Pre-Existing Intrarenal Parvovirus B19 Infection May Relate to Antibody-Mediated Rejection in Pediatric Kidney Transplant Patients
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
2.2. Histological Analysis
2.3. Viral Infection
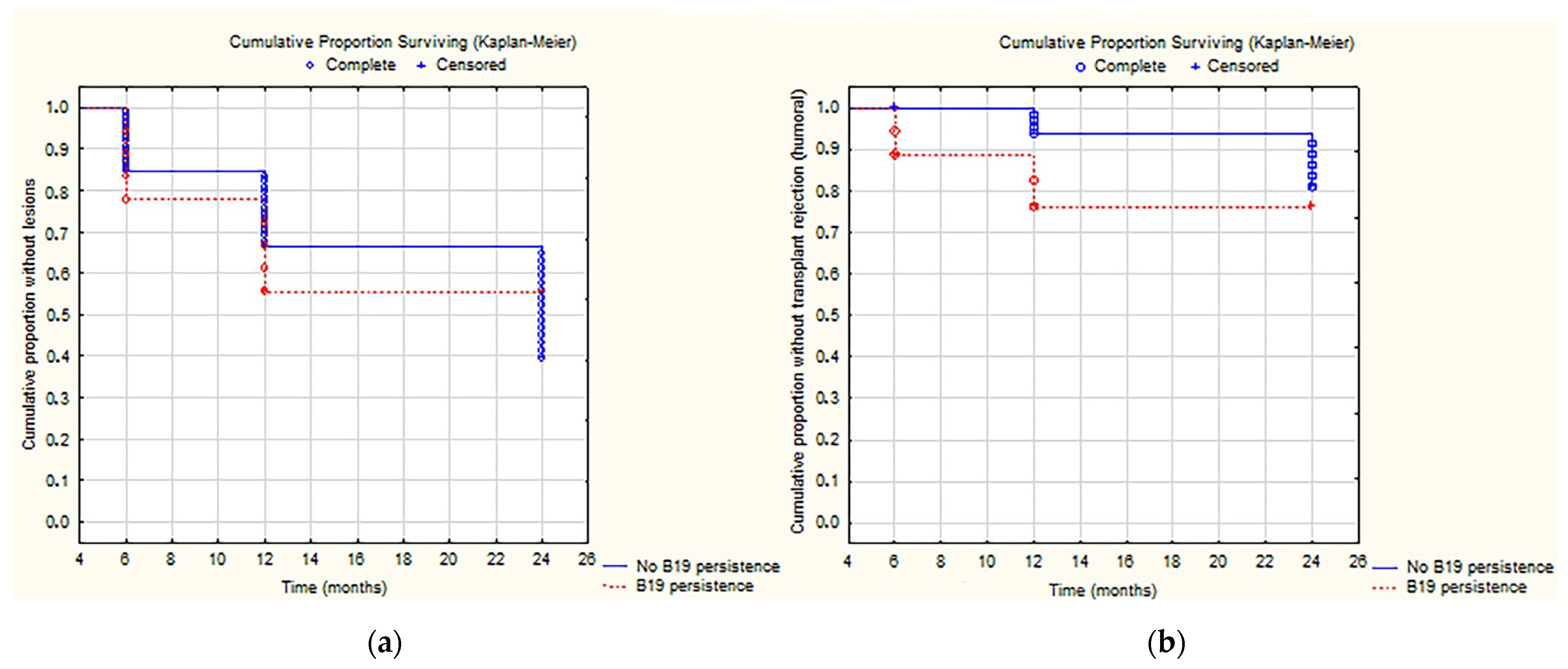
2.4. Correlation between Rejection and Viral Infection
2.5. In Situ Hybridization
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Histological Data
4.3. Virological Data
4.4. Detection of Viral Nucleic Acids in Allograft Biopsy Specimens
4.5. Donor-Specific Antibodies (DSA)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, J.M.; Dharnidharka, V.R. Viral surveillance and subclinical viral infection in pediatric kidney transplantation. Pediatr. Nephrol. 2015, 30, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Cukuranovic, J.; Ugrenovic, S.; Jovanovic, I.; Visnjic, M.; Stefanovic, V. Viral Infection in Renal Transplant Recipients. Sci. World J. 2012, 2012, 820621. [Google Scholar] [CrossRef] [PubMed]
- Pullerits, K.; Garland, S.; Rengarajan, S.; Guiver, M.; Chinnadurai, R.; Middleton, R.J.; Chukwu, C.A.; Kalra, P.A. Kidney Transplant-Associated Viral Infection Rates and Outcomes in a Single-Centre Cohort. Viruses 2022, 14, 2406. [Google Scholar] [CrossRef]
- Weikert, B.C.; Blumberg, E.A. Viral infection after renal transplantation: Surveillance and management. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 2), S76–S86. [Google Scholar] [CrossRef]
- Pour-Reza-Gholi, F.; Labibi, A.; Farrokhi, F.; Nafar, M.; Firouzan, A.; Einollahi, B. Signs and Symptoms of Cytomegalovirus Disease in Kidney Transplant Recipients. Transplant. Proc. 2005, 37, 3056–3058. [Google Scholar] [CrossRef]
- Kamal, M.; Govil, A.; Anand, M.; Abu Jawdeh, B.G.; Shah, S. Severe BK polyomavirus-induced hemorrhagic cystitis in a kidney transplant recipient with the absence of renal allograft involvement. Transpl. Infect. Dis. 2018, 20, e12814. [Google Scholar] [CrossRef]
- Barzon, L.; Murer, L.; Pacenti, M.; Biasolo, M.A.; Della Vella, M.; Ghirardo, G.; Gamba, P.; De Arias, A.E.; Zanon, G.F.; Palù, G. Detection of Viral DNA in kidney graft preservation and washing solutions Is Predictive of Posttransplant Infections in Pediatric Recipients. J. Infect. Dis. 2009, 200, 1425–1433. [Google Scholar] [CrossRef]
- Ambalathingal, G.R.; Francis, R.S.; Smyth, M.J.; Smith, C.; Khanna, R. BK Polyomavirus: Clinical Aspects, Immune Regulation, and Emerging Therapies. Clin. Microbiol. Rev. 2017, 30, 503–528. [Google Scholar] [CrossRef]
- Park, W.Y.; Kang, S.S.; Jin, K.; Park, S.B.; Choe, M.; Han, S. Long-term prognosis of BK virus-associated nephropathy in kidney transplant recipients. Kidney Res. Clin. Pract. 2018, 37, 167–173. [Google Scholar] [CrossRef]
- Dharnidharka, V.R.; Araya, C.E. Post-transplant lymphoproliferative disease. Pediatr. Nephrol. 2009, 24, 731–736. [Google Scholar] [CrossRef]
