An Update on the Molecular and Cellular Basis of Pharmacotherapy in Type 2 Diabetes Mellitus
Abstract
1. Introduction
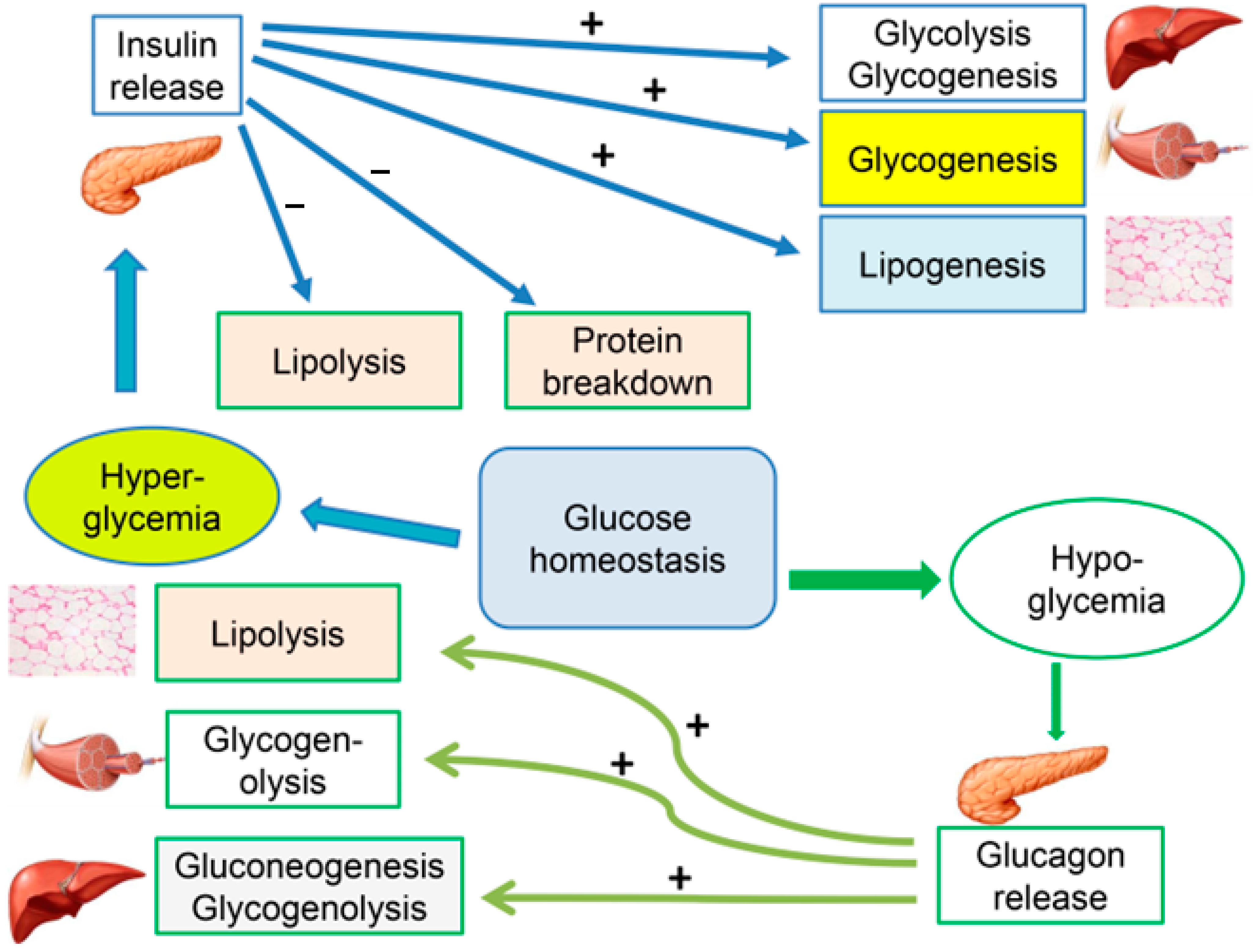
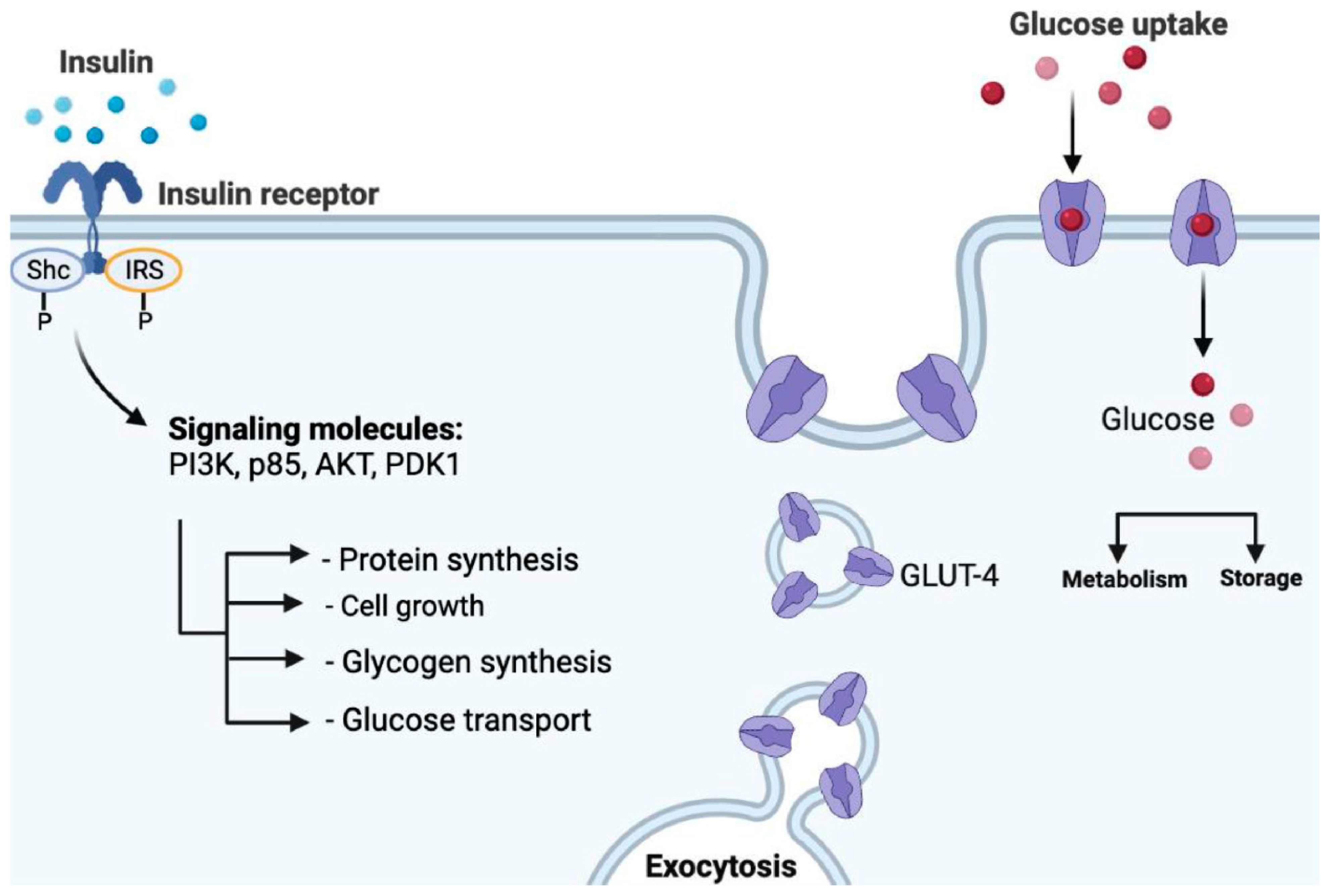
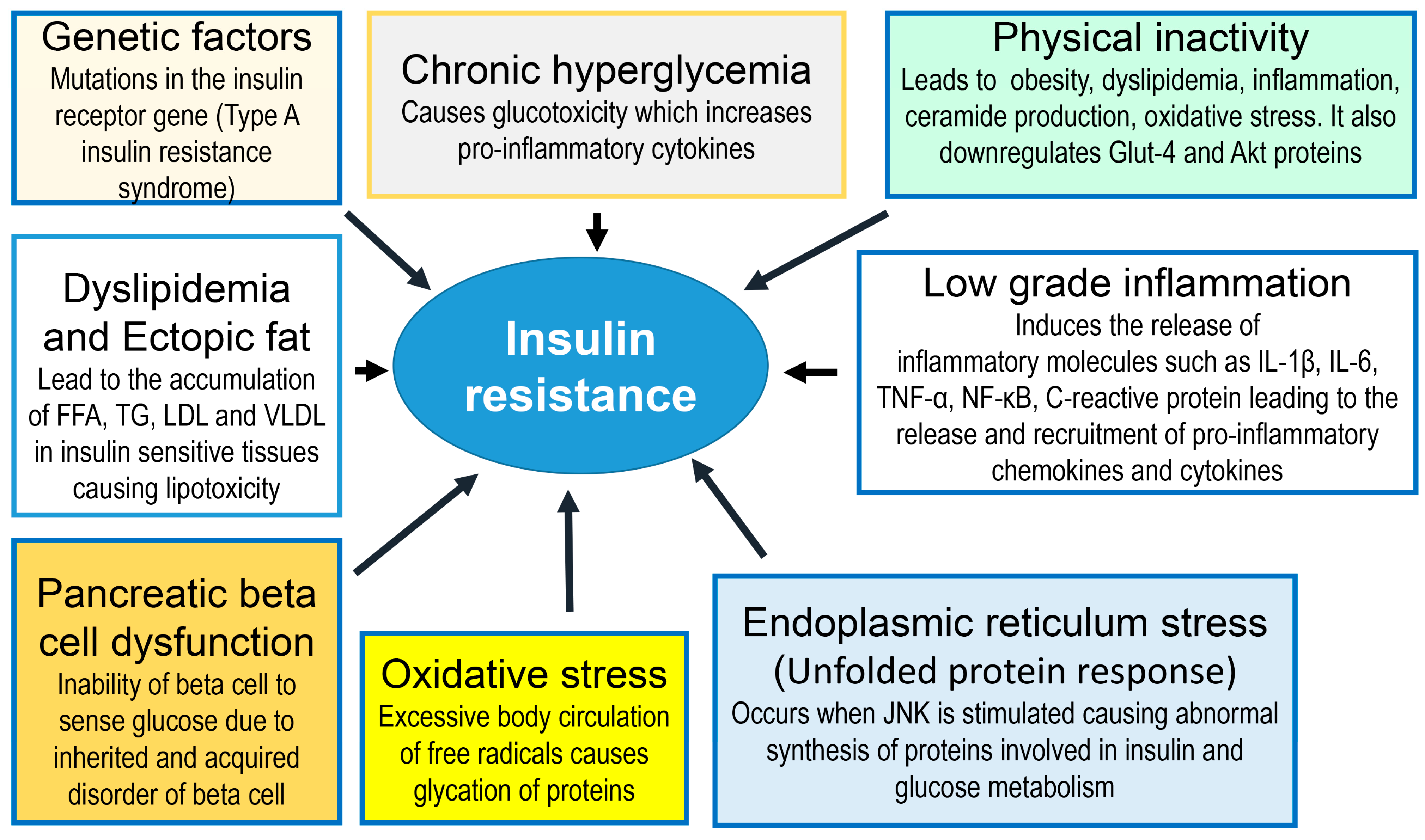
2. Insulin Resistance
3. Type 2 DM
4. Management of DM
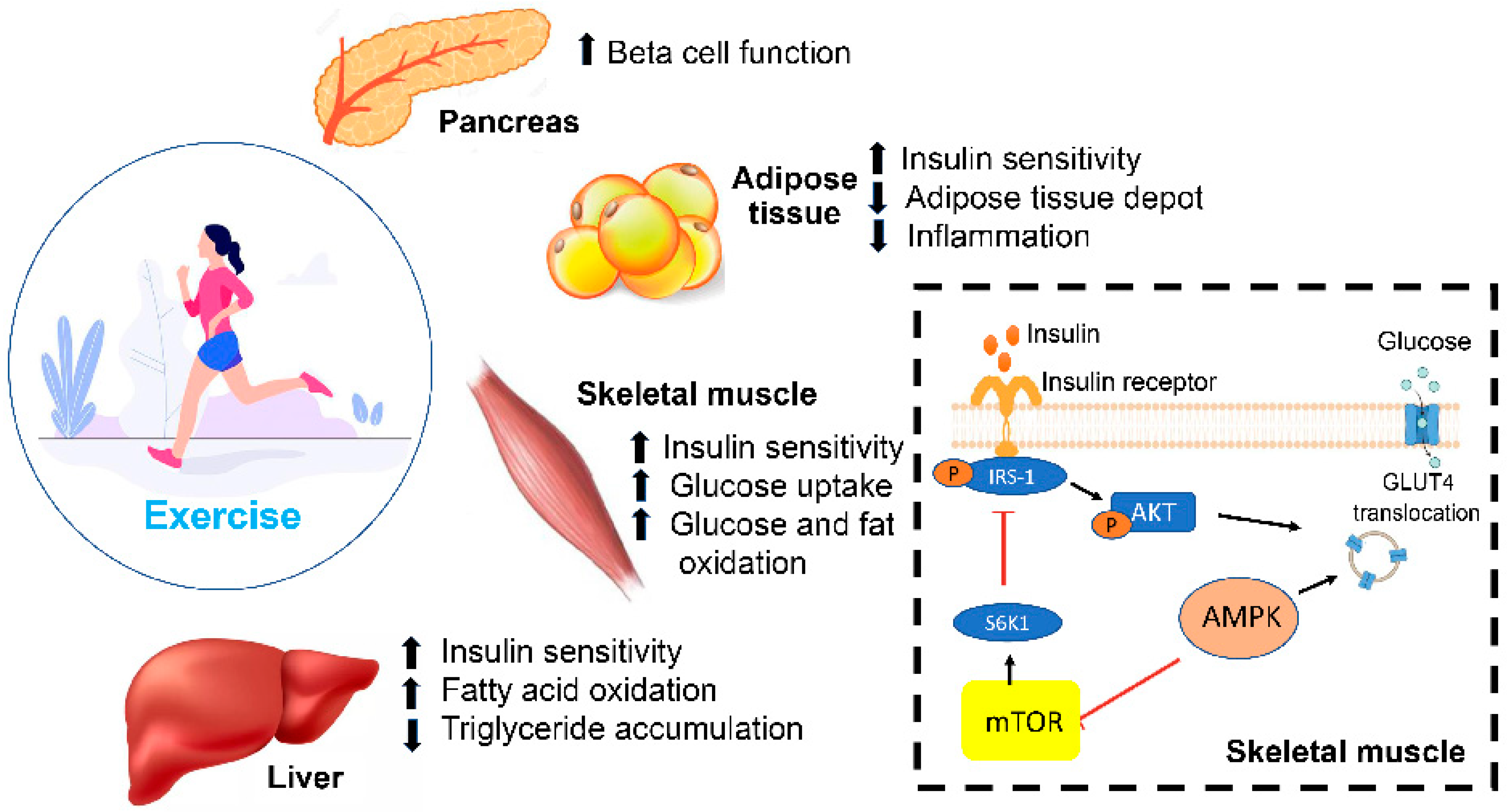
4.1. Lifestyle (Diet and Physical Activity)
4.2. Diet
4.3. Physical Activity
- Resistance exercises, which involve utilizing free weights and body weight exercises, have been shown to cause a threefold reduction in HbA1c in patients with T2DM when compared to inactive patients [90]. Another study showed that an 8-week weight-training protocol in patients with T2DM improved insulin and glucose responses upon oral glucose tolerance testing [91]. In addition, this type of training caused an increase in the skeletal muscle mass, which is believed to be due to enhanced muscle glycogen storage, leading to increased glucose uptake from the bloodstream. These findings support the benefit of implementing this type of training in a diabetes management plan.
- Aerobic training is another type of exercise that consists of the continuous movement of large muscles, such as in jogging and walking, for at least 30 min per day for 3–7 days weekly, as per the American Diabetes Association (ADA) guidelines [92]. Aerobic training is a well-established tool in improving HbA1c by improving the lipid metabolism and weight loss [93]. One study showed that in 60 adults with T2DM, 6 months of aerobic training caused a significant reduction in HbA1c and fasting insulin levels [94]. Another study showed that aerobic activity in diabetic patients improved glycemic control, insulin sensitivity and oxidative capacity compared to sedentary individuals [95].
- Combining both resistance and aerobic exercise may be the most effective approach to controlling glucose and lipid metabolism in T2DM, as per the current ADA guidelines. Cuff et al. showed that combining both types of exercises led to a significant increase in muscle glucose uptake and insulin sensitivity when compared to aerobic exercises alone [96]. Another distinguished study comparing the effects of both types of exercises alone and their combination in 915 adults showed that individuals utilizing both regimens had a more significant reduction in HbA1c [97].
5. Pharmacotherapy
6. Current Concepts on Insulin in T2DM
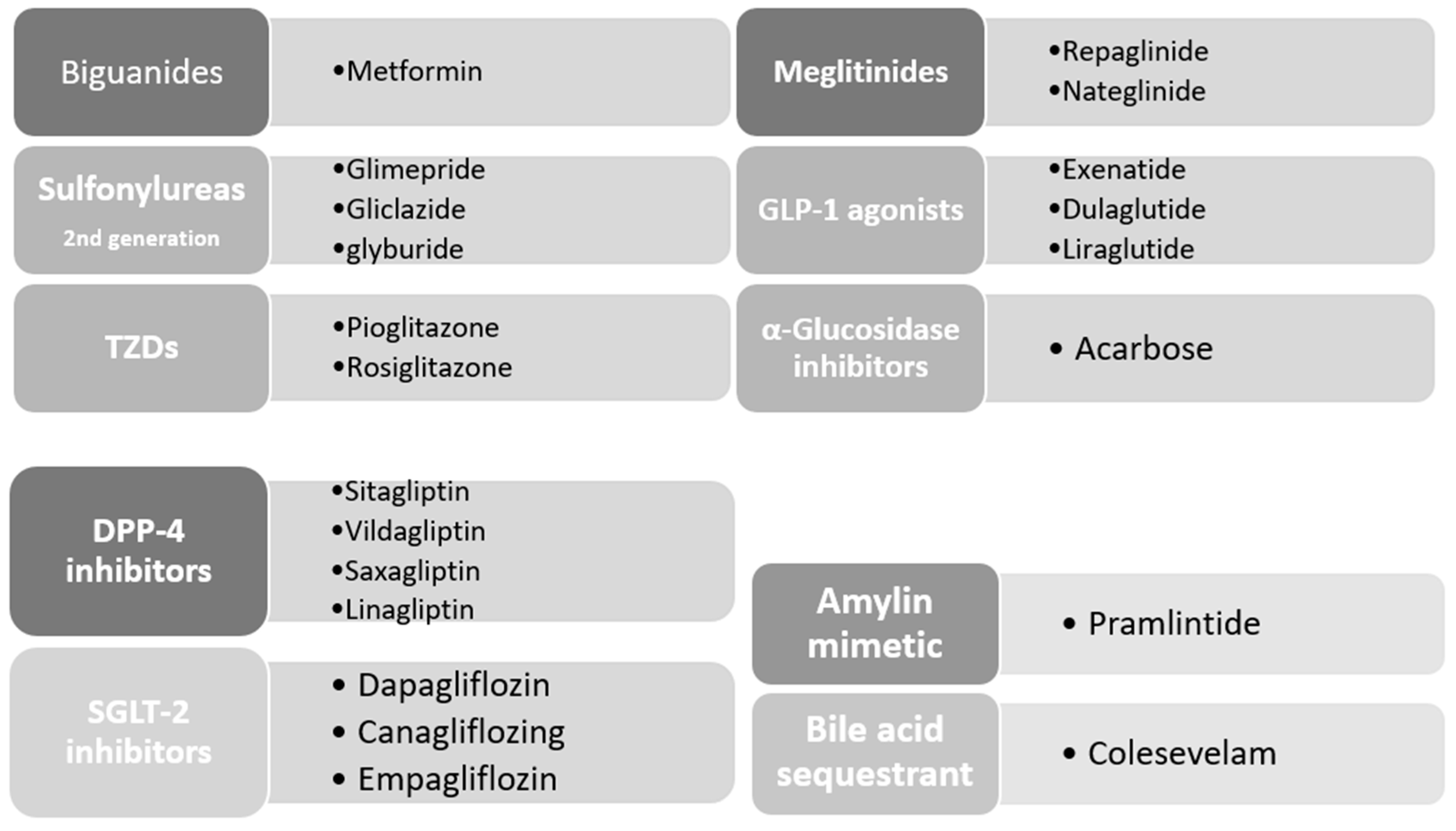
7. Oral Hypoglycemic Agents
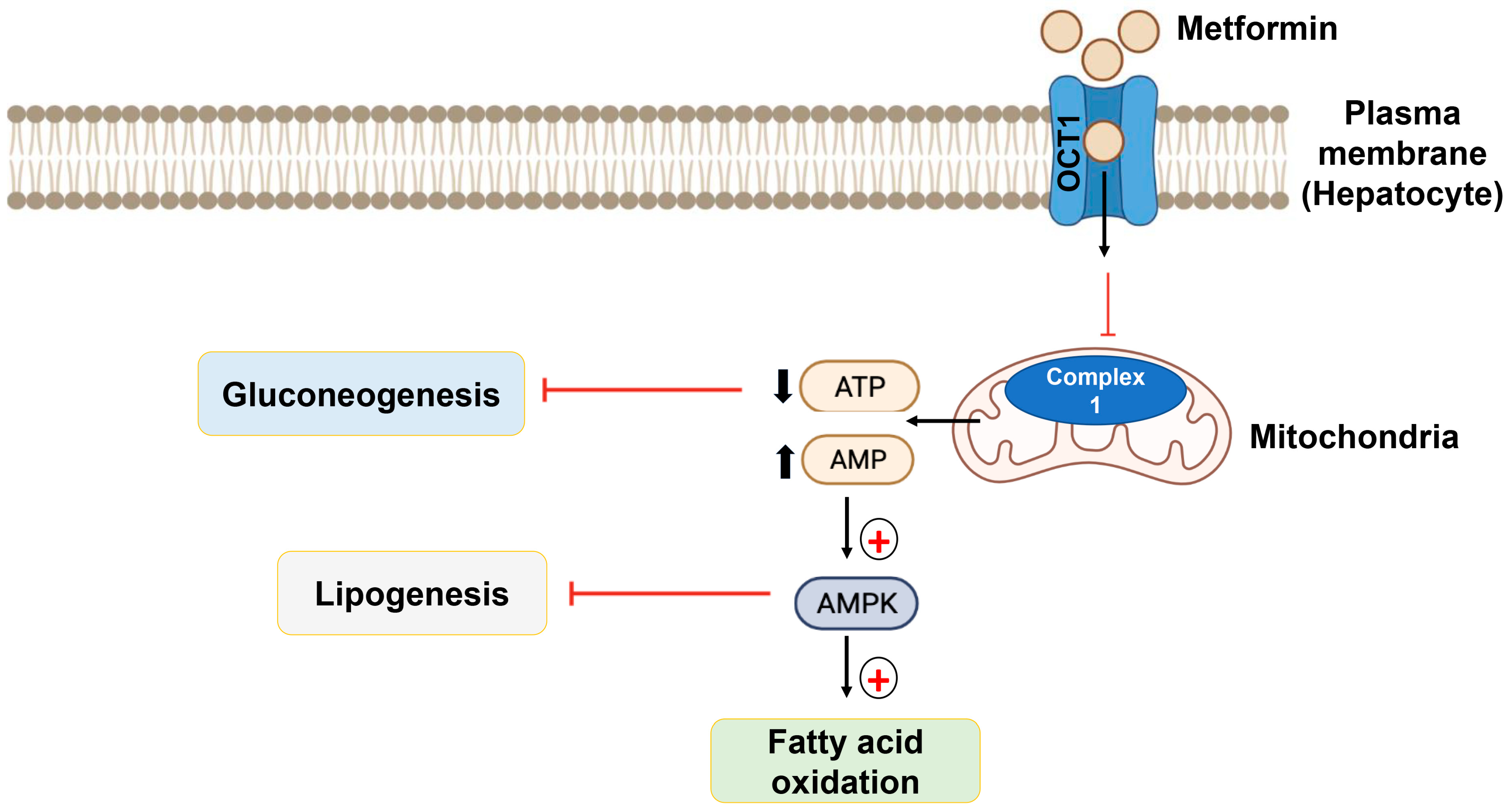
7.1. Biguanides
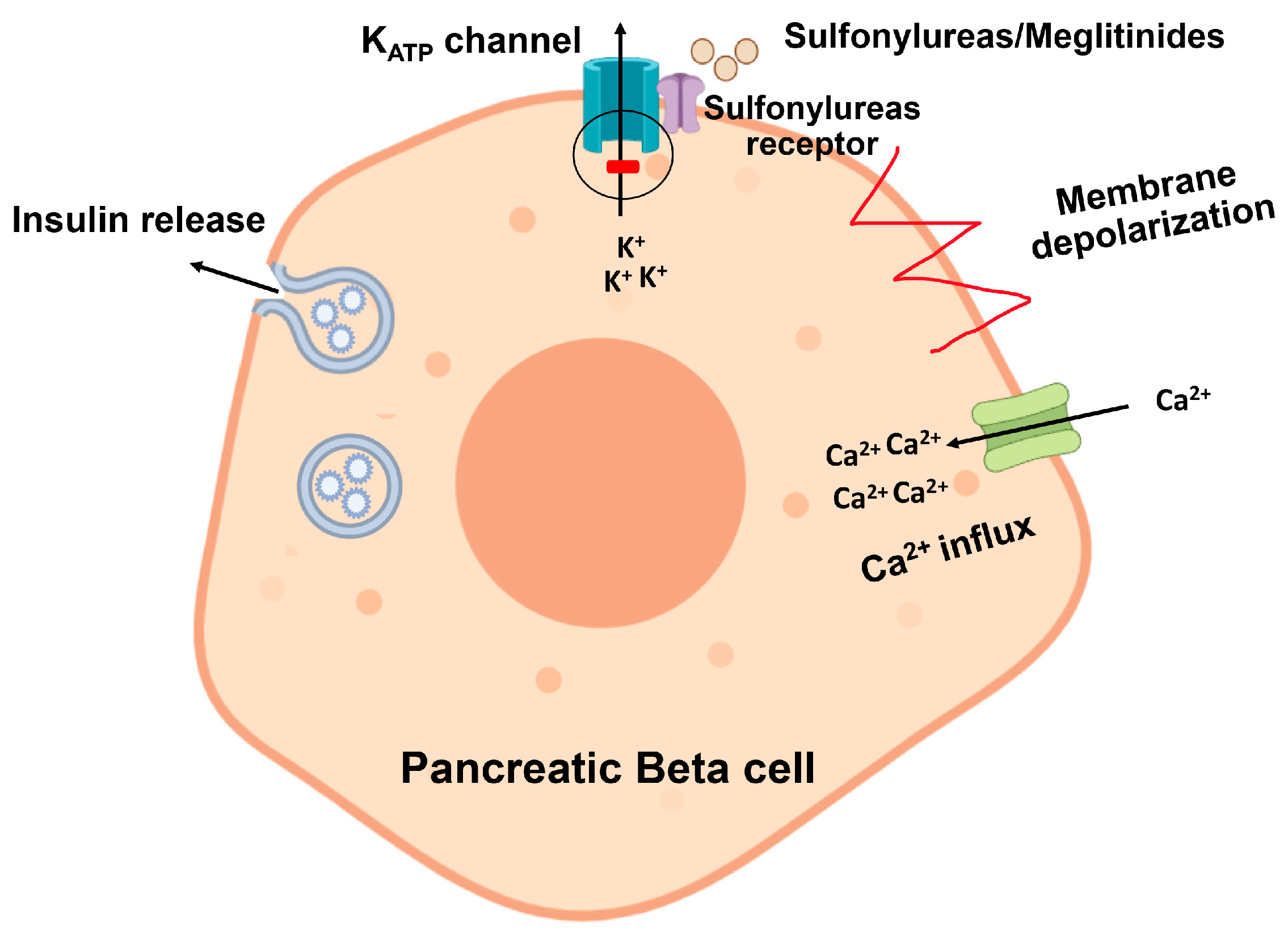
7.2. Sulfonylureas
7.3. Meglitinides
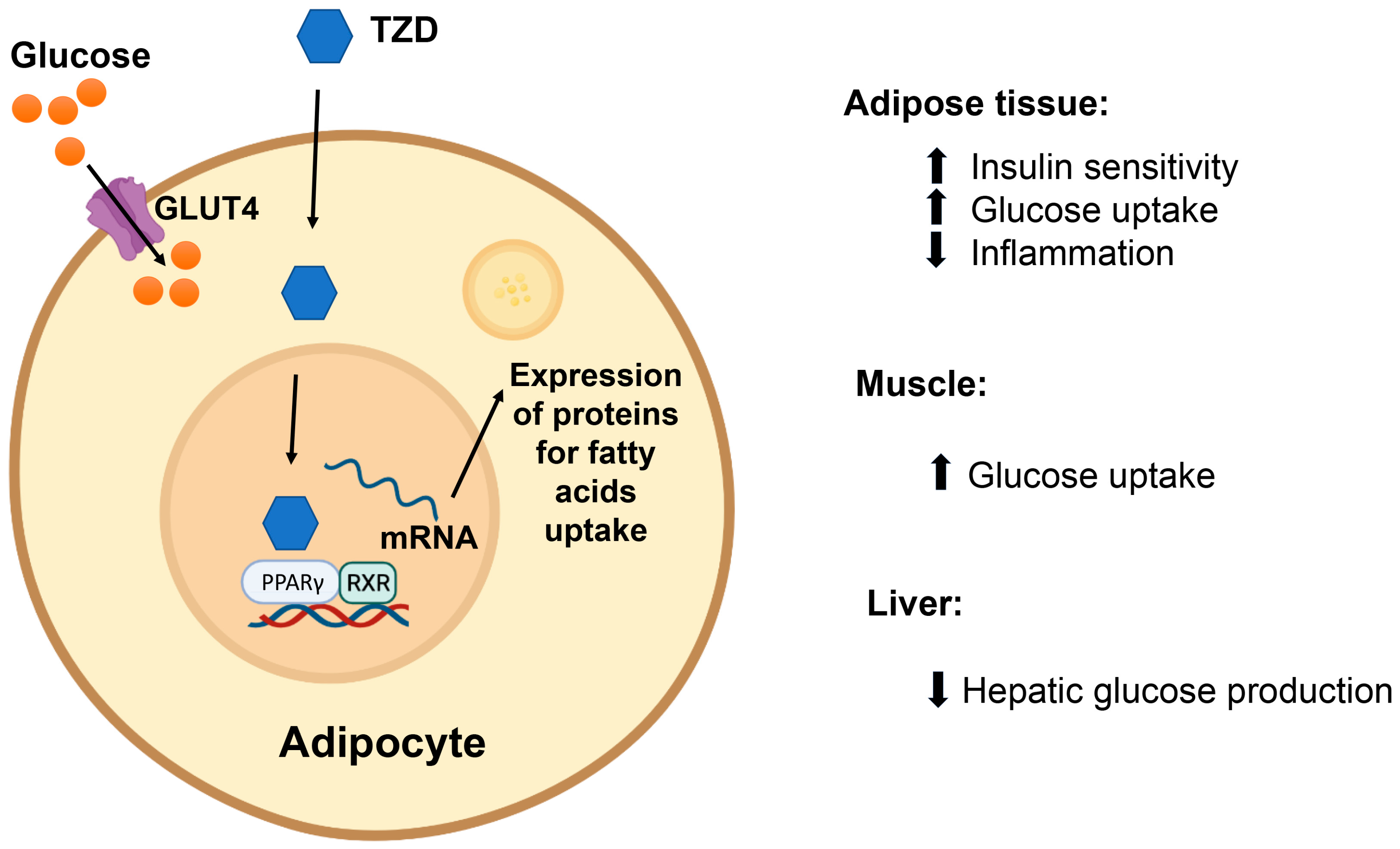
7.4. Thiazolidinediones
7.5. Glucagon-like Peptide-1 (GLP-1) Agonists
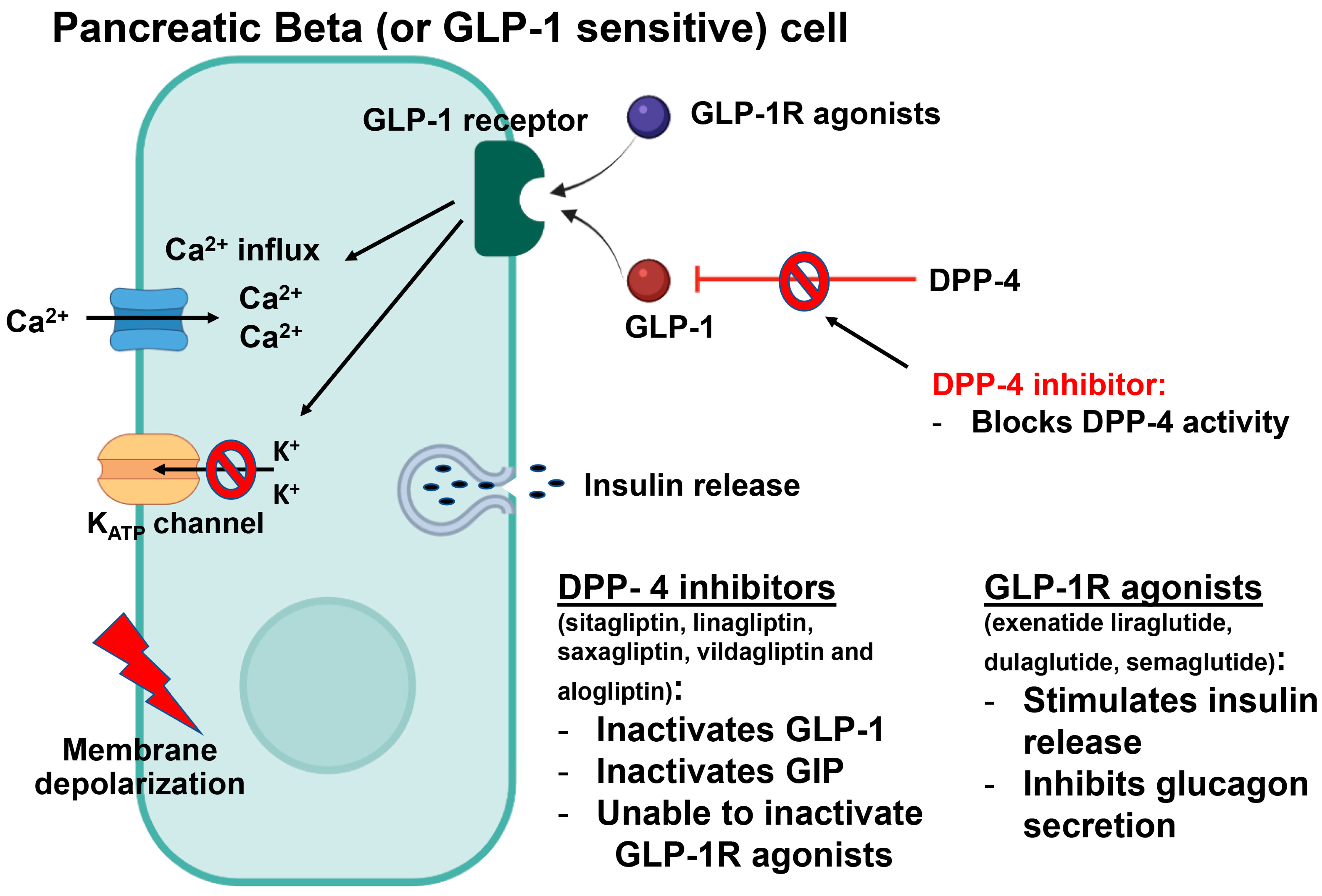
7.6. Dipeptidyl Peptidase-4 (DPP-4) Inhibitors
7.7. α-Glucosidase Inhibitors
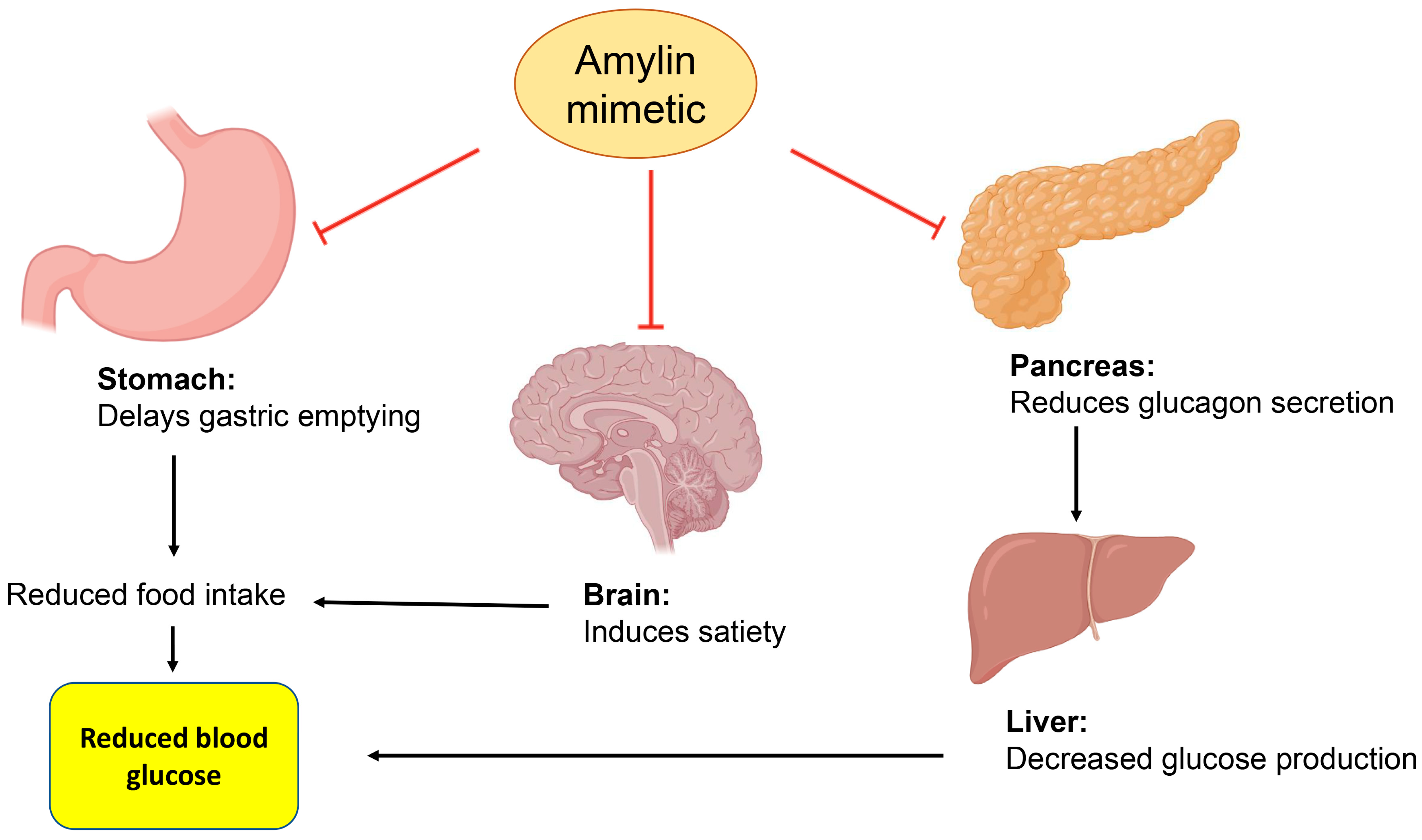
7.8. Amylin Mimetic
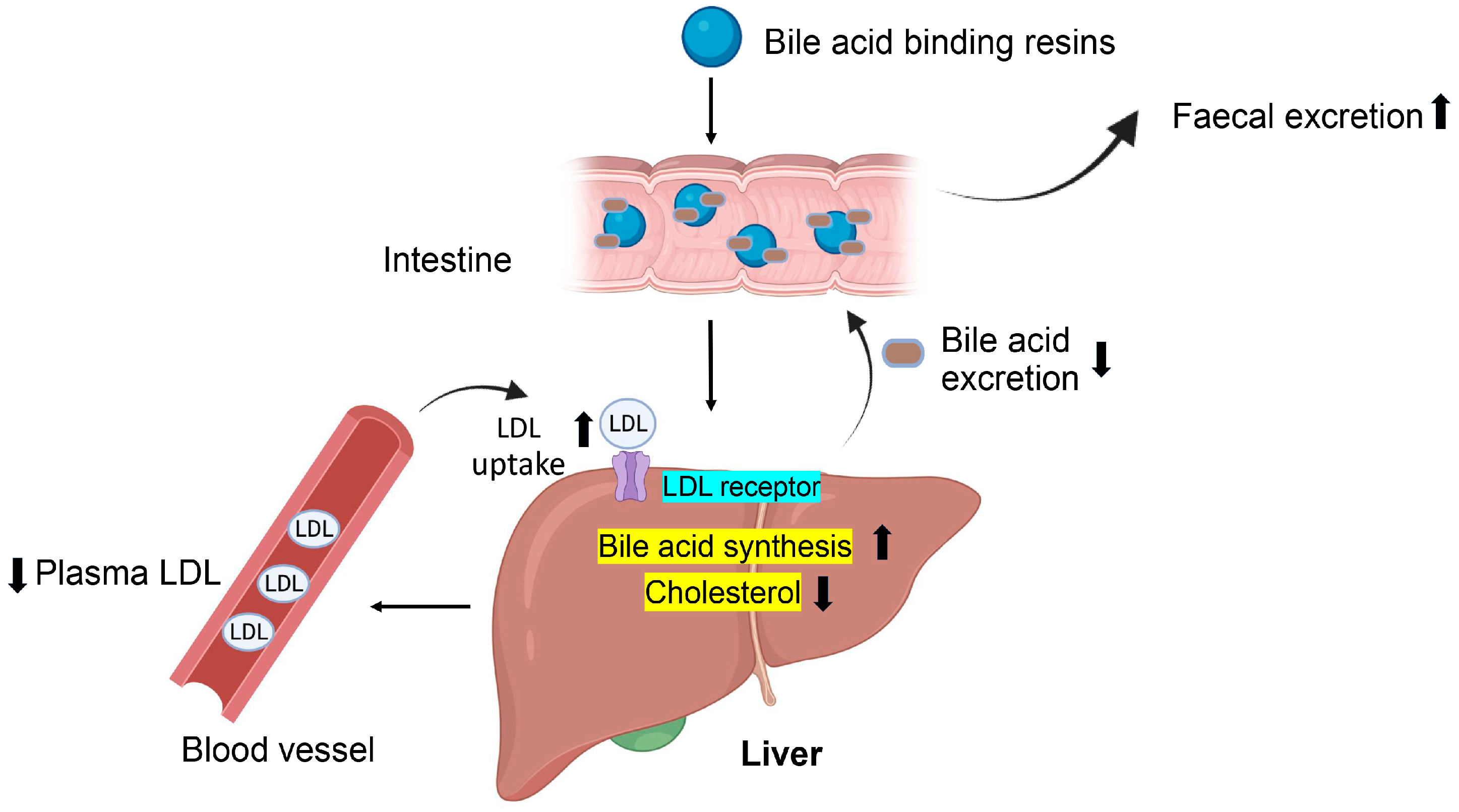
7.9. Bile Acid Binding Resins
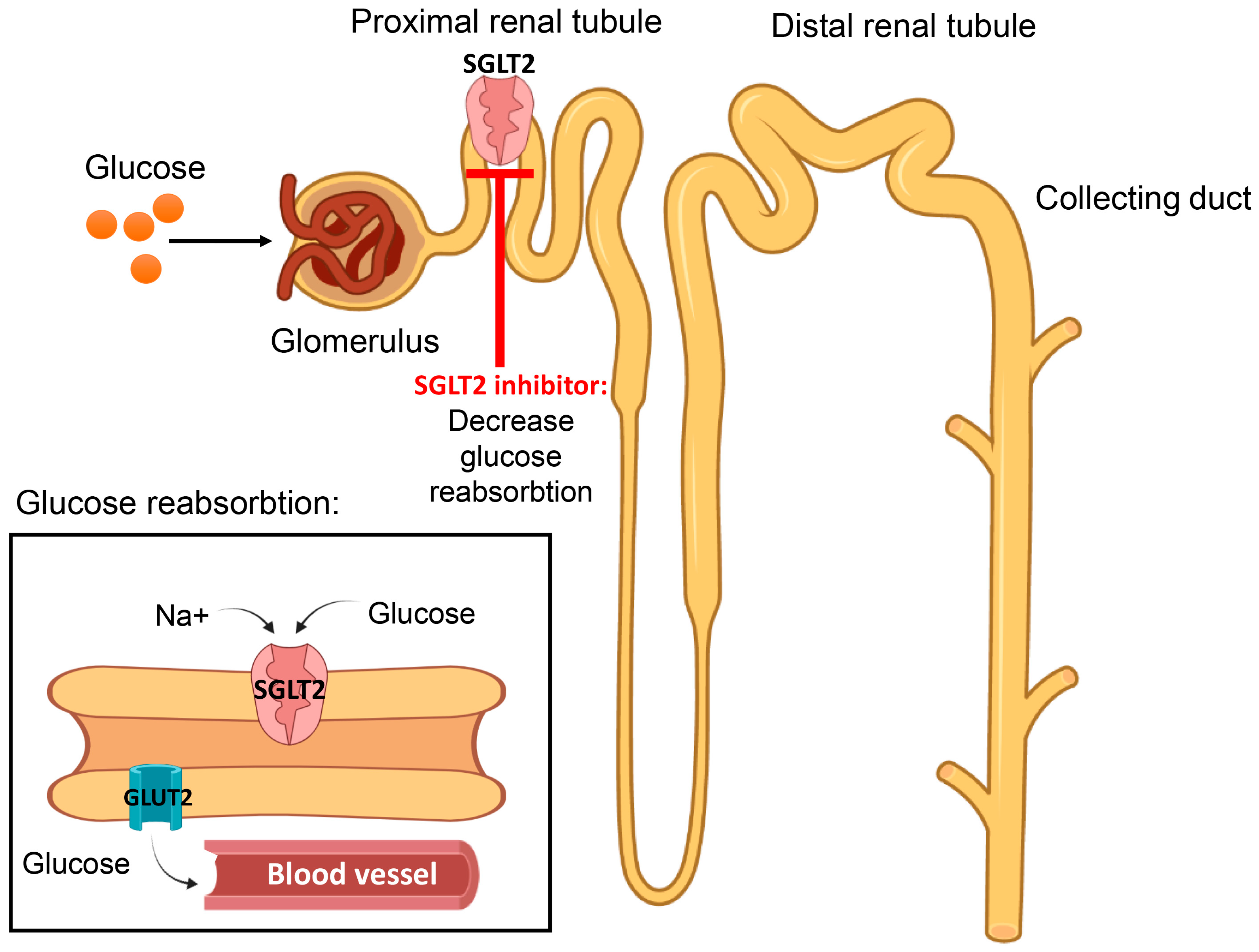
7.10. Sodium–Glucose Co-Transporter (SGLT) Inhibitors
8. Effectiveness of Different Classes of Anti-Diabetic Drugs on HbA1c
9. Anti-Diabetic Drugs That Have Been Suspended
10. New Directions for the Prevention and Management of Diabetes Mellitus
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- IDF Diabetes Atlas. International Diabetes Federation Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E. Diabetes mellitus-multifactorial in aetiology and global in prevalence. Arch. Physiol. Biochem. 2001, 109, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Schattner, P.; Dunn, E. An Update on the Etiology and Epidemiology of Diabetes Mellitus. Ann. N. Y. Acad. Sci. 2006, 1084, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabete Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J. Pediatr. 1994, 125, 177–188. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Maiorino, M.I.; Giugliano, D. Lifestyle approach for type 2 diabetes and metabolic syndrome. Curr. Atheroscler. Rep. 2008, 10, 523–528. [Google Scholar] [CrossRef]
- Gilmartin, A.B.H.; Ural, S.H.; Repke, J.T. Gestational Diabetes Mellitus. Rev. Obstet. Gynecol. 2008, 1, 129–134. [Google Scholar] [PubMed]
- Couch, S.C.; Philipson, E.H.; Bendel, R.B.; Pujda, L.M.; A Milvae, R.; Lammi-Keefe, C.J. Elevated Lipoprotein Lipids and Gestational Hormones in Women With Diet-Treated Gestational Diabetes Mellitus Compared to Healthy Pregnant Controls. J. Diabetes Its Complicat. 1998, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hedderson, M.M.; Quesenberry, C.P.; Feng, J.; Ferrara, A. Central Obesity Increases the Risk of Gestational Diabetes Partially Through Increasing Insulin Resistance. Obesity 2018, 27, 152–160. [Google Scholar] [CrossRef]
