Exploring a Nuclear-Selective Radioisotope Delivery System for Efficient Targeted Alpha Therapy
Abstract
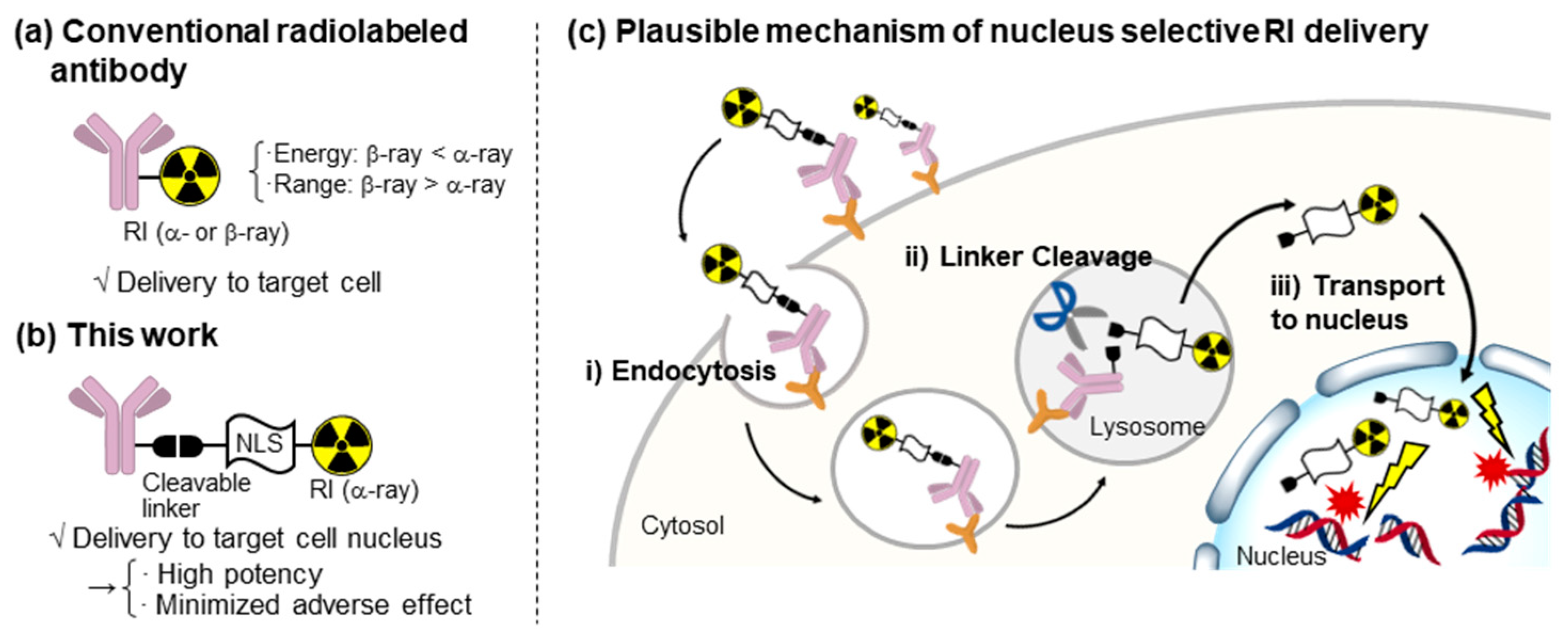
1. Introduction
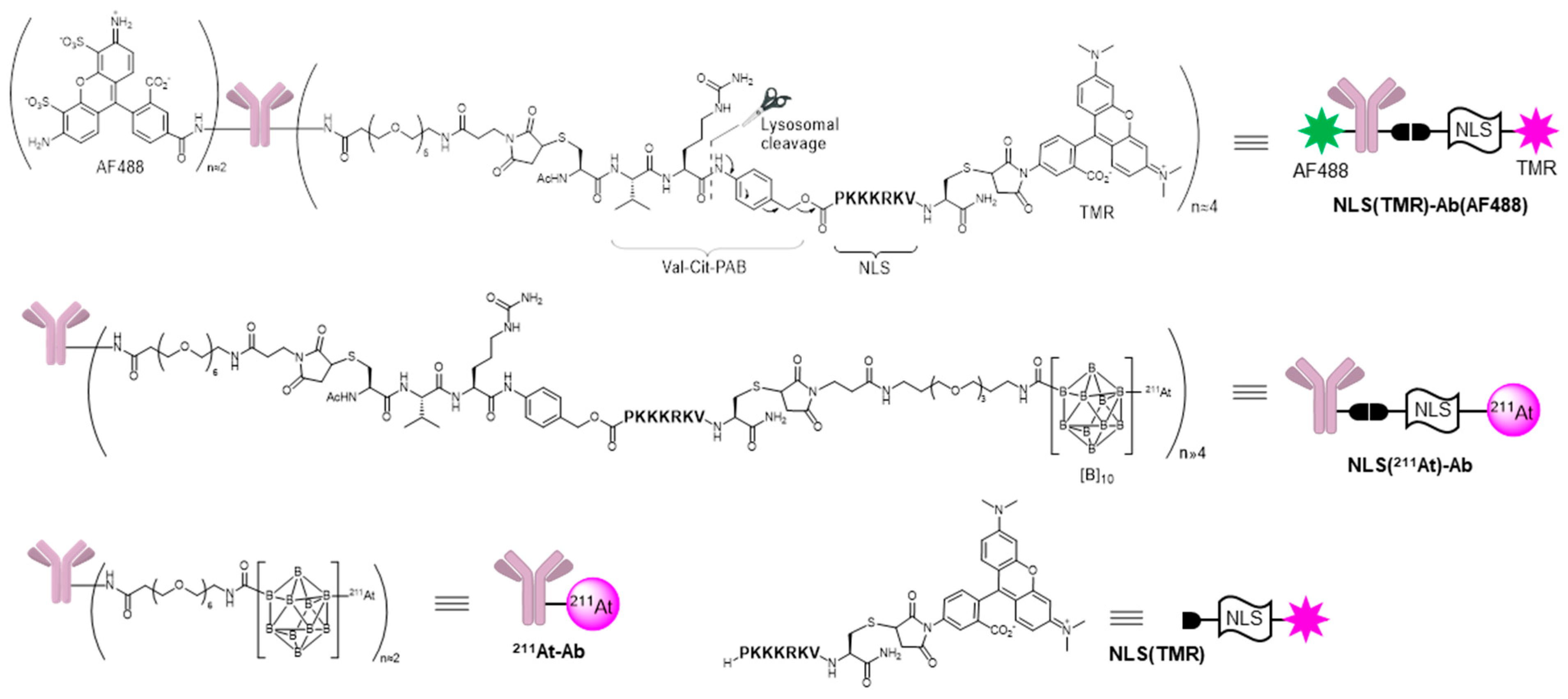
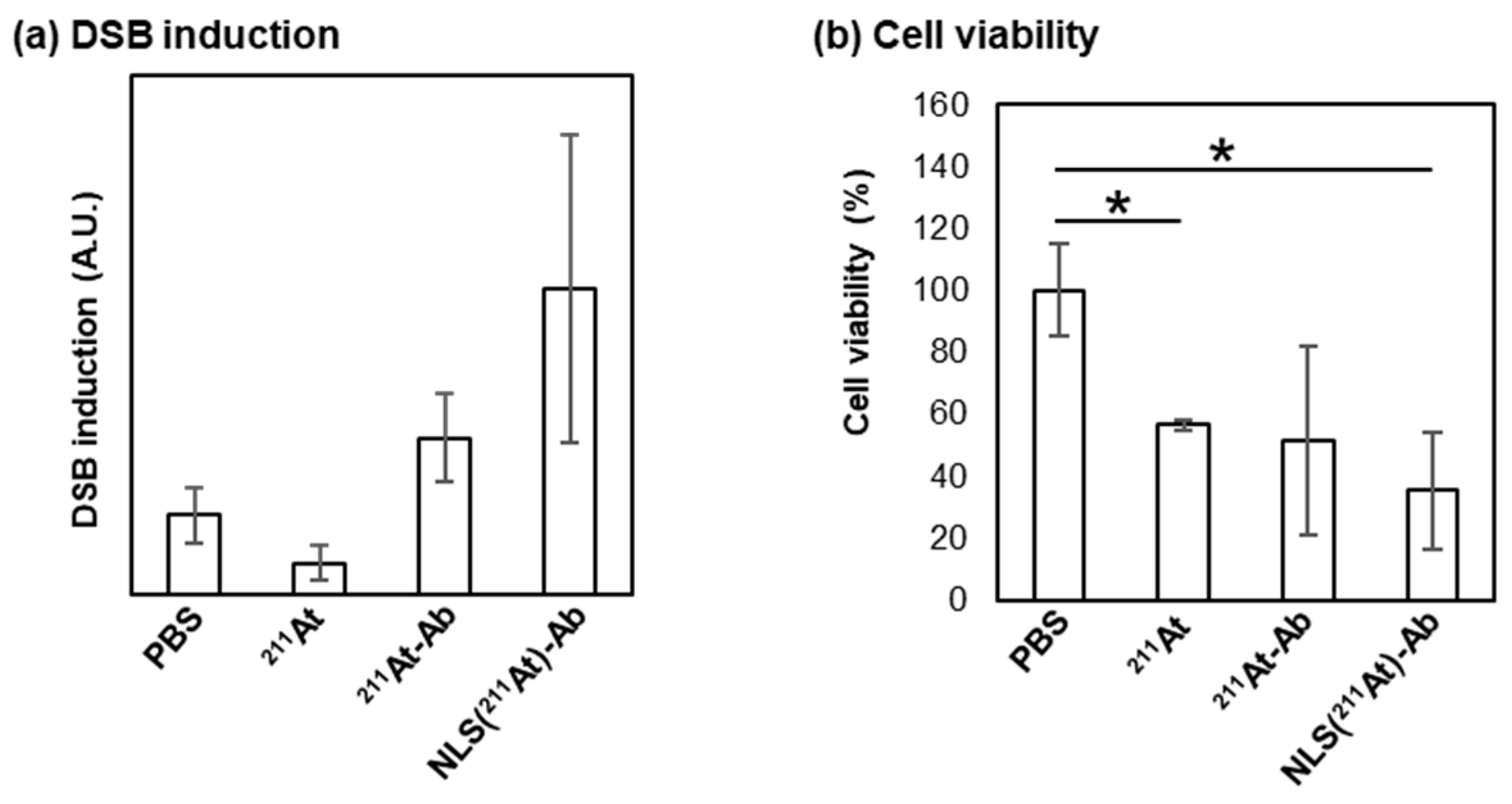
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Compounds
3.2. Fluorescent Imaging of NLS(TMR) Using Electroporation
3.3. Fluorescent Imaging of NLS(TMR)-Ab(AF488)
3.4. Protocol for Evaluation of DSB Induction
3.5. Protocol for Evaluation of Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody–drug conjugates for cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody–Drug Conjugates: A Comprehensive Review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Louzoun-Zada, S.; Jaber, Q.Z.; Fridman, M. Guiding Drugs to Target-Harboring Organelles: Stretching Drug-Delivery to a Higher Level of Resolution. Angew. Chem. Int. Ed. 2019, 58, 15584–15594. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Bodei, L.; McDevitt, M.R.; Nedrow, J.R. Radiopharmaceutical therapy in cancer: Clinical advances and challenges. Nat. Rev. Drug Discov. 2020, 19, 589–608. [Google Scholar] [CrossRef]
- Parakh, S.; Lee, S.T.; Gan, H.K.; Scott, A.M. Radiolabeled Antibodies for Cancer Imaging and Therapy. Cancers 2022, 14, 1454. [Google Scholar] [CrossRef]
- Knox, S.J.; Goris, M.L.; Trisler, K.; Negrin, R.; Davis, T.; Liles, T.M.; Grillo-López, A.; Chinn, P.; Varns, C.; Ning, S.C.; et al. Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin. Cancer Res. 1996, 2, 457–470. [Google Scholar] [PubMed]
