Non-Antigenic Modulation of Antigen Receptor (TCR) Cβ-FG Loop Modulates Signalling: Implications of External Factors Influencing T-Cell Responses
Abstract
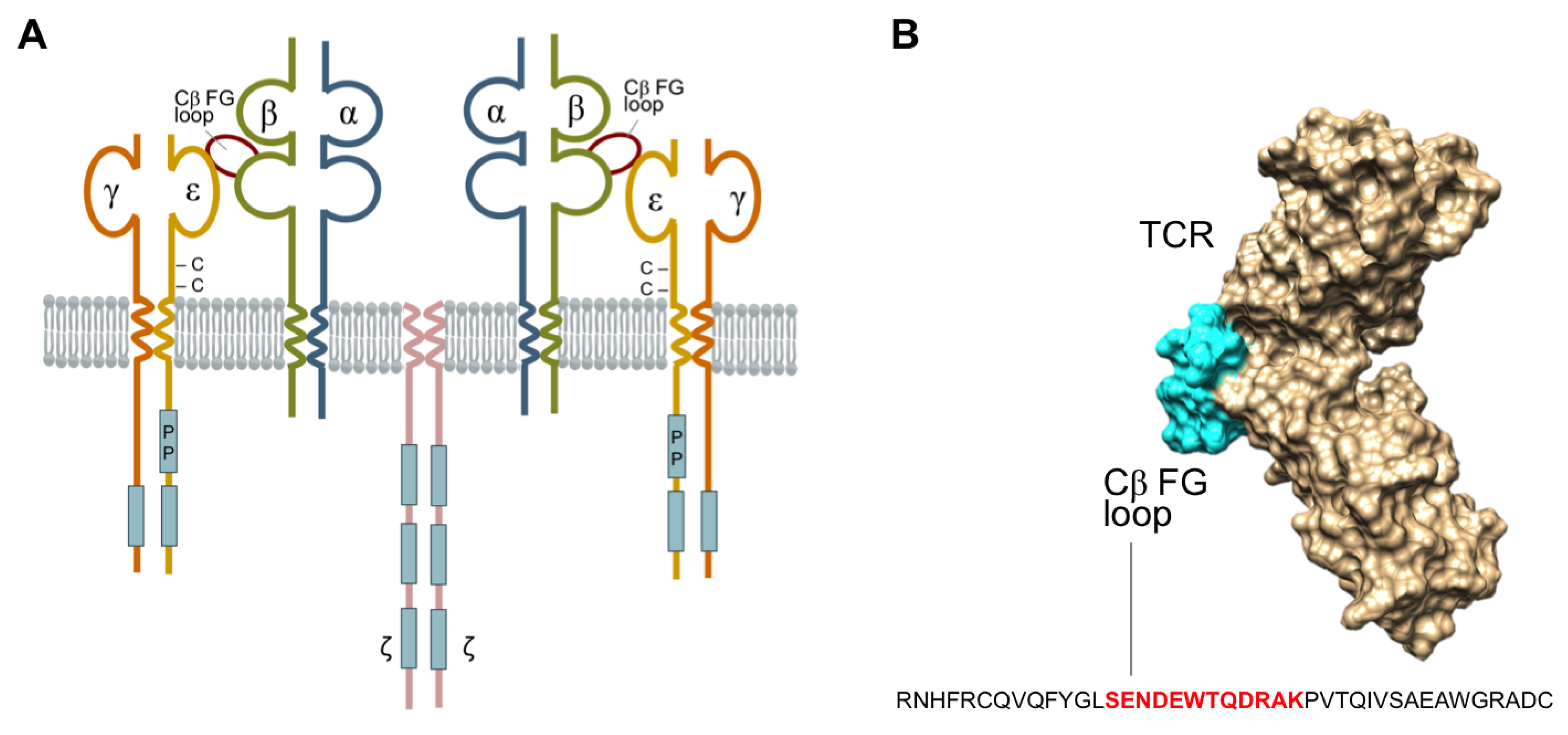
1. Introduction
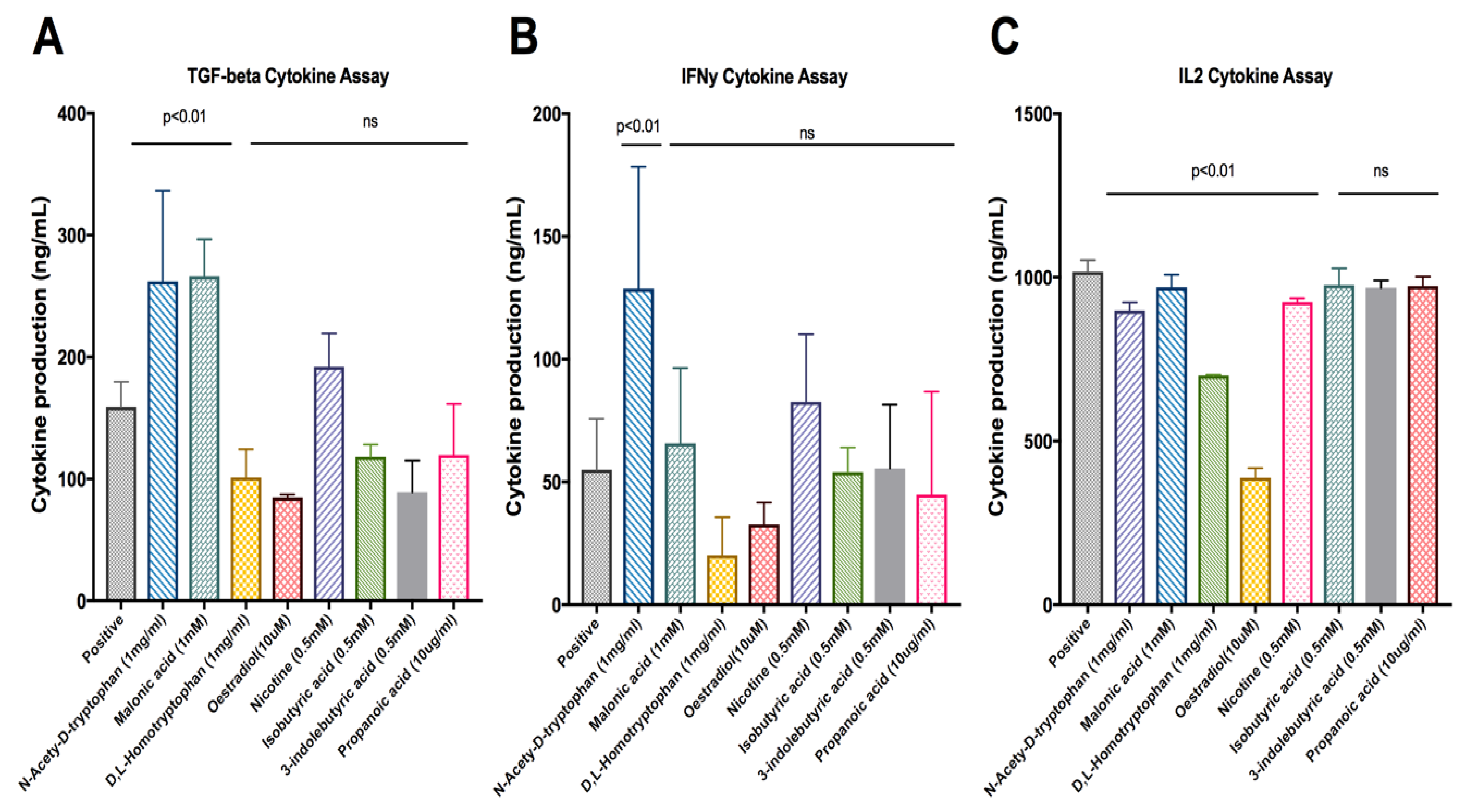
2. Results
3. Discussion
4. Methods and Materials
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.-H.; Reinherz, E.L. Structural basis of T cell recognition of peptides bound to MHC molecules. Mol. Immunol. 2002, 38, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Stewart-Jones, G.B.; McMichael, A.J.; Bell, J.I.; Stuart, D.I.; Jones, E.Y. A structural basis for immunodominant human T cell receptor recognition. Nat. Immunol. 2003, 4, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Touma, M.; Takeuchi, K.; Sun, Z.-Y.J.; Dave, V.P.; Kappes, D.J.; Wagner, G.; Reinherz, E.L. Distinctive CD3 heterodimeric ectodomain topologies maximize antigen-triggered activation of alpha beta T cell receptors. J. Immunol. 2010, 185, 2951–2959. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Feng, Y.; Mallis, R.J.; Li, X.; Keskin, D.B.; Hussey, R.E.; Brady, S.K.; Wang, J.-H.; Wagner, G.; Reinherz, E.L.; et al. Force-dependent transition in the T-cell receptor beta-subunit allosterically regulates peptide discrimination and pMHC bond lifetime. Proc. Natl. Acad. Sci. USA 2015, 112, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Touma, M.; Chang, H.C.; Sasada, T.; Handley, M.; Clayton, L.K.; Reinherz, E.L. The TCR C beta FG loop regulates alpha beta T cell development. J. Immunol. 2006, 176, 6812–6823. [Google Scholar] [CrossRef]
- Sasada, T.; Touma, M.; Chang, H.C.; Clayton, L.K.; Wang, J.H.; Reinherz, E.L. Involvement of the TCR Cbeta FG loop in thymic selection and T cell function. J. Exp. Med. 2002, 195, 1419–1431. [Google Scholar] [CrossRef]
- Touma, M.; Sun, Z.Y.; Clayton, L.K.; Marissen, W.E.; Kruisbeek, A.M.; Wagner, G.; Reinherz, E.L. Importance of the CD3gamma ectodomain terminal beta-strand and membrane proximal stalk in thymic development and receptor assembly. J. Immunol. 2007, 178, 3668–3679. [Google Scholar] [CrossRef]
