2. Results
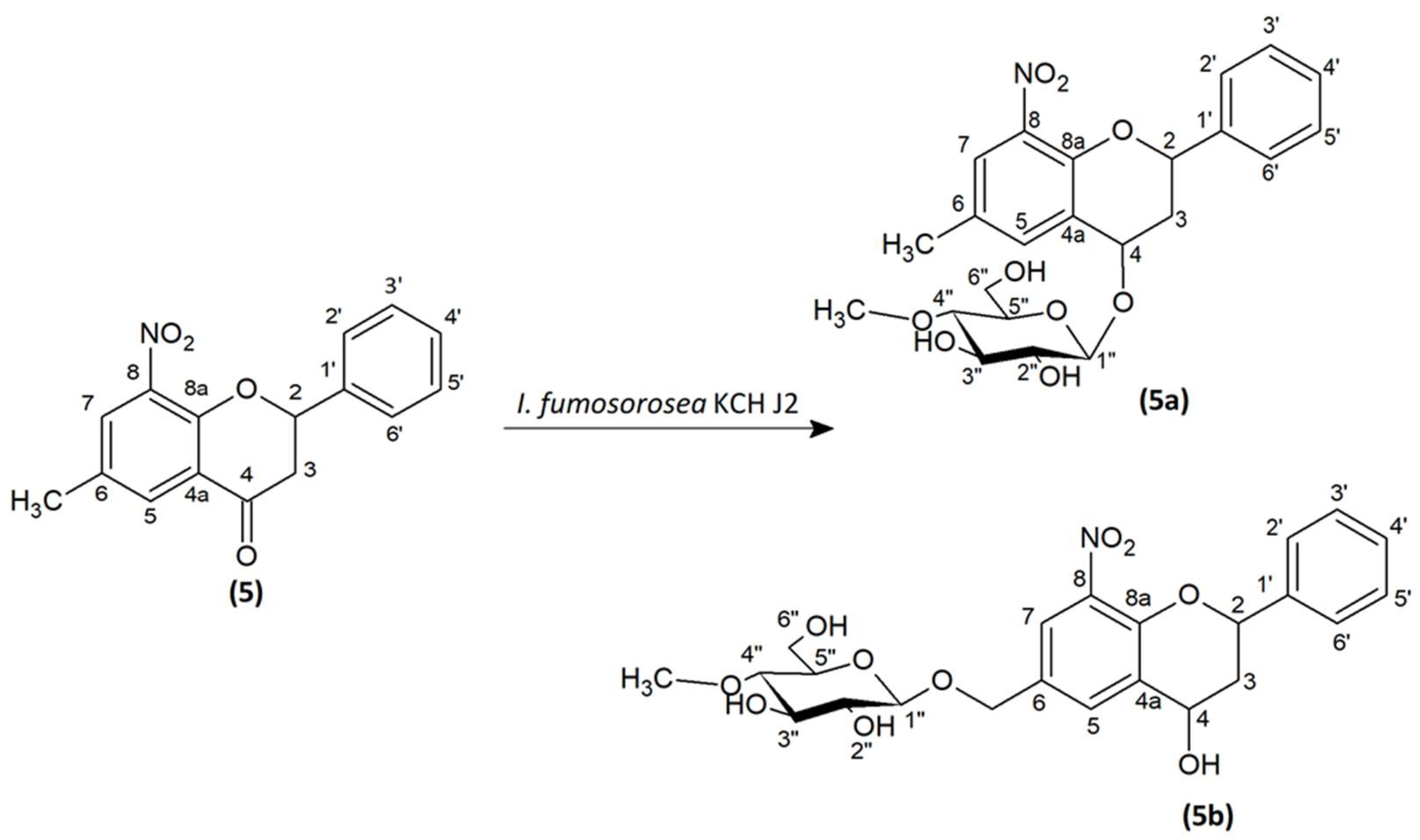
As a result of the biotransformation of substrates (5), (6), (7), (8), and (9) in cultures of B. bassiana KCH J1.5, I. fumosorosea KCH J2, and I. farinosa KCH J2.6, glycosidic derivatives of flavonoids were obtained. Only 2′-hydroxy-5′-methyl-3′-nitrochalcone (4) in the cultures of the chosen filamentous fungi was not biotransformed. The products were extracted from fungal cultures and purified using preparative thin-layer chromatography (pTLC). Obtained products were analyzed structurally with the use of Nuclear Magnetic Resonance (NMR), and masses were confirmed by Liquid Chromatography-Mass Spectrometry (LC-MS).
6-Methyl-8-nitro-2-phenylchromane 4-O-β-D-(4″-O-methyl)-glucopyranoside (5a). C23H27NO9, mp: 98–101 °C; HPLC Rt = 6.6 min; [α]D = −32.311 (0.38 w/v % in acetone); 1H NMR (600 MHz, acetone-d6) δ (ppm): 7.69 (1H, d, J = 1.6 Hz, H-7), 7.59 (1H, d, J = 2.1 Hz, H-5), 7.56–7.54 (2H, m, H-2′, H-6′), 7.43 (2H, dd, J = 10.4, 4.8 Hz, H-3′, H-5′), 7.37–7.33 (1H, m, H-4′), 5.55 (1H, dd, J = 12.1, 2.0 Hz, H-2), 5.06 (1H, t, J = 2.9 Hz, H-4), 4.50 (1H, d, J = 7.7 Hz, H-1″), 4.43 (1H, d, J = 3.9 Hz, 2″-OH), 4.25 (1H, d, J = 3.9 Hz, 3″-OH), 3.91 (1H, ddd, J = 11.4, 5.6, 2.1 Hz, H-6″), 3.86 (1H, m, 6″-OH), 3.72 (1H, dt, J = 11.7, 6.0 Hz, H-6″), 3.54 (3H, s, 4″-O-CH3), 3.50 (1H, dd, J = 8.9, 3.8 Hz, H-3″), 3.36 (1H, ddd, J = 9.8, 5.6, 2.1 Hz, H-5″), 3.30 (1H, ddd, J = 9.0, 8.0, 3.8 Hz, H-2″), 3.12 (1H, dd, J = 9.6, 9.0 Hz, H-4″), 2.60 (1H, dt, J = 14.4, 2.5 Hz, H-3ax), 2.37 (3H, s, -CH3), 2.19–2.13 (1H, m, H-3eq); 13C NMR (acetone-d6) δ (ppm): 147.27 (C-8a), 141.48 (C-1′), 137.48 (C-5), 130.03 (C-6), 129.33 (C-3′, C-5′), 128.78 (C-4′), 126.83 (C-2′, C-6′), 126.53 (C-7), 125.43 (C-8), 123.87 (C-4a), 101.49 (C-1″), 80.59 (C-4″), 78.11 (C-3″), 77.10 (C-5″), 75.29 (C-2″), 75.12 (C-2), 69.42 (C-4), 62.54 (C-6″), 60.52 (C4″-O-CH3), 36.72 (C-3), 20.09 (-CH3).
8-Nitroflavan-4-ol 6-methylene-O-β-D-(4″-O-methyl)-glucopyranoside (5b). C23H27NO10, mp: 177 °C; HPLC Rt = 11.6 min; [α]D = −37.348 (1.1 w/v % in acetone); 1H NMR (600 MHz, acetone-d6) δ (ppm): 7.87 (1H, d, J = 2.1 Hz, H-7), 7.71 (1H, d, J = 2.0 Hz, H-5), 7.54 (2H, d, J = 7.5 Hz, H-2′, H-6′), 7.45–7.42 (2H, m, H-3′, H-5′), 7.36 (1H, t, J = 7.5 Hz, H-4′), 5.49 (1H, dd, J = 11.8, 2.1 Hz, H-2), 4.90 (1H, s, H-4), 4.90 (1H, d, J = 12.1 Hz, 6-CH2-O), 4.68 (1H, d, J = 12.1 Hz, 6-CH2-O), 4.41 (1H, d, J = 7.8 Hz, H-1″), 4.29 (1H, s, 2″-OH), 3.83 (1H, d, J = 11.6 Hz, H-6″), 3.76–3.70 (1H, m, 6″-OH), 3.65 (1H, dd, J = 14.1, 8.8 Hz, H-6″), 3.53 (3H, s, 4″-O-CH3), 3.51 (1H, d, J = 9.0 Hz, H-3″), 3.31–3.25 (2H, m, H-5″, H-2″), 3.13–3.08 (1H, m, H-4″), 2.39–2.36 (1H, m, H-3ax), 2.23–2.18 (1H, m, H-3eq); 13C NMR (acetone-d6) δ (ppm): 148.21 (C-8a), 141.39 (C-1′), 139.96 (C-8), 137.40 (C-4a), 135.41 (C-5), 131.11 (C-6), 129.40 (C-3′, C-5′), 128.84 (C-4′), 126.78 (C-2′, C-6′), 124.95 (C-7), 103.29 (C-1″), 80.50 (C-4″), 78.09 (C-3″), 76.96 (C-5″), 75.24 (C-2), 75.15 (C-2″), 69.79 (6-CH2-O-), 63.10 (C-4), 62.44 (C-6″), 60.49 (4″-O-CH3), 38.83 (C-3).
