Synthesis and Evaluation of Clinically Translatable Targeted Microbubbles Using a Microfluidic Device for In Vivo Ultrasound Molecular Imaging
Abstract
1. Introduction
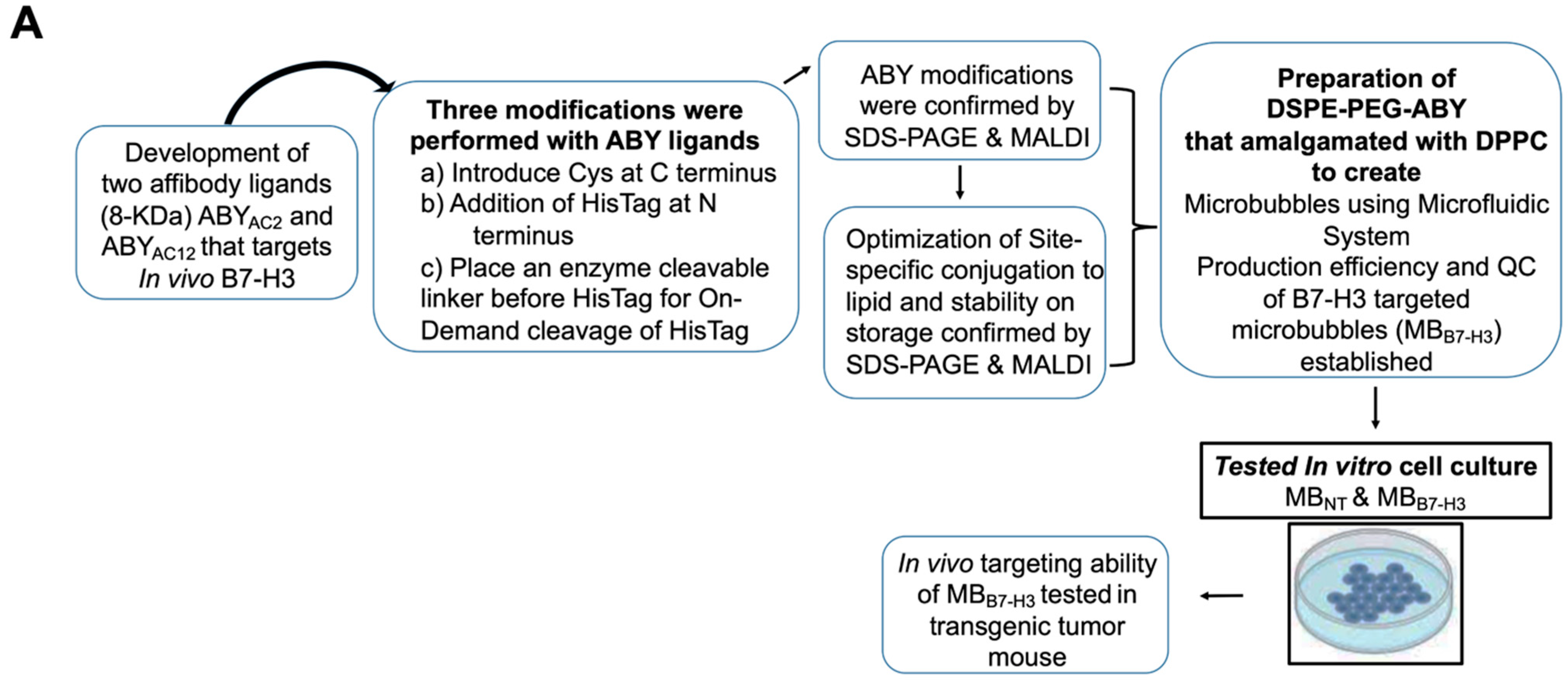
2. Results and Discussion
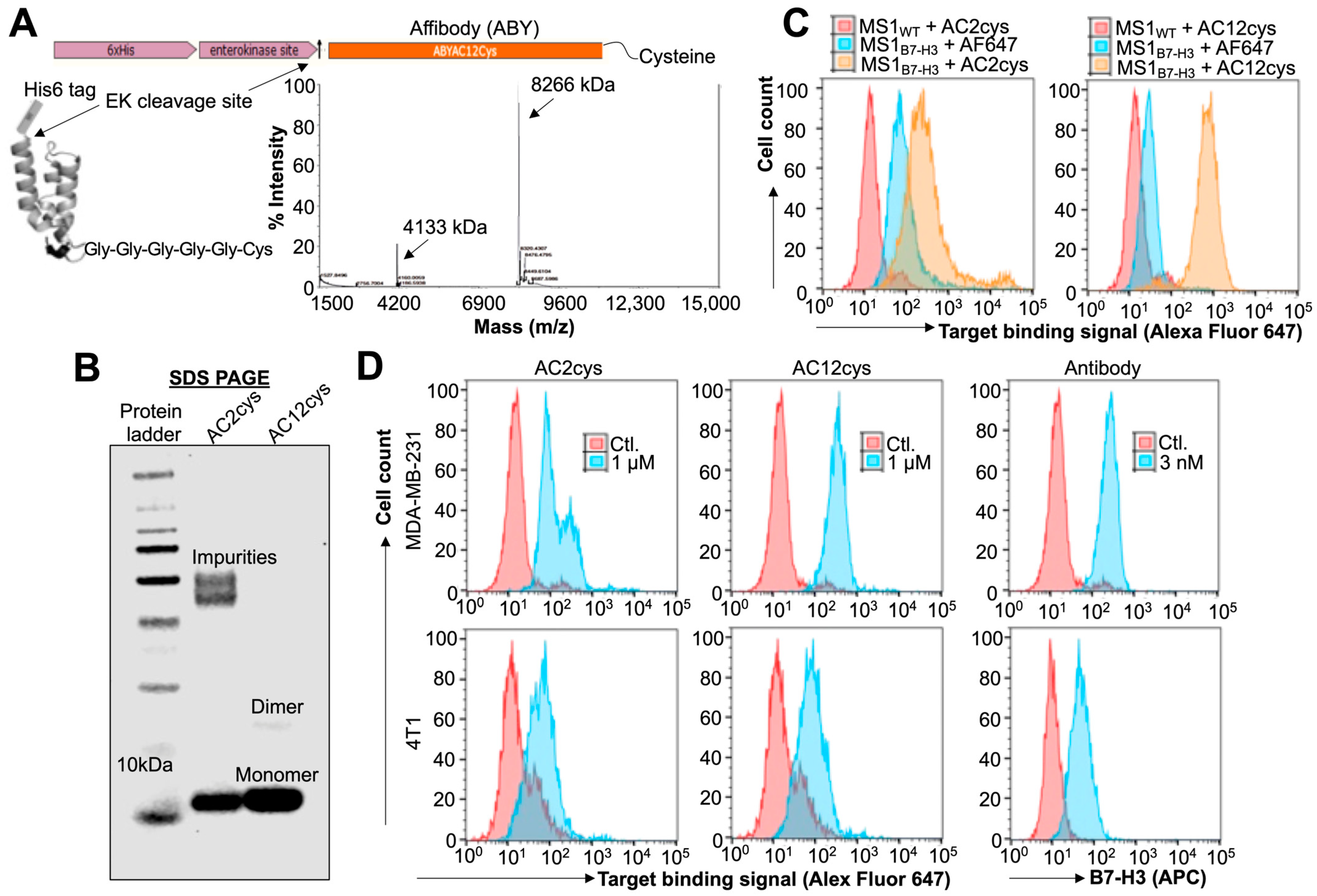
2.1. Expression and Target Binding Assessments of ABY Ligands after Site-Selective Protein Modifications
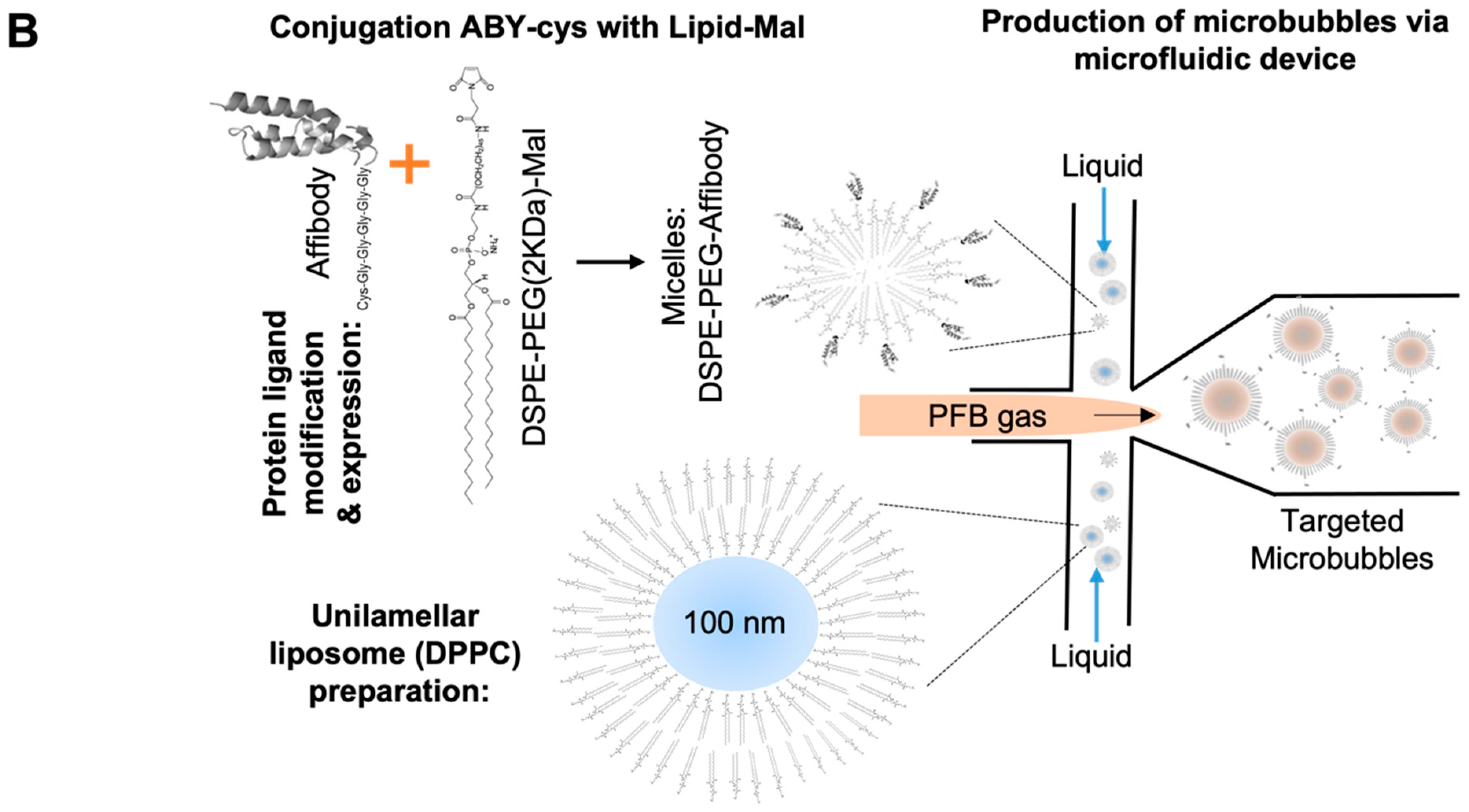
2.2. Bioconjugation Optimization and Stability of the ABY-Phospholipid Conjugates
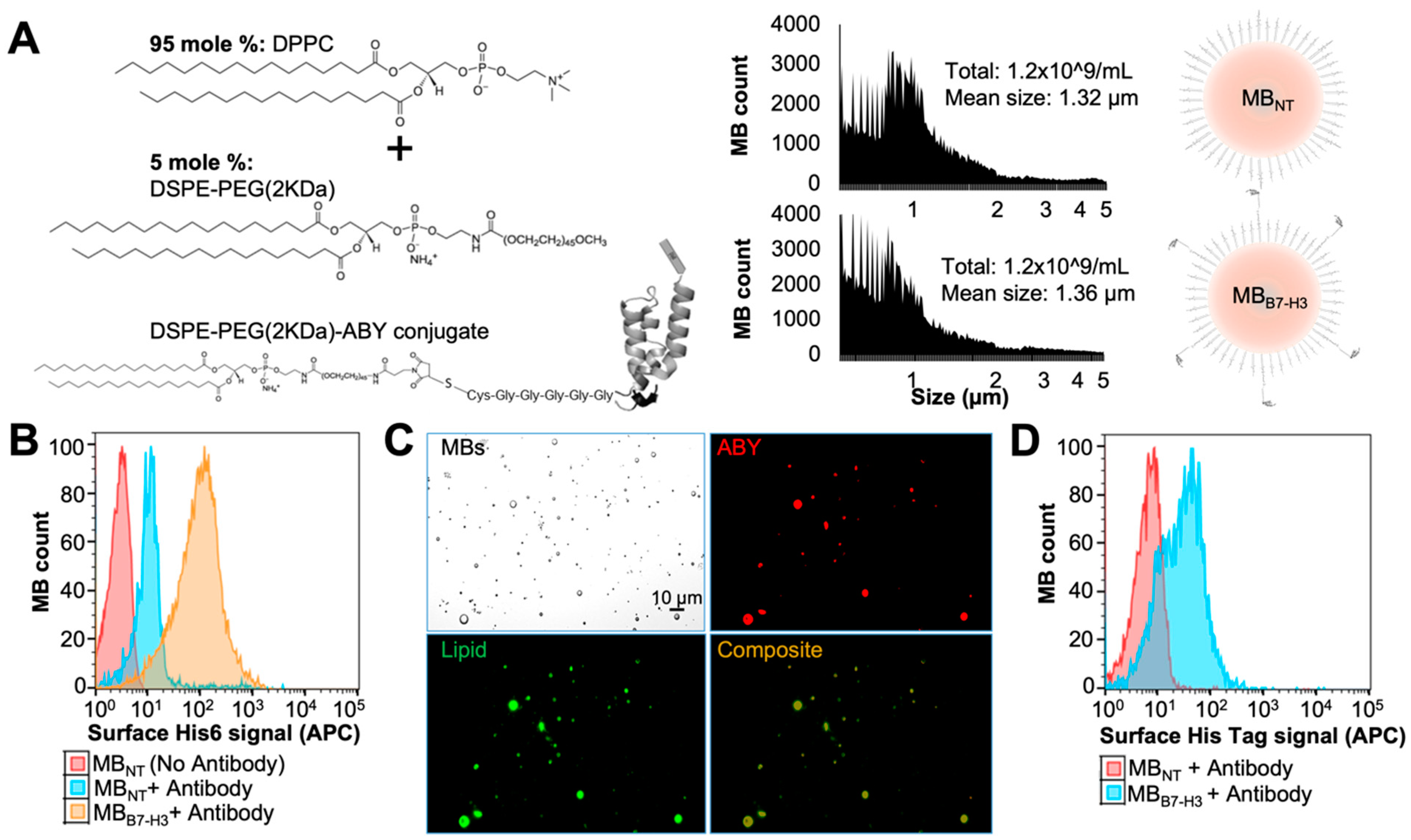
2.3. Preparation of Targeted MBB7-H3 with DSPE-PEG-ABY
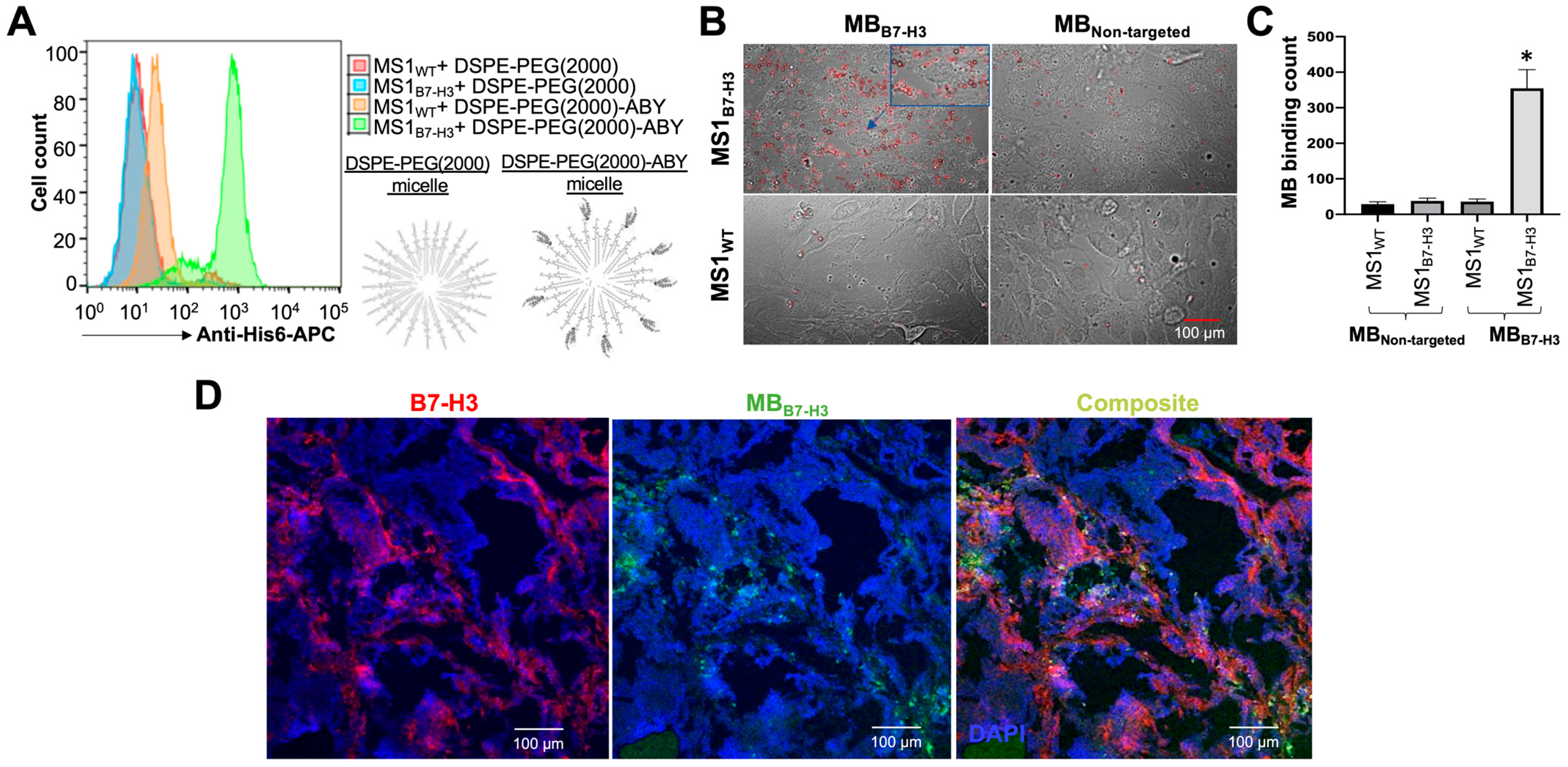
2.4. Binding Validation of MBB7-H3 Targeted to Endothelial Cells In Vitro
2.5. Binding Validation of MBB7-H3 Targeted to Tumor Vascular Endothelial Cells In Vivo
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Cell Culture
3.3. Protein Modification, Expression, and Purification
3.4. Biotinylation of ABY
3.5. His-Tag Removal by Enzymatic Cleavage
3.6. Phospholipid–Ligand Bioconjugation (Figure 1)
3.7. Flow Cytometry Analysis
3.8. Preparation of Targeted Microbubbles by a Microfluidic System
3.9. Validation of ABY Display on Targeted Microbubbles
3.10. In Vitro Binding Assay of MB to B7-H3
3.11. MBs Binding to B7-H3 Target Expressed in Mouse Tumor-Associated Blood Vessels after Intravenous Injection by Ex Vivo Immunostaining Analyses
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klibanov, A.L. Ultrasound Contrast: Gas Microbubbles in the Vasculature. Investig. Radiol. 2021, 56, 50–61. [Google Scholar] [CrossRef]
- Willmann, J.K.; Bonomo, L.; Testa, A.C.; Rinaldi, P.; Rindi, G.; Valluru, K.S.; Petrone, G.; Martini, M.; Lutz, A.M.; Gambhir, S.S. Ultrasound Molecular Imaging With BR55 in Patients With Breast and Ovarian Lesions: First-in-Human Results. J. Clin. Oncol. 2017, 35, 2133–2140. [Google Scholar] [CrossRef]
- Smeenge, M.; Tranquart, F.; Mannaerts, C.K.; de Reijke, T.M.; van de Vijver, M.J.; Laguna, M.P.; Pochon, S.; de la Rosette, J.; Wijkstra, H. First-in-Human Ultrasound Molecular Imaging With a VEGFR2-Specific Ultrasound Molecular Contrast Agent (BR55) in Prostate Cancer: A Safety and Feasibility Pilot Study. Investig. Radiol. 