- Sun, Z.Y.J.; Kim, S.T.; Kim, I.C.; Fahmy, A.; Reinherz, E.L.; Wagner, G. Solution structure of the CD3εδ ectodomain and comparison with CD3εγ as a basis for modeling T cell receptor topology and signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 16867–16872. [Google Scholar] [CrossRef]
- Degermann, S.; Sollami, G.; Karjalainen, K. Impaired NK1.1 T cell development in mice transgenic for a T cell receptor beta chain lacking the large, solvent-exposed cbeta FG loop. J. Exp. Med. 1999, 190, 1357–1362. [Google Scholar] [CrossRef]
- Kuhns, M.S.; Davis, M.M. Disruption of Extracellular Interactions Impairs T Cell Receptor-CD3 Complex Stability and Signaling. Immunity 2007, 26, 357–369. [Google Scholar] [CrossRef]
- Natarajan, A.; Nadarajah, V.; Felsovalyi, K.; Wang, W.; Jeyachandran, V.R.; Wasson, R.A.; Cardozo, T.; Bracken, C.; Krogsgaard, M. Structural Model of the Extracellular Assembly of the TCR-CD3 Complex. Cell Rep. 2016, 14, 2833–2845. [Google Scholar] [CrossRef]
- Dong, C.; Juedes, A.E.; Temann, U.-A.; Shresta, S.; Allison, J.P.; Ruddle, N.H.; Flavell, R.A. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature 2001, 409, 97. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Wong, M.T.; Ong, D.E.H.; Lim, F.S.H.; Teng, K.W.W.; McGovern, N.; Narayanan, S.; Ho, W.Q.; Cerny, D.; Tan, H.K.K.; Anicete, R.; et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016, 45, 442–456. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Locke, N.R.; Stankovic, S.; Funda, D.P.; Harrison, L.C. TCR gamma delta intraepithelial lymphocytes are required for self-tolerance. J. Immunol. 2006, 176, 6553–6559. [Google Scholar] [CrossRef]
- Midtvedt, K.; Fauchald, P.; Lien, B.; Hartmann, A.; Albrechtsen, D.; Bjerkely, B.L.; Leivestad, T.; Brekke, I.B. Individualized T cell monitored administration of ATG versus OKT3 in steroid-resistant kidney graft rejection. Clin. Transplant. 2003, 17, 69–74. [Google Scholar] [CrossRef]
