The Prognostic Value of Anti-PLA2R Antibodies Levels in Primary Membranous Nephropathy
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Population
4.2. Collection of Data
4.3. Statistical Analysis
4.4. Detection of Serum Anti-PLA2R Antibodies
4.5. PLA2R Immunohistochemistry from Renal Biopsy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cattran, D.C.; Brenchley, P.E. Membranous nephropathy: Integrating basic science into improved clinical management. Kidney Int. 2017, 91, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Keri, K.C.; Blumenthal, S.; Kulkarni, V.; Beck, L.; Chongkrairatanakul, T. Primary membranous nephropathy: Comprehensive review and historical perspective. Postgrad. Med. J. 2019, 95, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Ponticelli, C. Secondary Membranous Nephropathy. A Narrative Review. Front. Med. 2020, 7, 611317. [Google Scholar] [CrossRef]
- Beck, L.H., Jr.; Bonegio, R.G.; Lambeau, G.; Beck, D.M.; Powell, D.W.; Cummins, T.D.; Klein, J.B.; Salant, D.J. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N. Engl. J. Med. 2009, 361, 11–21. [Google Scholar] [CrossRef]
- Yeo, M.K.; Kim, Y.H.; Choi, D.E.; Choi, S.Y.; Kim, K.H.; Suh, K.S. The Usefulness of Phospholipase A2 Receptor and IgG4 Detection in Differentiation Primary Membranous Nephropathy From Secondary Membranous Nephropathy in Renal Biopsy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schwesinger, C.; Tomas, N.M.; Dehde, S.; Seifert, L.; Hermans-Borgmeyer, I.; Wiech, T.; Koch-Nolte, F.; Huber, T.B.; Zahner, G. A novel mouse model of phospholipase A2 receptor 1-associated membranous nephropathy mimics podocyte injury in patients. Kidney Int. 2020, 97, 913–919. [Google Scholar] [CrossRef]
- Hofstra, J.M.; Beck, L.H., Jr.; Beck, D.M.; Wetzels, J.F.; Salant, D.J. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 1286–1291. [Google Scholar] [CrossRef]
- Hofstra, J.M.; Debiec, H.; Short, C.D.; Pellé, T.; Kleta, R.; Mathieson, P.W.; Ronco, P.; Brenchley, P.E.; Wetzels, J.F. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1735–1743. [Google Scholar] [CrossRef]
- Oh, Y.J.; Yang, S.H.; Kim, D.K.; Kang, S.W.; Kim, Y.S. Autoantibodies against phospholipase A2 receptor in Korean patients with membranous nephropathy. PLoS ONE 2013, 8, e62151. [Google Scholar] [CrossRef]
- Pang, L.; Zhang, A.M.; Li, H.X.; Du, J.L.; Jiao, L.L.; Duan, N.; Liu, Y.; Yu, D. Serum anti-PLA2R antibody and glomerular PLA2R deposition in Chinese patients with membranous nephropathy: A cross-sectional study. Medicine 2017, 96, e7218. [Google Scholar] [CrossRef]