6-Methyl-8-nitroflavone 4′-O-β-D-(4″-O-methyl)-glucopyranoside (6a). C23H23NO10, mp: 140–143 °C; HPLC Rt = 6.7 min; [α]D = −12.707 (0.35 w/v % in acetone); 1H NMR (600 MHz, acetone-d6) δ (ppm): 8.34 (1H, dd, J = 2.3, 0.6 Hz, H-7), 8.24 (1H, dd, J = 2.2, 0.8 Hz, H-5), 8.11–8.08 (2H, m, H-2′, H-6′), 7.29–7.26 (2H, m, H-3′, H-5′), 6.93 (1H, s, H-3), 5.12 (1H, d, J = 7.8 Hz, H-1″), 4.75 (1H, d, J = 4.2 Hz, 2″-OH), 4.46 (1H, d, J = 3.9 Hz, 3″-OH), 4.16–4.13 (1H, m, 6″-OH), 3.87 (1H, ddd, J = 11.8, 5.1, 2.3 Hz, H-6″), 3.74–3.70 (1H, m, H-6″), 3.68–3.66 (1H, m, H-3″), 3.58–3.57 (4H, m, 4″-O-CH3, H-5″), 3.54–3.50 (1H, m, H-2″), 3.27–3.23 (1H, m, H-4″), 2.59 (3H, s, -CH3); 13C NMR (acetone-d6) δ (ppm): 179.09 (C=O), 163.80 (C-2), 161.81 (C-4′), 153.12 (C-8a), 139.88 (C-8), 136.11 (C-6), 131.58 (C-5), 131.36 (C-7), 129.17 (C-2′, C-6′), 125.75 (C-4a), 124.92 (C-1′), 117.77 (C-3′, C-5′), 106.73 (C-3), 101.14 (C-1″), 80.02 (C-4″), 77.97 (C-3″), 77.20 (C-5″), 74.86 (C-2″), 62.02 (C-6″), 60.58 (4″-O-CH3), 20.58 (-CH3).
8-Bromo-6-chloroflavanone 3′-O-β-D-(4″-O-methyl)-glucopyranoside (7a). C22H22BrClO8, mp: 97 °C; HPLC Rt = 13.6 min; [α]D = −60.024 (0.41 w/v % in acetone) 1H NMR (600 MHz, acetone-d6) δ (ppm): 7.91 (1H, d, J = 2.6 Hz, H-7), 7.77 (1H, d, J = 2.5 Hz, H-5), 7.38 (1H, td, J = 7.9, 3.0 Hz, H-5′), 7.32–7.29 (1H, m, H-2′), 7.25 (1H, d, J = 7.0 Hz, H-6′), 7.09 (1H, dd, J = 7.9, 2.1 Hz, H-4′), 5.81 (1H, dd, J = 12.6, 3.0 Hz, H-2), 5.01 (1H, d, J = 7.8 Hz, H-1″), 4.64 (1H, d, J = 4.2 Hz, 2″-OH), 4.39 (1H, d, J = 4.1 Hz, 3″-OH), 3.83 (1H, ddd, J = 11.6, 5.2, 2.2 Hz, H-6″), 3.73 (1H, dd, J = 7.9, 2.8 Hz, 6″-OH), 3.70–3.66 (1H, m, H-6″), 3.65–3.60 (1H, m, H-3″), 3.56 (3H, s, 4″-O-CH3), 3.50–3.46 (2H, m, H-2″, H-5″), 3.26 (2H, dd, J = 16.9, 12.5 Hz, H-3ax), 3.24–3.20 (1H, m, H-4″), 3.03 (1H, dd, J = 17.0, 3.1 Hz, H-3eq); 13C NMR (acetone-d6) δ (ppm): 190.26 (C=O), 158.93 (C-3′), 157.58 (C-8a), 140.87 (C-1′), 138.83 (C-7), 130.68 (C-5′), 127.35 (C-6), 126.12 (C-5), 123.68 (C-4a), 120.72 (C-6′), 117.43 (C-4′), 115.23 (C-2′), 113.60 (C-8), 101.35 (C-1″), 80.84 (C-2), 80.07 (C-4″), 78.08 (C-3″), 77.06 (C-2″), 74.94 (C-5″), 62.05 (C-6″), 60.55 (4″-O-CH3), 43.85 (C-3).
8-Bromo-6-chloroflavan-4-ol 4′-O-β-D-(4″-O-methyl)-glucopyranoside (8a). C22H24BrClO8, mp: 117 °C; HPLC Rt = 11.0 min; [α]D = −8.725 (0.4 w/v % in acetone); 1H NMR (600 MHz, acetone-d6) δ (ppm): 7.54 (1H, d, J = 2.5 Hz, H-7), 7.45 (2H, d, J = 8.6 Hz, H-2′, H-6′), 7.40 (1H, d, J = 2.5 Hz, H-5), 7.11 (2H, d, J = 8.7 Hz, H-3′, H-5′), 5.37 (1H, dd, J = 11.7, 2.2 Hz, H-2), 4.98 (1H, d, J = 7.8 Hz, H-1″), 4.85–4.81 (1H, m, H-4), 4.63 (1H, d, J = 4.2 Hz, 2″-OH), 4.38 (1H, d, J = 4.1 Hz, 3″-OH), 3.85 (1H, ddd, J = 11.7, 5.4, 2.2 Hz, H-6″), 3.76 (1H, dd, J = 7.1, 5.4 Hz, 6″-OH), 3.69 (1H, ddd, J = 7.1, 6.0, 4.3 Hz, H-6″), 3.66–3.61 (1H, m, H-3″), 3.57 (3H, s, 4″-O-CH3), 3.51–3.45 (2H, m, H-5″, H-2″), 3.24–3.20 (1H, m, H-4″), 2.29–2.25 (1H, m, H-3ax), 2.19–2.13 (1H, m, H-3eq); 13C NMR (acetone-d6) δ (ppm): 158.63 (C-4′), 151.36 (C-8a), 135.03 (C-1′), 132.58 (C-7), 130.45 (C-5), 128.78 (C-4a), 128.25 (C-2′, C-6′), 125.45 (C-6), 117.36 (C-3′, C-5′), 111.84 (C-8), 101.64 (C-1″), 80.12 (C-4″), 78.04 (C-3″), 77.06 (C-5″), 74.98 (C-2″), 74.91 (C-2), 63.44 (C-4), 62.11 (C-6″), 60.55 (4″-O-CH3), 38.57 (C-3).
