All-Trans Retinoic Acid-Responsive LGR6 Is Transiently Expressed during Myogenic Differentiation and Is Required for Myoblast Differentiation and Fusion
Abstract
1. Introduction
2. Results
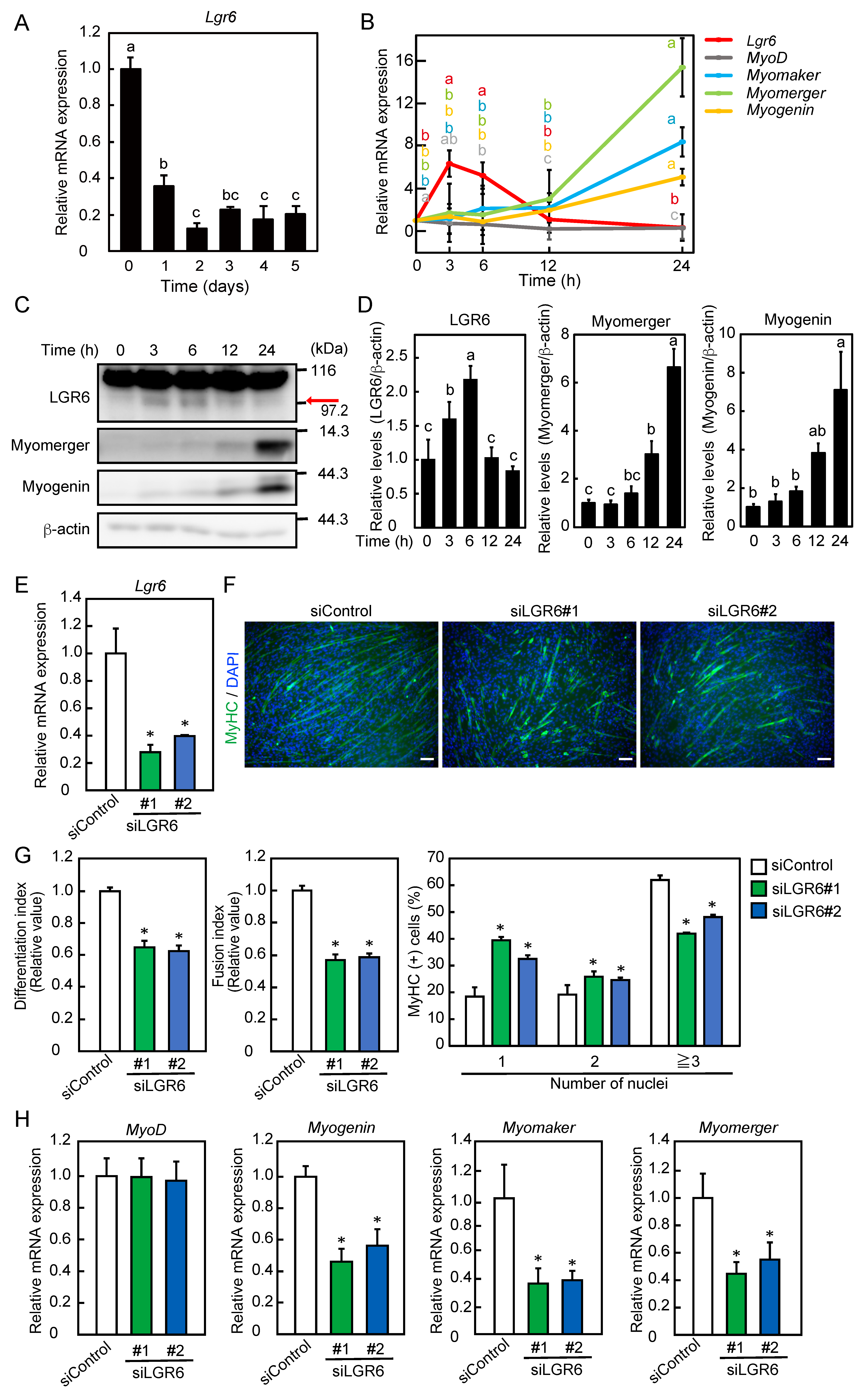
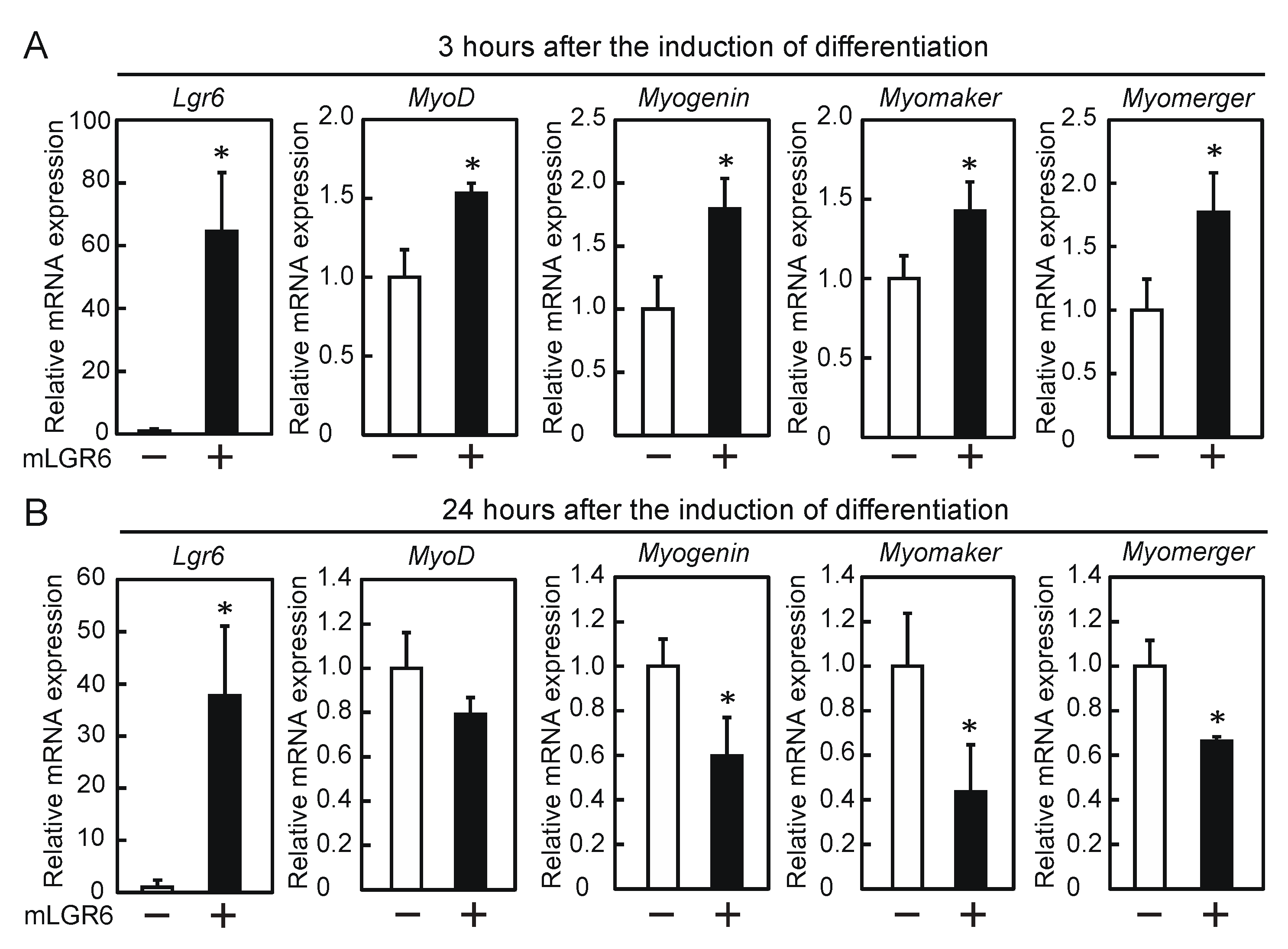
2.1. LGR6 Is Involved in the Promotion of Myogenic Differentiation
2.2. Transient Expression of LGR6 Appears to Be Required for the Promotion of Myogenic Differentiation
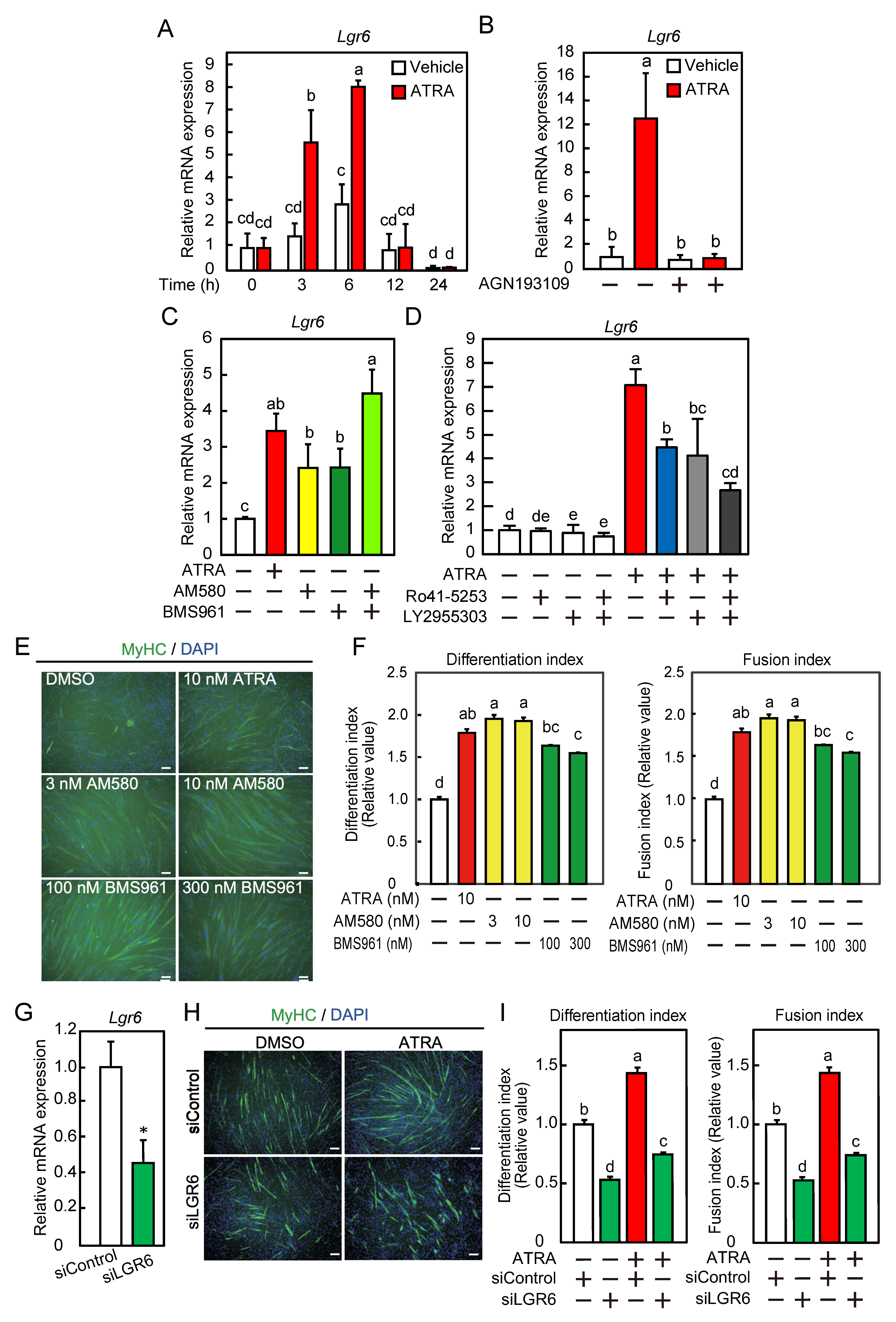
2.3. ATRA Is Required for Lgr6 mRNA Expression during Myogenic Differentiation
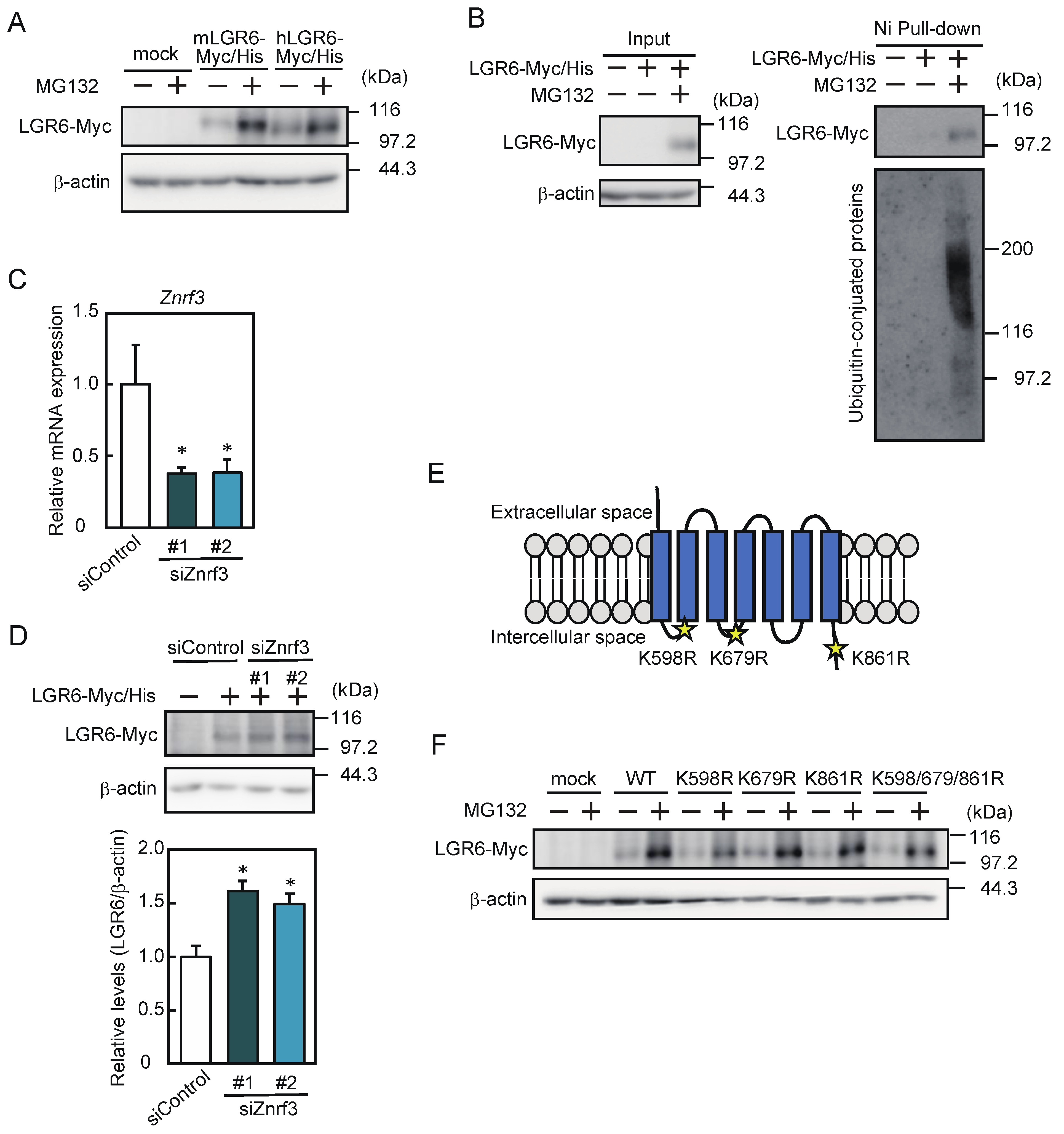
2.4. LGR6 Expression Is Downregulated by the Ubiquitin–Proteasome System
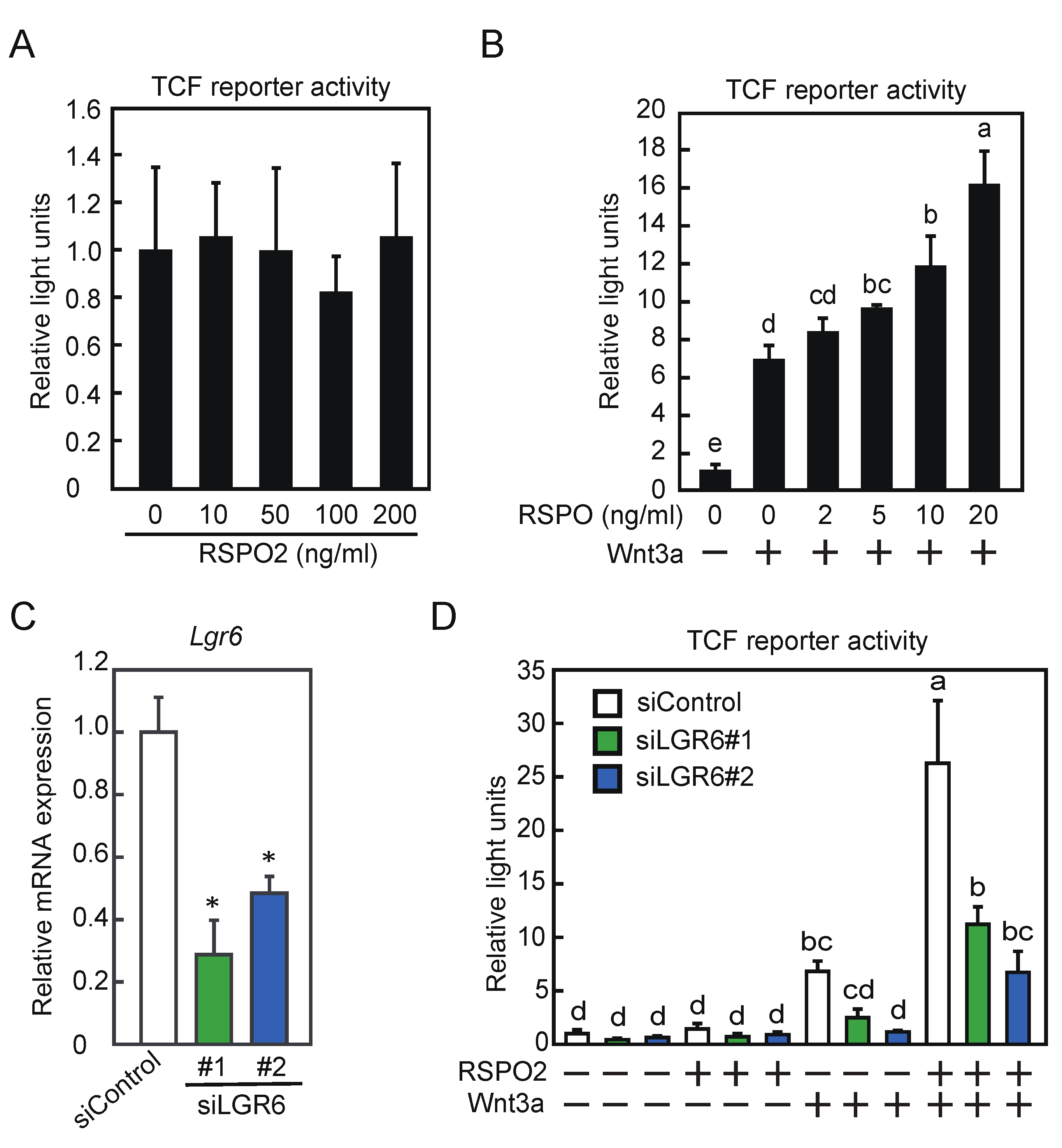
2.5. LGR6 Activates Wnt/β-Catenin Signaling
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Plasmids
4.3. Immunofluorescence Assay
4.4. siRNA-Mediated Knockdown
4.5. Transfection of Plasmids
4.6. Quantitative PCR
4.7. Western Blotting
4.8. Reporter Assay
4.9. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rai, M.; Nongthomba, U.; Grounds, M.D. Skeletal muscle degeneration and regeneration in mice and flies. Curr. Top. Dev. Biol. 2014, 108, 247–281. [Google Scholar] [CrossRef] [PubMed]
- Lehka, L. Mechanisms regulating myoblast fusion: A multilevel interplay. Semin. Cell Dev. Biol. 2020, 104, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Rawls, A.; Morris, J.H.; Rudnicki, M.; Braun, T.; Arnold, H.H.; Klein, W.H.; Olson, E.N. Myogenin’s functions do not overlap with those of MyoD or Myf-5 during mouse embryogenesis. Dev. Biol. 1995, 172, 37–50. [Google Scholar] [CrossRef]
