Pyroptosis: A Promising Mechanism Linking SARS-CoV-2 Infection to Adverse Pregnancy Outcomes
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Immunoglobulin Assessment and NLRP3 Quantification
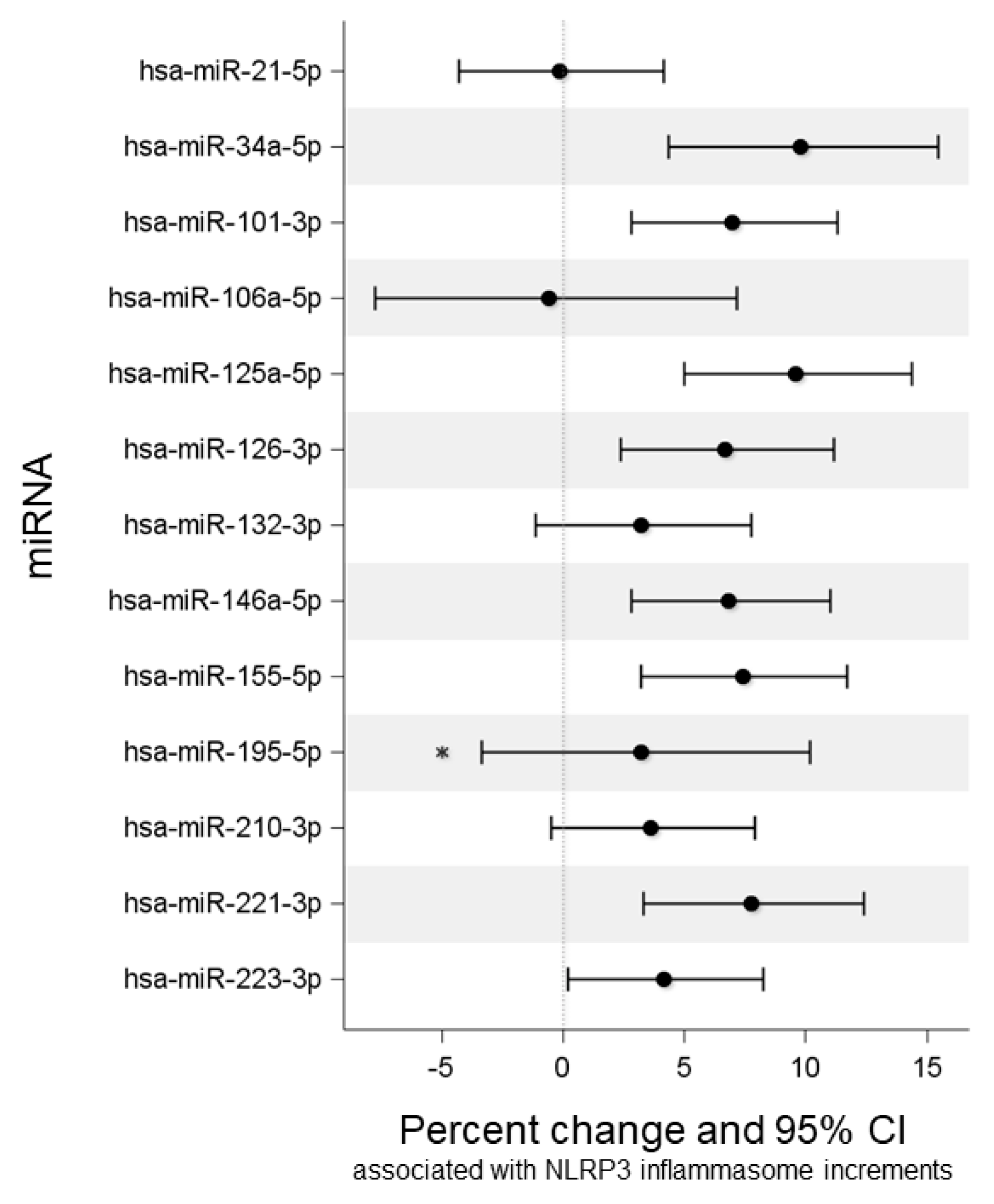
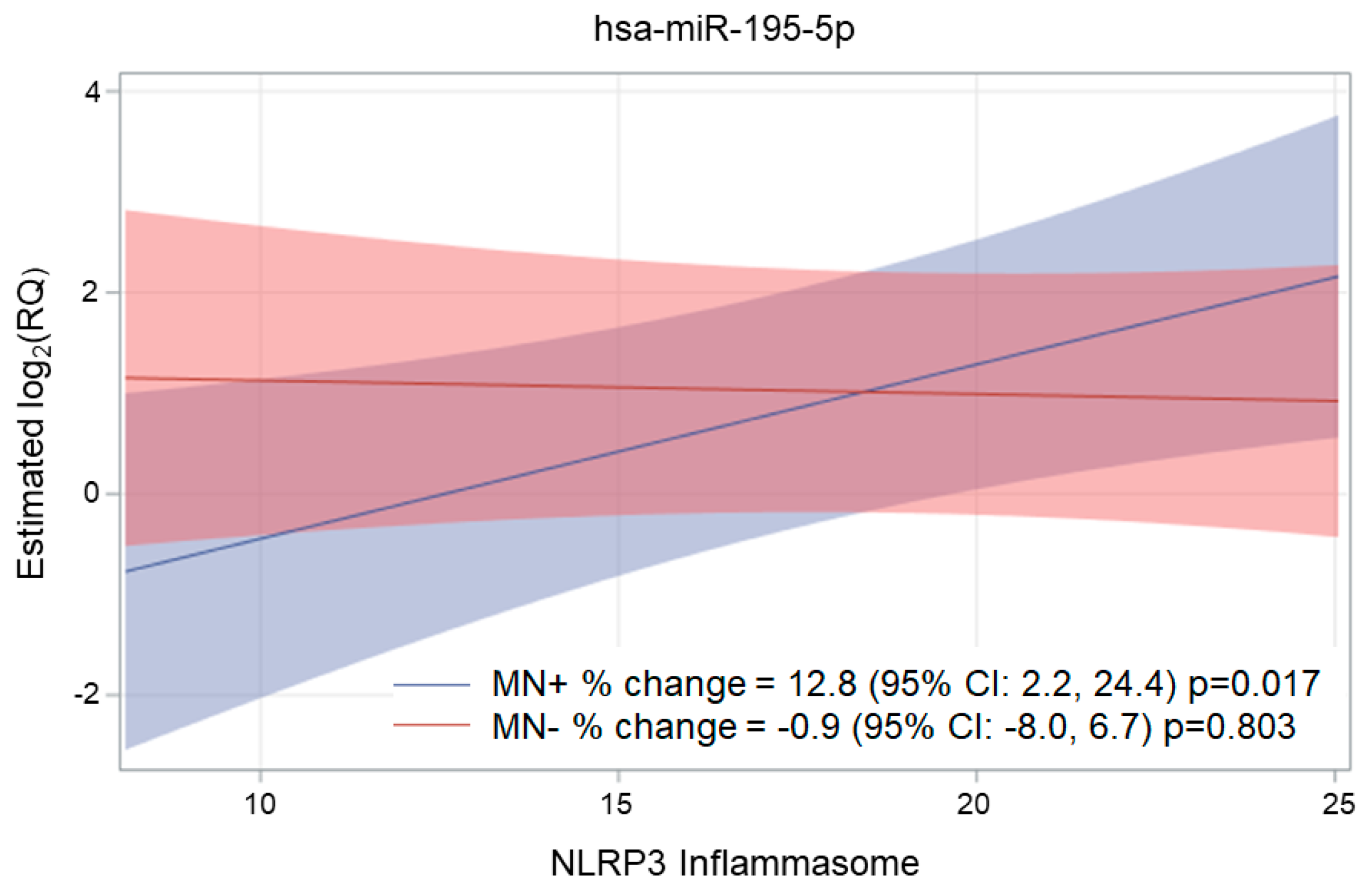
2.3. Association of NLRP3, Ig, and MN with miRNA Expression
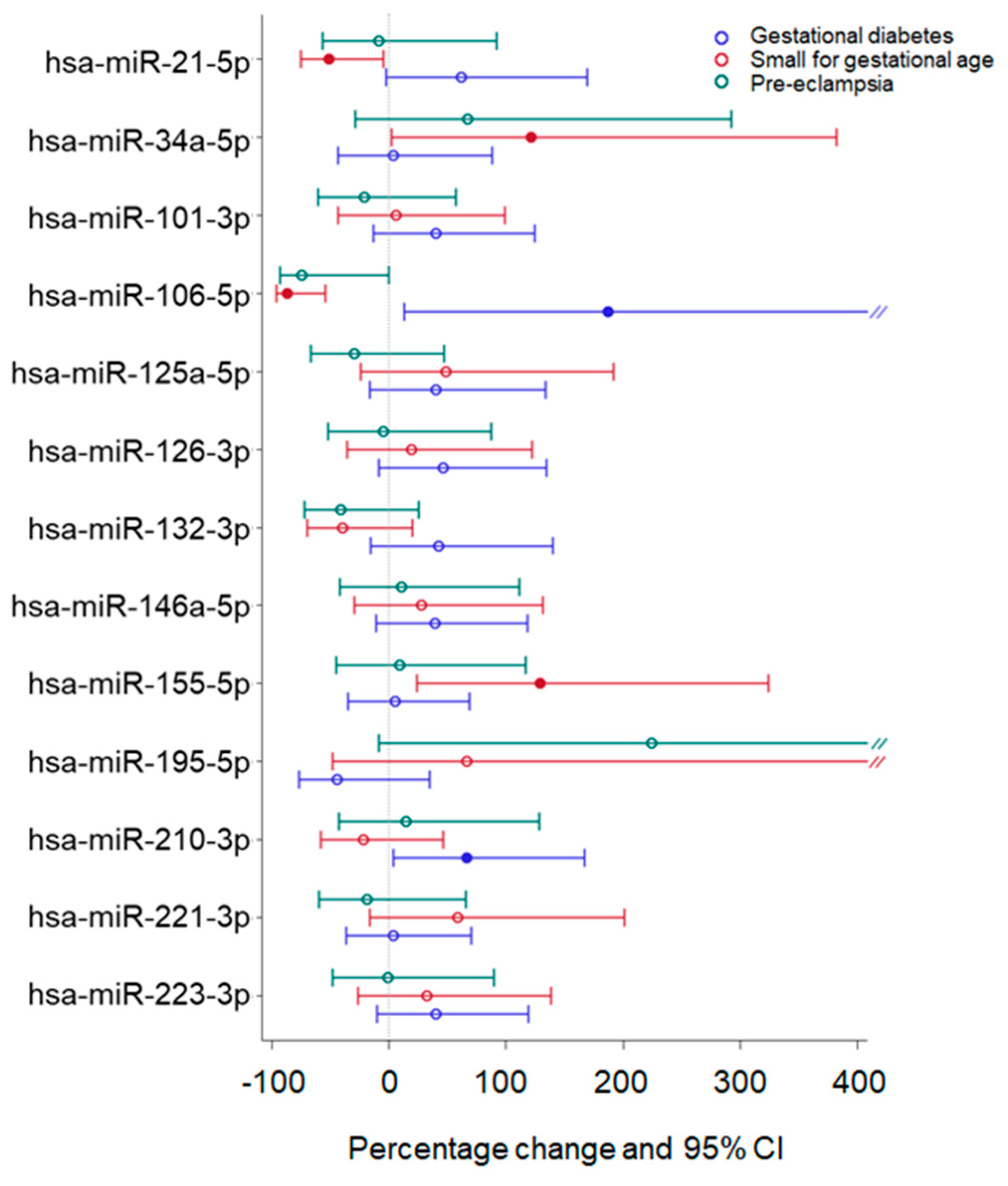
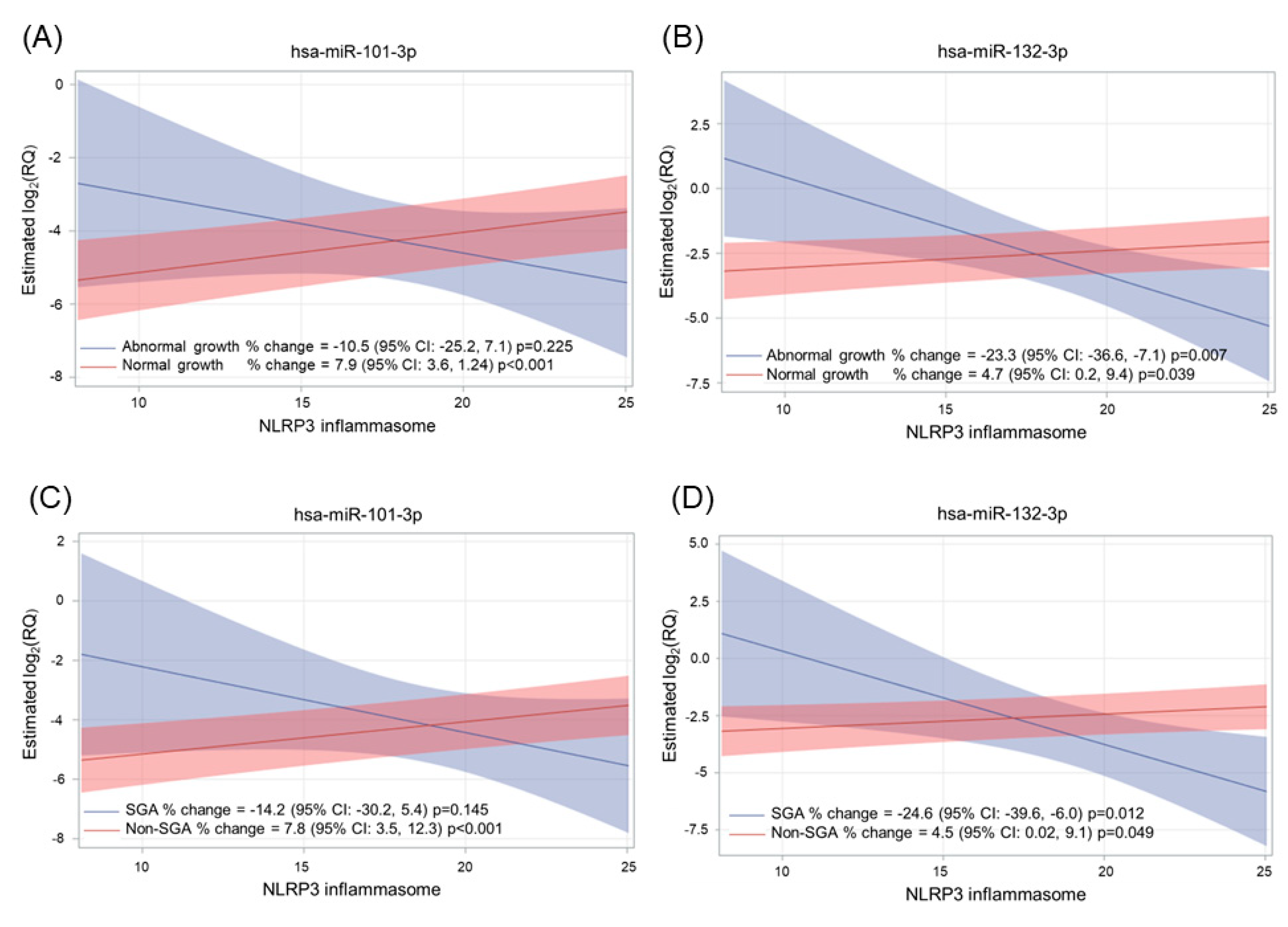
2.4. Effect of Adverse Pregnancy Outcomes on miRNA Expression
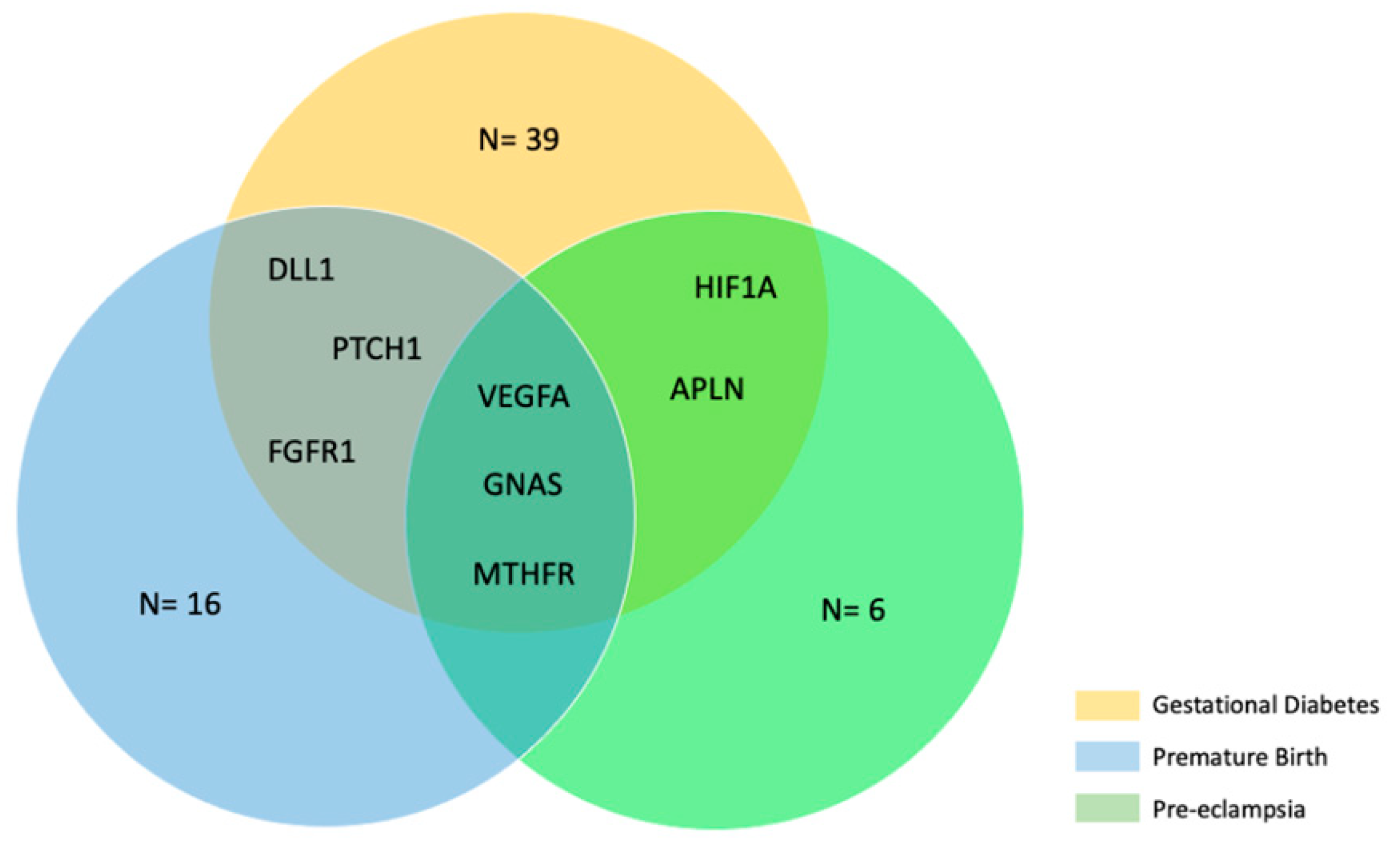
2.5. Bioinformatic Analysis
3. Discussion
4. Materials and Methods
4.1. Subject Enrollment and Blood Sample Collection
4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
4.3. Micro Neutralization CPE-Based Assay
4.4. miRNA Extraction and Analysis
4.5. Statistical Analysis
4.6. Bioinformatic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Orefice, R. Immunology and the immunological response in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yuan, F.; Dai, Z.; Wang, Z.; Zhu, Y.; Wang, B. Preeclampsia in systemic lupus erythematosus pregnancy: A systematic review and meta-analysis. Clin. Rheumatol. 2020, 39, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Gernaat, S.A.M.; Simard, J.F.; Wikström, A.K.; Svenungsson, E.; Arkema, E.V. Gestational Diabetes Mellitus Risk in Pregnant Women with Systemic Lupus Erythematosus. J. Rheumatol. 2022, 49, 465–469. [Google Scholar] [CrossRef]
- Merz, W.M.; Fischer-Betz, R.; Hellwig, K.; Lamprecht, G.; Gembruch, U. Pregnancy and Autoimmune Disease: Diseases of the Nervous System, Connective Tissue, and the Bowel. Dtsch. Arztebl. Int. 2022, 119, 145. [Google Scholar] [CrossRef]
- Silasi, M.; Cardenas, I.; Kwon, J.Y.; Racicot, K.; Aldo, P.; Mor, G. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015, 73, 199–213. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Villar, J.; Lindheimer, M. Maternal infection and risk of preeclampsia: Systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2008, 198, 7–22. [Google Scholar] [CrossRef]
- Adams Waldorf, K.M.; McAdams, R.M. Influence of infection during pregnancy on fetal development. Reproduction 2013, 146, R151–R162. [Google Scholar] [CrossRef]
- Wastnedge, E.A.N.; Reynolds, R.M.; van Boeckel, S.R.; Stock, S.J.; Denison, F.C.; Maybin, J.A.; Critchley, H.O.D. Pregnancy and COVID-19. Physiol. Rev. 2021, 101, 303–318. [Google Scholar] [CrossRef]