8-Bromo-6-chloroflavone 4′-O-β-D-(4″-O-methyl)-glucopyranoside (9a). C22H20BrClO8, mp: 160 °C; HPLC Rt = 11.1 min; [α]D = −17.181 (1.39 w/v % in acetone) 1H NMR (600 MHz, acetone-d6) δ (ppm): 8.12 (3H, dd, J = 5.7, 3.1 Hz, H-2′, H-6′, H-7), 8.01 (1H, d, J = 2.5 Hz, H-5), 7.27 (2H, d, J = 8.9 Hz, H-3, H-5′), 6.90 (1H, s, H-3), 5.12 (1H, d, J = 7.7 Hz, H-1″), 4.75 (1H, d, J = 0.7 Hz, 2″-OH), 4.47 (1H, d, J = 1.5 Hz, 3″-OH), 3.87 (1H, d, J = 11.5 Hz, H-6″), 3.84–3.82 (1H, m, 6″-OH), 3.73–3.70 (1H, m, H-6″), 3.66 (1H, t, J = 8.0 Hz, H-3″), 3.58 (3H, s, 4″-O-CH3), 3.56 (1H, d, J = 1.7 Hz, H-5″), 3.54–3.50 (1H, m, H-2″), 3.24 (1H, t, J = 9.3 Hz, H-4″); 13C NMR (acetone-d6) δ (ppm): 176.14 (C=O), 164.06 (C-2), 161.82 (C-4′), 152.40 (C-8a), 137.19 (C-7), 131.44 (C-6), 129.11 (C-2′, C-6′), 126.65 (C-4a), 125.38 (C-1′), 124.88 (C-5), 117.81 (C-3′, C-5′), 113.75 (C-8), 106.48 (C-3), 101.16 (C-1″), 80.05 (C-4″), 77.96 (C-3″), 77.22 (C-5″), 74.86 (C-2″), 62.05 (C-6″), 60.59 (4″-O-CH3).
2.1. Microbial Transformation of 2′-Hydroxy-5′-Methyl-3′-Nitrochalcone (4) in the Culture of Entomopathogenic Filamentous Fungi
The compound 2′-hydroxy-5′-methyl-3′-nitrochalcone (4) was subjected to small-scale biotransformation by B. bassiana KCH J1.5, I. fumosorosea KCH J2, and I. farinosa KCH J2.6. A High-Pressure Liquid Chromatography (HPLC) analysis showed no flavonoid derivative products in the obtained extracts.
2.2. Microbial Transformation of 6-Methyl-8-Nitroflavanone (5) in the Culture of I. fumosorosea KCH J2
6-Methyl-8-nitroflavanone (
5) was transformed microbiologically in the culture of
I. fumosorosea KCH J2 (
Figure 1). The process yielded two products 6-methyl-8-nitro-2-phenylchromane 4-
O-β-D-(4″-
O-methyl)-glucopyranoside (
5a) with 5.3% yield (4.3 mg) and 8-nitroflavan-4-ol 6-methylene-
O-β-D-(4″-
O-methyl)-glucopyranoside (
5b) with 14.8% yield (12.5 mg). The biotransformation took 10 days.
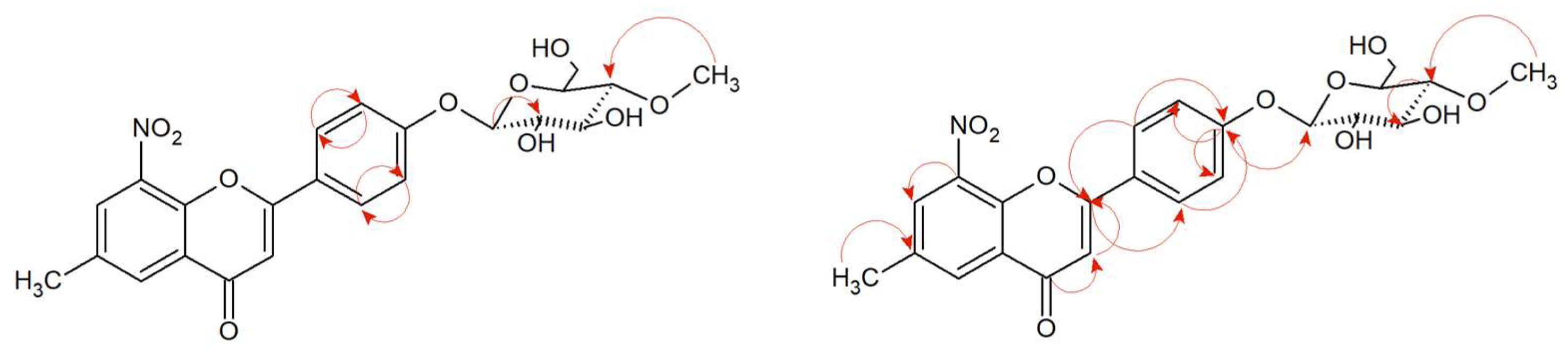
The structures of products (
5a) and (
5b) were determined by NMR spectroscopy using
1H NMR,
13C NMR, HMBC, and HSQC spectra and mass confirmation by LC-MS analysis (
Supplementary Materials Figures S34–S80). In product 6-methyl-8-nitro-2-phenylchromane 4-
O-β-D-(4″-
O-methyl)-glucopyranoside (
5a)
, the presence of glucose in position C-4 is confirmed by the lack of a characteristic signal from carbonyl carbon in the
13C NMR spectrum as it was in a substrate (
5). The NMR spectrum shows the signal of one proton at C-4, which proves the reduction of the carbonyl group at this position (δ = 5.06 ppm
1H NMR) and carbon δ = 69.42 ppm (
13C NMR) (
Supplementary Materials Figures S37 and S41). There is also a correlation between H-4 and C-1″ in the HMBC spectrum, which gives evidence that glucose is attached at the C-4 position (
Supplementary Materials Figure S55). The characteristic single proton doublet H-1″ present in the
1H NMR at δ = 4.50 ppm with the coupling constant
J = 7.7 Hz is evidence of
β-configuration of the glucose. What is more, the singlet from three protons at δ = 3.54 ppm (
1H NMR) and the presence of one signal from carbon at δ = 60.52 ppm (
13C NMR) shows that glucose is
O-methylated at the C-4″ position (
Supplementary Materials Figures S37, S38 and S41). There are two signals at δ = 7.69 ppm and δ = 7.59 ppm, which come from protons at carbons C-7 and C-5 in A ring of the flavonoid part. It evidences the substitution of additional groups in positions C-6 and C-8. The position of -CH
3 at C-6 confirms the correlation of the protons of this group with the C-5, C-6, and C-7 carbon in the HMBC spectrum (
Supplementary Materials Figure S53) Moreover, a doublet of doublets at δ = 5.55 ppm corresponds to one proton in C-2 position and a doublet of triplets at δ = 2.6 ppm and a multiplet at δ = 2.15 ppm are from 3ax and 3eq protons in C-3 position which is characteristic of flavanone structure (
Supplementary Materials Figures S49 and S51). Proton correlations through one bond (COSY) and multiple bonds (HMBC) are shown in
Figure 2 and
Supplementary Materials Figures S42–S46 and S52–S55.
In the case of product (
5b)
, the presence of the one proton attached to carbon C-2 at δ = 5.49 ppm in the
1H NMR spectrum (carbon at δ = 75.24 ppm
13C NMR) and the two protons: H-3ax at δ = 2.37 ppm and H-3eq at δ = 2.20 ppm (carbon C-3 at δ = 38.83 ppm
13C NMR) indicates the structure of the flavanone. Moreover, the correlation of these protons is visible in the COSY spectrum (marked with an arrow—
Supplementary Materials Figure S66). At δ = 4.90 ppm (
1H NMR), there is a signal from a single proton suggesting a reduction of the carbonyl group at the C-4 position. There is also a dehydrogenation of the -CH
3 group at the C-6 position to a -CH
2- group (two characteristic proton doublets at δ = 4.90 ppm and 4.68 ppm) (
Supplementary Materials Figure S71). In the HMBC spectrum is a correlation between these two protons with the carbons C-5, C-6, and C-7. Moreover, protons from the -CH
2- group correlate with the carbon C-1″ from the glucose molecule confirming its attachment in that position (
Supplementary Materials Figure S74). In the
1H NMR spectrum at δ = 4.41 ppm,
J = 7.8 Hz is a one-proton doublet H-1″ from the anomeric carbon atom C-1″. It evidences the presence of glucose in
β-configuration. At δ = 3.53 ppm in the
1H NMR is a signal from three protons from -CH
3 group, which is attached to the oxygen in the C-4″ position at the glucose molecule (
O-methylation) (
Supplementary Materials Figure S71). Proton correlations through one bond (COSY), and multiple bonds (HMBC) are shown in
Figure 3 and
Supplementary Materials Figures S64–S68 and S73–S80.