2017, 52, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Talu, E.; Hettiarachchi, K.; Zhao, S.; Powell, R.L.; Lee, A.P.; Longo, M.L.; Dayton, P.A. Tailoring the size distribution of ultrasound contrast agents: Possible method for improving sensitivity in molecular imaging. Mol. Imaging 2007, 6, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, R.H.; Armistead, F.J.; Batchelor, D.V.B.; Johnson, B.R.G.; Peyman, S.A.; Evans, S.D. Horizon: Microfluidic platform for the production of therapeutic microbubbles and nanobubbles. Rev. Sci. Instrum. 2021, 92, 74105. [Google Scholar] [CrossRef] [PubMed]
- Carugo, D.; Browning, R.J.; Iranmanesh, I.; Messaoudi, W.; Rademeyer, P.; Stride, E. Scaleable production of microbubbles using an ultrasound-modulated microfluidic device. J. Acoust. Soc. Am. 2021, 150, 1577. [Google Scholar] [CrossRef] [PubMed]
- Bachawal, S.; Bean, G.R.; Krings, G.; Wilson, K.E. Evaluation of ductal carcinoma in situ grade via triple-modal molecular imaging of B7-H3 expression. NPJ Breast Cancer 2020, 6, 14. [Google Scholar] [CrossRef]
- Mulvana, H.; Browning, R.J.; Luan, Y.; de Jong, N.; Tang, M.-X.; Eckersley, R.J.; Stride, E. Characterization of Contrast Agent Microbubbles for Ultrasound Imaging and Therapy Research. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2017, 64, 232–251. [Google Scholar] [CrossRef]
- Anderson, C.R.; Rychak, J.J.; Backer, M.; Backer, J.; Ley, K.; Klibanov, A.L. scVEGF microbubble ultrasound contrast agents: A novel probe for ultrasound molecular imaging of tumor angiogenesis. Investig. Radiol. 2010, 45, 579–585. [Google Scholar] [CrossRef]
- Yeh, J.S.-M.; Sennoga, C.A.; McConnell, E.; Eckersley, R.; Tang, M.-X.; Nourshargh, S.; Seddon, J.M.; Haskard, D.O.; Nihoyannopoulos, P. A Targeting Microbubble for Ultrasound Molecular Imaging. PLoS ONE 2015, 10, e0129681. [Google Scholar] [CrossRef]
- Bam, R.; Daryaei, I.; Abou-Elkacem, L.; Vilches-Moure, J.G.; Meuillet, E.J.; Lutz, A.; Marinelli, E.R.; Unger, E.C.; Gambhir, S.S.; Paulmurugan, R. Toward the Clinical Development and Validation of a Thy1-Targeted Ultrasound Contrast Agent for the Early Detection of Pancreatic Ductal Adenocarcinoma. Investig. Radiol. 2020, 55, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Punjabi, M.; Xu, L.; Ochoa-Espinosa, A.; Kosareva, A.; Wolff, T.; Murtaja, A.; Broisat, A.; Devoogdt, N.; Kaufmann, B.A. Ultrasound Molecular Imaging of Atherosclerosis With Nanobodies: Translatable Microbubble Targeting Murine and Human VCAM (Vascular Cell Adhesion Molecule) 1. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, J.; Gao, W. Site-selective protein modification with polymers for advanced biomedical applications. Biomaterials 2018, 178, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hong, K.; Kirpotin, D.B.; Colbern, G.; Shalaby, R.; Baselga, J.; Shao, Y.; Nielsen, U.B.; Marks, J.D.; Moore, D.; et al. Anti-HER2 immunoliposomes: Enhanced efficacy attributable to targeted delivery. Clin. Cancer Res. 2002, 8, 1172–1181. [Google Scholar]
- Pillai, R.; Marinelli, E.R.; Fan, H.; Nanjappan, P.; Song, B.; von Wronski, M.A.; Cherkaoui, S.; Tardy, I.; Pochon, S.; Schneider, M.; et al. A phospholipid-PEG2000 conjugate of a vascular endothelial growth factor receptor 2 (VEGFR2)-targeting heterodimer peptide for contrast-enhanced ultrasound imaging of angiogenesis. Bioconjugate Chem. 2010, 21, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Vidanapathirana, S.M.; Greineder, C.F. Site-Specific Modification of Single-Chain Affinity Ligands for Fluorescence Labeling, Radiolabeling, and Bioconjugation. In Peptide Conjugation; Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2355, pp. 163–173. [Google Scholar] [CrossRef]
- Snipstad, S.; Vikedal, K.; Maardalen, M.; Kurbatskaya, A.; Sulheim, E.; Davies, C.d.L. Ultrasound and microbubbles to beat barriers in tumors: Improving delivery of nanomedicine. Adv. Drug Deliv. Rev. 2021, 177, 113847. [Google Scholar] [CrossRef]
- Natarajan, A.; Xiong, C.Y.; Albrecht, H.; DeNardo, G.L.; DeNardo, S.J. Characterization of site-specific ScFv PEGylation for tumor-targeting pharmaceuticals. Bioconjugate Chem. 2005, 16, 113–121. [Google Scholar] [CrossRef]
- Peyman, S.A.; Abou-Saleh, R.H.; McLaughlan, J.R.; Ingram, N.; Johnson, B.R.G.; Critchley, K.; Freear, S.; Evans, J.A.; Markham, A.F.; Coletta, P.L.; et al. Expanding 3D geometry for enhanced on-chip microbubble production and single step formation of liposome modified microbubbles. Lab Chip 2012, 12, 4544–4552. [Google Scholar] [CrossRef]
- Yang, S.; Wei, W.; Zhao, Q. B7-H3, a checkpoint molecule, as a target for cancer immunotherapy. Int. J. Biol. Sci. 2020, 16, 1767–1773. [Google Scholar] [CrossRef]
- Segers, T.; Kruizinga, P.; Kok, M.P.; Lajoinie, G.; de Jong, N.; Versluis, M. Monodisperse Versus Polydisperse Ultrasound Contrast Agents: Non-Linear Response, Sensitivity, and Deep Tissue Imaging Potential. Ultrasound Med. Biol. 2018, 44, 1482–1492. [Google Scholar] [CrossRef]
