Cellulose Acetate-Supported Copper as an Efficient Sustainable Heterogenous Catalyst for Azide-Alkyne Cycloaddition Click Reactions in Water
Abstract
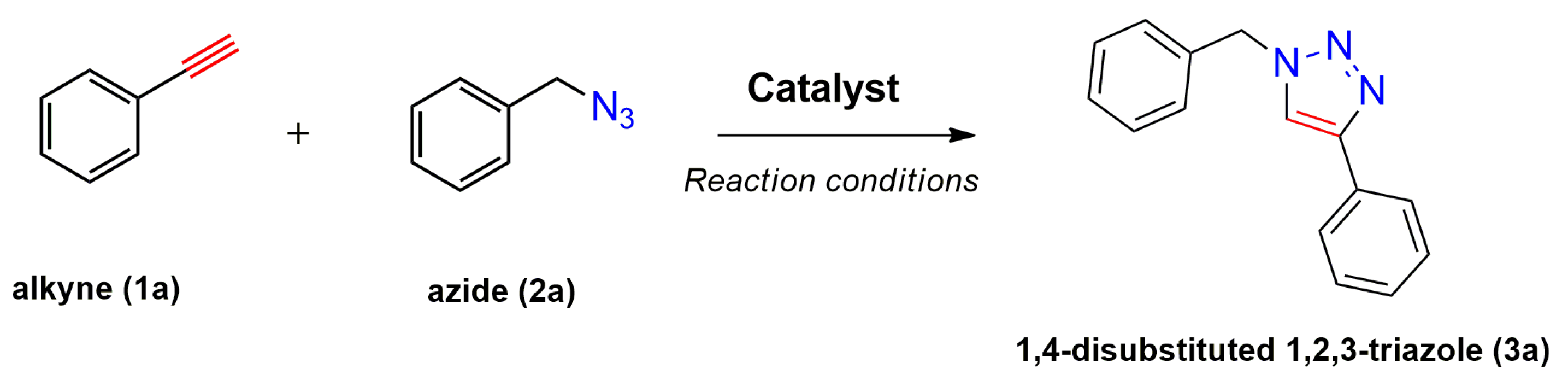
1. Introduction
2. Results and Discussion
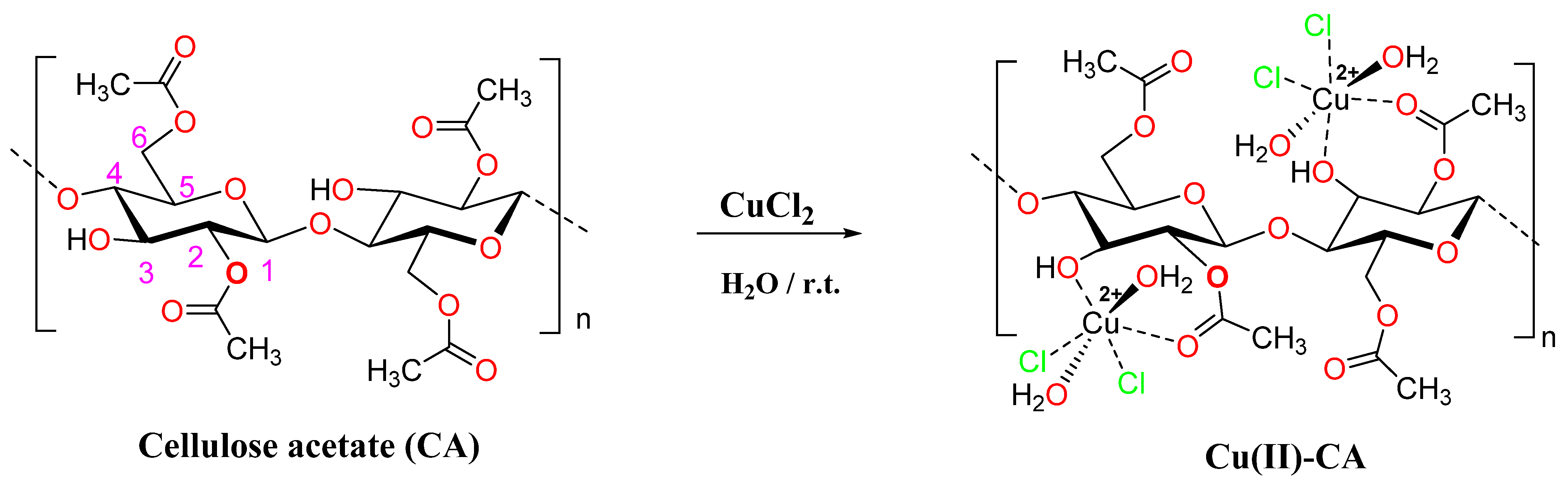
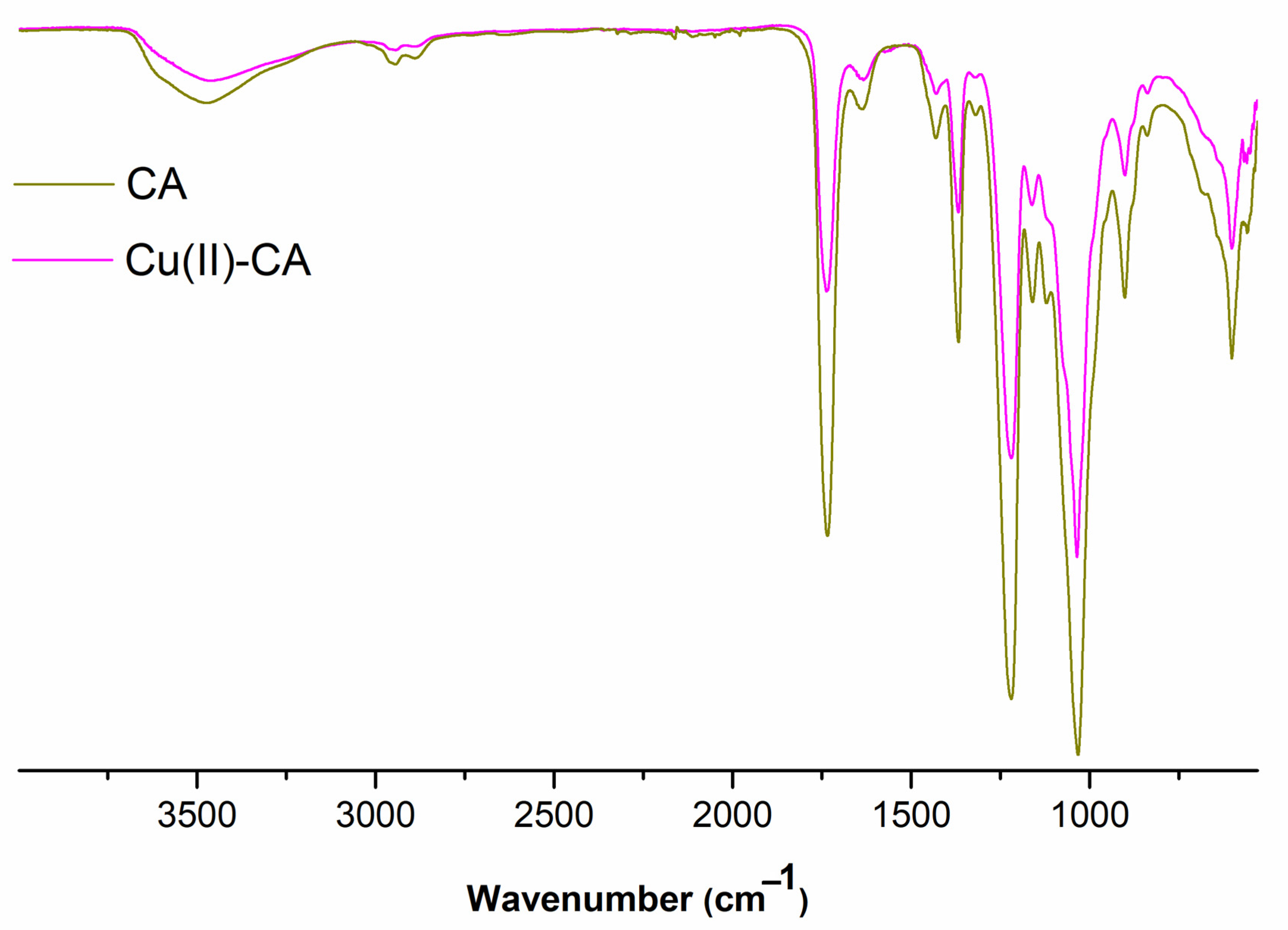
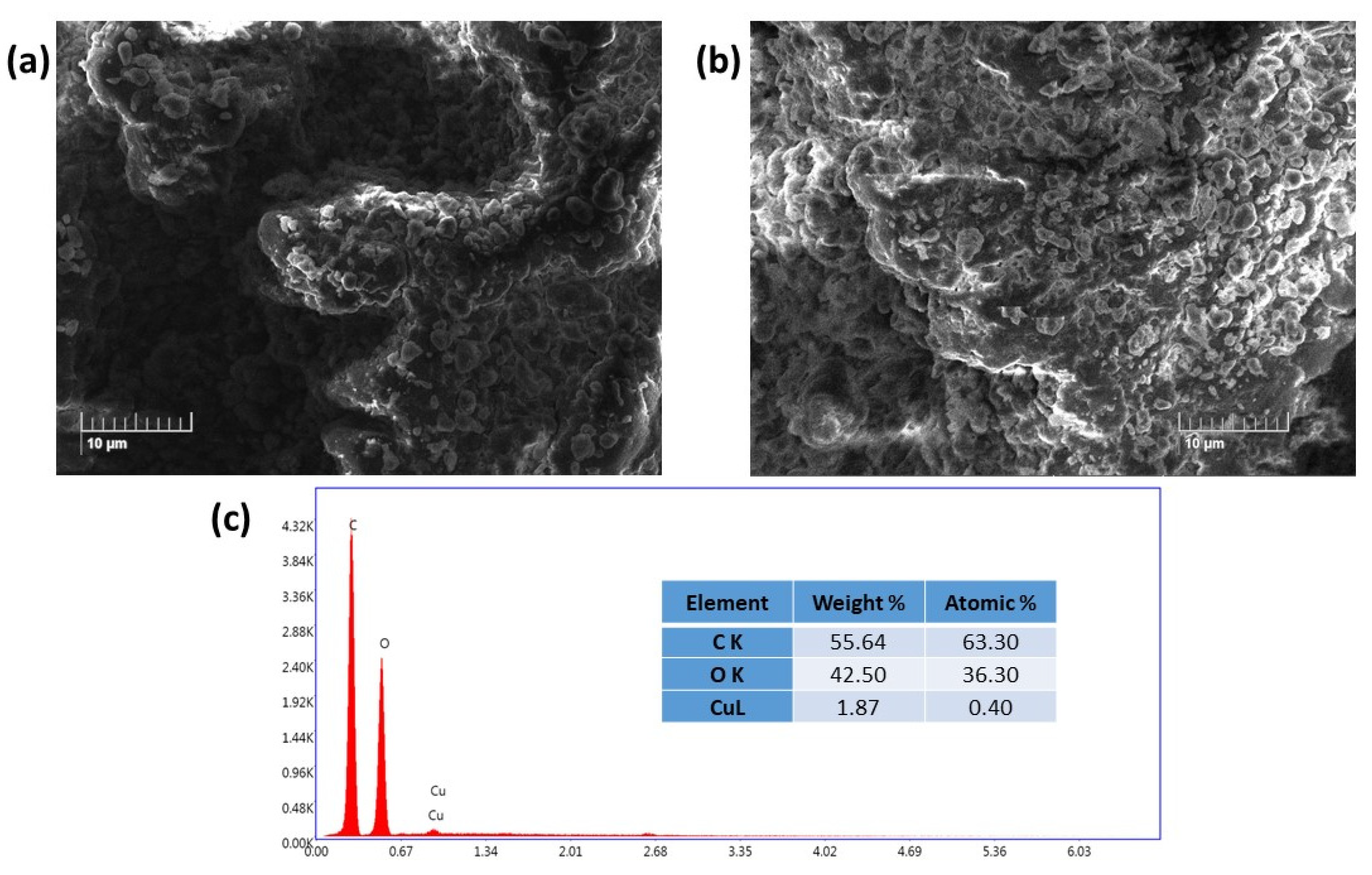
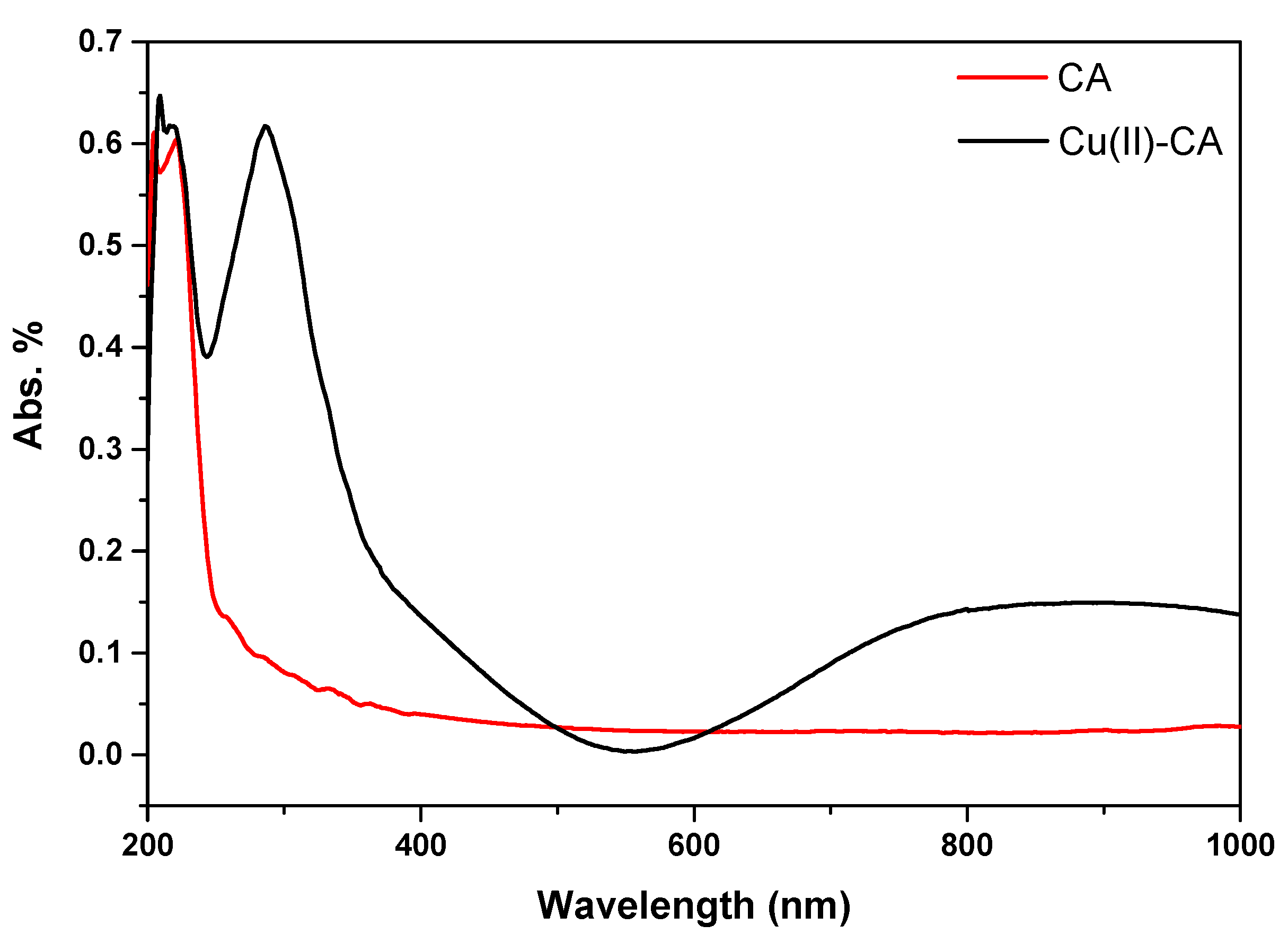
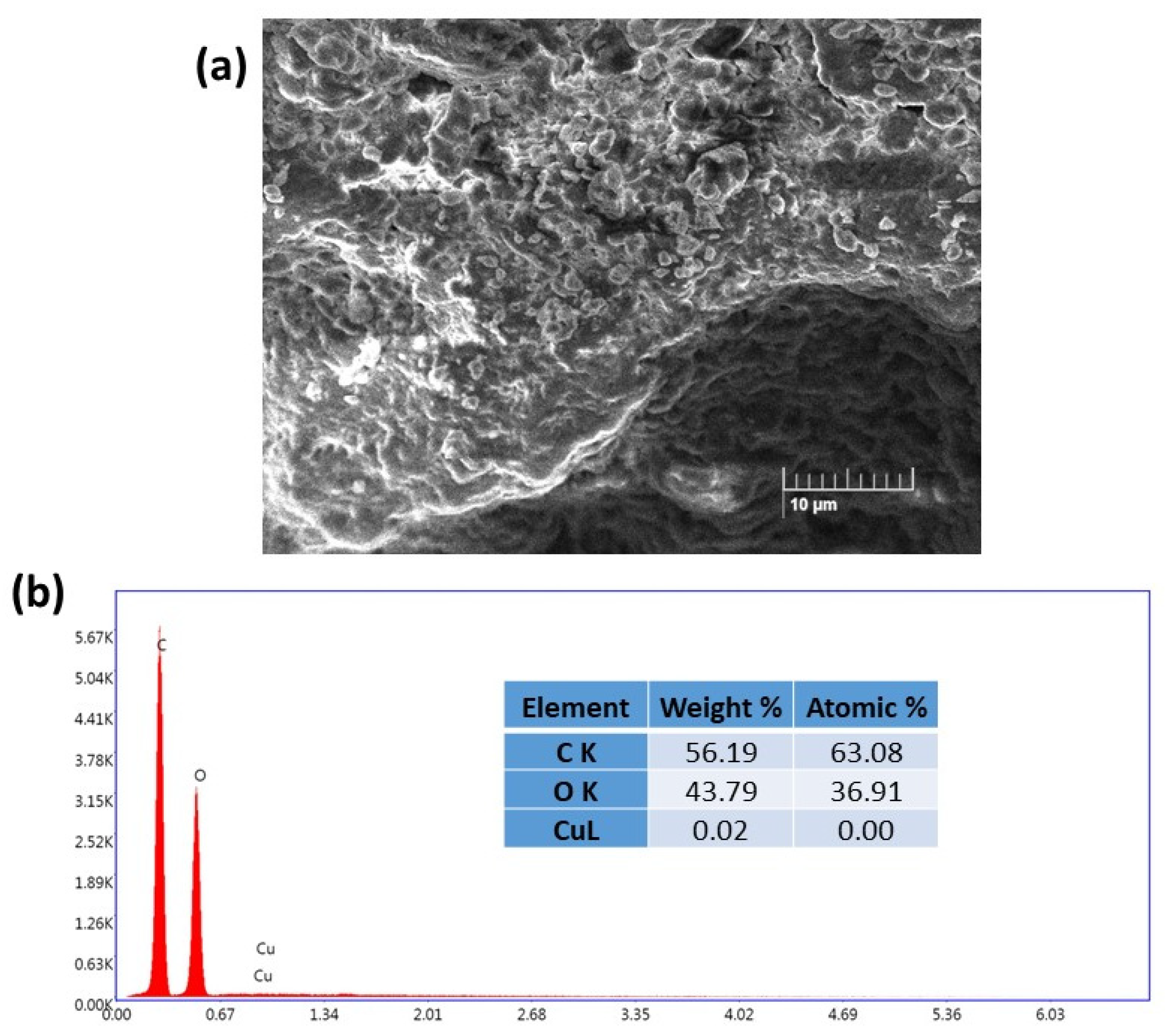
2.1. Characterization of the Cu(II)-Catalyst
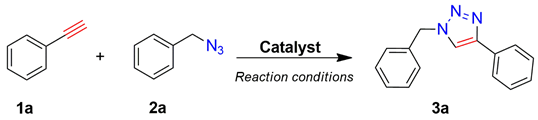
2.2. Catalytic Tests
2.3. Reusability of Cu(II)-CA Catalyst
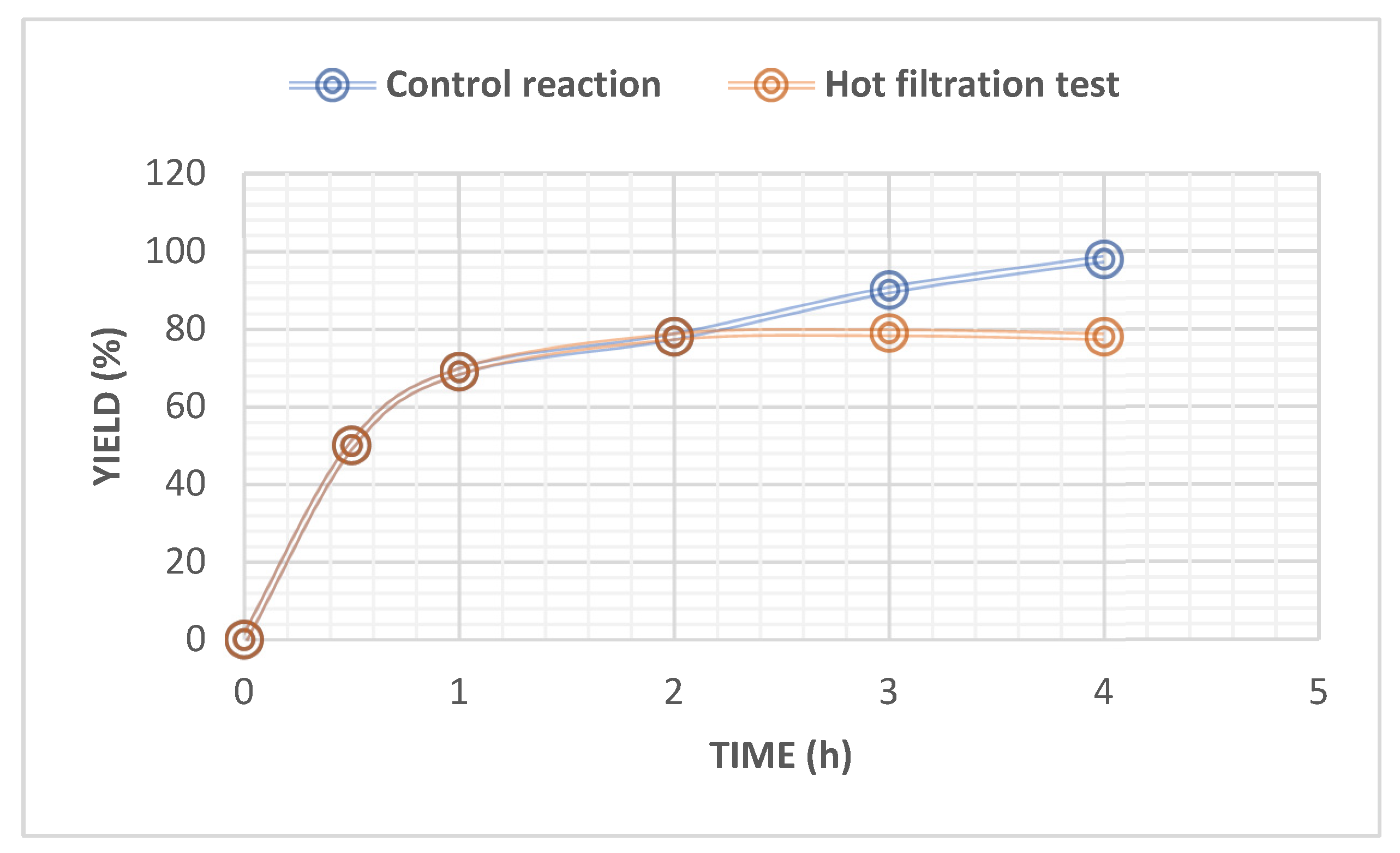
2.4. Heterogeneity Test
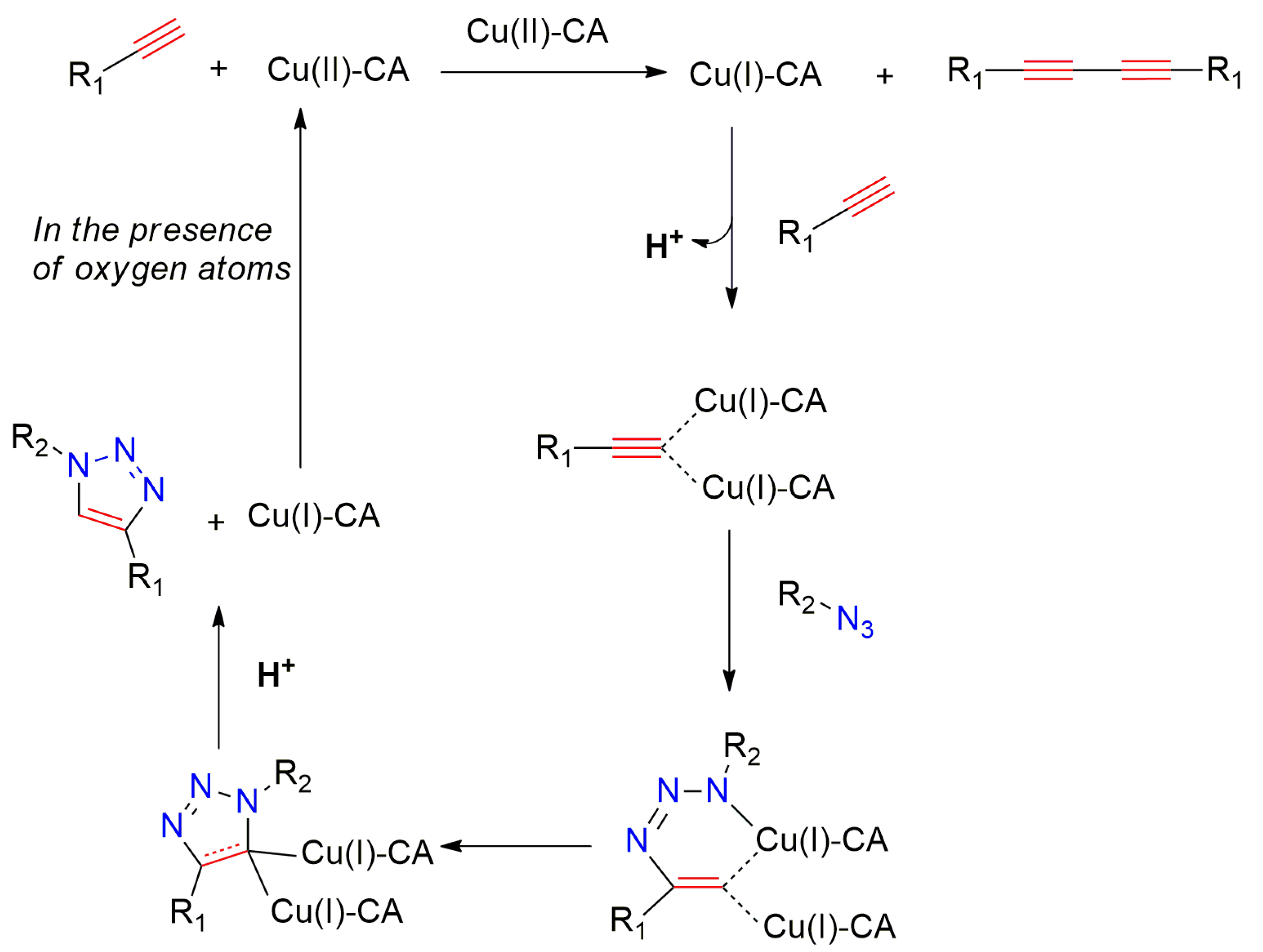
2.5. Mechanistic Studies
2.6. Comparison with Other Catalytic Methods
3. Materials and Methods
3.1. General Experimental Information
3.2. Preparation of the Cu(II)-Catalyst
3.3. Catalytic Synthesis of 1,2,3-Triazole Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, K.; Sun, Q.; Tang, Y.; Shan, C.; Aguila, B.; Wang, S.; Meng, X.; Ma, S.; Xiao, F.-S. Bio-Inspired Creation of Heterogeneous Reaction Vessels via Polymerization of Supramolecular Ion Pair. Nat. Commun. 2019, 10, 3059. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yip, A.C.K. Heterogeneous Catalysis: Enabling a Sustainable Future. Front. Catal. 2021, 1, 667675. [Google Scholar] [CrossRef]
- Chaouf, S.; El Barkany, S.; Amhamdi, H.; Jilal, I.; El Ouardi, Y.; Abou-salama, M.; Loutou, M.; El-Houssaine, A.; El Ouarghi, H.; El Idrissi, A. Low Degree of Substitution of Cellulose Acrylate Based Green Polyelectrolyte: Synthesis, Characterization and Application to the Removal of Cu (II) Ions and Colloidal Fe(OH)3 Turbidity. Mater. Today Proc. 2020, 31, S175–S182. [Google Scholar] [CrossRef]
- Essaghraoui, A.; Khatib, K.; Hamdaoui, B.; Brouillette, F.; Ablouh, E.-H.; Belfkira, A. Handsheet Coated by Polyvinyl Acetate as a Drug Release System. J. Pharm. Innov. 2021, 17, 599–609. [Google Scholar] [CrossRef]
- Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Choudhury, N.R. 3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review. Polymers 2019, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Li, H.; Li, B.; Liu, M.; Zhan, H. Characteristics and Properties of Cellulose Nanofibers Prepared by TEMPO Oxidation of Corn Husk. BioResources 2016, 11, 5276–5284. [Google Scholar] [CrossRef]
- Huang, T.; Kuboyama, K.; Fukuzumi, H.; Ougizawa, T. PMMA/TEMPO-Oxidized Cellulose Nanofiber Nanocomposite with Improved Mechanical Properties, High Transparency and Tunable Birefringence. Cellulose 2018, 25, 2393–2403. [Google Scholar] [CrossRef]
- Koga, H.; Saito, T.; Kitaoka, T.; Nogi, M.; Suganuma, K.; Isogai, A. Transparent, Conductive, and Printable Composites Consisting of TEMPO-Oxidized Nanocellulose and Carbon Nanotube. Biomacromolecules 2013, 14, 1160–1165. [Google Scholar] [CrossRef]
- Ahmad, H. Celluloses as Green Support of Palladium Nanoparticles for Application in Heterogeneous Catalysis: A Brief Review. J. Clust. Sci. 2021, 33, 421–438. [Google Scholar] [CrossRef]
- Kamel, S.; Khattab, T.A. Recent Advances in Cellulose Supported Metal Nanoparticles as Green and Sustainable Catalysis for Organic Synthesis. Cellulose 2021, 28, 4545–4574. [Google Scholar] [CrossRef]
- Riva, L.; Lotito, A.D.; Punta, C.; Sacchetti, A. Zinc- and Copper-Loaded Nanosponges from Cellulose Nanofibers Hydrogels: New Heterogeneous Catalysts for the Synthesis of Aromatic Acetals. Gels 2022, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.-E.; Lim, S.; Pang, Y.-L.; Ong, H.-C.; Lee, K.-T. Synthesis of Biomass as Heterogeneous Catalyst for Application in Biodiesel Production: State of the Art and Fundamental Review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Zarafshani, Z. Efficient Construction of Therapeutics, Bioconjugates, Biomaterials and Bioactive Surfaces Using Azide-Alkyne “Click” Chemistry. Adv. Drug Deliv. Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef]
