Analysis of the Ischemia-Modified Albumin as a Potential Biomarker for Cardiovascular Damage in Obstructive Sleep Apnea Patients with Acute Coronary Syndrome
Abstract
1. Introduction
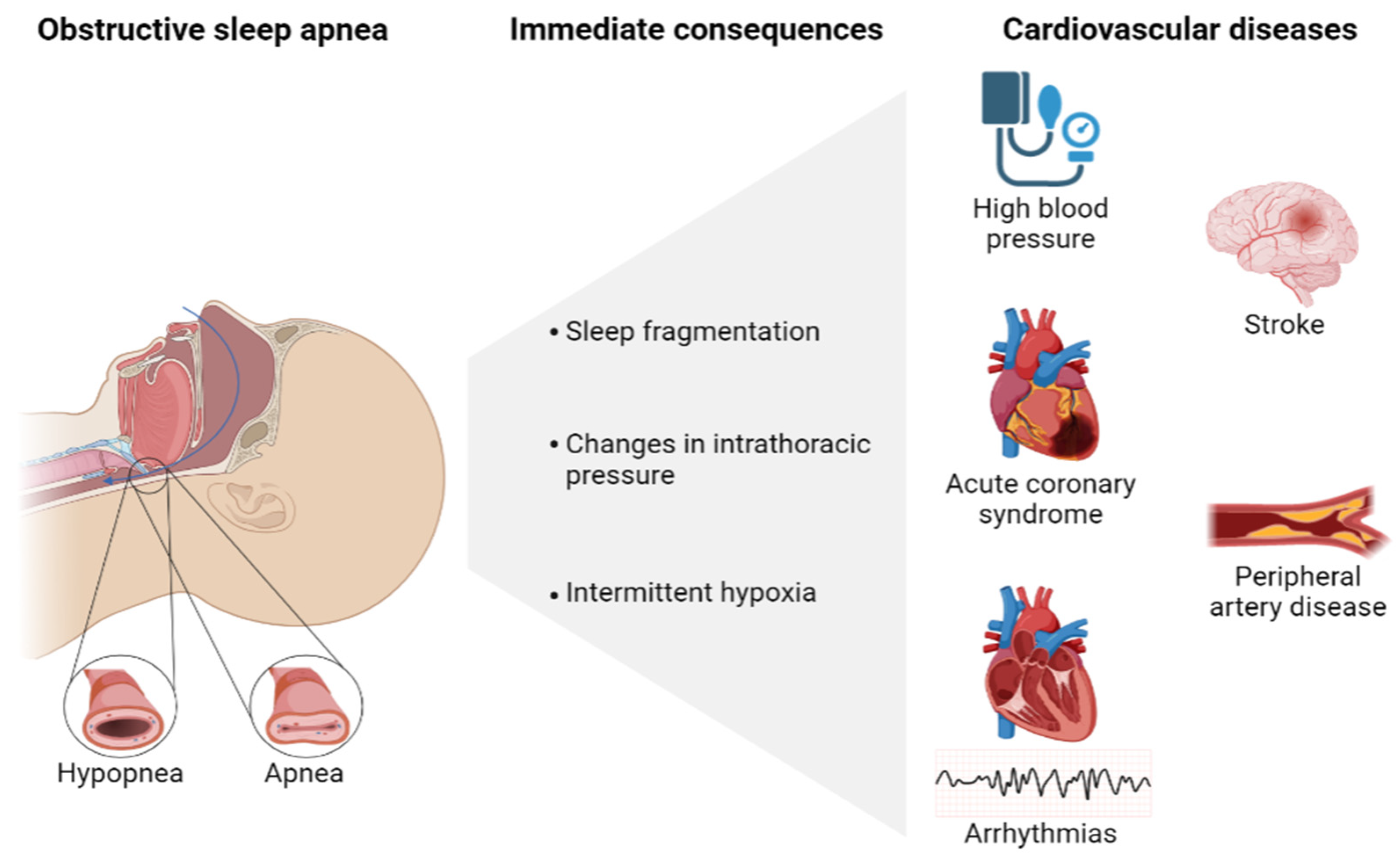
1.1. Role of Obstructive Sleep Apnea in the Development of Cardiovascular Disease
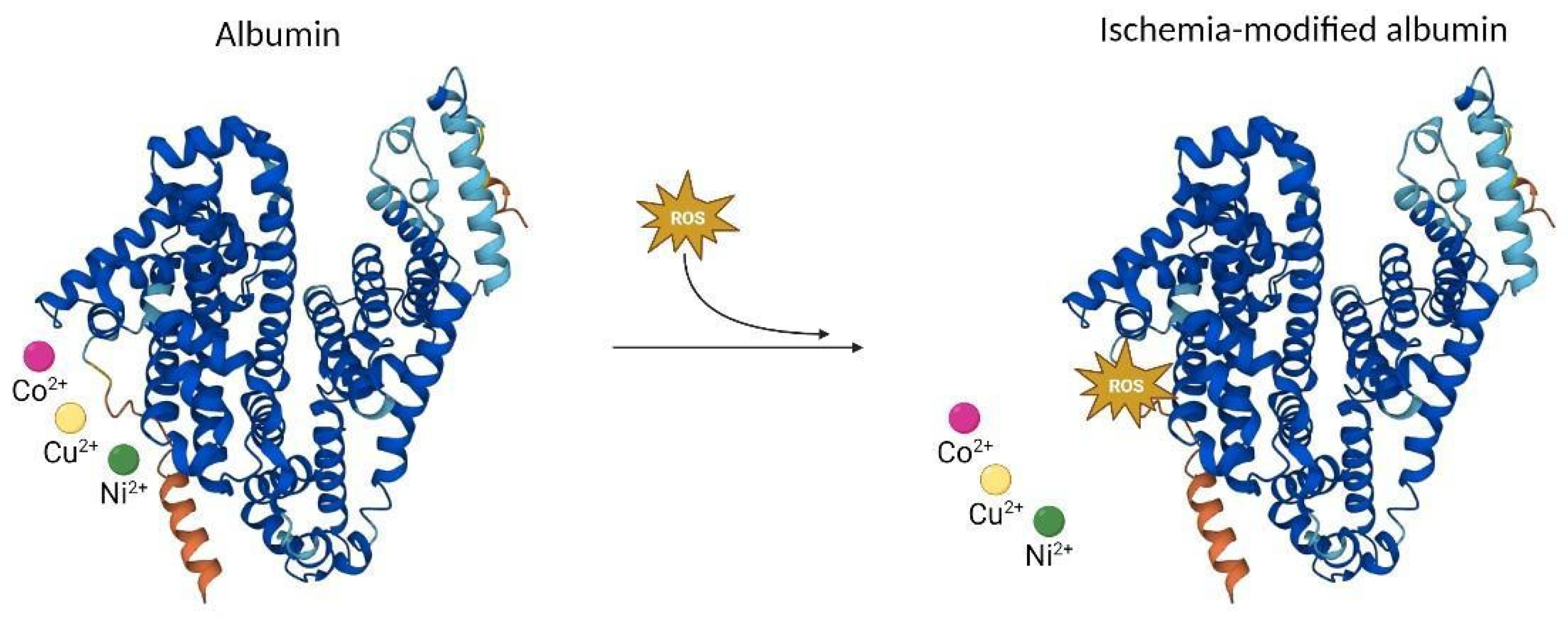
1.2. Obstructive Sleep Apnea Biomarkers
2. Results