2.3. Microbial Transformation of 6-Methyl-8-Nitroflavone (6) in the Culture of B. bassiana KCH J1.5
As a result of the biotransformation of the 6-methyl-8-nitroflavone (
6) in the culture of
B. bassiana KCH J1.5, the 6-methyl-8-nitroflavone 4′-
O-β-D-(4″-
O-methyl)-glucopyranoside (
6a) was formed (
Figure 4). After seven days of biotransformation, 4.2 mg of the product (
6a) was obtained (5%).
The structure of 6-methyl-8-nitroflavone 4′-
O-β-D-(4″-
O-methyl)-glucopyranoside (
6a) was determined based on NMR spectroscopy and LC-MS analysis (
Supplementary Materials Figures S98–S114). The carbon signal from C-1″ at δ = 101.14 ppm in
13C NMR spectrum with the characteristic attached proton doublet H-1” in the
1H NMR spectrum at δ = 5.12 ppm having the coupling constant
J = 7.8 Hz indicates the
β-configuration of the attached glucose molecule (
Supplementary Materials Figures S101 and S104). In the HMBC spectrum, a correlation between carbon C-4′ from the ring B of flavonoid and the proton at the C-1″ indicates the substitution of sugar molecule at the C-4′ position (
Supplementary Materials Figure S113). There is also a presence of the characteristic AA’BB’ coupling system with the signals from protons at C-2′ and C-6′ and signals from protons at C-3′ and C-5′, which confirms the para substitution. Moreover, the glucose is
O-methylated in position C-4″ (
Supplementary Materials Figure S100). A signal from the three protons from -O-CH
3 group located at δ = 3.57 ppm (
1H NMR) correlates with the carbon C-4″ (δ = 60.58 ppm,
13C NMR) in the HMBC spectrum (
Supplementary Materials Figure S114). The carbonyl group remained intact, evidencing the characteristic signal at the
13C NMR at δ = 176.09 ppm, additionally, a signal from this carbon (C-4) is correlated with the one proton at C-3 carbon in the HMBC spectrum (
Supplementary Materials Figures S104 and S113). The double bond between carbons C-2 and C-3 was not reduced, which confirms the correlation of C-2 with a proton at C-3 in the HMBC spectrum (
Supplementary Materials:
Figure S113). In
Figure 5, the key COSY and HMBC correlations are shown.
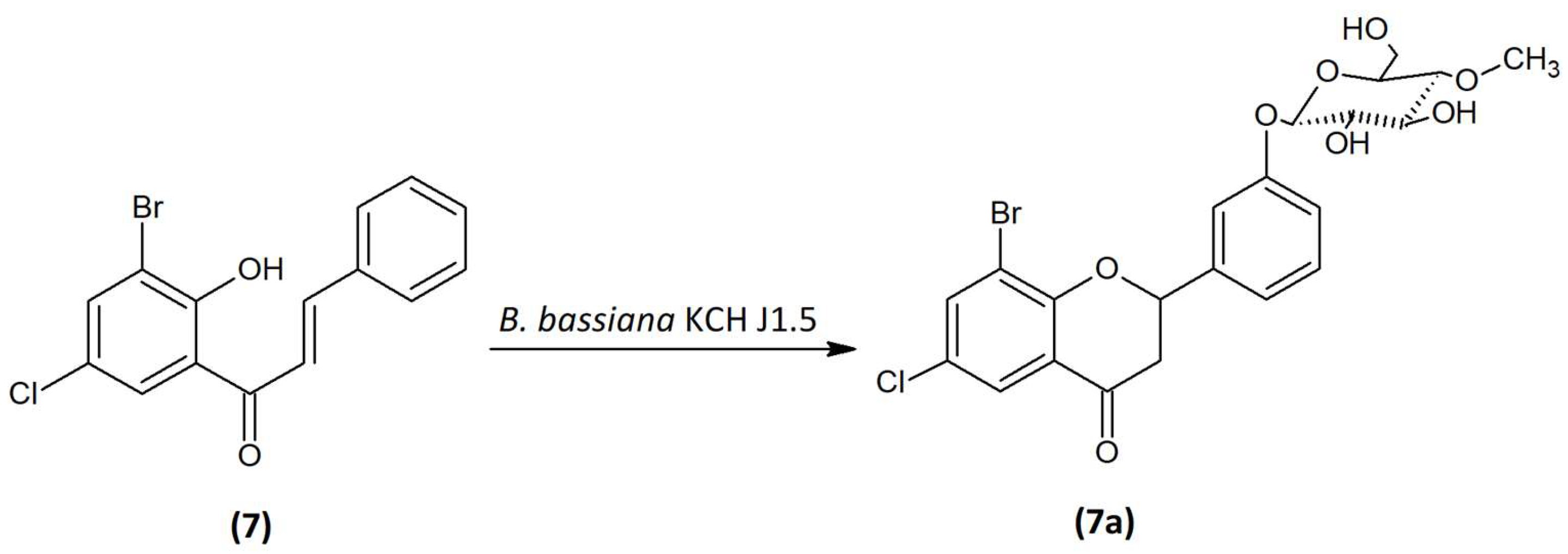
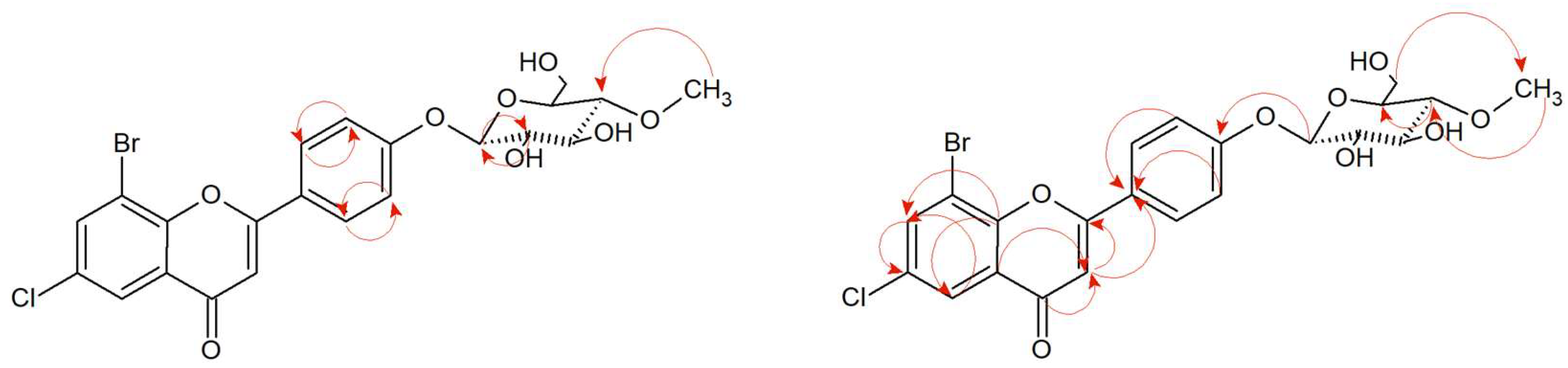
2.4. Microbial Transformation of 3′-Bromo-5′-Chloro-2′-Hydroxychalcone (7) in the Culture of B. bassiana KCH J1.5
3′-Bromo-5′-chloro-2′-hydroxychalcone (
7) was biotransformed by entomopathogenic fungi
B. bassiana KCH J1.5 into 8-bromo-6-chloroflavanone 3′-
O-β-D-(4″-
O-methyl)-glucopyranoside (
7a) with 6.24% yield (4.9 mg) (
Figure 6). The process took 10 days.
