C- and N-Phosphorylated Enamines—An Avenue to Heterocycles: NMR Spectroscopy
Abstract
1. Introduction
| Phosphorous chloride | ||||
| Coordination number | 3 | 4 | 5 | 6 |
| 31P NMR chemical shift, pm | +200 | +80 | −80 | −300 |
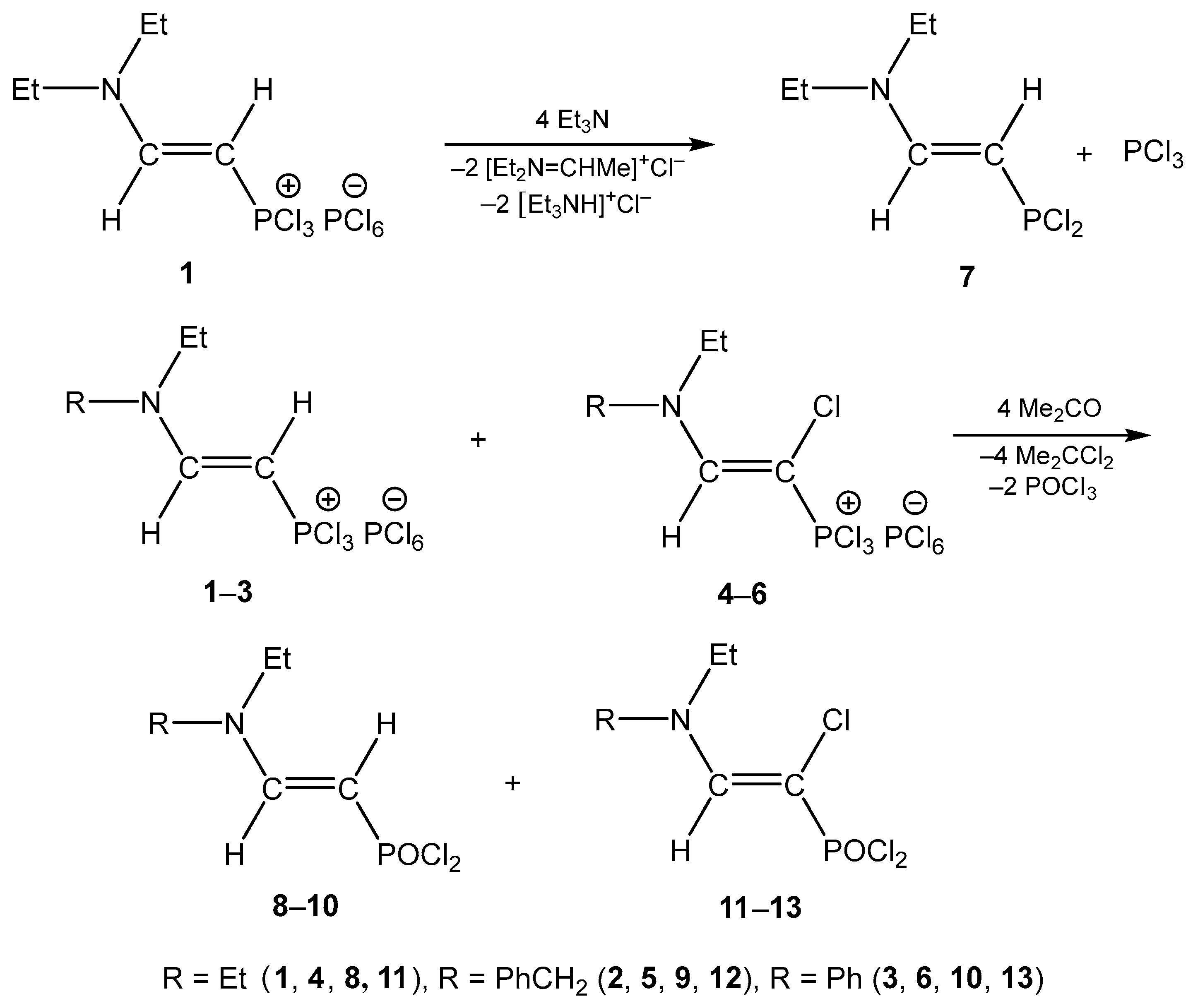
2. The Phosphorylated Tertiary Amines
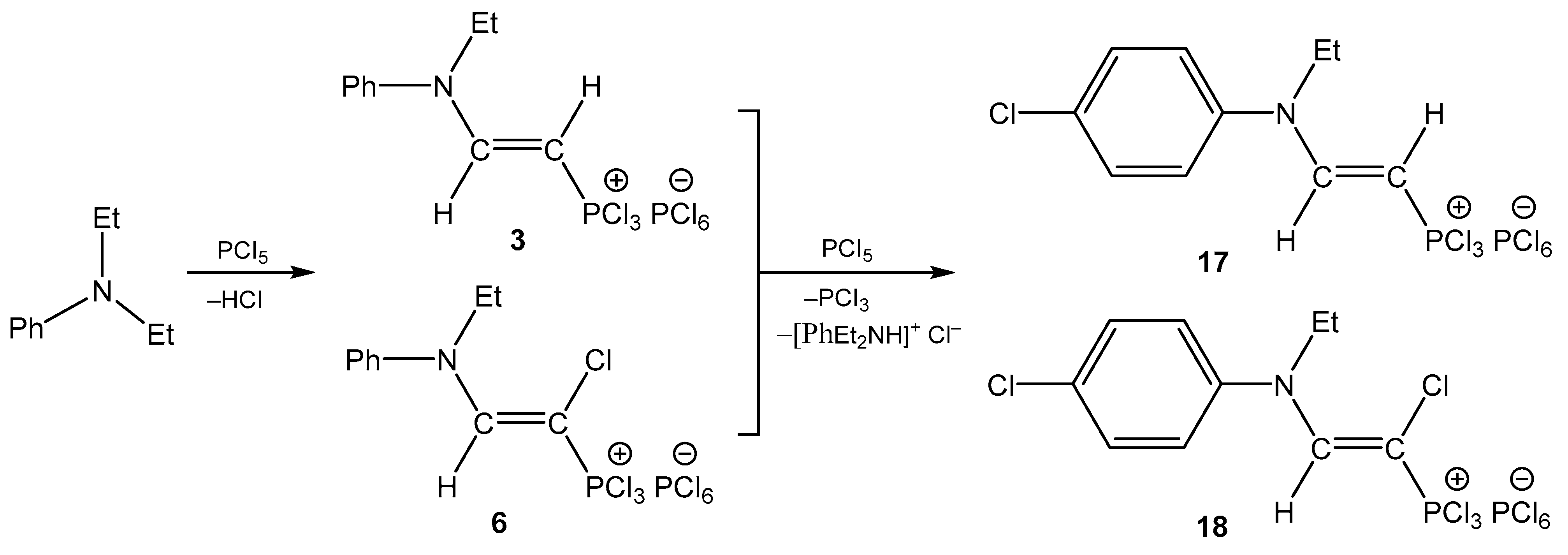
3. The Structure of the Reaction Products of Triethylamine with Organyltrichlorophosphonium Hexachlorophosphorates
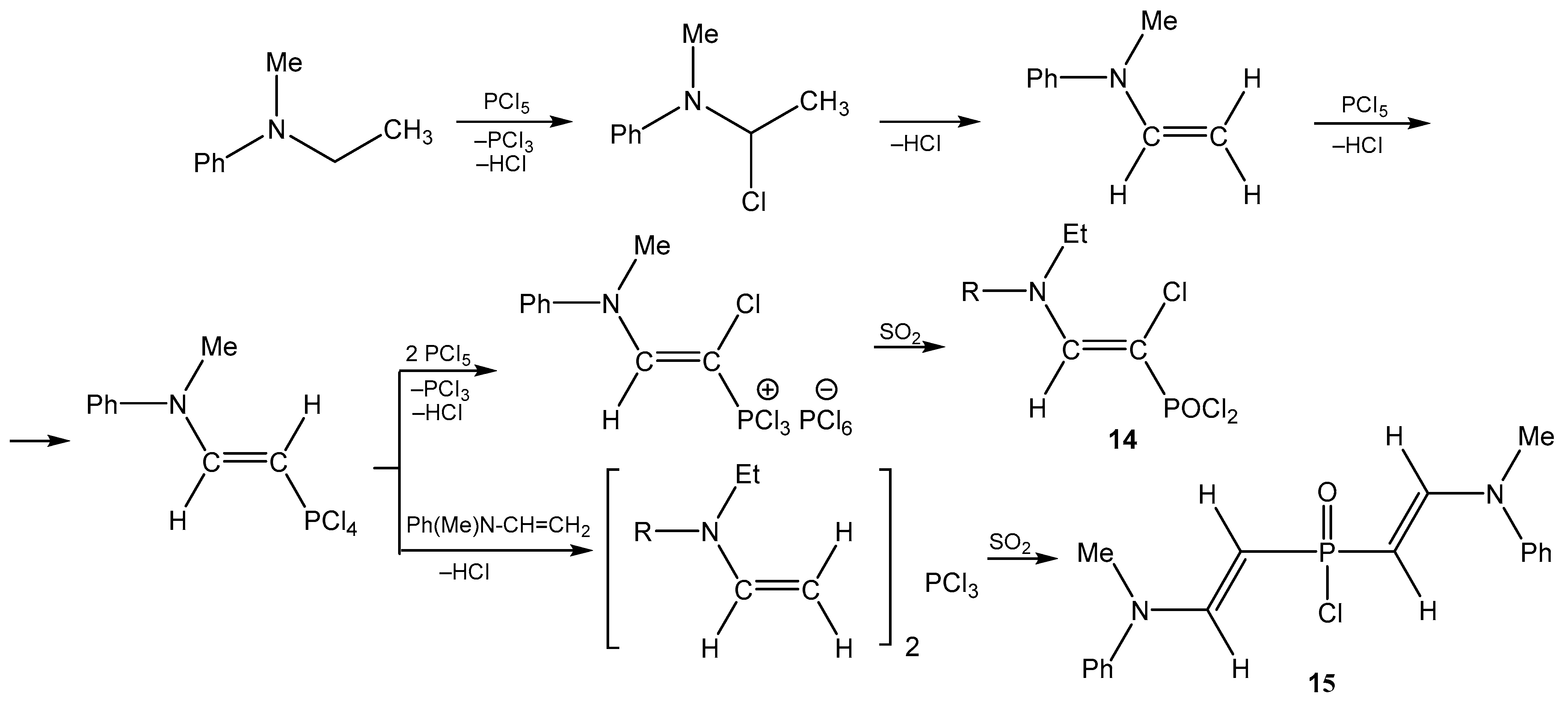
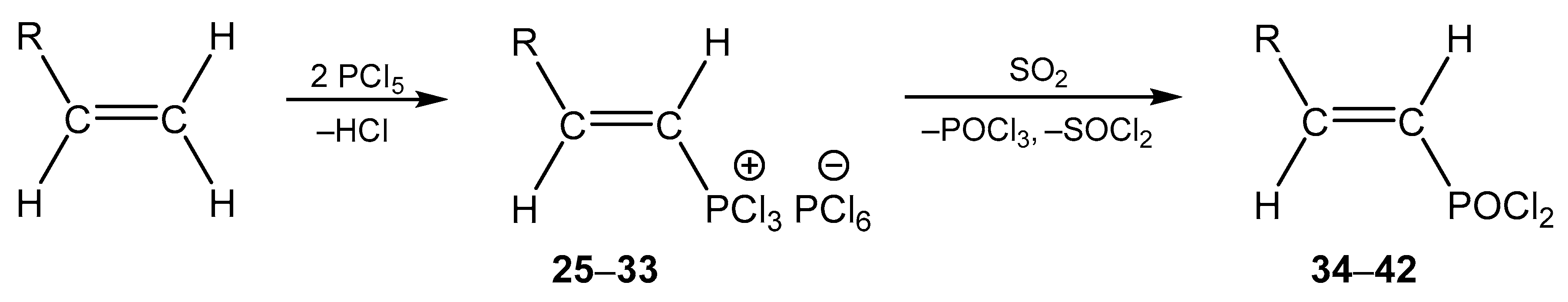
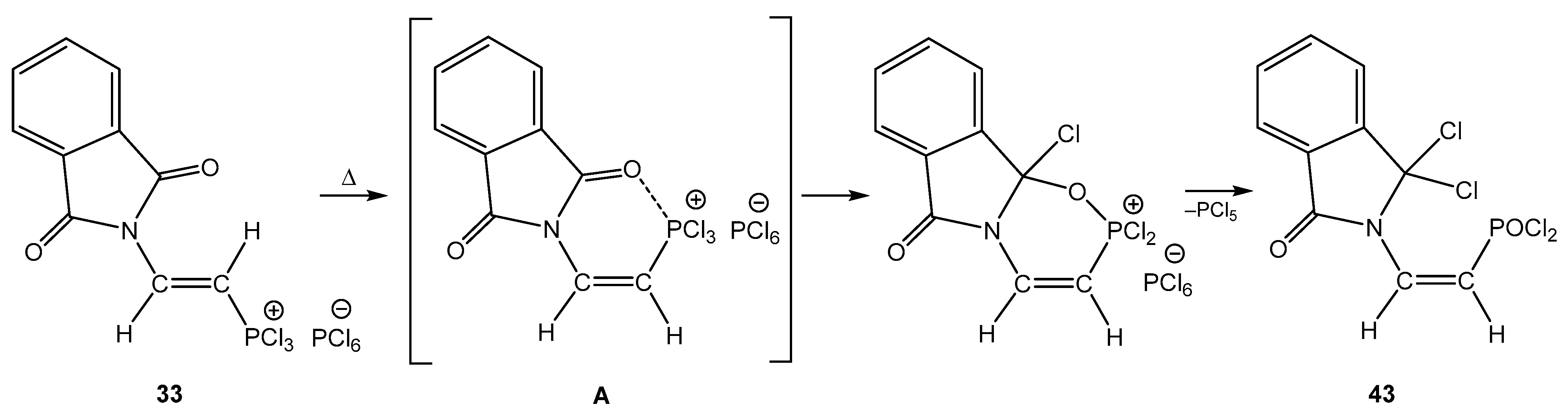
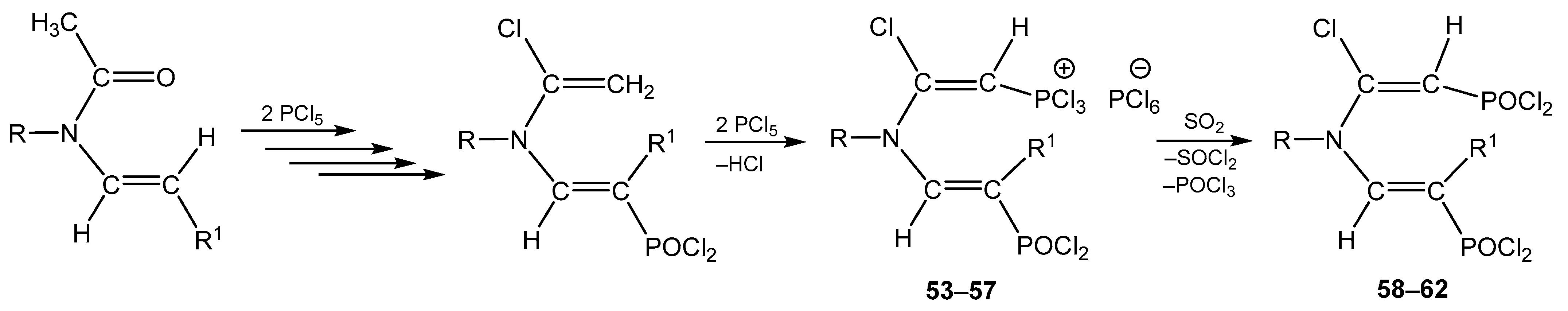
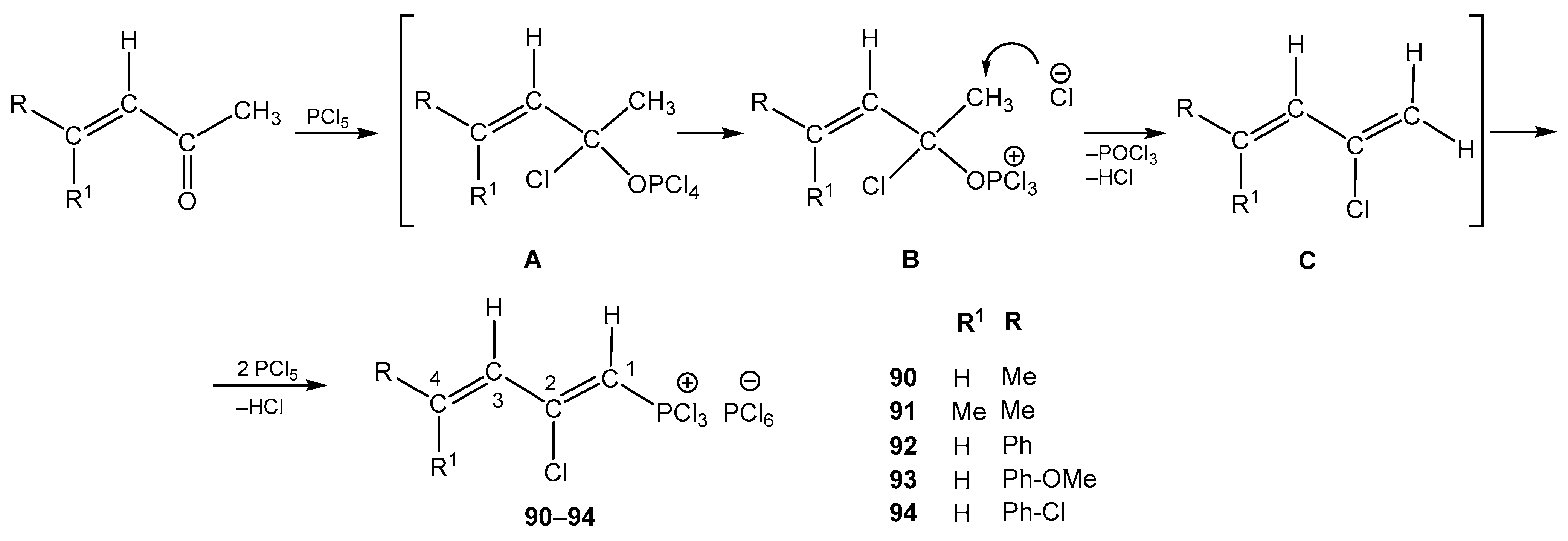
4. The Structural Aspects of the Phosphorylation Products of Enamides
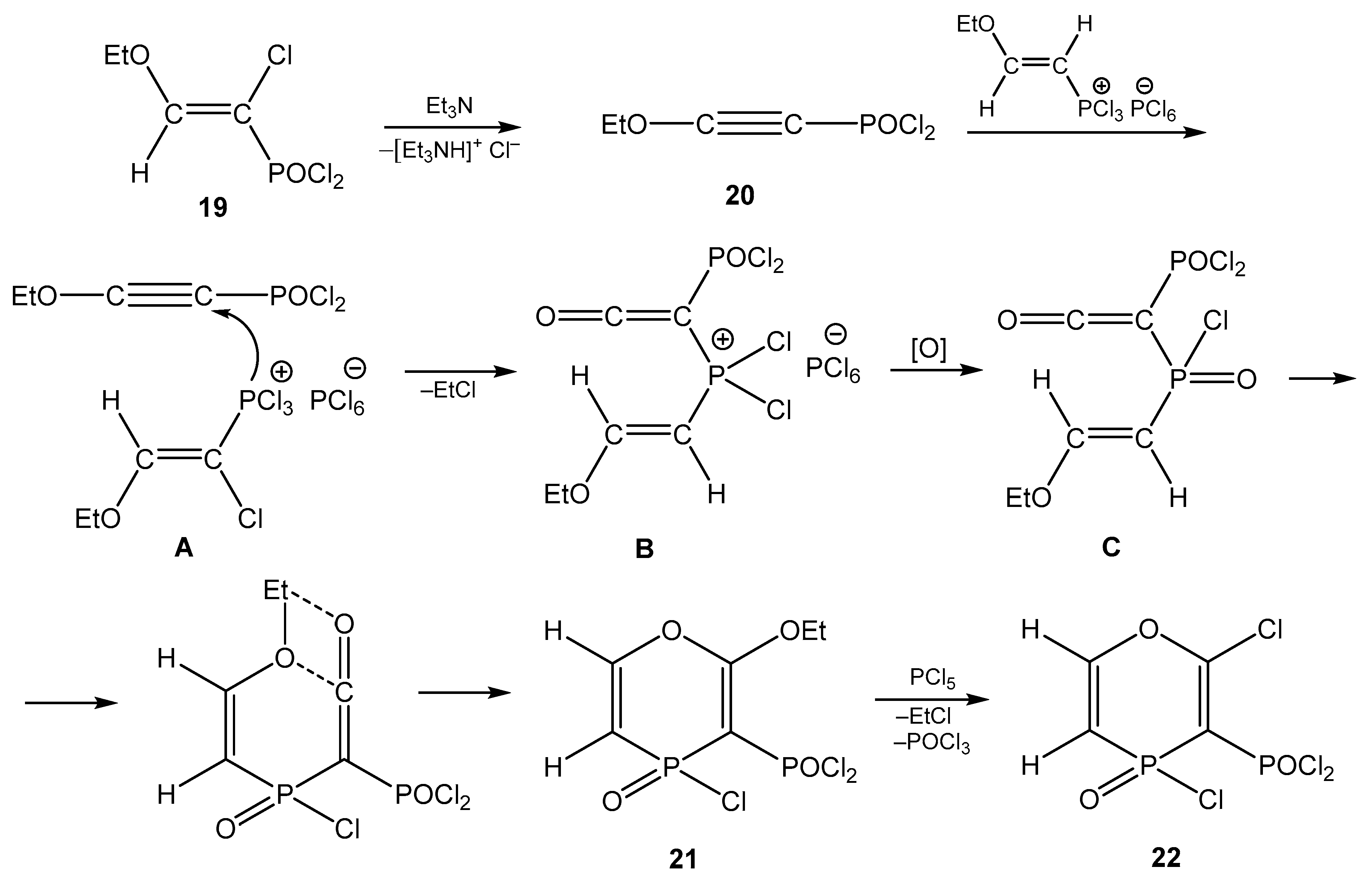
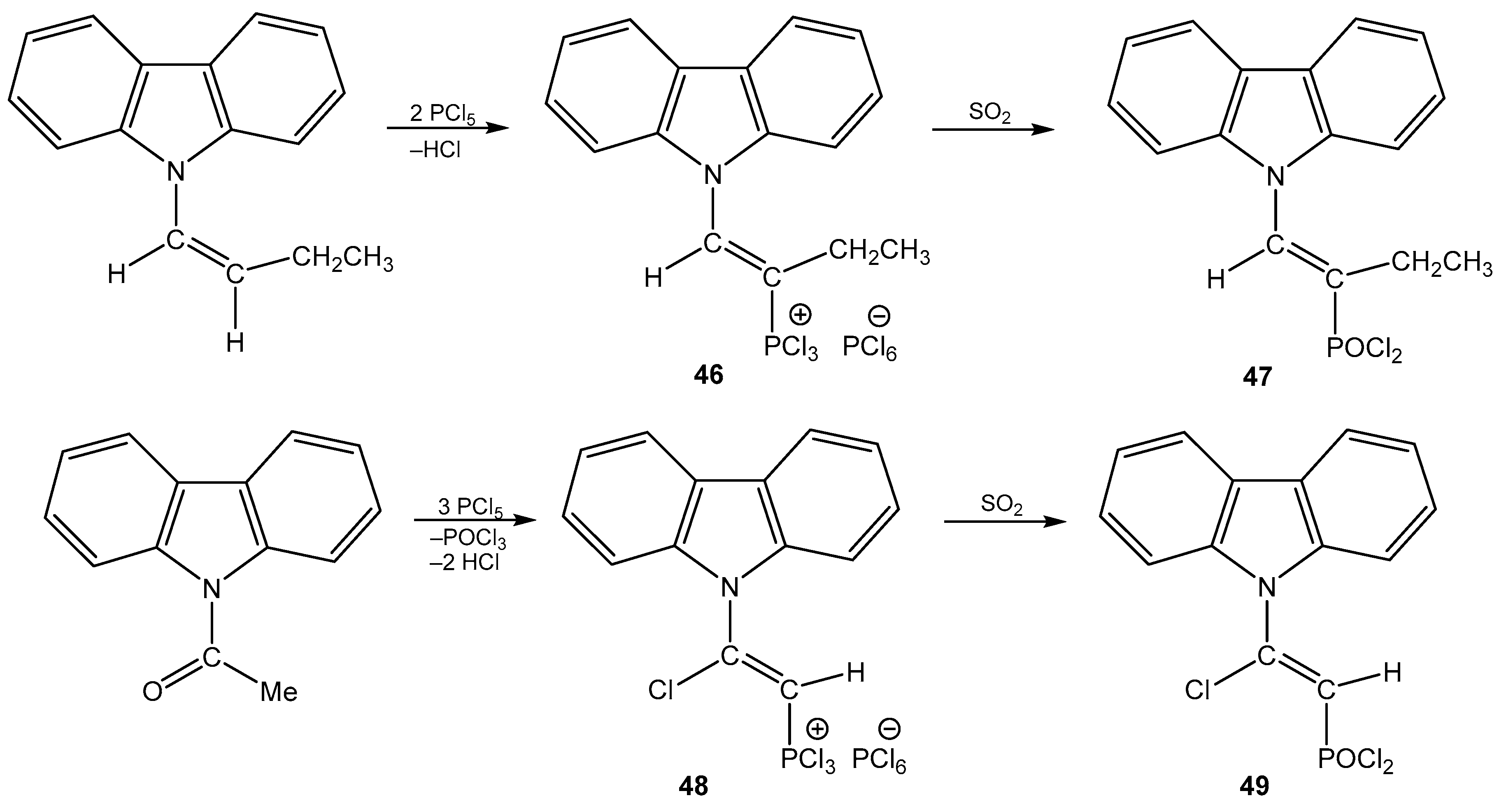
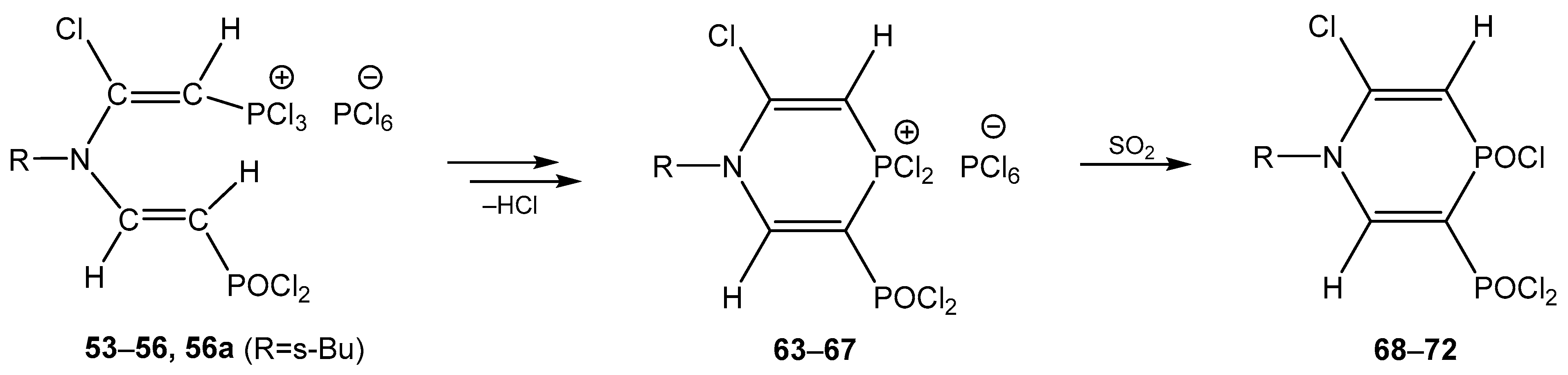
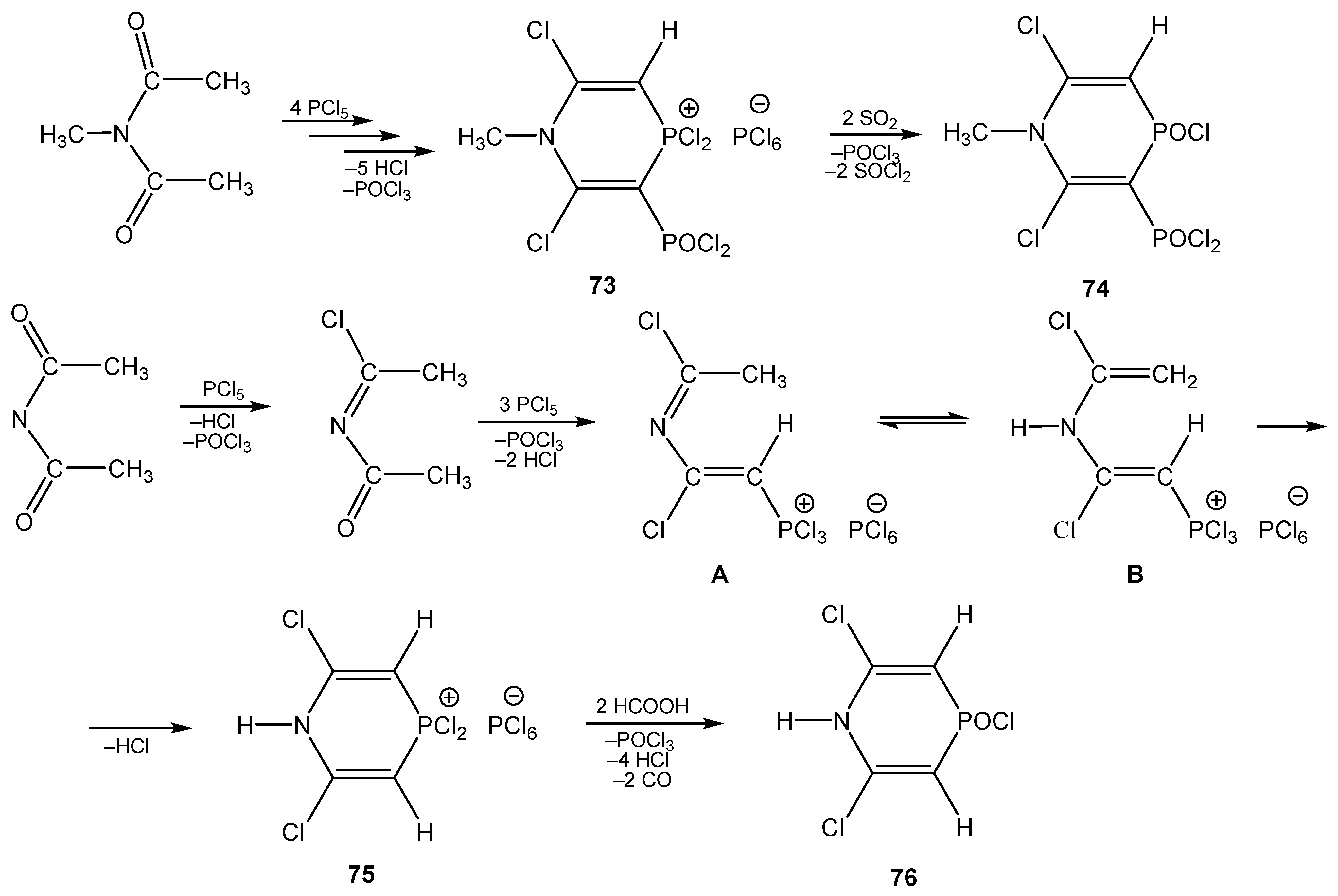
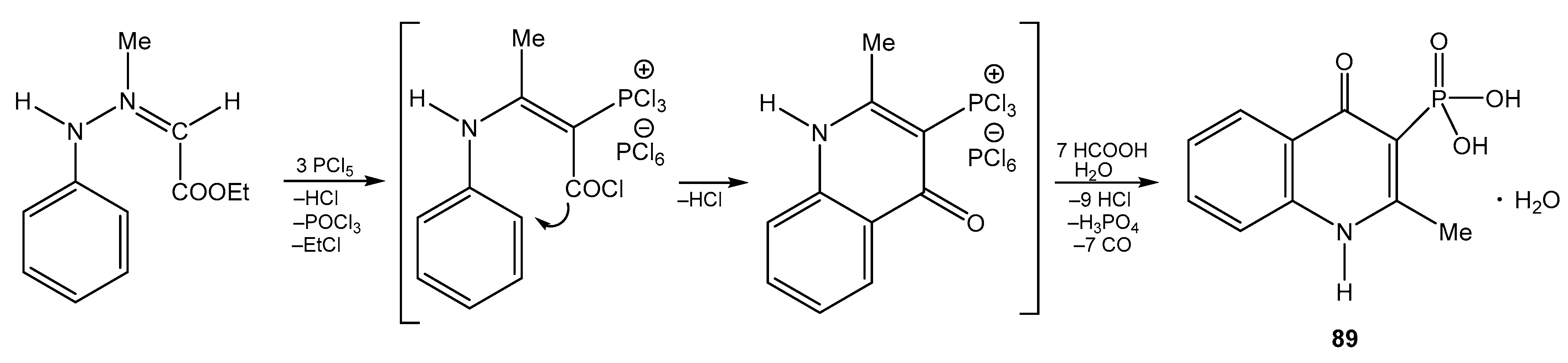
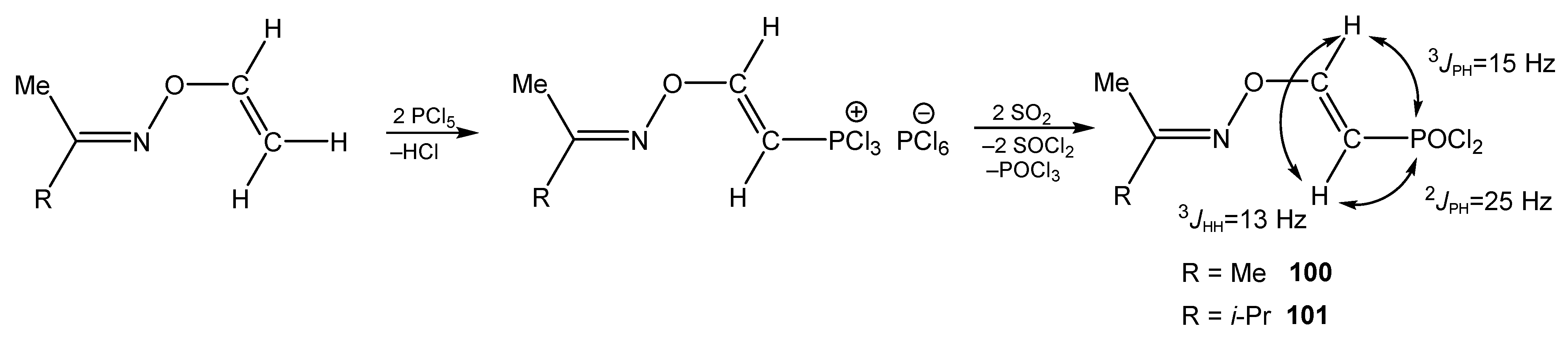
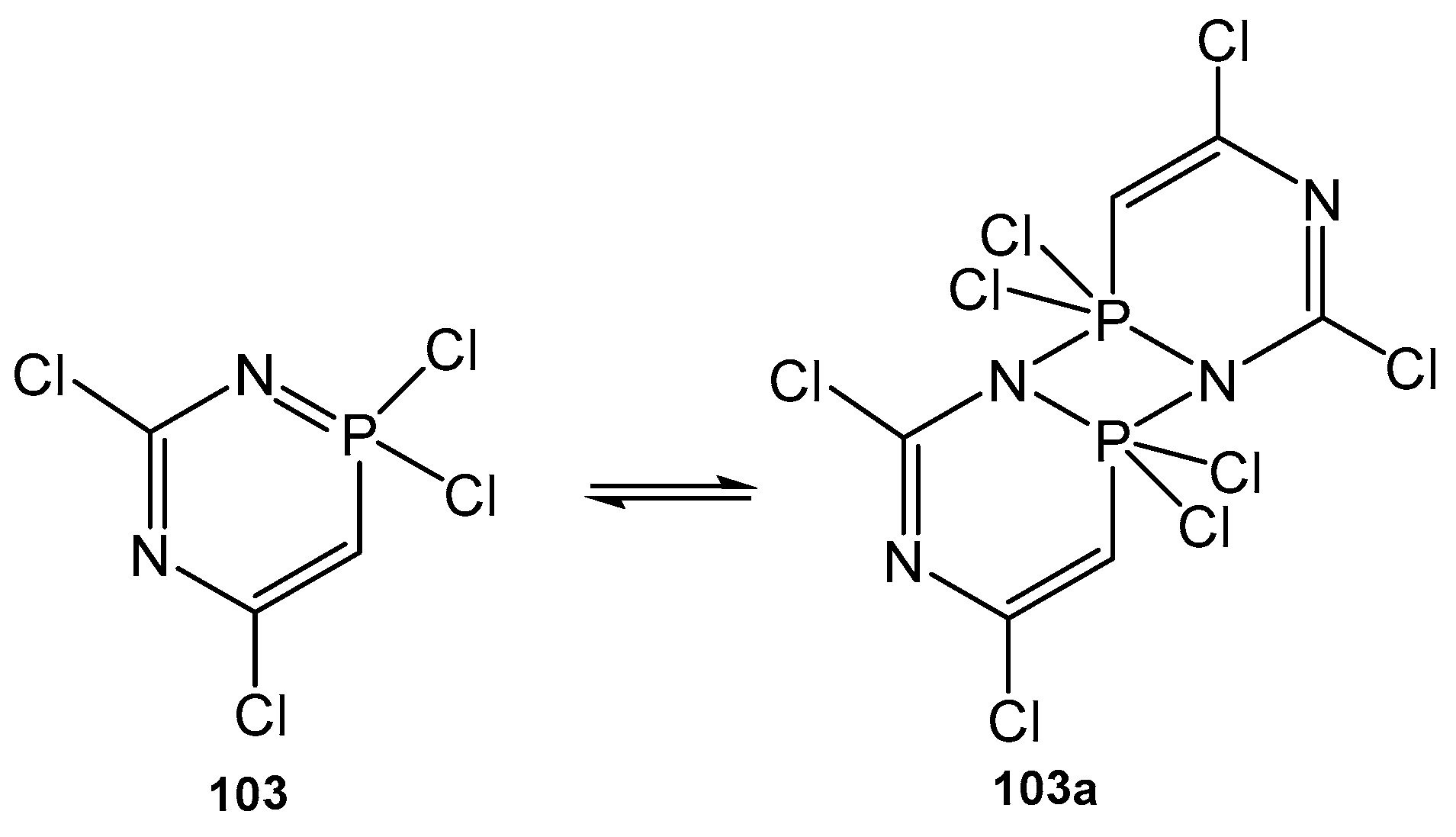
5. The Structure of Enamide-Derived Azaphosphorines
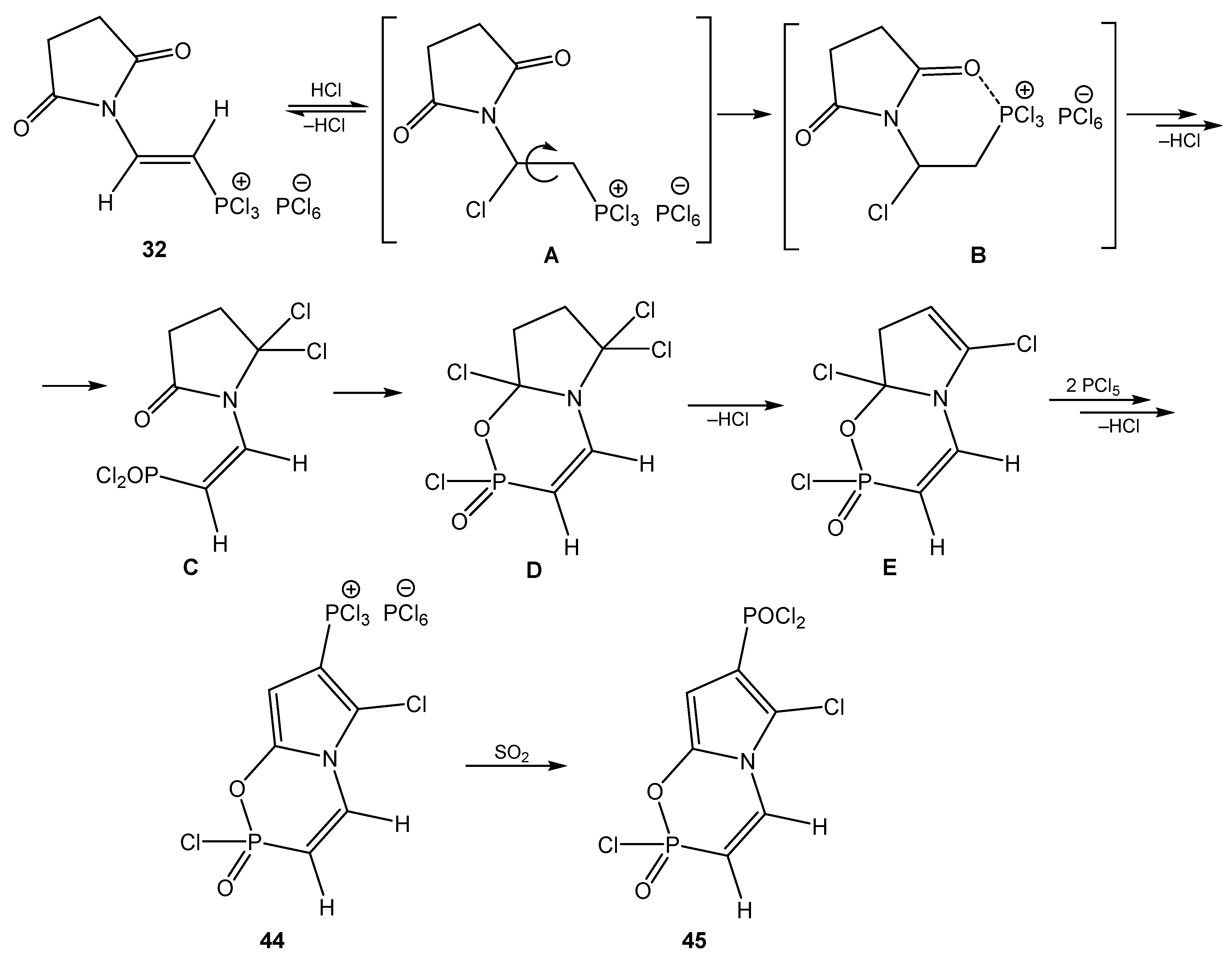
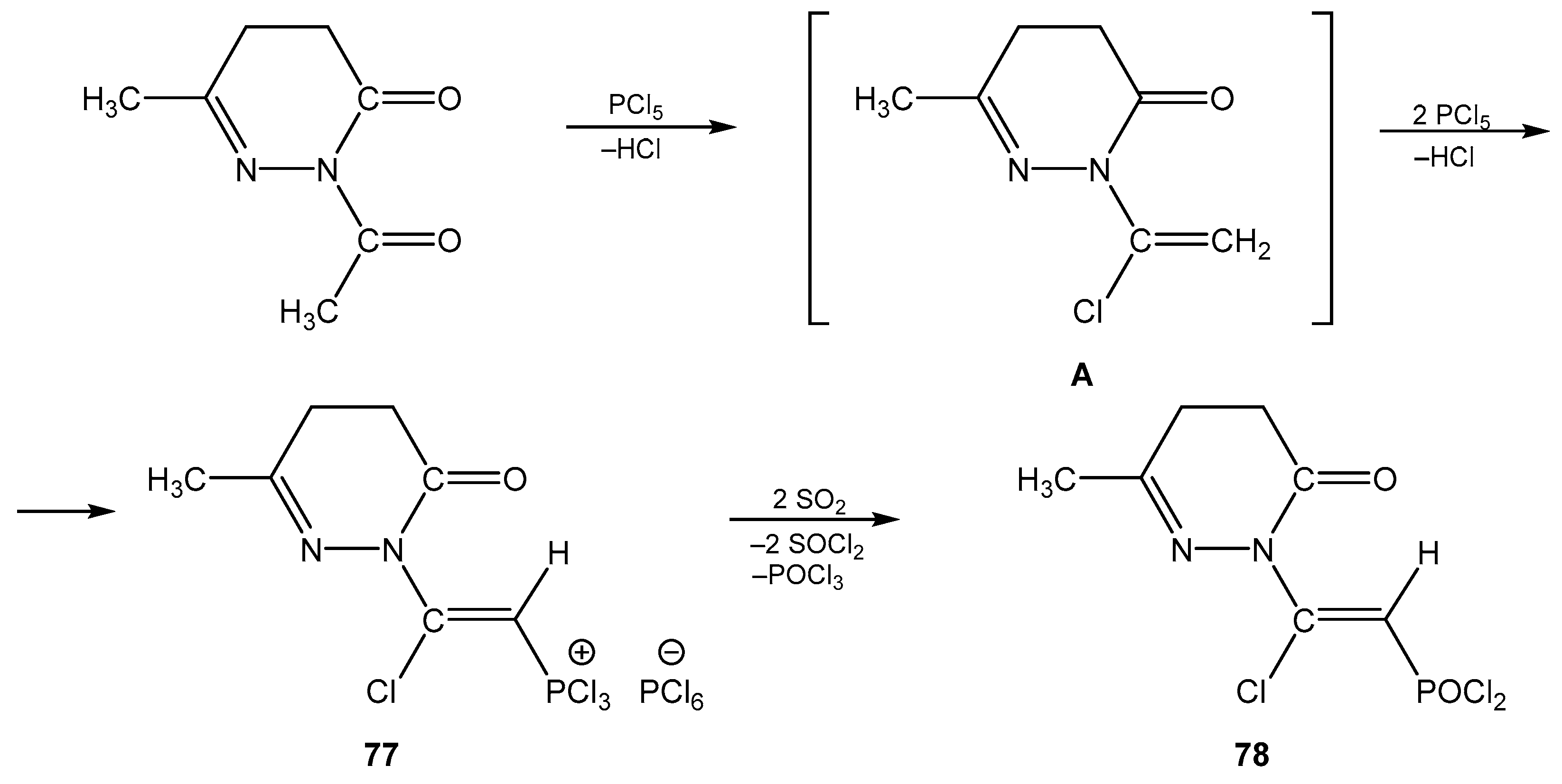
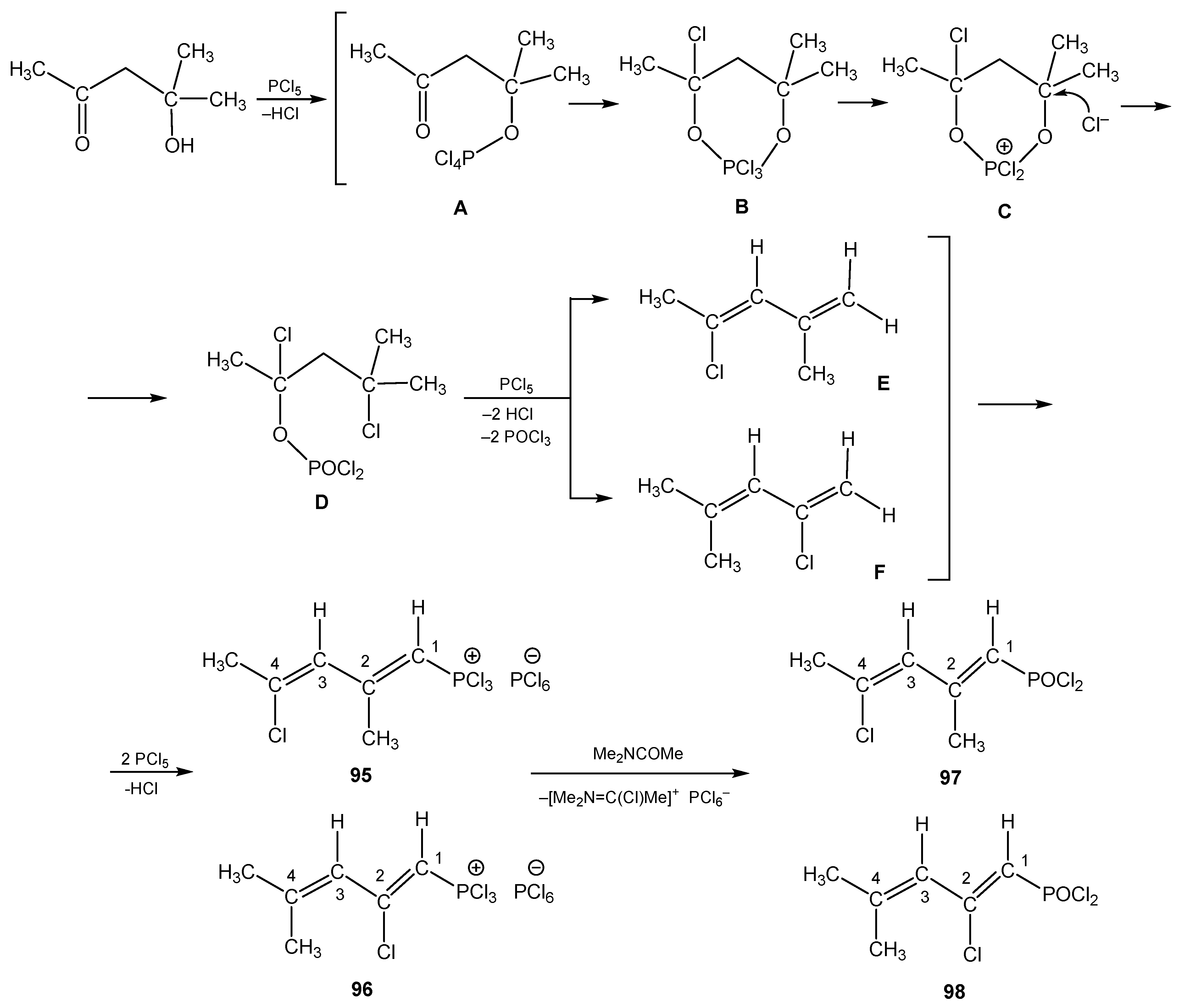
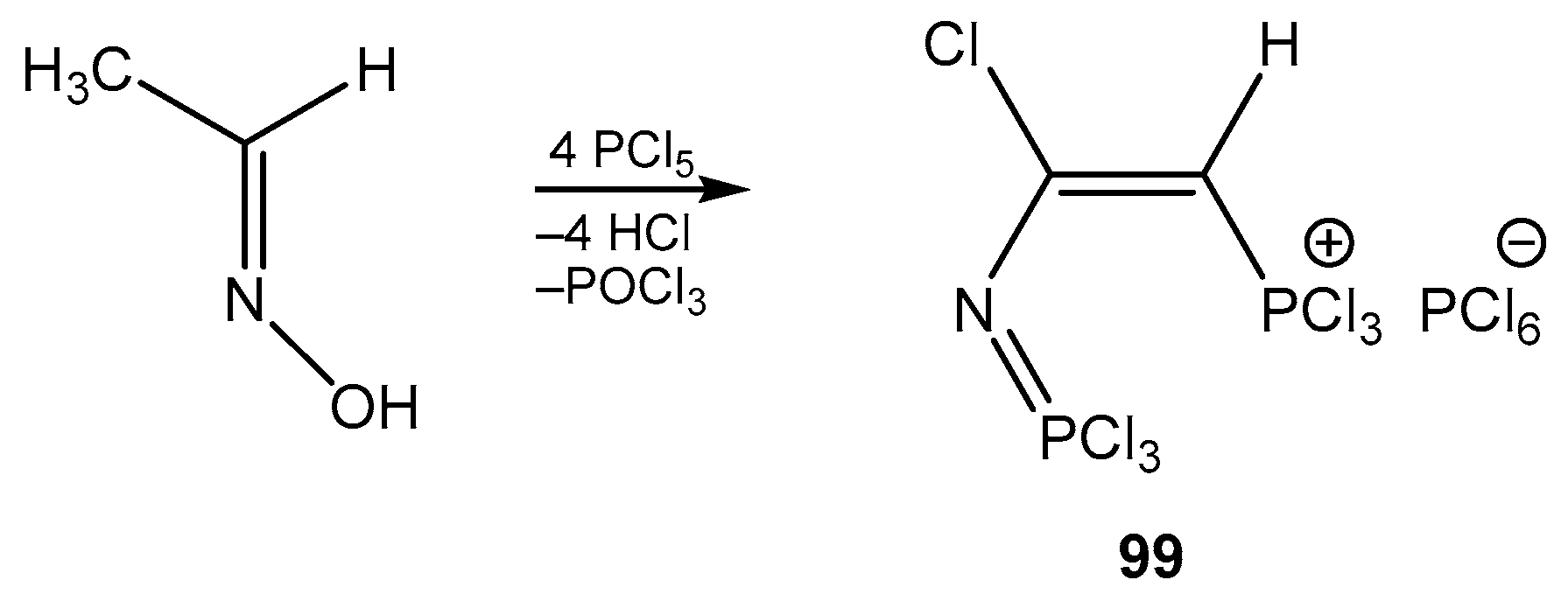
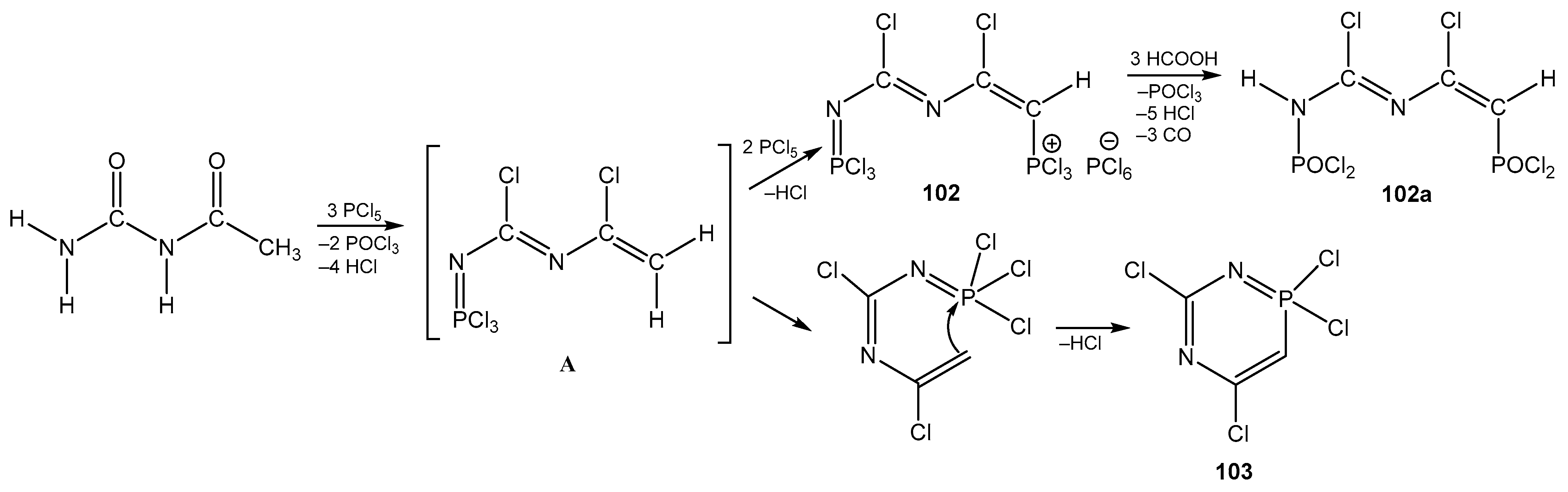
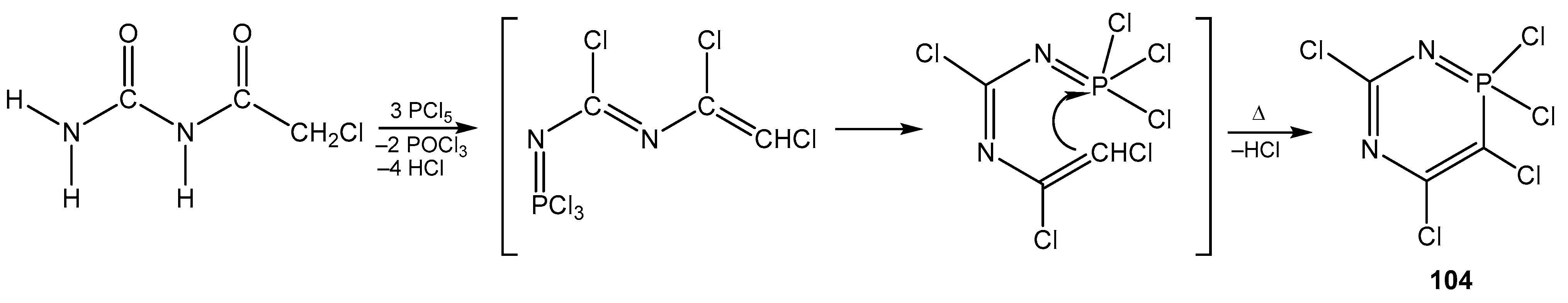
6. The Structural Features of Acetylurea-Derived Diazaphosphorines
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karl, D.M. Phosphorus, the staff of life. Nature 2000, 406, 31–33. [Google Scholar] [CrossRef] [PubMed]
- Demkowicz, S.; Rachon, J.; Dasko, M.; Kozak, W. Selected organophosphorus compounds with biological activity. Applications in medicine. RSC Adv. 2016, 6, 7101–7112. [Google Scholar] [CrossRef]
- Kochian, L.V. Rooting for more phosphorus. Nature 2012, 488, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Montchamp, J.-L. Phosphinate Chemistry in the 21st Century: A Viable Alternative to the Use of Phosphorus Trichloride in Organophosphorus Synthesis. Acc. Chem. Res. 2014, 47, 77–87. [Google Scholar] [CrossRef]
- Budnikova, Y.H. Opportunities and challenges for combining electro- and organometallic catalysis in C(sp2)-H phosphonation. Pure Appl. Chem. 2018, 91, 16. [Google Scholar] [CrossRef]
- Müller, C. Copper(I) complexes of low-coordinate phosphorus(III) compounds. In Copper(I) Chemistry of Phosphines, Functionalized Phosphines and Phosphorus; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. [Google Scholar] [CrossRef]
- Clausing, S.T.; Morales, D.; Orthaber, S.A. Preparation, photo- and electrochemical studies of a homoleptic imine-phosphaalkene Cu(I) complex. Inorg. Chim. Acta 2020, 513, 119958. [Google Scholar] [CrossRef]
- Zagidullin, A.A.; Sakhapov, I.F.; Miluykov, V.A.; Yakhvarov, D.G. Nickel Complexes in C-P Bond Formation. Molecules 2021, 26, 5283. [Google Scholar] [CrossRef]
- Larina, L.I. Organophosphorus Azoles Incorporating a Tetra-, penta-, and hexacoordinated Phosphorus atom: NMR spectroscopy and Quantum Chemistry. Molecules 2023, 28, 669. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Ma, J.-A. Stabilized Nucleophiles with Electron Deficient Alkenes, Alkynes, Allenes. In Comprehensive Organic Synthesis, 2nd ed.; Knochel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4, pp. 1–85. [Google Scholar] [CrossRef]
- Zarate, C.; Van Gemmeren, M.; Somerville, R.; Martin, R. Phenol Derivatives: Modern Electrophiles in Cross-Coupling Reactions. Adv. Organometall. Chem. 2016, 66, 143–222. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Trofimov, B.A. Organophosphorus chemistry based on elemental phosphorus: Advances and horizons. Russ. Chem. Rev. 2020, 89, 225–249. [Google Scholar] [CrossRef]
- Gafurov, Z.N.; Kagilev, A.A.; Kantyukov, A.O.; Sinyashin, O.G.; Yakhvarov, D.G. The role of organonickel reagents in organophosphorus chemistry. Coord. Chem. Rev. 2021, 438, 213889. [Google Scholar] [CrossRef]
- Rozinov, V.G. Chlorophosphorylated Enamines. Synthesis and Heterocyclization. Ph.D. Thesis, Irkutsk State University, Irkutsk, Russa, 1989; 420p. (In Russian). [Google Scholar]
- Mitrasov, Y.N.; Smolina, I.N.; Yegorova, G.Y. Reaction of allylalkanoates with phosphorus pentachloride. Russ. J. Gen. Chem. 2008, 78, 2154–2155. [Google Scholar] [CrossRef]
- Klapotke, T.M.; Krumm, B.; Moll, R. Synthesis and Structure of 2,2,2- Nitrilotriacetyl Chloride with a Flat NC3 Pyramide. Z. Naturforsch. 2013, 68, 735–738. [Google Scholar] [CrossRef]
- Mitrasov, Y.N.; Sosnov, D.A.; Kondrateva, O.V. Reaction of spiro[2.2]pentane with phosphorus pentachloride. Russ. J. Gen. Chem. 2013, 83, 1167. [Google Scholar] [CrossRef]
- Romero, A.H. Modified procedure for the synthesis of 2-chloroquinoline-3-carbaldehydes using phosphorus pentachloride. Synth. Commun. 2016, 46, 287–291. [Google Scholar] [CrossRef]
- Da Paixao Soares, F.; Groaz, E.; Herdewijn, P. Phosphorus Pentachloride Promoted gem-Dichlorination of 2′- and 3′-Deoxynucleosides. Molecules 2018, 23, 1457. [Google Scholar] [CrossRef]
- Salkeeva, L.K.; Muratbekova, A.A.; Minayeva, E.V.; Voitichek, P. Phosphorylation of glycoluryl derivatives with phosphorus pentachloride. Bull. Karag. Univer. Chem. Ser. 2021, 101, 12–18. [Google Scholar] [CrossRef]