- Seath, C.P.; Burley, G.A.; Watson, A.J.B. Determining the Origin of Rate-Independent Chemoselectivity in CuAAC Reactions: An Alkyne-Specific Shift in Rate-Determining Step. Angew. Chem. Int. Ed. 2017, 56, 3314–3318. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Katerina, I.S.; Lozan, T.T.; Nataliya, P.B.; Mauricio, A.P.; Irena, P.K. Developments in the Application of 1,2,3-Triazoles in Cancer Treatment. Recent Pat. Anti-Cancer Drug Discov. 2020, 15, 92–112. [Google Scholar]
- Nandikolla, A.; Srinivasarao, S.; Khetmalis, Y.M.; Kumar, B.K.; Murugesan, S.; Shetye, G.; Ma, R.; Franzblau, S.G.; Sekhar, K.V.G.C. Design, Synthesis and Biological Evaluation of Novel 1,2,3-Triazole Analogues of Imidazo-[1,2-a]-Pyridine-3-Carboxamide against Mycobacterium Tuberculosis. Toxicol. Vitr. 2021, 74, 105137. [Google Scholar] [CrossRef]
- Dumesic, J.A.; Huber, G.W.; Boudart, M. Principles of Heterogeneous Catalysis. In Handbook of Heterogeneous Catalysis; American Cancer Society: Atlanta, GA, USA, 2008; ISBN 978-3-527-61004-4. [Google Scholar]
- Norouzi, F.; Javanshir, S. Magnetic γFe2O3@Sh@Cu2O: An Efficient Solid-Phase Catalyst for Reducing Agent and Base-Free Click Synthesis of 1,4-Disubstituted-1,2,3-Triazoles. BMC Chem. 2020, 14, 1. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Introduction: Green Chemistry and Catalysis. In Green Chemistry and Catalysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 1–47. ISBN 978-3-527-61100-3. [Google Scholar]
- Singh, R.; Singh, G.; George, N.; Singh, G.; Gupta, S.; Singh, H.; Kaur, G.; Singh, J. Copper-Based Metal–Organic Frameworks (MOFs) as an Emerging Catalytic Framework for Click Chemistry. Catalysts 2023, 13, 130. [Google Scholar] [CrossRef]
- Schätz, A.; Grass, R.N.; Stark, W.J.; Reiser, O. TEMPO Supported on Magnetic C/Co-Nanoparticles: A Highly Active and Recyclable Organocatalyst. Chem. A Eur. J. 2008, 14, 8262–8266. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Cha, D.; Bouhrara, M.; Almana, N.; Polshettiwar, V. Fibrous Nano-Silica (KCC-1)-Supported Palladium Catalyst: Suzuki Coupling Reactions Under Sustainable Conditions. ChemSusChem 2012, 5, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, N.; Jones, C.W.; Weck, M. Rational Approach to Polymer-Supported Catalysts: Synergy between Catalytic Reaction Mechanism and Polymer Design. Acc. Chem. Res. 2008, 41, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- García-Garrido, S.E.; Francos, J.; Cadierno, V.; Basset, J.-M.; Polshettiwar, V. Chemistry by Nanocatalysis: First Example of a Solid-Supported RAPTA Complex for Organic Reactions in Aqueous Medium. ChemSusChem 2011, 4, 104–111. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Schaumlöffel, J.; Lösch, A.; De Cuyper, A.; Thiel, W.R. Dinuclear Copper Complex Immobilized on a Janus-Type Material as an Interfacial Heterogeneous Catalyst for Green Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 33091–33101. [Google Scholar] [CrossRef]
- Chassaing, S.; Bénéteau, V.; Pale, P. When CuAAC ‘Click Chemsitry’ goes heterogenous. Catal. Sci. Technol. 2016, 6, 923–957. [Google Scholar] [CrossRef]
- Bystrzanowska, M.; Petkov, P.; Tobiszewski, M. Ranking of Heterogeneous Catalysts Metals by Their Greenness. ACS Sustain. Chem. Eng. 2019, 7, 18434–18443. [Google Scholar] [CrossRef]
- Ablouh, E.-H.; Bahsis, L.; Sehaqui, H.; Anane, H.; Julve, M.; Stiriba, S.-E.; El Achaby, M. TEMPO-Oxidized-Cellulose Nanofibers-Immobilized Copper(II) Foam as an Efficient Heterogeneous Catalyst for the Azide-Alkyne Reaction in Water. Sustain. Chem. Pharm. 2022, 30, 100837. [Google Scholar] [CrossRef]
- Reis, D.T.; Ribeiro, I.H.S.; Pereira, D.H. DFT Study of the Application of Polymers Cellulose and Cellulose Acetate for Adsorption of Metal Ions (Cd2+, Cu2+ and Cr3+) Potentially Toxic. Polym. Bull. 2020, 77, 3443–3456. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, M.; Liu, R.; Li, Y.; Wang, D.; Tan, J.; Wu, R.; Huang, Y. Electrospun Membrane of Cellulose Acetate for Heavy Metal Ion Adsorption in Water Treatment. Carbohydr. Polym. 2011, 83, 743–748. [Google Scholar] [CrossRef]