3′-Bromo-5′-chloro-2′-hydroxychalcone (
7) was converted to the flavanone structure by the cyclization of the C ring and glycosylated at C-3′ position. The structure of the product was confirmed by NMR spectroscopy and LC-MS analysis (
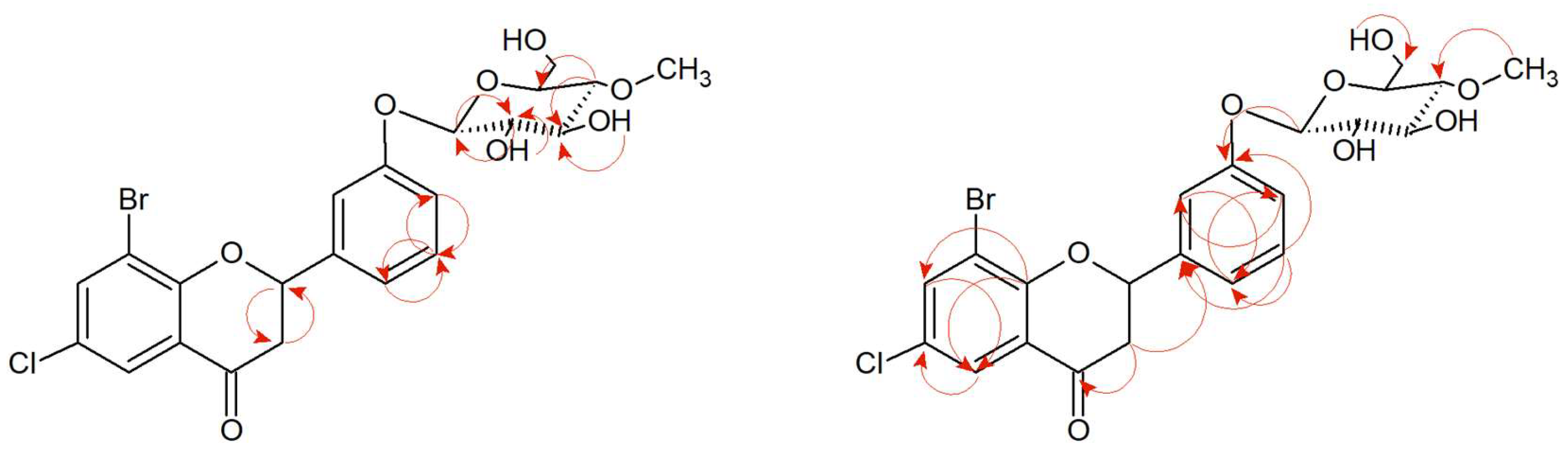
Supplementary Materials Figures S130–S150). The arrangement of signals from the protons belonging to the B ring of the flavonoid and their coupling in the COSY spectrum (
Figure 7 and
Supplementary Materials Figure S139) indicate the substitution of glucose at the C-3′ position. Another proof is also the correlation between the proton at C-1″ δ = 5.01 ppm (
1H NMR) and the carbon C-3′ δ = 158.93 ppm (
13C NMR) at the HMBC contour map (
Supplementary Materials Figure S147). Two doublets in A ring of the flavonoid H-7 (δ = 7.91 ppm,
J = 2.6 Hz) and H-5 (δ = 7.77 ppm,
J = 2.5 Hz) evidence the presence of substitution in positions C-6 and C-8 (
Supplementary Materials Figures S147–S149). In addition, there is a correlation between the carbonyl group (δ = 190.26 ppm,
13C NMR) and protons 3-axial and 3-equatorial (δ = 3.26 ppm and δ = 3.03,
1H NMR) at HMCB (
Supplementary Materials Figure S147). The presence of the one-proton doublet H-1” at the anomeric carbon C-1″at δ = 5.01 ppm in the
1H NMR spectrum with the coupling constant
J =7.8 Hz is characteristic of the
β-configuration of glucose molecule (
Supplementary Materials Figures S133 and S137). The attached glucose is
O-methylated as evidenced by the presence of 4″-O-C
H3 group in the
1H NMR δ = 3.56 ppm, and in the HMBC contour map, there is a correlation between the attached -CH
3 group in the C-4″ position and the carbon 4″ (δ = 80.07 ppm,
13C NMR) (
Figure 7 and
Supplementary Materials Figure S150). Other proton–proton correlations COSY and proton–carbon correlations HMBC are shown in
Figure 7.
2.5. Microbial Transformation of 8-Bromo-6-Chloroflavanone (8) in the Culture of I. fumosorosea KCH J2
Microbial transformation of 8-bromo-6-chloroflavanone (
8) in the culture of
I. fumosorosea KCH J2 allowed receiving 8-bromo-6-chloroflavan-4-ol 4′-
O-β-D-(4″-
O-methyl)-glucopyranoside (
8a) (
Figure 8) with 2.67% yield (2.1mg). The process took 10 days.
The analysis of NMR spectra confirmed the creation of the product (
8a). The
1H NMR spectrum shows two doublets coming from protons H-7 (δ = 7.54 ppm,
J = 2.5 Hz) and H-5 (δ = 7.40 ppm,
J = 2.5 Hz), evidencing the substitution at C-8 and C-6 positions, as in the case of the substrate (
8). The correlation is visible in the HMBC spectrum (
Figure 9 and
Supplementary Materials Figure S183). The characteristic AA′BB′ coupling system between signals from protons at C-2′ and C-6′ (δ = 7.45 ppm,
J = 8.6 Hz), and protons from C-3′ and C-5′ (δ = 7.11 ppm,
J = 8.7 Hz) suggesting additional substitution at the C-4′ position (
Supplementary Materials Figure S169). In the
13C NMR, there is no characteristic signal from the carbonyl group at the C-4 position, but on
1H NMR, there is a signal from the proton in the C-4 position δ = 4.83 ppm (δ = 63.44 ppm,
13C NMR) which proves the reduction of the carbonyl group (
Supplementary Materials Figure S170). According to HMBC spectra, there is a correlation between the proton in C-1″ and carbon C-4′, evidencing attachment of the glucose molecule in that position (
Figure 9,
Supplementary Materials Figure S183). The proton in anomeric carbon C-1″ δ = 4.98 ppm with the characteristic coupling constant
J = 7.8 Hz visualized in the
1H NMR spectrum evidencing
β-configuration of glucose. Additionally, the singlet coming from three protons at δ = 3.57 ppm (
1H NMR) and the presence of one carbon at δ = 60.55 ppm (
13C NMR) shows that glucose is
O-methylated at C-4″ position (
Supplementary Materials Figures S170, S180 and S181). In
Figure 9, the key COSY and HMBC correlations are shown.
