Follicle-Stimulating Hormone Biological Products: Does Potency Predict Clinical Efficacy?
Abstract
1. Introduction
2. Physiological Relevance and Functional Significance of FSH Heterogeneity
3. FSH Heterogeneity during Pubertal Development and during Ovarian Cycles
4. Biological Activity and Potency of hFSH Products
4.1. Measurement of Potency of hFSH Products
4.2. hFSH Products Used in Medically Assisted Reproduction
4.2.1. Urinary Gonadotropins
4.2.2. Follitropin Alfa Originator (GONAL-f®)
4.2.3. Follitropin Alfa Biosimilars
4.2.4. Follitropin Beta (Puregon®)
4.2.5. Follitropin Delta (Rekovelle®)
5. Effects of Glycoform Composition on the PK/PD of FSH Preparations
Glycoform Composition Is Related to the Clinical Effect
6. Effect of the Glycoform Composition of hFSH Preparations on Clinical Response
6.1. Efficacy Outcomes of hFSH Products
6.1.1. Urinary Gonadotropins versus Follitropin Alfa
6.1.2. Follitropin Alfa Originator versus Follitropin Alfa Biosimilars
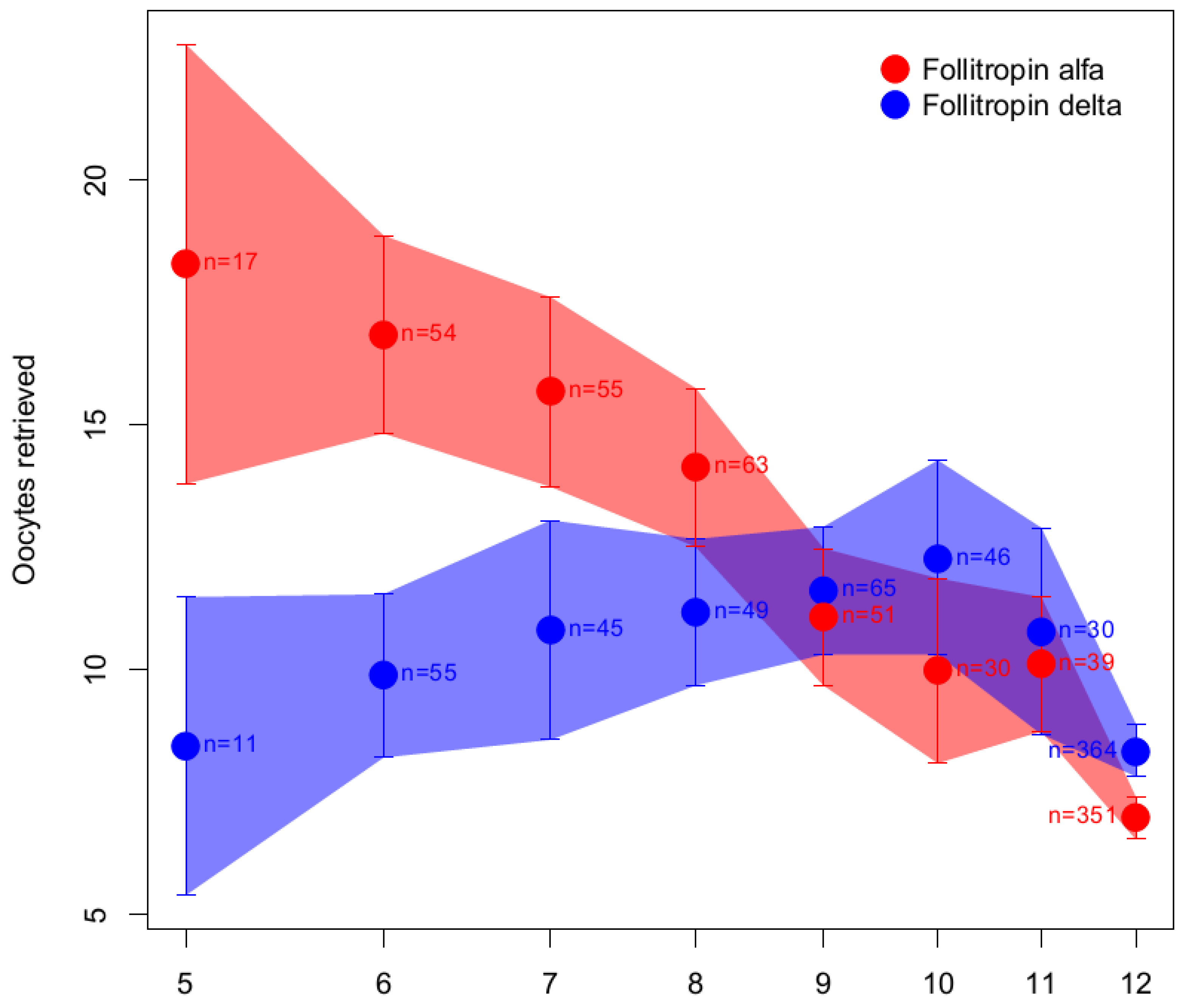
6.1.3. Follitropin Alfa Originator versus Follitropin Delta
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Marques, P.; Skorupskaite, K.; Rozario, K.S.; Anderson, R.A.; George, J.T. Physiology of GnRH and Gonadotropin Secretion. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK279070/ (accessed on 1 November 2022).
