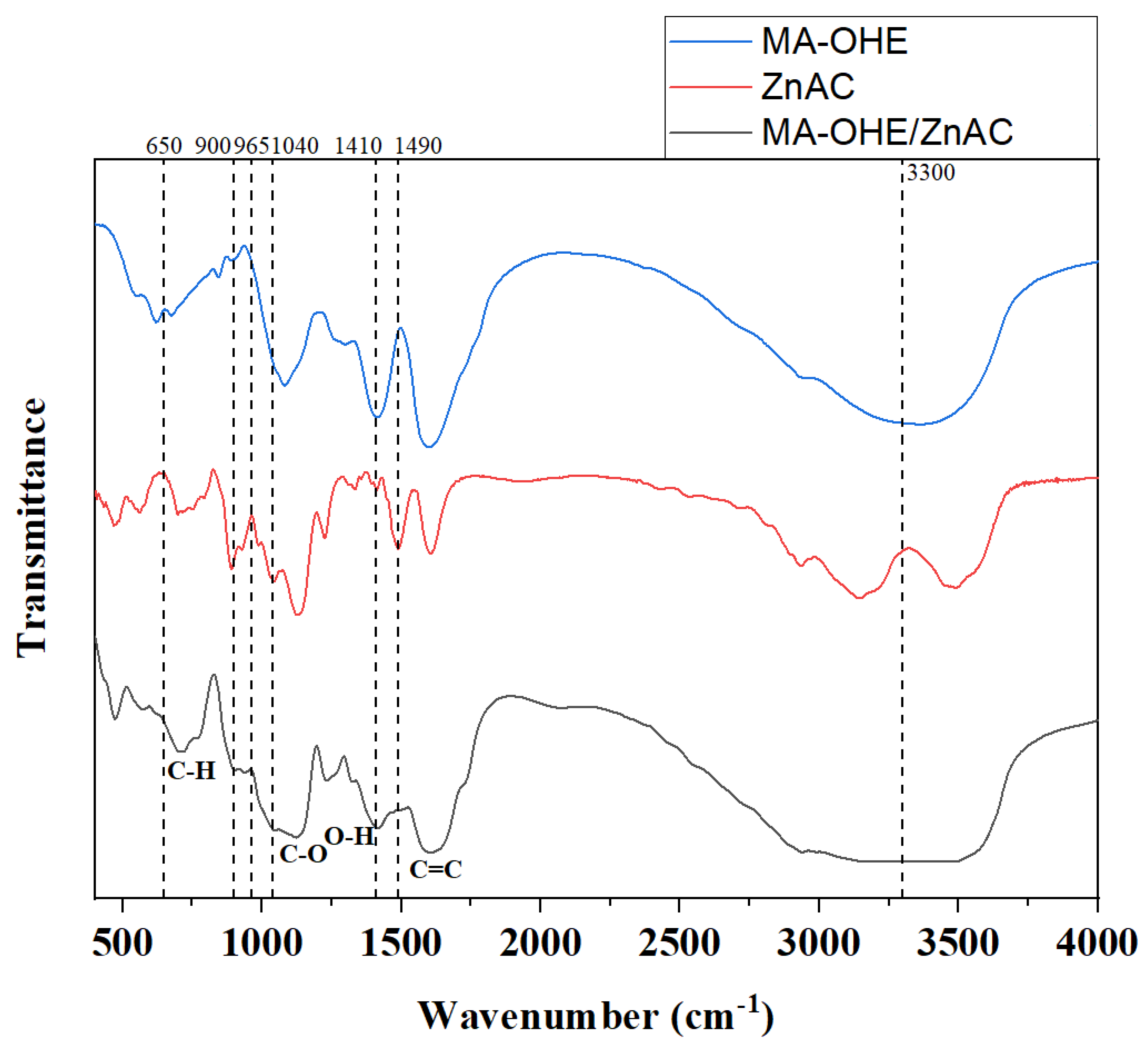
2.1. Preparation of MA-OHE/ZnAC
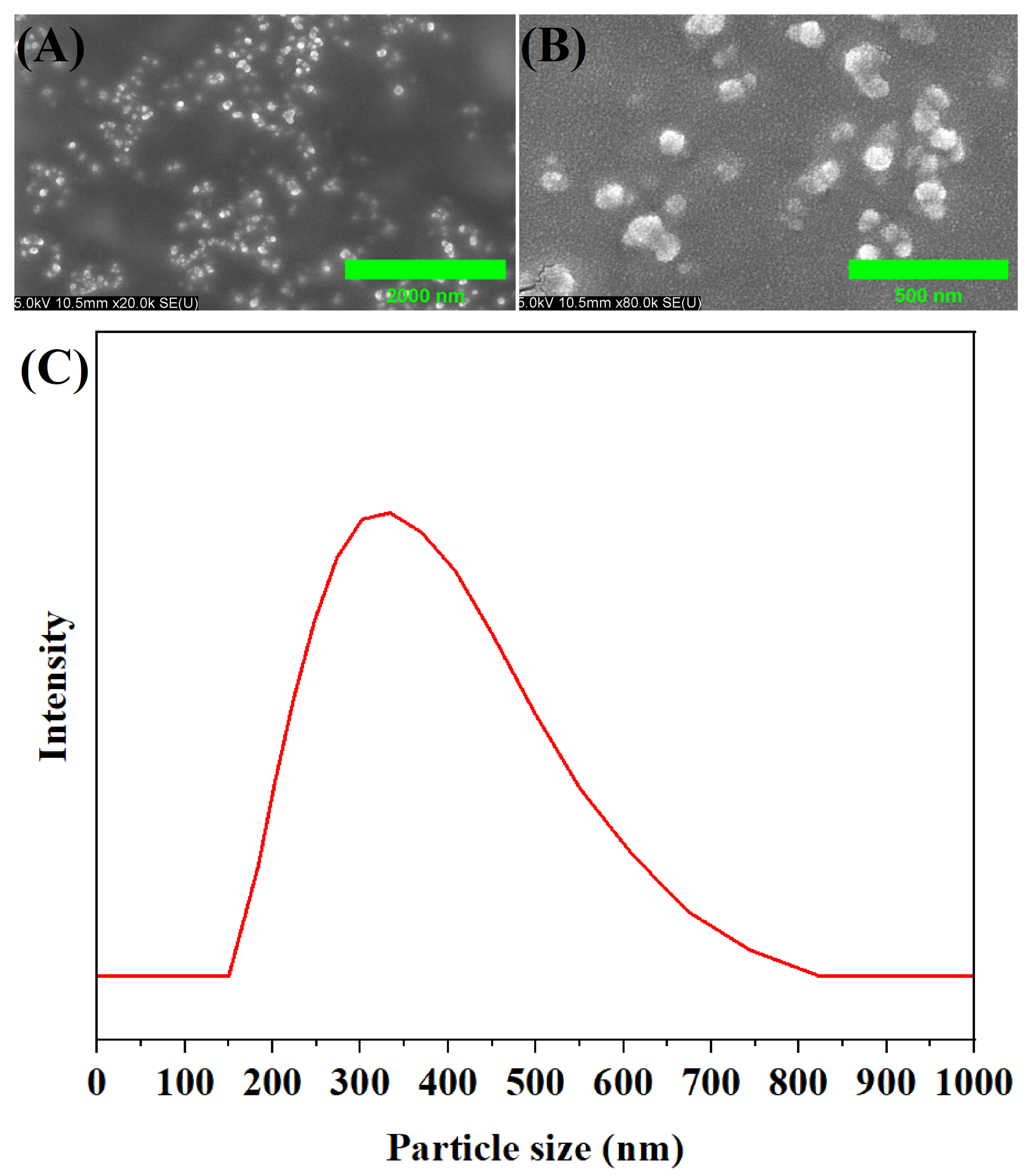
2.2. Morphological Structure of MA-OHE/ZnAC PE
2.3. Bioactive Compound Entrapment Efficiency
2.4. In Vitro Bioactive Compound Dissolution Test
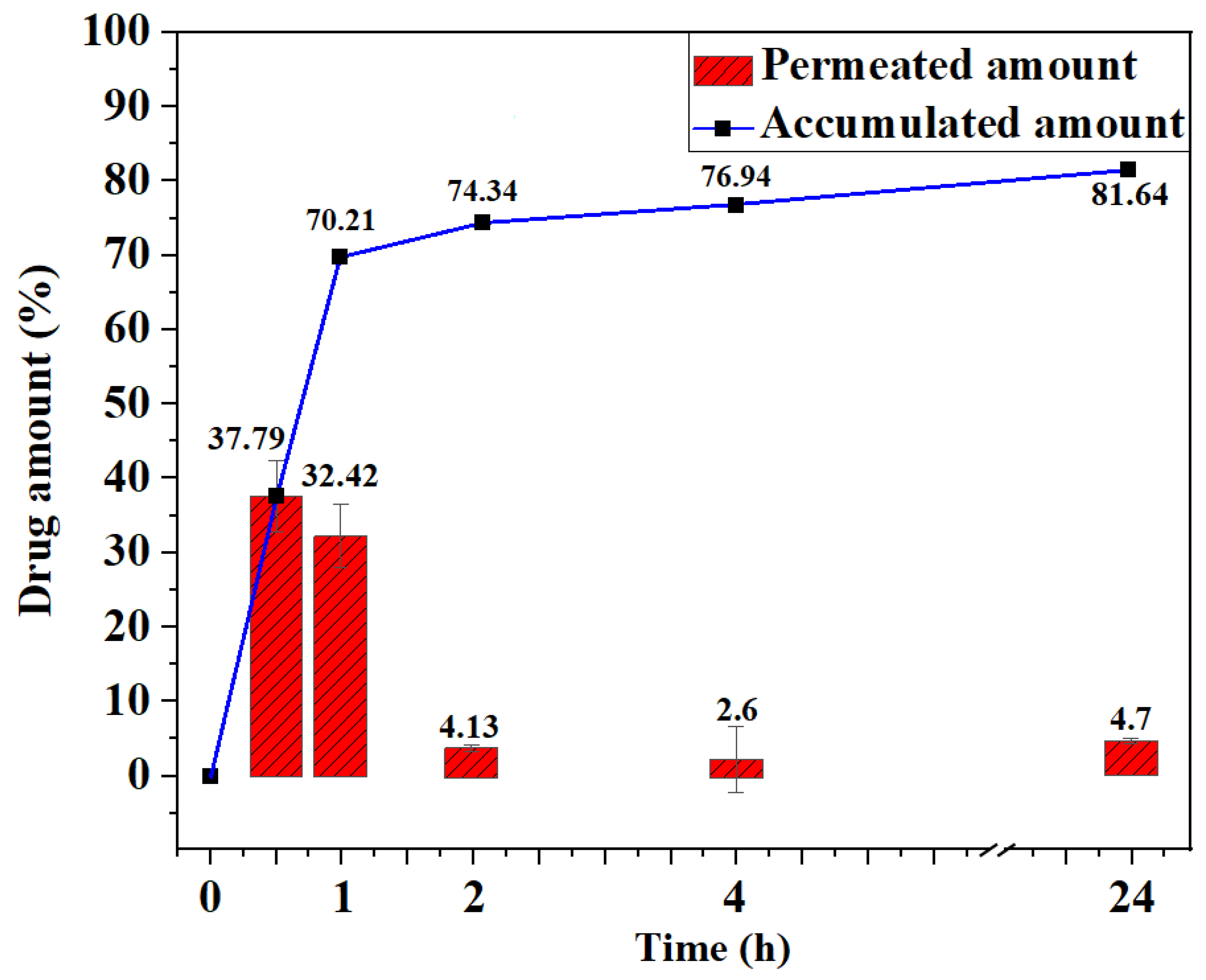
2.5. In Vitro Drug Permeation Kinetics
2.6. Antimicrobial Susceptibility Test
2.7. In Vitro Cell Viability Assay
3. Materials and Methods
3.1. Materials and Reagents
3.2. Formulation of MA-OHE Loaded Emulsion
3.2.1. Microwave-Assisted Extraction of O. humifusa (MA-OHE)
3.2.2. Synthesis of ZnAC and MA-OHE/ZnAC
3.2.3. Preparation of MA-OHE/ZnAC PE
3.3. Morphological Structure of MA-OHE/ZnAC PE
3.4. Bioactive Compound Entrapment Efficiency
3.5. In Vitro Bioactive Compound Dissolution Kinetic Analysis
3.6. In Vitro Drug Permeation Kinetics
3.7. Antimicrobial Susceptibility Test
3.7.1. Bacteria Culture
3.7.2. Determination of Minimum Inhibition Concentration (MIC)
3.8. In Vitro Cell Viability Test
3.8.1. Cell Culture
3.8.2. WST Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bojar, R.A.; Holland, K.T. Acne and propionibacterium acnes. Clin. Dermatol. 2004, 22, 375–379. [Google Scholar] [CrossRef]
- Chiller, K.; Selkin, B.A.; Murakawa, G.J. Skin microflora and bacterial infections of the skin. J. Investig. Dermatol. Symp. Proc. 2001, 6, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, M.; Kupperman, E.; Lautenbach, E.; Edelstein, P.H.; Margolis, D.J. Antibiotics, Acne, and Staphylococcus aureus Colonization. Arch. Dermatol. 2011, 147, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Kim, M.-G.; Lee, K.-Y. Antimicrobial effect of the extracts of cactus Chounnyouncho (Opuntia humifusa) against food borne pathogens. J. Korean Soc. Food. Sci. Nutr. 2004, 33, 1268–1272. [Google Scholar]
- Cha, M.-N.; Jun, H.-I.; Lee, W.-J.; Kim, M.-J.; Kim, M.-K.; Kim, Y.-S. Chemical composition and antioxidant activity of Korean cactus (Opuntia humifusa) fruit. Food Sci. Biotechnol. 2013, 22, 523–529. [Google Scholar] [CrossRef]
