Crosstalk of Immune Cells and Platelets in an Ovarian Cancer Microenvironment and Their Prognostic Significance
Abstract
1. Introduction
2. Tumor Development
2.1. Tumor-Associated Macrophages
2.2. Dendric Cell
2.3. Myeloid-Derived Suppressor Cells
2.4. Regulatory T Cells (Treg)
2.5. Tumor Infiltrating Lymphocytes (TIL)
2.6. NK Cells
3. Tumor Progression
3.1. Tumor-Associated Macrophages
3.2. Myeloid Derived Suppressor Cells
3.3. Tumor Infiltrating Lymphocytes (TIL)
3.4. Neutrophils
3.5. Platelets
4. Prognostic Significance of Immune Cell and Platelets in Ovarian Cancer
4.1. Tumor-Associated Macrophages
| Author/Year | Histology | Stage | Method | Impact | Findings |
|---|---|---|---|---|---|
| Tan et al., 2021 [207] | n.a | n.a | Meta-analysis | Detrimental | High density of M2 TAMs negatively correlated with OS. |
| Sue-A-Quan et al., 2021 [208] | CCOC | I-III | IHC | Detrimental | High density of CD68+ TAMs was associated with shorter DFS, RFS and OS. |
| Macciò et al., 2020 [209] | EOC | IIIC-IV | FC | Beneficial | High density of M1 TAMs, and high M1/M2 ratio correlated with longer OS, PFS and PFI. |
| Badmann et al., 2020 [210] | EOC | I-IV | IHC, IF | Detrimental | Infiltration of MDR1+ CD163 + CD68+ M2 TAMs was associated with poor prognosis. |
| Yuan et al., 2017 [204] | n.a | I-IV | Meta-analysis | Detrimental | Higher M1/M2 TAMs was associated with a favorable OS and PFS. High density of CD163+ M2 TAMs correlated with poor disease outcome. |
| Yin et al., 2016 [123] | EOC | III-IV | IHC | Detrimental | High density of EGF-secreting M2-like TAMs correlated with poor disease outcome. |
| Zhang et al., 2014 [205] | EOC | I-IV | IHC, IF, FC | Beneficial | High M1/M2 TAMs ratio correlated with improved prognosis. |
| Reinartz et al., 2014 [31] | HGSOC | I-IV | FC | Detrimental | High density of CD163+ TAMs correlated with poor RFS and OS. |
| He et al., 2013 [40] | EOC | II-IV | IHC, IF | Detrimental | High density of M2 TAMs negatively correlated with OS. |
4.2. Dendritic Cells
| Author/Year | Histology | Stage | Method | Impact | Findings |
|---|---|---|---|---|---|
| Hensler et al., 2020 [211] | HGSOC | III-IV | IHC | Beneficial | High density of mature DC-LAMP+ DCs in peritoneal metastasis correlated with improved disease outcome. |
| Mastelic-Gavillet et al., 2020 [213] | n.a | I-IV | FC | Beneficial | High expression of CLEC9A (cDC1s marker) in tissues was associated with better OS. |
| Truxova et al., 2018 [212] | HGSOC | I-IV | IHC | Beneficial | High density of tumor-infiltrating DC-LAMP+ mDCs correlated with cytotoxic activity and favorable OS. |
| Zhang et al., 2015 [218] | EOC | I-IV | IHC | Beneficial | High density of CD1a+ mDCs correlated with improve OS. |
| Labidi-Galy et al., 2012 [214] | n.a | n.a | IHC | Detrimental | CD4 + BDCA2 + CD123+ pDC in tumors correlated with poor disease outcome. |
| Conrad et al., 2012 [60] | EOC | II-IV | IHC, FC | Detrimental | High density of HLA-DR + CD123+ pDCs and ICOS+ FOXP3+ Treg cells correlated with poor disease outcome. |
4.3. Myeloid-Derived Suppressor Cells
| Author/Year | Histology | Stage | Method | Impact | Findings |
|---|---|---|---|---|---|
| Okła et al., 2020 [225] | EOC | I-IV | IHC | None | High density of PD-L1+ MDSCs was not associated. with disease outcome. |
| Komura et al. 2020 [141] | n.a | n.a | IHC, FC | Detrimental | MDSC increases the stem cell-like properties and tumor PD-L1 expression by PGE2 production. |
| Okła et al., 2019 [222] | EOC | I-IV | FC | Detrimental | High density of M-MDSCs in peritoneal fluid correlated with poor disease outcome. |
| Lee et al., 2019 [220] | HGSOC, OCCC | III-IV | FC | Beneficial | Patients with gBRCAm mutation correlated with low level of MDSC in peripheral blood and better disease outcome. |
| Li et al., 2018 [221] | EOC | I-IV | FC | Detrimental | Metformin inhibition of CD39+/CD73+ MDSCs improve antitumor T-cell immunity. |
| Taki et al., 2018 [226] | HGSOC | III-IV | IHC, FC | Detrimental | High MDSC infiltration correlated with high CXCL1/2 level and short OS. |
| Santegoets et al., 2018 [224] | EOC | n.a | FC | Detrimental | High density of M-MDSC and low M-MDSC/DC ratio are associated with poor disease outcome. |
| Horikawa et al., 2017 [146] | HGSOC | III-IV | IHC, FC | Detrimental | High VEGF expression induced MDSCs and correlated with poor prognosis. |
| Cui et al., 2013 [142] | HGSOC | I-IV | IHC, FC | Detrimental | High density of MDSC, significantly correlated with poor disease outcome. |
| Obermajer et al., 2011 [135] | n.a | III-IV | FC | Detrimental | High CXCL12 levels correlated. with accumulation of MDSCs in malignant ascites and poor disease outcome. |
4.4. Lymphocytes
| Author/Year | Histology | Stage | Method | Impact | Findings |
|---|---|---|---|---|---|
| Hao et al., 2020 [232] | HGSOC | I-IV | Meta-analyses | Beneficial | CD3+, CD4+, CD8+, and CD103+ TILs positively correlated with PFS, OS. |
| Henriksen et al., 2020 [245] | HGSOC | I-IV | IHC | Beneficial | High density of CD8+ TILs and PD-L1 expression correlated with favorable prognostic outcome. |
| Zhu et al., 2020 [246] | EOC | II-IV | Retrospective analysis | Beneficial | High density of CD38+ was associated with longer DFS and increased OS. |
| Fucikova et al., 2019 [247] | HGSOC | I-IV | IHC, FC | Detrimental | High density of PD-1+ TIM-3+ CD8+ T cells correlated with poor disease outcome. |
| Truxova et al., 2018 [212] | HGSOC | I-IV | IHC | Beneficial | High density of CD8+ T cells and CD20+ B cells correlated with improve survival. |
| Zhou et al., 2018 [235] | EOC | III-IV | FC, miRNA microarray | Detrimental | High Treg cells/Th17 cells ratio, derived by TAMs correlated with disease progression and metastasis potential. |
| Li et al., 2017 [248] | EOC | III-IV | Meta-analysis | Beneficial | Intraepithelial CD3+ and CD8+ CD103 + TILs correlated with improved survival. |
| Wang et al., 2017 [249] | HGSOC | I-IV | IHC | Beneficial | CD3+ or CD8+ TILs were positively correlated with longer OS. |
| Montfort et al., 2017 [250] | HGSOC | III-IV | IHC, FC | Beneficial | High density of memory 20+ B cells in omental metastasis correlated with cytolytic favorable disease outcome. |
| Darb-Esfahan et al., 2016 [251] | HGSOC | I-IV | IHC | Beneficial | High density of CD3+ T cells and high expression of PD-L1 correlated with PFS and OS. |
| Santoiemma et al., 2016 [238] | EOC | I-IV | IHC | Beneficial | High density of CD8+ T cells and CD20+ B cells TILs positively correlated with OS |
| Lundgren et al., 2016 [241] | EOC | I-IV | IHC | Detrimental | High density of CD138+ plasma cells correlated with poor OS. |
| Kroeger et al., 2016 [240] | HGSOC | I-IV | IHC, FC | Beneficial | TLS with high density of CD8+, CD4+ T cell, CD20+ B cells and plasma cells (CD20− CD38, CD138, and CD79a) correlated improve disease outcome. |
| Zhang et al., 2015 [218] | EOC | I-IV | IHC | Beneficial | High density of CD45RO+ T cells correlated with higher survival rates |
| Shang et al., 2015 [237] | n.a | I-IV | Meta-analysis | None | FoxP3 + Tregs cells did not correlated with OS. |
| Knutson et al., 2015 [252] | EOC | I-IV | IF | Beneficial | High CD8+ T cells and CD4+/Treg cells ratio was associated with improved OS. |
| Webb et al., 2014 [253] | EOC | I-III | IHC, FC | Beneficial | Intraepithelial CD103+, CD8+ TILs correlated with improved survival. |
| Hermans et al., 2014 [234] | EOC | I-IV | IHC | Beneficial/ Detrimental | CD8+ TILs positively correlated with OS. Treg cells lymphoid aggregates correlated with reduce OS. |
| Iglesia et al., 2014 [254] | n.a | n.a | Retrospective analysis | Beneficial | BCR gene segments correlated with improved prognosis. |
| Bachmayr-Heyda et al., 2013 [255] | EOC | II-IV | IHC | Beneficial | High densities of CD8+ TILs correlated with better OS. |
| Hwang et al., 2012 [256] | n.a | n.a | Meta-analyses | Beneficial | Intraepithelial CD3+ CD8+ TILs correlated with a improve survival. |
| Nielsen et al., 2012 [239] | HGSOC | II-IV | IHC, FC | Beneficial | The presence of both CD20+ and CD8+ TIL correlated with increased patient survival. Additionally, CD27-CD20+ memory B cells correlated with cytolytic immune response and favorable prognosis. |
| Callahan et al., 2010 [257] | EOC | IIIB-IV | IHC | Beneficial | Tumor cell expression of HLA-CD8 + TILs correlated with improved survival. |
| Barnett et al., 2010 [258] | EOC | I-IV | IHC, IF | Beneficial | CD8+ TILs positively correlated with improved disease outcome. |
| Leffers et al., 2009 [233] | EOC | I–IV | IHC | Beneficial | High densities of CD8+, CD45R0+, Treg and a high ratio CD8+/FoxP3+ Treg correlated with DSS PFS, OS. |
| Milne et al., 2009 [236] | HGSOC | I-IV | IHC | Beneficial | High density of CD3+, CD8+, Treg and CD20+ B cells correlated with improved disease outcome |
| Stumpf et al., 2009 [259] | SOC | III | IHC | Beneficial | Intraepithelial CD3+ CD8+ correlated with improved DFS and OS |
| Hamanishi et al., 2007 [230] | EOC | I-IV | IHC | Detrimental | Intraepithelial CD8+ TILs count was poor prognostic factor for PFS and OS. |
| Kryczek et al., 2007 [43] | EOC | I-IV | IF | Detrimental | Treg cells and TAMs B7-H4, correlated with poor disease outcome. |
| Sato et al., 2005 [227] | EOC | I-IV | IHC | Beneficial | High density of intraepithelial CD8+ T cells and high CD8+/Treg cells ratio correlated with favorable outcome. |
| Curiel et al., 2004 [83] | n.a | I-IV | IF, FC | Detrimental | Treg cells in tumor and malignant ascites were associated with poor survival. |
4.5. NK Cells
4.6. Neutrophils
| Author/Year | Histology | Stage | Method | Impact | Findings |
|---|---|---|---|---|---|
| Kim et al., 2022 [273] | HGSOC | I-IV | ELISA | Detrimental | High circulating levels of NET markers (histone−DNA complex, cell free DNA, and neutrophil elastase) and prekallikrein, correlated with poor OS. |
| Marchetti et al., 2021 [275] | HGSOC | III-IV | Retrospective study | Detrimental | High NLR correlated with negative PFS and OS. |
| Henriksen et al., 2020 [245] | EOC | I-IV | FC | Detrimental | High NLR was associated with lack of response to chemotherapy and a poor prognosis. |
| He et al., 2020 [276] | SOC | I-IV | Retrospective study | Detrimental | Early onset neutropenia correlated with chemosensitivity and favorable PFS and OS. |