- Crowe, S.J.; Cushing, H.; Homans, J. Experimental Hypophysectomy. Johns Hopkins Hosp. Bull. 1910, 21, 127–167. [Google Scholar]
- Lunenfeld, B. Historical perspectives in gonadotrophin therapy. Hum Reprod Update 2004, 10, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Aschner, B. Ueber die Beziehung zwischen Hypophysis und Genitale. Arch. Gynäkol. 1912, 97, 200–227. [Google Scholar] [CrossRef]
- Smith, P.E.; Engle, E.T. Experimental evidence of the role of anterior pituitary in development and regulation of gonads. Am. J. Anat. 1927, 40, 159. [Google Scholar] [CrossRef]
- Zondek, B. Ueber die Funktion des Ovariums. Z. Geburtsh. Gynäkol. 1926, 90, 327. [Google Scholar]
- Smith, P.E. Hypophysectomy and replacement therapy in the rat. Am. J. Anat. 1930, 45, 205–274. [Google Scholar] [CrossRef]
- Zondek, B. Weitere Untersuchungen zur Darstellung. Biologie und Klinik des Hypophysenvorderlappenhormons (Prolan). Zentralbl. Gynäkol. 1929, 14, 834–848. [Google Scholar]
- Fevold, H.L.; Hisaw, F.L.; Leonard, S.L. The gonad stimulating and the luteinizing hormones of the anterior lobe of the hypophesis. Am. J. Physiol. 1931, 97, 291–301. [Google Scholar] [CrossRef]
- Ascheim, S.; Zondek, B. Hypophysenvorderlappen hormone und ovarialhormone im Harn von Schwangeren. Klin. Wochenschr. 1927, 6, 13–21. [Google Scholar] [CrossRef]
- Bassett, R.; Lispi, M.; Ceccarelli, D.; Grimaldi, L.; Mancinelli, M.; Martelli, F.; Van Dorsselaer, A. Analytical identification of additional impurities in urinary-derived gonadotrophins. Reprod. Biomed. Online 2009, 19, 300–313. [Google Scholar] [CrossRef]
- Lunenfeld, B.; Bilger, W.; Longobardi, S.; Alam, V.; D’Hooghe, T.; Sunkara, S.K. The development of gonadotropins for clinical use in the treatment of infertility. Front. Endocrinol. 2019, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Bassett, R.M.; Driebergen, R. Continued improvements in the quality and consistency of follitropin alfa, recombinant human FSH. Reprod. Biomed. Online 2005, 10, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N.; Devroey, P.; Arce, J.C. Clinical outcome following stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF: A randomized assessor-blind controlled trial. Hum. Reprod. 2006, 21, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.J.; Mol, B.W.; Longobardi, S.; Orvieto, R.; Venetis, C.A.; Lispi, M.; Storr, A.; D’Hooghe, T. Biosimilar recombinant follitropin alfa preparations versus the reference product (Gonal-F®) in couples undergoing assisted reproductive technology treatment: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2021, 19, 51. [Google Scholar] [CrossRef]
- Koechling, W.; Plaksin, D.; Croston, G.E.; Jeppesen, J.V.; Macklon, K.T.; Andersen, C.Y. Comparative pharmacology of a new recombinant FSH expressed by a human cell line. Endocr. Connect. 2017, 6, 297–305. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Audino, M.C.; Palinsky, W.; Broly, H.; Bierau, H. Current views on N-glycolylneuraminic acid in therapeutic recombinant proteins. Trends Pharmacol. Sci. 2021, 42, 943–956. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Satwekar, A.; Cutillo, F.; Ciampolillo, C.; Palinsky, W.; Longobardi, S. In-vivo biological activity and glycosylation analysis of a biosimilar recombinant human follicle-stimulating hormone product (Bemfola) compared with its reference medicinal product (GONAL-f). PLoS ONE 2017, 12, e0184139. [Google Scholar] [CrossRef] [PubMed]
- Olsson, H.; Sandstrom, R.; Grundemar, L. Different pharmacokinetic and pharmacodynamic properties of recombinant follicle-stimulating hormone (rFSH) derived from a human cell line compared with rFSH from a non-human cell line. J. Clin. Pharmacol. 2014, 54, 1299–1307. [Google Scholar] [CrossRef]
- Selman, H.; Pacchiarotti, A.; El-Danasouri, I. Ovarian stimulation protocols based on follicle-stimulating hormone glycosylation pattern: Impact on oocyte quality and clinical outcome. Fertil. Steril. 2010, 94, 1782–1786. [Google Scholar] [CrossRef]
- Bousfield, G.R.; Harvey, D.J. Follicle-stimulating hormone glycobiology. Endocrinology 2019, 160, 1515–1535. [Google Scholar] [CrossRef]