- Potgieter, M.; Bester, J.; Kell, D.B.; Pretorius, E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol. Rev. 2015, 39, 567–591. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Bouman, A.; Heineman, M.J.; Faas, M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update 2005, 11, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Guo, W.; Chen, W.; Fu, L.; Wang, J.; Tian, Y.; Xiao, X.; Kang, T.; Huang, W.; Deng, W. Nicotine Promotes Proliferation of Human Nasopharyngeal Carcinoma Cells by Regulating α7AChR, ERK, HIF-1α and VEGF/PEDF Signaling. PLoS ONE 2012, 7, e43898. [Google Scholar] [CrossRef] [PubMed]
- Maret, A.; Coudert, J.D.; Garidou, L.; Foucras, G.; Gourdy, P.; Krust, A.; Dupont, S.; Chambon, P.; Druet, P.; Bayard, F.; et al. Estradiol enhances primary antigen specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur. J. Immunol. 2003, 33, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Nordman, J.C.; Muldoon, P.; Clark, S.; Damaj, M.I.; Kabbani, N. The alpha4 nicotinic receptor promotes CD4+ T-cell proliferation and a helper T-cell immune response. Mol. Pharmacol. 2014, 85, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Razani-Boroujerdi, S.; Boyd, R.T.; Dávila-García, M.I.; Nandi, J.S.; Mishra, N.C.; Singh, S.P.; Pena-Philippides, J.C.; Langley, R.; Sopori, M.L. T Cells Express α7-Nicotinic Acetylcholine Receptor Subunits That Require a Functional TCR and Leukocyte-Specific Protein Tyrosine Kinase for Nicotine-Induced Ca2+ Response. J. Immunol. 2007, 179, 2889–2898. [Google Scholar] [CrossRef]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with PyRx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [CrossRef]
- Brylinski, M.; Skolnick, J. A threading-based method (FINDSITE) for ligand-binding site prediction and functional annotation. Proc. Natl. Acad. Sci. USA 2008, 105, 129–134. [Google Scholar] [CrossRef]
- Call, M.E.; Pyrdol, J.; Wiedmann, M.; Wucherpfennig, K.W. The organizing principle in the formation of the T cell receptor-CD3 complex. Cell 2002, 111, 967–979. [Google Scholar] [CrossRef]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Keustermans, G.C.; Hoeks, S.B.; Meerding, J.M.; Prakken, B.J.; de Jager, W. Cytokine assays: An assessment of the preparation and treatment of blood and tissue samples. Methods 2013, 61, 10–17. [Google Scholar] [CrossRef]