- Langeveld, S.A.G.; Meijlink, B.; Beekers, I.; Olthof, M.; van der Steen, A.F.W.; de Jong, N.; Kooiman, K. Theranostic Microbubbles with Homogeneous Ligand Distribution for Higher Binding Efficacy. Pharmaceutics 2022, 14, 311. [Google Scholar] [CrossRef]
- Langeveld, S.A.G.; Schwieger, C.; Beekers, I.; Blaffert, J.; van Rooij, T.; Blume, A.; Kooiman, K. Ligand Distribution and Lipid Phase Behavior in Phospholipid-Coated Microbubbles and Monolayers. Langmuir 2020, 36, 3221–3233. [Google Scholar] [CrossRef]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-gamma production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Hofmeyer, K.A.; Ray, A.; Zang, X. The contrasting role of B7-H3. Proc. Natl. Acad. Sci. USA 2008, 105, 10277–10278. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cheung, I.Y.; Guo, H.F.; Cheung, N.K. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: Potential implications for immune based therapy of human solid tumors. Cancer Res. 2009, 69, 6275–6281. [Google Scholar] [CrossRef]
- Lee, Y.H.; Martin-Orozco, N.; Zheng, P.; Li, J.; Zhang, P.; Tan, H.; Park, H.J.; Jeong, M.; Chang, S.H.; Kim, B.S.; et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017, 27, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Thompson, R.H.; Al-Ahmadie, H.A.; Serio, A.M.; Reuter, V.E.; Eastham, J.A.; Scardino, P.T.; Sharma, P.; Allison, J.P. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 19458–19463. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.J.; Sheinin, Y.; Lohse, C.M.; Kuntz, S.M.; Frigola, X.; Inman, B.A.; Krambeck, A.E.; McKenney, M.E.; Karnes, R.J.; Blute, M.L.; et al. B7-H3 ligand expression by prostate cancer: A novel marker of prognosis and potential target for therapy. Cancer Res. 2007, 67, 7893–7900. [Google Scholar] [CrossRef] [PubMed]
- Crispen, P.L.; Sheinin, Y.; Roth, T.J.; Lohse, C.M.; Kuntz, S.M.; Frigola, X.; Thompson, R.H.; Boorjian, S.A.; Dong, H.; Leibovich, B.C.; et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin. Cancer Res. 2008, 14, 5150–5157. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Sullivan, P.S.; Soslow, R.A.; Waitz, R.; Reuter, V.E.; Wilton, A.; Thaler, H.T.; Arul, M.; Slovin, S.F.; Wei, J.; et al. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod. Pathol. 2010, 23, 1104–1112. [Google Scholar] [CrossRef]