- Dias, J.A.; Ulloa-Aguirre, A. New human follitropin preparations: How glycan structural differences may affect biochemical and biological function and clinical effect. Front. Endocrinol. 2021, 12, 636038. [Google Scholar] [CrossRef] [PubMed]
- Manzi, L.; Sepe, N.; Migliaccio, W.; Lanzoni, L.; Iozzino, L.; D’Angelo, F.; Colarusso, L.; Montenegro, S.; Palmese, A.; D’Hooghe, T.; et al. Comparative assessment of the structural features of originator recombinant human follitropin alfa versus recombinant human follitropin alfa biosimilar preparations approved in non-European regions. Int. J. Mol. Sci. 2022, 23, 6762. [Google Scholar] [CrossRef] [PubMed]
- Bousfield, G.R.; May, J.V.; Davis, J.S.; Dias, J.A.; Kumar, T.R. In vivo and in vitro impact of carbohydrate variation on human follicle-stimulating hormone function. Front. Endocrinol. 2018, 9, 216. [Google Scholar] [CrossRef]
- Vaitukaitis, J.L.; Ross, G.T.; Braunstein, G.D.; Rayford, P.L. Gonadotropins and their subunits: Basic and clinical studies. Recent Progr. Horm. Res. 1976, 32, 289–331. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.S.; Kumar, T.R.; May, J.V.; Bousfield, G.R. Naturally occurring follicle-stimulating hormone glycosylation variants. J. Glycom. Lipidom. 2014, 4, e117. [Google Scholar] [CrossRef]
- Wide, L.; Eriksson, K. Dynamic changes in glycosylation and glycan composition of serum FSH and LH during natural ovarian stimulation. Ups J. Med. Sci. 2013, 118, 153–164. [Google Scholar] [CrossRef]
- Wide, L.; Eriksson, K. Molecular size and charge as dimensions to identify and characterize circulating glycoforms of human FSH, LH and TSH. Ups J. Med. Sci. 2017, 122, 217–223. [Google Scholar] [CrossRef]
- Wide, L.; Eriksson, K. Low-glycosylated forms of both FSH and LH play major roles in the natural ovarian stimulation. Ups J. Med. Sci. 2018, 123, 100–108. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Midgley, A.R., Jr.; Beitins, I.Z.; Padmanabhan, V. Follicle-stimulating isohormones: Characterization and physiological relevance. Endocr. Rev. 1995, 16, 765–787. [Google Scholar] [CrossRef]
- Kagawa, Y.; Takasaki, S.; Utsumi, J.; Hosoi, K.; Shimizu, H.; Kochibe, N.; Kobata, A. Comparative study of the asparagine-linked sugar chains of natural human interferon-beta 1 and recombinant human interferon-beta 1 produced by three different mammalian cells. J. Biol. Chem. 1988, 263, 17508–17515. [Google Scholar] [CrossRef]
- Svensson, E.C.; Soreghan, B.; Paulson, J.C. Organization of the beta-galactoside alpha 2,6-sialyltransferase gene. Evidence for the transcriptional regulation of terminal glycosylation. J. Biol. Chem. 1990, 265, 20863–20868. [Google Scholar] [CrossRef]
- Takeuchi, M.; Takasaki, S.; Miyazaki, H.; Kato, T.; Hoshi, S.; Kochibe, N.; Kobata, A. Comparative study of the asparagine-linked sugar chains of human erythropoietins purified from urine and the culture medium of recombinant Chinese hamster ovary cells. J. Biol. Chem. 1988, 263, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, I.; Plaksin, D.; White, R. Recombinant FSH Including Alpha 2,3- and Alpha 2,6-Sialylation. EUR Patent EP,226,866,6A1, 16 April 2009. [Google Scholar]
- Campo, S.; Andreone, L.; Ambao, V.; Urrutia, M.; Calandra, R.S.; Rulli, S.B. Hormonal regulation of follicle-stimulating hormone glycosylation in males. Front. Endocrinol. 2019, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Green, E.D.; Baenziger, J.U. Asparagine-linked oligosaccharides on lutropin, follitropin, and thyrotropin. I. Structural elucidation of the sulfated and sialylated oligosaccharides on bovine, ovine, and human pituitary glycoprotein hormones. J. Biol. Chem. 1988, 263, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Byrd-Leotis, L.; Cummings, R.D.; Steinhauer, D.A. The interplay between the host receptor and influenza virus hemagglutinin and neuraminidase. Int. J. Mol. Sci. 2017, 18, 1541. [Google Scholar] [CrossRef]
- Perlman, S.; van den Hazel, B.; Christiansen, J.; Gram-Nielsen, S.; Jeppesen, C.B.; Andersen, K.V.; Halkier, T.; Okkels, S.; Schambye, H.T. Glycosylation of an N-terminal extension prolongs the half-life and increases the in vivo activity of follicle stimulating hormone. J. Clin. Endocrinol. Metab. 2003, 88, 3227–3235. [Google Scholar] [CrossRef]