- Elsaddig, M.; Khalil, A. Effects of the COVID pandemic on pregnancy outcomes. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 73, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Liu, Y.Y.; Wu, C.H.; Wang, C.Y.; Wang, C.H.; Long, C.Y. Impact of COVID-19 on Pregnancy. Int. J. Med. Sci. 2021, 18, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.Q.; Bilodeau-Bertrand, M.; Liu, S.; Auger, N. The impact of COVID-19 on pregnancy outcomes: A systematic review and meta-analysis. CMAJ 2021, 193, E540–E548. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Anderson, F.L.; von Herrmann, K.M.; Andrew, A.S.; Kuras, Y.I.; Young, A.L.; Scherzer, C.R.; Hickey, W.F.; Lee, S.L.; Havrda, M.C. Plasma-borne indicators of inflammasome activity in Parkinson’s disease patients. NPJ Park. Dis. 2021, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Yang, L.; Pandeya, A.; Cui, J.; Zhang, Y.; Li, Z. Pyroptosis-Induced Inflammation and Tissue Damage. J. Mol. Biol. 2022, 434, 167301. [Google Scholar] [CrossRef]
- Declercq, J.; De Leeuw, E.; Lambrecht, B.N. Inflammasomes and IL-1 family cytokines in SARS-CoV-2 infection: From prognostic marker to therapeutic agent. Cytokine 2022, 157, 155934. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef]
- Cheng, S.B.; Nakashima, A.; Huber, W.J.; Davis, S.; Banerjee, S.; Huang, Z.; Saito, S.; Sadovsky, Y.; Sharma, S. Pyroptosis is a critical inflammatory pathway in the placenta from early onset preeclampsia and in human trophoblasts exposed to hypoxia and endoplasmic reticulum stressors. Cell Death Dis. 2019, 10, 927. [Google Scholar] [CrossRef]
- Yu, S.Y.; Li, X.L. Pyroptosis and inflammasomes in obstetrical and gynecological diseases. Gynecol. Endocrinol. 2021, 37, 385–391. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Tong, Y.; Zhang, X.; Zhao, J.; Gao, X.; Yong, J.; Wang, H. Isoliquiritin ameliorates depression by suppressing NLRP3-mediated pyroptosis via miRNA-27a/SYK/NF-κB axis. J. Neuroinflammation 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zhang, T.; Zhang, C.; Ji, H.; Tong, X.; Xia, R.; Wang, W.; Ma, Z.; Shi, X. Exosomal miR-30d-5p of neutrophils induces M1 macrophage polarization and primes macrophage pyroptosis in sepsis-related acute lung injury. Crit. Care 2021, 25, 356. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.X.; Mao, Y.; Cao, Q.; Chen, Y.; Zhou, L.B.; Li, S.; Chen, H.; Chen, J.H.; Zhou, G.P.; Jin, R. Exosome-mediated pyroptosis of miR-93-TXNIP-NLRP3 leads to functional difference between M1 and M2 macrophages in sepsis-induced acute kidney injury. J. Cell. Mol. Med. 2021, 25, 4786–4799. [Google Scholar] [CrossRef] [PubMed]