- Quinn, M.E.; Goh, Q.; Kurosaka, M.; Gamage, D.G.; Petrany, M.J.; Prasad, V.; Millay, D.P. Myomerger induces fusion of non-fusogenic cells and is required for skeletal muscle development. Nat. Commun. 2017, 8, 15665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.H.; Huang, J.; Bi, Y.; Su, Y.; Tang, Y.; He, B.-C.; He, Y.; Luo, J.; Wang, Y.; Chen, L.; et al. Activation of RXR and RAR signaling promotes myogenic differentiation of myoblastic C2C12 cells. Differentiation 2009, 78, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.M.; Tajrishi, M.M.; Kumar, A. Signaling mechanisms in mammalian myoblast fusion. Sci. Signal. 2013, 6, re2. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Zhang, R.; Qin, X.; Zhao, J. All-trans retinoic acid regulates sheep primary myoblast proliferation and differentiation in vitro. Domest. Anim. Endocrinol. 2020, 71, 106394. [Google Scholar] [CrossRef]
- von Maltzahn, J.; Chang, N.C.; Bentzinger, C.F.; Rudnicki, M.A. Wnt signaling in myogenesis. Trends Cell Biol. 2012, 22, 602–609. [Google Scholar] [CrossRef]
- Ren, Q.; Chen, J.; Liu, Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021, 9, 670960. [Google Scholar] [CrossRef]
- Tanaka, S.; Terada, K.; Nohno, T. Canonical Wnt signaling is involved in switching from cell proliferation to myogenic differentiation of mouse myoblast cells. J. Mol. Signal. 2011, 6, 12. [Google Scholar] [CrossRef]
- Kim, K.A.; Kakitani, M.; Zhao, J.; Oshima, T.; Tang, T.; Binnerts, M.; Liu, Y.; Boyle, B.; Park, E.; Emtage, P.; et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 2005, 309, 1256–1259. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.S.; Turcotte, T.J.; Smith, P.F.; Choi, S.; Yoon, J.K. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 2006, 281, 13247–13257. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.Y.; Kudo, M.; Chen, T.; Nakabayashi, K.; Bhalla, A.; van der Spec, P.J.; van Duin, M.; Hsueh, A.J. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): Identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol. Endocrinol. 2000, 14, 1257–1271. [Google Scholar] [CrossRef]
- Raslan, A.A.; Yoon, J.K. R-spondins: Multi-mode WNT signaling regulators in adult stem cells. Int. J. Biochem. Cell Biol. 2019, 106, 26–34. [Google Scholar] [CrossRef]
- Hao, H.X.; Xie, Y.; Zhang, Y.; Charlar, O.; Oster, E.; Avello, M.; Lei, H.; Mickanin, X.; Liu, D.; Ruffner, H. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 2012, 485, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, S.; Sun, T.; Tchorz, J.S. The RSPO-LGR4/5-ZNRF3/RNF43 module in liver homeostasis, regeneration, and disease. Hepatology 2022, 76, 888–899. [Google Scholar] [CrossRef]
- Han, X.H.; Jin, Y.R.; Tan, L.; Kosciuk, T.; Lee, J.-S.; Yoon, J.K. Regulation of the follistatin gene by RSPO-LGR4 signaling via activation of the WNT/β-catenin pathway in skeletal myogenesis. Mol. Cell. Biol. 2014, 34, 752–764. [Google Scholar] [CrossRef]
- Leung, C.; Murad, K.B.A.; Tan, A.L.T.; Yada, S.; Sagiraju, S.; Bode, P.K.; Barker, N. Lgr5 marks adult progenitor cells contributing to skeletal muscle regeneration and sarcoma formation. Cell Rep. 2020, 33, 108535. [Google Scholar] [CrossRef]
- Kitakaze, T.; Yoshikawa, M.; Kobayashi, Y.; Kimura, N.; Goshima, N.; Ishikawa, T.; Ogata, Y.; Yamashita, Y.; Ashida, H.; Harada, N. Extracellular transglutaminase 2 induces myotube hypertrophy through G protein-coupled receptor 56. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118563. [Google Scholar] [CrossRef]