- Amed, S.; Oram, R. Maturity-Onset Diabetes of the Young (MODY): Making the Right Diagnosis to Optimize Treatment. Can. J. Diabetes 2016, 40, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Anık, A.; Çatlı, G.; Abacı, A.; Böber, E. Maturity-onset diabetes of the young (MODY): An update. J. Pediatr. Endocrinol. Metab. 2015, 28, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Timsit, J.; Saint-Martin, C.; Dubois-Laforgue, D.; Bellanne-Chantelot, C. Searching for Maturity-Onset Diabetes of the Young (MODY): When and What for? Can. J. Diabetes 2016, 40, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Goran, M.I.; Bergman, R.N.; Cruz, M.L.; Watanabe, R. Insulin resistance and associated compensatory responses in african-american and Hispanic children. Diabetes Care 2002, 25, 2184–2190. [Google Scholar] [CrossRef]
- Von Ah Morano, A.E.; Dorneles, G.P.; Peres, A.; Lira, F.S. The role of glucose homeostasis on immune function in response to exercise: The impact of low or higher energetic conditions. J. Cell Physiol. 2020, 235, 3169–3188. [Google Scholar] [CrossRef]
- Wen, S.; Wang, C.; Gong, M.; Zhou, L. An overview of energy and metabolic regulation. Sci. China Life Sci. 2018, 62, 771–790. [Google Scholar] [CrossRef]
- White, M.; Kahn, C. The insulin signaling system. J. Biol. Chem. 1994, 269, 1–4. [Google Scholar] [CrossRef]
- Lee, J.; Pilch, P.F. The insulin receptor: Structure, function, and signaling. Am. J. Physiol. 1994, 266, C319–C334. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int. J. Obes. 2008, 32, S52–S54. [Google Scholar] [CrossRef]
- Goodyear, L.J.; Giorgino, F.; Sherman, L.A.; Carey, J.; Smith, R.J.; Dohm, G.L. Insulin receptor phosphorylation, insulin receptor substrate-1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J. Clin. Investig. 1995, 95, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Bjornholm, M.; Kawano, Y.; Lehtihet, M.; Zierath, J.R. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes 1997, 46, 524–527. [Google Scholar] [CrossRef]
- Zaid, H.; Antonescu, C.N.; Randhawa, V.K.; Klip, A. Insulin action on glucose transporters through molecular switches, tracks and tethers. Biochem. J. 2008, 413, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty sweet years of GLUT4. J. Biol. Chem. 2019, 294, 11369–11381. [Google Scholar] [CrossRef]
- Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000, 106, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Herzberg-Schäfer, S.A.; Heni, M.; Stefan, N.; Häring, H.-U.; Fritsche, A.E. Impairment of GLP1-induced insulin secretion: Role of genetic background, insulin resistance and hyperglycaemia. Diabetes Obes. Metab. 2012, 14 (Suppl. 3), 85–90. [Google Scholar] [CrossRef] [PubMed]
- de Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef]
- Field, A.E.; Coakley, E.H.; Must, A.; Spadano, J.L.; Laird, N.; Dietz, W.H.; Rimm, E.; Colditz, G. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch. Intern. Med. 2001, 161, 1581–1586. [Google Scholar] [CrossRef]
- Greenberg, A.S.; McDaniel, M.L. Identifying the links between obesity, insulin resistance and beta-cell function: Potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur. J. Clin. Investig. 2002, 32 (Suppl. 3), 24–34. [Google Scholar] [CrossRef]
- Unger, R.H. Lipotoxic diseases. Annu. Rev. Med. 2002, 53, 319–336. [Google Scholar] [CrossRef]
- Adeghate, E. An update on the biology and physiology of resistin. Cell. Mol. Life Sci. 2004, 61, 2485–2496. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E. Visfatin: Structure, function and relation to diabetes mellitus and other dysfunctions. Curr. Med. Chem. 2008, 15, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Pathophysiology of type 2 diabetes. Acta Clin. Belg. 2003, 58, 335–341. [Google Scholar] [CrossRef]
- Bastard, J.-P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Dludla, P.V.; Mabhida, S.E.; Ziqubu, K.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Hanser, S.; Basson, A.K.; Pheiffer, C.; Kengne, A.P. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J. Diabetes 2023, 14, 130–146. [Google Scholar] [CrossRef]
- Nauck, M.; Stockmann, F.; Ebert, R.; Creutzfeldt, W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986, 29, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Fehmann, H.C.; Goke, R.; Goke, B. Cell and molecular biology of the incretin hormones glucagon-like peptide-I and glucose-dependent insulin releasing polypeptide. Endocr. Rev. 1995, 16, 390–410. [Google Scholar] [CrossRef]
- Gautier, J.; Fetita, S.; Sobngwi, E.; Salaün-Martin, C. Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes. Diabetes Metab. 2005, 31, 233–242. [Google Scholar] [CrossRef]