- Sprangers, B.; Riella, L.V.; Dierickx, D. Posttransplant Lymphoproliferative Disorder Following Kidney Transplantation: A Review. Am. J. Kidney Dis. 2021, 78, 272–281. [Google Scholar] [CrossRef]
- Reinke, P.; Fietze, E.; Ode-Hakim, S.; Prösch, S.; Lippert, J.; Ewert, R.; Volk, H.D. Late-acute renal allograft rejection and symptomless cytomegalovirus infection. Lancet 1994, 344, 1737–1738. [Google Scholar] [CrossRef] [PubMed]
- Sawinski, D.; Forde, K.A.; Trofe-Clark, J.; Patel, P.; Olivera, B.; Goral, S.; Bloom, R.D. Persistent BK viremia Does Not Increase Intermediate-Term Graft Loss but Is Associated with De Novo Donor-Specific Antibodies. J. Am. Soc. Nephrol. 2015, 26, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Babel, N.; Schwarzmann, F.; Prang, N.; Jaeger, M.; Wolf, H.; Kern, F.; Volk, H.D.; Reinke, P. Association between Epstein-Barr virus infection and Late Acute Transplant Rejection in Long-Term Transplant Patients. Transplantation 2001, 72, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bajwa, R. Parvovirus Infection-Related Anemia after Kidney Transplantation. Case Rep. Transplant. 2020, 2020, 6437392. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Khoury, N.J. Epidemiology of parvovirus B19 and anemia among kidney transplant recipients: A meta-analysis. Urol. Ann. 2020, 12, 241–247. [Google Scholar] [CrossRef]
- Waldman, M.; Kopp, J.B. Parvovirus B19 and the Kidney. Clin. J. Am. Soc. Nephrol. 2007, 2 (Suppl. 1), S47–S56. [Google Scholar] [CrossRef]
- Barzon, L.; Murer, L.; Pacenti, M.; Biasolo, M.A.; Della Vella, M.; Benetti, E.; Zanon, G.F.; Palù, G. Investigation of intrarenal viral Infections in Kidney Transplant Recipients Unveils an Association between Parvovirus B19 and Chronic Allograft Injury. J. Infect. Dis. 2009, 199, 372–380. [Google Scholar] [CrossRef]
- Eid, A.J.; Brown, R.A.; Patel, R.; Razonable, R.R. Parvovirus B19 infection after Transplantation: A Review of 98 Cases. Clin. Infect. Dis. 2006, 43, 40–48. [Google Scholar] [CrossRef]
- Jordan, S.C.; Toyoda, M.; Kahwaji, J.; Vo, A.A. Clinical Aspects of Intravenous Immunoglobulin Use in Solid Organ Transplant Recipients. Am. J. Transplant. 2011, 11, 196–202. [Google Scholar] [CrossRef]
- Puliyanda, D.P.; Jordan, S.C.; Kim, I.K.; Patel, M.; Murthy, A.; Huang, E.; Zhang, X.; Reinsmoen, N.; Kamil, E.S.; Toyoda, M. Use of Rituximab for persistent EBV DNAemia, and Its effect on donor-specific antibody development in pediatric renal transplant recipients: A case series. Pediatr. Transplant. 2021, 25, e14113. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.J.; Kuten, S.; Knight, R.J.; A Graviss, E.; Nguyen, D.; Gaber, A.O. Incidence and Factors Associated with De Novo DSA After BK Viremia in Renal Transplant Recipients. Clin. transplants 2016, 32, 103–109. [Google Scholar]