- Lee, Y.H.; Sharma, A.R.; Jagga, S.; Lee, S.S.; Nam, J.S. Differential expression patterns of Rspondin family and leucine-rich repeat-containing G-protein coupled receptors in chondrocytes and osteoblasts. Cell J. 2021, 22, 437–449. [Google Scholar] [CrossRef]
- Khedgikar, V.; Charles, J.F.; Lehoczky, J.A. Mouse LGR6 regulates osteogenesis in vitro and in vivo through differential ligand use. Bone 2022, 155, 116267. [Google Scholar] [CrossRef]
- Doherty, L.; Wan, M.; Peterson, A.; Youngstrom, D.W.; King, J.S.; Kalajzic, I.; Hankenson, K.D.; Sanjay, A. Wnt-associated adult stem cell marker Lgr6 is required for osteogenesis and fracture healing. Bone 2023, 169, 116681. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ma, C.; Feng, J.Q.; Jing, Y. The Emerging Role of Cell Transdifferentiation in skeletal development and diseases. Int. J. Mol. Sci. 2022, 23, 5974. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Liang, S.G.; Hsueh, A.J. Characterization of two LGR genes homologous to gonadotropin and thyrotropin receptors with extracellular leucine-rich repeats and a G protein-coupled, seven-transmembrane region. Mol. Endocrinol. 1998, 12, 1830–1845. [Google Scholar] [CrossRef] [PubMed]
- Koubek, E.J.; Santy, L.C. Actin up: An overview of the Rac GEF Dock1/Dock180 and its role in cytoskeleton rearrangement. Cells 2022, 11, 3565. [Google Scholar] [CrossRef]
- Vartanian, A.D.; Chabanais, J.; Carrion, C.; Maftah, A.; Germot, A. Downregulation of POFUT1 impairs secondary myogenic fusion through a reduced NFATc2/IL-4 signaling pathway. Int. J. Mol. Sci. 2019, 20, 4396. [Google Scholar] [CrossRef] [PubMed]