- Atmani, H.; Zazouli, S.; Ezzahra Bakkardouch, F.; Laallam, L.; Jouaiti, A. Insights into Interactions of Cellulose Acetate and Metal Ions (Zn2+, Cu2+, and Ag+) in Aqueous Media Using DFT Study. Comput. Theor. Chem. 2021, 1202, 113322. [Google Scholar] [CrossRef]
- Khan, J.; Siddiq, M.; Akram, B.; Ashraf, M.A. In-Situ Synthesis of CuO Nanoparticles in P(NIPAM-Co-AAA) Microgel, Structural Characterization, Catalytic and Biological Applications. Arab. J. Chem. 2018, 11, 897–909. [Google Scholar] [CrossRef]
- Culica, M.E.; Chibac-Scutaru, A.L.; Melinte, V.; Coseri, S. Cellulose Acetate Incorporating Organically Functionalized CeO2 NPs: Efficient Materials for UV Filtering Applications. Materials 2020, 13, 2955. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, O.; Bhagat, M.; Gopalakrishnan, C.; Arunachalam, K.D. Ultrafine Dispersed CuO Nanoparticles and Their Antibacterial Activity. J. Exp. Nanosci. 2008, 3, 185–193. [Google Scholar] [CrossRef]
- Kong, A.; Wang, H.; Yang, X.; Hou, Y.; Shan, Y. A Facile Direct Route to Synthesize Large-Pore Mesoporous Silica Incorporating High CuO Loading with Special Catalytic Property. Microporous Mesoporous Mater. 2009, 118, 348–353. [Google Scholar] [CrossRef]
- Sindhu, K.S.; Anilkumar, G. Recent Advances and Applications of Glaser Coupling Employing Greener Protocols. RSC Adv. 2014, 4, 27867–27887. [Google Scholar] [CrossRef]
- Kuang, G.-C.; Michaels, H.A.; Simmons, J.T.; Clark, R.J.; Zhu, L. Chelation-Assisted, Copper(II)-Acetate-Accelerated Azide−Alkyne Cycloaddition. J. Org. Chem. 2010, 75, 6540–6548. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, W.S.; Michaels, H.A.; Simmons, J.T.; Clark, R.J.; Dalal, N.S.; Zhu, L. Apparent Copper(II)-Accelerated Azide−Alkyne Cycloaddition. Org. Lett. 2009, 11, 4954–4957. [Google Scholar] [CrossRef]
- Iacobucci, C.; Reale, S.; Gal, J.-F.; De Angelis, F. Dinuclear Copper Intermediates in Copper(I)-Catalyzed Azide–Alkyne Cycloaddition Directly Observed by Electrospray Ionization Mass Spectrometry. Angew. Chem. Int. Ed. 2015, 54, 3065–3068. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef]
