High Adherence of Oral Streptococcus to Polylactic Acid Might Explain Implant Infections Associated with PLA Mesh Implantation
Abstract
1. Introduction
2. Results
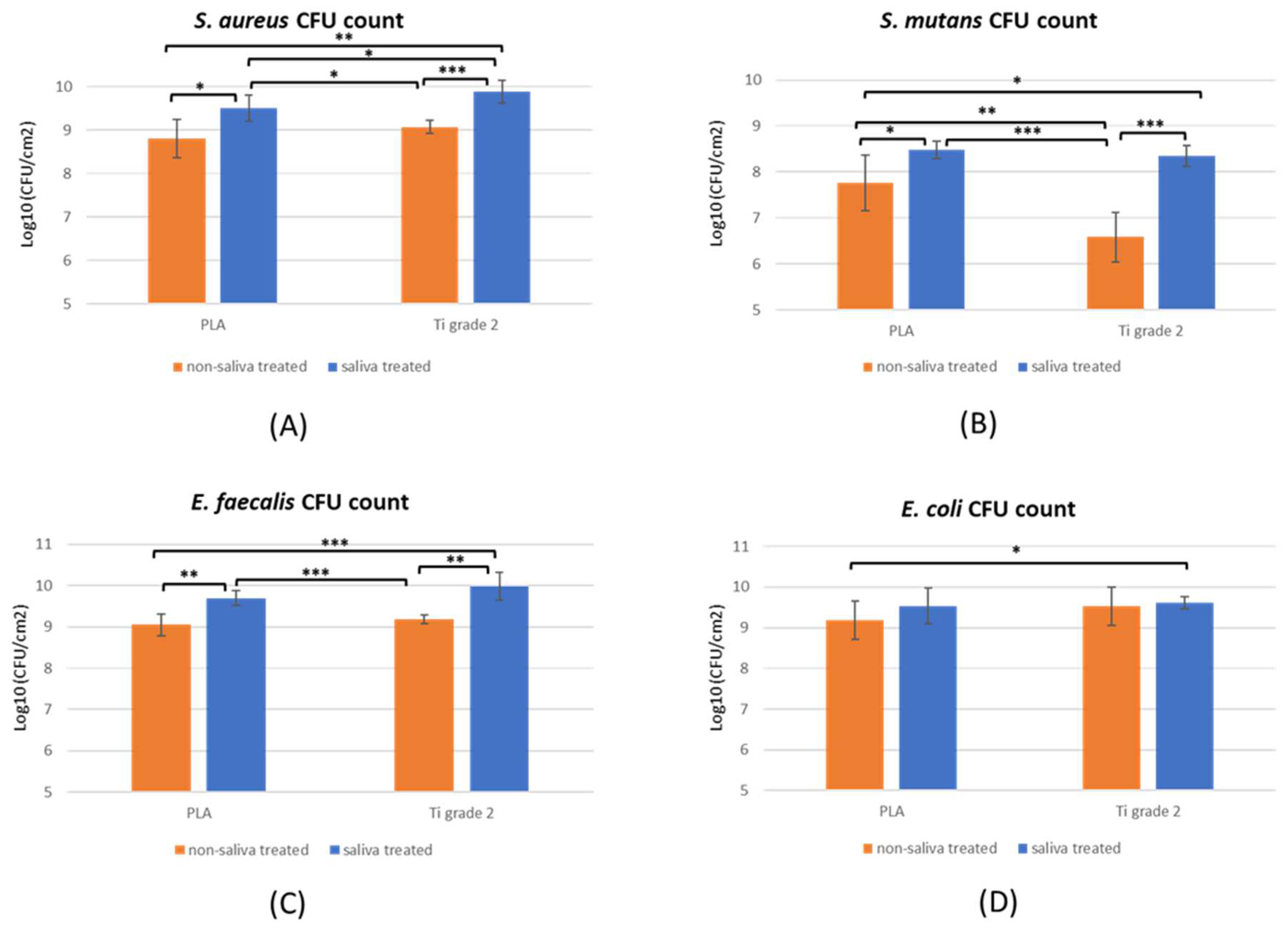
2.1. Biofilm Formation
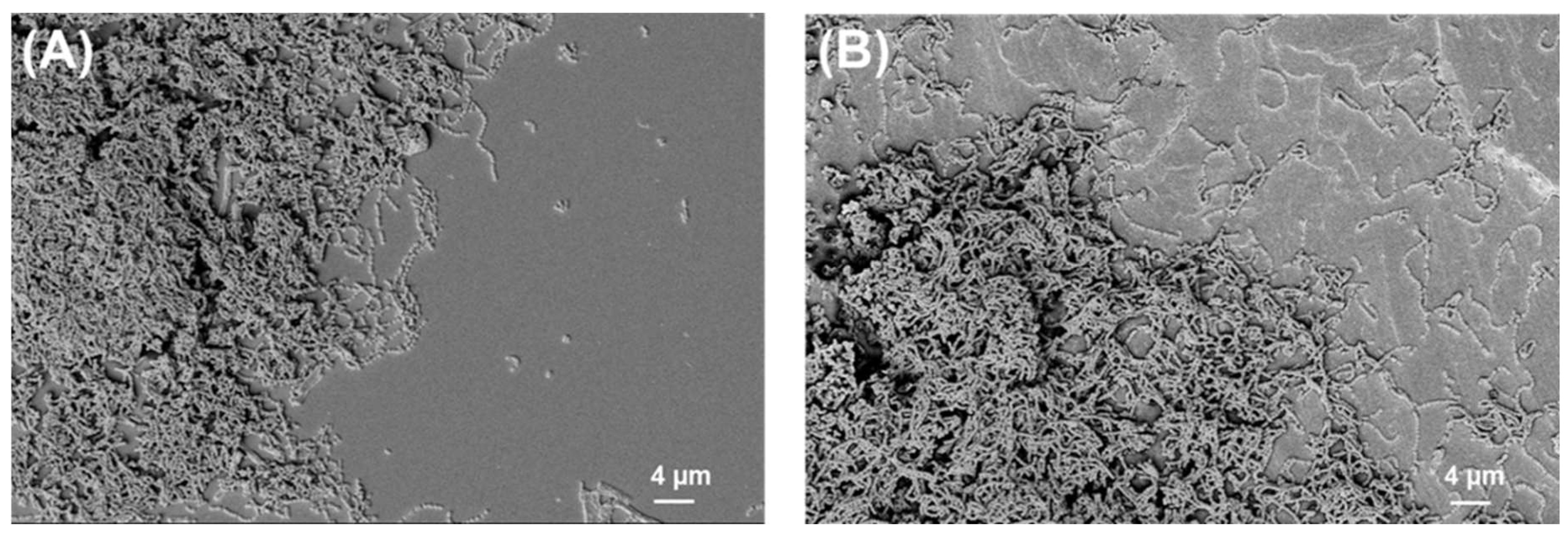
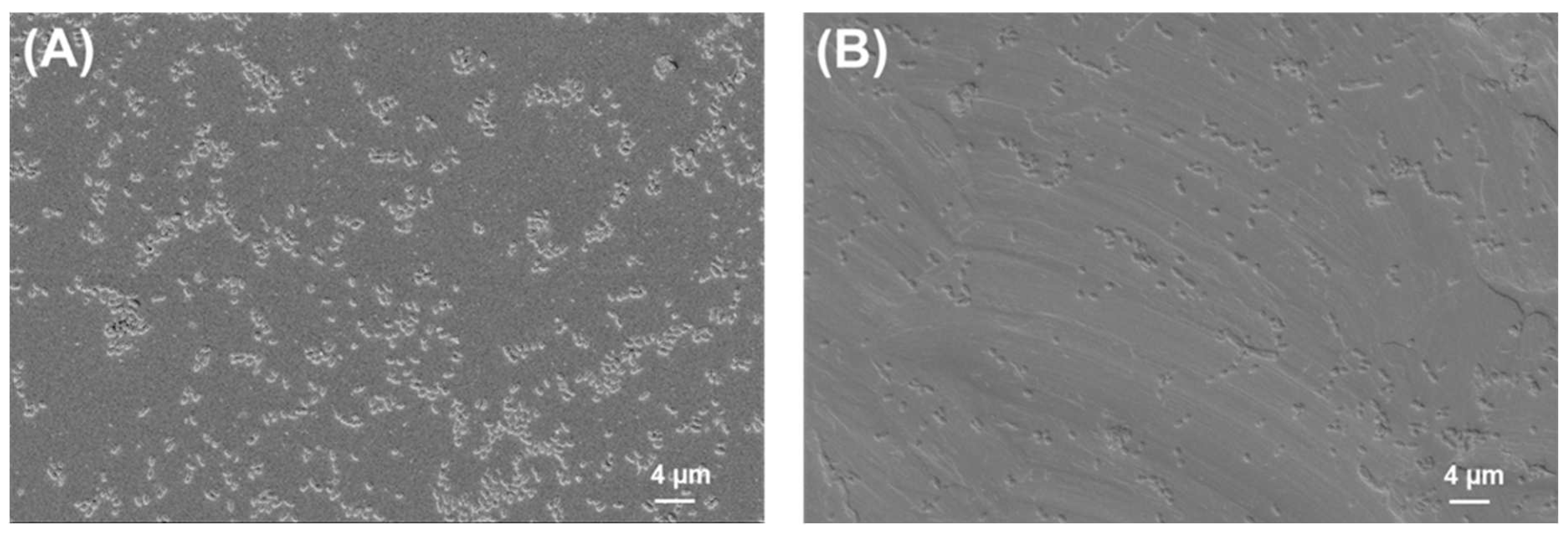
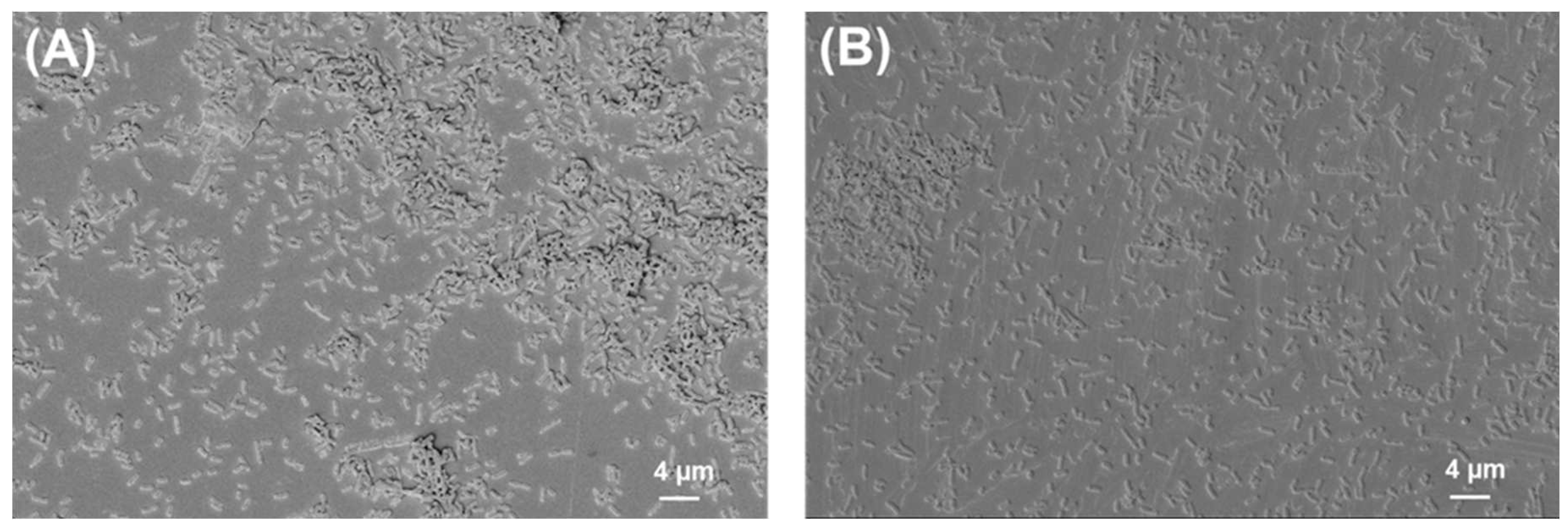
2.2. SEM Analysis of Biofilms
3. Discussion
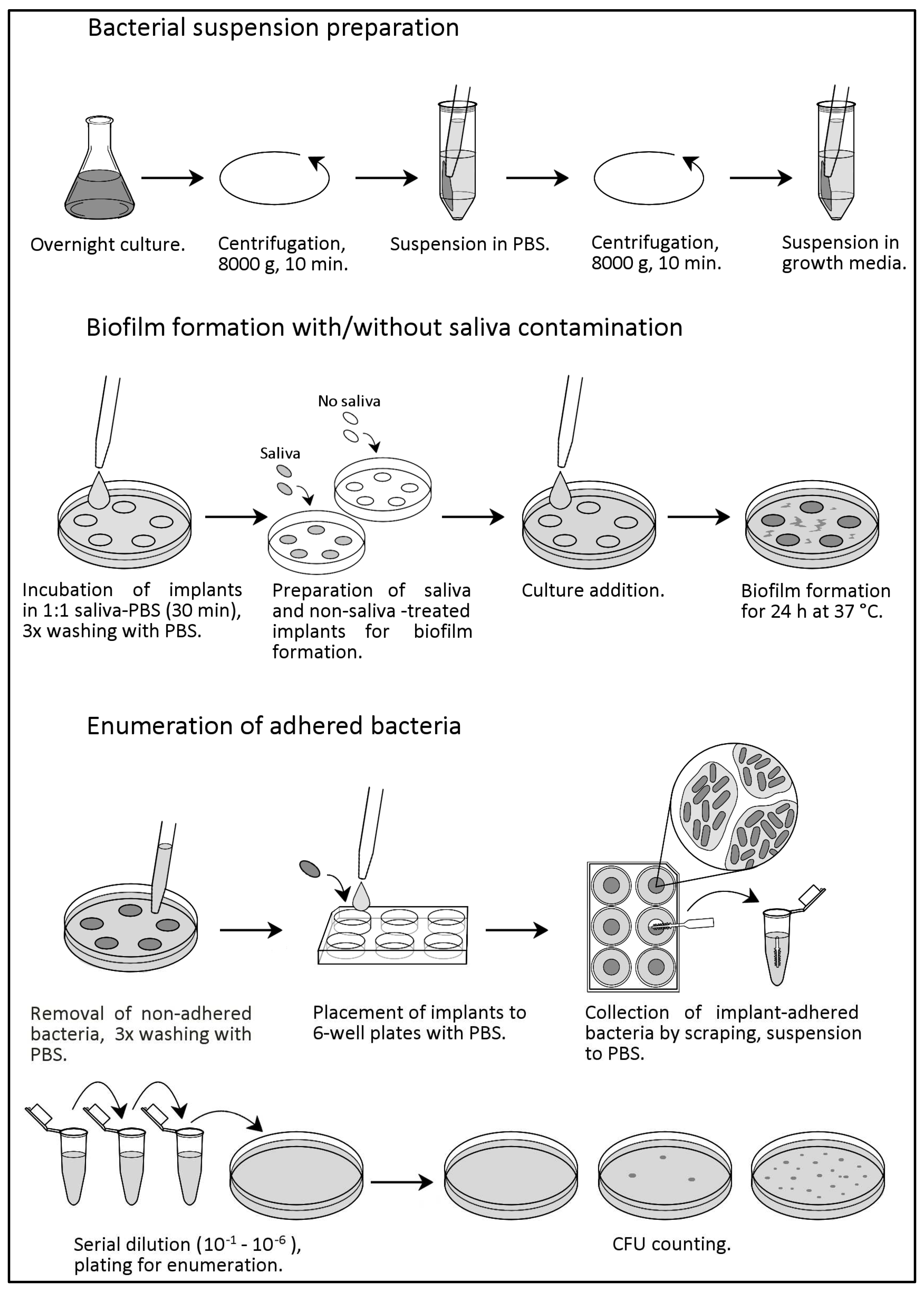
4. Materials and Methods
4.1. PLA and Titanium Implant Preparation
4.2. Bacterial Suspension Preparation
4.3. Saliva Contamination
4.4. Biofilm Formation and Enumeration of Adhered Bacteria
4.5. Preparation of Samples for Scanning Electron Microscopy (SEM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Agrawal, C.M.; Barber, F.A.; Burkhart, S.S. Orthopaedic Applications for PLA-PGA Biodegradable Polymers. Arthrosc. J. Arthrosc. Relat. Surg. 1998, 14, 726–737. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Dedukh, N.V.; Makarov, V.B.; Pavlov, A.D. Polylactide-Based Biomaterial and Its Use as Bone Implants (Analytical Literature Review). Pain Jt. Spine 2019, 9, 28–35. [Google Scholar] [CrossRef]
- Parra, M.; Moya, M.P.; Rebolledo, C.; Haidar, Z.S.; Alister, J.P.; Olate, S. PLA/PGA and Its Co-Polymers in Alveolar Bone Regeneration. A Systematic Review PLA/PGA y Sus Copolímeros En Regeneración Ósea Alveolar. Una Revisión Sistemática. Int. J. Odontostomatol. 