- Murer, L.; Zacchello, G.; Bianchi, D.; Dall’Amico, R.; Montini, G.; Andreetta, B.; Perini, M.; Dossi, E.C.G.A.; Zanon, G.; Zacchello, F. Thrombotic Microangiopathy Associated with Parvovirus B 19 Infection after Renal Transplantation. J. Am. Soc. Nephrol. 2000, 11, 1132–1137. [Google Scholar] [CrossRef]
- von Kietzell, K.; Pozzuto, T.; Heilbronn, R.; Grössl, T.; Fechner, H.; Weger, S. Antibody-mediated Enhancement of Parvovirus B19 Uptake into Endothelial Cells Mediated by a Receptor for Complement Factor C1q. J. Virol. 2014, 88, 8102–8115. [Google Scholar] [CrossRef]
- Tzang, B.-S.; Tsai, C.-C.; Chiu, C.-C.; Shi, J.-Y.; Hsu, T.-C. Up-regulation of adhesion molecule expression and induction of TNF-α on vascular endothelial cells by antibody against human parvovirus B19 VP1 unique region protein. Clin. Chim. Acta 2008, 395, 77–83. [Google Scholar] [CrossRef]
- Zhou, F. Molecular Mechanisms of IFN-γ to Up-Regulate MHC Class I Antigen Processing and Presentation. Int. Rev. Immunol. 2009, 28, 239–260. [Google Scholar] [CrossRef]
- Dhanda, S.K.; Vir, P.; Raghava, G.P. Designing of interferon-gamma inducing MHC class-II binders. Biol. Direct 2013, 8, 30. [Google Scholar] [CrossRef]
- Raemer, P.C.; Haemmerling, S.; Giese, T.; Canaday, D.H.; Katus, H.A.; Dengler, T.J.; Shankar, S.G.V. Endothelial Progenitor Cells Possess Monocyte-like Antigen-presenting and T-cell-Co-stimulatory Capacity. Transplantation 2009, 87, 340–349, Erratum in Transplantation 2010, 90, 694. [Google Scholar] [CrossRef]
- Abrahimi, P.; Qin, L.; Chang, W.G.; Bothwell, A.L.; Tellides, G.; Saltzman, W.M.; Pober, J.S. Blocking MHC class II on human endothelium mitigates acute rejection. J. Clin. Investig. 2016, 1, e85293. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, C.; Lyu, J.; Wu, X.; Wang, R.; Huang, H.; Wu, J.; Chen, J.; Peng, W. Donor-Derived Human Parvovirus B19 Infection in Kidney Transplantation. Front. Cell. Infect. Microbiol. 2021, 11, 753970. [Google Scholar] [CrossRef]
- Eid, A.J.; Ardura, M.I. The AST Infectious Diseases Community of Practice Human parvovirus B19 in solid organ transplantation: Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019, 33, e13535. [Google Scholar] [CrossRef] [PubMed]
- Crabol, Y.; Terrier, B.; Rozenberg, F.; Pestre, V.; Legendre, C.; Hermine, O.; Montagnier-Petrissans, C.; Guillevin, L.; Mouthon, L.; Loïc, G.; et al. Intravenous immunoglobulin Therapy for Pure Red Cell Aplasia Related to Human Parvovirus B19 Infection: A Retrospective Study of 10 Patients and Review of the Literature. Clin. Infect. Dis. 2013, 56, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Ruenroengbun, N.; Sapankaew, T.; Chaiyakittisopon, K.; Phoompoung, P.; Ngamprasertchai, T. Efficacy and Safety of Antiviral Agents in Preventing Allograft Rejection Following CMV Prophylaxis in High-Risk Kidney Transplantation: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol. 2022, 12, 865735. [Google Scholar] [CrossRef] [PubMed]