- Murao, A.; Aziz, M.; Wang, H.; Brenner, M.; Wang, P. Release mechanisms of major DAMPs. Apoptosis 2021, 26, 152. [Google Scholar] [CrossRef]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [Google Scholar] [CrossRef]
- Guarda, G.; Zenger, M.; Yazdi, A.S.; Schroder, K.; Ferrero, I.; Menu, P.; Tardivel, A.; Mattmann, C.; Tschopp, J. Differential expression of NLRP3 among hematopoietic cells. J. Immunol. 2011, 186, 2529–2534. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Baroja-Mazo, A.; Martín-Sánchez, F.; Gomez, A.I.; Martínez, C.M.; Amores-Iniesta, J.; Compan, V.; Barberà-Cremades, M.; Yagüe, J.; Ruiz-Ortiz, E.; Antón, J.; et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat. Immunol. 2014, 15, 738–748. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, Q.; Zhao, G.; Jiang, J.; Li, Y.; Guo, Z. miR-146a-5p Attenuates Allergic Airway Inflammation by Inhibiting the NLRP3 Inflammasome Activation in Macrophages. Int. Arch. Allergy Immunol. 2022, 183, 919–930. [Google Scholar] [CrossRef]
- Gao, X.R.; Ge, J.; Li, W.Y.; Zhou, W.C.; Xu, L.; Geng, D. qin miR-34a carried by adipocyte exosomes inhibits the polarization of M1 macrophages in mouse osteolysis model. J. Biomed. Mater. Res. A 2021, 109, 994–1003. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Q.; Yu, J.; Cao, X.; Xu, Z. miR-125a-5p inhibits the expression of NLRP3 by targeting CCL4 in human vascular smooth muscle cells treated with ox-LDL. Exp. Ther. Med. 2019, 18, 1645–1652. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Pu, L.; Zhuang, Z. miR-155-5p/FOXO3a promotes pulmonary fibrosis in rats by mediating NLRP3 inflammasome activation. Immunopharmacol. Immunotoxicol. 2022, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Cypryk, W.; Nyman, T.A.; Matikainen, S. From Inflammasome to Exosome-Does Extracellular Vesicle Secretion Constitute an Inflammasome-Dependent Immune Response? Front. Immunol. 2018, 9, 2188. [Google Scholar] [CrossRef] [PubMed]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488. [Google Scholar] [CrossRef]