- Eigler, T.; Zarfati, G.; Amzallag, E.; Sinha, S.; Segev, N.; Zabary, Y.; Zaritsky, A.; Shakked, A.; Umansky, K.-B.; Schejter, E.D.; et al. ERK1/2 inhibition promotes robust myotube growth via CaMKII activation resulting in myoblast-to-myotube fusion. Dev. Cell 2021, 56, 3349–3363.e6. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Li, L.; Yu, R.T.; Downes, M.; Evans, R.; Hulin, J.-A.; Makarenkova, H.P.; Meech, R. β-Catenin is essential for differentiation of primary myoblasts via cooperation with MyoD and α-catenin. Development 2019, 146, dev167080. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; You, W.; Wang, Y.; Shan, T. The regulatory role of Myomaker and Myomixer-Myomerger-Minion in muscle development and regeneration. Cell. Mol. Life Sci. 2020, 77, 1551–1569. [Google Scholar] [CrossRef]
- Pan, Y.C.; Wang, X.W.; Teng, H.F.; Wu, Y.J.; Chang, H.C.; Chen, S.L. Wnt3a signal pathways activate MyoD expression by targeting cis-elements inside and outside its distal enhancer. Biosci. Rep. 2015, 35, e00180. [Google Scholar] [CrossRef]
- Ganassi, M.; Badodi, S.; Ortuste Quiroga, H.P.; Zammit, P.S.; Hinits, Y.; Hughes, S.M. Myogenin promotes myocyte fusion to balance fibre number and size. Nat. Commun. 2018, 9, 4232. [Google Scholar] [CrossRef]
- Millay, D.P.; O'Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vashisht, A.A.; O'Rourke, J.; Corbel, S.Y.; Moran, R.; Romeo, A.; Miraglia, L.; Durrant, E.; Schmedt, C. The microprotein Minion controls cell fusion and muscle formation. Nat. Commun. 2017, 8, 15664. [Google Scholar] [CrossRef] [PubMed]