| Singel et al., 2019 [277] | EOC | III-IV | IF | Detrimental | High ascites mtDNA and neutrophil elastase was associated with reduced PFS. |
| Posabella et al., 2019 [278] | HGSOC | II-IV | IHC | Beneficial | High density of CD66b+ neutrophils was associated with chemosensitivity and longer RFS. |
| Chen et al., 2018 [279] | n.a | n.a | Meta-analysis | Detrimental | High NLR ratio correlated negatively with PFS and OS. |
| Komura et al., 2018 [262] | EOC | I–IV | Retrospective study | Detrimental | Pre-treatment neutrophilia and high NLR correlated with poor disease outcome. |
| Huang et al., 2017 [280] | EOC | I-IV | Meta-analysis | Detrimental | Elevated pre-treatment NLR correlated with poor disease outcome. |
4.7. Platelets
| Author/Year | Histology | Stage | Method | Impact | Findings |
|---|---|---|---|---|---|
| Kim et al., 2022 [291] | EOC | III-IV | Retrospective study | Detrimental | Reactive thrombocytosis after cytoreductive surgery and chemotherapy was associated with poor OS and PFS. |
| Canzler et al., 2020 [292] | EOC | I-IV | Retrospective study | Detrimental | Patients with pretreatment thrombocytosis responded less to treatment and corelated with worse OS and PFS. |
| Ye et al., 2019 [282] | EOC | I-IV | Meta-analysis | Detrimental | Pre-treatment thrombocytosis corelated with worse OS and PFS. |
| Yildirim et al., 2015 [269] | n.a | I-IV | Retrospective study | Detrimental | Preoperative high PLR ratio may help identify OC in patients with adnexal masses. |
| Ma et al., 2014 [293] | EOC | I-IV | Retrospective study | Detrimental | Thrombocytosis and MAR correlated negatively with OS. |
| Allensworth et al., 2013 [281] | n.a | I-IV | Retrospective study | Detrimental | Preoperative thrombocytosis correlated with worse DFS and OS |
| Lee et al., 2011 [284] | EOC | III-IV | Retrospective study | Detrimental | Preoperative and post-chemotherapy thrombocytosis correlated with poor prognosis. |
| Asher et al., 2011 [285] | EOC | I-IV | Retrospective study | Detrimental | Pre-surgery high PLR ratio was associated with poor OS. |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, C.R.; Collins, C.; Stokes-Lampard, H.; Rose, P.; Wilson, S.; Clements, A.; Mant, D.; Kehoe, S.T.; Austoker, J. Identifying symptoms of ovarian cancer: A qualitative and quantitative study. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 1008–1014. [Google Scholar] [CrossRef]
- Hamilton, W.; Peters, T.; Bankhead, C.; Sharp, D. Risk of ovarian cancer in women with symptoms in primary care: Population based case-control study. BMJ 2009, 339, b2998. [Google Scholar] [CrossRef] [PubMed]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Kurman, R.J.; Shih, I.-M. The Origin and Pathogenesis of Epithelial Ovarian Cancer: A Proposed Unifying Theory. Am. J. Surg. Pathol. 2010, 34, 433–443. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Yap, T.A.; Carden, C.P.; Kaye, S.B. Beyond chemotherapy: Targeted therapies in ovarian cancer. Nat. Rev. Cancer 2009, 9, 167–181. [Google Scholar] [CrossRef]
- Huang, L.; Cronin, K.A.; Johnson, K.A.; Mariotto, A.B.; Feuer, E.J. Improved survival time: What can survival cure models tell us about population-based survival improvements in late-stage colorectal, ovarian, and testicular cancer? Cancer 2008, 112, 2289–2300. [Google Scholar] [CrossRef]
- Braun, A.; Anders, H.-J.; Gudermann, T.; Mammadova-Bach, E. Platelet-Cancer Interplay: Molecular Mechanisms and New Therapeutic Avenues. Front. Oncol. 2021, 11, 665534. [Google Scholar] [CrossRef]
- Pradeep, S.; Kim, S.W.; Wu, S.Y.; Nishimura, M.; Chaluvally-Raghavan, P.; Miyake, T.; Pecot, C.V.; Kim, S.-J.; Choi, H.J.; Bischoff, F.Z.; et al. Hematogenous Metastasis of Ovarian Cancer: Rethinking Mode of Spread. Cancer Cell 2014, 26, 77–91. [Google Scholar] [CrossRef]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef]
- Rasmussen, C.B.; Kjaer, S.K.; Albieri, V.; Bandera, E.V.; Doherty, J.A.; Høgdall, E.; Webb, P.M.; Jordan, S.J.; Rossing, M.A.; Wicklund, K.G.; et al. Pelvic Inflammatory Disease and the Risk of Ovarian Cancer and Borderline Ovarian Tumors: A Pooled Analysis of 13 Case-Control Studies. Am. J. Epidemiol. 2017, 185, 8–20. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C. Inflammation and ovarian cancer. Cytokine 2012, 58, 133–147. [Google Scholar] [CrossRef]
- Savant, S.S.; Sriramkumar, S.; O’hagan, H.M. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers 2018, 10, 251. [Google Scholar] [CrossRef]
- Charbonneau, B.; Goode, E.L.; Kalli, K.R.; Knutson, K.L.; DeRycke, M.S. The Immune System in the Pathogenesis of Ovarian Cancer. Crit. Rev. Immunol. 2013, 33, 137–164. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef]
- Mills, C.D. Anatomy of a Discovery: M1 and M2 Macrophages. Front. Immunol. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yung, M.M.H.; Ngan, H.Y.S.; Chan, K.K.L.; Chan, D.W. The Impact of the Tumor Microenvironment on Macrophage Polarization in Cancer Metastatic Progression. Int. J. Mol. Sci. 2021, 22, 6560. [Google Scholar] [CrossRef] [PubMed]
- Sica, A. Role of tumour-associated macrophages in cancer-related inflammation. Exp. Oncol. 2010, 32, 153–158. [Google Scholar] [PubMed]
- Ramanathan, S.; Jagannathan, N. Tumor Associated Macrophage: A Review on the Phenotypes, Traits and Functions. Iran. J. Cancer Prev. 2014, 7, 1–8. [Google Scholar]
- Van Dalen, F.J.; van Stevendaal, M.H.M.E.; Fennemann, F.L.; Verdoes, M.; Ilina, O. Molecular repolarisation of tumour-associated macrophages. Molecules 2019, 24, 9. [Google Scholar] [CrossRef]
- Roy, A.; Li, S. Modifying the tumor microenvironment using nanoparticle therapeutics. WIREs Nanomed. Nanobiotechnol. 2016, 8, 891–908. [Google Scholar] [CrossRef]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Ostuni, R.; Kratochvill, F.; Murray, P.J.; Natoli, G. Macrophages and cancer: From mechanisms to therapeutic implications. Trends Immunol. 2015, 36, 229–239. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Singhal, S.; Stadanlick, J.; Annunziata, M.J.; Rao, A.S.; Pratik, S.; Brien, S.O.; Moon, E.K.; Cantu, E.; Danet-Desnoyers, G.; Ra, H.; et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci. Transl. Med. 2019, 11, eaat1500. [Google Scholar] [CrossRef]
- Reinartz, S.; Schumann, T.; Finkernagel, F.; Wortmann, A.; Jansen, J.M.; Meissner, W.; Krause, M.; Schwörer, A.; Wagner, U.; Müller-Brüsselbach, S.; et al. Mixed-polarization phenotype of ascites-associated macrophages in human ovarian carcinoma: Correlation of CD163 expression, cytokine levels and early relapse. Int. J. Cancer 2014, 134, 32–42. [Google Scholar] [CrossRef]
- Thibault, B.; Castells, M.; Delord, J.-P.; Couderc, B. Ovarian cancer microenvironment: Implications for cancer dissemination and chemoresistance acquisition. Cancer Metastasis Rev. 2014, 33, 17–39. [Google Scholar] [CrossRef]
- Hagemann, T.; Wilson, J.; Burke, F.; Kulbe, H.; Li, N.F.; Plüddemann, A.; Charles, K.; Gordon, S.; Balkwill, F.R. Ovarian Cancer Cells Polarize Macrophages toward A Tumor-Associated Phenotype. J. Immunol. 2006, 176, 5023–5032. [Google Scholar] [CrossRef]
- Chambers, S.K.; Kacinski, B.M.; Ivins, C.M.; Carcangiu, M.L. Overexpression of epithelial macrophage colony-stimulating factor (CSF-1) and CSF-1 receptor: A poor prognostic factor in epithelial ovarian cancer, contrasted with a protective effect of stromal CSF-1. Clin. Cancer Res. 1997, 3, 999–1007. [Google Scholar]
- Cai, D.L.; Jin, L.-P. Immune Cell Population in Ovarian Tumor Microenvironment. J. Cancer 2017, 8, 2915–2923. [Google Scholar] [CrossRef]
- Drakes, M.L.; Stiff, P.J. Regulation of Ovarian Cancer Prognosis by Immune Cells in the Tumor Microenvironment. Cancers 2018, 10, 302. [Google Scholar] [CrossRef]
- Moughon, D.L.; He, H.; Schokrpur, S.; Jiang, Z.K.; Yaqoob, M.; David, J.; Lin, C.; Iruela-Arispe, M.L.; Dorigo, O.; Wu, L. Macrophage Blockade Using CSF1R Inhibitors Reverses the Vascular Leakage Underlying Malignant Ascites in Late-Stage Epithelial Ovarian Cancer. Cancer Res. 2015, 75, 4742–4752. [Google Scholar] [CrossRef]
- Duluc, D.; Delneste, Y.; Tan, F.; Moles, M.-P.; Grimaud, L.; Lenoir, J.; Preisser, L.; Anegon, I.; Catala, L.; Ifrah, N.; et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007, 110, 4319–4330. [Google Scholar] [CrossRef]
- Allavena, P.; Chieppa, M.; Bianchi, G.; Solinas, G.; Fabbri, M.; Laskarin, G.; Mantovani, A. Engagement of the Mannose Receptor by Tumoral Mucins Activates an Immune Suppressive Phenotype in Human Tumor-Associated Macrophages. Clin. Dev. Immunol. 2010, 2010, 547179. [Google Scholar] [CrossRef]
- He, Y.-F.; Zhang, M.-Y.; Wu, X.; Sun, X.-J.; Xu, T.; He, Q.-Z.; Di, W. High MUC2 Expression in Ovarian Cancer Is Inversely Associated with the M1/M2 Ratio of Tumor-Associated Macrophages and Patient Survival Time. PLoS ONE 2013, 8, e79769. [Google Scholar] [CrossRef]
- Li, H.; Fan, X.; Houghton, J. Tumor microenvironment: The role of the tumor stroma in cancer. J. Cell. Biochem. 2007, 101, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Quiceno, D.G.; Zabaleta, J.; Ortiz, B.; Zea, A.H.; Piazuelo, M.B.; Delgado, A.; Correa, P.; Brayer, J.; Sotomayor, E.M.; et al. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004, 64, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Wei, S.; Zhu, G.; Myers, L.; Mottram, P.; Cheng, P.; Chen, L.; Coukos, G.; Zou, W. Relationship between B7-H4, Regulatory T Cells, and Patient Outcome in Human Ovarian Carcinoma. Cancer Res. 2007, 67, 8900–8905. [Google Scholar] [CrossRef] [PubMed]
- Ying, X.; Wu, Q.; Wu, X.; Zhu, Q.; Wang, X.; Jiang, L.; Chen, X.; Wang, X. Epithelial ovarian cancer-secreted exosomal miR-222-3p induces polarization of tumor-associated macrophages. Oncotarget 2016, 7, 43076–43087. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Timmerman, J.M.; Levy, R. Dendritic Cell Vaccine. In Encyclopedic Dictionary of Genetics, Genomics and Proteomics; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Riboldi, E.; Musso, T.; Moroni, E.; Urbinati, C.; Bernasconi, S.; Rusnati, M.; Adorini, L.; Presta, M.; Sozzani, S. Cutting Edge: Proangiogenic Properties of Alternatively. J. Immunol. 2005, 175, 2788–2792. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Sisirak, V.; Meeus, P.; Gobert, M.; Treilleux, I.; Bajard, A.; Combes, J.-D.; Faget, J.; Mithieux, F.; Cassignol, A.; et al. Quantitative and Functional Alterations of Plasmacytoid Dendritic Cells Contribute to Immune Tolerance in Ovarian Cancer. Cancer Res. 2011, 71, 5423–5434. [Google Scholar] [CrossRef]