- Lemke, D.; Pfenning, P.N.; Sahm, F.; Klein, A.C.; Kempf, T.; Warnken, U.; Schnolzer, M.; Tudoran, R.; Weller, M.; Platten, M.; et al. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin. Cancer Res. 2012, 18, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.; Chen, W.; Shan, B.; Ding, Y.; Zhang, G.; Cao, N.; Liu, L.; Zhang, Y. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS ONE 2013, 8, e70689. [Google Scholar] [CrossRef]
- Yamato, I.; Sho, M.; Nomi, T.; Akahori, T.; Shimada, K.; Hotta, K.; Kanehiro, H.; Konishi, N.; Yagita, H.; Nakajima, Y. Clinical importance of B7-H3 expression in human pancreatic cancer. Br. J. Cancer 2009, 101, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, A.; Corrias, M.V.; Castriconi, R.; Dondero, A.; Mosconi, M.; Gambini, C.; Moretta, A.; Moretta, L.; Bottino, C. Small round blue cell tumours: Diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology 2008, 53, 73–80. [Google Scholar] [CrossRef]
- Zhou, Z.; Luther, N.; Ibrahim, G.M.; Hawkins, C.; Vibhakar, R.; Handler, M.H.; Souweidane, M.M. B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. J. Neuro-Oncol. 2013, 111, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Calabro, L.; Sigalotti, L.; Fonsatti, E.; Bertocci, E.; Di Giacomo, A.M.; Danielli, R.; Cutaia, O.; Colizzi, F.; Covre, A.; Mutti, L.; et al. Expression and regulation of B7-H3 immunoregulatory receptor, in human mesothelial and mesothelioma cells: Immunotherapeutic implications. J. Cell. Physiol. 2011, 226, 2595–2600. [Google Scholar] [CrossRef]
- Stern, L.A.; Lown, P.S.; Kobe, A.C.; Abou-Elkacem, L.; Willmann, J.K.; Hackel, B.J. Cellular-Based Selections Aid Yeast-Display Discovery of Genuine Cell-Binding Ligands: Targeting Oncology Vascular Biomarker CD276. ACS Comb. Sci. 2019, 21, 207–222. [Google Scholar] [CrossRef]
- Shen, B.Q.; Xu, K.; Liu, L.; Raab, H.; Bhakta, S.; Kenrick, M.; Parsons-Reponte, K.L.; Tien, J.; Yu, S.F.; Mai, E.; et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 2012, 30, 184–189. [Google Scholar] [CrossRef]
- Wall, A.; Wills, A.G.; Forte, N.; Bahou, C.; Bonin, L.; Nicholls, K.; Ma, M.T.; Chudasama, V.; Baker, J.R. One-pot thiol-amine bioconjugation to maleimides: Simultaneous stabilisation and dual functionalisation. Chem. Sci. 2020, 11, 11455–11460. [Google Scholar] [CrossRef]
- Lim, S.B.; Rubinstein, I.; Onyuksel, H. Freeze drying of peptide drugs self-associated with long-circulating, biocompatible and biodegradable sterically stabilized phospholipid nanomicelles. Int. J. Pharm. 2008, 356, 345–350. [Google Scholar] [CrossRef]