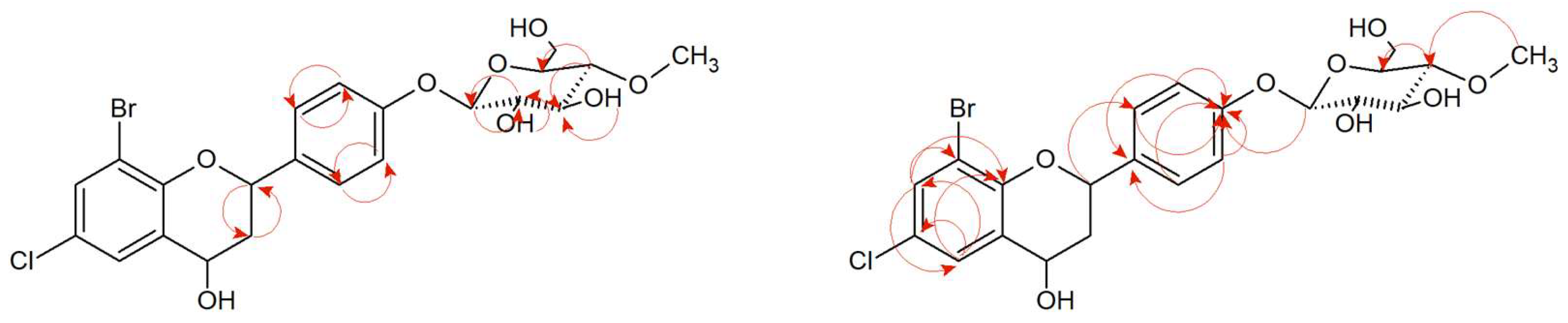
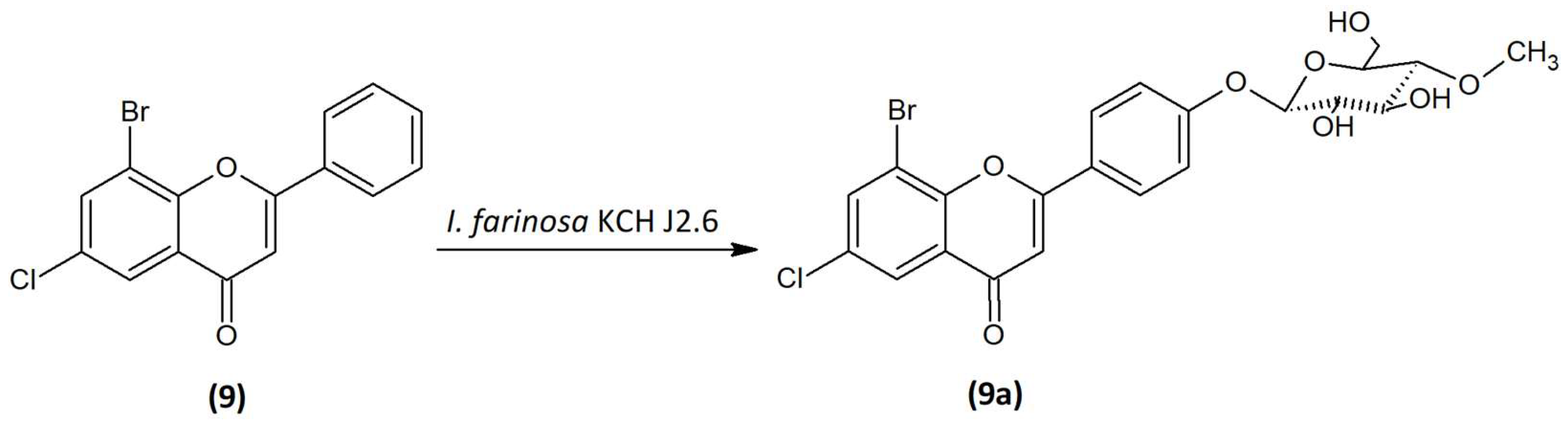
2.6. Microbial Transformation of 8-Bromo-6-Chloroflavone (9) in the Culture of I. farinosa KCH J2.6
The 8-bromo-6-chloroflavone (
9) was biotransformed by enthomopathogenic fungi
I. farinosa KCH J2.6 into 8-bromo-6-chloroflavone 4′-
O-β-D-(4″-
O-methyl)-glucopyranoside (
9a) (
Figure 10) with 19.59% yield (15.4 mg). The process took 10 days.
The obtained product (
9a) was an
O-methylglycosylated 8-bromo-6-chloroflavone derivative. The accurate NMR and LC-MS analysis confirming the structure and mass of the obtained product is presented in
Supplementary Materials Figures S197–S213. The characteristic signals from the attached glucose molecule were shown in the
1H NMR and
13C NMR spectra (
Supplementary Materials Figures S200 and S201 and
Figure S204). The attachment of glucose to the 8-bromo-6-chloroflavone (
9) was confirmed by the presence of a proton doublet from the proton H-1″ at the anomeric carbon C-1″ at δ = 5.12 ppm in the
1H NMR spectrum with the characteristic coupling constant
J = 7.7 Hz (
Supplementary Materials Figures S200). This coupling constant corresponds to the
β-configuration of glucose. A singlet coming from three protons at δ = 3.58 ppm in the
1H NMR and the carbon at δ = 60.59 ppm in the
13C NMR spectrum gives proof of appearance of a -O-CH
3 group. What’s more, this signal is correlated in the HMBC spectrum with the C-4″ signal at δ = 80.05 ppm, which proves the
O-methylation at the C-4″ hydroxyl group of the glucose (
Supplementary Materials Figures S201 and S213). The presence of the characteristic AA′BB′ coupling system in the flavonoid B ring from protons at C-2′ and C6′ and from the C-3′ and C-5′ confirmed the substitution at C-4′ (
Figure 11,
Supplementary Materials Figure S206). In the HMBC spectrum, a proton at the carbon C-1″ (δ = 5.12 ppm) derived from sugar moiety corresponding with the carbon C-4′ (δ = 161.82 ppm) coming from flavonoid part, proving substitution with the glucose molecule at this position (
Figure 11,
Supplementary Materials Figure S212). The presence of one-proton signals from C-5 and C-7 in the
1H NMR spectrum (Supplementary materials
Figures S199 and S212) confirms the preservation of the arrangement of substituents at the C-8 and C-6 position as in the substrate (
9). Other proton–proton correlations COSY and proton–carbon correlations HMBC are shown in
Figure 11.
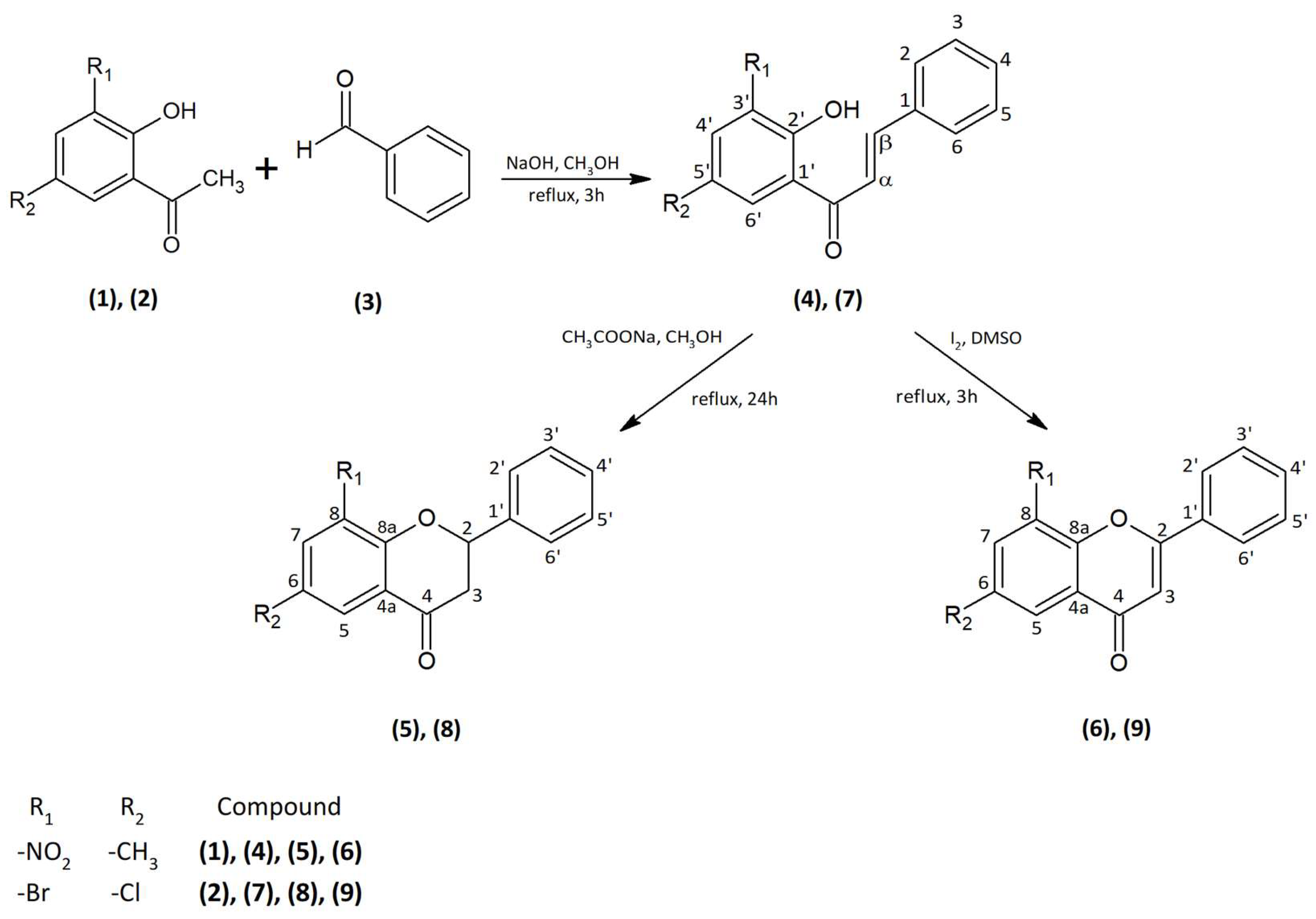
4. Materials and Methods
4.1. Substrates
The substrates 2′-hydroxy-5′-methyl-3′-nitrochalcone (
4), 6-methyl-8-nitroflavanone (
5), 6-methyl-8-nitroflavone (
6), 3′-bromo-5′-chloro-2′-hydroxychalcone (
7), 8-bromo-6-chloroflavanone (
8), and 8-bromo-6-chloroflavone (
9) were synthesized according to the reaction presented in
Figure 12. The 2′-hydroxy-5′-methyl-3′-nitroacetophenone (
1) and 3′-bromo-5′-chloro-2′-hydroxyacetophenone (
2), and the benzaldehyde (
3) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
The first step was the synthesis of chalcones (
4) and (
7) carried out by the Claisen–Schmidt condensation (
Figure 12). The reaction of the appropriately substituted acetophenone (
1) and (
2) with benzaldehyde (
3) allowed to obtain 2′-hydroxy-5′-methyl-3′-nitrochalcone (
4) (75% yield) and 3′-bromo-5′-chloro-2′-hydroxychalcone (
7) (60% yield). Substrates to the synthesis were dissolved in methanol under alkaline conditions (NaOH) with the addition of water. The reaction was carried out for 3 h at the boiling point of the reactants under reflux. The second step was the cyclization of chalcones (
4) and (
7) into flavanones (
5) (42% yield) and (
8) (77% yield) using sodium acetate dissolved in methanol under reflux via 24 h (
Figure 12). The last step was to obtain flavones (
6) (90% yield) and (
9) (94% yield) by reacting appropriate chalcones with iodine dissolved in dimethyl sulfoxide (DMSO) for 3 h under reflux at 125 °C in oil bath (
Figure 12).