- Farr, R.J.; Rootes, C.L.; Rowntree, L.C.; Nguyen, T.H.O.; Hensen, L.; Kedzierski, L.; Cheng, A.C.; Kedzierska, K.; Au, G.G.; Marsh, G.A.; et al. Altered microRNA expression in COVID-19 patients enables identification of SARS-CoV-2 infection. PLoS Pathog. 2021, 17, e1009759. [Google Scholar] [CrossRef]
- Nersisyan, S.; Engibaryan, N.; Gorbonos, A.; Kirdey, K.; Makhonin, A.; Tonevitsky, A. Potential role of cellular miRNAs in coronavirus-host interplay. PeerJ 2020, 8, e9994. [Google Scholar] [CrossRef]
- Kim, W.R.; Park, E.G.; Kang, K.W.; Lee, S.M.; Kim, B.; Kim, H.S. Expression Analyses of MicroRNAs in Hamster Lung Tissues Infected by SARS-CoV-2. Mol. Cells 2020, 43, 953–963. [Google Scholar] [CrossRef]
- Zeng, H.; He, D.; Xie, H.; Zhao, Y.; Peng, Z.; Deng, H.; Hu, J.; Jiang, B.; Liu, N. H19 regulates angiogenic capacity of extravillous trophoblasts by H19/miR-106a-5p/VEGFA axis. Arch. Gynecol. Obstet. 2020, 301, 671–679. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, X.; Jiang, Z.; Ge, Z. Identification of underlying mechanisms and hub gene-miRNA networks of the genomic subgroups in preeclampsia development. Medicine 2022, 101, E29569. [Google Scholar] [CrossRef]
- Wang, S.; Shi, M.; Li, J.; Zhang, Y.; Wang, W.; Xu, P.; Li, Y. Endothelial cell–derived exosomal circHIPK3 promotes the proliferation of vascular smooth muscle cells induced by high glucose via the miR-106a-5p/Foxo1/Vcam1 pathway. Aging 2021, 13, 25241. [Google Scholar] [CrossRef] [PubMed]
- Wander, P.L.; Boyko, E.J.; Hevner, K.; Parikh, V.J.; Tadesse, M.G.; Sorensen, T.K.; Williams, M.A.; Enquobahrie, D.A. Circulating early- and mid-pregnancy microRNAs and risk of gestational diabetes. Diabetes Res. Clin. Pract. 2017, 132, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. First-Trimester Screening for Fetal Growth Restriction and Small-for-Gestational-Age Pregnancies without Preeclampsia Using Cardiovascular Disease-Associated MicroRNA Biomarkers. Biomedicines 2022, 10, 718. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Liu, G.; Chen, G.; Guo, D.; Xu, L.; Hang, M.; Jin, M. Apelin protects against sepsis-induced cardiomyopathy by inhibiting the TLR4 and NLRP3 signaling pathways. Int. J. Mol. Med. 2018, 42, 1161–1167. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, Y.; Wang, X.D.; Luo, E.F.; Li, Y.P.; Wang, B.L.; Chen, Y.F. BDNF corrects NLRP3 inflammasome-induced pyroptosis and glucose metabolism reprogramming through KLF2/HK1 pathway in vascular endothelial cells. Cell. Signal. 2021, 78, 109843. [Google Scholar] [CrossRef]