- Bohme, H.; Viene, H.L. (Eds.) Iminium Salts in Organic Chemistry; Part 1, Methods and Results; Wiley: New York, NY, USA, 1976; p. 619. [Google Scholar]
- Kukhar, V.P.; Shevchenko, M.V. Amidation of trivalent phosphorus acid chlorides with bis(dialkylamino)methanes. Russ. J. Gen. Chem. 1982, 52, 562–566. [Google Scholar]
- Shevchenko, M.V.; Kukhar, V.P. Amidation of Halogenated Phosphorus Derivatives with Formic Acid Orthomorpholide. Russ. J. Gen. Chem. 1984, 54, 1499–1503. [Google Scholar]
- Larina, L.I.; Rozinov, V.G.; Komarova, T.N. Studies of phosphorylation reaction of tertiary amines by NMR spectroscopy. Russ. Bull. Chem. 2013, 62, 39–46. [Google Scholar] [CrossRef]
- Pensionerova, G.A.; Rozinov, V.G.; Glukhikh, V.I. The phosphorylation of tertiary amines with phosphorus pentachloride. Russ. J. Gen. Chem. 1978, 48, 2796. [Google Scholar]
- Rozinov, V.G.; Pensionerova, G.A.; Donskikh, V.I.; Kalabina, A.V.; Domnina, E.S.; Skvortsova, G.G. Unsaturated organophosphorus compounds based on 1-vinylbenzotriazole. Russ. J. Gen. Chem. 1983, 53, 697–698. (In Russian) [Google Scholar]
- Rozinov, V.G.; Pensionerova, G.A.; Donskih, V.I.; Sergienko, L.M.; Korostova, S.E.; Mikhaleva, A.I.; Dolgushin, G.V. Reaction of alkyl- and phenylsubstituted N-vinylpyrroles with phosphorus pentachlorides. Russ. J. Gen. Chem. 1986, 56, 790–804. [Google Scholar]
- Larina, L.I.; Rozinov, V.G.; Dmitrichenko, M.Y.; Eskova, L.A. NMR investigation of chlorophosphorylation products of N-vinylazoles. Magn. Reson. Chem. 2009, 47, 149–157. [Google Scholar] [CrossRef]
- Rozinov, V.G.; Kolbina, V.E.; Donskikh, V.I. Reaction of divinyl ether with phosphorus pentachloride. Russ. J. Gen. Chem. 1987, 57, 847–849. [Google Scholar]
- Fridland, S.V.; Malkov, Y.K. Reactions and Investigation Methods of Organic Compounds; Knunyants, I.L., Melnikova, N.N., Grapova, A.F., Simonova, V.D., Eds.; Chemistry: Moscow, Russia, 1986; pp. 106–149. [Google Scholar]
- Larina, L.I.; Komarova, T.N.; Rozinov, V.G. NMR study of reactions of organyltrichlorophosphonium hexachlorophosphorates with triethylamine. Russ. Chem. Bull. 2015, 64, 732–737. [Google Scholar] [CrossRef]
- Moskva, V.V.; Novruzov, S.A.; Ismailov, V.M.; Razumov, A.I.; Zykova, T.N.; Akhmedov, S.T. Derivatives of substituted vinylphosphonic acids. 21. Transformations of substituted vinylphosphonates under the action of nucleophilic reagents. Russ. J. Gen. Chem. 1976, 46, 534–542. [Google Scholar]
- Sultana, T.; Khan, M.W. A facile synthesis of 5-Iodo-6-substituted Pyrimidines from Uracil-6-Carboxylic acid (Orotic acid). Dhaka Univ. J. Pharm. Sci. 2014, 12, 97–102. [Google Scholar] [CrossRef]
- Khan, W.; Haque, E.; Ahmed, F. An expeditious synthesis of 6-Amido-(1H,3H)-Pyrimidine- 2,4-Diones from Uracil-6-Carboxylic Acid. Dhaka Univ. J. Pharm. Sci. 2015, 13, 23–29. [Google Scholar] [CrossRef]
- Liu, Y.T.; Song, S.M.; Yin, D.W. Solvent-free green synthesis of 4-amino-5-substituted-1,2,4-triazole-3-thione by Grinding. Adv. Mater. Res. 2015, 1088, 367–370. [Google Scholar] [CrossRef]
- Zhang, D.-L.; Li, C.-K.; Zeng, R.-S.; Shoberu, A.; Zou, J.-P. Manganese(III)-mediated selective phosphorylation of enamides: Direct synthesis of β-phosphoryl enamides. Org. Chem. Front. 2019, 6, 236–240. [Google Scholar] [CrossRef]
- Lei, T.; Liang, G.; Cheng, Y.Y.; Chen, B.; Tung, C.H.; Wu, L.Z. Cobaloxime Catalysis for Enamine Phosphorylation with Hydrogen Evolution. Org. Lett. 2020, 22, 5385–5389. [Google Scholar] [CrossRef] [PubMed]
- Rozinov, V.G.; Izhboldina, L.P.; Malysheva, S.F.; Donskikh, V.I.; Kalabina, A.V. Phosphorylation of N-vinylacetanilide with phosphorus pentachloride. Russ. J. Gen. Chem. 1983, 53, 934–935. (In Russian) [Google Scholar]
- Rozinov, V.G.; Izhboldina, L.P.; Pensionerova, G.A.; Donskih, V.I.; Malysheva, S.F.; Glukhikh, V.I.; Sergienko, L.M.; Ratovskiy, G.V. Phosphor-containing enamines. II. Phosphorylation of N-vinyl-substituted tertiary amides, lactams and cyclic imides with phosphorus pentachloride. Russ. J. Gen. Chem. 1984, 54, 1051–1060. (In Russian) [Google Scholar]
- Rozinov, V.G.; Izhboldina, L.P.; Donskih, V.I.; Dolgushin, G.V.; Ratovskiy, G.V. Thermal transformations of phosphorylated N-vinylphthalimide. Russ. J. Gen. Chem. 1988, 58, 942–943. [Google Scholar] [CrossRef]
- Rozinov, V.G.; Dmitrichenko, M.Y.; Kolbina, V.E.; Dolgushin, G.V. Phosphorus-containing enamines from acyclic and cyclic amides. II. Phosphorylation of acetamides, their homologs, and lactams. Russ. J. Gen. Chem. 1999, 69, 1377–1385. [Google Scholar]
- Rozinov, V.G.; Pensionerova, G.A.; Izhboldina, L.P.; Donskih, V.I. Reaction of β-substituted enamines with phosphorus pentachloride. Russ. J. Gen. Chem. 1985, 55, 2626–2627. [Google Scholar]
- Pensionerova, G.A.; Rozinov, V.G.; Kalabina, A.V.; Donskih, V.I.; Glukhikh, V.I.; Ratovskiy, G.V.; Dmitrichenko, M.Y. Phosphorylation of N,N-disubstituted amides with phosphorus pentachloride. Russ. J. Gen. Chem. 1982, 52, 2491–2499. [Google Scholar]
- Pensionerova, G.A.; Rozinov, V.G.; Glukhikh, V.I. Phosphorylation of tertiary amides with phosphorus pentachloride. Russ. J. Gen. Chem. 1981, 51, 1201–1202. [Google Scholar]