2.1. Patient Characteristics
2.2. Cardiovascular Parameters Related to the Severity of Acute Coronary Syndrome
2.3. Serum Levels of Ischemia-Modified Albumin Are Increased in Patients with Moderate–Severe Obstructive Sleep Apnea
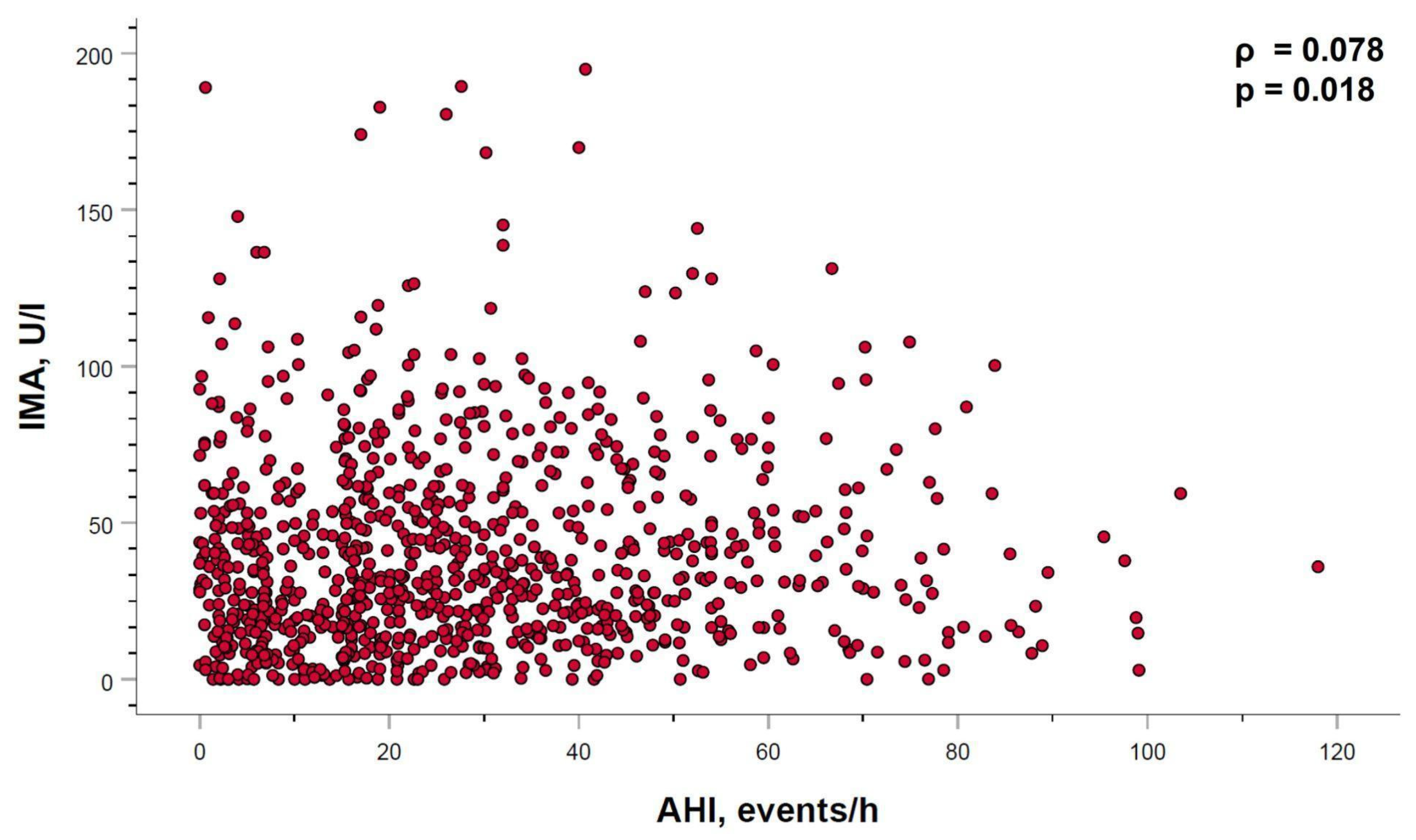
2.4. Relation between Ischemia-Modified Albumin Levels and Sleep Parameters
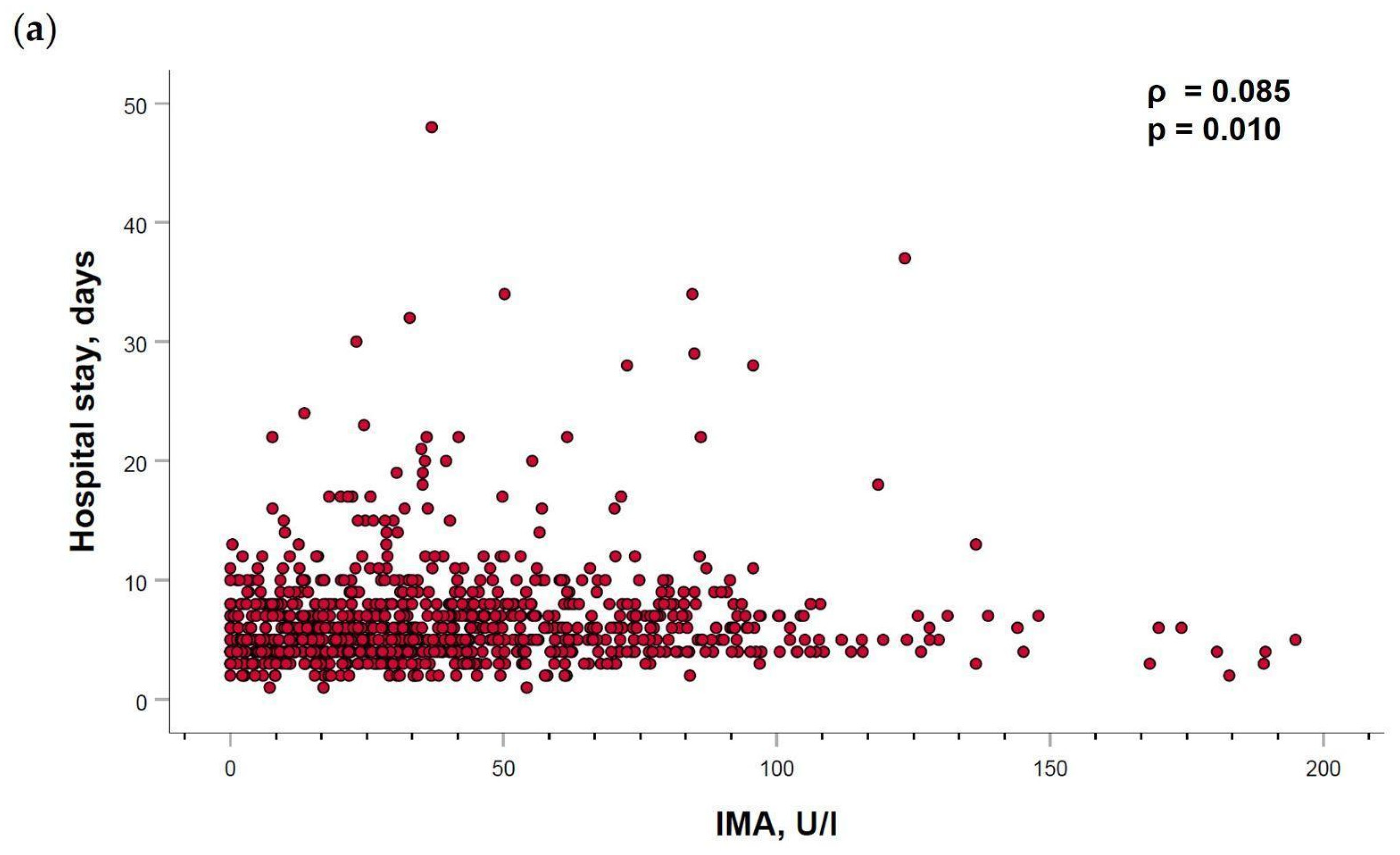
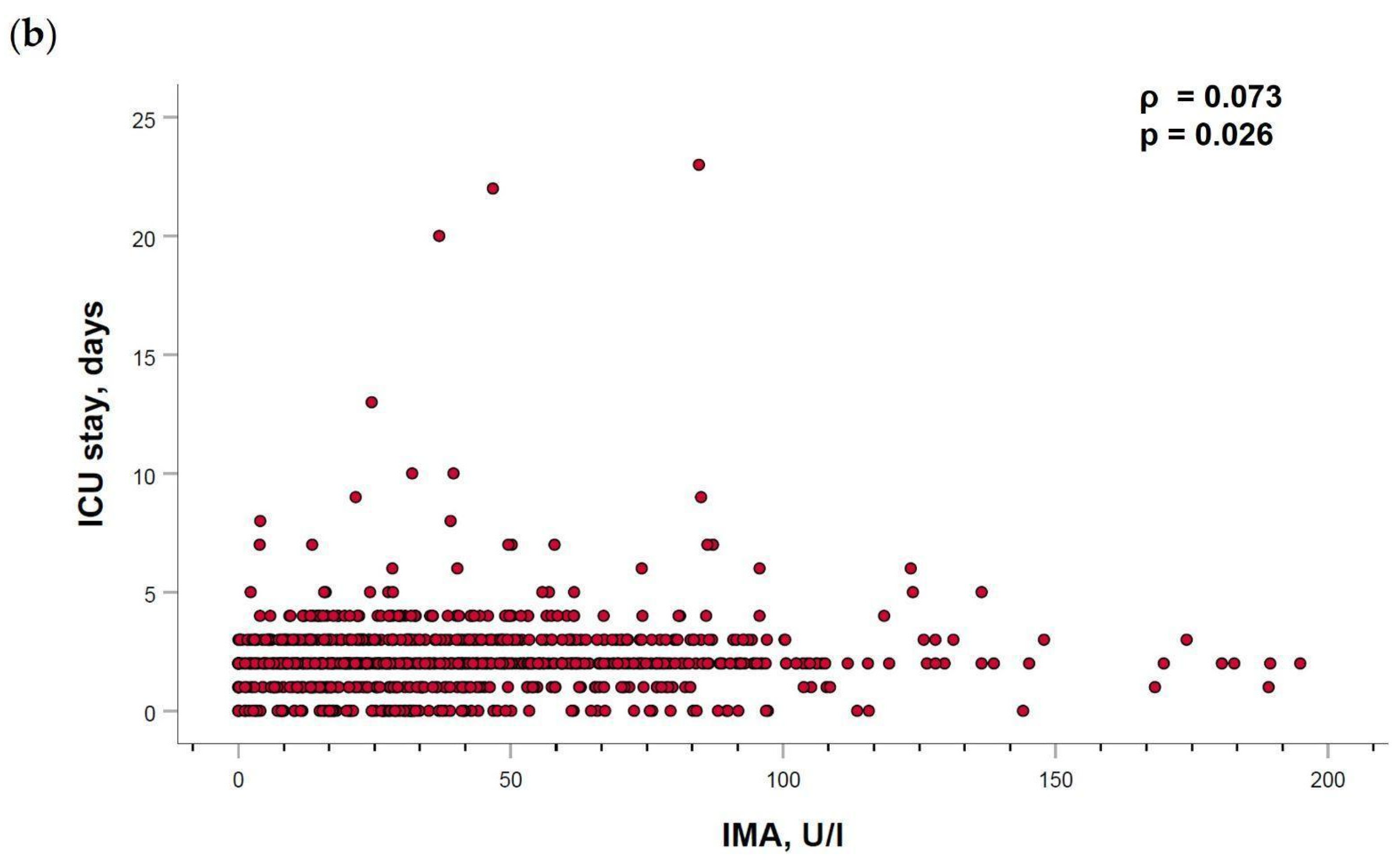