- Aiello, F.B.; Calabrese, F.; Rigotti, P.; Furian, L.; Marino, S.; Cusinato, R.; Valente, M. Acute rejection and graft survival in renal transplanted patients with viral diseases. Mod. Pathol. 2004, 17, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-L.; Wu, B.-S.; Lien, T.-J.; Yang, A.-H.; Yang, C.-Y. BK Polyomavirus Nephropathy in Kidney Transplantation: Balancing Rejection and Infection. Viruses 2021, 13, 487. [Google Scholar] [CrossRef]
- Cotiguala, L.; Masood, A.; Park, J.M.; Samaniego-Picota, M.D.; Kaul, D.R.; Naik, A.S. Increasing net immunosuppression after BK polyoma virus infection. Transpl. Infect. Dis. 2021, 23, e13472. [Google Scholar] [CrossRef]
- Dieplinger, G.; Everly, M.; Briley, K.; Haisch, C.; Bolin, P.; Maldonado, A.; Kendrick, W.; Kendrick, S.; Morgan, C.; Terasaki, P.; et al. Onset and progression of de novo donor-specific anti-human leukocyte antigen antibodies after BK polyomavirus and preemptive immunosuppression reduction. Transpl. Infect. Dis. 2015, 17, 848–858. [Google Scholar] [CrossRef]
- Cheungpasitporn, W.; Kremers, W.K.; Lorenz, E.; Amer, H.; Cosio, F.G.; Stegall, M.D.; Gandhi, M.J.; Schinstock, C.A. De novo donor-specific antibody following BK nephropathy: The incidence and association with antibody-mediated rejection. Clin. Transplant. 2018, 32, e13194. [Google Scholar] [CrossRef]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Mian, A.N.; Schwartz, G.J. Measurement and Estimation of Glomerular Filtration Rate in Children. Adv. Chronic Kidney Dis. 2017, 24, 348–356. [Google Scholar] [CrossRef]
- Pirsch, J.; Bekersky, I.; Vincenti, F.; Boswell, G.; Woodle, E.S.; Alak, A.; Kruelle, M.; Fass, N.; Facklam, D.; Mekki, Q. Coadministration of Tacrolimus and Mycophenolate Mofetil in Stable Kidney Transplant Patients: Pharmacokinetics and Tolerability. J. Clin. Pharmacol. 2000, 40, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.D.; Dmitrewski, J. Multicenter randomized trial comparing tacrolimus (FK506) and cyclosporine in the prevention of renal allograft rejection: A report of the Europe an Tacrolimus Multicenter Renal Study Group. Transplantation 1997, 64, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Pirsch, J.D.; Miller, J. A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation: FK506 Kidney Transplant Study Group. Transplantation 1997, 63, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Montini, G.; Murer, L.; Ghio, L.; Pietrobon, B.; Ginevri, F.; Ferraresso, M.; Cardillo, M.; Scalamogna, M.; Perfumo, F.; Edefonti, A.; et al. One-year results of basiliximab induction and tacrolimus associated with sequential steroid and MMF treatment in pediatric kidney transplant recipient. Transpl. Int. 2005, 18, 36–42. [Google Scholar] [CrossRef]