- Rozinov, V.G.; Izhboldina, L.P.; Donskih, V.I.; Dolgushin, G.V. Bisphosphorylated N-vinyl-N-chlorovinylamine on the base of N-vinyl-N-butylacetamide. Russ. J. Gen. Chem. 1984, 54, 222–223. [Google Scholar]
- Rozinov, V.G.; Izhboldina, L.P.; Donskih, V.I.; Kalabina, A.V. Reaction of phosphorus pentachloride with enamides—A new source of 1,4-azaphosphorins. Russ. J. Gen. Chem. 1987, 57, 478–479. [Google Scholar]
- Rozinov, V.G.; Izhboldina, L.P.; Donskih, V.I.; Ratovskiy, G.V.; Sergienko, L.M.; Dolgushin, G.V.; Dmitrichenko, M.Y. Phosphor-containing enamines. V. Features of the formation of 1,4-azaphosphorines during phosphorylation of enamides. Russ. J. Gen. Chem. 1989, 59, 997–1018. [Google Scholar]
- Dmitrichenko, M.Y.; Donskih, V.I.; Rozinov, V.G.; Dolgushin, G.V.; Feshin, V.P.; Kalabina, A.V. Phosphorylation of N-methyl-N,N-diacetamide. Russ. J. Gen. Chem. 1987, 57, 1912–1913. [Google Scholar]
- Dmitrichenko, M.Y.; Rozinov, V.G.; Zinchenko, S.V.; Zherebtsov, A.A. 1,4-Azaphosphorine from N,N-diacetamide. Russ. J. Gen. Chem. 1990, 60, 1919–1920. [Google Scholar] [CrossRef]
- Larina, L.I.; Elokhina, V.N.; Yaroshenko, T.I.; Nakhmanovich, A.S.; Dolgushin, G.V. 1H, 13C, 15N and 19F NMR study of acetylation products of heterocyclic thiosemicarbazones. Magn. Reson. Chem. 2007, 45, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Larina, L.I.; Lopyrev, V.A. Nitroazoles: Synthesis, Structure and Applications; Springer: New York, NY, USA, 2009; 446p. [Google Scholar]
- Larina, L.I. Tautomerism and Structure of azoles: Nuclear Magnetic Resonance Spectroscopy. Adv. Heterocycl. Chem. 2018, 124, 233–321. [Google Scholar] [CrossRef]
- Larina, L.I. The structure of biologically active functionalized azoles: NMR spectroscopy and quantum chemistry. Magnetochemistry 2022, 8, 52. [Google Scholar] [CrossRef]
- Larina, L.I. Nuclear Quadrupole Resonance Spectroscopy: Tautomerism and structure of functional azoles. Crystals 2019, 9, 366. [Google Scholar] [CrossRef]
- Dmitrichenko, M.Y.; Rozinov, V.G.; Eliseeva, G.D.; Ratovskiy, G.V.; Kushnarev, D.F.; Zhilyakov, A.V. Phosphorylation of N-acetylhydropyridazine. Russ. J. Gen. Chem. 1997, 67, 162. [Google Scholar]
- Kolbina, V.E.; Dolgushin, G.V.; Rozinov, V.G. Phosphorylated amino-1,3-pentendienes. Russ. J. Gen. Chem. 1992, 62, 471–472. [Google Scholar]
- Borisov, A.A.; Rozinov, V.G.; Bohzenkov, G.V.; Larina, L.I. Enamino ketones in the synthesis of phosphorus-containing heterocycles. Russ. J. Gen. Chem. 2004, 74, 1822–1823. [Google Scholar] [CrossRef]
- Borisov, A.A.; Kolbina, V.E.; Dmitrichenko, M.Y.; Larina, L.I.; Rozinov, V.G. Phosphorus-containing quinolone derived from phenylaminocrotonate. Russ. J. Gen. Chem. 2005, 75, 1676–1677. [Google Scholar] [CrossRef]
- Larina, L.I.; Komarova, T.N.; Rozinov, V.G. C-phosphorylation of unsaturated ketones with phosphorus pentachloride. Russ. J. Gen. Chem. 2016, 86, 512–514. [Google Scholar] [CrossRef]
- Larina, L.I.; Komarova, T.N.; Rozinov, V.G. Phosphorus-containing dienes derived from diacetone alcohol: Synthesis and study by NMR spectroscopy. Russ. Chem. Bull. 2015, 64, 2744–2746. [Google Scholar] [CrossRef]
- Rozinov, V.G.; Kolbina, V.E.; Dmitrichenko, M.Y. Features of phosphorylation of oximes and amides under mild conditions. Russ. J. Gen. Chem. 1997, 67, 519–520. [Google Scholar]
- Rozinov, V.G.; Dmitrichenko, M.Y.; Larina, L.I.; Schmidt, E.Y.; Michaleva, A.I. Unsaturated organophosphorus compounds based on O-vinyl oximes. Russ. J. Gen. Chem. 2001, 71, 1041–1042. [Google Scholar] [CrossRef]
- Sokolov, F.; Brusko, V.; Safin, D.; Cherkasov, R.; Zabirov, N. Coordination diversity of N-phosphorylated amides and ureas towards viiib group cations. Transit. Met. Chem. New Res. 2008, 101–150. Available online: https://dspace.kpfu.ru/xmlui/handle/net/141420 (accessed on 12 April 2023).
- Kantlehner, W.; Kreß, R.; Mezger, J.; Ziegler, G. Orthoamide und Iminiumsalze, LXXXVIII. Synthese N,N,N′,N′,N″,N″-persubstituierter Guanidiniumsalze aus N,N′-persubstituierten Harnstoff/Säurechlorid-Addukten. Z. Naturforsch. B 2015, 70, 9–27. [Google Scholar] [CrossRef]
- Bhagat, V.; O’Brien, E.; Zhou, J.; Becker, M.L. Caddisfly inspired phosphorylated poly(ester-urea)-based degradable bone adhesives. Biomacromolecules 2016, 17, 3016–3024. [Google Scholar] [CrossRef]
- Bhagat, V.; Matthew, L.; Becker, M.L. Degradable adhesives for surgery and tissue engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef]
- Hawkins, L.J.; Wang, M.; Zhang, B.; Xiao, Q.; Wang, H.; Storey, K.B. Glucose and urea metabolic enzymes are differentially phosphorylated during freezing, anoxia, and dehydration exposures in a freeze tolerant frog. Comparat. Biochem. Physiol. Part D 2019, 30, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bakibaev, A.A.; Zhumanov, K.B.; Panshina, S.Y.; Gorbin, S.I.; Malkov, V.S.; Tsoy, I.G.; Massalimova, B.K.; Matniyazova, G.K.; Baybazarova, E.A. Synthesis methods of phosphorylated carbamide containing acyclic and heterocyclic compounds. Bull. Karag. Univer. Chem. Ser. 2019, 95, 115–117. [Google Scholar] [CrossRef]