- Poulin, P. A single-species approach considering additional physiological information for prediction of hepatic clearance of glycoprotein derivate therapeutics. Clin. Pharmacokinet. 2011, 50, 665–674. [Google Scholar] [CrossRef]
- D’Antonio, M.; Borrelli, F.; Datola, A.; Bucci, R.; Mascia, M.; Polletta, P.; Piscitelli, D.; Papoian, R. Biological characterization of recombinant human follicle stimulating hormone isoforms. Hum. Reprod. 1999, 14, 1160–1167. [Google Scholar] [CrossRef]
- Zambrano, E.; Olivares, A.; Mendez, J.P.; Guerrero, L.; Diaz-Cueto, L.; Veldhuis, J.D.; Ulloa-Aguirre, A. Dynamics of basal and gonadotropin-releasing hormone-releasable serum follicle-stimulating hormone charge isoform distribution throughout the human menstrual cycle. J. Clin. Endocrinol. Metab. 1995, 80, 1647–1656. [Google Scholar] [CrossRef]
- Phillips, D.J.; Albertsson-Wikland, K.; Eriksson, K.; Wide, L. Changes in the isoforms of luteinizing hormone and follicle-stimulating hormone during puberty in normal children. J. Clin. Endocrinol. Metab. 1997, 82, 3103–3106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; May, J.; Butnev, V.; Shuai, B.; May, J.V.; Bousfield, G.R.; Kumar, T.R. Evaluation of in vivo bioactivities of recombinant hypo-(FSH(21/18)) and fully-(FSH(24)) glycosylated human FSH glycoforms in Fshb null mice. Mol. Cell Endocrinol. 2016, 437, 224–236. [Google Scholar] [CrossRef]
- Bousfield, G.R.; Butnev, V.Y.; Rueda-Santos, M.A.; Brown, A.; Hall, A.S.; Harvey, D.J. Macro- and micro-heterogeneity in pituitary and urinary follicle-stimulating hormone glycosylation. J. Glycom. Lipidom. 2014, 4, 125. [Google Scholar] [CrossRef]
- Jiang, C.; Hou, X.; Wang, C.; May, J.V.; Butnev, V.Y.; Bousfield, G.R.; Davis, J.S. Hypoglycosylated hFSH has greater bioactivity than fully glycosylated recombinant hFSH in human granulosa cells. J. Clin. Endocrinol. Metab. 2015, 100, E852–E860. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. ICH: E6 (R2) Good Clinical Practice–Scientific Guideline. 2016. Available online: https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice (accessed on 1 November 2022).
- Steelman, S.L.; Pohley, F.M. Assay of the follicle stimulating hormone based on the augmentation with human chorionic gonadotropin. Endocrinology 1953, 53, 604–616. [Google Scholar] [CrossRef]
- le Cotonnec, J.Y.; Porchet, H.C.; Beltrami, V.; Khan, A.; Toon, S.; Rowland, M. Clinical pharmacology of recombinant human follicle-stimulating hormone (FSH). I. Comparative pharmacokinetics with urinary human FSH. Fertil. Steril. 1994, 61, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Driebergen, R.; Baer, G. Quantification of follicle stimulating hormone (follitropin alfa): Is in vivo bioassay still relevant in the recombinant age? Curr. Med. Res. Opin. 2003, 19, 41–46. [Google Scholar] [CrossRef]
- Nevelli, F.; Palmese, A.; Gleixner, R.; Peroglio, F.; D’Acunto, C.W.; Dadone, A.; D’Hooghe, T.; Lispi, M. Biological assay to determine gonadotropin potency: From in-vivo to in-vitro sustainable method. Int. J. Med. Sci. 2023, 24, 8040. [Google Scholar] [CrossRef]
- Nevelli, F.; Peroglio, F.; Gleixner, R.; Dadone, A.; Palmese, A.; Lispi, M.; D’Hooghe, T.; D’Acunto, C.W. P-635. The impact of force-degraded variants of recombinant human follicle stimulating hormone alfa (r-hFSH alfa) on in-vitro and in-vivo biological activity. Hum. Reprod. 2022, 37, 584. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA/CHMP/836351/2022; Opinion of the Committee for Medicinal Products for Human Use on a Type II Variation to the Terms of the Marketing Authorisation; European Medicines Agency: Amsterdam, The Netherlands, 27 October 2022. [Google Scholar]
- European Medicines Agency. ICH Q5E Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process: Comparability of Biotechnological/Biological Products–Scientific Guideline. 2005. Available online: https://www.ema.europa.eu/en/ich-q5e-biotechnological-biological-products-subject-changes-their-manufacturing-process (accessed on 1 November 2022).