- Buteau, J. GLP-1 receptor signaling: Effects on pancreatic beta-cell proliferation and survival. Diabetes Metab. 2008, 34 (Suppl. 2), S73–S77. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Singh, J.; Rashed, H.; Tariq, S.; Zilahi, E.; Adeghate, E. Mechanism of the beneficial and protective effects of exenatide in diabetic rats. J. Endocrinol. 2014, 220, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Singh, J.; Kalász, H.; Tekes, K.; Adeghate, E. Medicinal Chemistry and Applications of Incretins and DPP-4 Inhibitors in the Treatment of Type 2 Diabetes Mellitus. Open Med. Chem. J. 2011, 5 (Suppl. 2), 82–92. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Surampudi, P.N.; John-Kalarickal, J.; Fonseca, V.A. Emerging concepts in the pathophysiology of type 2 diabetes mellitus. Mt. Sinai J. Med. J. Transl. Pers. Med. 2009, 76, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Westermark, P.; Johnson, K.H.; O’Brien, T.; Betsholtz, C. Islet amyloid polypeptide? A novel controversy in diabetes research. Diabetologia 1992, 35, 297–303. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Yang, J.; Wang, L.; Liu, Y.; Wang, W.; Zhu, L.; Wang, W.; Yang, J.; Chen, F. Case Report: A Chinese Family of Type A Insulin Resistance Syndrome With Diabetes Mellitus, With a Novel Heterozygous Missense Mutation of the Insulin Receptor Gene. Front. Endocrinol. 2022, 13, 895424. [Google Scholar] [CrossRef]
- Kawahito, S.; Kitahata, H.; Oshita, S. Problems associated with glucose toxicity: Role of hyperglycemia-induced oxidative stress. World J. Gastroenterol. 2009, 15, 4137–4142. [Google Scholar] [CrossRef]
- Lotfy, M.; Adeghate, J.; Kalasz, H.; Singh, J.; Adeghate, E. Chronic complications of diabetes mellitus: A mini review. Curr. Diabetes Rev. 2017, 13, 3–10. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pathophysiology of Physical Inactivity-Dependent Insulin Resistance: A Theoretical Mechanistic Review Emphasizing Clinical Evidence. J. Diabetes Res. 2021, 2021, 7796727. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef]
- Cerf, M.E. High fat programming of beta cell compensation, exhaustion, death and dysfunction. Pediatr. Diabetes 2014, 16, 71–78. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. Mechanisms for Insulin Resistance: Common Threads and Missing Links. Cell 2012, 148, 852–871. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, S.-Y.; Choi, C.S. Insulin Resistance: From Mechanisms to Therapeutic Strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, U.; Cao, Q.; Yilmaz, E.; Lee, A.-H.; Iwakoshi, N.N.; Özdelen, E.; Tuncman, G.; Görgün, C.; Glimcher, L.H.; Hotamisligil, G.S. Endoplasmic Reticulum Stress Links Obesity, Insulin Action, and Type 2 Diabetes. Science 2004, 306, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Olokoba, A.B.; Obateru, O.A.; Olokoba, L.B. Type 2 Diabetes Mellitus: A Review of Current Trends. Oman Med. J. 2012, 27, 269–273. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, S.G.; Shaper, A.G.; Perry, I.J. Smoking as a Modifiable Risk Factor for Type 2 Diabetes in Middle-Aged Men. Diabetes Care 2001, 24, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gibbons, L.W.; Mitchell, T.L.; Kampert, J.B.; Blair, S.N. Alcohol intake and incidence of type 2 diabetes in men. Diabetes Care 2000, 23, 18–22. [Google Scholar] [CrossRef]
- Elhefnawy, M.E.; Ghadzi, S.M.S.; Harun, S.N. Predictors Associated with Type 2 Diabetes Mellitus Complications over Time: A Literature Review. J. Vasc. Dis. 2022, 1, 13–23. [Google Scholar] [CrossRef]
- Adeghate, E.A.; Kalász, H.; Al Jaberi, S.; Adeghate, J.; Tekes, K. Tackling type 2 diabetes-associated cardiovascular and renal comorbidities: A key challenge for drug development. Expert Opin. Investig. Drugs 2020, 30, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ebady, S.A.; Arami, M.A.; Shafigh, M.H. Investigation on the relationship between diabetes mellitus type 2 and cognitive impairment. Diabetes Res. Clin. Pract. 2008, 82, 305–309. [Google Scholar] [CrossRef]
- Chávez-Reyes, J.; Escárcega-González, C.E.; Chavira-Suárez, E.; León-Buitimea, A.; Vázquez-León, P.; Morones-Ramírez, J.R.; Villalón, C.M.; Quintanar-Stephano, A.; Marichal-Cancino, B.A. Susceptibility for Some Infectious Diseases in Patients With Diabetes: The Key Role of Glycemia. Front. Public Health 2021, 9, 559595. [Google Scholar] [CrossRef]
- Akinpelu, O.V.; Mujica-Mota, M.; Daniel, S.J. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope 2013, 124, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Majety, P.; Orquera, F.A.L.; Edem, D.; Hamdy, O. Pharmacological approaches to the prevention of type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1118848. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Al Jaberi, S.; Cohen, A.; Saeed, Z.; Ojha, S.; Singh, J.; Adeghate, E. Obesity: Molecular Mechanisms, Epidemiology, Complications and Pharmacotherapy. Cell. Biochem. Mech. Obes. 2021, v23, 249–266. [Google Scholar]
- Tuso, P. Prediabetes and lifestyle modification: Time to prevent a preventable disease. Perm. J. 2014, 18, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Deed, G.; Barlow, J.; Kawol, D.; Kilov, G.; Sharma, A.; Hwa, L.Y. Diet and diabetes. Aust. Fam. Physician 2015, 44, 192–196. [Google Scholar] [PubMed]