- Kesseli, F.P.; Lauer, C.S.; Baker, I.; Mirica, K.A.; Van Citters, W. Identification of a calcium phosphoserine coordination network in an adhesive organo-apatitic bone cement system. Acta Biomater. 2020, 105, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Shokri, M.; Dalili, F.; Kharaziha, M.; Eslaminejad, B.M.; Tafti, H.A. Strong and bioactive bioinspired biomaterials, next generation of bone adhesives. Adv. Coll. Interface Sci. 2022, 305, 102706. [Google Scholar] [CrossRef] [PubMed]
- Bingol, H.B.; Bender, J.C.M.E.; Opsteen, J.A.; Leeuwenburgh, S.C.G. Bone adhesive materials: From bench to bedside. Mater. Today Bio 2023, 19, 100599. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, X.; Ma, C.; Xia, W.; Hu, L.; Dong, X.; Xiong, Y. Electrochemically oxidative phosphating of aldehydes and ketones. J. Org. Chem. 2023, 88, 4264–4272. [Google Scholar] [CrossRef]
- Rozinov, V.G.; Dmitrichenko, M.Y.; Donskih, V.I.; Dolgushin, G.V.; Feshin, V.P. 1,3,4-Diazaphosphorine from N-acetylurea. Russ. J. Gen. Chem. 1987, 57, 228–229. [Google Scholar]
- Dmitrichenko, M.Y.; Rozinov, V.G.; Donskih, V.I.; Ratovskiy, G.V.; Sergienko, L.M.; Dolgushin, G.V.; Valeev, R.B. Phosphorus-containing enureas. I. Phosphorylation of N-acetyl-N,N’-dimethylureas with phosphorus pentachloride. Russ. J. Gen. Chem. 1988, 58, 2252–2261. [Google Scholar]
- Dmitrichenko, M.Y.; Dolgushin, G.V.; Rozinov, V.G.; Donskih, V.I. Phosphorylation of N-methyl-N’-acetylurea. Russ. J. Gen. Chem. 1990, 60, 462–463. [Google Scholar]
- Dmitrichenko, M.Y.; Rozinov, V.G.; Donskih, V.I.; Ratovskiy, G.V.; Dolgushin, G.V.; Vitkovsky, V.Y.; Shilin, S.V. 1,5,2-Diazaphosphorines. II. Synthesis and peculiarities of cyclization of phosphorus-containing azabutadienes on the base of acetyl- and chloroacetylureas. Russ. J. Gen. Chem. 1991, 61, 643–656. [Google Scholar]
- Dmitrichenko, M.Y.; Rozinov, V.G.; Donskih, V.I.; Ratovskiy, G.V.; Efremov, V.G.; Dolgushin, G.V. 1,5,2-Diazaphosphorines. III. Phosphorus-containing azabutadienes and heterocycles from N-methyl-N’-acetylurea. Russ. J. Gen. Chem. 1992, 62, 306–318. [Google Scholar]
- Dmitrichenko, M.Y.; Rozinov, V.G.; Ratovskiy, G.V.; Dolgushin, G.V.; Donskih, V.I.; Sergienko, L.M. 1,5,2-Diazaphosphorines. IV. Structure and chemical transformation of 2,2,4,6-tetrachloro-2,2-dihydro-1,5,2-diazaphosphorine. Russ. J. Gen. Chem. 1993, 63, 1976–1989. [Google Scholar]
- Dmitrichenko, M.Y.; Donskih, V.I.; Rozinov, V.G.; Dolgushin, G.V.; Ratovskiy, G.V.; Efremov, V.G.; Rubkina, V.V. Phosphorus-containing chloroethenylchloroformamidines from N-acetylureas. Russ. J. Gen. Chem. 1989, 59, 227–228. [Google Scholar]
- Dmitrichenko, M.Y.; Rozinov, V.G.; Ratovskiy, G.V.; Dolgushin, G.V.; Donskih, V.I.; Sergienko, L.M. 1,5,2-Diazaphosphorines. V. Features of amination of heterocycles of varying degrees of unsaturation. Russ. J. Gen. Chem. 1995, 65, 426–430. [Google Scholar]
- Larina, L.I.; Dmitrichenko, M.Y.; Rozinov, V.G.; Benedyuk, A.V. Phosphorylation of N-acetyl-N,N’-ethyleneurea with phosphorus pentachloride. Russ. J. Gen. Chem. 2005, 75, 484–485. [Google Scholar] [CrossRef]
- Larina, L.I.; Rozinov, V.G.; Chernyshev, K.A. The Products of Phosphorylation of N,N-Dialkylureas and Dialkylcyanamides with Phosphorus Pentachloride. NMR Spectroscopy Study. Russ. J. Gen. Chem. 2012, 82, 72–76. [Google Scholar] [CrossRef]
- Chernyshev, K.A.; Larina, L.I.; Rozinov, V.G.; Krivdin, L.B. Quantum-chemical calculations of NMR chemical shifts of organic molecules: IX. Electronic structure of [dimethylamino(chloro)methylideneamino]trichlorophosphonium hexachlorophosphate: Azomethine or azophosphine? Russ. J. Org. Chem. 2013, 49, 195–197. [Google Scholar] [CrossRef]
- Dmitrichenko, M.Y.; Rozinov, V.G.; Donskih, V.I. Synthesis of 1-methyl-2,2,2-trichloro-3-(1,1,2,2-tetrachloroethyl)-1,3-diaza-2-phosphetidinone. Russ. J. Gen. Chem. 1989, 59, 715–716. [Google Scholar]
- Turchaninov, V.K.; Dolgushin, G.V.; Dmitrichenko, M.Y.; Larina, L.I. AM1 study of photoelectron spectra. 9. 2,2,4,6-Tetrachloro-2,2-dihydro-1,5,2-diazaphosphorinine. Russ. Chem. Bull. 1996, 45, 781–785. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wagberg, L. Phosphorylated 619 Cellulose Nanofibrils: A Renewable Nanomaterial for the Preparation of Intrinsically 620 Flame-Retardant Materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef]
- Long, H.; Huang, C.; Zheng, Y.-T.; Li, Z.-Y.; Jie, L.-H.; Song, J.; Zhu, S.; Xu, H.-C. Electrochemical C–H phosphorylation of arenes in continuous flow suitable for late-stage functionalization. Nat. Commun. 2021, 12, 6629–6636. [Google Scholar] [CrossRef]
- Rol, F.; Sillard, C.; Bardet, M.; Yarava, J.R.; Emsley, L.; Gablin, C.; Leonard, D.; Belgacem, N.; Bras, J. Cellulose phosphorylation comparison and analysis of phosphorate position on cellulose fibers. Carbohydr. Polym. 2020, 229, 115294–115332. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, J.; Hu, Y.; Chen, Q.; Jiu, Y.; Li, S.; Maruoka, K.; Huo, Y.; Zhang, H.-L. Visible-Light-Induced α-C(sp 3)–H Phosphinylation of Unactivated Ethers under Photocatalyst- and Additive-Free Conditions. J. Org. Chem. 2022, 87, 11281–11291. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Yan, Z.; Liu, L.L. Facile synthesis of the dicyanophosphide anion via electrochemical activation of white phosphorus: An avenue to organophosphorus compounds. J. Amer. Chem. Soc. 2022, 144, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Bonfant, G.; Balestri, D.; Perego, J.; Comotti, A.; Bracco, S.; Koepf, M.; Gennari, M.; Marchio, L. Phosphine oxide porous organic polymers incorporating cobalt(II) ions: Synthesis, characterization, and investigation of H 2 production. ASC Omega 2022, 7, 6104–6112. [Google Scholar] [CrossRef]