- Park, K.; Choi, H.-S.; Hong, Y.H.; Jung, E.Y.; Suh, H.J. Cactus cladodes (Opuntia humifusa) extract minimizes the effects of UV irradiation on keratinocytes and hairless mice. Pharm. Biol. 2017, 55, 1032–1040. [Google Scholar] [CrossRef]
- Moon, J.-Y.; Ngoc, L.T.N.; Chae, M.; Tran, V.V.; Lee, Y.-C. Effects of microwave-assisted Opuntia humifusa extract in inhibiting the impacts of particulate matter on human keratinocyte skin cell. Antioxidants 2020, 9, 271. [Google Scholar] [CrossRef]
- Moradi Alvand, Z.; Rajabi, H.R.; Mirzaei, A.; Masoumiasl, A. Ultrasonic and microwave assisted extraction as rapid and efficient techniques for plant mediated synthesis of quantum dots: Green synthesis, characterization of zinc telluride and comparison study of some biological activities. New J. Chem. 2019, 43, 15126–15138. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Kim, S.-H.; Bae, I.-S.; Lee, H.U.; Moon, J.-Y.; Lee, Y.-C. Microwave-assisted Opuntia humifusa extract containing multifunctional antioxidant carbon nanodots. Carbon Lett. 2023, 1–10. [Google Scholar] [CrossRef]
- Troxler, R.; von Graevenitz, A.; Funke, G.; Wiedemann, B.; Stock, I. Natural antibiotic susceptibility of Listeria species: L. grayi, L. innocua, L. ivanovii, L. monocytogenes, L. seeligeri and L. welshimeri strains. Clin. Microbiol. Infect. 2000, 6, 525–535. [Google Scholar] [CrossRef]
- Machado, D.G.; Bettio, L.E.B.; Cunha, M.P.; Capra, J.C.; Dalmarco, J.B.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: Involvement of the monoaminergic system. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 642–650. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Babakir-Mina, M. Investigation of rosemary herbal extracts (Rosmarinus officinalis) and their potential effects on immunity. Phytother. Res. 2020, 34, 1829–1837. [Google Scholar] [CrossRef]
- Vora, J.; Srivastava, A.; Modi, H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform. Med. Unlocked 2018, 13, 128–132. [Google Scholar] [CrossRef]