- Brunetti, L.; Kalabalik, J. Management of type-2 diabetes mellitus in adults: Focus on individualizing non-insulin therapies. P T Peer-Rev. J. Formul. Manag. 2012, 37, 687–696. [Google Scholar]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Yamaoka, K.; Nemoto, A.; Tango, T. Comparison of the Effectiveness of Lifestyle Modification with Other Treatments on the Incidence of Type 2 Diabetes in People at High Risk: A Network Meta-Analysis. Nutrients 2019, 11, 1373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fowler, M.J. Diabetes Treatment, Part 1: Diet and Exercise. Clin. Diabetes 2007, 25, 105. [Google Scholar] [CrossRef]
- Marín-Aguilar, F.; Pavillard, L.E.; Giampieri, F.; Bullón, P.; Cordero, M.D. Adenosine Monophosphate (AMP)-Activated Protein Kinase: A New Target for Nutraceutical Compounds. Int. J. Mol. Sci. 2017, 18, 288. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Ramachandran, A.; Yancy, W.S., Jr.; Forouhi, N.G. Nutritional basis of type 2 diabetes remission. BMJ 2021, 374, n1449. [Google Scholar] [CrossRef]
- Taylor, R. Type 2 diabetes and remission: Practical management guided by pathophysiology. J. Intern. Med. 2020, 289, 754–770. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Lifestyle Management: Standards of Medical Care in Diabetes-2018. Diabetes Care, 2018; 41, (Suppl. 1), S38–S50.
- Cespedes, E.M.; Hu, F.B.; Tinker, L.; Rosner, B.; Redline, S.; Garcia, L.; Hingle, M.; van Horn, L.; Howard, B.V.; Levitan, E.B.; et al. Multiple Healthful Dietary Patterns and Type 2 Diabetes in the Women’s Health Initiative. Am. J. Epidemiol. 2016, 183, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Maiorino, M.I.; Ciotola, M.; Di Palo, C.; Scognamiglio, P.; Gicchino, M.; Petrizzo, M.; Saccomanno, F.; Beneduce, F.; Ceriello, A.; et al. Effects of a Mediterranean-Style Diet on the Need for Antihyperglycemic Drug Therapy in Patients With Newly Diagnosed Type 2 Diabetes: A Randomized Trial. Ann. Intern Med. 2009, 151, 306–314. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Campbell, A.P. DASH Eating Plan: An Eating Pattern for Diabetes Management. Diabetes Spectr. 2017, 30, 76–81. [Google Scholar] [CrossRef]
- Franz, M.J.; MacLeod, J.; Evert, A.; Brown, C.; Gradwell, E.; Handu, D.; Reppert, A.; Robinson, M. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Systematic Review of Evidence for Medical Nutrition Therapy Effectiveness and Recommendations for Integration into the Nutrition Care Process. J. Acad. Nutr. Diet. 2017, 117, 1659–1679. [Google Scholar] [CrossRef]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. Nutrition Therapy Recommendations for the Management of Adults With Diabetes. Diabetes Care 2013, 36, 3821–3842. [Google Scholar] [CrossRef]
- Delahanty, L.M.; Nathan, D.M.; Lachin, J.M.; Hu, F.B.; Cleary, P.A.; Ziegler, G.K.; Wylie-Rosett, J.; Wexler, D.J.; Diabetes Control and Complications Trial/Epidemiology of Diabetes. Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the Diabetes Control and Complications Trial. Am. J. Clin. Nutr. 2009, 89, 518–524. [Google Scholar] [CrossRef] [PubMed]
- DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: Dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002, 325, 746. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S. Response to Comment on: Wheeler et al. Macronutrients, Food Groups, and Eating Patterns in the Management of Diabetes: A Systematic Review of the Literature, 2010. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.; Waugh, N.; Robertson, A. Protein restriction for diabetic renal disease. Cochrane Database Syst. Rev. 2007, 2007, Cd002181. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine, The National Academies. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 617S–625S. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef]
- Dunstan, D.W.; Daly, R.M.; Owen, N.; Jolley, D.; de Courten, M.; Shaw, J.; Zimmet, P. High-Intensity Resistance Training Improves Glycemic Control in Older Patients With Type 2 Diabetes. Diabetes Care 2002, 25, 1729–1736. [Google Scholar] [CrossRef]
- Dunstan, D.; Puddey, I.; Beilin, L.; Burke, V.; Morton, A.; Stanton, K. Effects of a short-term circuit weight training program on glycaemic control in NIDDM. Diabetes Res. Clin. Pract. 1998, 40, 53–61. [Google Scholar] [CrossRef]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Zanuso, S.; Jimenez, A.; Pugliese, G.; Corigliano, G.; Balducci, S. Exercise for the management of type 2 diabetes: A review of the evidence. Acta Diabetol. 2009, 47, 15–22. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Iliadis, F.; Angelopoulou, N.; Perrea, D.; Ampatzidis, G.; Liapis, C.; Alevizos, M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur. J. Prev. Cardiol. 2007, 14, 837–843. [Google Scholar] [CrossRef]
- Boulé, N.G.; Kenny, G.P.; Haddad, E.; Wells, G.A.; Sigal, R.J. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in Type 2 diabetes mellitus. Diabetologia 2003, 46, 1071–1081. [Google Scholar] [PubMed]