2019, 13, 258–265. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, P.; Ray, S.; Kalia, V.C. Challenges and Opportunities for Customizing Polyhydroxyalkanoates. Indian J. Microbiol. 2015, 55, 235. [Google Scholar] [CrossRef]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; van der Heide, E. In Silico Contact Pressure of Metal-on-Metal Total Hip Implant with Different Materials Subjected to Gait Loading. Metals 2022, 12, 1241. [Google Scholar] [CrossRef]
- Ju, G.S.; Son, K.M.; Choi, W.Y.; Cheon, J.S. Clinical Usefulness of Fixation of Absorbable Implants with Cyanoacrylate in Comminuted Fractures of the Maxilla. Arch. Craniofac. Surg. 2019, 20, 233–238. [Google Scholar] [CrossRef]
- Konofaos, P.; Goubran, S.; Wallace, R.D. The Role of Resorbable Mesh as a Fixation Device in Craniosynostosis. J. Craniofac. Surg. 2016, 27, 105–108. [Google Scholar] [CrossRef]
- Oliva, A.; Miele, M.C.; Al Ismail, D.; Di Timoteo, F.; De Angelis, M.; Rosa, L.; Cutone, A.; Venditti, M.; Mascellino, M.T.; Valenti, P.; et al. Challenges in the Microbiological Diagnosis of Implant-Associated Infections: A Summary of the Current Knowledge. Front. Microbiol. 2021, 12, 3236. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of Orthopedic Implants with Emphasis on Bacterial Adhesion Process and Techniques Used in Studying Bacterial-Material Interactions. Biomatter 2012, 2, 176. [Google Scholar] [CrossRef]