- Rather, L.J.; Shahid ul, I.; Mohammad, F. Acacia nilotica (L.): A review of its traditional uses, phytochemistry, and pharmacology. Sustain. Chem. Pharm. 2015, 2, 12–30. [Google Scholar] [CrossRef]
- Tewari, D.; Pandey, H.K.; Sah, A.N.; Meena, H.; Chander, V.; Singh, R.; Singh, P. Phytochemical, Antioxidant and Antidepressant Evaluation of Ocimum basilicum, O. tenuiflorum, O. kilimandscharicum Grown in India. J. Biol. Act. Prod. Nat. 2015, 5, 120–131. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind. Crops Prod. 2018, 126, 434–439. [Google Scholar] [CrossRef]
- Lalla, J.K.; Nandedkar, S.Y.; Paranjape, M.H.; Talreja, N.B. Clinical trials of ayurvedic formulations in the treatment of acne vulgaris. J. Ethnopharmacol. 2001, 78, 99–102. [Google Scholar] [CrossRef]
- Winters, W.D.; Benavides, R.; Clouse, W.J. Effects of aloe extracts on human normal and tumor cells in vitro. Econ. Bot. 1981, 35, 89–95. [Google Scholar] [CrossRef]
- Olaleye, M.T.; Bello-Michael, C.O. Comparative antimicrobial activities of Aloe vera gel and leaf. Afr. J. Biotechnol. 2005, 4, 1413–1414. [Google Scholar]
- Hamilton-Miller, J.M.T. Anti-cariogenic properties of tea (Camellia sinensis). J. Med. Microbiol. 2001, 50, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Shim, J.S.; Lee, J.S.; Kim, J.K.; Yang, I.S.; Chung, M.-S.; Kim, K.H. Inhibition of Pathogenic Bacterial Adhesion by Acidic Polysaccharide from Green Tea (Camellia sinensis). J. Agric. Food Chem. 2006, 54, 8717–8723. [Google Scholar] [CrossRef] [PubMed]
- Yam, T.S.; Shah, S.; Hamilton-Miller, J.M.T. Microbiological activity of whole and fractionated crude extracts of tea (Camellia sinensis), and of tea components. FEMS Microbiol. Lett. 1997, 152, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kotnis, M.S.; Patel, P.; Menon, S.N.; Sane, R.T. Renoprotective effect of Hemidesmus indicus, a herbal drug used in gentamicin-induced renal toxicity. Nephrology 2004, 9, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Jayaveera, K.; Kumar, C.; Sanjay, U.P.; Swamy, B.; Kumar, D. Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. Trop. J. Pharm. Res. 2007, 6, 717–723. [Google Scholar] [CrossRef]