- Kaminski, M.S.; Tuck, M.; Estes, J.; Kolstad, A.; Ross, C.W.; Zasadny, K.; Regan, D.; Kison, P.; Fisher, S.; Kroll, S.; et al. 131I-Tositumomab Therapy as Initial Treatment for Follicular Lymphoma. N. Engl. J. Med. 2005, 352, 441–449. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2021, 13, 49. [Google Scholar] [CrossRef]
- Pouget, J.-P.; Constanzo, J. Revisiting the Radiobiology of Targeted Alpha Therapy. Front. Med. 2021, 8, 692436. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Liu, Y.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Fukuda, M.; Shinohara, A.; Hatazawa, J. Enhancement of 211At Uptake via the Sodium Iodide Symporter by the Addition of Ascorbic Acid in Targeted α-Therapy of Thyroid Cancer. J. Nucl. Med. 2019, 60, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Kaneda-Nakashima, K.; Zhang, Z.; Manabe, Y.; Shimoyama, A.; Kabayama, K.; Watabe, T.; Kanai, Y.; Ooe, K.; Toyoshima, A.; Shirakami, Y.; et al. α-Emitting cancer therapy using 211At-AAMT targeting LAT1. Cancer Sci. 2021, 112, 1132–1140. [Google Scholar] [CrossRef]
- Shirakami, Y.; Watabe, T.; Obata, H.; Kaneda, K.; Ooe, K.; Liu, Y.; Teramoto, T.; Toyoshima, A.; Shinohara, A.; Shimosegawa, E.; et al. Synthesis of [211At]4-astato-L-phenylalanine by dihydroxyboryl-astatine substitution reaction in aqueous solution. Sci. Rep. 2021, 11, 12982. [Google Scholar] [CrossRef]
- Huang, X.; Kaneda-Nakashima, K.; Kadonaga, Y.; Kabayama, K.; Shimoyama, A.; Ooe, K.; Kato, H.; Toyoshima, A.; Shinohara, A.; Haba, H.; et al. Astatine-211-Labeled Gold Nanoparticles for Targeted Alpha-Particle Therapy via Intravenous Injection. Pharmaceutics 2022, 14, 2705. [Google Scholar] [CrossRef]
- Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Kadonaga, Y.; Ooe, K.; Wang, Y.; Haba, H.; Toyoshima, A.; Cardinale, J.; Giesel, F.L.; et al. Targeted α-therapy using astatine (211At)-labeled PSMA1, 5, and 6: A preclinical evaluation as a novel compound. Eur. J. Nucl. Med. Mol. Imag. 2023, 50, 849–858. [Google Scholar] [CrossRef]
- Wilbur, D.S.; Chyan, M.-K.; Hamlin, D.K.; Vessella, R.L.; Wedge, T.J.; Hawthorne, M.F. Reagents for Astatination of Biomolecules. 2. Conjugation of Anionic Boron Cage Pendant Groups to a Protein Provides a Method for Direct Labeling that is Stable to in Vivo Deastatination. Bioconjugate Chem. 2007, 18, 1226–1240. [Google Scholar] [CrossRef]
- Wilbur, D.S.; Chyan, M.-K.; Nakamae, H.; Chen, Y.; Hamlin, D.K.; Santos, E.B.; Kornblit, B.T.; Sandmaier, B.M. Reagents for Astatination of Biomolecules. 6. An Intact Antibody Conjugated with a Maleimido-closo-Decaborate(2-) Reagent via Sulfhydryl Groups Had Considerably Higher Kidney Concentrations than the Same Antibody Conjugated with an Isothiocyanato-closo-Decaborate(2-) Reagent via Lysine Amines. Bioconjugate Chem. 2012, 23, 409–420. [Google Scholar]
- Li, H.K.; Morokoshi, Y.; Nagatsu, K.; Kamada, T.; Hasegawa, S. Locoregional therapy with α-emitting trastuzumab against peritoneal metastasis of human epidermal growth factor receptor 2-positive gastric cancer in mice. Cancer Sci. 2017, 108, 1648–1656. [Google Scholar] [CrossRef]
- Fujiki, K.; Kanayama, Y.; Yano, S.; Sato, N.; Yokokita, T.; Ahmadi, P.; Watanabe, Y.; Haba, H.; Tanaka, K. 211At-labeled immunoconjugate via a one-pot three-component double click strategy: Practical access to α-emission cancer radiotherapeutics. Chem. Sci. 2019, 10, 1936–1944. [Google Scholar] [CrossRef]