- Cuff, D.J.; Meneilly, G.S.; Martin, A.; Ignaszewski, A.; Tildesley, H.D.; Frohlich, J.J. Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care 2003, 26, 2977–2982. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Missbach, B.; Dias, S.; Koenig, J.; Hoffmann, G. Impact of different training modalities on glycaemic control and blood lipids in patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetologia 2014, 57, 1789–1797. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Jelleyman, C.; Yates, T.; O’Donovan, G.; Gray, L.J.; King, J.A.; Khunti, K.; Davies, M.J. The effects of high-intensity interval training on glucose regulation and insulin resistance: A meta-analysis. Obes. Rev. 2015, 16, 942–961. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Bailey, C.J.; Del Prato, S.; Barnett, A.H. Management of type 2 diabetes: New and future developments in treatment. Lancet 2011, 378, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Lorenzati, B.; Zucco, C.; Miglietta, S.; Lamberti, F.; Bruno, G. Oral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of ActionOral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of Action. Pharmaceuticals 2010, 3, 3005–3020. [Google Scholar] [CrossRef]
- Chatterjee, S.; Davies, M.J. Current management of diabetes mellitus and future directions in care. Postgrad. Med. J. 2015, 91, 612–621. [Google Scholar] [CrossRef]
- Leroith, D.; Biessels, G.J.; Braithwaite, S.S.; Casanueva, F.F.; Draznin, B.; Halter, J.B.; Hirsch, I.B.; McDonnell, M.; Molitch, M.E.E.; Murad, M.H.; et al. Treatment of diabetes in older adults: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1520–1574. [Google Scholar] [CrossRef]
- American Diabetes Association. Nutrition recommendations and principles for people with diabetes mellitus. Diabetes Care 2000, 23 (Suppl. 1), S43–S46. [Google Scholar]
- Balakumar, P.; Maung-U, K.; Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 2016, 113 Pt 1, 600–609. [Google Scholar] [CrossRef]
- Rosenfeld, C.R. Insulin therapy in type 2 diabetes mellitus: History drives patient care toward a better future. J. Am. Osteopat. Assoc. 2013, 113, S4–S5. [Google Scholar]
- Jermendy, G. Intensive insulin therapy in type 2 diabetes mellitus. Orv. Hetil. 2012, 153, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Tabet, E. The introduction of insulin in type 2 diabetes mellitus. Aust. Fam. Physician 2015, 44, 278–283. [Google Scholar]
- Nathan, D.M. Diabetes: Advances in Diagnosis and Treatment. JAMA 2015, 314, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, F.R. Type 1 diabetes mellitus. Pediatr. Rev. 2003, 24, 291–300. [Google Scholar] [CrossRef]
- DeWitt, D.E.; Hirsch, I.B. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: Scientific review. JAMA 2003, 289, 2254–2264. [Google Scholar] [CrossRef]
- Setji, T.L.; Hong, B.D.; Feinglos, M.N. Technosphere insulin: Inhaled prandial insulin. Expert Opin. Biol. Ther. 2015, 16, 111–117. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Garcia, N.S.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Song, R. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care 2016, 39, 187–189. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fuji, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Ryan, K.K.; Li, B.; Grayson, B.E.; Matter, E.K.; Woods, S.C.; Seeley, R.J. A role for central nervous system PPAR-gamma in the regulation of energy balance. Nat. Med. 2011, 17, 623–626. [Google Scholar] [CrossRef]
- Park, K.S.; Ciaraldi, T.P.; Abrams-Carter, L.; Mudaliar, S.; Nikoulina, S.E.; Henry, R.R. PPAR-gamma gene expression is elevated in skeletal muscle of obese and type II diabetic subjects. Diabetes 1997, 46, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Gause-Nilsson, I.; Xu, J.; Sonesson, C.; Johnsson, E. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and concomitant heart failure. J. Diabetes Complicat. 2017, 31, 1215–1221. [Google Scholar] [CrossRef]
- Proks, P.; Reimann, F.; Green, N.; Gribble, F.; Ashcroft, F. Sulfonylurea Stimulation of Insulin Secretion. Diabetes 2002, 51 (Suppl. 1), S368–S376. [Google Scholar] [CrossRef]
- Becker, M.; Galler, A.; Raile, K. Meglitinide Analogues in Adolescent Patients With HNF1A-MODY (MODY 3). Pediatrics 2014, 133, e775–e779. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Fukushima, M.; Yabe, D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010, 1, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Lynn, F.C.; Pamir, N.; Ng, E.H.; McIntosh, C.H.; Kieffer, T.J.; Pederson, R.A. Defective Glucose-Dependent Insulinotropic Polypeptide Receptor Expression in Diabetic Fatty Zucker Rats. Diabetes 2001, 50, 1004–1011. [Google Scholar] [CrossRef]
- Lynn, F.C.; Thompson, S.A.; Pospisilik, J.A.; Ehses, J.A.; Hinke, S.A.; Pamir, N.; Mclntosh, C.H.S.; Pederson, R.A. A novel pathway for regulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta cells. FASEB J. 2003, 17, 91–93. [Google Scholar] [CrossRef]