- Mary, N.K.; Achuthan, C.R.; Babu, B.H.; Padikkala, J. In vitro antioxidant and antithrombotic activity of Hemidesmus indicus (L) R.Br. J. Ethnopharmacol. 2003, 87, 187–191. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I.; Owais, M. Evaluation of anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and synergy of some bioactive plant extracts. Biotechnol. J. 2006, 1, 1093–1102. [Google Scholar] [CrossRef]
- Brendlé, J. Organic–inorganic hybrids having a talc-like structure as suitable hosts to guest a wide range of species. Dalton Trans. 2018, 47, 2925–2932. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef]
- Chun, H.-S.; Park, D.; Eun Lim, S.; Jeong, K.-H.; Park, J.-S.; Park, H.-J.; Kang, S.; Kang, K.S.; Park, H.G.; An, H.-R.; et al. Two zinc-aminoclays’ in-vitro cytotoxicity assessment in HeLa cells and in-vivo embryotoxicity assay in zebrafish. Ecotox. Environ. Safe 2017, 137, 103–112. [Google Scholar] [CrossRef]
- Datta, K.K.R.; Achari, A.; Eswaramoorthy, M. Aminoclay: A functional layered material with multifaceted applications. J. Mater. Chem. A 2013, 1, 6707–6718. [Google Scholar] [CrossRef]
- Yang, L.; Shao, Y.; Han, H.-K. Improved pH-dependent drug release and oral exposure of telmisartan, a poorly soluble drug through the formation of drug-aminoclay complex. Int. J. Pharm. 2014, 471, 258–263. [Google Scholar] [CrossRef]
- Saboo, S.; Kestur, U.S.; Flaherty, D.P.; Taylor, L.S. Congruent release of drug and polymer from amorphous solid dispersions: Insights into the role of drug-polymer hydrogen bonding, surface crystallization, and glass transition. Mol. Pharm. 2020, 17, 1261–1275. [Google Scholar] [CrossRef]
- Wang, S.; Cao, H.; Zhong, Y.; Yang, Y.; Shao, Z. A novel aminoclay–curcumin hybrid for enhanced chemotherapy. J. Mater. Chem. B 2016, 4, 4295–4301. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloid Surf. A-Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Gonzalez Ortiz, D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current trends in pickering emulsions: Particle morphology and applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Tai, Z.; Huang, Y.; Zhu, Q.; Wu, W.; Yi, T.; Chen, Z.; Lu, Y. Utility of pickering emulsions in improved oral drug delivery. Drug Discov. Today 2020, 25, 2038–2045. [Google Scholar] [CrossRef]
- Albert, C.; Beladjine, M.; Tsapis, N.; Fattal, E.; Agnely, F.; Huang, N. Pickering emulsions: Preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J. Control. Release 2019, 309, 302–332. [Google Scholar] [CrossRef]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-grade pickering emulsions for encapsulation and delivery of bioactives. Trends. Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Dai, H.; Wu, J.; Zhang, H.; Chen, Y.; Ma, L.; Huang, H.; Huang, Y.; Zhang, Y. Recent advances on cellulose nanocrystals for pickering emulsions: Development and challenge. Trends Food Sci. Technol. 2020, 102, 16–29. [Google Scholar] [CrossRef]
- Martel-Estrada, S.-A.; Morales-Cardona, A.-I.; Vargas-Requena, C.-L.; Rubio-Lara, J.-A.; Martínez-Pérez, C.-A.; Jimenez-Vega, F. Delivery systems in nanocosmeceuticals. Rev. Adv. Mater. Sci. 2022, 61, 901–930. [Google Scholar] [CrossRef]
- Kim, S.J.; Shin, M.K.; Back, J.H.; Koh, J.S. Pore volume is most highly correlated with the visual assessment of skin pores. Skin Res. Technol. 2014, 20, 429–434. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, K.; Liu, C.; Li, Y.; Lu, C.; Wang, P. Fabrication of starch-based nanospheres to stabilize pickering emulsion. Carbohydr. Polym. 2012, 88, 1358–1363. [Google Scholar] [CrossRef]
- Huo, J.; Marcello, M.; Garai, A.; Bradshaw, D. MOF-Polymer Composite Microcapsules Derived from Pickering Emulsions. Adv. Mater. 2013, 25, 2717–2722. [Google Scholar] [CrossRef]
- Burgos-Díaz, C.; Opazo-Navarrete, M.; Soto-Añual, M.; Leal-Calderón, F.; Bustamante, M. Food-grade Pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020, 134, 109244. [Google Scholar] [CrossRef]