- Nolte, C.; Mayer, P.; Straub, B.F. Isolation of a Copper(I) Triazolide: A “Click” Intermediate. Angew. Chem. Int. Ed. 2007, 46, 2101–2103. [Google Scholar] [CrossRef] [PubMed]
- Albano, G.; Aronica, L.A. Acyl Sonogashira Cross-Coupling: State of the Art and Application to the Synthesis of Heterocyclic Compounds. Catalysts 2020, 10, 25. [Google Scholar] [CrossRef]
- Berg, R.; Straub, B.F. Advancements in the Mechanistic Understanding of the Copper-Catalyzed Azide–Alkyne Cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Tolentino, D.R.; Melaimi, M.; Bertrand, G. Isolation of Bis(Copper) Key Intermediates in Cu-Catalyzed Azide-Alkyne “Click Reaction”. Sci. Adv. 2015, 1, e1500304. [Google Scholar] [CrossRef]
- Bahsis, L.; Ablouh, E.-H.; Anane, H.; Taourirte, M.; Julve, M.; Stiriba, S.-E. Cu(II)-Alginate-Based Superporous Hydrogel Catalyst for Click Chemistry Azide–Alkyne Cycloaddition Type Reactions in Water. RSC Adv. 2020, 10, 32821–32832. [Google Scholar] [CrossRef]
- Rajender Reddy, K.; Rajgopal, K.; Lakshmi Kantam, M. Copper-Alginates: A Biopolymer Supported Cu(II) Catalyst for 1,3-Dipolar Cycloaddition of Alkynes with Azides and Oxidative Coupling of 2-Naphthols and Phenols in Water. Catal. Lett. 2007, 114, 36–40. [Google Scholar] [CrossRef]
- Bahsis, L.; El Ayouchia, H.B.; Anane, H.; Benhamou, K.; Kaddami, H.; Julve, M.; Stiriba, S.-E. Cellulose-copper as Bio-Supported Recyclable Catalyst for the Clickable Azide-Alkyne [3+2] Cycloaddition Reaction in Water. Int. J. Biol. Macromol. 2018, 119, 849–856. [Google Scholar] [CrossRef]
- Mandal, B.H.; Rahman, M.L.; Yusoff, M.M.; Chong, K.F.; Sarkar, S.M. Bio-Waste Corn-Cob Cellulose Supported Poly(Hydroxamic Acid) Copper Complex for Huisgen Reaction: Waste to Wealth Approach. Carbohydr. Polym. 2017, 156, 175–181. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Copper on Chitosan: A Recyclable Heterogeneous Catalyst for Azide–Alkyne Cycloaddition Reactions in Water. Green Chem. 2013, 15, 1839–1843. [Google Scholar] [CrossRef]