| Rank | Compounds | IL2 | INF-Gamma | TGF-Beta |
|---|---|---|---|---|
| 1 | Nicotine | Stimulated | No activity | No activity |
| 2 | Aspartame | Stimulated | No activity | No activity |
| 3 | Estradiol | Stimulated | No activity | No activity |
| 4 | Sucrose | Stimulated | No activity | No activity |
| 5 | Sucralfate | Not tested | Not tested | Not tested |
| 6 | Diethylene glycol monoethyl ether (NF) | Not tested | Not tested | Not tested |
| 7 | Ethoxyl-Ethanol | Not tested | Not tested | Not tested |
| 8 | 3,6,9,12,15-Pentaoxaheptadecane | Not tested | Not tested | Not tested |
| 9 | Ethane | Not tested | Not tested | Not tested |
| 10 | 3,6,9,12,15,18-HEXAOXAICOSANE | Not tested | Not tested | Not tested |
| 11 | Carbitol | Not tested | Not tested | Not tested |
| 12 | Platinum compounds | Inhibited | No activity | No activity |
| 13 | 18-Crown-6-tantalum(V)pentachloride | Not tested | Not tested | Not tested |
| 14 | 2,2′-(Ethane-1,2-diylbis(oxy))diethanol | Not tested | Not tested | Not tested |
| 15 | D,L-Homotryptophan | Stimulated | No activity | No activity |
| 16 | Malonic | No activity | No activity | Inhibited |
| 17 | Isobutyric | No activity | No activity | No activity |
| 18 | Propanoic | No activity | No activity | No activity |
| 19 | N-Acetyl-D-tryptophan | Stimulated | Inhibited | Inhibited |
| 20 | Butanoate | Not tested | Not tested | Not tested |
| 21 | Butanediol | Stimulated | No activity | Stimulated |
| 22 | Hexanoate | Not tested | Not tested | Not tested |
| 23 | Pentanoate | Not tested | Not tested | Not tested |
| 24 | Glycerol | Stimulated | No activity | Stimulated |
| 25 | Propanediol | Not tested | Not tested | Not tested |
| 26 | Indole-3-butanoate | Not tested | Not tested | Not tested |
| 27 | Indole-3-butyrate | No activity | No activity | No activity |
| Title | Docking Score a (kcal/mol) | MMGBSA dG Bind b (kcal/mol) | Interacting Residues c | Biological Activity |
|---|---|---|---|---|
| estradiol | −0.4 | −31.3 | GLU219 TRP223 GLN225 | Active |
| sucrose | −5.0 | −29.7 | ASP221 GLU222 TRP223 | Active |