- Kalderon, D.; Roberts, B.L.; Richardson, W.D.; Smith, A.E. A short amino acid sequence able to specify nuclear location. Cell 1984, 39, 499–509. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. The Chaotropic Effect as an Assembly Motif in Chemistry. Angew. Chem. Int. Ed. 2018, 57, 13968–13981. [Google Scholar] [CrossRef] [PubMed]
- Barba-Bon, A.; Salluce, G.; Lostalé-Seijo, I.; Assaf, K.I.; Hennig, A.; Montenegro, J.; Nau, W.M. Boron clusters as broadband membrane carriers. Nature 2022, 603, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kieu, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Dalla Pozza, E.; Dando, I.; Biondani, G.; Brandi, J.; Costanzo, C.; Zoratti, E.; Fassan, M.; Boschi, F.; Melisi, D.; Cecconi, D.; et al. Pancreatic ductal adenocarcinoma cell lines display a plastic ability to bi-directionally convert into cancer stem cells. Int. J. Oncol. 2015, 46, 1099–1108. [Google Scholar] [CrossRef]
- Dubowchik, G.M.; Firestone, R.A.; Padilla, L.; Willner, D.; Hofstead, S.J.; Mosure, K.; Knipe, J.O.; Lasch, S.J.; Trail, P.A. Cathepsin B-Labile Dipeptide Linkers for Lysosomal Release of Doxorubicin from Internalizing Immunoconjugates: Model Studies of Enzymatic Drug Release and Antigen-Specific In Vitro Anticancer Activity. Bioconjug. Chem. 2002, 13, 855–869. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Pilling, R.L.; Knoth, W.H. Bis (triethylammonium) Decahydrodecaborate (2−). Inorg. Synth. 1967, 9, 16–19. [Google Scholar]