- Leikina, E.; Gamage, D.G.; Prasad, V.; Goykhberg, H.; Crowe, M.; Diao, J.; Kozlov, M.M.; Chernomordik, L.V.; Millay, D.P. Myomaker and myomerger work independently to control distinct steps of membrane remodeling during myoblast fusion. Dev. Cell 2018, 46, 767–780.e7. [Google Scholar] [CrossRef]
- Varshavsky, A. The ubiquitin system, autophagy, and regulated protein degradation. Annu. Rev. Biochem. 2017, 86, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Wu, L.; Tu, J.; Yu, W.; Toh, Y.; Carmon, K.S.; Liu, Q.J. Unlike LGR4, LGR5 potentiates Wnt-β-catenin signaling without sequestering E3 ligases. Sci. Signal. 2020, 13, eaaz4051. [Google Scholar] [CrossRef]
- Huang, P.Y.; Kandyba, E.; Jabouille, A.; Sjolund, J.; Kumar, A.; Halliwill, K.; McCreery, M.; DelRosario, R.; Kang, H.C.; Wong, C.E. Lgr6 is a stem cell marker in mouse skin squamous cell carcinoma. Nat. Genet. 2017, 49, 1624–1632. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Zhou, Y.M.; Tang, D.B.; Zhou, N.; Zheng, W.-W.; Tang, Z.-H.; Duan, C.-W.; Zheng, L.; Chen, J. LGR6 promotes osteogenesis by activating the Wnt/β-catenin signaling pathway. Biochem. Biophys. Res. Commun. 2019, 519, 1–7. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, R.; Wang, Y.; Zhu, L.; Zhang, X. Down-regulation of LGR6 promotes bone fracture recovery using bone marrow stromal cell. Biomed. Pharmacother. 2018, 99, 629–637. [Google Scholar] [CrossRef]
- Keidel, S.; LeMotte, P.; Apfel, C. Different agonist- and antagonist-induced conformational changes in retinoic acid receptors analyzed by protease mapping. Mol. Cell. Biol. 1994, 14, 287–298. [Google Scholar] [CrossRef]