| aspartame | −2.1 | −27.9 | GLU222 TRP223 LYS229 | Active |
| butanediol | −0.9 | −21.1 | GLU219 GLU222 TRP223 | Active |
| indolebutyrate | −1.6 | −20.9 | PHE121 ARG187 ALA228 | Inactive |
| nicotine | 0.9 | −20.6 | Active | |
| homotryptophan | −2.0 | −18.3 | GLU222 TRP223 LYS229 | Active |
| NAD_tryptophan | −1.7 | −17.3 | GLU124 ARG187 | Active |
| homotryptophan | −0.3 | −17.2 | GLU222 | Active |
| aspartame | 0.4 | −16.7 | GLU219 TRP223 LYS229 | Active |
| nicotine | −2.4 | −15.3 | GLU222 LYS229 | Active |
| nicotine | −1.7 | −14.4 | GLU219 | Active |
| glycerol | −1.7 | −13.8 | GLN225 LYS229 | Active |
| malonic | 0.0 | −9.7 | GLN225 LYS229 | Active |
| propanoic | −0.4 | −5.5 | LYS229 | Inactive |
| isobutyrate | −0.8 | −4.5 | GLN233 | Inactive |
| malonic | −2.0 | 0.6 | Inactive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolios, N.; Pham, S.; Hou, G.; Du, J.; Quek, C.; Hibbs, D. Non-Antigenic Modulation of Antigen Receptor (TCR) Cβ-FG Loop Modulates Signalling: Implications of External Factors Influencing T-Cell Responses. Int. J. Mol. Sci. 2023, 24, 9334. https://doi.org/10.3390/ijms24119334
Manolios N, Pham S, Hou G, Du J, Quek C, Hibbs D. Non-Antigenic Modulation of Antigen Receptor (TCR) Cβ-FG Loop Modulates Signalling: Implications of External Factors Influencing T-Cell Responses. International Journal of Molecular Sciences. 2023; 24(11):9334. https://doi.org/10.3390/ijms24119334
Chicago/Turabian StyleManolios, Nicholas, Son Pham, Guojiang Hou, Jonathan Du, Camelia Quek, and David Hibbs. 2023. "Non-Antigenic Modulation of Antigen Receptor (TCR) Cβ-FG Loop Modulates Signalling: Implications of External Factors Influencing T-Cell Responses" International Journal of Molecular Sciences 24, no. 11: 9334. https://doi.org/10.3390/ijms24119334
APA StyleManolios, N., Pham, S., Hou, G., Du, J., Quek, C., & Hibbs, D. (2023). Non-Antigenic Modulation of Antigen Receptor (TCR) Cβ-FG Loop Modulates Signalling: Implications of External Factors Influencing T-Cell Responses. International Journal of Molecular Sciences, 24(11), 9334. https://doi.org/10.3390/ijms24119334