2′-Hydroxy-5′-methyl-3′-nitrochalcone (4). C16H13NO4, mp: 158 °C; HPLC Rt = 18.2 min; 1H NMR (600 MHz, acetone-d6) δ (ppm): 13.50 (1H, s, -OH), 8.44 (1H, d, J = 1.6 Hz, H-6′), 8.05 (2H, dd, J = 8.6, 6.9 Hz, H-4′, H-α), 7.98 (1H, d, J = 15.5 Hz, H-β), 7.90–7.87 (2H, m, H-2, H-6), 7.53–7.48 (5H, m, H-3, H-4, H-5), 2.44 (3H, s, -CH3); 13C NMR (acetone-d6) δ (ppm): 194.60 (C=O), 155.23 (C-1′), 147.59 (C-β), 139.16 (C-3′), 136.98 (C-6′), 135.50 (C-1), 132.23 (C-4), 132.14 (C-4′), 130.12 (C-2, C-6), 129.97 (C-3, C-5), 129.35 (C-5′), 123.82 (C-2′), 121.82 (C-α), 20.08 (-CH3)
6-Methyl-8-nitroflavanone (5). C16H13NO4, mp: 179–180 °C; HPLC Rt = 18.1 min; [α]D = 0.108 (0.5 w/v % in acetone); 1H NMR (600 MHz, acetone-d6) δ (ppm): 8.02 (1H, d, J = 1.9 Hz, H-7), 7.96–7.95 (1H, m, H-5), 7.61 (2H, d, J = 7.4 Hz, H-2′, H-6′), 7.47 (2H, t, J = 7.5 Hz, H-3′, H-5′), 7.41 (1H t, J = 7.4 Hz, H-4′), 5.87 (1H, dd, J = 12.7, 2.9 Hz, H-2), 3.30 (1H, dd, J = 16.9, 12.7 Hz, H-3ax), 3.07 (1H, dd, J = 16.9, 3.0 Hz, H-3eq), 2.44 (3H, s, -CH3); 13C NMR (acetone-d6) δ (ppm): 190.29 (C=O), 152.95 (C-6), 140.62 (C-8), 139.39 (C-1′), 132.30 (C-5), 132.09 (C-7), 131.87 (C-8a), 129.57 (C-3′, C-5′), 129.53 (C-4′), 127.10 (C-2′, C-6′), 123.97 (4a), 81.36 (C-2), 44.35 (C-3), 20.06 (-CH3)
6-Methyl-8-nitroflavone (6). C16H11NO4, mp: 219 °C; HPLC Rt = 17.1 min; 1H NMR (600 MHz; acetone-d6) δ (ppm): 8.35 (1H, dd, J = 2.2, 0.5 Hz, H-7), 8.24 (1H, dd, J = 2.2, 0.8 Hz, H-5), 8.15 (2H, ddd, J = 5.7, 4.3, 2.5 Hz, H-2′, H-6′), 7.65–7.61 (3H, m, H-3′, H-4′, H-5′), 7.01 (1H, s, H-3), 2.59 (3H, s, -CH3); 13C NMR (acetone-d6) δ (ppm): 176.23 (C=O), 163.90 (C-2), 147.56 (C-8a), 139.61 (C-8), 136.24 (C-6), 133.00 (C-4′), 131.92 (C-1′), 131.57 (C-5), 131.53 (C-7), 130.11 (C-3′, C-5′), 127.45 (C-2′, C-6′), 126.31 (C-4a), 107.97 (C-3), 20.60 (-CH3)
3′-Bromo-5′-chloro-2′-hydroxychalcone (7). C15H10BrClO2, mp: 133–135 °C; HPLC Rt = 19.9 min; 1H NMR (600 MHz, acetone-d6) δ (ppm): 13.63 (1H, s, -OH), 8.40 (1H, d, J = 2.4 Hz, H-6′), 8.14 (1H, d, J = 15.4 Hz, H-α), 8.03 (1H, d, J = 15.4 Hz, H-β), 7.94 (2H, dd, J = 7.6, 1.7 Hz, H-2, H-6), 7.90 (1H, d, J = 2.5 Hz, H-4′), 7.54–7.48 (3H, m, H-3, H-4, H-5); 13C NMR (acetone-d6) δ (ppm): 194.21 (C=O), 159.79 (C-1′), 148.25 (C-β), 139.40 (C-4′), 135.44 (C-1), 132.33 (C-4), 130.34 (C-2, C-6), 130.13 (C-6′), 129.94 (C-3, C-5), 124.46 (C-5′), 122.09 (C-2′), 120.75 (C-α), 113.07 (C-3′)
8-Bromo-6-chloroflavanone (8). C15H10BrClO2, mp: 117–119 °C; HPLC Rt = 18.6 min; [α]D = -5.331 (0.325 w/v % in acetone); 1H NMR (600 MHz, acetone-d6) δ (ppm): 7.91 (1H, d, J = 2.6 Hz, H-7), 7.77 (1H, d, J = 2.6 Hz, H-5), 7.64–7.61 (2H, m, H-2′, H-6′), 7.50–7.46 (2H, m, H-3′, H-5′), 7.44–7.40 (1H, m, H-4′), 5.84 (1H, dd, J = 12.7, 3.0 Hz, H-2), 3.27 (1H, dd, J = 16.9, 12.7 Hz, H-3ax), 3.03 (1H, dd, J = 16.9, 3.0 Hz, H-3eq); 13C NMR (acetone-d6) δ (ppm): 190.27 (C=O), 157.66 (C-8a), 139.43 (C-1′), 138.81 (C-7), 129.61 (C-3′, C-4′, C-5′), 127.32 (C-6), 127.22 (C-2′, C-6′), 126.14 (C-5), 123.70 (C-4a), 113.58 (C-8),81.05 (C-2), 43.87 (C-3)
8-Bromo-6-chloroflavone (9). C15H8BrClO2, mp: 186–189 °C; HPLC Rt = 19.2 min; 1H NMR (600 MHz, acetone-d6) δ (ppm): 8.19–8.17 (2H, m, H-2′, H-6′), 8.14 (1H, d, J = 2.5 Hz, H-7), 8.03 (1H, d, J = 2.5 Hz, H-5), 7.66–7.62 (3H, m, H-4′, H-3′, H-5′), 7.00 (1H, s, H-3); 13C NMR (acetone-d6) δ (ppm): 176.30 (C=O), 164.21 (C-2), 152.51 (C-8a), 137.38 (C-7), 133.05 (C-4′), 132.06 (C-1′), 131.56 (C-6), 130.16 (C-3′, C-5′), 127.43 (C-2′, C-6′), 126.69 (C-4a), 124.90 (C-5), 113.90 (C-8), 107.72 (C-3)
4.2. Microorganisms
In the biotransformation process, three strains of entomopathogenic filamentous fungi
B. bassiana KCH J1.5,
I. fumosorosea KCH J2, and
I. farinosa KCH J2.6 were used. The microorganisms belong to the Department of Food Chemistry and Biocatalysis of the Wrocław University of Environmental and Life Sciences in Poland. The methods of isolation of entomopathogenic filamentous fungi, reproduction, and genetic identification were described in our previous papers [
23,
52,
53].