- Mondal, A.; Thiel, N.O.; Dore, R.; Feringa, B.L. P-chirogenic phosphorus compounds by stereoselective Pd-catalysed arylation of phosphoramidites. Nat. Catal. 2022, 5, 10–19. [Google Scholar] [CrossRef]






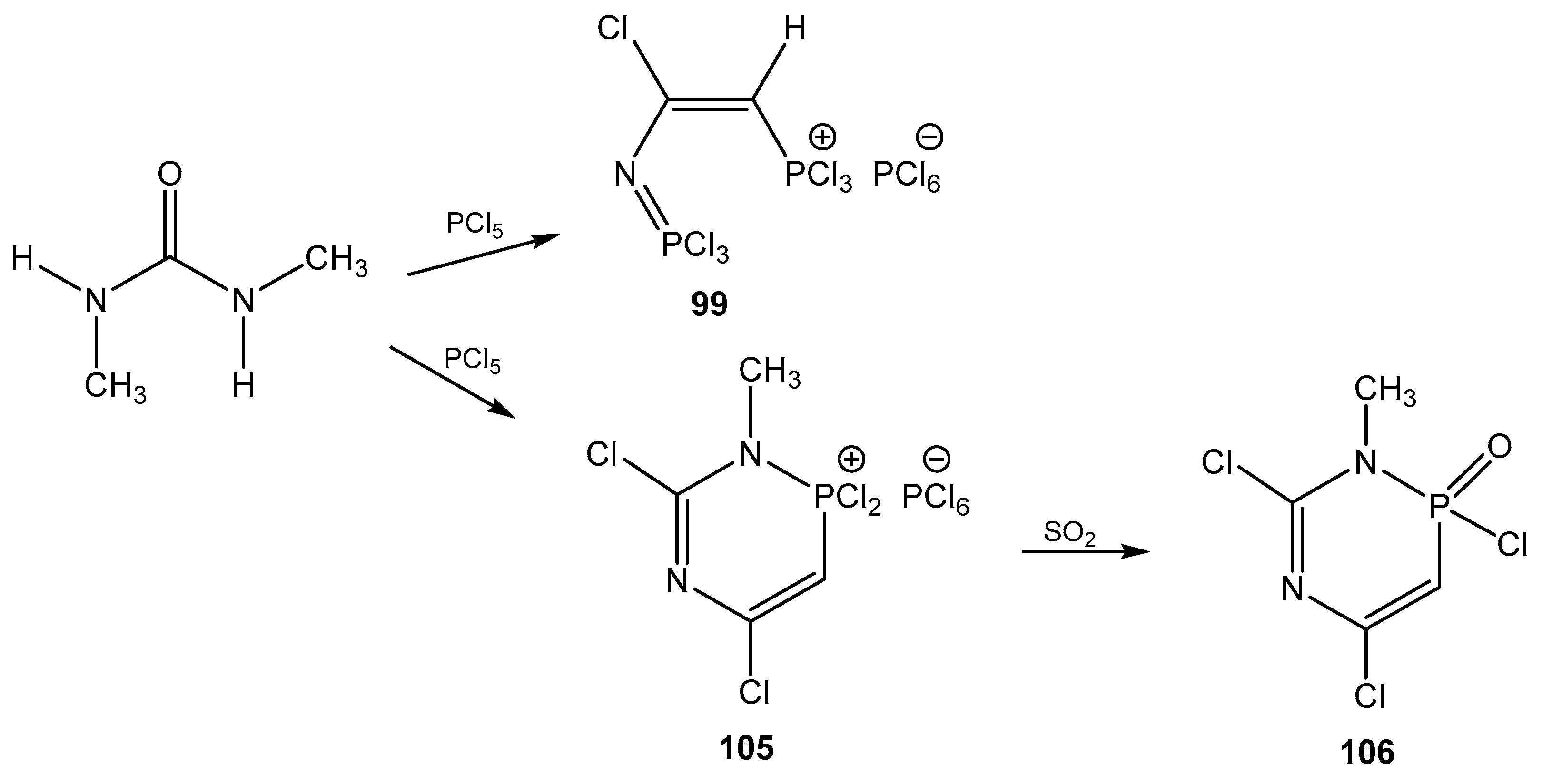



| No. | Structure | 1H a | 13C | 31P b |
|---|---|---|---|---|
| 1 | 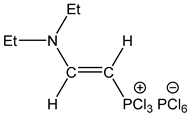 | 8.00 dd, 1H, N–CH= 3JPH = 25.9, 3JHH = 13.7 5.65 dd, 1H, P–CH= 2JPH = 33.7, 3JHH = 13.7 | 153.25 d, N–CH=, 2JPC = 47.3 81.47 d, P–CH=, 1JPC = 195.4 | 89.9 dd 2JPH = 33.7 3JPH = 25.9 |
| 2 |  | 7.81 dd, 1H, N–CH= 3JPH = 23.8, 3JHH = 13.9 5.50 dd, 1H, P–CH= 2JPH = 34.0, 3JHH = 13.9 | 155.12 d, N–CH=, 2JPC = 48.1 80.92 d, P–CH=, 1JPC = 200.2 | 80.1 dd 2JPH = 34.0 3JPH = 23.8 |
| 3 | 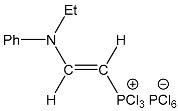 | 7.60 dd, 1H, N–CH= 3JPH = 21.4, 3JHH = 13.9 4.55 dd, 1H, P–CH= 2JPH = 34.3, 3JHH = 13.9 | 150.40 d, N–CH=, 2JPC = 52.7 80.29 d, P–CH=, 1JPC = 200.8 | 79.8 dd 2JPH = 34.3 3JPH = 21.4 |
| 4 | 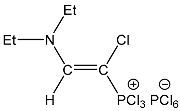 | 7.50 d, 1H, N–CH= 3JPH = 14.1 | 158.11 d, N–CH=, 2JPC = 37.5 77.26 d, P–CH=, 1JPC = 187.1 | 80.7 d 3JPH = 14.1 |
| 5 | 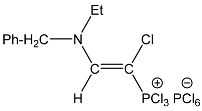 | 7.65 d, 1H, N–CH= 3JPH = 13.9 | 157.20 d, N–CH=, 2JPC = 35.4 77.82 d, P–CH=, 1JPC = 179.5 | 82.0 d 3JPH = 13.9 |
| 6 | 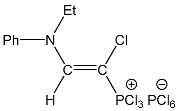 | 7.50 d, 1H, N–CH= 3JPH = 13.7 | 159.21 d, N–CH=, 2JPC = 30.9 75.64 d, P–CH=, 1JPC = 159.6 | 72.0 d 3JPH = 13.7 |
| 7 |  | 7.25 dd, 1H, N–CH= 3JPH = 18.3, 3JHH = 13.1 5.36 dd, 1H, P–CH= 2JPH = 9.0, 3JHH = 13.1 3.27 m 4H, CH2 , 1.29 m 3HCH3 | 156.80 d, N–CH=, 2JPC = 31.0 85.11 d, P–CH=, 1JPC = 201.2 50.67 s, CH2 14.86 s, CH3 | 160.0 dd PCl2 2JPH = 9.0 3JPH = 18.3 |
| 8 | 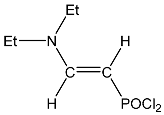 | 7.06 dd, 1H, N–CH= 3JPH = 21.5, 3JHH = 13.7 5.12 dd, 1H, P–CH= 2JPH = 25.8, 3JHH = 13.7 3.51 m 4H, CH2, 1.27 m 6H, CH3 | 150.88 d, N–CH=, 2JPC = 38.9 85.45 d, P–CH=, 1JPC = 208.9 43.51 s, CH2 13.84 s, CH3 | 35.0 dd POCl2 2JPH = 25.8 3JPH = 21.5 |
| 9 |  | 7.25 dd, 1H, N–CH= 3JPH = 21.2, 3JHH = 14.2 7.1–7.3 m, 5H, Ph 5.20 dd, 1H, P–CH= 2JPH = 25.5, 3JHH = 14.2 4.62 s, 2H, CH2-Ph 3.54 m, 2H, CH2 1.28 m, 3H, CH3 | 149.27 d, N–CH=, 2JPC = 37.1 86.23 d, P–CH=, 1JPC = 208.9 125.20 m, Ph 51.60 s, CH2-Ph 49.70 s, CH2 15.23 s, CH3 | 36.2 dd POCl2 2JPH = 25.5 3JPH = 21.2 |
| 10 | 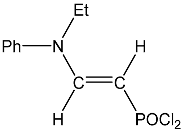 | 7.20 dd, 1H, N–CH= 3JPH = 21.8, 3JHH = 14.1 7.1–7.3 m, 5H, Ph 4.95 dd, 1H, P–CH= 2JPH = 26.4, 3JHH = 14.1 3.73 m, 2H, CH2 1.25 m, 3H, CH3 | 150.10 d, N–CH=, 2JPC = 32.8 85.32 d, P–CH=, 1JPC = 201.4 120.40 m, Ph 48.34 s, CH2 15.00 s, CH3 | 37.0 dd POCl2 2JPH = 26.4 3JPH = 21.8 |
| 11 | 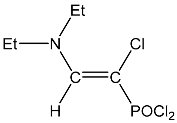 | 7.15 d, 1H, N–CH= 3JPH = 11.6 3.45 m, 4H, CH2 1.18 m, 6H, CH3 | 145.19 d, N–CH=, 2JPC = 39.4 1JCH=165.7 88.64 d, P–CH=, 1JPC = 212.5 46.91 s, CH2 14.84 s, CH3 | 36.1 d POCl2 3JPH = 11.6 |
| 12 |  | 7.34 d, 1H, N–CH= 3JPH = 12.1 7.2–7.4 m, 5H, Ph 5.37 s, 2H, CH2-Ph 3.53 m, 2H, CH2 1.23 m, 3H, CH3 | 148.95 d, N–CH=, 2JPC = 39.8 86.02 d, P–CH=, 1JPC = 210.3 124.24 m, Ph 54.26 s, CH2 -Ph 49.15 s, CH2 14.65 s, CH3 | 35.9 d POCl2 3JPH = 12.1 |
| 13 |  | 7.51 d, 1H, N–CH= 3JPH = 11.1 7.1–7.3 m, 5H, Ph 3.55 m, 2H, CH2 1.20 m, 3H, CH3 | 151.44 d, N–CH=, 2JPC = 33.5 84.57 d, P–CH=, 1JPC = 200.4 127.40 m, Ph 51.34 s, CH2 15.07 s, CH3 | 34.8 d POCl2 3JPH = 11.1 |
| No. | Structure | 1H | 13C | 31P |
|---|---|---|---|---|
| 14 | 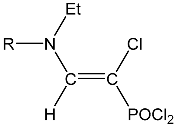 | 7.47 d, 1H, N–CH= 3JPH = 11.8 7.3 m, 5H, Ph 3.02 s, 3H, CH3 | 148.40 d, N–CH=, 2JPC = 30.5 123.4 m Ph 81.37 d, P–CH=, 1JPC = 201.0 18.07 CH3 | 34.2 d POCl2 3JPH = 11.8 |
| 15 |  | 7.63 dd, 1H, N–CH= 3JPH = 16.4, 3JHH = 12.0 7.18 m, 5H, Ph 6.68 dd, 1H, P–CH= 3JHH = 12.0, 2JPH = 5.1 3.11 s, 3H, CH3 | 150.20 d, N–CH=, 2JPC = 36.9 123.40 m Ph 88.11 d, P–CH=, 1JPC = 140.9 15.12 CH3 | 35.6 tt POCl 3JPH = 16.4 2JPH = 5.1 |
| 16 | 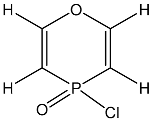 | 7.59 dd, 1H, O–CH= 3JPH = 31.4, 3JHH = 6.4 5.68 dd, 1H, P–CH= 3JHH = 6.4, 2JPH = 1.5 | 149.27 d, O–CH = 120.23 d, P–CH= 1JPC = 120.9 | 10.7 tt POCl 3JPH = 31.4 2JPH = 1.5 |
| 17 |  | 7.28 dd, 1H, N–CH= 3JPH = 22.9, 3JHH = 13.8 7.30 m, 4H, Ph 4.90 dd, 1H, P–CH= 2JPH = 33.6, 3JHH = 13.8 3.53 m, 2H, CH2 1.21 m, 3H, CH3 | 156.10 d, N–CH=, 2JPC = 32.8 129.24 m Ph 85.32 d, P–CH=, 1JPC = 201.4 48.34 CH2 15.00 CH3 | 82.7 dd 2JPH = 33.6 3JPH = 22.9 −297.9 br s |
| 18 | 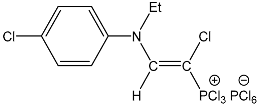 | 7.45 d, 1H, N–CH= 3JPH = 14.5 7.24 m, 4H, Ph 3.41 m, 2H, CH2 1.16 m, 3H, CH3 | 145.19 d, N–CH=, 2JPC = 39.6 86.64 d, P–CH=, 1JPC = 200.5 46.91 CH2 14.84 CH3 | 74.0 d PCl3 3JPH = 14.5 −298.0 br s |
| No. | Structure | 1H | 13C | 31P |
|---|---|---|---|---|