- Choi, W.C.; Choi, H.G.; Kim, J.N.; Lee, M.C.; Shin, D.H.; Kim, S.H.; Kim, C.K.; Jo, D.I. The Efficacy of Bioabsorbable Mesh in Craniofacial Trauma Surgery. Arch. Craniofac. Surg. 2016, 17, 135. [Google Scholar] [CrossRef]
- Eppley, B.L.; Morales, L.; Wood, R.; Pensler, J.; Goldstein, J.; Havlik, R.J.; Habal, M.; Losken, A.; Williams, J.K.; Burstein, F.; et al. Resorbable PLLA-PGA Plate and Screw Fixation in Pediatric Craniofacial Surgery: Clinical Experience in 1883 Patients. Plast. Reconstr. Surg. 2004, 114, 850–856. [Google Scholar] [CrossRef]
- Sarfraz, S.; Mäntynen, P.H.; Laurila, M.; Suojanen, J.; Saarnio, J.; Rossi, S.; Horelli, J.; Kaakinen, M.; Leikola, J.; Reunanen, J. Effect of Surface Tooling Techniques of Medical Titanium Implants on Bacterial Biofilm Formation In Vitro. Materials 2022, 15, 3228. [Google Scholar] [CrossRef]
- Sarfraz, S.; Mäntynen, P.H.; Laurila, M.; Rossi, S.; Leikola, J.; Kaakinen, M.; Suojanen, J.; Reunanen, J. Comparison of Titanium and PEEK Medical Plastic Implant Materials for Their Bacterial Biofilm Formation Properties. Polymers 2022, 14, 3862. [Google Scholar] [CrossRef]
- Hjerppe, J.; Rodas, S.; Korvala, J.; Pesonen, P.; Kaisanlahti, A.; Özcan, M.; Suojanen, J.; Reunanen, J. Surface Roughness and Streptococcus Mutans Adhesion on Metallic and Ceramic Fixed Prosthodontic Materials after Scaling. Materials 2021, 14, 1027. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the Oral Cavity and Approaches for Biofilm Management by Surface Modifications. Clin. Oral. Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Gareb, B.; Van Bakelen, N.B.; Vissink, A.; Bos, R.R.M.; Van Minnen, B. Titanium or Biodegradable Osteosynthesis in Maxillofacial Surgery? In Vitro and In Vivo Performances. Polymers 2022, 14, 2782. [Google Scholar] [CrossRef]
- Laine, P.; Kontio, R.; Lindqvist, C.; Suuronen, R. Are There Any Complications with Bioabsorbable Fixation Devices?: A 10 Year Review in Orthognathic Surgery. Int. J. Oral. Maxillofac. Surg. 2004, 33, 240–244. [Google Scholar] [CrossRef]
- Cheung, L.K.; Chow, L.K.; Chiu, W.K. A Randomized Controlled Trial of Resorbable versus Titanium Fixation for Orthognathic Surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2004, 98, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Tuovinen, V.; Suuronen, R.; Teittinen, M.; Nurmenniemi, P. Comparison of the Stability of Bioabsorbable and Titanium Osteosynthesis Materials for Rigid Internal Fixation in Orthognathic Surgery. A Prospective Randomized Controlled Study in 101 Patients with 192 Osteotomies. Int. J. Oral. Maxillofac. Surg. 2010, 39, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, I.; Igawa, K.; Nagata, J.; Yoshida, M.; Ogawa, Y.; Ichiki, T.; Yokota, R.; Takamori, K.; Kashima, K.; Sakoda, S. Comparison of Material-Related Complications after Bilateral Sagittal Split Mandibular