- ZhuGe, D.L.; Javaid, H.M.A.; Sahar, N.E.; Zhao, Y.Z.; Huh, J.Y. Fibroblast growth factor 2 exacerbates inflammation in adipocytes through NLRP3 inflammasome activation. Arch. Pharm. Res. 2020, 43, 1311–1324. [Google Scholar] [CrossRef]
- Yuan, D.; Guan, S.X.; Wang, Z.; Ni, H.L.; Ding, D.L.; Xu, W.B.; Li, G.M. HIF-1α aggravated traumatic brain injury by NLRP3 inflammasome-mediated pyroptosis and activation of microglia. J. Chem. Neuroanat. 2021, 116, 101994. [Google Scholar] [CrossRef]
- Marneros, A.G. VEGF-A and the NLRP3 Inflammasome in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2016, 854, 79–85. [Google Scholar] [CrossRef]
- Fang, M.; Li, B.; Li, X.; Wang, Y.; Zhuang, Y. MicroRNA-29b regulates pyroptosis involving calcific aortic valve disease through the STAT3/SOCS1 pathway. Int. J. Cardiol. 2022, 371, 319–328. [Google Scholar] [CrossRef]
- Romacho, T.; Valencia, I.; Ramos-González, M.; Vallejo, S.; López-Esteban, M.; Lorenzo, O.; Cannata, P.; Romero, A.; San Hipólito-Luengo, A.; Gómez-Cerezo, J.F.; et al. Visfatin/eNampt induces endothelial dysfunction in vivo: A role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci. Rep. 2020, 10, 5386. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, J.; Dai, Z.; Long, X.; Jin, J.; Yang, Q.; Lin, C.; Yang, Y.; Chen, Y.; Zhu, J. Long non-coding RNA LINC01347 suppresses trophoblast cell migration, invasion and EMT by regulating miR-101-3p/PTEN/AKT axis. Reprod. Biol. 2022, 22, 100670. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Liu, Z.; Zheng, R.; Chai, J.; Jiang, S. miR-132-3p Modulates DUSP9-Dependent p38/JNK Signaling Pathways to Enhance Inflammation in the Amnion Leading to Labor. Int. J. Mol. Sci. 2022, 23, 1864. [Google Scholar] [CrossRef] [PubMed]
- Akgör, U.; Ayaz, L.; Çayan, F. Expression levels of maternal plasma microRNAs in preeclamptic pregnancies. J. Obstet. Gynaecol. 2021, 41, 910–914. [Google Scholar] [CrossRef]
- Gillet, V.; Ouellet, A.; Stepanov, Y.; Rodosthenous, R.S.; Croft, E.K.; Brennan, K.; Abdelouahab, N.; Baccarelli, A.; Takser, L. miRNA Profiles in Extracellular Vesicles from Serum Early in Pregnancies Complicated by Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2019, 104, 5157–5169. [Google Scholar] [CrossRef]