| 19 | 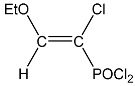 | 7.34 d, 1H, CH= 3JPH = 18.3 4.50 m, 2H, CH2 1.52 m, 3H, CH3 | 135.30 s, CH= 125.76 d, CCl 1JPC = 92.2 42.54 s, OCH2 15.32 s, CH3 | 80.1 d 3JPH = 18.3 |
| 20 | 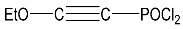 | 4.42 m, 2H, CH2 1.50 m, 3H, CH3 | 102.30 CO 94.30 CP 44.50 OCH2 13.40 CH3 | 6.0 s |
| 21 | 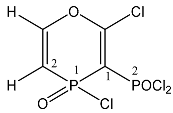 | 8.15 ddd, 1H, O–CH= 3JP1H = 57.2, 5JP2H = 19.1 3JHH = 11.0 7.59 ddd, 1H, P–CH= 4JP2H = 40.4, 2JP1H = 27.0 3JHH = 11.0 4.30 m, 2H, CH2 1.55 m, 3H, CH3 | 138.5 d, C-2 1JP1C2 = 101.2 106.4 dd, C-1 1JP1C1 = 157.7 1JP2C1 = 67.5 | 28.3 ddd, P1 3JP1H = 57.2, 2JPP = 56.5, 2JP1H = 27.0 21.4 ddd P2 2JPP = 56.5, 4JP2H = 40.4 5JP2H = 19.1 |
| 22 | 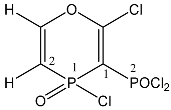 | 8.20 ddd, 1H, O–CH= 3JP1H = 62.0, 5JP2H = 16.8 3JHH = 11.0 7.50 d, 1H, P–CH= 4JP2H = 36.6, 2JP1H = 27.5 3JHH = 11.0 | 139.1 d, C-2 1JP1C2 = 100.0 108.7 dd, C-1 1JP1C1 = 150.0 1JP2C1 = 68.0 | 25.6 ddd P1 3JP1H = 62.0 2JPP = 48.8, 2JP1H = 27.5 21.9 ddd P2 2JPP = 48.8, 4JP2H = 36.6 5JP2H = 16.8 |
| 23 | 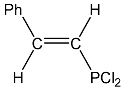 | 7.58 dd, 1H, P–CH= 3JHH = 15.3, 2JPH = 9.2 7.50 m, 5H, Ph 7.30 dd, 1H, Ph–CH= 3JPH = 19.1, 3JHH = 15.3 | 142.2 d, C-P 1JPC = 98.0 112.4 d, C-Ph 2JPC = 35.0 | 162.8 dd PCl2 3JPH = 19.1 2JPH = 9.2 |
| 24 |  | 7.50 m, 5H, Ph 7.40 d, 1H, Ph–CH= 3JPH = 22.1 | 142.2 d, C-P 1JPC = 99.0 118.3 d, C-Ph 2JPC = 33.0 | 157.8 d PCl2 3JPH = 22.1 |
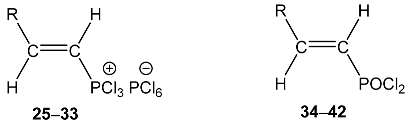 | |||||
|---|---|---|---|---|---|
| R | No. | 31P a, | No. | 1H | 31P, POCl2 |
 | 25 | 94.1 dd 2JPH = 40.2 3JPH = 25.9 | 34 | 8.50 dd, 1H, N–CH= 3JPH = 21.8, 3JHH = 15.0 7.32 m, 5H, Ph 4.75 dd, 1H, P–CH= 2JPH = 27.8, 3JHH = 15.0 1.91 3H, CH3 | 34.7 dd 2JPH = 27.8 3JPH = 21.8 |
 | 26 | 93.7 dd 2JPH = 40.0 3JPH = 26.0 | 35 | 8.54 dd, 1H, N–CH= 3JPH = 21.9, 3JHH = 15.0 7.35 m, 10H, Ph 5.12 dd, 1H, P–CH= 2JPH = 27.8, 3JHH = 15.0 | 33.7 dd 2JPH = 27.8 3JPH = 21.9 |
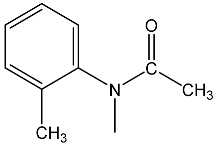 | 27 | 94.0 dd 2JPH = 39.6 3JPH = 26.6 | 36 | 7.81 dd, 1H, N–CH= 3JPH = 21.9, 3JHH = 15.2 7.20 m, 4H, Ph 5.00 dd, 1H, P–CH= 2JPH = 27.8, 3JHH = 15.2 2.15, 3H, CH3 1.98 3H, CH3 | 34.7 dd 2JPH = 27.8 3JPH = 21.9 |
 | 28 | 94.0 dd 2JPH = 40.0 3JPH = 26.1 | 37 | 8.32 dd, 1H, N–CH= 3JPH = 21.5, 3JHH = 15.1 7.33 m, 4H, Ph 4.88 dd, 1H, P–CH= 2JPH = 28.5, 3JHH = 15.1 4.15 3H, OMe; 1.96 3H, CH3 | 34.1 dd 2JPH = 28.5 3JPH = 21.5 |
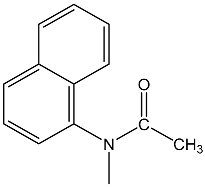 | 29 | 93.5 dd 2JPH = 40.0 3JPH = 26.0 | 38 | 8.77 dd, 1H, N–CH= 3JPH = 21.6, 3JHH = 14.6 8.1–7.4 m, 7H, Napht 4.72 dd, 1H, P–CH= 2JPH = 27.9, 3JHH = 14.6 1.88 3H, CH3 | 33.7 dd 2JPH = 27.9 3JPH = 21.6 |
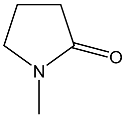 | 30 | 92.1 dd 2JPH = 39.7 3JPH = 26.8 | 39 | 7.84 dd, 1H, N–CH= 3JPH = 21.7, 3JHH = 15.1 5.30 dd, 1H, P–CH= 2JPH = 26.9, 3JHH = 15.1 3.53 m, 2H, NCH2 2.49 m, 2H, CH2CO 2.1 m, 2H, CH2 | 33.9 dd 2JPH = 26.9 3JPH = 21.7 |
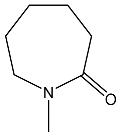 | 31 | 92.5 dd 2JPH = 40.4 3JPH = 26.3 | 40 | 8.12 dd, 1H, N–CH= 3JPH = 24.0, 3JHH = 15.2 5.41 dd, 1H, P–CH= 2JPH = 26.5, 3JHH = 15.2 3.55 m, 2H, NCH2 2.58 m, 2H, CH2CO 1.6 m, 6H, CH2 | 37.3 dd 2JPH = 26.5 3JPH = 24.0 |
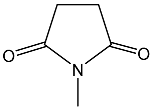 | 32 | 98.9 dd 2JPH = 40.0 3JPH = 29.4 | 41 | 7.53 dd, 1H, N–CH= 3JPH = 26.2, 3JHH = 15.6 7.31 dd, 1H, P–CH= 2JPH = 30.5, 3JHH = 15.6 2.84 m, 4H, CH2CO | 33.1 dd 2JPH = 30.5 3JPH = 26.2 |
 | 33 | 94.0 dd 2JPH = 39.6 3JPH = 26.6 | 42 | 7.64 dd, 1H, N–CH= 3JPH = 25.4, 3JHH = 15.6 7.85 m, 4H, Ph 7.12 dd, 1H, P–CH= 2JPH = 30.1, 3JHH = 15.6 | 34.4 dd 2JPH = 30.1 3JPH = 25.4 |
| No. | R | 1H | 13C | 31P |
|---|---|---|---|---|
| 43 | 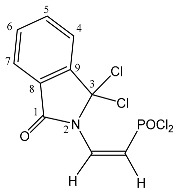 | 7.80 m, 4H, H-(4–7) 7.10 dd, 1H, N–CH= 3JPH = 57.1, 3JHH = 10.5 6.28 dd, 1H, P–CH= 2JPH = 26.2, 3JHH = 10.5 | 157.70 C-1 140.98 C-9 131.14 C-4 128.20 d, N–CH= 2JPC = 8.5 127.53 C-7 121.05 C-8 119.64 C-6 118.57 C-5 114.55 d, P–CH= 1JPC = 151.4 86.77 C-3 | 23.3 dd 2JPH = 26.2 3JPH = 57.1 |
| 44 | 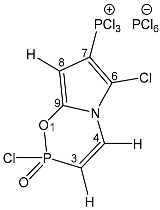 | 7.78 dd, 1H, N–CH= 3JPH = 40.8, 3JHH = 10.0 6.44 dd, 1H, P–CH= 2JPH = 20.0, 3JHH = 10.0 6.22 d, 1H, O–CH= 3JHH = 6.0 | 142.60 dd, C-9 2JPC = 23.3 d, 3JPC = 10.0 133.40 d, C-4, 2JPC = 6.0 117.84 d, C-6, 2JPC = 16.2 113.75 d, C-7, 1JPC = 185.3 101.50 dd, C-8 2JPC = 16.6 d, 3JPC = 5.5 | 83.5 dd 3JPH = 6.0 5JPP = 2.8 11.1 ddd POCl 3JPH = 40.8 2JPH = 20.0 4JPH = 2.8 −297.7 br s |
| 45 | 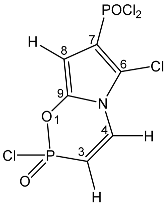 | 7.67 dd, 1H, N–CH= 3JPH = 40.5, 3JHH = 10.5 6.17 dd, 1H, P–CH= 2JPH = 18.6, 3JHH = 10.5 6.23 d, 1H, O–CH= 3JHH = 6.1 | 139.82 dd, C-9 2JPC = 25.6 d, 3JPC = 11.0 135.29 d, C-4 2JPC = 6.1 115.95 d, C-6, 2JPC = 18.3 114.80 d, C-7, 1JPC = 194.7 96.50 dd, C-8 2JPC = 14.6 d, 3JPC = 4.5 | 18.6 dd POCl2 3JPH = 6.1 5JPP = 2.8 10.4 ddd POCl 3JPH = 40.5 2JPH = 18.6 4JPH = 2.8 |
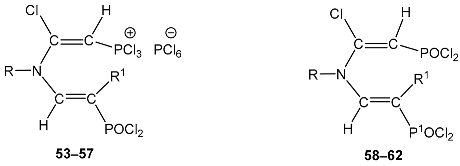 | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. | R1 | R | 31P, POCl2 | 31P a, | No. | 1H | 31P, P1OCl2 | 31P, POCl2 |
| 53 | H | Pr | 31.2 dd 2JPH = 23.4 3JPH = 23.4 | 82.7 d 2JPH = 32.2 | 58 | 7.50 dd, 1H, N–CH= 3JPH = 23.0, 3JHH = 15.0 5.75 dd, 1H, P–CH= 2JPH = 17.0 5.48 dd, 1H, P1–CH= 2JPH = 23.0, 3JHH = 15.0 3.64 t, NCH2, 3JHH = 6.7 1.49 m, 2H, CH2 0.98 t, 3H, CH3, 3JHH = 6.9 | 33.7 dd 2JPH = 23.0 3JPH = 23.0 | 25.4 d 2JPH = 17.0 |