- Hughes, N.; Bleisch, T.J.; Jones, S.A.; Richardson, T.I.; Doti, R.A.; Wang, Y.; Stout, S.L.; Durst, G.L.; Chambers, M.G.; Oskins, J.L.; et al. Identification of potent and selective retinoic acid receptor gamma (RARγ) antagonists for the treatment of osteoarthritis pain using structure based drug design. Bioorg. Med. Chem. Lett. 2016, 26, 3274–3277. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; de Paiva, C.S.; Yu, Z.; de Souza, R.G.; Li, D.-Q.; Pflugfelder, S.C. Goblet cell-produced retinoic acid suppresses CD86 expression and IL-12 production in bone marrow-derived cells. Int. Immunol. 2018, 30, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Ren, Y.; Wang, X.; Lyu, Y.; Xie, B.; Sun, Y.; Yuan, X.; Liu, H.; Yang, W.; et al. Derivation of totipotent-like stem cells with blastocyst-like structure forming potential. Cell Res. 2022, 32, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Iturbide, A.; Segura, M.L.R.T.; Noll, C.; Schorpp, K.; Rothenaigner, I.; Ruiz-Morales, E.R.; Lubatti, G.; Agami, A.; Hadian, K.; Scialdone, A.; et al. Retinoic acid signaling is critical during the totipotency window in early mammalian development. Nat. Struct. Mol. Biol. 2021, 28, 521–532. [Google Scholar] [CrossRef]
- Rocco, A.D.; Uchibe, K.; Larmour, C.; Berger, R.; Liu, M.; Barton, E.R.; Iwamoto, M. Selective retinoic acid receptor γ agonists promote repair of injured skeletal muscle in mouse. Am. J. Pathol. 2015, 185, 2495–2504. [Google Scholar] [CrossRef]
- Suzuki, A.; Pelikan, R.C.; Iwata, J. WNT/β-Catenin signaling regulates multiple steps of myogenesis by regulating step-specific targets. Mol. Cell. Biol. 2015, 35, 1763–1776. [Google Scholar] [CrossRef]
