Infection-Related Cryoglobulinemic Glomerulonephritis with Serum Anti-Factor B Antibodies Identified and Staining for NAPlr/Plasmin Activity Due to Infective Endocarditis
Abstract
1. Introduction
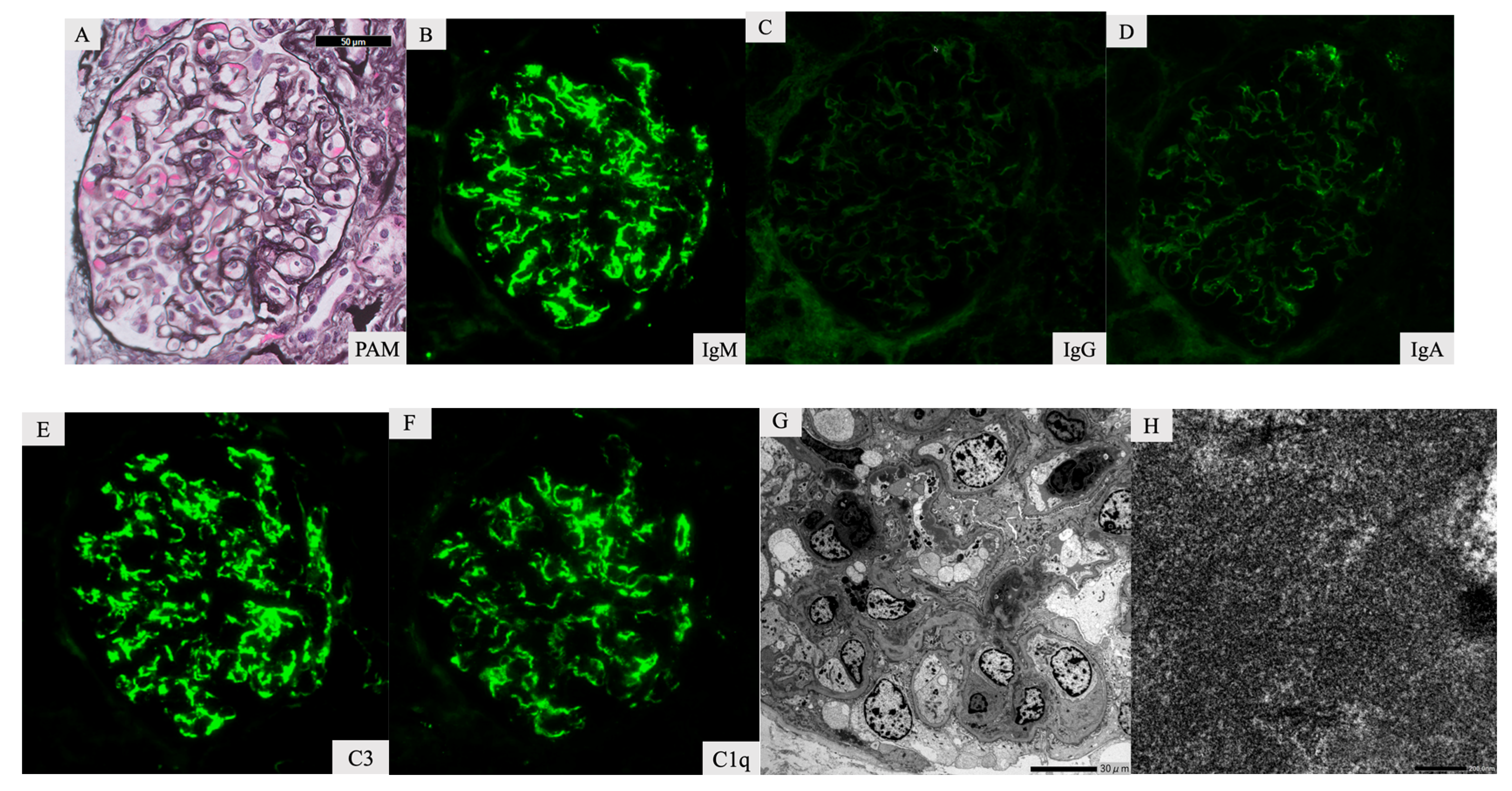
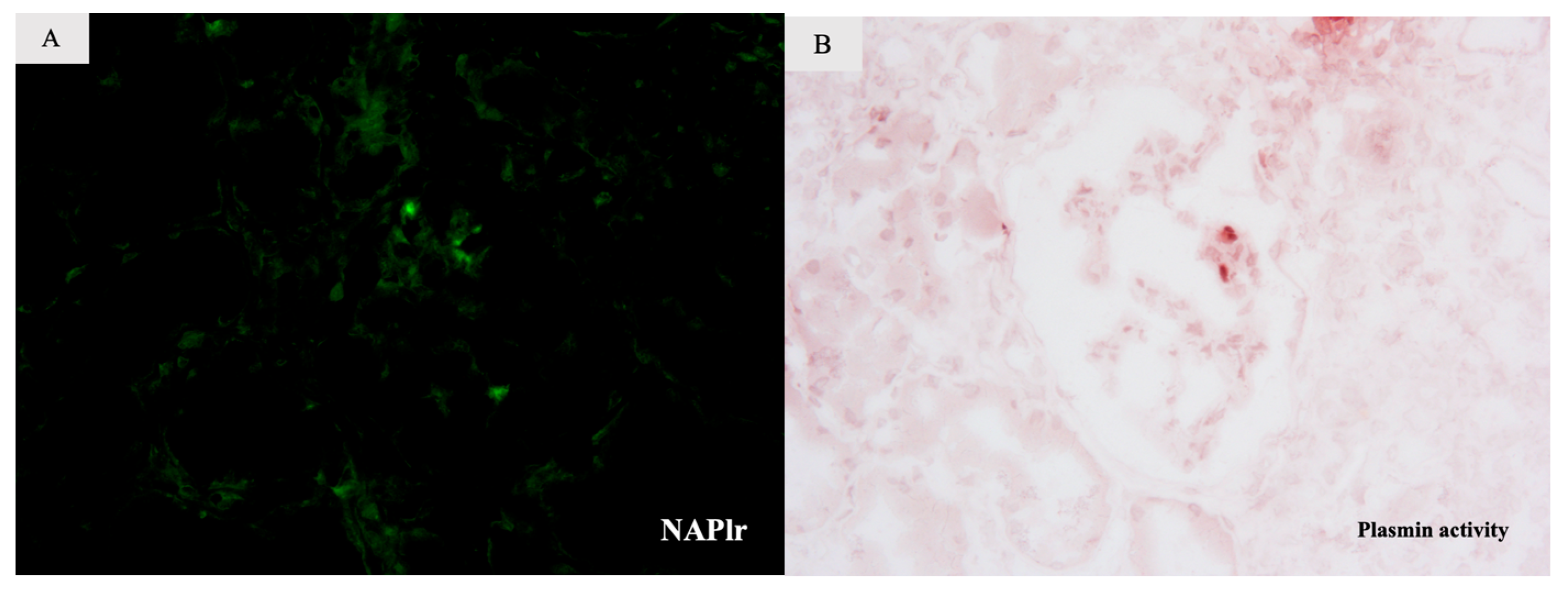
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Nasr, S.H.; Radhakrishnan, J.; D’Agati, V.D. Bacterial infection-related glomerulonephritis in adults. Kidney Int. 2013, 83, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, M.A.; Chang, A. Infection-Related Glomerulonephritis. Glomerular Dis. 2021, 1, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, N.; Yamada, M.; Fujino, M.; Oda, T. Nephritis-associated plasmin receptor (NAPlr): An essential inducer of C3-dominant glomerular injury and a potential key diagnostic biomarker of infection-related glomerulonephritis (IRGN). Int. J. Mol. Sci. 2022, 23, 9974. [Google Scholar] [CrossRef] [PubMed]
- Chauvet, S.; Berthaud, R.; Devriese, M.; Mignotet, M.; Martins, P.V.; Robe-Rybkine, T.; Miteva, M.A.; Gyulkhandanyan, A.; Ryckewaert, A.; Louillet, F.; et al. Anti-factor B antibodies and acute postinfectious GN in children. J. Am. Soc. Nephrol. 2020, 31, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Stone, J.H.; Cid, M.C.; Bosch, X. The cryoglobulinaemias. Lancet 2012, 379, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, M.A.; Lassman, C.R. Hepatitis C-associated cryoglobulinemic glomerulonephritis with crystalline deposits. Am. J. Kidney Dis. 2013, 62, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Yoshizawa, N.; Yamakami, K.; Tamura, K.; Kuroki, A.; Sugisaki, T.; Sawanobori, E.; Higashida, K.; Ohtomo, Y.; Hotta, O.; et al. Localization of nephritis-associated plasmin receptor in acute poststreptococcal glomerulonephritis. Hum. Pathol. 