- van de Logt, A.E.; Hofstra, J.M.; Wetzels, J.F. Serum anti-PLA2R antibodies can be initially absent in idiopathic membranous nephropathy: Seroconversion after prolonged follow-up. Kidney Int. 2015, 87, 1263–1264. [Google Scholar] [CrossRef]
- Guerry, M.J.; Vanhille, P.; Ronco, P.; Debiec, H. Serum anti-PLA2R antibodies may be present before clinical manifestations of membranous nephropathy. Kidney Int. 2016, 89, 1399. [Google Scholar] [CrossRef]
- Ramachandran, R.; Kumar, V.; Nada, R.; Jha, V. Serial monitoring of anti-PLA2R in initial PLA2R-negative patients with primary membranous nephropathy. Kidney Int. 2015, 88, 1198–1199. [Google Scholar] [CrossRef]
- Hill, P.A.; McRae, J.L.; Dwyer, K.M. PLA2R and membranous nephropathy: A 3 year prospective Australian study. Nephrology 2016, 21, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Porcelli, B.; Guarnieri, A.; Ferretti, F.; Garosi, G.; Terzuoli, L.; Cinci, F.; Tabucchi, A.; Tampoia, M.; Abbracciavento, L.; Villani, C.; et al. Diagnostic accuracy of anti-phospholipase A2 receptor (PLA2R) antibodies in idiopathic membranous nephropathy: An Italian experience. J. Nephrol. 2020, 34, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Ronco, P.; Debiec, H. Pathophysiological advances in membranous nephropathy: Time for a shift in patient’s care. Lancet 2015, 385, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Radice, A.; Trezzi, B.; Maggiore, U.; Pregnolato, F.; Stellato, T.; Napodano, P.; Rolla, D.; Pesce, G.; D’Amico, M.; Santoro, D.; et al. Clinical usefulness of autoantibodies to M-type phospholipase A2 receptor (PLA2R) for monitoring disease activity in idiopathic membranous nephropathy (IMN). Autoimmun. Rev. 2016, 15, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Pourcine, F.; Dahan, K.; Mihout, F.; Cachanado, M.; Brocheriou, I.; Debiec, H.; Ronco, P. Prognostic value of PLA2R autoimmunity detected by measurement of anti-PLA2R antibodies combined with detection of PLA2R antigen in membranous nephropathy: A single-centre study over 14 years. PLoS ONE 2017, 12, e0173201. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.A.; Abdul Hamid, M.A.; Cohen Tervaert, J.W.; Damoiseaux, J.G.; van Paassen, P.; Limburg Renal Registry. Anti-PLA2R Antibodies as a Prognostic Factor in PLA2R-Related Membranous Nephropathy. Am. J. Nephrol. 