- Zhu, F. Starch based Pickering emulsions: Fabrication, properties, and applications. Trends. Food Sci. Technol. 2019, 85, 129–137. [Google Scholar] [CrossRef]
- Patil, A.J.; Muthusamy, E.; Mann, S. Synthesis and Self-Assembly of Organoclay-Wrapped Biomolecules. Angew. Chem. Int. Ed. 2004, 43, 4928–4933. [Google Scholar] [CrossRef]
- Nourabi, A.; Tabibiazar, M.; Mashhadi, H.; Mahmoudzadeh, M. Characterization of pickering emulsion stabilized by colloidal sodium caseinate nanoparticles prepared using complexation and antisolvent method. LWT 2023, 180, 114686. [Google Scholar] [CrossRef]
- Margraf, T.; Karnopp, A.R.; Rosso, N.D.; Granato, D. Comparison between Folin-Ciocalteu and Prussian Blue assays to estimate the total phenolic content of juices and teas using 96-well microplates. J. Food Sci. 2015, 80, C2397–C2403. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. In vitro dissolution testing strategies for nanoparticulate drug delivery systems: Recent developments and challenges. Drug Deliv. Transl. Res. 2013, 3, 409–415. [Google Scholar] [CrossRef]
- Ng, S.-F.; Rouse, J.J.; Sanderson, F.D.; Meidan, V.; Eccleston, G.M. Validation of a Static Franz Diffusion Cell System for In Vitro Permeation Studies. AAPS PharmSciTech 2010, 11, 1432–1441. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed.; NCCLS Publication: Berwyn, PA, USA, 2008; Volume 28. [Google Scholar]
- Dutta, D.; Markhoff, J.; Suter, N.; Rezwan, K.; Brüggemann, D. Effect of Collagen Nanofibers and Silanization on the Interaction of HaCaT Keratinocytes and 3T3 Fibroblasts with Alumina Nanopores. ACS Appl. Bio Mater. 2021, 4, 1852–1862. [Google Scholar] [CrossRef]
- Cho, W.-C.; Shin, Y.-K.; Na, Y.-S.; Ryu, M.-H.; Ku, J.-L.; Kang, Y.-K. The role of novel fusion genes in human GIST cell lines derived from imatinib-resistant GIST patients: A therapeutic potential of fusion gene. Biochem. Biophys. Res. Commun. 2020, 529, 699–706. [Google Scholar] [CrossRef]


| Medicinal Plants | Bacteria | Additional Effects | References |
|---|---|---|---|
| Rosmarinus officinalis | P. acnes S. aureus Kocuria sp. | Anti-depressant effect Immunomodulatory function | [11,12,13] |