- Boyle, C.N.; Lutz, T.A.; Le Foll, C. Amylin—Its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol. Metab. 2017, 8, 203–210. [Google Scholar] [CrossRef]
- Schmitz, O.; Brock, B.; Rungby, J. Amylin Agonists: A Novel Approach in the Treatment of Diabetes. Diabetes 2004, 53, S233–S238. [Google Scholar] [CrossRef] [PubMed]
- Adeghate, E.; Kalász, H. Amylin Analogues in the Treatment of Diabetes Mellitus: Medicinal Chemistry and Structural Basis of its Function. Open Med. Chem. J. 2011, 5, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.S.; Lakshmi, M.J.; Sharavana, G.; Sathaiah, G.; Sreerama, Y.N.; Baskaran, V. Lactucaxanthin-a potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits alpha-amylase and alpha-glucosidase activity in vitro and in diabetic rats. Food Funct. 2017, 8, 1124–1131. [Google Scholar] [CrossRef]
- Adeghate, E.; Kalasz, H.; Veress, G.; Tekes, K. Medicinal Chemistry of Drugs Used in Diabetic Cardiomyopathy. Curr. Med. Chem. 2010, 17, 517–551. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Mannucci, E.; Ahrén, B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-a review and meta analysis. Diabetes Obes. Metab. 2012, 14, 762–767. [Google Scholar] [CrossRef]
- Kobayashi, K.; Yokoh, H.; Sato, Y.; Takemoto, M.; Uchida, D.; Kanatsuka, A.; Kuribayashi, N.; Terano, T.; Hashimoto, N.; Sakurai, K.; et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin compared with alpha-glucosidase inhibitor in Japanese patients with type 2 diabetes inadequately controlled on sulfonylurea alone (SUCCESS-2): A multicenter, randomized, open-label, non-inferiority trial. Diabetes Obes. Metab. 2014, 16, 761–765. [Google Scholar]
- Shahani, S.; Shahani, L. Use of insulin in diabetes: A century of treatment. Hong Kong Med. J. 2015, 21, 553–559. [Google Scholar] [CrossRef]
- Steineck, I.; Ranjan, A.; Norgaard, K.; Schmidt, S. Sensor-Augmented Insulin Pumps and Hypoglycemia Prevention in Type 1 Diabetes. J. Diabetes Sci. Technol. 2016, 11, 50–58. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014, 37 (Suppl. 1), S14–S80. [Google Scholar] [CrossRef] [PubMed]
- Leahy, J.L. Insulin therapy in type 2 diabetes mellitus. Endocrinol. Metab. Clin. North Am. 2012, 41, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Ilkova, H.; Glaser, B.; Tunçkale, A.; Bagriaçik, N.; Cerasi, E. Induction of Long-Term Glycemic Control in Newly Diagnosed Type 2 Diabetic Patients by Transient Intensive Insulin Treatment. Diabetes Care 1997, 20, 1353–1356. [Google Scholar] [CrossRef]
- Li, Y.; Xu, W.; Liao, Z.; Yao, B.; Chen, X.; Huang, Z.; Hu, G.; Weng, J. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care 2004, 27, 2597–2602. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Lim, P.C.; Chong, C.P. What’s next after metformin? focus on sulphonylurea: Add-on or combination therapy. Pharm. Pract. 2015, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Tran, L.; Zielinski, A.; Roach, A.H.; Jende, J.A.; Householder, A.M.; Cole, E.E.; Atway, S.A.; Amornyard, M.; Accursi, M.L.; Shieh, S.W.; et al. Pharmacologic treatment of type 2 diabetes: Oral medications. Ann. Pharmacother. 2015, 49, 540–556. [Google Scholar] [CrossRef]
- Eldor, R.; Raz, I. Diabetes therapy--focus on Asia: Second-line therapy debate: Insulin/secretagogues. Diabetes/Metab. Res. Rev. 2012, 28, 85–89. [Google Scholar] [CrossRef]
- Lau, D.C.; Teoh, H. Impact of Current and Emerging Glucose-Lowering Drugs on Body Weight in Type 2 Diabetes. Can. J. Diabetes 2015, 39, S148–S154. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meininger, G.; Sheng, D.; Terranella, L.; Stein, P.P. Sitagliptin Study 024 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: A randomized, double-blind, non-inferiority trial. Diabetes Obes. Metab. 2007, 9, 194–205. [Google Scholar] [PubMed]
- Scott, L.J. Repaglinide: A review of its use in type 2 diabetes mellitus. Drugs 2012, 72, 249–272. [Google Scholar] [CrossRef] [PubMed]
- Skliros, N.P.; Vlachopoulos, C.; Tousoulis, D. Treatment of diabetes: Crossing to the other side. Hell. J. Cardiol. 2016, 57, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Cao, Y.; Paudel, S.; Yoon, G.; Cheon, S.H.; Bae, G.U.; Jin, L.T.; Kim, Y.K.; Kim, S.-N. Antidiabetic effect of SN158 through PPARalpha/gamma dual activation in ob/ob mice. Chem. Biol. Interact. 2017, 268, 24–30. [Google Scholar] [CrossRef]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; López Machado, A.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef]
- Scheen, A.J. Dulaglutide for the treatment of type 2 diabetes. Expert Opin. Biol. Ther. 2017, 17, 485–496. [Google Scholar] [CrossRef]
- Wysham, C.H.; Lin, J.; Kuritzky, L. Safety and efficacy of a glucagon-like peptide-1 receptor agonist added to basal insulin therapy versus basal insulin with or without a rapid-acting insulin in patients with type 2 diabetes: Results of a meta-analysis. Postgrad. Med. 2017, 129, 1–10. [Google Scholar] [CrossRef]