- Manh, T.-P.V.; Bertho, N.; Hosmalin, A.; Schwartz-Cornil, I.; Dalod, M. Investigating Evolutionary Conservation of Dendritic Cell Subset Identity and Functions. Front. Immunol. 2015, 6, 260. [Google Scholar] [CrossRef]
- Volovitz, I.; Melzer, S.; Amar, S.; Bocsi, J.; Bloch, M.; Efroni, S.; Ram, Z.; Tárnok, A. Dendritic Cells in the Context of Human Tumors: Biology and Experimental Tools. Int. Rev. Immunol. 2016, 35, 116–135. [Google Scholar] [CrossRef]
- Strioga, M.; Schijns, V.; Powell, D.J.; Pasukoniene, V.; Dobrovolskiene, N.; Michalek, J. Dendritic cells and their role in tumor immunosurveillance. J. Endotoxin Res. 2013, 19, 98–111. [Google Scholar] [CrossRef]
- Braun, A.; Worbs, T.; Moschovakis, G.L.; Halle, S.; Hoffmann, K.; Bölter, J.; Münk, A.; Forster, R. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat. Immunol. 2011, 12, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Comerford, I.; Harata-Lee, Y.; Bunting, M.D.; Gregor, C.; Kara, E.E.; McColl, S.R. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013, 24, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Diao, J.; Zhao, J.; Winter, E.; Cattral, M.S. Tumors Suppress In Situ Proliferation of Cytotoxic T Cells by Promoting Differentiation of Gr-1+ Conventional Dendritic Cells through IL-6. J. Immunol. 2011, 186, 5058–5067. [Google Scholar] [CrossRef] [PubMed]
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the Tumor Myeloid Compartment Reveals Rare Activating Antigen-Presenting Cells Critical for T Cell Immunity. Cancer Cell 2014, 26, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Martinek, J.; Wu, T.-C.; Cadena, D.; Banchereau, J.; Palucka, K. Interplay between Dendritic Cells and Cancer Cells, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 348. [Google Scholar] [CrossRef]
- Lin, H.; Wei, S.; Hurt, E.M.; Green, M.D.; Zhao, L.; Vatan, L.; Szeliga, W.; Herbst, R.; Harms, P.W.; Fecher, L.A.; et al. Host expression of PD-L1 determines efficacy of PD-L1 pathway blockade-mediated tumor regression. J. Clin. Investig. 2018, 128, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hou, M.; Ye, F.; Lv, W.; Xie, X. Ovarian Cancer Cells Induce Peripheral Mature Dendritic Cells to Differentiate into Macrophagelike Cells In Vitro. Int. J. Gynecol. Cancer 2009, 19, 1487–1493. [Google Scholar] [CrossRef]
- Scarlett, U.K.; Rutkowski, M.R.; Rauwerdink, A.M.; Fields, J.; Escovar-Fadul, X.; Baird, J.; Cubillos-Ruiz, J.R.; Jacobs, A.C.; Gonzalez, J.L.; Weaver, J.; et al. Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J. Exp. Med. 2012, 209, 495–506. [Google Scholar] [CrossRef]
- Conrad, C.; Gregorio, J.; Wang, Y.-H.; Ito, T.; Meller, S.; Hanabuchi, S.; Anderson, S.; Atkinson, N.; Ramirez, P.T.; Liu, Y.-J.; et al. Plasmacytoid Dendritic Cells Promote Immunosuppression in Ovarian Cancer via ICOS Costimulation of Foxp3+ T-Regulatory Cells. Cancer Res. 2012, 72, 5240–5249. [Google Scholar] [CrossRef]
- Liefers-Visser, J.; Meijering, R.; Reyners, A.; van der Zee, A.; de Jong, S. IGF system targeted therapy: Therapeutic opportunities for ovarian cancer. Cancer Treat. Rev. 2017, 60, 90–99. [Google Scholar] [CrossRef]
- Huang, C.-T.; Chang, M.-C.; Chen, Y.-L.; Chen, T.-C.; Chen, C.-A.; Cheng, W.-F. Insulin-like growth factors inhibit dendritic cell-mediated anti-tumor immunity through regulating ERK1/2 phosphorylation and p38 dephosphorylation. Cancer Lett. 2015, 359, 117–126. [Google Scholar] [CrossRef]
- Somri-Gannam, L.; Meisel-Sharon, S.; Hantisteanu, S.; Groisman, G.; Limonad, O.; Hallak, M.; Bruchim, I. IGF1R Axis Inhibition Restores Dendritic Cell Antitumor Response in Ovarian Cancer. Transl. Oncol. 2020, 13, 100790. [Google Scholar] [CrossRef]
- Tanyi, J.L.; Bobisse, S.; Ophir, E.; Tuyaerts, S.; Roberti, A.; Genolet, R.; Baumgartner, P.; Stevenson, B.J.; Iseli, C.; Dangaj, D.; et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 2018, 10, eaao5931. [Google Scholar] [CrossRef]
- Krempski, J.; Karyampudi, L.; Behrens, M.D.; Erskine, C.L.; Hartmann, L.; Dong, H.; Goode, E.L.; Kalli, K.R.; Knutson, K.L. Tumor-Infiltrating Programmed Death Receptor-1+ Dendritic Cells Mediate Immune Suppression in Ovarian Cancer. J. Immunol. 2011, 186, 6905–6913. [Google Scholar] [CrossRef]
- Dolcetti, L.; Peranzoni, E.; Ugel, S.; Marigo, I.; Gomez, A.F.; Mesa, C.; Geilich, M.; Winkels, G.; Traggiai, E.; Casati, A.; et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 2010, 40, 22–35. [Google Scholar] [CrossRef]
- Atretkhany, K.-S.N.; Drutskaya, M.S. Myeloid-derived suppressor cells and proinflammatory cytokines as targets for cancer therapy. Biochemistry 2016, 81, 1274–1283. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Obermajer, N.; Muthuswamy, R.; Lesnock, J.; Edwards, R.P.; Kalinski, P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011, 118, 5498–5505. [Google Scholar] [CrossRef]
- Wong, J.L.; Obermajer, N.; Odunsi, K.; Edwards, R.P.; Kalinski, P. Synergistic COX2 Induction by IFNγ and TNFα Self-Limits Type-1 Immunity in the Human Tumor Microenvironment. Cancer Immunol. Res. 2016, 4, 303–311. [Google Scholar] [CrossRef]
- Wouters, M.; Dijkgraaf, E.M.; Kuijjer, M.; Jordanova, E.S.; Hollema, H.; Welters, M.; Van Der Hoeven, J.; Daemen, T.; Kroep, J.; Nijman, H.W.; et al. Interleukin-6 receptor and its ligand interleukin-6 are opposite markers for survival and infiltration with mature myeloid cells in ovarian cancer. Oncoimmunology 2014, 3, e962397. [Google Scholar] [CrossRef]
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef]
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Mahnke, K.; Enk, A.H. Myeloid derived suppressor cells and their role in tolerance induction in cancer. J. Dermatol. Sci. 2010, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pan, P.-Y.; Li, Q.; Sato, A.I.; Levy, D.E.; Bromberg, J.; Divino, C.M.; Chen, S.-H. Gr-1+ CD115+ Immature Myeloid Suppressor Cells Mediate the Development of Tumor-Induced T Regulatory Cells and T-Cell Anergy in Tumor-Bearing Host. Cancer Res. 2006, 66, 1123–1131. [Google Scholar] [CrossRef]
- Hanson, E.M.; Clements, V.K.; Sinha, P.; Ilkovitch, D.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells Down-Regulate L-Selectin Expression on CD4+ and CD8+ T Cells. J. Immunol. 2009, 183, 937–944. [Google Scholar] [CrossRef]
- Neumann, K.; Ostmann, A.; Breda, P.C.; Ochel, A.; Tacke, F.; Paust, H.-J.; Panzer, U.; Tiegs, G. The co-inhibitory molecule PD-L1 contributes to regulatory T cell-mediated protection in murine crescentic glomerulonephritis. Sci. Rep. 2019, 9, 2038. [Google Scholar] [CrossRef]
- Groth, C.; Hu, X.; Weber, R.; Fleming, V.; Altevogt, P.; Utikal, J.; Umansky, V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br. J. Cancer 2019, 120, 16–25. [Google Scholar] [CrossRef]
- Juneja, V.R.; McGuire, K.A.; Manguso, R.T.; LaFleur, M.W.; Collins, N.; Haining, W.N.; Freeman, G.J.; Sharpe, A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017, 214, 895–904. [Google Scholar] [CrossRef]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-Mediated Inhibition in Regulation of T Cell Responses: Mechanisms and Manipulation in Tumor Immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef]
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371. [Google Scholar] [CrossRef]
- Curiel, T.J.; Coukos, G.; Zou, L.; Alvarez, X.; Cheng, P.; Mottram, P.; Evdemon-Hogan, M.; Conejo-Garcia, J.R.; Zhang, L.; Burow, M.; et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med. 2004, 10, 942–949. [Google Scholar] [CrossRef]
- Wolf, D.; Wolf, A.M.; Rumpold, H.; Fiegl, H.; Zeimet, A.G.; Muller-Holzner, E.; Deibl, M.; Gastl, G.; Gunsilius, E.; Marth, C. The Expression of the Regulatory T Cell-Specific Forkhead Box Transcription Factor FoxP3 Is Associated with Poor Prognosis in Ovarian Cancer. Clin. Cancer Res. 2005, 11, 8326–8331. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.B.; Goedegebuure, P.S.; Belt, B.A.; Flaherty, B.; Sankpal, N.; Gillanders, W.E.; Eberlein, T.J.; Hsieh, C.-S.; Linehan, D.C. Disruption of CCR5-Dependent Homing of Regulatory T Cells Inhibits Tumor Growth in a Murine Model of Pancreatic Cancer. J. Immunol. 2009, 182, 1746–1755. [Google Scholar] [CrossRef] [PubMed]
- Fialová, A.; Partlová, S.; Sojka, L.; Hromádková, H.; Brtnický, T.; Fučíková, J.; Kocián, P.; Rob, L.; Bartůňková, J.; Špíšek, R. Dynamics of T-cell infiltration during the course of ovarian cancer: The gradual shift from a Th17 effector cell response to a predominant infiltration by regulatory T-cells. Int. J. Cancer 2013, 132, 1070–1079. [Google Scholar] [CrossRef] [PubMed]
- Jonuleit, H.; Schmitt, E.; Schuler, G.; Knop, J.; Enk, A.H. Induction of Interleukin 10—Producing, Nonproliferating Cd4+ T Cells with Regulatory Properties by Repetitive Stimulation with Allogeneic Immature Human Dendritic Cells. J. Exp. Med. 2000, 192, 1213–1222. [Google Scholar] [CrossRef]
- Facciabene, A.; Peng, X.; Hagemann, I.S.; Balint, K.; Barchetti, A.; Wang, L.-P.; Gimotty, P.A.; Gilks, C.B.; Lal, P.; Zhang, L.; et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 2011, 475, 226–230. [Google Scholar] [CrossRef]
- Barnett, B.; Kryczek, I.; Cheng, P.; Zou, W.; Curiel, T.J. Regulatory T Cells in Ovarian Cancer: Biology and Therapeutic Potential. Am. J. Reprod. Immunol. 2005, 54, 369–377. [Google Scholar] [CrossRef]
- Friedline, R.H.; Brown, D.S.; Nguyen, H.; Kornfeld, H.; Lee, J.; Zhang, Y.; Appleby, M.; Der, S.D.; Kang, J.; Chambers, C.A. CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 2009, 206, 421–434. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.-F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef]
- Wang, R.; Wan, Q.; Kozhaya, L.; Fujii, H.; Unutmaz, D. Identification of a Regulatory T Cell Specific Cell Surface Molecule that Mediates Suppressive Signals and Induces Foxp3 Expression. PLoS ONE 2008, 3, e2705. [Google Scholar] [CrossRef]
- Mandapathil, M.; Szczepanski, M.J.; Szajnik, M.; Ren, J.; Lenzner, D.E.; Jackson, E.K.; Gorelik, E.; Lang, S.; Johnson, J.T.; Whiteside, T.L. Increased Ectonucleotidase Expression and Activity in Regulatory T Cells of Patients with Head and Neck Cancer. Clin. Cancer Res. 2009, 15, 6348–6357. [Google Scholar] [CrossRef]