- Ravasco, J.M.J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. A Eur. J. 2019, 25, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, S.D.; Reid, R.; Robinson, L.; Ashley, G.W.; Santi, D.V. Long-term stabilization of maleimide-thiol conjugates. Bioconjugate Chem. 2015, 26, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, R.H.; Peyman, S.A.; Johnson, B.R.; Marston, G.; Ingram, N.; Bushby, R.; Coletta, P.L.; Markham, A.F.; Evans, S.D. The influence of intercalating perfluorohexane into lipid shells on nano and microbubble stability. Soft Matter 2016, 12, 7223–7230. [Google Scholar] [CrossRef] [PubMed]
- Abou-Saleh, R.H.; Swain, M.; Evans, S.D.; Thomson, N.H. Poly(ethylene glycol) lipid-shelled microbubbles: Abundance, stability, and mechanical properties. Langmuir 2014, 30, 5557–5563. [Google Scholar] [CrossRef]
- Ferrara, K.W.; Borden, M.A.; Zhang, H. Lipid-shelled vehicles: Engineering for ultrasound molecular imaging and drug delivery. Acc. Chem. Res. 2009, 42, 881–892. [Google Scholar] [CrossRef]
- Borden, M.A.; Martinez, G.V.; Ricker, J.; Tsvetkova, N.; Longo, M.; Gillies, R.J.; Dayton, P.A.; Ferrara, K.W. Lateral phase separation in lipid-coated microbubbles. Langmuir 2006, 22, 4291–4297. [Google Scholar] [CrossRef]
- Winther, J.R.; Thorpe, C. Quantification of thiols and disulfides. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 838–846. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Catalysis of imido group hydrolysis in a maleimide conjugate. Bioorganic Med. Chem. Lett. 2007, 17, 6286–6289. [Google Scholar] [CrossRef]
- Takalkar, A.M.; Klibanov, A.L.; Rychak, J.J.; Lindner, J.R.; Ley, K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J. Control. Release 2004, 96, 473–482. [Google Scholar] [CrossRef]
- Ahmed, M.; Gustafsson, B.; Aldi, S.; Dusart, P.; Egri, G.; Butler, L.M.; Bone, D.; Dähne, L.; Hedin, U.; Caidahl, K. Molecular Imaging of a New Multimodal Microbubble for Adhesion Molecule Targeting. Cell. Mol. Bioeng. 2019, 12, 15–32. [Google Scholar] [CrossRef]
- Wang, S.; Hossack, J.A.; Klibanov, A.L. Targeting of microbubbles: Contrast agents for ultrasound molecular imaging. J. Drug Target. 2018, 26, 420–434. [Google Scholar] [CrossRef]