2010, 41, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Vivekanantham, A.; Patel, R.; Jenkins, P.; Cleary, G.; Porter, D.; Khawaja, F.; McCarthy, E. A “cat”-astrophic case of Bartonella infective endocarditis causing secondary cryoglobulinemia: A case report. BMC Rheumatol. 2022, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Babiker, A.; El Hag, M.I.; Perez, C. Bartonella infectious endocarditis associated with cryoglobulinemia and multifocal proliferative glomerulonephritis. Open Forum Infect. Dis. 2018, 5, ofy186. [Google Scholar] [CrossRef] [PubMed]
- Guinault, D.; Ribes, D.; Delas, A.; Milongo, D.; Abravanel, F.; Puissant-Lubrano, B.; Izopet, J.; Kamar, N. Hepatitis E virus-induced cryoglobulinemic glomerulonephritis in a non-immunocompromised person. Am. J. Kidney Dis. 2016, 67, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, M.A.; Al-Rabadi, L.; Chalasani, M.; Smith, M.; Kakani, S.; Revelo, M.P.; Meehan, S.M. Staphylococcal infection-related glomerulonephritis with cryoglobulinemic features. Kidney Int. Rep. 2018, 3, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Yoshizawa, N.; Yamakami, K.; Sakurai, Y.; Takechi, H.; Yamamoto, K.; Oshima, N.; Kumagai, H. The role of nephritis-associated plasmin receptor (NAPlr) in glomerulonephritis associated with streptococcal infection. J. Biomed. Biotechnol. 2012, 2012, 417675. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Oda, T. Glomerular deposition of nephritis-associated plasmin receptor (NAPlr) and related plasmin activity: Key diagnostic biomarkers of bacterial infection-related glomerulonephritis. Int. J. Mol. Sci. 2020, 21, 2595. [Google Scholar] [CrossRef] [PubMed]