- Stagg, J.; Divisekera, U.; Duret, H.; Sparwasser, T.; Teng, M.W.; Darcy, P.K.; Smyth, M.J. CD73-Deficient Mice Have Increased Antitumor Immunity and Are Resistant to Experimental Metastasis. Cancer Res. 2011, 71, 2892–2900. [Google Scholar] [CrossRef]
- Feng, L.; Sun, X.; Csizmadia, E.; Han, L.; Bian, S.; Murakami, T.; Wang, X.; Robson, S.C.; Wu, Y. Vascular CD39/ENTPD1 Directly Promotes Tumor Cell Growth by Scavenging Extracellular Adenosine Triphosphate. Neoplasia 2011, 13, 206–216. [Google Scholar] [CrossRef]
- Goding, J.W. Ecto-enzymes: Physiology meets pathology. J. Leukoc. Biol. 2000, 67, 285–311. [Google Scholar] [CrossRef]
- Scholzen, A.; Mittag, D.; Rogerson, S.J.; Cooke, B.M.; Plebanski, M. Plasmodium falciparum—Mediated Induction of Human CD25hiFoxp3hi CD4 T Cells Is Independent of Direct TCR Stimulation and Requires IL-2, IL-10 and TGFβ. PLoS Pathog. 2009, 5, e1000543. [Google Scholar] [CrossRef]
- Chen, X.; Subleski, J.J.; Hamano, R.; Howard, O.M.Z.; Wiltrout, R.H.; Oppenheim, J.J. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+ FOXP3+ regulatory T cells in human peripheral blood. Eur. J. Immunol. 2010, 40, 1099–1106. [Google Scholar] [CrossRef]
- Mercer, F.; Kozhaya, L.; Unutmaz, D. Expression and Function of TNF and IL-1 Receptors on Human Regulatory T Cells. PLoS ONE 2010, 5, e8639. [Google Scholar] [CrossRef]
- Kulbe, H.; Chakravarty, P.; Leinster, D.A.; Charles, K.A.; Kwong, J.; Thompson, R.G.; Coward, J.I.; Schioppa, T.; Robinson, S.C.; Gallagher, W.M.; et al. A Dynamic Inflammatory Cytokine Network in the Human Ovarian Cancer Microenvironment. Cancer Res. 2012, 72, 66–75. [Google Scholar] [CrossRef]
- Govindaraj, C.; Scalzo-Inguanti, K.; Madondo, M.; Hallo, J.; Flanagan, K.; Quinn, M.; Plebanski, M. Impaired Th1 immunity in ovarian cancer patients is mediated by TNFR2+ Tregs within the tumor microenvironment. Clin. Immunol. 2013, 149, 97–110. [Google Scholar] [CrossRef]
- Santoiemma, P.P.; Powell, D.J., Jr. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol. Ther. 2015, 16, 807–820. [Google Scholar] [CrossRef]
- Hara, H.; Kobayashi, A.; Yoshida, K.; Ohashi, M.; Ohnami, S.; Uchida, E.; Higashihara, E.; Yoshida, T.; Aoki, K. Local interferon-alpha gene therapy elicits systemic immunity in a syngeneic pancreatic cancer model in hamster. Cancer Sci. 2007, 98, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lang, J. Programmed Death-1 Pathway Blockade Produces a Synergistic Antitumor Effect: Combined Application in Ovarian Cancer. J. Gynecol. Oncol. 2017, 28, e64. [Google Scholar] [CrossRef] [PubMed]
- Wylie, B.; Macri, C.; Mintern, J.; Waithman, J. Dendritic Cells and Cancer: From Biology to Therapeutic Intervention. Cancers 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.R.; De Palma, M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat. Commun. 2019, 10, 5408. [Google Scholar] [CrossRef]
- Thomas, S.; Prendergast, G.C. Cancer Vaccines: A Brief Overview. Methods Mol. Biol. 2016, 1403, 755–761. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.S.; Bekkers, R.; Ottevanger, N.; Jansen, J.H.; Massuger, L.; Dolstra, H. Harnessing natural killer cells for the treatment of ovarian cancer. Gynecol. Oncol. 2020, 157, 810–816. [Google Scholar] [CrossRef]
- Morvan, M.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Krockenberger, M.; Dombrowski, Y.; Weidler, C.; Ossadnik, M.; Hönig, A.; Häusler, S.; Voigt, H.; Becker, J.C.; Leng, L.; Steinle, A.; et al. Macrophage Migration Inhibitory Factor Contributes to the Immune Escape of Ovarian Cancer by Down-Regulating NKG2D. J. Immunol. 2008, 180, 7338–7348. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Y.; Chen, L.; Xu, B.; Wu, C.; Jiang, J. B7-H6 Expression Correlates with Cancer Progression and Patient’s Survival in Human Ovarian Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 9428–9433. [Google Scholar]
- Pesce, S.; Tabellini, G.; Cantoni, C.; Patrizi, O.; Coltrini, D.; Rampinelli, F.; Matta, J.; Vivier, E.; Moretta, A.; Parolini, S.; et al. B7-H6-mediated downregulation of NKp30 in NK cells contributes to ovarian carcinoma immune escape. Oncoimmunology 2015, 4, e1001224. [Google Scholar] [CrossRef]
- Wong, J.L.; Berk, E.; Edwards, R.P.; Kalinski, P. IL-18-Primed Helper NK Cells Collaborate with Dendritic Cells to Promote Recruitment of Effector CD8+ T Cells to the Tumor Microenvironment. Cancer Res. 2013, 73, 4653–4662. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Sousa, C.R. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Tan, D.S.; Agarwal, R.; Kaye, S.B. Mechanisms of Transcoelomic Metastasis in Ovarian Cancer. Lancet Oncol. 2006, 7, 925–934. [Google Scholar] [CrossRef]
- Eisenhauer, E. Real-world evidence in the treatment of ovarian cancer. Ann. Oncol. 2017, 28, viii61–viii65. [Google Scholar] [CrossRef]
- Kastelein, A.W.; Vos, L.M.C.; van Baal, J.O.A.M.; Koning, J.J.; Hira, V.V.V.; Nieuwland, R.; van Driel, W.J.; Uz, Z.; van Gulik, T.M.; van Rheenen, J.; et al. Poor perfusion of the microvasculature in peritoneal metastases of ovarian cancer. Clin. Exp. Metastasis 2020, 37, 293–304. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited—The role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128 (Suppl. S8), 2527–2535. [Google Scholar] [CrossRef]
- Rickard, B.P.; Conrad, C.; Sorrin, A.J.; Ruhi, M.K.; Reader, J.C.; Huang, S.A.; Franco, W.; Scarcelli, G.; Polacheck, W.J.; Roque, D.M.; et al. Malignant Ascites in Ovarian Cancer: Cellular, Acellular, and Biophysical Determinants of Molecular Characteristics and Therapy Response. Cancers 2021, 13, 4318. [Google Scholar] [CrossRef]
- Motohara, T.; Masuda, K.; Morotti, M.; Zheng, Y.; El-Sahhar, S.; Chong, K.Y.; Wietek, N.; Alsaadi, A.; Carrami, E.M.; Hu, Z.; et al. An evolving story of the metastatic voyage of ovarian cancer cells: Cellular and molecular orchestration of the adipose-rich metastatic microenvironment. Oncogene 2019, 38, 2885–2898. [Google Scholar] [CrossRef]
- Barbolina, M.V. Molecular Mechanisms Regulating Organ-Specific Metastases in Epithelial Ovarian Carcinoma. Cancers 2018, 10, 444. [Google Scholar] [CrossRef]
- Gasparri, M.L.; Savone, D.; Besharat, R.A.; Farooqi, A.A.; Bellati, F.; Ruscito, I.; Panici, P.B.; Papadia, A. Circulating tumor cells as trigger to hematogenous spreads and potential biomarkers to predict the prognosis in ovarian cancer. Tumor Biol. 2016, 37, 71–75. [Google Scholar] [CrossRef]
- Yin, M.; Li, X.; Tan, S.; Zhou, H.J.; Ji, W.; Bellone, S.; Xu, X.; Zhang, H.; Santin, A.D.; Lou, G.; et al. Tumor-associated macrophages drive spheroid formation during early transcoelomic metastasis of ovarian cancer. J. Clin. Investig. 2016, 126, 4157–4173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, D.; Chen, Y.H.; Xia, H. Peritoneal resident macrophages in tumor metastasis and immunotherapy. Front. Cell Dev. Biol. 2022, 10, 948952. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, H.; Li, M.; Li, B.; Gao, W.; Shao, Q.; Fan, B.; Zhao, F.; Wang, Q.; Xie, Q.; et al. Increased metabolites of 5-lipoxygenase from hypoxic ovarian cancer cells promote tumor-associated macrophage infiltration. Oncogene 2014, 34, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.O.; Freedman, R.S. Defective Antitumor Function of Monocyte-Derived Macrophages from Epithelial Ovarian Cancer Patients. Clin. Cancer Res. 2006, 12, 1515–1524. [Google Scholar] [CrossRef]
- Robinson-Smith, T.M.; Isaacsohn, I.; Mercer, C.A.; Zhou, M.; Van Rooijen, N.; Husseinzadeh, N.; McFarland-Mancini, M.M.; Drew, A.F. Macrophages Mediate Inflammation-Enhanced Metastasis of Ovarian Tumors in Mice. Cancer Res. 2007, 67, 5708–5716. [Google Scholar] [CrossRef]
- Hagemann, T.; Wilson, J.; Kulbe, H.; Li, N.F.; Leinster, D.A.; Charles, K.; Klemm, F.; Pukrop, T.; Binder, C.; Balkwill, F.R. Macrophages Induce Invasiveness of Epithelial Cancer Cells via NF-κB and JNK. J. Immunol. 2005, 175, 1197–1205. [Google Scholar] [CrossRef]
- Hagemann, T.; Robinson, S.C.; Thompson, R.G.; Charles, K.; Kulbe, H.; Balkwill, F.R. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol. Cancer Ther. 2007, 6, 1993–2002. [Google Scholar] [CrossRef]
- Lane, D.; Matte, I.; Laplante, C.; Garde-Granger, P.; Carignan, A.; Bessette, P.; Rancourt, C.; Piché, A. CCL18 from ascites promotes ovarian cancer cell migration through proline-rich tyrosine kinase 2 signaling. Mol. Cancer 2016, 15, 58. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Yu, H.; Yin, Q.; Li, M.; Shi, L.; Zhang, W.; Li, D.; Li, L. CCL18 from tumor-cells promotes epithelial ovarian cancer metastasis via mTOR signaling pathway. Mol. Carcinog. 2016, 55, 1688–1699. [Google Scholar] [CrossRef]
- Neyen, C.; Plüddemann, A.; Mukhopadhyay, S.; Maniati, E.; Bossard, M.; Gordon, S.; Hagemann, T. Macrophage Scavenger Receptor A Promotes Tumor Progression in Murine Models of Ovarian and Pancreatic Cancer. J. Immunol. 2013, 190, 3798–3805. [Google Scholar] [CrossRef]
- Hagemann, T.; Lawrence, T.; McNeish, I.; Charles, K.A.; Kulbe, H.; Thompson, R.G.; Robinson, S.C.; Balkwill, F.R. “Re-educating” tumor-associated macrophages by targeting NF-κB. J. Exp. Med. 2008, 205, 1261–1268. [Google Scholar] [CrossRef]
- Browning, L.; Patel, M.R.; Horvath, E.B.; Tawara, K.; Jorcyk, C.L. IL-6 and ovarian cancer: Inflammatory cytokines in promotion of metastasis. Cancer Manag. Res. 2018, 10, 6685–6693. [Google Scholar] [CrossRef]
- Obermajer, N.; Muthuswamy, R.; Odunsi, K.; Edwards, R.P.; Kalinski, P. PGE2-Induced CXCL12 Production and CXCR4 Expression Controls the Accumulation of Human MDSCs in Ovarian Cancer Environment. Cancer Res. 2011, 71, 7463–7470. [Google Scholar] [CrossRef]
- Kajiyama, H.; Shibata, K.; Terauchi, M.; Ino, K.; Nawa, A.; Kikkawa, F. Involvement of SDF-1α/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int. J. Cancer 2008, 122, 91–99. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, X.; Wang, K.; Wu, L.; Duan, T. Interaction of monocytes/macrophages with ovarian cancer cells promotes angiogenesisin vitro. Cancer Sci. 2013, 104, 516–523. [Google Scholar] [CrossRef]
- Zhang, W.; Tian, J.; Hao, Q. HMGB1 combining with tumor-associated macrophages enhanced lymphangiogenesis in human epithelial ovarian cancer. Tumor Biol. 2014, 35, 2175–2186. [Google Scholar] [CrossRef]