- Li, H.; Zhou, L.; Zhi, Z.; Lv, X.; Wei, Z.; Zhang, X.; Tang, W.; Tong, M. Lipopolysaccharide upregulates miR-132/212 in Hirschsprung-associated enterocolitis, facilitating pyroptosis by activating NLRP3 inflammasome via targeting Sirtuin 1 (SIRT1). Aging 2020, 12, 18588–18602. [Google Scholar] [CrossRef]
- Accurti, V.; Gambitta, B.; Iodice, S.; Manenti, A.; Boito, S.; Dapporto, F.; Leonardi, M.; Molesti, E.; Fabietti, I.; Montomoli, E.; et al. SARS-CoV-2 Seroconversion and Pregnancy Outcomes in a Population of Pregnant Women Recruited in Milan, Italy, between April 2020 and October 2020. Int. J. Environ. Res. Public Health 2022, 19, 16720. [Google Scholar] [CrossRef] [PubMed]
- Manenti, A.; Maggetti, M.; Casa, E.; Martinuzzi, D.; Torelli, A.; Trombetta, C.M.; Marchi, S.; Montomoli, E. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J. Med. Virol. 2020, 92, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Su, F.; Kong, F.; Guo, Q.; Wang, X.; Cui, H.; Li, Q.; Zhang, W.; Li, L.; Li, A. miR-101-3p contributes to the progression of preeclampsia by suppressing WDR5-mediated proliferation and invasion of trophoblast. J. Obstet. Gynaecol. Res. 2023, 49, 141–153. [Google Scholar] [CrossRef]
- Banerjee, S.; Cui, H.; Xie, N.; Tan, Z.; Yang, S.; Icyuz, M.; Thannickal, V.J.; Abraham, E.; Liu, G. miR-125a-5p regulates differential activation of macrophages and inflammation. J. Biol. Chem. 2013, 288, 35428–35436. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Kotlabova, K.; Krofta, L. A History of Preterm Delivery Is Associated with Aberrant Postpartal MicroRNA Expression Profiles in Mothers with an Absence of Other Pregnancy-Related Complications. Int. J. Mol. Sci. 2021, 22, 4033. [Google Scholar] [CrossRef]
- Peng, H.Y.; Li, H.P.; Li, M.Q. High glucose induces dysfunction of human umbilical vein endothelial cells by upregulating miR-137 in gestational diabetes mellitus. Microvasc. Res. 2018, 118, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.S.Q.; Casselman, R.C.; Tayade, C.; Smith, G.N. Differential expression of plasma microRNA in preeclamptic patients at delivery and 1 year postpartum. Am. J. Obstet. Gynecol. 2015, 213, 367.e1–367.e9. [Google Scholar] [CrossRef]
- Xue, F.; Yang, J.; Li, Q.; Zhou, H. Down-regulation of microRNA-34a-5p promotes trophoblast cell migration and invasion via targetting Smad4. Biosci. Rep. 2019, 39, BSR20181631. [Google Scholar] [CrossRef]