2015, 42, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bech, A.P.; Hofstra, J.M.; Brenchley, P.E.; Wetzels, J.F. Association of anti-PLA2R antibodies with outcomes after immunosuppressive therapy in idiopathic membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2014, 9, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Choi, Y.W.; Kim, S.Y.; Moon, J.Y.; Ihm, C.G.; Lee, T.W.; Jeong, K.H.; Yang, S.H.; Kim, Y.S.; Oh, Y.J. Anti-Phospholipase A2 Receptor Antibody as Prognostic Indicator in Idiopathic Membranous Nephropathy. Am. J. Nephrol. 2015, 42, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.J.; Shen, Q.; Wang, H.M.; Tang, S.; Wang, X.Y. The association of anti-PLA2R with clinical manifestations and outcomes in idiopathic membranous nephropathy: A meta-analysis. Int. Urol. Nephrol. 2020, 52, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Fan, T.T.; Wang, Y.Y.; Zhang, L.; Song, L.; Zhang, L. Relationship between renal tissues phospholipase A2 receptor and its serum antibody and clinical condition and prognosis of idiopathic membranous nephropathy: A meta-analysis. BMC Nephrol. 2019, 20, 444. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wan, J.; Chen, Y.; Pan, Y. Serum anti-phospholipase A2 receptor (PLA2R) antibody detected at diagnosis as a predictor for clinical remission in patients with primary membranous nephropathy: A meta-analysis. BMC Nephrol. 2019, 20, 360. [Google Scholar] [CrossRef]
- Qin, H.Z.; Zhang, M.C.; Le, W.B.; Ren, Q.; Chen, D.C.; Zeng, C.H.; Liu, L.; Zuo, K.; Xu, F.; Liu, Z.H. Combined Assessment of Phospholipase A2 Receptor Autoantibodies and Glomerular Deposits in Membranous Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 3195–3203. [Google Scholar] [CrossRef]
- Cattran, D.C.; Pei, Y.; Greenwood, C.M.; Ponticelli, C.; Passerini, P.; Honkanen, E. Validation of a predictive model of idiopathic membranous nephropathy: Its clinical and research implications. Kidney Int. 1997, 51, 901–907. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Glassock, R.J.; Nath, K.A.; Sethi, S.; Fervenza, F.C. A Proposal for a Serology-Based Approach to Membranous Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 421–430. [Google Scholar] [CrossRef]
- Wei, S.Y.; Wang, Y.X.; Li, J.S.; Zhao, S.L.; Diao, T.T.; Wang, Y.; Wang, C.; Qin, Y.; Cao, Y.; Wei, Q.; et al. Serum Anti-PLA2R Antibody Predicts Treatment Outcome in Idiopathic Membranous Nephropathy. Am. J. Nephrol. 2016, 43, 129–140. [Google Scholar] [CrossRef]