- Bruno, A.; Mortara, L.; Baci, D.; Noonan, D.M.; Albini, A. Myeloid Derived Suppressor Cells Interactions with Natural Killer Cells and Pro-Angiogenic Activities: Roles in Tumor Progression. Front. Immunol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Mabuchi, S.; Komura, N.; Sasano, T.; Shimura, K.; Yokoi, E.; Kozasa, K.; Kuroda, H.; Takahashi, R.; Kawano, M.; Matsumoto, Y.; et al. Pretreatment tumor-related leukocytosis misleads positron emission tomography-computed tomography during lymph node staging in gynecological malignancies. Nat. Commun. 2020, 11, 1364. [Google Scholar] [CrossRef]
- Komura, N.; Mabuchi, S.; Shimura, K.; Yokoi, E.; Kozasa, K.; Kuroda, H.; Takahashi, R.; Sasano, T.; Kawano, M.; Matsumoto, Y.; et al. The role of myeloid-derived suppressor cells in increasing cancer stem-like cells and promoting PD-L1 expression in epithelial ovarian cancer. Cancer Immunol. Immunother. 2020, 69, 2477–2499. [Google Scholar] [CrossRef]
- Cui, T.X.; Kryczek, I.; Zhao, L.; Zhao, E.; Kuick, R.; Roh, M.H.; Vatan, L.; Szeliga, W.; Mao, Y.; Thomas, D.G.; et al. Myeloid-Derived Suppressor Cells Enhance Stemness of Cancer Cells by Inducing MicroRNA101 and Suppressing the Corepressor CtBP2. Immunity 2013, 39, 611–621. [Google Scholar] [CrossRef]
- Kassim, S.K.; El-Salahy, E.M.; Fayed, S.T.; Helal, S.A.; Helal, T.; Azzam, E.E.-D.; Khalifa, A. Vascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patients. Clin. Biochem. 2004, 37, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Bouzin, C.; Brouet, A.; De Vriese, J.; DeWever, J.; Feron, O. Effects of Vascular Endothelial Growth Factor on the Lymphocyte-Endothelium Interactions: Identification of Caveolin-1 and Nitric Oxide as Control Points of Endothelial Cell Anergy1. J. Immunol. 2007, 178, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Santoro, S.P.; Wang, L.-P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, N.; Abiko, K.; Matsumura, N.; Hamanishi, J.; Baba, T.; Yamaguchi, K.; Yoshioka, Y.; Koshiyama, M.; Konishi, I. Expression of Vascular Endothelial Growth Factor in Ovarian Cancer Inhibits Tumor Immunity through the Accumulation of Myeloid-Derived Suppressor Cells. Clin. Cancer Res. 2017, 23, 587–599. [Google Scholar] [CrossRef]
- Patel, S.; Fu, S.; Mastio, J.; Dominguez, G.A.; Purohit, A.; Kossenkov, A.; Lin, C.; Alicea-Torres, K.; Sehgal, M.; Nefedova, Y.; et al. Unique pattern of neutrophil migration and function during tumor progression. Nat. Immunol. 2018, 19, 1236–1247. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Jablonska, J.; Leschner, S.; Westphal, K.; Lienenklaus, S.; Weiss, S. Neutrophils Responsive to Endogenous IFN-ß Regulate Tumor Angiogenesis and Growth in a Mouse Tumor Model. J. Clin. Invest. 2010, 120, 1151–1164. [Google Scholar] [CrossRef]
- Piccard, H.; Muschel, R.; Opdenakker, G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit. Rev. Oncol. 2012, 82, 296–309. [Google Scholar] [CrossRef]
- Singhal, S.; Bhojnagarwala, P.S.; O’Brien, S.; Moon, E.K.; Garfall, A.L.; Rao, A.S.; Quatromoni, J.G.; Stephen, T.L.; Litzky, L.; Deshpande, C.; et al. Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 2016, 30, 120–135. [Google Scholar] [CrossRef]
- Cohen, C.A.; Shea, A.A.; Heffron, C.L.; Schmelz, E.M.; Roberts, P.C. The Parity-Associated Microenvironmental Niche in the Omental Fat Band Is Refractory to Ovarian Cancer Metastasis. Cancer Prev. Res. 2013, 6, 1182–1193. [Google Scholar] [CrossRef]
- Fruhman, G.J. Neutrophil Mobilization into Peritoneal Fluid. Blood 1960, 16, 1753–1761. [Google Scholar] [CrossRef]
- Buscher, K.; Wang, H.; Zhang, X.; Striewski, P.; Wirth, B.; Saggu, G.; Lütke-Enking, S.; Mayadas, T.N.; Ley, K.; Sorokin, L.; et al. Protection from septic peritonitis by rapid neutrophil recruitment through omental high endothelial venules. Nat. Commun. 2016, 7, 10828. [Google Scholar] [CrossRef]
- Lee, W.; Ko, S.Y.; Mohamed, M.S.; Kenny, H.A.; Lengyel, E.; Naora, H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J. Exp. Med. 2019, 216, 176–194. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Costa, S.; Bevilacqua, D.; Cassatella, M.A.; Scapini, P. Recent advances on the crosstalk between neutrophils and B or T lymphocytes. Immunology 2019, 156, 23–32. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, W. Neutrophils diminish T-cell immunity to foster gastric cancer progression: The role of GM-CSF/PD-L1/PD-1 signaling pathway. Gut 2017, 66, 1878–1880. [Google Scholar] [CrossRef]
- Charles, K.A.; Kulbe, H.; Soper, R.; Escorcio-Correia, M.; Lawrence, T.; Schultheis, A.; Chakravarty, P.; Thompson, R.G.; Kollias, G.; Smyth, J.F.; et al. The tumor-promoting actions of TNF-α involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Investig. 2009, 119, 3011–3023. [Google Scholar] [CrossRef]
- Emmons, T.R.; Giridharan, T.; Singel, K.L.; Khan, A.N.H.; Ricciuti, J.; Howard, K.; Toro, S.L.S.-D.; Debreceni, I.L.; Aarts, C.E.; Brouwer, M.C.; et al. Mechanisms Driving Neutrophil-Induced T-cell Immunoparalysis in Ovarian Cancer. Cancer Immunol. Res. 2021, 9, 790–810. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Klausen, C.; Leung, P.C.K. Hydrogen Peroxide Mediates EGF-Induced Down-Regulation of E-Cadherin Expression via p38 MAPK and Snail in Human Ovarian Cancer Cells. Mol. Endocrinol. 2010, 24, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, A.; Okitaka, M.; Takagi, S.; Takami, M.; Sato, S.; Nishio, M.; Okumura, S.; Fujita, N. A critical role of platelet TGF-β release in podoplanin-mediated tumour invasion and metastasis. Sci. Rep. 2017, 7, 42186. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cui, W.; Pei, Y.; Xu, D. Platelets promote invasion and induce epithelial to mesenchymal transition in ovarian cancer cells by TGF-β signaling pathway. Gynecol. Oncol. 2019, 153, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.J.; Kwon, Y.W.; Jang, I.H.; Kim, D.K.; Lee, S.I.; Choi, E.J.; Kim, K.-H.; Suh, D.-S.; Lee, J.H.; Choi, K.U.; et al. Autotaxin Regulates Maintenance of Ovarian Cancer Stem Cells through Lysophosphatidic Acid-Mediated Autocrine Mechanism. Stem Cells 2016, 34, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, S.; Lieber, S.; Pesek, J.; Brandt, D.T.; Asafova, A.; Finkernagel, F.; Watzer, B.; Nockher, W.A.; Nist, A.; Stiewe, T.; et al. Cell type-selective pathways and clinical associations of lysophosphatidic acid biosynthesis and signaling in the ovarian cancer microenvironment. Mol. Oncol. 2019, 13, 185–201. [Google Scholar] [CrossRef]
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591. [Google Scholar] [CrossRef]
- Lin, R.J.; Afshar-Kharghan, V.; Schafer, A.I. Paraneoplastic thrombocytosis: The secrets of tumor self-promotion. Blood 2014, 124, 184–187. [Google Scholar] [CrossRef]
- Eder, A.M.; Sasagawa, T.; Mao, M.; Aoki, J.; Mills, G.B. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: Role of phospholipase D and phospholipase A2. Clin. Cancer Res. 2000, 6, 2482–2491. [Google Scholar]
- Safaee, M.; Clark, A.J.; Ivan, M.E.; Oh, M.C.; Bloch, O.; Sun, M.Z.; Oh, T.; Parsa, A.T. CD97 is a multifunctional leukocyte receptor with distinct roles in human cancers. Int. J. Oncol. 2013, 43, 1343–1350. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets guide the formation of early metastatic niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–E3061. [Google Scholar] [CrossRef]
- Orellana, R.; Kato, S.; Erices, R.; Bravo, M.L.; Gonzalez, P.; Oliva, B.; Cubillos, S.; Valdivia, A.; Ibañez, C.; Brañes, J.; et al. Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer 2015, 15, 290. [Google Scholar] [CrossRef]
- Haemmerle, M.; Taylor, M.L.; Gutschner, T.; Pradeep, S.; Cho, M.S.; Sheng, J.; Lyons, Y.M.; Nagaraja, A.S.; Dood, R.L.; Wen, Y.; et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat. Commun. 2017, 8, 310. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, A.; Simon-Saez, I.; Perales, S.; Garrido-Navas, C.; Russo, A.; de Miguel-Perez, D.; Puche-Sanz, I.; Alaminos, C.; Ceron, J.; Lorente, J.A.; et al. Exchange of cellular components between platelets and tumor cells: Impact on tumor cells behavior. Theranostics 2022, 12, 2150–2161. [Google Scholar] [CrossRef]
- Egan, K.; Cooke, N.; Kenny, D. Living in shear: Platelets protect cancer cells from shear induced damage. Clin. Exp. Metastasis 2014, 31, 697–704. [Google Scholar] [CrossRef]
- Palumbo, J.S.; Talmage, K.E.; Massari, J.V.; La Jeunesse, C.M.; Flick, M.J.; Kombrinck, K.W.; Jirousková, M.; Degen, J.L. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005, 105, 178–185. [Google Scholar] [CrossRef]
- Placke, T.; Salih, H.R.; Kopp, H.-G. GITR Ligand Provided by Thrombopoietic Cells Inhibits NK Cell Antitumor Activity. J. Immunol. 2012, 189, 154–160. [Google Scholar] [CrossRef]
- Kopp, H.-G.; Placke, T.; Salih, H.R. Platelet-Derived Transforming Growth Factor-β Down-Regulates NKG2D Thereby Inhibiting Natural Killer Cell Antitumor Reactivity. Cancer Res. 2009, 69, 7775–7783. [Google Scholar] [CrossRef]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Abu Saadeh, F.; Norris, L.; O’toole, S.; Gleeson, N. Venous thromboembolism in ovarian cancer: Incidence, risk factors and impact on survival. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 214–218. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Dubois, A.; Karavas, A.N.; Trappe, R.; Aminossadati, B.; Schmalfeldt, B.; Pfisterer, J.; Sehouli, J. Incidence of Venous Thromboembolism in Patients with Ovarian Cancer Undergoing Platinum/Paclitaxel-Containing First-Line Chemotherapy: An Exploratory Analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group. J. Clin. Oncol. 2008, 26, 2683–2689. [Google Scholar] [CrossRef]
- Weeks, K.S.; Herbach, E.; McDonald, M.; Charlton, M.; Schweizer, M.L. Meta-Analysis of VTE Risk: Ovarian Cancer Patients by Stage, Histology, Cytoreduction, and Ascites at Diagnosis. Obstet. Gynecol. Int. 2020, 2020, 2374716. [Google Scholar] [CrossRef] [PubMed]
- Buergy, D.; Wenz, F.; Groden, C.; Brockmann, M.A. Tumor-platelet interaction in solid tumors. Int. J. Cancer 2012, 130, 2747–2760. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kaneko, M.; Sata, M.; Fujita, N.; Tsuruo, T.; Osawa, M. Enhanced Expression of Aggrus (T1alpha/Podoplanin), a Platelet-Aggregation-Inducing Factor in Lung Squamous Cell Carcinoma. Tumor Biol. 2005, 26, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Mitrugno, A.; Williams, D.; Kerrigan, S.W.; Moran, N. A Novel and Essential Role for Fc g RIIa in Cancer Cell—Induced Platelet Activation. Blood 2016, 123, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Noh, K.; Haemmerle, M.; Li, D.; Park, H.; Hu, Q.; Hisamatsu, T.; Mitamura, T.; Mak, S.L.C.; Kunapuli, S.; et al. Role of ADP receptors on platelets in the growth of ovarian cancer. Blood 2017, 130, 1235–1242. [Google Scholar] [CrossRef]