| 54 | H | Bu | 30.7 dd 2JPH = 22.4 3JPH = 22.4 | 81.5 d 2JPH = 30.3 | 59 | 7.73 dd, 1H, N–CH= 3JPH = 21.8, 3JHH = 14.8 5.78 d, 1H, P–CH= 2JPH = 17.1 5.40 dd, 1H, P1–CH= 2JPH = 23.9, 3JHH = 14.8 3.62 t, NCH2, 3JHH = 6.8 1.44 m, 4H, (CH2)2 0.92 t, 3H, CH3, 3JHH = 6.7 | 32.5 dd 2JPH = 23.9 3JPH = 21.8 | 24.1 d 2JPH = 17.1 |
| 55 | H | PhCH2 | 30.2 dd 2JPH = 24.2 3JPH = 23.4 | 81.0 d 2JPH = 30.6 | 60 | 7.54 dd, 1H, N–CH= 3JPH = 26.0, 3JHH = 15.2 7.28 m, 5H, Ph 5.69 d, 1H, P–CH= 2JPH = 16.2 5.48 dd, 1H, P1–CH= 2JPH = 26.0, 3JHH = 15.2 4.68 s, 2H, NCH2 | 33.0 dd 2JPH = 26.0 3JPH = 26.0 | 24.7 d 2JPH = 16.2 |
| 56 | H | Ph | 30.0 dd 2JPH = 22.5 3JPH = 22.5 | 83.1 d 2JPH = 31.8 | 61 | 7.70 dd, 1H, N–CH= 3JPH = 20.3, 3JHH = 14.8 7.22 m, 5H, Ph 5.70 d, 1H, P–CH= 2JPH = 18.0 5.47 dd, 1H, P1–CH= 2JPH = 25.0, 3JHH = 14.8 | 33.4 dd 2JPH = 25.0 3JPH = 20.3 | 26.0 d 2JPH = 18.0 |
| 57 | Et | PhCH2 | 31.3 dd 3JPH = 28.6 CH2 (Et) 3JPH = 19.7 | 75.5 d 2JPH = 30.5 | 62 | 7.49 d, 1H, N–CH= 3JPH = 21.6 7.20 m, 5H, Ph 5.62 d, 1H, P–CH= 2JPH = 18.7 4.23 s, 2H, NCH2 3.28 d, 2H, CH2 3JPH = 28.6 1.70 3H, CH3 | 38.7 dd 3JPH = 28.6 CH2 (Et) 3JPH = 21.6 | 24.5 d 2JPH = 18.7 |
| No. | R | 1H | 31P, POCl2 | 31P a, /POCl | 2JPP |
|---|---|---|---|---|---|
| 63 | Pr | 9.65 dd, 1H, N–CH= 3JPH = 45.1, 3JPH = 22.9 8.25 dd, 1H, P–CH=, 4JPH = 10.4 3.64 t, NCH2, 3JHH = 6.6 1.49 m, 2H, CH2 0.98 t, 3H, CH3, 3JHH = 6.8 | 19.8 ddd 2JPP = 25.9 3JPH = 22.9 4JPH = 10.4 | 46.8 dd 3JPH = 45.1 2JPP = 25.9 | 25.9 |
| 64 | Bu | 9.90 dd, 1H, N–CH= 3JPH = 45.5, 3JPH = 22.3 8.28 d, 1H, P–CH=, 4JPH = 10.1 4.18 t, NCH2, 3JHH = 6.6 1.44 m, 1.72 m, 4H, (CH2)2 0.90 t, 3H, CH3, 3JHH = 6.7 | 20.3 ddd 2JPP = 25.5 3JPH = 22.3 4JPH = 10.1 | 46.2 dd 3JPH = 45.5 2JPP = 25.5 | 25.5 |
| 65 | PhCH2 | 9.85 dd, 1H, N–CH= 3JPH = 45.0, 3JPH = 22.3 8.02 d, 1H, P–CH=, 4JPH = 9.9 7.27 m, 5H, Ph 4.47 s, 2H, NCH2 | 19.5 ddd 2JPP = 25.6 3JPH = 22.3 4JPH = 9.9 | 46.4 dd 3JPH = 45.0 2JPP = 25.6 | 25.6 |
| 66 | Ph | 8.77 dd, 1H, N–CH= 3JPH = 44.6, 3JPH = 24.3 7.21 d, 1H, P–CH=, 4JPH = 9.6 7.34 m, 5H, Ph | 20.0 ddd 2JPP = 25.0 3JPH = 24.8 4JPH = 9.6 | 48.1 dd 3JPH = 44.6 2JPP = 25.0 | 25.0 |
| 67 | s-Bu | 8.85 dd, 1H, N–CH= 3JPH = 45.0, 3JPH = 26.0 7.40 d, 1H, P–CH=, 4JPH = 10.5 3.34 m, 1H, NCH 1.5 m, 2H, CH2 1.15 m, 6H, CH3 | 20.6 ddd 2JPP = 26.0 3JPH = 26.0 4JPH = 10.5 | 45.5 dd 3JPH = 45.0 2JPP = 26.0 | 26.0 |
| 68 | Pr | 8.12 dd, 1H, N–CH= 3JPH = 33.0, 3JPH = 23.9 6.68 d, 1H, P–CH=, 4JPH = 10.9 3.40 t, NCH2, 3JHH = 6.4 1.53 m, 2H, CH2 1.04 t, 3H, CH3, 3JHH = 6.7 | 22.6 ddd 2JPP = 25.9 3JPH = 23.9 4JPH = 10.9 | 10.9 dd 3JPH = 33.0 2JPP = 25.9 | 25.9 |
| 69 | Bu | 8.07 dd, 1H, N–CH= 3JPH = 32.9, 3JPH = 23.5 6.22 d, 1H, P–CH=, 4JPH = 10.3 4.05 t, NCH2, 3JHH = 6.5 1.40 m, 1.75 m, 4H, (CH2)2 0.92 t, 3H, CH3, 3JHH = 6.4 | 22.1 ddd 2JPP = 24.3 3JPH = 23.5 4JPH = 10.3 | 10.2 dd 3JPH = 32.9 2JPP = 24.3 | 24.3 |
| 70 | PhCH2 | 8.07 dd, 1H, N–CH= 3JPH = 32.4, 3JPH = 22.9 7.48 m, 5H, Ph 6.24 d, 1H, P–CH=, 4JPH = 10.2 4.24 s, 2H, NCH2 | 25.5 ddd 2JPP = 24.4 3JPH = 22.9 4JPH = 10.2 | 13.5 dd 2JPP = 24.4 3JPH = 32.4 | 24.4 |
| 71 | Ph | 7.95 dd, 1H, N–CH= 3JPH = 32.6, 3JPH = 22.8 7.44 m, 5H, Ph 6.18 d, 1H, P–CH=, 4JPH = 9.9 | 25.8 ddd 2JPP = 24.1 3JPH = 22.8 4JPH = 9.9 | 13.9 dd 2JPP = 24.1 3JPH = 32.6 | 24.1 |
| 72 | s-Bu | 7.90 dd, 1H, N–CH= 3JPH = 32.2, 3JPH = 23.4 6.47 d, 1H, P–CH=, 4JPH = 10.8 3.44 m, 1H, NCH 1.5 m, 2H, CH2 1.13 m, 6H, CH3 | 24.0 ddd 2JPP = 24.6 3JPH = 23.4 4JPH = 10.8 | 13.1 dd 2JPP = 24.6 3JPH = 32.2 | 24.6 |
| No. | 1H/13C | 31P, POCl2 | 31P, |
|---|---|---|---|
| 73 | 7.34 d, 1H, CH=, 4JPH = 9.3 4.32 s, CH3 140.8 d, CCl=, 2JPC = 14.0 108.47 dd, P-C-P, 1JPC = 152.6 (POCl2) 1JPC = 101.3 (POCl) 106.20 dd, CH=, 1JPC = 124.4, 3JPC = 8.5 42.15 s, CH3 | 21.8 dd 2JPP = 21.5 4JPH = 9.3 | 49.2 d 2JPP = 21.5 |
| 74 | 6.24 d, 1H, CH=, 4JPH = 8.5 3.80 s, CH3 148.8 d, CCl=, 2JPC = 12.0 110.24 dd, P-C-P, 1JPC = 149.6 (POCl2) 1JPC = 102.5 (POCl) 104.34 ddd, CH=, 1JCH = 180.6, 1JPC = 118.2, 3JPC = 9.2 43.67 s, CH3 | 17.2 dd 2JPP = 21.2 4JPH = 8.5 | 9.0 d 2JPP = 21.2 |
| 75 | 11.37 br s, 1H, NH 5.98 d, 2H, CH=, 2JPH = 2.2 145.20 d, CCl=, 2JPC = 16.1 82.31 dd, CH=, 1JCH = 187.0, 1JPC = 110.8 | - | 49.9 t 2JPH = 2.2 |
| 76 | 10.60 br s, 1H, NH 5.72 d, 2H, CH=, 2JPH = 1.1 143.70 d, CCl=, 2JPC = 14.2 80.44 dd, CH=, 1JCH = 180.0, 1JPC = 103.6 | - | 25.8 t 2JPH = 1.1 |
| No. | Structure | 1H | 13C | 31P |
|---|---|---|---|---|
| 88 | 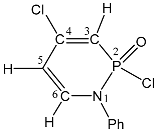 | 7.07–7.20 m, 5H, Ph 6.44 dd, 1H, H-6 3JPH = 23.9, 3JHH = 8.1 5.85 dd, 1H, H-3, 3JPH = 11.4, 4JHH = 2.2 5.36 ddd, 1H, H-5, 3JHH = 8.1, 4JHH = 2.2, 4JPH = 2.2 | 147.5, 129.8, 120.0, 127.2 Ph 139.5 d, C-6, 2JPC = 2.0 138.3 s, C-Cl, 107, d, C-3, 1JPC = 149.6 105.6 d, C-5, 3JPC = 11.6 | 21.6 dd 3JPH = 23.9 2JPH = 11.4 |
| 89 | 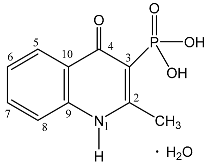 | 13.39 s, 1H, NH 8.22 d, 1H, H-5, 3JHH = 8.2 7.89 dd, 1H, H-7, 3JHH = 8.2, 3JHH = 7.6 7.78 d, 1H, H-8, 3JHH = 8.2 7.59 dd, 1H, H-6, 3JHH = 8.2, 3JHH = 7.6 3.8 br s, 2H, OH 2.83 s, 3H, CH3 | 176.03 d, C-4, 2JPC = 7.7 157.24 d, C-2, 2JPC = 13.8 138.66 s, C-9, 133.91 s, C-7, 126.05 s, C-5, 124.87 s, C-6, 121.17 d, C-10, 3JPC = 11.1 118.86 s, C-8, 109.39 d, C-3, 1JPC = 161.0 20.42 s, CH3 | 11.6 s |