| Acacia nilotica | P. acnes Kocuria sp. B. subtilis | Wounds healing effect Gastrointestinal-disorder treatment Anti-cancer | [14,15] |
| Ocimum tenuiflorum | P. acnes Kocuria sp. B. subtilis | Anti-depressant effect Anti-insecticide effect | [14,16,17] |
| Aloe vera | S. aureus T. mentagrophytes | Anti-inflammatory effect Anti-cancer effect | [18,19,20] |
| Camellia sinensis | P. acnes | Anti-inflammatory Anti-cariogenic | [21,22,23] |
| Hemidesmus indicus | S. epidermis S. aureus | Anti-thrombotic effect Renoprotective effect | [24,25,26,27] |
| Compounds | Microbes | MIC Values |
|---|---|---|
| MA-OHE | S. aureus | 0.75 mg/mL |
| MA-OHE | C. acnes | 7.5 mg/mL |
| ZnAC | S. aureus | 0.1 mg/mL |
| ZnAC | C. acnes | 0.05 mg/mL |
| MA-OHE/ZnAC | S. aureus | 0.1 mg/mL |
| MA-OHE/ZnAC | C. acnes | 0.025 mg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Bae, I.-S.; Lee, H.U.; Moon, J.-Y.; Lee, Y.-C. A Bioactive Compound-Loaded Zinc-Aminoclay Encapsulated, Pickering Emulsion System for Treating Acne-Inducing Microbes. Int. J. Mol. Sci. 2023, 24, 9669. https://doi.org/10.3390/ijms24119669
Kim S-H, Bae I-S, Lee HU, Moon J-Y, Lee Y-C. A Bioactive Compound-Loaded Zinc-Aminoclay Encapsulated, Pickering Emulsion System for Treating Acne-Inducing Microbes. International Journal of Molecular Sciences. 2023; 24(11):9669. https://doi.org/10.3390/ijms24119669
Chicago/Turabian StyleKim, Seong-Hyeon, In-Sun Bae, Hyun Uk Lee, Ju-Young Moon, and Young-Chul Lee. 2023. "A Bioactive Compound-Loaded Zinc-Aminoclay Encapsulated, Pickering Emulsion System for Treating Acne-Inducing Microbes" International Journal of Molecular Sciences 24, no. 11: 9669. https://doi.org/10.3390/ijms24119669
APA StyleKim, S.-H., Bae, I.-S., Lee, H. U., Moon, J.-Y., & Lee, Y.-C. (2023). A Bioactive Compound-Loaded Zinc-Aminoclay Encapsulated, Pickering Emulsion System for Treating Acne-Inducing Microbes. International Journal of Molecular Sciences, 24(11), 9669. https://doi.org/10.3390/ijms24119669