- Brakemeier, S.; Pfau, A.; Zukunft, B.; Budde, K.; Nickel, P. Prophylaxis and treatment of Pneumocystis Jirovecii pneumonia after solid organ transplantation. Pharmacol. Res. 2018, 134, 61–67. [Google Scholar] [CrossRef]
- Rodrigo, E.; Chedid, M.F.; Segundo, D.S.; Millán, J.C.S.; López-Hoyos, M. Acute Rejection Following Kidney Transplantation: State-of-the-Art and Future Perspectives. Curr. Pharm. Des. 2020, 26, 3468–3496. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. Special Issue: KDIGO clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am. J. Transplant. 2009, 9, S1–S155. [CrossRef]
- Liu, W.; Ittmann, M.; Liu, J.; Schoentag, R.; Tierno, P.; Greco, M.; Sidhu, G.; Nierodzik, M.; Wieczorek, R. Human parvovirus B19 in bone marrows from adults with acquired immunodeficiency syndrome: A comparative study using in situ hybridization and immunohistochemistry. Hum. Pathol. 1997, 28, 760–766. [Google Scholar] [CrossRef]
- Yamamoto, T.; Watarai, Y.; Takeda, A.; Tsujita, M.; Hiramitsu, T.; Goto, N.; Narumi, S.; Katayama, A.; Morozumi, K.; Uchida, K.; et al. De Novo Anti-HLA DSA Characteristics and Subclinical Antibody-Mediated Kidney Allograft Injury. Transplantation 2016, 100, 2194–2202. [Google Scholar] [CrossRef]

| Patients enrolled | 106 |
| Age (years) | 11 (r. 5–16) |
| Weight kg | 26.9 ± 20.72 (r. 15.2–46.9) |
| Sex | |
| Male | 67 (63.2%) |
| Female | 39 (36.8%) |
| Donor | |
| Living | 30 (28.3%) |
| Not living | 76 (71.7%) |
| Kidney Transplant | |
| First | 87 |
| Second | 18 |
| D/R weight ratio kg | 1.7 (r. 1.0–3.4) |
| (D/R) Mismatch HLA | 3 (r. 3–4) |
| BIOPSIES | 6 Months | 12 Months | 24 Months | Total |
|---|---|---|---|---|
| Banff1 | 72 | 54 | 27 | 153 |
| ABMR | 8 | 6 | 2 | 16 |
| TCMR | 10 | 10 | 16 | 36 |
| IF/TA | 2 | 2 | 5 | 9 |
| Other diagnosis | 0 | 1 | 3 | 4 |
| Total | 92 | 73 | 53 | 218 |
| Detection of Viral DNA on Blood | Biopsies Results | p-Value | |
|---|---|---|---|
| 6 Months of Follow-Up | |||
| Banff1 | TCMR–ABMR–IF/TA | ||
| Total | 72 (78%) | 20 (22%) | |
| Any virus | 23 (32%) | 1 (5%) | 0.02 |
| CMV | 3 (4%) | 0 (0%) | 1 |
| EBV | 8 (11%) | 1 (5%) | 0.7 |
| BKV | 11 (15%) | 0 (0%) | 0.1 |
| Parvovirus B19 | 3 (4%) | 0 (0%) | 1 |
| 12 Months of Follow-Up | |||
| Total | 54 (75%) | 18 (25%) | |
| Any virus | 14 (26%) | 5 (28%) | 1 |
| CMV | 1 (2%) | 0 (0%) | 1 |
| EBV | 9 (17%) | 2 (11%) | 0.7 |
| BKV | 7 (13%) | 3 (17%) | 0.7 |
| Parvovirus B19 | 1 (2%) | 0 (0%) | 1 |
| 24 Months of Follow-Up | |||
| Total | 27 (54%) | 23 (46%) | |
| Any virus | 4 (15%) | 9 (39%) | 0.6 |
| CMV | 0 (0%) | 0 (0%) | 1 |
| EBV | 2 (7%) | 5 (22%) | 0.2 |
| BKV | 3 (11%) | 5 (22%) | 0.5 |
| Parvovirus B19 | 0 (0%) | 0 (0%) | 1 |
| Detection of Viral DNA on Bioptic Sample | Biopsies Results | p-Value | |