- Pergoli, L.; Cantone, L.; Favero, C.; Angelici, L.; Iodice, S.; Pinatel, E.; Hoxha, M.; Dioni, L.; Letizia, M.; Albetti, B.; et al. Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation. Part. Fibre Toxicol. 2017, 14, 32. [Google Scholar] [CrossRef]
- Ferrari, L.; Iodice, S.; Cantone, L.; Solazzo, G.; Dioni, L.; Hoxha, M.; Vicenzi, M.; Mozzoni, P.; Bergamaschi, E.; Persico, N.; et al. Extracellular vesicles and their miRNA contents counterbalance the pro-inflammatory effect of air pollution during physiological pregnancy: A focus on Syncytin-1 positive vesicles. Environ. Int. 2022, 169, 107502. [Google Scholar] [CrossRef] [PubMed]
- Fabietti, I.; Nardi, T.; Favero, C.; Dioni, L.; Cantone, L.; Pergoli, L.; Hoxha, M.; Pinatel, E.; Mosca, F.; Bollati, V.; et al. Extracellular Vesicles and Their miRNA Content in Amniotic and Tracheal Fluids of Fetuses with Severe Congenital Diaphragmatic Hernia Undergoing Fetal Intervention. Cells 2021, 10, 1493. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, S.; Liu, H.; Meng, Q.; Ma, X.; Liu, H.; Wang, L.; Jiang, W. NcRI: A manually curated database for experimentally validated non-coding RNAs in inflammation. BMC Genom. 2020, 21, 1–5. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Piñero, J.; Saüch, J.; Sanz, F.; Furlong, L.I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 2021, 19, 2960–2967. [Google Scholar] [CrossRef]


| Test | First Trimester (T0) 1 | Peripartum (T1) |
|---|---|---|
| n (%) | n (%) | |
| Any Positivity (IgG, IgM, IgA) | 40 | 97 |
| Positive IgG antibodies | 40 (100%) | 97 (100%) |
| High Positive | 4 (10.0%) | 9 (9.3%) |
| Positive | 22 (55.0%) | 51 (52.6%) |
| Low Positive | 14 (35.0%) | 37 (38.1%) |
| Microneutralization (n = 137) | 27 (67.5%) | 79 (81.4%) |
| Microneutralization assay titers | ||
| 10 | 13 (32.5%) | 28 (35.4%) |
| 20 | 8 (29.6%) | 22 (27.8%) |
| 40 | 5 (18.5%) | 16 (20.3%) |
| 80 | 1 (3.7%) | 9 (11.4%) |
| 160 | 0 (0.0%) | 2 (2.5%) |
| 320 | 0 (0%) | 2 (2.5%) |
| Positive IgM antibodies | 12 (30.0%) | 52 (53.6%) |
| High Positive | 0 (0%) | 1 (1.0%) |
| Positive | 3 (7.5%) | 20 (20.6%) |
| Low Positive | 9 (22.5%) | 31 (32.0%) |
| Positive IgA antibodies | 3 (7.5%) | 23 (10.0%) |
| High Positive | 0 (0%) | 1 (4.3%) |
| Positive | 0 (0%) | 5 (21.7%) |
| Low Positive | 3 (7.5%) | 17 (23.7%) |