- Zhou, M.; Mok, M.T.; Sun, H.; Chan, A.W.; Huang, Y.; Cheng, A.S.; Xu, G. The anti-diabetic drug exenatide, a glucagon-like peptide-1 receptor agonist, counteracts hepatocarcinogenesis through cAMP-PKA-EGFR-STAT3 axis. Oncogene 2017, 36, 4135–4149. [Google Scholar] [CrossRef]
- Cao, L.; Li, D.; Feng, P.; Li, L.; Xue, G.-F.; Li, G.; Hölscher, C. A novel dual GLP-1 and GIP incretin receptor agonist is neuroprotective in a mouse model of Parkinson’s disease by reducing chronic inflammation in the brain. Neuroreport 2016, 27, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Jermendy, G. Incretin-based antidiabetic treatment and diseases of the pancreas (pancreatitis, pancreas carcinoma). Orv. Hetil. 2016, 157, 523–528. [Google Scholar] [CrossRef]
- Garber, A.; Henry, R.; Ratner, R.; Garcia-Hernandez, P.A.; Rodriguez-Pattzi, H.; Olvera-Alvarez, I.; Hale, P.M.; Zdravkovic, M.; Bode, B. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009, 373, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Hayashino, Y.; Akai, Y.; Yabuta, M.; Tsujii, S. Dipeptidyl peptidase-4 inhibitors as preferable oral hypoglycemic agents in terms of treatment satisfaction: Results from a multicenter, 12-week, open label, randomized controlled study in Japan (PREFERENCE 4 study). J. Diabetes Investig. 2017, 9, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, G.; Hall, G.M. Diabetes mellitus: New drugs for a new epidemic. Br. J. Anaesth. 2011, 107, 65–73. [Google Scholar] [CrossRef]
- Forst, T.; Uhlig-Laske, B.; Ring, A.; Graefe-Mody, U.; Friedrich, C.; Herbach, K.; Woerle, H.-J.; Dugi, K.A. Linagliptin (BI 1356), a potent and selective DPP-4 inhibitor, is safe and efficacious in combination with metformin in patients with inadequately controlled Type 2 diabetes. Diabete Med. 2010, 27, 1409–1419. [Google Scholar] [CrossRef]
- Amori, R.E.; Lau, J.; Pittas, A.G. Efficacy and safety of incretin therapy in type 2 diabetes: Systematic review and meta-analysis. JAMA 2007, 298, 194–206. [Google Scholar] [CrossRef]
- Liu, M.; Hoskins, A.; Verma, N.; Bers, D.M.; Despa, S.; Despa, F. Amylin and diabetic cardiomyopathy—Amylin-induced sarcolemmal Ca2+ leak is independent of diabetic remodeling of myocardium. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 1923–1930. [Google Scholar] [CrossRef]
- Nyholm, B.; Brock, B.; Ørskov, L.; Schmitz, O. Amylin receptor agonists: A novel pharmacological approach in the management of insulin-treated diabetes mellitus. Expert Opin. Investig. Drugs 2001, 10, 1641–1652. [Google Scholar] [CrossRef]
- Hansen, M.; Sonne, D.P.; Mikkelsen, K.H.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Effect of bile acid sequestrants on glycaemic control: Protocol for a systematic review with meta-analysis of randomised controlled trials. BMJ Open 2012, 2, e001803. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Sonne, D.P.; Mikkelsen, K.H.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: A systematic review with meta-analysis of randomized controlled trials. J. Diabetes Complicat. 2017, 31, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Hermes-DeSantis, E.R. The Role of Colesevelam Hydrochloride in Hypercholesterolemia and Type 2 Diabetes Mellitus. Ann. Pharmacother. 2010, 44, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Steen, O.; Goldenberg, R.M. The Role of Sodium-Glucose Cotransporter 2 Inhibitors in the Management of Type 2 Diabetes. Can. J. Diabetes 2017, 41, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.R. The kidney and type 2 diabetes mellitus: Therapeutic implications of SGLT2 inhibitors. Postgrad. Med. 2016, 128, 290–298. [Google Scholar] [CrossRef]
- Bailey, C.J.; Tahrani, A.A.; Barnett, A.H. Future glucose-lowering drugs for type 2 diabetes. Lancet Diabetes Endocrinol. 2016, 4, 350–359. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacodynamics, Efficacy and Safety of Sodium–Glucose Co-Transporter Type 2 (SGLT2) Inhibitors for the Treatment of Type 2 Diabetes Mellitus. Drugs 2014, 75, 33–59. [Google Scholar] [CrossRef]
- Stenlöf, K.; Cefalu, W.T.; Kim, K.; Alba, M.; Usiskin, K.; Tong, C.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes. Metab. 2013, 15, 372–382. [Google Scholar] [CrossRef]
- Bailey, C.J.; Gross, J.L.; Hennicken, D.; Iqbal, N.; Mansfield, T.A.; List, J.F. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: A randomized, double-blind, placebo-controlled 102-week trial. BMC Med. 2013, 11, 43. [Google Scholar] [CrossRef]
- Bagnasco, A.; Di Giacomo, P.; Mora, R.D.R.D.; Catania, G.; Turci, C.; Rocco, G.; Sasso, L. Factors influencing self-management in patients with type 2 diabetes: A quantitative systematic review protocol. J. Adv. Nurs. 2013, 70, 187–200. [Google Scholar] [CrossRef]