- Ben El Ayouchia, H.; El Mouli, H.; Bahsis, L.; Anane, H.; Laamari, R.; Gómez-García, C.J.; Julve, M.; Stiriba, S.-E. Hyperbranched Polyethylenimine-Supported Copper(II) Ions as a Macroliganted Homogenous Catalyst for Strict Click Reactions of Azides and Alkynes in Water. J. Organomet. Chem. 2019, 898, 120881. [Google Scholar] [CrossRef]
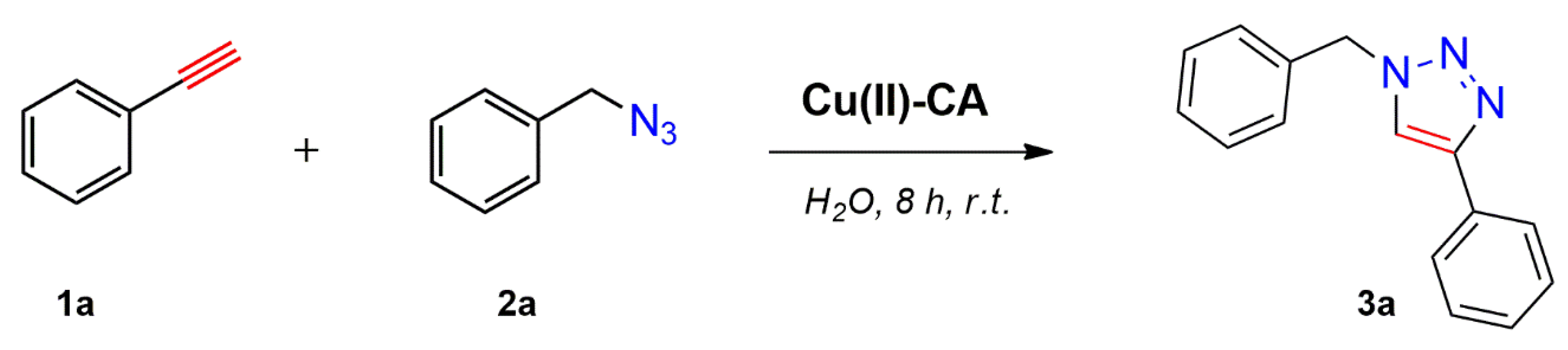
| Entry | Catalyst | Loading (mol%) | Time (h) | Yield (%) b |
|---|---|---|---|---|
| 1 | Neat | - | 24 | 0 |
| 2 | CuCl2·2H2O | 5 | 24 | 52 |
| 3 | CA | 20 c | 24 | 0 |
| 4 | Cu(II)-CA | 10 | 24 | 99 |
| 5 | Cu(II)-CA | 10 | 12 | 96 |
| 6 | Cu(II)-CA | 10 | 8 | 93 |
| 7 | Cu(II)-CA | 20 | 8 | 94 |
| 8 | Cu(II)-CA | 5 | 8 | 91 |
| 9 | Cu(II)-CA | 2 | 8 | 68 |
| 10 c | Cu(II)-CA | 5 | 0.5 | 50 |
| 11 d | Cu(II)-CA | 5 | 2 | 78 |
| 12 c | Cu(II)-CA | 5 | 4 | 98 |
| Entry | Alkynes | Azides | Product | Yield (%) b |
|---|---|---|---|---|
| 1 | 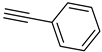 |  | 3a | 91 |
| 2 | 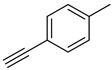 | 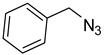 | 3b | 93 |
| 3 | 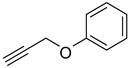 |  | 3c | 97 |
| 4 | 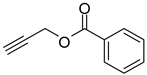 | 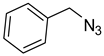 | 3d | 97 |
| 5 | 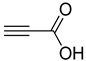 | 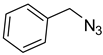 | 3e | 90 |
| 6 |  | 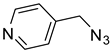 | 3f | 94 |
| 7 | 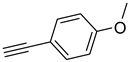 | 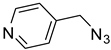 | 3g | 94 |
| 8 | 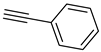 | 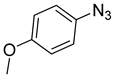 | 3h | 91 |
| 9 | 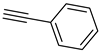 | 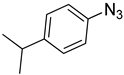 | 3i | 92 |
| Run | Cu(II)-CA b |
|---|---|
| 1 | 91 |
| 2 | 85 |
| 3 | 78 |
| 4 | 62 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst | [Cu] Loading (mol%) | Conditions | Time (h) | TON d | TOF e | Yield (%) | Ref. |