- Zheng, G.; Cochella, L.; Liu, J.; Hobert, O.; Li, W.H. Temporal and spatial regulation of microRNA activity with photoactivatable cantimirs. ACS Chem. Biol. 2011, 6, 1332–1338. [Google Scholar] [CrossRef]
- Wilbur, D.S.; Chyan, M.K.; Hamlin, D.K.; Perry, M.A. Reagents for astatination of biomolecules. 3. Comparison of closo-decaborate (2−) and closo-dodecaborate (2−) moieties as reactive groups for labeling with astatine-211. Bioconjug. Chem. 2009, 20, 591–602. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iizuka, Y.; Manabe, Y.; Ooe, K.; Toyoshima, A.; Yin, X.; Haba, H.; Kabayama, K.; Fukase, K. Exploring a Nuclear-Selective Radioisotope Delivery System for Efficient Targeted Alpha Therapy. Int. J. Mol. Sci. 2023, 24, 9593. https://doi.org/10.3390/ijms24119593
Iizuka Y, Manabe Y, Ooe K, Toyoshima A, Yin X, Haba H, Kabayama K, Fukase K. Exploring a Nuclear-Selective Radioisotope Delivery System for Efficient Targeted Alpha Therapy. International Journal of Molecular Sciences. 2023; 24(11):9593. https://doi.org/10.3390/ijms24119593
Chicago/Turabian StyleIizuka, Yuki, Yoshiyuki Manabe, Kazuhiro Ooe, Atsushi Toyoshima, Xiaojie Yin, Hiromitsu Haba, Kazuya Kabayama, and Koichi Fukase. 2023. "Exploring a Nuclear-Selective Radioisotope Delivery System for Efficient Targeted Alpha Therapy" International Journal of Molecular Sciences 24, no. 11: 9593. https://doi.org/10.3390/ijms24119593
APA StyleIizuka, Y., Manabe, Y., Ooe, K., Toyoshima, A., Yin, X., Haba, H., Kabayama, K., & Fukase, K. (2023). Exploring a Nuclear-Selective Radioisotope Delivery System for Efficient Targeted Alpha Therapy. International Journal of Molecular Sciences, 24(11), 9593. https://doi.org/10.3390/ijms24119593