- Deng, L.; Huang, Q.; Wang, J.; Luo, K.; Liu, J.; Yan, W.; Jiang, F.; Xu, G. Efficacy and Safety of Different Immunosuppressive Therapies in Patients with Membranous Nephropathy and High PLA2R Antibody Titer. Front. Pharmacol. 2022, 12, 786334. [Google Scholar] [CrossRef]
- Song, E.J.; Jeong, K.H.; Yang, Y.A.; Lim, J.H.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Kim, Y.L.; Park, S.H. Anti-phospholipase A2 receptor antibody as a prognostic marker in patients with primary membranous nephropathy. Kidney Res. Clin. Pract. 2018, 37, 248–256. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, Q.; Sheng, H.; Li, T.; Liu, X.; Yang, X.; Lin, B.; Zhou, X.; Jin, J.; Wang, L.; et al. Quantitative detection of anti-PLA2R antibodies targeting different epitopes and its clinical application in primary membranous nephropathy. Clin. Chem. Lab. Med. 2022, 61, 251–259. [Google Scholar] [CrossRef]
- Hoxha, E.; Harendza, S.; Pinnschmidt, H.; Panzer, U.; Stahl, R.A. PLA2R antibody levels and clinical outcome in patients with membranous nephropathy and non-nephrotic range proteinuria under treatment with inhibitors of the renin-angiotensin system. PLoS ONE 2014, 9, e110681. [Google Scholar] [CrossRef] [PubMed]
- Kanigicherla, D.; Gummadova, J.; McKenzie, E.A.; Roberts, S.A.; Harris, S.; Nikam, M.; Poulton, K.; McWilliam, L.; Short, C.D.; Venning, M.; et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013, 83, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Hoxha, E.; Harendza, S.; Pinnschmidt, H.; Panzer, U.; Stahl, R.A. M-type phospholipase A2 receptor autoantibodies and renal function in patients with primary membranous nephropathy. Clin. J. Am. Soc. Nephrol. 2014, 9, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Dähnrich, C.; Komorowski, L.; Probst, C.; Seitz-Polski, B.; Esnault, V.; Wetzels, J.F.; Hofstra, J.M.; Hoxha, E.; Stahl, R.A.; Lambeau, G.; et al. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin. Chim. Acta 2013, 421, 213–218. [Google Scholar] [CrossRef] [PubMed]
| Variable | Median | IQR |
|---|---|---|
| Baseline (n = 41) | ||
| Creatinine (mg/dL) | 0.89 | 0.73–1.13 |
| Albumin (g/dL) | 2.70 | 2.25–3.00 |
| Proteinuria (g/24-h) | 7.80 | 6.20–10.90 |
| Anti-PLA2R (RU/mL) | 78 | 35–183 |
| eGFR (mL/min) | 89 | 64–103 |
| One year after diagnosis (n = 32) | ||
| Creatinine (mg/dL) | 0.98 | 0.82–1.25 |
| Albumin (g/dL) | 3.05 | 2.48–3.40 |