- Sakurai, M.; Matsumoto, K.; Gosho, M.; Sakata, A.; Hosokawa, Y.; Tenjimbayashi, Y.; Katoh, T.; Shikama, A.; Komiya, H.; Michikami, H.; et al. Expression of Tissue Factor in Epithelial Ovarian Carcinoma Is Involved in the Development of Venous Thromboembolism. Int. J. Gynecol. Cancer 2017, 27, 37–43. [Google Scholar] [CrossRef]
- Yokota, N.; Koizume, S.; Miyagi, E.; Hirahara, F.; Nakamura, Y.; Kikuchi, K.; Ruf, W.; Sakuma, Y.; Tsuchiya, E.; Miyagi, Y. Self-production of tissue factor-coagulation factor VII complex by ovarian cancer cells. Br. J. Cancer 2009, 101, 2023–2029. [Google Scholar] [CrossRef]
- Mackman, N. Role of Tissue Factor in Hemostasis, Thrombosis, and Vascular Development. Arter. Thromb. Vasc. Biol. 2004, 24, 1015–1022. [Google Scholar] [CrossRef]
- Cohen, J.G.; Prendergast, E.; Geddings, J.E.; Walts, A.E.; Agadjanian, H.; Hisada, Y.; Karlan, B.Y.; Mackman, N.; Walsh, C.S. Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol. Oncol. 2017, 146, 146–152. [Google Scholar] [CrossRef]
- Zhong, Y.-C.; Zhang, T.; Di, W.; Li, W.-P. Thrombin promotes epithelial ovarian cancer cell invasion by inducing epithelial-mesenchymal transition. J. Gynecol. Oncol. 2013, 24, 265–272. [Google Scholar] [CrossRef]
- Alexander, E.T.; Minton, A.R.; Peters, M.C.; van Ryn, J.; Gilmour, S.K. Thrombin inhibition and cisplatin block tumor progression in ovarian cancer by alleviating the immunosuppressive microenvironment. Oncotarget 2016, 7, 85291–85305. [Google Scholar] [CrossRef]
- De Candia, E. Mechanisms of platelet activation by thrombin: A short history. Thromb. Res. 2012, 129, 250–256. [Google Scholar] [CrossRef]
- Möhle, R.; Green, D.; Moore, M.A.S.; Nachman, R.L.; Rafii, S. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc. Natl. Acad. Sci. USA 1997, 94, 663–668. [Google Scholar] [CrossRef]
- Holmes, C.E.; Levis, J.E.; Ornstein, D.L. Activated platelets enhance ovarian cancer cell invasion in a cellular model of metastasis. Clin. Exp. Metastasis 2009, 26, 653–661. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, X. Platelets are associated with xenograft tumor growth and the clinical malignancy of ovarian cancer through an angiogenesis-dependent mechanism. Mol. Med. Rep. 2015, 11, 2449–2458. [Google Scholar] [CrossRef]
- Erices, R.; Cubillos, S.; Aravena, R.; Santoro, F.; Marquez, M.; Orellana, R.; Ramírez, C.; González, P.; Fuenzalida, P.; Bravo, M.L.; et al. Diabetic concentrations of metformin inhibit platelet-mediated ovarian cancer cell progression. Oncotarget 2017, 8, 20865–20880. [Google Scholar] [CrossRef]
- Haemmerle, M.; Bottsford-Miller, J.; Pradeep, S.; Taylor, M.L.; Choi, H.-J.; Hansen, J.M.; Dalton, H.J.; Stone, R.L.; Cho, M.S.; Nick, A.M.; et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J. Clin. Investig. 2016, 126, 1885–1896. [Google Scholar] [CrossRef]
- Kawamura, K.; Komohara, Y.; Takaishi, K.; Katabuchi, H.; Takeya, M. Detection of M2 macrophages and colony-stimulating factor 1 expression in serous and mucinous ovarian epithelial tumors. Pathol. Int. 2009, 59, 300–305. [Google Scholar] [CrossRef]
- Wang, X.; Deavers, M.; Patenia, R.; Bassett, R.L.; Mueller, P.; Ma, Q.; Wang, E.; Freedman, R.S. Monocyte/macrophage and T-cell infiltrates in peritoneum of patients with ovarian cancer or benign pelvic disease. J. Transl. Med. 2006, 4, 30. [Google Scholar] [CrossRef]
- Lan, C.; Huang, X.; Lin, S.; Huang, H.; Cai, Q.; Wan, T.; Lu, J.; Liu, J. Expression of M2-Polarized Macrophages is Associated with Poor Prognosis for Advanced Epithelial Ovarian Cancer. Technol. Cancer Res. Treat. 2013, 12, 259–267. [Google Scholar] [CrossRef]
- Yafei, Z.; Jun, G.; Guolan, G. Correlation between Macrophage Infiltration and Prognosis of Ovarian Cancer—A Preliminary Study. Biomed. Res. 2016, 27, 305–312. [Google Scholar]
- Yuan, X.; Zhang, J.; Li, D.; Mao, Y.; Mo, F.; Du, W.; Ma, X. Prognostic significance of tumor-associated macrophages in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2017, 147, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, Y.; Sun, X.; Li, Q.; Wang, W.; Zhao, A.; Di, W. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Le Page, C.; Marineau, A.; Bonza, P.K.; Rahimi, K.; Cyr, L.; Labouba, I.; Madore, J.; Delvoye, N.; Mes-Masson, A.-M.; Provencher, D.M.; et al. BTN3A2 Expression in Epithelial Ovarian Cancer Is Associated with Higher Tumor Infiltrating T Cells and a Better Prognosis. PLoS ONE 2012, 7, e38541. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, H.; Xu, J.; Mo, Y.; Dai, F. Integrated analysis of tumor-associated macrophage infiltration and prognosis in ovarian cancer. Aging 2021, 13, 23210–23232. [Google Scholar] [CrossRef]
- Sue-A-Quan, R.; Patel, P.G.; Shakfa, N.; Nyi, M.-P.N.; Afriyie-Asante, A.; Kang, E.Y.; Köbel, M.; Koti, M. Prognostic significance of T cells, PD-L1 immune checkpoint and tumour associated macrophages in clear cell carcinoma of the ovary. Gynecol. Oncol. 2021, 162, 421–430. [Google Scholar] [CrossRef]
- Macciò, A.; Gramignano, G.; Cherchi, M.C.; Tanca, L.; Melis, L.; Madeddu, C. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci. Rep. 2020, 10, 6096. [Google Scholar] [CrossRef]
- Badmann, S.; Heublein, S.; Mayr, D.; Reischer, A.; Liao, Y.; Kolben, T.; Beyer, S.; Hester, A.; Zeder-Goess, C.; Burges, A.; et al. M2 Macrophages Infiltrating Epithelial Ovarian Cancer Express MDR1: A Feature That May Account for the Poor Prognosis. Cells 2020, 9, 1224. [Google Scholar] [CrossRef]
- Hensler, M.; Kasikova, L.; Fiser, K.; Rakova, J.; Skapa, P.; Laco, J.; Lanickova, T.; Pecen, L.; Truxova, I.; Vosahlikova, S.; et al. M2-like macrophages dictate clinically relevant immunosuppression in metastatic ovarian cancer. J. Immunother. Cancer 2020, 8, e000979. [Google Scholar] [CrossRef]
- Truxova, I.; Kasikova, L.; Hensler, M.; Skapa, P.; Laco, J.; Pecen, L.; Belicova, L.; Praznovec, I.; Halaska, M.J.; Brtnicky, T.; et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J. Immunother. Cancer 2018, 6, 139. [Google Scholar] [CrossRef]
- Mastelic-Gavillet, B.; Sarivalasis, A.; Lozano, L.E.; Wyss, T.; Inoges, S.; de Vries, I.J.M.; Dartiguenave, F.; Jichlinski, P.; Derrè, L.; Coukos, G.; et al. Quantitative and qualitative impairments in dendritic cell subsets of patients with ovarian or prostate cancer. Eur. J. Cancer 2020, 135, 173–182. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Treilleux, I.; Goddard-Leon, S.; Combes, J.-D.; Blay, J.-Y.; Ray-Coquard, I.; Caux, C.; Bendriss-Vermare, N. Plasmacytoid dendritic cells infiltrating ovarian cancer are associated with poor prognosis. Oncoimmunology 2012, 1, 380–382. [Google Scholar] [CrossRef]
- Zhang, X.; He, T.; Li, Y.; Chen, L.; Liu, H.; Wu, Y.; Guo, H. Dendritic Cell Vaccines in Ovarian Cancer. Front. Immunol. 2021, 11, 613773. [Google Scholar] [CrossRef]
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103+ Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938. [Google Scholar] [CrossRef]
- Scarlett, U.K.; Cubillos-Ruiz, J.R.; Nesbeth, Y.C.; Martinez, D.G.; Engle, X.; Gewirtz, A.T.; Ahonen, C.L.; Conejo-Garcia, J.R. In Situ Stimulation of CD40 and Toll-like Receptor 3 Transforms Ovarian Cancer—Infiltrating Dendritic Cells from Immunosuppressive to Immunostimulatory Cells. Cancer Res. 2009, 69, 7329–7337. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, C.; Yang, H.; Qiu, H.; Li, J.; Liu, Y.; Qin, L.; Wang, L.; Hao, S.; et al. Infiltration of dendritic cells and T lymphocytes predicts favorable outcome in epithelial ovarian cancer. Cancer Gene Ther. 2015, 22, 198–206. [Google Scholar] [CrossRef]
- Baert, T.; Vankerckhoven, A.; Riva, M.; Van Hoylandt, A.; Thirion, G.; Holger, G.; Mathivet, T.; Vergote, I.; Coosemans, A. Myeloid Derived Suppressor Cells: Key Drivers of Immunosuppression in Ovarian Cancer. Front. Immunol. 2019, 10, 1273. [Google Scholar] [CrossRef]
- Lee, J.-M.; Botesteanu, D.-A.; Tomita, Y.; Yuno, A.; Lee, M.-J.; Kohn, E.C.; Annunziata, C.M.; Matulonis, U.; Macdonald, L.A.; Nair, J.R.; et al. Patients with BRCA mutated ovarian cancer may have fewer circulating MDSC and more peripheral CD8+ T cells compared with women with BRCA wild-type disease during the early disease course. Oncol. Lett. 2019, 18, 3914–3924. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Li, J.; Fan, Z.; Yang, L.; Zhang, Z.; Zhang, C.; Yue, D.; Qin, G.; Zhang, T.; et al. Metformin-Induced Reduction of CD39 and CD73 Blocks Myeloid-Derived Suppressor Cell Activity in Patients with Ovarian Cancer. Cancer Res. 2018, 78, 1779–1791. [Google Scholar] [CrossRef]
- Okła, K.; Czerwonka, A.; Wawruszak, A.; Bobiński, M.; Bilska, M.; Tarkowski, R.; Bednarek, W.; Wertel, I.; Kotarski, J. Clinical Relevance and Immunosuppressive Pattern of Circulating and Infiltrating Subsets of Myeloid-Derived Suppressor Cells (MDSCs) in Epithelial Ovarian Cancer. Front. Immunol. 2019, 10, 691. [Google Scholar] [CrossRef]
- Wu, L.; Deng, Z.; Peng, Y.; Han, L.; Liu, J.; Wang, L.; Li, B.; Zhao, J.; Jiao, S.; Wei, H. Ascites-derived IL-6 and IL-10 synergistically expand CD14+ HLA-DR-/low myeloid-derived suppressor cells in ovarian cancer patients. Oncotarget 2017, 8, 76843–76856. [Google Scholar] [CrossRef]
- Santegoets, S.J.A.M.; de Groot, A.F.; Dijkgraaf, E.M.; Carnaz Simões, A.M.; van der Noord, V.E.; van Ham, J.J.; Welters, M.J.P.; Kroep, J.R.; van der Burg, S.H. The blood mMDSC to DC ratio is a sensitive and easy to assess independent predictive factor for epithelial ovarian cancer survival. OncoImmunology 2018, 7, e1465166. [Google Scholar] [CrossRef]