- Lispi, M.; Bassett, R.; Crisci, C.; Mancinelli, M.; Martelli, F.; Ceccarelli, D.; De Bellis, C.; Mendola, D. Comparative assessment of the consistency and quality of a highly purified FSH extracted from human urine (urofollitropin) and a recombinant human FSH (follitropin alpha). Reprod. Biomed. Online 2006, 13, 179–193. [Google Scholar] [CrossRef]
- Waldman, S.A. Does potency predict clinical efficacy? Illustration through an antihistamine model. Ann. Allergy Asthma Immunol. 2002, 89, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Bosch, E.; Vidal, C.; Labarta, E.; Simon, C.; Remohi, J.; Pellicer, A. Highly purified hMG versus recombinant FSH in ovarian hyperstimulation with GnRH antagonists--a randomized study. Hum. Reprod. 2008, 23, 2346–2351. [Google Scholar] [CrossRef] [PubMed]
- Devroey, P.; Pellicer, A.; Nyboe Andersen, A.; Arce, J.C.; Menopur in GnRH Antagonist Cycles with Single Embryo Transfer Trial Group. A randomized assessor-blind trial comparing highly purified hMG and recombinant FSH in a GnRH antagonist cycle with compulsory single-blastocyst transfer. Fertil. Steril. 2012, 97, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Group, E.A.I.S. Efficacy and safety of highly purified menotropin versus recombinant follicle-stimulating hormone in in vitro fertilization/intracytoplasmic sperm injection cycles: A randomized, comparative trial. Fertil. Steril. 2002, 78, 520–528. [Google Scholar] [CrossRef]
- Hompes, P.G.; Broekmans, F.J.; Hoozemans, D.A.; Schats, R. Effectiveness of highly purified human menopausal gonadotropin vs. recombinant follicle-stimulating hormone in first-cycle in vitro fertilization-intracytoplasmic sperm injection patients. Fertil. Steril. 2008, 89, 1685–1693. [Google Scholar] [CrossRef]
- Witz, C.A.; Daftary, G.S.; Doody, K.J.; Park, J.K.; Seifu, Y.; Yankov, V.I.; Heiser, P.W. Randomized, assessor-blinded trial comparing highly purified human menotropin and recombinant follicle-stimulating hormone in high responders undergoing intracytoplasmic sperm injection. Fertil. Steril. 2020, 114, 321–330. [Google Scholar] [CrossRef]
- de Mora, F.; Fauser, B. Biosimilars to recombinant human FSH medicines: Comparable efficacy and safety to the original biologic. Reprod. Biomed. Online 2017, 35, 81–86. [Google Scholar] [CrossRef]
- Grampp, G.; Ramanan, S. The Diversity of Biosimilar Design and Development: Implications for Policies and Stakeholders. BioDrugs 2015, 29, 365–372. [Google Scholar] [CrossRef]
- European Medicines Agency. Puregon: EPAR Product Information. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/puregon-epar-product-information_en.pdf (accessed on 2 January 2023).
- Olijve, W.; de Boer, W.; Mulders, J.W.; van Wezenbeek, P.M. Molecular biology and biochemistry of human recombinant follicle stimulating hormone (Puregon). Mol. Hum. Reprod. 1996, 2, 371–382. [Google Scholar] [CrossRef]
- Arce, J.C.; Larsson, P.; Garcia-Velasco, J.A. Establishing the follitropin delta dose that provides a comparable ovarian response to 150 IU/day follitropin alfa. Reprod. Biomed. Online 2020, 41, 616–622. [Google Scholar] [CrossRef]
- European Medicines Agency. Rekovelle Summary of Product Characteristics. 2017. Available online: https://www.ema.europa.eu/en/documents/product-information/rekovelle-epar-product-information_en.pdf (accessed on 1 November 2022).
- Nyboe Andersen, A.; Nelson, S.M.; Fauser, B.C.; Garcia-Velasco, J.A.; Klein, B.M.; Arce, J.C.; Group, E.-S. Individualized versus conventional ovarian stimulation for in vitro fertilization: A multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil. Steril. 2017, 107, 387–396.e384. [Google Scholar] [CrossRef] [PubMed]
- de Leeuw, R.; Mulders, J.; Voortman, G.; Rombout, F.; Damm, J.; Kloosterboer, L. Structure-function relationship of recombinant follicle stimulating hormone (Puregon). Mol. Hum. Reprod. 1996, 2, 361–369. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. GONAL-f Summary of Product Characteristics. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/gonal-f-epar-product-information_en.pdf (accessed on 1 November 2022).
- Ulloa-Aguirre, A.; Timossi, C. Biochemical and functional aspects of gonadotrophin-releasing hormone and gonadotrophins. Reprod. Biomed. Online 2000, 1, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Mannaerts, B.M.; Rombout, F.; Out, H.J.; Coelingh Bennink, H. Clinical profiling of recombinant follicle stimulating hormone (rFSH.; Puregon): Relationship between serum FSH and efficacy. Hum. Reprod. Update 1996, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chappel, S.C. Heterogeneity of follicle stimulating hormone: Control and physiological function. Hum. Reprod. Update 1995, 1, 479–487. [Google Scholar] [CrossRef]
- Palmese, A.; Satwekar, A.; Melchiorre, M.; Colarusso, L.; Lispi, M. In-depth glycosylation profiling of r-hFSH-alfa and r-hFSH-delta in comparison with human pituitary follicle stimulating hormone (hFSH). Fertil. Steril. 2022, 118, e283. [Google Scholar] [CrossRef]
- Brokso Kyhl, L.E.; Hesse, C.; Larsson, P.; Bruzelius, K.; Mannaerts, B. First-in-human trial assessing the pharmacokinetic-pharmacodynamic profile of a novel recombinant human chorionic gonadotropin in healthy women and men of reproductive age. Clin. Transl. Sci. 2021, 14, 1590–1599. [Google Scholar] [CrossRef]
- Frydman, R.; Howles, C.M.; Truong, F. A double-blind, randomized study to compare recombinant human follicle stimulating hormone (FSH.; Gonal-F) with highly purified urinary FSH (Metrodin) HP) in women undergoing assisted reproductive techniques including intracytoplasmic sperm injection. The French Multicentre Trialists. Hum. Reprod. 2000, 15, 520–525. [Google Scholar] [CrossRef]
- The ESHRE Guideline Group on Ovarian Stimulation; Bosch, E.; Broer, S.; Griesinger, G.; Grynberg, M.; Humaidan, P.; Kolibianakis, E.; Kunicki, M.; La Marca, A.; Lainas, G.; et al. ESHRE guideline: Ovarian stimulation for IVF/ICSI. Hum. Reprod. Open 2020, 2020, hoaa009. [Google Scholar]
- European Medicines Agency. Guideline on Non-Clinical and Clinical Development of Similar Biological Medicinal Products Containing Recombinant Human Follicle Stimulating Hormone (r-hFSH). 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-clinical-development-similar-biological-medicinal-products-containing_en.pdf (accessed on 1 November 2022).