|---|---|---|---|
| 6 Months of Follow-Up | |||
| Banff1 | TCMR–ABMR–IF/TA | ||
| Total | 72 (78%) | 20 (22%) | |
| Any virus | 17 (24%) | 5 (25%) | 1 |
| CMV | 0 (0%) | 0 (0%) | 1 |
| EBV | 6 (8%) | 1 (5%) | 1 |
| BKV | 6 (8%) | 1 (5%) | 1 |
| Parvovirus B19 | 17 (24%) | 6 (30%) | 0.6 |
| 12 Months of Follow-Up | |||
| Total | 54 (75%) | 18 (25%) | |
| Any virus | 22 (41%) | 10 (58%) | 0.3 |
| CMV | 1 (1%) | 0 (0%) | 1 |
| EBV | 6 (11%) | 3 (17%) | 0.7 |
| BKV | 3 (6%) | 2 (11%) | 0.6 |
| Parvovirus B19 | 14 (26%) | 7 (39%) | 0.4 |
| 24 Months of Follow-Up | |||
| Total | 27 (54%) | 23 (46%) | |
| Any virus | 10 (37%) | 11 (48%) | 0.6 |
| CMV | 0 (0%) | 0 (0%) | 1 |
| EBV | 6 (22%) | 7 (30%) | 0.5 |
| BKV | 1 (4%) | 4 (17%) | 0.2 |
| Parvovirus B19 | 4 (15%) | 3 (13%) | 1 |
| Detection of Viral DNA on Blood | TCMR (n = 36) | AMBR (n = 16) | p-Value |
| Any virus | 7 (19%) | 4 (25%) | 0.7 |
| CMV | 0 (0%) | 0 (0%) | 1 |
| EBV | 6 (17%) | 2 (13%) | 1 |
| BKV | 2 (5%) | 3 (19%) | 0.16 |
| Parvovirus B19 | 0 (0%) | 0 (0%) | 1 |
| Detection of Viral DNA on Histological Sample | TCMR (n = 36) | AMBR (n = 16) | p-Value |
| Any virus | 13 (36%) | 11 (69%) | p < 0.05 |
| CMV | 0 (0%) | 0 (0%) | 1 |
| EBV | 6 (17%) | 2 (13%) | 1 |
| BKV | 3 (8%) | 2 (13%) | 0.6 |
| Parvovirus B19 | 7 (19%) | 8 (50%) | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertazza Partigiani, N.; Negrisolo, S.; Carraro, A.; Marzenta, D.; Manaresi, E.; Gallinella, G.; Barzon, L.; Benetti, E. Pre-Existing Intrarenal Parvovirus B19 Infection May Relate to Antibody-Mediated Rejection in Pediatric Kidney Transplant Patients. Int. J. Mol. Sci. 2023, 24, 9147. https://doi.org/10.3390/ijms24119147
Bertazza Partigiani N, Negrisolo S, Carraro A, Marzenta D, Manaresi E, Gallinella G, Barzon L, Benetti E. Pre-Existing Intrarenal Parvovirus B19 Infection May Relate to Antibody-Mediated Rejection in Pediatric Kidney Transplant Patients. International Journal of Molecular Sciences. 2023; 24(11):9147. https://doi.org/10.3390/ijms24119147
Chicago/Turabian StyleBertazza Partigiani, Nicola, Susanna Negrisolo, Andrea Carraro, Diana Marzenta, Elisabetta Manaresi, Giorgio Gallinella, Luisa Barzon, and Elisa Benetti. 2023. "Pre-Existing Intrarenal Parvovirus B19 Infection May Relate to Antibody-Mediated Rejection in Pediatric Kidney Transplant Patients" International Journal of Molecular Sciences 24, no. 11: 9147. https://doi.org/10.3390/ijms24119147
APA StyleBertazza Partigiani, N., Negrisolo, S., Carraro, A., Marzenta, D., Manaresi, E., Gallinella, G., Barzon, L., & Benetti, E. (2023). Pre-Existing Intrarenal Parvovirus B19 Infection May Relate to Antibody-Mediated Rejection in Pediatric Kidney Transplant Patients. International Journal of Molecular Sciences, 24(11), 9147. https://doi.org/10.3390/ijms24119147