Setback Surgery: Biodegradable versus Titanium Miniplates. J. Oral. Maxillofac. Surg. 2012, 70, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, M.; Jin, X.; Xu, J.; Lu, J.; Zhang, C.; Tian, T.; Teng, L. Complications of Absorbable Fixation in Maxillofacial Surgery: A Meta-Analysis. PLoS ONE 2013, 8, e67449. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.C.L.; Souto-Souza, D.; de Souza, G.M.; Magesty, R.A.; de Cassia Ávila, B.; Galvão, E.L.; Falci, S.G.M. Comparison between Resorbable Plates vs. Titanium Plates for Treatment of Zygomatic Fractures: A Systematic Review with Meta-Analysis. Oral. Maxillofac. Surg. 2021, 25, 289–301. [Google Scholar] [CrossRef]
- Pontell, M.E.; Niklinska, E.B.; Braun, S.A.; Jaeger, N.; Kelly, K.J.; Golinko, M.S. Resorbable Versus Titanium Rigid Fixation for Pediatric Mandibular Fractures: A Systematic Review, Institutional Experience and Comparative Analysis. Craniomaxillofac. Trauma. Reconstr. 2022, 15, 189–200. [Google Scholar] [CrossRef]
- Lee, H.B.; Oh, J.S.; Kim, S.G.; Kim, H.K.; Moon, S.Y.; Kim, Y.K.; Yun, P.Y.; Son, J.S. Comparison of Titanium and Biodegradable Miniplates for Fixation of Mandibular Fractures. J. Oral Maxillofac. Surg. 2010, 68, 2065–2069. [Google Scholar] [CrossRef]
- Wood, R.J.; Petronio, J.A.; Graupman, P.C.; Shell, C.D.; Gear, A.J.L. New Resorbable Plate and Screw System in Pediatric Craniofacial Surgery. J. Craniofac. Surg. 2012, 23, 845–849. [Google Scholar] [CrossRef]