| No. | R1 | R | 1H | 13C | 31P |
|---|---|---|---|---|---|
| 90 | H | Me | 7.80 dd, 1H, H-3 3JHH = 15.2, 4JPH = 2.9 7.21 d, 1H, H-4, 3JHH = 15.2 5.30 d, 1H, H-1, 2JPH = 56.4 2.10 s, 3H, CH3 | 143.50 d, C-2, 2JPC = 27.8 133.85 s, C-4, 128.67, d, C-3, 3JPC = 9.8 118.20 d, C-1, 1JPC = 116.3 21.28 s CH3 | 84.3 dd 2JPH = 56.4 4JPH = 2.9 |
| 91 | Me | Me | 7.73 d, 1H, H-3, 4JPH = 2.7 5.61 d, 1H, H-1, 2JPH = 56.2 2.08 s, 3H, CH3 1.98 s, 3H, CH3 | 142.05 d, C-2, 2JPC = 26.7 139.75 s, C-4, 124.51, d, C-3, 3JPC = 7.2 116.50 d, C-1, 1JPC = 114.9 29.27 s CH3 21.66 s CH3 | 85.7 dd 2JPH = 56.2 4JPH = 2.7 |
| 92 | H | Ph | 7.75 dd, 1H, H-3 3JHH = 14.8, 4JPH = 3.1 7.55–7.40 m, 5H, Ph 7.15 d, 1H, H-4, 3JHH = 14.8 5.87 d, 1H, H-1, 2JPH = 41.0 | 144.65 d, C-2, 2JPC = 26.0 140.90 s, i-Ph 136.86 s, C-4 128.98 s, m-Ph, p-Ph 127.69 s, o-Ph 127.48 d, C-3, 3JPC = 8.9 117.27 d, C-1, 1JPC = 115.5 | 89.1 dd 2JPH = 41.0 4JPH = 3.1 |
| 93 | H | p-MeO-Ph | 7.80 dd, 1H, H-3 3JHH = 14.9, 4JPH = 3.0 7.60, d 2H, m-Ph, 3JHH = 8.7 7.19 d, 1H, H-4, 3JHH = 14.9 6.92, d 2H, o-Ph, 3JHH = 8.7 5.00 d, 1H, H-1, 2JPH = 42.7 4.23 s, OMe | 159.27 s, C-OMe 144.25 d, C-2, 2JPC = 26.9 136.95 s, C-4 133.10 s, i-Ph 129.36 s, o-Ph 128.44 d, C-3, 3JPC = 8.2 119.54 d, C-1, 1JPC = 117.1 114.32 s, m-Ph 49.36 s, OMe | 84.6 dd 2JPH = 42.7 4JPH = 3.0 |
| 94 | H | p-Cl-Ph | 7.77 dd, 1H, H-3 3JHH = 14.5, 4JPH = 2.7 7.63, d 2H, m-Ph, 3JHH = 8.3 7.16 d, 1H, H-4. 3JHH = 14.5 6.76, d 2H, o-Ph, 3JHH = 8.3 5.63 d, 1H, H-1, 2JPH = 41.2 | 143.09 d, C-2, 2JPC = 27.6 139.00 s, i-Ph 136.29 s, C-4 134.60 s, C-Cl 129.24 s, m-Ph 129.18 s, o-Ph 128.51 d, C-3, 3JPC = 7.8 119.34 d, C-1, 1JPC = 116.9 | 88.7 dd 2JPH = 41.2 4JPH = 2.7 |
| No. | Structure | 1H | 13C | 31P |
|---|---|---|---|---|
| 103 |  | 5.93 d, H-3 2JPH 14.0 | 163.15 d, C-4, JPC 21.2 160.90 d, C-6, 2JPC 6.2 88.02 d, C-3, JPC 112.0 | 53.4 d 2JPH 14.0 |
| 104 |  | - | 160.15 d, C-4, JPC 37.8 157.73 d, C-6, 2JPC 4.9 97.27 d, C-3, JPC 132.8 | 44.7 s |
| 105 | 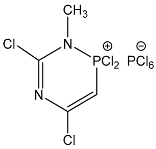 | 5.88 d, H-3 2JPH 42.2 3.45 d, CH3 3JPH 6.9 | 158.15 C-4, 2JPC 23.4 156.90 C-6, 2JPC 6.8 82.20 C-3, 1JPC 116.0 | 64.4 dq 2JPH 42.2 3JPH 6.9 –297.5 br s |
| 106 | 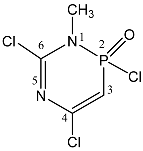 | 6.08 d, H-3 2JPH 12.8 3.38 d, CH3 3JPH 6.6 | 166.45 C-4, 2JPC 24.2 159.70 C-6, 2JPC 6.2 85.50 C-3, 1JPC 112.0 | 21.5 dq 2JPH 12.8 3JPH 6.6 |
| 107 |  | 6.84 d, CHCl2 4JPH 1.0 3.10 d, CH3 3JPH 24.4 | 147.7 d, C=O, 2JPC 17.5 95.8 d, C-Cl, 2JPC 6.1 76.5 d, CHCl, 3JPC 7.3 28.9 d, CH3, 2JPC 2.5 | −69.1 qd 3JPH 24.4 4JPH 1.0 |
| No. | Structure | 1H | 31P |
|---|---|---|---|
| 108 | 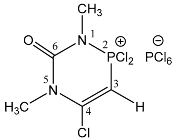 | 6.83 d, H-3, 2JPH = 10.6 3.90 s, 5-CH3 3.78 d, 1-CH3 3JPH = 9.9 | 58.3 dq 2JPH = 10.6, 3JPH = 9.9 −294.7 br s, |
| 109 | 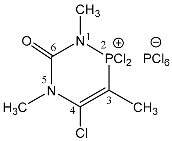 | 3.68 s, 5-CH3 3.37 d, 1-CH3 3JPH = 9.6 1.85 d, 3-CH3 3JPH = 6.7 | 61.2 qq 3JPH = 9.6, 3JPH = 6.7 −297.6 br s, |
| 110 |  | 3.57 s, 5-CH3 3.30 d, 1-CH3 3JPH = 12.2 2.85 dd, 3-CH2 3JPH = 10.0, 3JHH = 8.2 1.75 d, 3-CH3 3JHH = 8.2 | 60.8 qt 3JPH = 12.2, 3JPH = 10.0 −297.9 br s, |
| 111 | 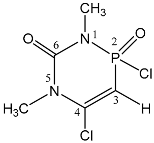 | 5.71 d, H-3 2JPH = 6.8 3.50 s, 5-CH3 3.19 d, 1-CH3 3JPH = 7.3 | 17.1 qd 3JPH = 7.3, 2JPH = 6.8 |
| 112 |  | 3.35 s, 5-CH3 3.04 d, 1-CH3 3JPH = 7.6 1.96 d, 3-CH3 3JPH = 15.1 | 18.8 qq 3JPH = 15.1, 3JPH = 7.6 |
| 113 | 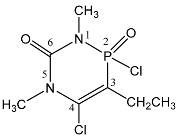 | 3.48 s, 5-CH3 3.32 d, 1-CH3 3JPH = 10.0 2.85 dd, 3-CH2 3JPH = 9.0, 3JHH = 7.8 1.75 d, 3-CH3 3JHH = 7.8 | 19.2 qt 3JPH = 10.0, 3JPH = 9.0 |
| 114 | 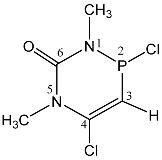 | 5.70 d, H-3 2JPH = 61.4 3.42 s, CH3 3.14 d, CH3 3JPH = 14.2 | 100.5 dq 2JPH = 61.4, 3JPH = 14.2 |
| No. | Structure | 1H | 31P | 13C |
|---|---|---|---|---|
| 117 | 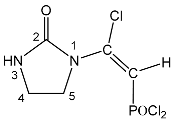 | 7.14 d, = CH 2JPH = 21.1 6.38 br s, NH 4.10 t, 5-CH2 3JHH = 8.4 3.52 t, 4-CH2 3JHH = 8.4 | 27.9 d 2JPH = 21.1 | 155.98 s, C = O 143.89 d, C-Cl, 2JPC = 6.5 103.28 d, = CH 1JPC = 176.4 45.75 s, 5-CH2 36.38 s, 4-CH2 |
| 118 | 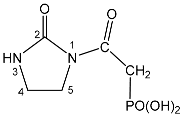 | 7.64 br s, NH 3.75 t, 5-CH2 3JHH = 8.2 3.57 d, CH2 2JPH = 21.8 3.28 t, 4-CH2 3JHH = 8.2 | 16.0 t 2JPH = 21.8 | 165.70 d, C = O 2JPC = 6.9 155.80 s, 2-C = O 42.21 s, 5-CH2 35.33 s, 4-CH2 35.28 d CH2 1JPC = 126.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larina, L. C- and N-Phosphorylated Enamines—An Avenue to Heterocycles: NMR Spectroscopy. Int. J. Mol. Sci. 2023, 24, 9646. https://doi.org/10.3390/ijms24119646
Larina L. C- and N-Phosphorylated Enamines—An Avenue to Heterocycles: NMR Spectroscopy. International Journal of Molecular Sciences. 2023; 24(11):9646. https://doi.org/10.3390/ijms24119646
Chicago/Turabian StyleLarina, Lyudmila. 2023. "C- and N-Phosphorylated Enamines—An Avenue to Heterocycles: NMR Spectroscopy" International Journal of Molecular Sciences 24, no. 11: 9646. https://doi.org/10.3390/ijms24119646
APA StyleLarina, L. (2023). C- and N-Phosphorylated Enamines—An Avenue to Heterocycles: NMR Spectroscopy. International Journal of Molecular Sciences, 24(11), 9646. https://doi.org/10.3390/ijms24119646