| miRNA | βNLRP3 (SE) | Percentage Change | 95% LCI | 95% UCI | p-Value | FDR p-Value |
|---|---|---|---|---|---|---|
| miR-21-5p | −0.002 (0.031) | −0.1 | −4.3 | 4.2 | 0.945 | 0.945 |
| miR-34a-5p | 0.135 (0.037) | 9.8 | 4.4 | 15.5 | <0.001 | 0.002 |
| miR-101-3p | 0.097 (0.029) | 7 | 2.8 | 11.3 | 0.001 | 0.002 |
| miR-106a-5p | −0.008 (0.055) | −0.6 | −7.8 | 7.2 | 0.881 | 0.945 |
| miR-125a-5p | 0.132 (0.031) | 9.6 | 5 | 14.4 | <0.001 | <0.001 |
| miR-126-3p | 0.093 (0.030) | 6.7 | 2.4 | 11.2 | 0.002 | 0.004 |
| miR-132-3p | 0.046 (0.032) | 3.2 | −1.1 | 7.8 | 0.147 | 0.187 |
| miR-146a-5p | 0.095 (0.028) | 6.8 | 2.8 | 11 | 0.001 | 0.002 |
| miR-155-5p | 0.103 (0.029) | 7.4 | 3.2 | 11.7 | <0.001 | 0.002 |
| miR-195-5p | 0.045 (0.048) | 3.2 | −3.4 | 10.2 | 0.346 * | 0.404 |
| miR-210-3p | 0.052 (0.03) | 3.6 | −0.5 | 7.9 | 0.083 | 0.116 |
| miR-221-3p | 0.108 (0.031) | 7.8 | 3.3 | 12.4 | 0.001 | 0.002 |
| miR-223-5p | 0.059 (0.028) | 4.2 | 0.2 | 8.3 | 0.039 | 0.061 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monti, P.; Solazzo, G.; Accurti, V.; Gambitta, B.; Iodice, S.; Boito, S.; Cantone, L.; Manenti, A.; Dioni, L.; Montomoli, E.; et al. Pyroptosis: A Promising Mechanism Linking SARS-CoV-2 Infection to Adverse Pregnancy Outcomes. Int. J. Mol. Sci. 2023, 24, 9278. https://doi.org/10.3390/ijms24119278
Monti P, Solazzo G, Accurti V, Gambitta B, Iodice S, Boito S, Cantone L, Manenti A, Dioni L, Montomoli E, et al. Pyroptosis: A Promising Mechanism Linking SARS-CoV-2 Infection to Adverse Pregnancy Outcomes. International Journal of Molecular Sciences. 2023; 24(11):9278. https://doi.org/10.3390/ijms24119278
Chicago/Turabian StyleMonti, Paola, Giulia Solazzo, Veronica Accurti, Bianca Gambitta, Simona Iodice, Simona Boito, Laura Cantone, Alessandro Manenti, Laura Dioni, Emanuele Montomoli, and et al. 2023. "Pyroptosis: A Promising Mechanism Linking SARS-CoV-2 Infection to Adverse Pregnancy Outcomes" International Journal of Molecular Sciences 24, no. 11: 9278. https://doi.org/10.3390/ijms24119278
APA StyleMonti, P., Solazzo, G., Accurti, V., Gambitta, B., Iodice, S., Boito, S., Cantone, L., Manenti, A., Dioni, L., Montomoli, E., Persico, N., & Bollati, V. (2023). Pyroptosis: A Promising Mechanism Linking SARS-CoV-2 Infection to Adverse Pregnancy Outcomes. International Journal of Molecular Sciences, 24(11), 9278. https://doi.org/10.3390/ijms24119278