- Bam, R.; Laffey, M.; Nottberg, K.; Lown, P.S.; Hackel, B.J.; Wilson, K.E. Affibody-Indocyanine Green Based Contrast Agent for Photoacoustic and Fluorescence Molecular Imaging of B7-H3 Expression in Breast Cancer. Bioconjugate Chem. 2019, 30, 1677–1689. [Google Scholar] [CrossRef]
- Vraka, I.; Panourgias, E.; Sifakis, E.; Koureas, A.; Galanis, P.; Dellaportas, D.; Gouliamos, A.; Antoniou, A. Correlation Between Contrast-enhanced Ultrasound Characteristics (Qualitative and Quantitative) and Pathological Prognostic Factors in Breast Cancer. Vivo 2018, 32, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Becerra, J.A.; Song, K.-H.; Martinez, P.; Borden, M.A. Microbubble Size and Dose Effects on Pharmacokinetics. ACS Biomater. Sci. Eng. 2022, 8, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Ergen, C.; Heymann, F.; Al Rawashdeh, W.E.; Gremse, F.; Bartneck, M.; Panzer, U.; Pola, R.; Pechar, M.; Storm, G.; Mohr, N.; et al. Targeting distinct myeloid cell populations in vivo using polymers, liposomes and microbubbles. Biomaterials 2017, 114, 106–120. [Google Scholar] [CrossRef] [PubMed]
- van Hoeve, W.; de Vargas Serrano, M.; Te Winkel, L.; Forsberg, F.; Dave, J.K.; Sarkar, K.; Wessner, C.E.; Eisenbrey, J.R. Improved Sensitivity of Ultrasound-Based Subharmonic Aided Pressure Estimation Using Monodisperse Microbubbles. J. Ultrasound Med. 2021, 41, 1781–1789. [Google Scholar] [CrossRef]
- Garg, S.; Thomas, A.A.; Borden, M.A. The effect of lipid monolayer in-plane rigidity on in vivo microbubble circulation persistence. Biomaterials 2013, 34, 6862–6870. [Google Scholar] [CrossRef]
- Wright, T.A.; Rahman, M.S.; Bennett, C.; Johnson, M.R.; Fischesser, H.; Ram, N.; Tyler, A.; Page, R.C.; Konkolewicz, D. Hydrolytically Stable Maleimide-End-Functionalized Polymers for Site-Specific Protein Conjugation. Bioconjugate Chem. 2021, 32, 2447–2456. [Google Scholar] [CrossRef]
- Badescu, G.; Bryant, P.; Swierkosz, J.; Khayrzad, F.; Pawlisz, E.; Farys, M.; Cong, Y.; Muroni, M.; Rumpf, N.; Brocchini, S.; et al. A new reagent for stable thiol-specific conjugation. Bioconjugate Chem. 2014, 25, 460–469. [Google Scholar] [CrossRef]
- Bam, R.; Lown, P.S.; Stern, L.A.; Sharma, K.; Wilson, K.E.; Bean, G.R.; Lutz, A.M.; Paulmurugan, R.; Hackel, B.J.; Dahl, J.; et al. Efficacy of Affibody-Based Ultrasound Molecular Imaging of Vascular B7-H3 for Breast Cancer Detection. Clin. Cancer Res. 2020, 26, 2140–2150. [Google Scholar] [CrossRef]