- Kohatsu, K.; Suzuki, T.; Yazawa, M.; Yahagi, K.; Ichikawa, D.; Koike, J.; Oda, T.; Shibagaki, Y. Granulomatosis with polyangiitis induced by infection. Kidney Int. Rep. 2018, 4, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Ekbom, A.; Brandt, L.; Askling, J. What is the significance in routine care of c-ANCA/PR3-ANCA in the absence of systemic vasculitis? A case series. Clin. Exp. Rheumatol. 2008, 26 (Suppl. 49), S53–S56. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toishi, T.; Oda, T.; Hamano, A.; Sugihara, S.; Inoue, T.; Kawaji, A.; Nagaoka, K.; Matsunami, M.; Fukuda, J.; Ohara, M.; et al. Infection-Related Cryoglobulinemic Glomerulonephritis with Serum Anti-Factor B Antibodies Identified and Staining for NAPlr/Plasmin Activity Due to Infective Endocarditis. Int. J. Mol. Sci. 2023, 24, 9369. https://doi.org/10.3390/ijms24119369
Toishi T, Oda T, Hamano A, Sugihara S, Inoue T, Kawaji A, Nagaoka K, Matsunami M, Fukuda J, Ohara M, et al. Infection-Related Cryoglobulinemic Glomerulonephritis with Serum Anti-Factor B Antibodies Identified and Staining for NAPlr/Plasmin Activity Due to Infective Endocarditis. International Journal of Molecular Sciences. 2023; 24(11):9369. https://doi.org/10.3390/ijms24119369
Chicago/Turabian StyleToishi, Takumi, Takashi Oda, Atsuro Hamano, Shinnosuke Sugihara, Tomohiko Inoue, Atsuro Kawaji, Kanako Nagaoka, Masatoshi Matsunami, Junko Fukuda, Mamiko Ohara, and et al. 2023. "Infection-Related Cryoglobulinemic Glomerulonephritis with Serum Anti-Factor B Antibodies Identified and Staining for NAPlr/Plasmin Activity Due to Infective Endocarditis" International Journal of Molecular Sciences 24, no. 11: 9369. https://doi.org/10.3390/ijms24119369
APA StyleToishi, T., Oda, T., Hamano, A., Sugihara, S., Inoue, T., Kawaji, A., Nagaoka, K., Matsunami, M., Fukuda, J., Ohara, M., & Suzuki, T. (2023). Infection-Related Cryoglobulinemic Glomerulonephritis with Serum Anti-Factor B Antibodies Identified and Staining for NAPlr/Plasmin Activity Due to Infective Endocarditis. International Journal of Molecular Sciences, 24(11), 9369. https://doi.org/10.3390/ijms24119369