- Adeghate, E.; Mohsin, S.; Adi, F.; Ahmed, F.; Yahya, A.; Kalász, H.; Tekes, K.; Adeghate, E.A. An update of SGLT1 and SGLT2 inhibitors in early phase diabetes-type 2 clinical trials. Expert Opin. Investig. Drugs 2019, 28, 811–820. [Google Scholar] [CrossRef]
- Babai, S.; Auclert, L.; Le-Louët, H. Safety data and withdrawal of hepatotoxic drugs. Therapies 2021, 76, 715–723. [Google Scholar] [CrossRef]
- Qato, D.M.; Trivedi, A.N.; Mor, V.; Dore, D. Disparities in Discontinuing Rosiglitazone Following the 2007 FDA Safety Alert. Med. Care 2016, 54, 406–413. [Google Scholar] [CrossRef]
- Halegoua-De Marzio, D.; Navarro, V.J. Chapter 29-Hepatotoxicity of Cardiovascular and Antidiabetic Drugs. In Drug-Induced Liver Disease, 3rd ed.; Kaplowitz, N., DeLeve, L.D., Eds.; Academic Press: Boston, MA, USA, 2013; pp. 519–540. [Google Scholar]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in Antidiabetic Drug Discovery: FDA Approved Drugs, New Drugs in Clinical Trials and Global Sales. Front. Pharmacol. 2022, 12, 807548. [Google Scholar] [CrossRef] [PubMed]
- Gardner, D.S.; Tai, E.S. Clinical features and treatment of maturity onset diabetes of the young (MODY). Diabetes Metab. Syndr. Obes. Targets Ther. 2012, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Sathananthan, A.; Vella, A. Personalized pharmacotherapy for Type 2 diabetes mellitus. Pers. Med. 2009, 6, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Semiz, S.; Dujic, T.; Causevic, A. Pharmacogenetics and personalized treatment of type 2 diabetes. Biochem. Med. 2013, 23, 154–171. [Google Scholar] [CrossRef]
| Drugs | Organ Targeted | Mechanism | References |
|---|---|---|---|
| TZD and biguanides | Adipose tissue Skeletal muscle | ↓ Insulin resistance | [102,103,104,105,106,107] |
| TZD and biguanides | Liver | ↓ Gluconeogenesis | [55] |
| SGLT2 inhibitors | Kidney | Glucose elimination in urine | [108] |
| SU and meglitinides | Pancreas | Insulin secretagogues | [109,110] |
| GLP-1R agonists | Pancreas | Improve response to glucose | [111,112,113] |
| Pramlintide | Pancreas | ↓ Glucagon secretion | [114,115,116] |
| Pramlintide | Stomach | Delays gastric emptying | [115] |
| α-glucosidase inhibitors | Small intestine | Slows absorption of starch | [117,118] |
| DPP-4 inhibitors | Plasma | ↓ Incretin breakdown | [119,120] |
| Insulin Type | Onset of Action (h) | Peak of Action (h) | Duration of Action (h) | Maximal Duration (h) |
|---|---|---|---|---|
| Rapid-acting | ||||
| Lispro | ¼ to ½ | 1 to 2 | 3 to 5 | 4 to 6 |
| Aspart | ¼ to ½ | 1 to 2 | 3 to 6 | 5 to 8 |
| Glulisine | 0.25 to 0.5 | 0.5 to 1 | 3 to 4 | 4 |
| Short-acting | ||||
| Regular | ½ to 1 | 2 to 4 | 3 to 6 | 6 to 8 |
| Intermediate-acting | ||||
| NPH human | 2 to 4 | 8 to 12 | 12 to 20 | 14 to 22 |
| Long-acting | ||||
| Glargine | 1 to 2 | None | 19 to 24 | 24 |
| Detemir | 3 to 4 | 6 to 8 | 20 to 24 | 24 |
| Degludec | 1 | 9 | 24 to 42 | 42 |
| Insulin combinations | ||||
| Protamine/Lispro | 0.25 to 0.4 | 0.5 to 3 | 14 to 24 | 24 |
| Protamine/Aspart | 0.1 to 0.2 | 1 to 4 | 18 to 24 | 24 |
| Daily Dosage | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Daily Dosage | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Daily Dosage | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Daily Dosage | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Daily Dosage | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Weekly Dosage | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Daily Dosage | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Daily Dosage. | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Daily Dosage. | Advantages | Side Effects | Contraindications |
|---|---|---|---|
|
|
|
|
| Daily Dosage | Advantages | Side Effects | Contra-Indications |
|---|---|---|---|
|
|
|
|
| Class of Drug | Expected Reduction in HbA1c | Contraindications |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahgoub, M.O.; Ali, I.I.; Adeghate, J.O.; Tekes, K.; Kalász, H.; Adeghate, E.A. An Update on the Molecular and Cellular Basis of Pharmacotherapy in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 9328. https://doi.org/10.3390/ijms24119328
Mahgoub MO, Ali II, Adeghate JO, Tekes K, Kalász H, Adeghate EA. An Update on the Molecular and Cellular Basis of Pharmacotherapy in Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2023; 24(11):9328. https://doi.org/10.3390/ijms24119328
Chicago/Turabian StyleMahgoub, Mohamed Omer, Ifrah Ismail Ali, Jennifer O. Adeghate, Kornélia Tekes, Huba Kalász, and Ernest A. Adeghate. 2023. "An Update on the Molecular and Cellular Basis of Pharmacotherapy in Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 24, no. 11: 9328. https://doi.org/10.3390/ijms24119328
APA StyleMahgoub, M. O., Ali, I. I., Adeghate, J. O., Tekes, K., Kalász, H., & Adeghate, E. A. (2023). An Update on the Molecular and Cellular Basis of Pharmacotherapy in Type 2 Diabetes Mellitus. International Journal of Molecular Sciences, 24(11), 9328. https://doi.org/10.3390/ijms24119328