- Allegra, A.; Marino, A.; Volpes, A.; Coffaro, F.; Scaglione, P.; Gullo, S.; La Marca, A. A randomized controlled trial investigating the use of a predictive nomogram for the selection of the FSH starting dose in IVF/ICSI cycles. Reprod. Biomed. Online 2017, 34, 429–438. [Google Scholar] [CrossRef]
- Shavit, T.; Shalom-Paz, E.; Samara, N.; Aslih, N.; Michaeli, M.; Ellenbogen, A. Comparison between stimulation with highly purified hMG or recombinant FSH in patients undergoing IVF with GnRH antagonist protocol. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2016, 32, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Grynberg, M.; Cedrin-Durnerin, I.; Raguideau, F.; Herquelot, E.; Luciani, L.; Porte, F.; Verpillat, P.; Helwig, C.; Schwarze, J.E.; Paillet, S.; et al. Comparative effectiveness of gonadotropins used for ovarian stimulation during assisted reproductive technologies (ART) in France: A real-world observational study from the French nationwide claims database (SNDS). Best Pract. Research. Clin. Obstet. Gynaecol. 2022, 102308. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, O.; Klein, B.M.; Arce, J.C.; Japanese Follitropin Delta Phase 2 Trial Group. Randomized, assessor-blind, antimullerian hormone-stratified, dose-response trial in Japanese in vitro fertilization/intracytoplasmic sperm injection patients undergoing controlled ovarian stimulation with follitropin delta. Fertil. Steril. 2021, 115, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Zhang, Y.; Liang, X.; Ho, T.; Huang, H.Y.; Kim, S.H.; Goethberg, M.; Mannaerts, B.; Arce, J.C. A randomised controlled trial to clinically validate follitropin delta in its individualised dosing regimen for ovarian stimulation in Asian IVF/ICSI patients. Hum. Reprod. 2021, 36, 2452–2462. [Google Scholar] [CrossRef]
- Longobardi, S.; D’Hooghe, T. Comment by Longobardi and D’Hooghe Regarding Article “Individualized versus Conventional Ovarian Stimulation for in vitro Fertilization: A Multicenter, Randomized, Controlled, Assessor-Blinded, Phase 3 Noninferiority Trial”. Fertil Steril Dial. 2017. Available online: www.fertstertdialog.com/posts/12852-23086 (accessed on 1 November 2022).
- Montenegro Gouveia, S.; Lispi, M.; D’Hooghe, T.M. Comparison between follitropin-delta and follitropin-alfa for ovarian stimulation in context of ART is only scientifically sound and clinically relevant if individualization of starting dose is allowed in both arms! Reprod. Biomed. Online 2022, 45, 623–624. [Google Scholar] [CrossRef]
- Arce, J.C.; Andersen, A.N.; Fernández-Sánchez, M.; Visnova, H.; Bosch, E.; García-Velasco, J.A.; Barri, P.; de Sutter, P.; Klein, B.M.; Fauser, B.C. Ovarian response to recombinant human follicle-stimulating hormone: A randomized, antimüllerian hormone-stratified, dose-response trial in women undergoing in vitro fertilization/intracytoplasmic sperm injection. Fertil. Steril. 2014, 102, 1633–1640.e1635. [Google Scholar] [CrossRef]
- Batista, A.R.; Akbas, E.; Coulidiaty, G.V.; Schwarze, J.-E.; Kohlhepp, F.; Shanker Gupta, S.S.; Velthuis, E.; Kharbat, A.; Lispi, M.; D’Hooghe, T.; et al. Regarding Article “Randomized, Assessor-Blinded Trial Comparing Highly Purified Human Menotropin and Recombinant Follicle-Stimulating Hormone. Fertil Steril Dial. 2021. Available online: https://www.fertstertdialog.com/documents/comment-by-batista-et-al-regarding-article-randomized-assessor-blinded-trial-comparing-highly-purified-human-menotropin-and-recombinant-follicle-stimulating-hormone (accessed on 1 November 2022).
- National Cancer Institute. Definition of Mechanism of Action–NCI Dictionary of Cancer Terms. 2011. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/mechanism-of-action (accessed on 1 November 2022).
- European Medicines Agency. ICH Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products–Scientific Guideline. 1999. Available online: https://www.ema.europa.eu/en/ich-q6b-specifications-test-procedures-acceptance-criteria-biotechnologicalbiological-products (accessed on 1 November 2022).