- Okła, K.; Rajtak, A.; Czerwonka, A.; Bobiński, M.; Wawruszak, A.; Tarkowski, R.; Bednarek, W.; Szumiło, J.; Kotarski, J. Accumulation of blood-circulating PD-L1-expressing M-MDSCs and monocytes/macrophages in pretreatment ovarian cancer patients is associated with soluble PD-L1. J. Transl. Med. 2020, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Taki, M.; Abiko, K.; Baba, T.; Hamanishi, J.; Yamaguchi, K.; Murakami, R.; Yamanoi, K.; Horikawa, N.; Hosoe, Y.; Nakamura, E.; et al. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat. Commun. 2018, 9, 1685. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- James, F.R.; Jiminez-Linan, M.; Alsop, J.; Mack, M.; Song, H.; Brenton, J.D.; Pharoah, P.D.P.; Ali, H.R. Association between tumour infiltrating lymphocytes, histotype and clinical outcome in epithelial ovarian cancer. BMC Cancer 2017, 17, 657. [Google Scholar] [CrossRef] [PubMed]
- Bösmüller, H.-C.; Wagner, P.; Peper, J.K.; Schuster, H.; Pham, D.L.; Greif, K.; Beschorner, C.; Rammensee, H.-G.; Stevanović, S.; Fend, F.; et al. Combined Immunoscore of CD103 and CD3 Identifies Long-Term Survivors in High-Grade Serous Ovarian Cancer. Int. J. Gynecol. Cancer 2016, 26, 671–679. [Google Scholar] [CrossRef]
- Hamanishi, J.; Mandai, M.; Iwasaki, M.; Okazaki, T.; Tanaka, Y.; Yamaguchi, K.; Higuchi, T.; Yagi, H.; Takakura, K.; Minato, N.; et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 3360–3365. [Google Scholar] [CrossRef]
- Clarke, B.; Tinker, A.V.; Lee, C.H.; Subramanian, S.; van de Rijn, M.; Turbin, D.; Kalloger, S.; Han, G.; Ceballos, K.; Cadungog, M.G.; et al. Intraepithelial T cells and prognosis in ovarian carcinoma: Novel associations with stage, tumor type, and BRCA1 loss. Mod. Pathol. 2009, 22, 393–402. [Google Scholar] [CrossRef]
- Hao, J.; Yu, H.; Zhang, T.; An, R.; Xue, Y. Prognostic impact of tumor-infiltrating lymphocytes in high grade serous ovarian cancer: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 1758835920967241. [Google Scholar] [CrossRef]
- Leffers, N.; Gooden, M.J.M.; de Jong, R.A.; Hoogeboom, B.-N.; ten Hoor, K.A.; Hollema, H.; Boezen, H.M.; van der Zee, A.G.J.; Daemen, T.; Nijman, H.W. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2009, 58, 449–459. [Google Scholar] [CrossRef]
- Hermans, C.; Anz, D.; Engel, J.; Kirchner, T.; Endres, S.; Mayr, D. Analysis of FoxP3+ T-Regulatory Cells and CD8+ T-Cells in Ovarian Carcinoma: Location and Tumor Infiltration Patterns Are Key Prognostic Markers. PLoS ONE 2014, 9, e111757. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.; Wu, X.; Zhang, T.; Zhu, Q.; Wang, X.; Wang, H.; Wang, K.; Lin, Y.; Wang, X. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol. Res. 2018, 6, 1578–1592. [Google Scholar] [CrossRef]
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE 2009, 4, e6412. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.-J.; Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef] [PubMed]
- Santoiemma, P.P.; Reyes, C.; Wang, L.-P.; McLane, M.W.; Feldman, M.D.; Tanyi, J.L.; Powell, D.J. Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol. Oncol. 2016, 143, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20+ Tumor-Infiltrating Lymphocytes Have an Atypical CD27− Memory Phenotype and Together with CD8+ T Cells Promote Favorable Prognosis in Ovarian Cancer. Clin. Cancer Res. 2012, 18, 3281–3292. [Google Scholar] [CrossRef]
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 3005–3015. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Berntsson, J.; Nodin, B.; Micke, P.; Jirström, K. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J. Ovarian Res. 2016, 9, 21. [Google Scholar] [CrossRef]
- Alwosaibai, K.; Aalmri, S.; Mashhour, M.; Ghandorah, S.; Alshangiti, A.; Azam, F.; Selwi, W.; Gharaibeh, L.; Alatawi, Y.; Alruwaii, Z.; et al. PD-L1 is highly expressed in ovarian cancer and associated with cancer stem cells populations expressing CD44 and other stem cell markers. BMC Cancer 2023, 23, 13. [Google Scholar] [CrossRef]
- Buderath, P.; Mairinger, F.; Mairinger, E.; Böhm, K.; Mach, P.; Schmid, K.W.; Kimmig, R.; Kasimir-Bauer, S.; Bankfalvi, A.; Westerwick, D.; et al. Prognostic significance of PD-1 and PD-L1 positive tumor-infiltrating immune cells in ovarian carcinoma. Int. J. Gynecol. Cancer 2019, 29, 1389–1395. [Google Scholar] [CrossRef]
- Wang, L. Prognostic effect of programmed death-ligand 1 (PD-L1) in ovarian cancer: A systematic review, meta-analysis and bioinformatics study. J. Ovarian Res. 2019, 12, 37. [Google Scholar] [CrossRef]
- Henriksen, J.R.; Donskov, F.; Waldstrøm, M.; Jakobsen, A.; Hjortkjaer, M.; Petersen, C.B.; Steffensen, K.D. Favorable prognostic impact of Natural Killer cells and T cells in high-grade serous ovarian carcinoma. Acta Oncol. 2020, 59, 652–659. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Z.; Jiang, Z.; Liu, Y.; Zhou, J. CD38 Predicts Favorable Prognosis by Enhancing Immune Infiltration and Antitumor Immunity in the Epithelial Ovarian Cancer Microenvironment. Front. Genet. 2020, 11, 369. [Google Scholar] [CrossRef]
- Fucikova, J.; Rakova, J.; Hensler, M.; Kasikova, L.; Belicova, L.; Hladikova, K.; Truxova, I.; Skapa, P.; Laco, J.; Pecen, L.; et al. TIM-3 Dictates Functional Orientation of the Immune Infiltrate in Ovarian Cancer. Clin. Cancer Res. 2019, 25, 4820–4831. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Chen, R.; Bai, Y.; Lu, X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget 2017, 8, 15621–15631. [Google Scholar] [CrossRef]
- Wang, Q.; Lou, W.; Di, W.; Wu, X. Prognostic value of tumor PD-L1 expression combined with CD8+ tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int. Immunopharmacol. 2017, 52, 7–14. [Google Scholar] [CrossRef]
- Montfort, A.; Pearce, O.; Maniati, E.; Vincent, B.G.; Bixby, L.; Böhm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A Strong B-Cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin. Cancer Res. 2017, 23, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Darb-Esfahani, S.; Kunze, C.A.; Kulbe, H.; Sehouli, J.; Wienert, S.; Lindner, J.; Budczies, J.; Bockmayr, M.; Dietel, M.; Denkert, C.; et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016, 7, 1486–1499. [Google Scholar] [CrossRef]
- Knutson, K.L.; Maurer, M.J.; Preston, C.C.; Moysich, K.B.; Goergen, K.; Hawthorne, K.M.; Cunningham, J.M.; Odunsi, K.; Hartmann, L.C.; Kalli, K.R.; et al. Regulatory T cells, inherited variation, and clinical outcome in epithelial ovarian cancer. Cancer Immunol. Immunother. 2015, 64, 1495–1504. [Google Scholar] [CrossRef]
- Webb, J.R.; Milne, K.; Watson, P.; Deleeuw, R.J.; Nelson, B.H. Tumor-Infiltrating Lymphocytes Expressing the Tissue Resident Memory Marker CD103 Are Associated with Increased Survival in High-Grade Serous Ovarian Cancer. Clin. Cancer Res. 2014, 20, 434–444. [Google Scholar] [CrossRef]
- Iglesia, M.D.; Vincent, B.G.; Parker, J.S.; Hoadley, K.A.; Carey, L.A.; Perou, C.M.; Serody, J.S. Prognostic B-cell Signatures Using mRNA-Seq in Patients with Subtype-Specific Breast and Ovarian Cancer. Clin. Cancer Res. 2014, 20, 3818–3829. [Google Scholar] [CrossRef] [PubMed]
- Bachmayr-Heyda, A.; Aust, S.; Heinze, G.; Polterauer, S.; Grimm, C.; Braicu, E.I.; Sehouli, J.; Lambrechts, S.; Vergote, I.; Mahner, S.; et al. Prognostic impact of tumor infiltrating CD8+ T cells in association with cell proliferation in ovarian cancer patients—A study of the OVCAD consortium. BMC Cancer 2013, 13, 422. [Google Scholar] [CrossRef]
- Hwang, W.-T.; Adams, S.F.; Tahirovic, E.; Hagemann, I.S.; Coukos, G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: A meta-analysis. Gynecol. Oncol. 2012, 124, 192–198. [Google Scholar] [CrossRef]
- Callahan, M.J.; Nagymanyoki, Z.; Bonome, T.; Johnson, M.E.; Litkouhi, B.; Sullivan, E.H.; Hirsch, M.S.; Matulonis, U.A.; Liu, J.; Birrer, M.J.; et al. Increased HLA-DMB Expression in the Tumor Epithelium Is Associated with Increased CTL Infiltration and Improved Prognosis in Advanced-Stage Serous Ovarian Cancer. Clin. Cancer Res. 2010, 14, 7667–7673. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.C.; Bean, S.M.; Whitaker, R.S.; Kondoh, E.; Baba, T.; Fujii, S.; Marks, J.R.; Dressman, H.K.; Murphy, S.K.; Berchuck, A. Ovarian cancer tumor infiltrating T-regulatory (Treg) cells are associated with a metastatic phenotype. Gynecol. Oncol. 2010, 116, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, M.P.H.; Hasenburg, A.; Riener, M.-O.; Jutting, U.; Wang, C.; Shen, Y.; Orlowskavolk, M.; Fisch, P.; Wang, Z.; Gitsch, G.; et al. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: Relevance of clonal selection of T lymphocytes. Br. J. Cancer 2009, 101, 1513–1521. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.S.; Maas, R.J.; Van Der Meer, J.; Cany, J.; Van Der Steen, S.; Jansen, J.H.; Miller, J.S.; Bekkers, R.; Hobo, W.; Massuger, L.; et al. Peritoneal NK cells are responsive to IL-15 and percentages are correlated with outcome in advanced ovarian cancer patients. Oncotarget 2018, 9, 34810–34820. [Google Scholar] [CrossRef]
- Henriksen, J.R.; Nederby, L.; Donskov, F.; Waldstrøm, M.; Adimi, P.; Jakobsen, A.; Steffensen, K.D. Blood natural killer cells during treatment in recurrent ovarian cancer. Acta Oncol. 2020, 59, 1365–1373. [Google Scholar] [CrossRef]
- Komura, N.; Mabuchi, S.; Yokoi, E.; Kozasa, K.; Kuroda, H.; Sasano, T.; Matsumoto, Y.; Kimura, T. Comparison of clinical utility between neutrophil count and neutrophil-lymphocyte ratio in patients with ovarian cancer: A single institutional experience and a literature review. Int. J. Clin. Oncol. 2018, 23, 104–113. [Google Scholar] [CrossRef]
- Bishara, S.; Griffin, M.; Cargill, A.; Bali, A.; Gore, M.; Kaye, S.; Shepherd, J.; Van Trappen, P. Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 71–75. [Google Scholar] [CrossRef]
- Cho, H.; Hur, H.W.; Kim, S.W.; Kim, S.H.; Kim, J.H.; Kim, Y.T.; Lee, K. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol. Immunother. 2009, 58, 15–23. [Google Scholar] [CrossRef]
- Badora-Rybicka, A.; Nowara, E.; Starzyczny-Słota, D. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio before chemotherapy as potential prognostic factors in patients with newly diagnosed epithelial ovarian cancer. ESMO Open 2016, 1, e000039. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Jin, C.; Zheng, H.-M.; Zhou, K.; Shi, B.-B.; Zhang, Q.; Zheng, F.-Y.; Lin, F. A novel prognostic inflammation score predicts outcomes in patients with ovarian cancer. Clin. Chim. Acta 2016, 456, 163–169. [Google Scholar] [CrossRef]
- Van Nguyen, J.M.; Ferguson, S.E.; Bernardini, M.Q.; May, T.; LaFramboise, S.; Hogen, L.; Bouchard-Fortier, G. Preoperative neutrophil-to-lymphocyte ratio predicts 30 day postoperative morbidity and survival after primary surgery for ovarian cancer. Int. J. Gynecol. Cancer 2020, 30, 1378–1383. [Google Scholar] [CrossRef]