- Ogawa, M.; Yamaji, R.; Higashimura, Y.; Harada, N.; Ashida, H.; Nakano, Y.; Inui, H. 17β-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor α. J. Biol. Chem. 2011, 286, 41455–41465. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitakaze, T.; Tatsumi, R.; Yamaguchi, M.; Kubota, M.; Nakatsuji, A.; Harada, N.; Yamaji, R. All-Trans Retinoic Acid-Responsive LGR6 Is Transiently Expressed during Myogenic Differentiation and Is Required for Myoblast Differentiation and Fusion. Int. J. Mol. Sci. 2023, 24, 9035. https://doi.org/10.3390/ijms24109035
Kitakaze T, Tatsumi R, Yamaguchi M, Kubota M, Nakatsuji A, Harada N, Yamaji R. All-Trans Retinoic Acid-Responsive LGR6 Is Transiently Expressed during Myogenic Differentiation and Is Required for Myoblast Differentiation and Fusion. International Journal of Molecular Sciences. 2023; 24(10):9035. https://doi.org/10.3390/ijms24109035
Chicago/Turabian StyleKitakaze, Tomoya, Rina Tatsumi, Mayu Yamaguchi, Mai Kubota, Aino Nakatsuji, Naoki Harada, and Ryoichi Yamaji. 2023. "All-Trans Retinoic Acid-Responsive LGR6 Is Transiently Expressed during Myogenic Differentiation and Is Required for Myoblast Differentiation and Fusion" International Journal of Molecular Sciences 24, no. 10: 9035. https://doi.org/10.3390/ijms24109035
APA StyleKitakaze, T., Tatsumi, R., Yamaguchi, M., Kubota, M., Nakatsuji, A., Harada, N., & Yamaji, R. (2023). All-Trans Retinoic Acid-Responsive LGR6 Is Transiently Expressed during Myogenic Differentiation and Is Required for Myoblast Differentiation and Fusion. International Journal of Molecular Sciences, 24(10), 9035. https://doi.org/10.3390/ijms24109035