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef]
- Eichler, J. Protein glycosylation. Curr. Biol. 2019, 29, R229–R231. [Google Scholar] [CrossRef]
- European Pharmacopoeia. EP10.0; Follitropin European Pharmacopoeia. European Directorate for the Quality of Medicines & Healthcare: Strasbourg, France, 2020. [Google Scholar]
- National Institute for Biological Standards and Control (NIBSC). WHO International Standards. 2022. Available online: https://www.nibsc.org/standardisation/international_standards.aspx (accessed on 1 November 2022).
- World Health Organization. WHO Expert Committee on Biological Standardization; Technical Report No. 565; World Health Organization: Geneva, Switzerland, 1975. [Google Scholar]
- International Union of Pure and Applied Chemistry (IUPAC). Compendium of Chemical Terminology, The Gold Book, 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]

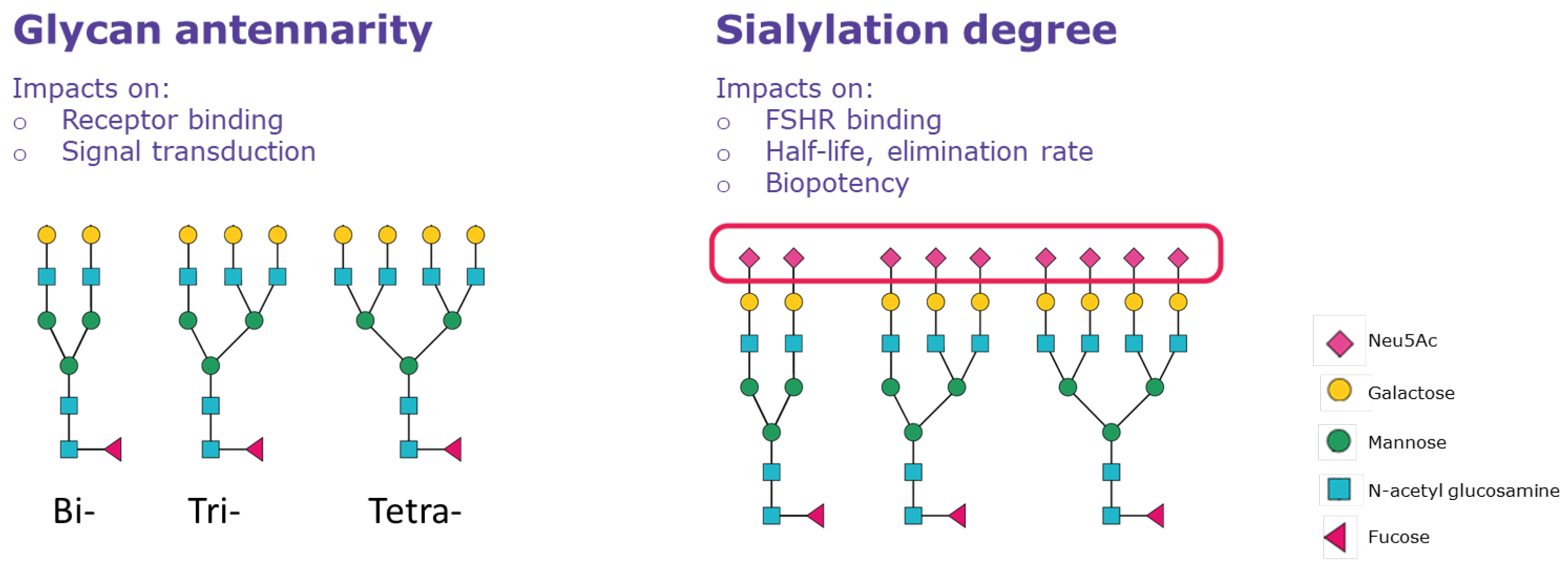
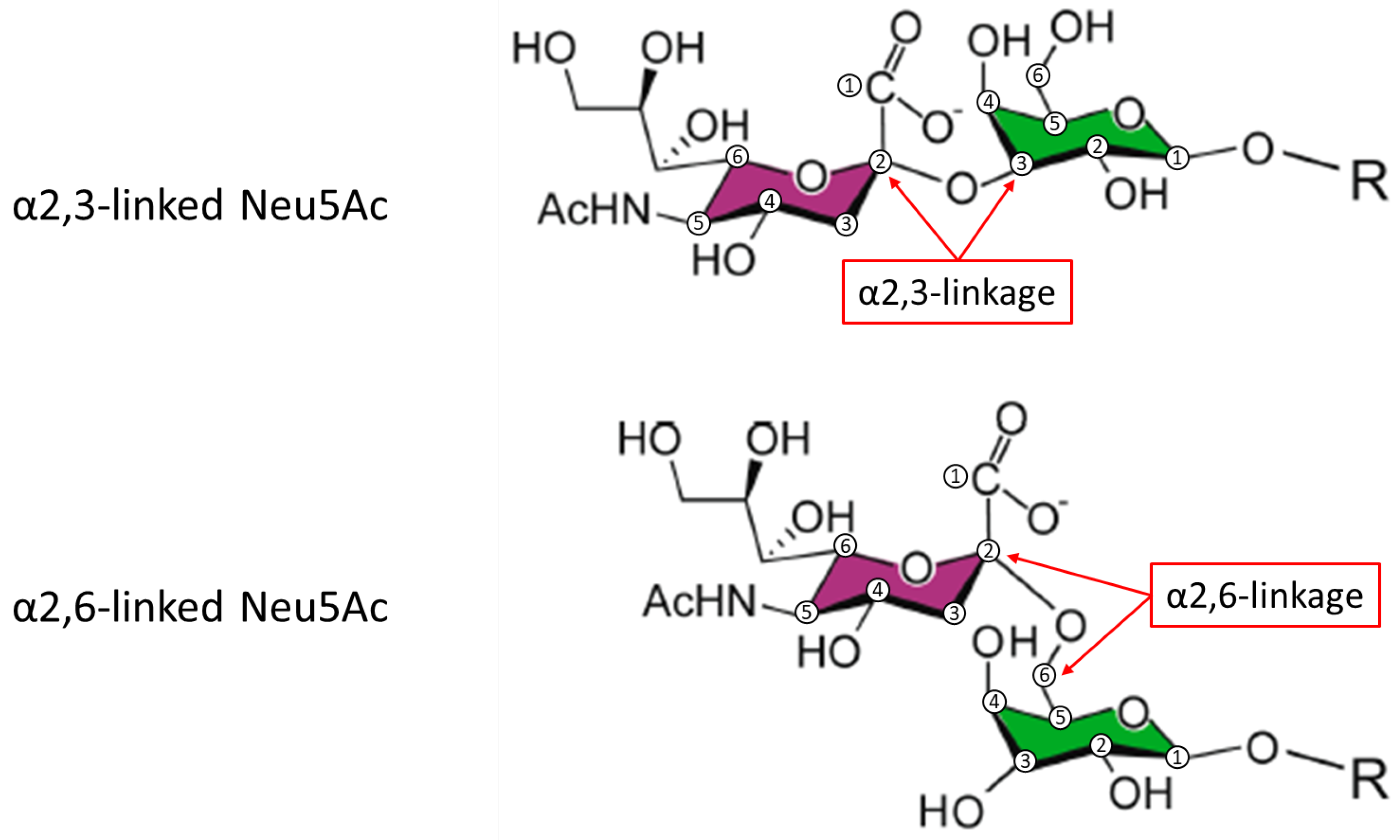

| Heterogeneity | Structural Feature | Glycoform Properties |
|---|---|---|
| Macro-heterogeneity | β-subunit glycan occupancy | FSH18,21 ↑ FSHR affinity ↑ in vitro biological activity ↓ half-life ↑ plasma clearance FSH24 ↓ FSHR affinity ↓ in vitro biological activity ↑ half-life ↓ plasma clearance |
| Micro-heterogeneity | Terminal branches antennarity | High degree of antennarity ↓ FSHR binding (delayed receptor response) Low degree of antennarity ↑ FSHR binding (rapid receptor response) |
| Sialylation (acidity/sulfation) | More acidic forms ↓ FSHR binding ↑ half-life ↓ elimination rate Less acidic forms ↑ FSHR binding ↓ half-life ↑ elimination rate |