- Nakamura, K.; Nagasaka, T.; Nishida, T.; Haruma, T.; Ogawa, C.; Kusumoto, T.; Seki, N.; Hiramatsu, Y. Neutrophil to lymphocyte ratio in the pre-treatment phase of final-line chemotherapy predicts the outcome of patients with recurrent ovarian cancer. Oncol. Lett. 2016, 11, 3975–3981. [Google Scholar] [CrossRef]
- Yildirim, M.; Cendek, B.D.; Avsar, A.F. Differentiation between benign and malignant ovarian masses in the preoperative period using neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios. Mol. Clin. Oncol. 2015, 3, 317–321. [Google Scholar] [CrossRef]
- Yildirim, M.A.; Seckin, K.D.; Togrul, C.; Baser, E.; Karsli, M.F.; Gungor, T.; Gulerman, H.C. Roles of Neutrophil/Lymphocyte and Platelet/Lymphocyte Ratios in the Early Diagnosis of Malignant Ovarian Masses. Asian Pac. J. Cancer Prev. 2014, 15, 6881–6885. [Google Scholar] [CrossRef]
- Abu-Shawer, O.; Hirmas, N.; Alhouri, A.; Massad, A.; Alsibai, B.; Sultan, H.; Hammo, H.; Souleiman, M.; Shebli, Y.; Al-Hussaini, M. Hematologic markers of distant metastases and poor prognosis in gynecological cancers. BMC Cancer 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Khalifa, C.; Defour, J.-P.; Latinne, D.; Van Pel, M.-C.; De Kock, M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res. Notes 2017, 10, 12. [Google Scholar] [CrossRef]
- Kim, J.G.; Kim, S.I.; Song, S.H.; Gu, J.-Y.; Lee, M.; Kim, H.K. Diagnostic and prognostic role of circulating neutrophil extracellular trap markers and prekallikrein in patients with high-grade serous ovarian cancer. Front. Oncol. 2022, 12, 992056. [Google Scholar] [CrossRef]
- Muqaku, B.; Pils, D.; Mader, J.C.; Aust, S.; Mangold, A.; Muqaku, L.; Slany, A.; Del Favero, G.; Gerner, C. Neutrophil Extracellular Trap Formation Correlates with Favorable Overall Survival in High Grade Ovarian Cancer. Cancers 2020, 12, 505. [Google Scholar] [CrossRef]
- Marchetti, C.; D’indinosante, M.; Bottoni, C.; Di Ilio, C.; Di Berardino, S.; Costantini, B.; Minucci, A.; Vertechy, L.; Scambia, G.; Fagotti, A. NLR and BRCA mutational status in patients with high grade serous advanced ovarian cancer. Sci. Rep. 2021, 11, 11125. [Google Scholar] [CrossRef]
- He, Y.; Li, T.; Liu, J.; Ou, Q.; Zhou, J. Early onset neutropenia: A useful predictor of chemosensitivity and favorable prognosis in patients with serous ovarian cancer. BMC Cancer 2020, 20, 116. [Google Scholar] [CrossRef]
- Singel, K.; Grzankowski, K.S.; Khan, A.N.M.N.H.; Grimm, M.J.; D’auria, A.C.; Morrell, K.; Eng, K.H.; Hylander, B.; Mayor, P.C.; Emmons, T.R.; et al. Mitochondrial DNA in the tumour microenvironment activates neutrophils and is associated with worse outcomes in patients with advanced epithelial ovarian cancer. Br. J. Cancer 2019, 120, 207–217. [Google Scholar] [CrossRef]
- Posabella, A.; Köhn, P.; Lalos, A.; Wilhelm, A.; Mechera, R.; Soysal, S.; Muenst, S.; Güth, U.; Stadlmann, S.; Terracciano, L.; et al. High density of CD66b in primary high-grade ovarian cancer independently predicts response to chemotherapy. J. Cancer Res. Clin. Oncol. 2020, 146, 127–136. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, L.; Yang, Y.; Long, Y.; Li, X.; Wang, Y. Prognostic Role of Neutrophil to Lymphocyte Ratio in Ovarian Cancer: A Meta-Analysis. Technol. Cancer Res. Treat. 2018, 17, 1533033818791500. [Google Scholar] [CrossRef]
- Huang, Q.-T.; Zhou, L.; Zeng, W.-J.; Ma, Q.-Q.; Wang, W.; Zhong, M.; Yu, Y.-H. Prognostic Significance of Neutrophil-To-Lymphocyte Ratio in Ovarian Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Cell. Physiol. Biochem. 2017, 41, 2411–2418. [Google Scholar] [CrossRef]
- Allensworth, S.; Langstraat, C.; Martin, J.; Lemens, M.; McGree, M.; Weaver, A.; Dowdy, S.; Podratz, K.; Bakkum-Gamez, J. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol. Oncol. 2013, 130, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Cheng, J.; Ye, M.; Liu, D.; Zhang, Y. Association of pretreatment thrombocytosis with prognosis in ovarian cancer: A systematic review and meta-analysis. J. Gynecol. Oncol. 2019, 30, e5. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wen, H.; Bi, R.; Duan, Y.; Yang, W.; Wu, X. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer 2016, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, S.W.; Nam, E.J.; Yim, G.W.; Kim, S.; Kim, Y.T. The impact of pretreatment thrombocytosis and persistent thrombocytosis after adjuvant chemotherapy in patients with advanced epithelial ovarian cancer. Gynecol. Oncol. 2011, 122, 238–241. [Google Scholar] [CrossRef]
- Asher, V.; Lee, J.; Innamaa, A.; Bali, A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin. Transl. Oncol. 2011, 13, 499–503. [Google Scholar] [CrossRef]
- Ceran, M.U.; Tasdemir, U.; Colak, E.; Güngör, T. Can complete blood count inflammatory parameters in epithelial ovarian cancer contribute to prognosis?—A survival analysis. J. Ovarian Res. 2019, 12, 16. [Google Scholar] [CrossRef]
- Winarno, G.N.A.; Pasaribu, M.; Susanto, H.; Nisa, A.S.; Harsono, A.B.; Yuseran, H.; Suardi, D.; Trianasari, N. The Platelet to Lymphocyte and Neutrophil to Lymphocyte Ratios in Predicting Response to Platinum-Based Chemotherapy for Epithelial Ovarian Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1561–1566. [Google Scholar] [CrossRef]
- Eo, W.K.; Chang, H.J.; Kwon, S.H.; Koh, S.B.; Kim, Y.O.; Ji, Y.I.; Kim, H.-B.; Lee, J.Y.; Suh, D.S.; Kim, K.H.; et al. The Lymphocyte-Monocyte Ratio Predicts Patient Survival and Aggressiveness of Ovarian Cancer. J. Cancer 2016, 7, 289–296. [Google Scholar] [CrossRef]
- Polat, M.; Senol, T.; Ozkaya, E.; Pakay, G.O.; Cikman, M.S.; Konukcu, B.; Ozten, M.A.; Karateke, A. Neutrophil to lymphocyte and platelet to lymphocyte ratios increase in ovarian tumors in the presence of frank stromal invasion. Clin. Transl. Oncol. 2016, 18, 457–463. [Google Scholar] [CrossRef]
- Wang, N.; Li, C.; Yang, Y.; Guan, Y.; Wang, F.; Wang, Y.; Zhao, W. The Use of Platelet/Lymphocyte Ratio and Cancer Antigen 125 Combined with Magnetic Resonance Diffusion-Weighted Imaging in Diagnosis of Recurrent Ovarian Cancer and Neuropathic Pain. World Neurosurg. 2021, 149, 502–510. [Google Scholar] [CrossRef]
- Kim, M.-S.; Baek, S.H.; Noh, J.J.; Shim, J.I.; Kang, J.H.; Jeong, S.Y.; Choi, C.H.; Kim, T.-J.; Lee, J.-W.; Lee, Y.-Y. Role of reactive thrombocytosis after primary cytoreductive surgery in advanced ovarian cancer. Front. Oncol. 2022, 12, 926878. [Google Scholar] [CrossRef]
- Canzler, U.; Lück, H.-J.; Neuser, P.; Sehouli, J.; Burges, A.; Harter, P.; Schmalfeldt, B.; Aminossadati, B.; Mahner, S.; Kommoss, S.; et al. Prognostic role of thrombocytosis in recurrent ovarian cancer: A pooled analysis of the AGO Study Group. Arch. Gynecol. Obstet. 2020, 301, 1267–1274. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Sheng, H.; Tian, W.; Qi, Z.; Teng, F.; Xue, F. Prognostic significance of thrombocytosis, platelet parameters and aggregation rates in epithelial ovarian cancer. J. Obstet. Gynaecol. Res. 2014, 40, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kandalaft, L.E.; Motz, G.T.; Duraiswamy, J.; Coukos, G. Tumor immune surveillance and ovarian cancer. Cancer Metastasis Rev. 2011, 30, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Yigit, R.; Massuger, L.F.; Figdor, C.; Torensma, R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol. Oncol. 2010, 117, 366–372. [Google Scholar] [CrossRef]
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural Innate and Adaptive Immunity to Cancer. Annu. Rev. Immunol. 2011, 29, 235–271. [Google Scholar] [CrossRef]
- Rosen, D.G.; Wang, L.; Atkinson, J.N.; Yu, Y.; Lu, K.H.; Diamandis, E.P.; Hellstrom, I.; Mok, S.C.; Liu, J.; Bast, R.C. Potential markers that complement expression of CA125 in epithelial ovarian cancer. Gynecol. Oncol. 2005, 99, 267–277. [Google Scholar] [CrossRef]
- Terry, K.L.; Sluss, P.M.; Skates, S.J.; Mok, S.C.; Ye, B.; Vitonis, A.F.; Cramer, D.W. Blood and Urine Markers for Ovarian Cancer: A Comprehensive Review. Dis. Markers 2004, 20, 53–70. [Google Scholar] [CrossRef]
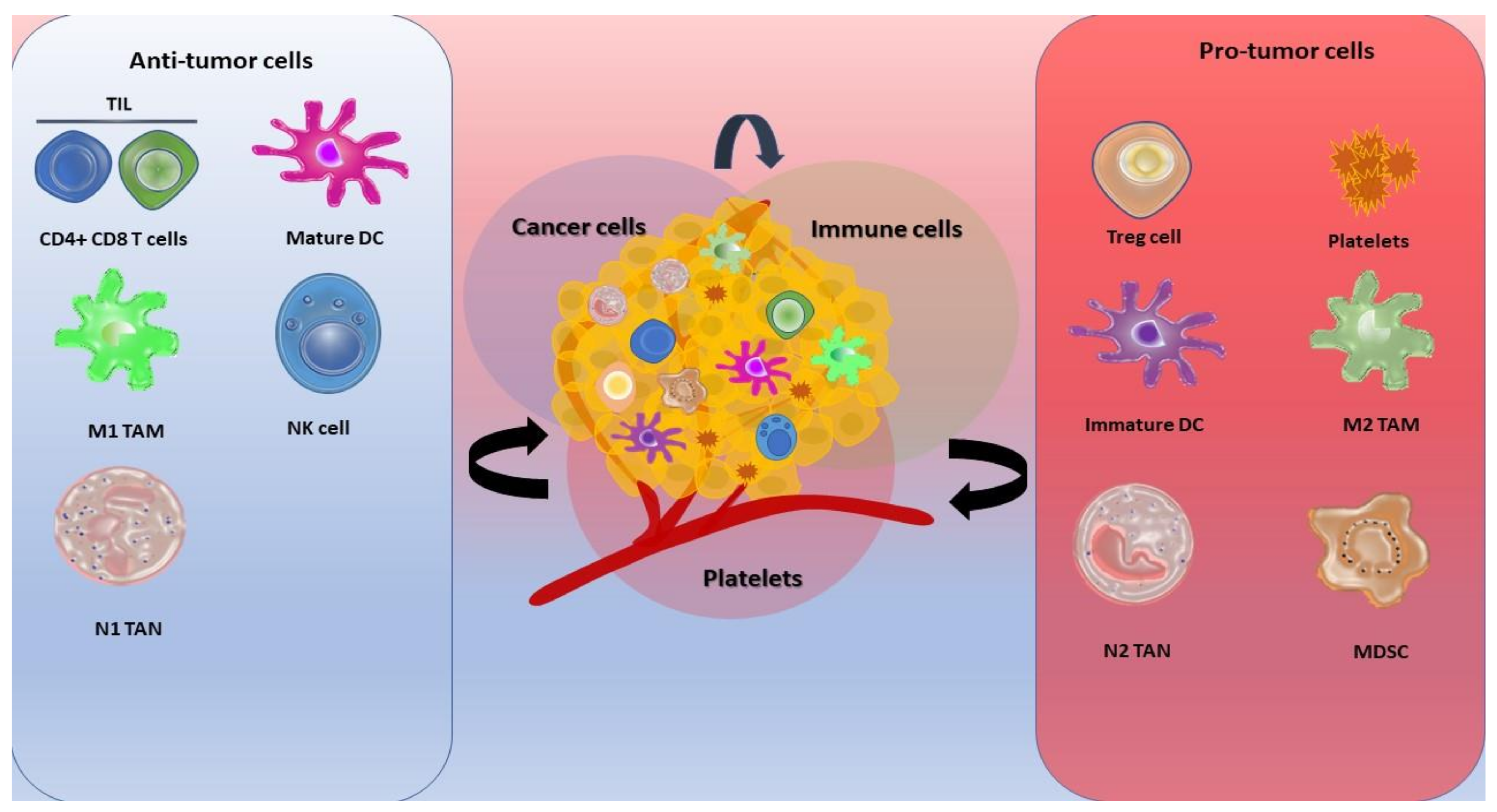
| Author/Year | Histology | Stage | Method | Impact | Findings |
|---|---|---|---|---|---|
| Webb et al., 2014 [253] | EOC | I-III | IHC, FC | Beneficial | High density of CD103+ NK cells correlated with favorable disease outcome. |
| Henriksen et al., 2020 [261] | EOC | n.a | FC | Detrimental | A decreased level of NK cell count in recurrent metastasis during chemotherapy is associated with unfavorable prognostic impact. |
| Henriksen et al., 2020 [245] | HGSOC | I-IV | IHC | Beneficial | High density of CD57+ NK cells correlated with favorable OS. |
| Krockenberger et al., 2008 [110] | EOC | n.a | IHC, IF | Detrimental | High density of MIF inhibits NK cells and correlated with poor prognosis |
| Hoogstad-van Evert et al., 2018 [260] | HGSOC | III-IV | FC | Beneficial | High density of CD103+ NK cells correlated with favorable disease outcome. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pankowska, K.A.; Będkowska, G.E.; Chociej-Stypułkowska, J.; Rusak, M.; Dąbrowska, M.; Osada, J. Crosstalk of Immune Cells and Platelets in an Ovarian Cancer Microenvironment and Their Prognostic Significance. Int. J. Mol. Sci. 2023, 24, 9279. https://doi.org/10.3390/ijms24119279
Pankowska KA, Będkowska GE, Chociej-Stypułkowska J, Rusak M, Dąbrowska M, Osada J. Crosstalk of Immune Cells and Platelets in an Ovarian Cancer Microenvironment and Their Prognostic Significance. International Journal of Molecular Sciences. 2023; 24(11):9279. https://doi.org/10.3390/ijms24119279
Chicago/Turabian StylePankowska, Katarzyna Aneta, Grażyna Ewa Będkowska, Joanna Chociej-Stypułkowska, Małgorzata Rusak, Milena Dąbrowska, and Joanna Osada. 2023. "Crosstalk of Immune Cells and Platelets in an Ovarian Cancer Microenvironment and Their Prognostic Significance" International Journal of Molecular Sciences 24, no. 11: 9279. https://doi.org/10.3390/ijms24119279
APA StylePankowska, K. A., Będkowska, G. E., Chociej-Stypułkowska, J., Rusak, M., Dąbrowska, M., & Osada, J. (2023). Crosstalk of Immune Cells and Platelets in an Ovarian Cancer Microenvironment and Their Prognostic Significance. International Journal of Molecular Sciences, 24(11), 9279. https://doi.org/10.3390/ijms24119279