| 1 | Cu(II)-AHG a | 2 | H2O, r.t. | 24 | 24 | 1 | 95 | [47] |
| 2 | Cu(II)-AD b | 2 | H2O, r.t. | 48 | 23 | 0.48 | 93 | |
| 3 | Cu(II)-Alginate | 21 | H2O, r.t. | 18 | 4.6 | 0.26 | 98 | [48] |
| 4 | Cu(II)-Cellulose | 1.2 | H2O, r.t. | 12 | 40 | 3.33 | 96 | [49] |
| 5 | Cu(II)-Poly(hydroxamic acid) | 0.1 | H2O, 50 °C | 4 | 910 | 227.5 | 91 | [50] |
| 6 | CuSO4-Chitosan | n.d c | H2O, r.t. | 4 | - | - | 99 | [51] |
| 7 | Cu(II)-Polyethylenimine | 5 | H2O, r.t. | 24 | 12 | 0.5 | 98 | [52] |
| 8 | Cu(II)-CA | 5 | H2O, r.t. | 8 | 18.2 | 2.28 | 91 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiriba, S.-E.; Bahsis, L.; Benhadria, E.; Oudghiri, K.; Taourirte, M.; Julve, M. Cellulose Acetate-Supported Copper as an Efficient Sustainable Heterogenous Catalyst for Azide-Alkyne Cycloaddition Click Reactions in Water. Int. J. Mol. Sci. 2023, 24, 9301. https://doi.org/10.3390/ijms24119301
Stiriba S-E, Bahsis L, Benhadria E, Oudghiri K, Taourirte M, Julve M. Cellulose Acetate-Supported Copper as an Efficient Sustainable Heterogenous Catalyst for Azide-Alkyne Cycloaddition Click Reactions in Water. International Journal of Molecular Sciences. 2023; 24(11):9301. https://doi.org/10.3390/ijms24119301
Chicago/Turabian StyleStiriba, Salah-Eddine, Lahoucine Bahsis, Elhouceine Benhadria, Khaoula Oudghiri, Moha Taourirte, and Miguel Julve. 2023. "Cellulose Acetate-Supported Copper as an Efficient Sustainable Heterogenous Catalyst for Azide-Alkyne Cycloaddition Click Reactions in Water" International Journal of Molecular Sciences 24, no. 11: 9301. https://doi.org/10.3390/ijms24119301
APA StyleStiriba, S.-E., Bahsis, L., Benhadria, E., Oudghiri, K., Taourirte, M., & Julve, M. (2023). Cellulose Acetate-Supported Copper as an Efficient Sustainable Heterogenous Catalyst for Azide-Alkyne Cycloaddition Click Reactions in Water. International Journal of Molecular Sciences, 24(11), 9301. https://doi.org/10.3390/ijms24119301