- Jugniot, N.; Bam, R.; Paulmurugan, R. Expression and purification of a native Thy1-single-chain variable fragment for use in molecular imaging. Sci. Rep. 2021, 11, 23026. [Google Scholar] [CrossRef] [PubMed]
- Vuković, L.; Khatib, F.A.; Drake, S.P.; Madriaga, A.; Brandenburg, K.S.; Král, P.; Onyuksel, H. Structure and dynamics of highly PEG-ylated sterically stabilized micelles in aqueous media. J. Am. Chem. Soc. 2011, 133, 13481–13488. [Google Scholar] [CrossRef] [PubMed]
- Püspöki, Z.; Sage, D.; Ward, J.P.; Unser, M. SpotCaliper: Fast wavelet-based spot detection with accurate size estimation. Bioinformatics 2016, 32, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Gage, G.J.; Kipke, D.R.; Shain, W. Whole animal perfusion fixation for rodents. J. Vis. Exp. 2012, e3564. [Google Scholar] [CrossRef]
- Abou-Elkacem, L.; Wang, H.; Chowdhury, S.M.; Kimura, R.H.; Bachawal, S.V.; Gambhir, S.S.; Tian, L.; Willmann, J.K. Thy1-Targeted Microbubbles for Ultrasound Molecular Imaging of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2018, 24, 1574–1585. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bam, R.; Natarajan, A.; Tabesh, F.; Paulmurugan, R.; Dahl, J.J. Synthesis and Evaluation of Clinically Translatable Targeted Microbubbles Using a Microfluidic Device for In Vivo Ultrasound Molecular Imaging. Int. J. Mol. Sci. 2023, 24, 9048. https://doi.org/10.3390/ijms24109048
Bam R, Natarajan A, Tabesh F, Paulmurugan R, Dahl JJ. Synthesis and Evaluation of Clinically Translatable Targeted Microbubbles Using a Microfluidic Device for In Vivo Ultrasound Molecular Imaging. International Journal of Molecular Sciences. 2023; 24(10):9048. https://doi.org/10.3390/ijms24109048
Chicago/Turabian StyleBam, Rakesh, Arutselvan Natarajan, Farbod Tabesh, Ramasamy Paulmurugan, and Jeremy J. Dahl. 2023. "Synthesis and Evaluation of Clinically Translatable Targeted Microbubbles Using a Microfluidic Device for In Vivo Ultrasound Molecular Imaging" International Journal of Molecular Sciences 24, no. 10: 9048. https://doi.org/10.3390/ijms24109048
APA StyleBam, R., Natarajan, A., Tabesh, F., Paulmurugan, R., & Dahl, J. J. (2023). Synthesis and Evaluation of Clinically Translatable Targeted Microbubbles Using a Microfluidic Device for In Vivo Ultrasound Molecular Imaging. International Journal of Molecular Sciences, 24(10), 9048. https://doi.org/10.3390/ijms24109048