4.3. Analysis
Analytical and preparative TLC (Thin Layer Chromatography) was used to assess the course of synthesis as well as biotransformation and product isolation. Analytical TLC was used to monitor the course of chemical syntheses and the progress of biotransformation. For this purpose, TLC Silica gel 60/Kieselguhr F254 (0.2 mm thick) aluminum sheets 20 cm × 20 cm (Merck, Darmstadt, Germany) were used. The eluent consisted of cyclohexane (Chempur, Piekary Śląskie, Poland): Ethyl acetate (Chempur, Piekary Śląskie, Poland) in the ratio 9:1 and 4:1 v/v was used for the analysis of chemical syntheses. On the other hand, the mixture of chloroform (Chempur, Piekary Śląskie, Poland): Methanol (Chempur, Piekary Śląskie, Poland) in a ratio of 9:1 v/v was used to monitor the course of biotransformation. In both cases, the plates were observed under a UV lamp using a wavelength of λ = 254 nm and λ = 365 nm. Preparative TLC was used to separate the product mixture on scale-up biotransformation. For this purpose, preparative TLC Silica plates (Analtech, Gehrden, Germany) (0.5, 1, and 2 mm thick) were used with the eluent consisting of chloroform (Chempur, Piekary Śląskie, Poland) and methanol (Chempur, Piekary Śląskie, Poland) in the ratio (9:1 v/v). The products were observed under a UV lamp using a wavelength of λ = 254 nm and λ = 365 nm. Then, products were extracted thrice with 15 mL of ethyl acetate (Chempur, Piekary Śląskie, Poland). The extracts were filtered and evaporated in a vacuum evaporator.
HPLC chromatography was performed to check the biotransformation’s progress and determine the retention times of their substrates and products. Dionex Ultimate 3000 instrument (Thermo Fisher Scientific, Waltham, MA, USA) with a DAD-3000 diode array detector using an analytical octadecylsilica (ODS) 2 column (4.6 mm × 250 mm, Waters, Milford, MA, USA) and pre-column. The eluent was a mixture of 0.1% aqueous acid formic acid
v/
v (A) and acetonitrile (B). The gradient program was as follows: initial conditions—32.5% B in A, 4 min—40% B in A, 8 min—40% B in A, 10 min—45% B in A, 15 min—95% B in A, 18 min—95% B in A, 19 min—32.5% B in A, and 23 min—32.5% B in A. The flow rate was 1 mL/min, the injection volume was 5 µL, and the detection wavelength was 280 nm [
22]. Data were collected using Chromeleon software version 7.2 (Thermo Fisher Scientific, Waltham, MA, USA).
For the NMR analysis of substrates and biotransformation products, 1H NMR, 13C NMR, COSY, HSQC, and HMBC spectra were performed using the DRX AvanceTM 600 MHz NMR spectrometer (Bruker, Billerica, MA, USA). All samples were dissolved in 0.7 mL of deuterated acetone.
The mass of the obtained biotransformation substrates and products was confirmed using LC-MS analysis, LC-MS 8045 SHIMADZU Triple Quadrupole Liquid Chromatograph Mass Spectrometer with electrospray ionization (ESI) source (Shimadzu, Kyoto, Japan). Analyses were conducted using method “product ion scan”. Only a specific ion with a known molecular mass (determined by previous NMR analysis) was searched in each sample with a pure compound. The separation was achieved on the Kinetex column (2.6 µm C18 100 Å, 100 mm × 3 mm, Phenomenex, Torrance, CA, USA) operated at 30 °C. The mobile phase was a mixture of 0.1% aqueous formic acid v/v (A) and acetonitrile (B). The flow rate was 0.4 mL/min, and the injection volume was 5 µL. The gradient program was as follows: Initial conditions—80% B in A, 6.5 min—100% B, 7 min—80% B in A. The principal operating parameters for the LC-MS were set as follows: nebulizing gas flow: 3 L/min, heating gas flow: 10 L/min, interface temperature: 300 °C, drying gas flow: 10 L/min, data acquisition range, m/z 100–1000 Da, positive ionization mode. Data were collected with LabSolutions version 5.97 (Shimadzu, Kyoto, Japan) software.
Optical rotation was measured using digital polarimeter P-2000-Na (ABL&E-JASCO, Kraków, Poland).
4.4. Small-Scale Biotransformation
Three entomopathogenic filamentous fungi
B. bassiana KCH J1.5,
I. fumosorosea KCH J2, and
I. farinosa KCH J2.6 were used for small-scale biotransformation. Each of the six substrates underwent biotransformation by all the microorganisms above-mentioned. These fungi have been chosen for their ability to biotransform flavonoid compounds (production of glycoside derivatives) based on previous screening studies conducted by our team in the Department of Food Chemistry and Biocatalysis [
47,
54]. The purpose of this part of the research was to select the appropriate microorganism and biotransformation time of substrates (
4), (
5), (
6), (
7), (
8), and (
9) for further scale-up studies.
Small-scale biotransformation was carried out in 300 mL flat-bottomed conical flasks (Erlenmeyer flasks) containing 100 mL of modified Sabouraud medium (1% aminobac, 3% sucrose per 1 L of water). The flasks were inoculated with about 1 mL of a culture of entomopathogenic filamentous fungi and then shaken for 72 h at 140 rpm and 25 °C. Next, 10 mg of the substrate was added to each flask and shaken again at 140 rpm at 25 °C. Samples were collected after 3, 7, and 10 days of the experiment. After that, samples were extracted with ethyl acetate in the ratio of 1:1 ethyl acetate: medium, and collected in a separate flask. Extracts were dried with magnesium sulfate (MgSO4), filtered, and concentrated in a vacuum evaporator. Samples for HPLC analysis from obtained extracts were prepared. The dried extracts were suspended in 1 mL of acetonitrile and analyzed by HPLC. The results of this analysis made it possible to select the appropriate biotransformation time and strain for the increased scale of the experiment so that the process would run as efficiently as possible.
4.5. Scale-Up Biotransformation
The scale-up biotransformation was carried out in a 2-L flask containing 500 mL of modified Sabouraud’s medium (same as for small scale). The scale-up biotransformation was used to obtain more products for further analysis.
To the flask with a prepared sterile medium 1 mL of preincubation culture of entomopathogenic filamentous fungi was added. Then, the flask was incubated for 72 h at 25 °C with shaking at 145 rpm. After this time, 50 mg of substrate dissolved in 2 mL of DMSO was added to the flask. The time of scale-up biotransformation was based on previous small-scale studies for a given microorganism and substrate. The next step was to extract the obtained products three times using 350 mL of ethyl acetate. The combined extracts were then dried with MgSO4, filtered, and evaporated to dryness. The obtained samples were separated using preparative TLC plates. For this purpose, the extract was dissolved in approx. two mL of THF and applied to the plate. In a chromatography chamber, the products were separated using chloroform: methanol mixture (9:1, v/v) as the eluent. TLC plates were visualized under a UV lamp (254 nm and 365 nm), and separated fractions were removed from the plate. Fractions were extracted three times with 15 mL ethyl acetate for 30 min. The extracts were filtered and evaporated to dryness in a vacuum evaporator. Then, the fractions prepared this way were dissolved in 0.7 mL of deuterated acetone and subjected to NMR analysis.