| Follitropin Alfa | Follitropin Beta | Follitropin Delta | Urinary Products | |
|---|---|---|---|---|
| FSH content (quantity of protein by chromatographic methods) | Dosed in μg | NA | Dosed in μg | NA |
| Biopotency of specimen according to biological activity Steelman–Pohley bioassay | Dosed in IU | Dosed in IU | NA | Dosed in IU |
| Specific bioactivity * expressed in IU/mg FSH | 13,636 IU/mg | 10,000 IU/mg | NA | NA |
| Filling process | Filled-by-mass (μg of FSH protein) and labelled in μg and IU | Filled by IU | Filled-by-mass (μg of FSH protein) | Filled by IU |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lispi, M.; Humaidan, P.; Bousfield, G.R.; D’Hooghe, T.; Ulloa-Aguirre, A. Follicle-Stimulating Hormone Biological Products: Does Potency Predict Clinical Efficacy? Int. J. Mol. Sci. 2023, 24, 9020. https://doi.org/10.3390/ijms24109020
Lispi M, Humaidan P, Bousfield GR, D’Hooghe T, Ulloa-Aguirre A. Follicle-Stimulating Hormone Biological Products: Does Potency Predict Clinical Efficacy? International Journal of Molecular Sciences. 2023; 24(10):9020. https://doi.org/10.3390/ijms24109020
Chicago/Turabian StyleLispi, Monica, Peter Humaidan, George R. Bousfield, Thomas D’Hooghe, and Alfredo Ulloa-Aguirre. 2023. "Follicle-Stimulating Hormone Biological Products: Does Potency Predict Clinical Efficacy?" International Journal of Molecular Sciences 24, no. 10: 9020. https://doi.org/10.3390/ijms24109020
APA StyleLispi, M., Humaidan, P., Bousfield, G. R., D’Hooghe, T., & Ulloa-Aguirre, A. (2023). Follicle-Stimulating Hormone Biological Products: Does Potency Predict Clinical Efficacy? International Journal of Molecular Sciences, 24(10), 9020. https://doi.org/10.3390/ijms24109020