- Kalmar, C.L.; Bushold, J.; Carlson, A.R.; Zapatero, Z.D.; Kosyk, M.S.; Swanson, J.W.; Taylor, J.A.; Bartlett, S.P. Safety of Contemporary Resorbable Fixation Systems for Craniofacial Reconstruction in Pediatric Patients. Plast. Reconstr. Surg. 2021, 148, 838–848. [Google Scholar] [CrossRef]
- Serlo, W.S.; Ylikontiola, L.P.; Vesala, A.L.; Kaarela, O.I.; Iber, T.; Sándor, G.K.B.; Ashammakhi, N. Effective Correction of Frontal Cranial Deformities Using Biodegradable Fixation on the Inner Surface of the Cranial Bones during Infancy. Childs Nerv. Syst. 2007, 23, 1439–1445. [Google Scholar] [CrossRef]
- Mudenur, C.; Boruah, P.; Kumar, A.; Katiyar, V. Prodigiosin-Loaded Poly(Lactic Acid) to Combat the Biofilm-Associated Infections. ACS Appl. Bio Mater. 2021, 5, 2143–2151. [Google Scholar] [CrossRef]
- Campoccia, D.; Visai, L.; Renò, F.; Cangini, I.; Rizzi, M.; Poggi, A.; Montanaro, L.; Rimondini, L.; Arciola, C.R. Bacterial Adhesion to Poly-(D,L)Lactic Acid Blended with Vitamin E: Toward Gentle Anti-Infective Biomaterials. J. Biomed. Mater. Res. A 2015, 103, 1447–1458. [Google Scholar] [CrossRef]
- Boschetto, F.; Doan, H.N.; Vo, P.P.; Zanocco, M.; Yamamoto, K.; Zhu, W.; Adachi, T.; Kinashi, K.; Marin, E.; Pezzotti, G. Bacteriostatic Behavior of PLA-BaTiO3 Composite Fibers Synthesized by Centrifugal Spinning and Subjected to Aging Test. Molecules 2021, 26, 2918. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarfraz, S.; Tamminen, A.-M.; Leikola, J.; Salmi, S.; Kaakinen, M.; Sorsa, T.; Suojanen, J.; Reunanen, J. High Adherence of Oral Streptococcus to Polylactic Acid Might Explain Implant Infections Associated with PLA Mesh Implantation. Int. J. Mol. Sci. 2023, 24, 9504. https://doi.org/10.3390/ijms24119504
Sarfraz S, Tamminen A-M, Leikola J, Salmi S, Kaakinen M, Sorsa T, Suojanen J, Reunanen J. High Adherence of Oral Streptococcus to Polylactic Acid Might Explain Implant Infections Associated with PLA Mesh Implantation. International Journal of Molecular Sciences. 2023; 24(11):9504. https://doi.org/10.3390/ijms24119504
Chicago/Turabian StyleSarfraz, Sonia, Anni-Maria Tamminen, Junnu Leikola, Sonja Salmi, Mika Kaakinen, Timo Sorsa, Juho Suojanen, and Justus Reunanen. 2023. "High Adherence of Oral Streptococcus to Polylactic Acid Might Explain Implant Infections Associated with PLA Mesh Implantation" International Journal of Molecular Sciences 24, no. 11: 9504. https://doi.org/10.3390/ijms24119504
APA StyleSarfraz, S., Tamminen, A.-M., Leikola, J., Salmi, S., Kaakinen, M., Sorsa, T., Suojanen, J., & Reunanen, J. (2023). High Adherence of Oral Streptococcus to Polylactic Acid Might Explain Implant Infections Associated with PLA Mesh Implantation. International Journal of Molecular Sciences, 24(11), 9504. https://doi.org/10.3390/ijms24119504