| Proteinuria (g/24-h) | 4.85 | 2.01–11.55 |
| eGFR (mL/min) | 78 | 55–103 |
| Kendall Correlation | ||||
|---|---|---|---|---|
| Variable | Kendall’s Tau | p-Value | Adjusted p-Value | |
| At diagnosis | ||||
| Creatinine | 0.102 | 0.406 | 0.575 | |
| Albumin | −0.142 | 0.252 | 0.444 | |
| 24 h Proteinuria | −0.054 | 0.667 | 0.810 | |
| eGFR | −0.005 | 0.976 | 0.976 | |
| One year after diagnosis | ||||
| Creatinine | −0.165 | 0.261 | 0.444 | |
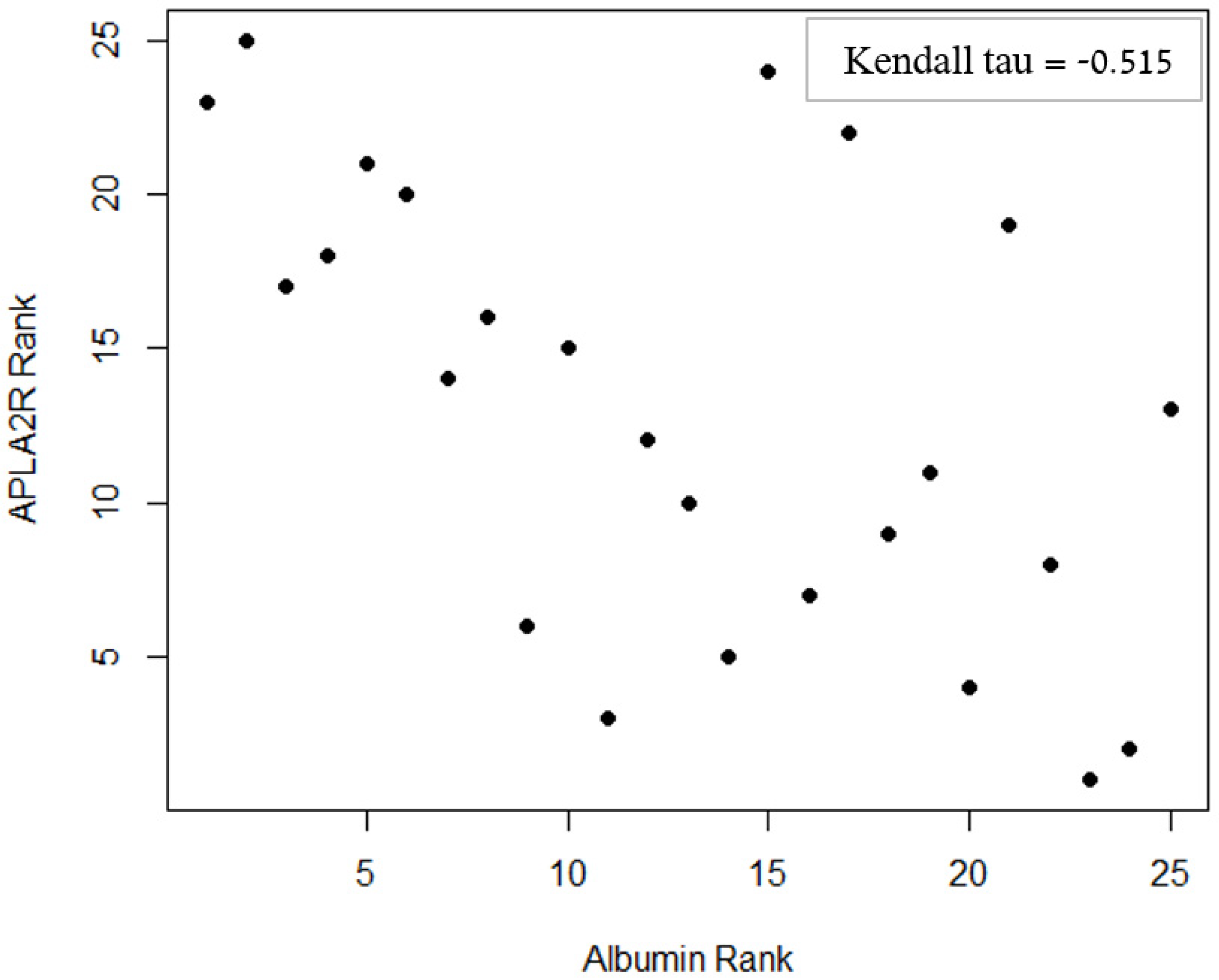
| Albumin | −0.515 * | <0.001 * | 0.003 * | |
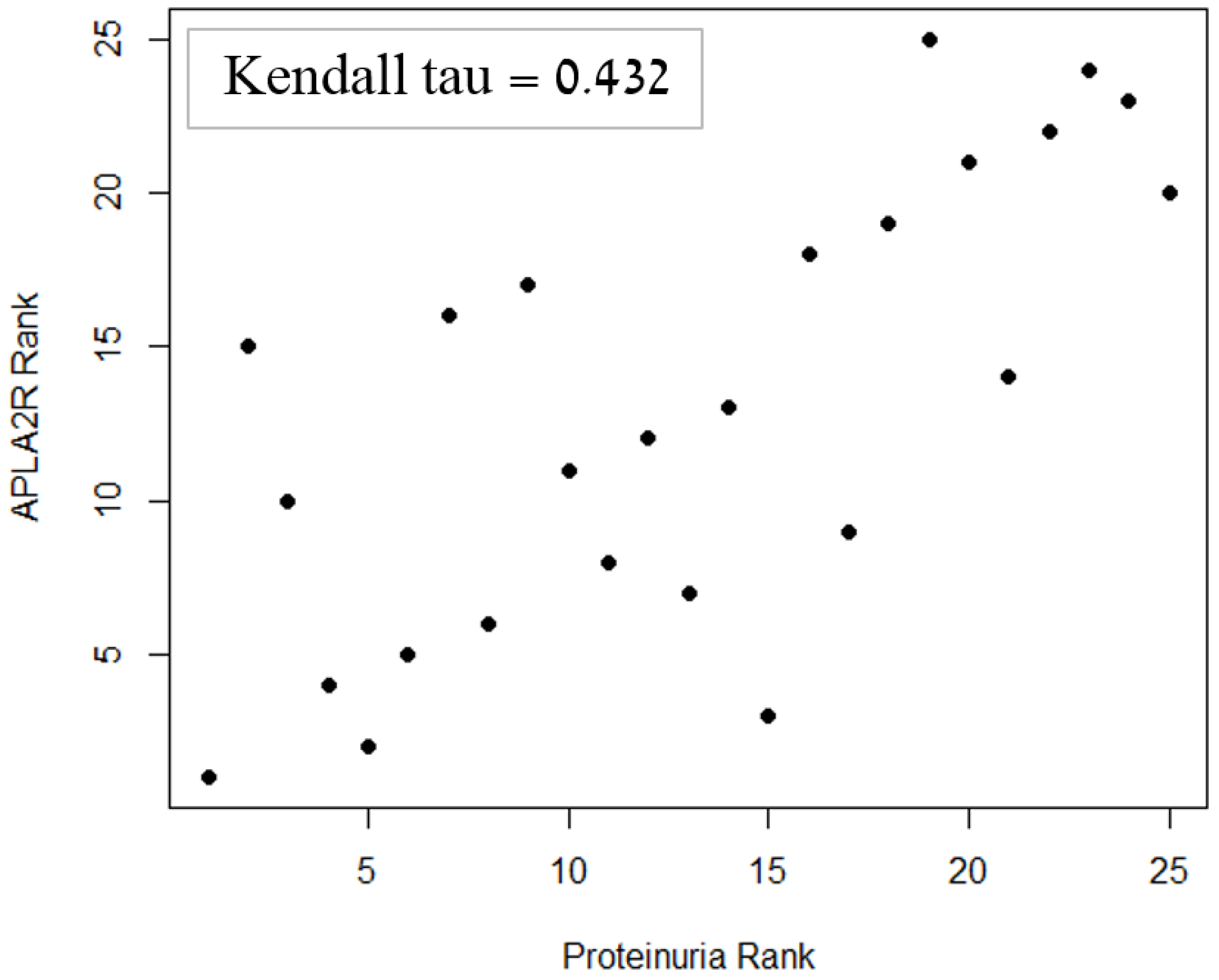
| 24 h Proteinuria | 0.432 * | 0.003 * | 0.017 * | |
| eGFR | 0.167 | 0.252 | 0.444 | |
| Mann–Whitney U-test | ||||
| Variable | Median [IQR] Anti-PLA2R levels | p-value | Adjusted p-value | |
| Hypertension (at diagnosis) | Yes | 88 [42–257] | 0.337 | 0.521 |
| No | 66 [29–120] | |||
| Leg edema (at diagnosis) | Yes | 81 [32–190] | 0.873 | 0.969 |
| No | 76 [49–201] | |||
| Remission (after 1 year) | Yes | 27 [23–87] | 0.009 | 0.034 * |
| No | 91 [58–278] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukuy, O.L.; Cohen, R.; Gilburd, B.; Zeruya, E.; Weinstein, T.; Agur, T.; Dinour, D.; Beckerman, P.; Volkov, A.; Nissan, J.; et al. The Prognostic Value of Anti-PLA2R Antibodies Levels in Primary Membranous Nephropathy. Int. J. Mol. Sci. 2023, 24, 9051. https://doi.org/10.3390/ijms24109051
Kukuy OL, Cohen R, Gilburd B, Zeruya E, Weinstein T, Agur T, Dinour D, Beckerman P, Volkov A, Nissan J, et al. The Prognostic Value of Anti-PLA2R Antibodies Levels in Primary Membranous Nephropathy. International Journal of Molecular Sciences. 2023; 24(10):9051. https://doi.org/10.3390/ijms24109051
Chicago/Turabian StyleKukuy, Olga Lesya, Ron Cohen, Boris Gilburd, Eleanor Zeruya, Talia Weinstein, Timna Agur, Dganit Dinour, Pazit Beckerman, Alexander Volkov, Johnatan Nissan, and et al. 2023. "The Prognostic Value of Anti-PLA2R Antibodies Levels in Primary Membranous Nephropathy" International Journal of Molecular Sciences 24, no. 10: 9051. https://doi.org/10.3390/ijms24109051
APA StyleKukuy, O. L., Cohen, R., Gilburd, B., Zeruya, E., Weinstein, T., Agur, T., Dinour, D., Beckerman, P., Volkov, A., Nissan, J., Davidson, T., Amital, H., Shoenfeld, Y., & Shovman, O. (2023). The Prognostic Value of Anti-PLA2R Antibodies Levels in Primary Membranous Nephropathy. International Journal of Molecular Sciences, 24(10), 9051. https://doi.org/10.3390/ijms24109051