2.5. Ischemia-Modified Albumin Levels Correlation with Cardiovascular Parameters
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Procedures
4.2.1. General Procedures
4.2.2. Sleep Study Methodology
4.2.3. Blood Sample Determination
4.2.4. Cardiovascular Parameters
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABSU | absorbance units |
| ACS | acute coronary syndrome |
| AHI | apnea hypopnea index |
| ALT | alanine aminotransferase |
| APTT | activated partial thromboplastin clotting time |
| ARAII | angiotensin II receptor antagonists |
| AST | aspartate aminotransferase |
| ACEI | angiotensin convertase enzyme inhibitors |
| BMI | body mass index |
| BP | blood pressure |
| CK | creatine kinase |
| CK-MB | creatine kinase isoform 2 |
| CPAP | continuous positive airway pressure |
| CRP | C-reactive protein |
| CV | cardiovascular |
| EDS | excessive daytime sleepiness |
| EDTA | ethylene diamine tetra-acetic acid |
| ELISA | enzyme-linked immunosorbent assay |
| ESS | Epworth sleepiness scale |
| FDA | Food and Drug Administration |
| HBP | high blood pressure |
| HDL | high density lipoprotein |
| Hs-cTnT | high sensitive cardiac troponin T |
| ICU | intensive care unit |
| IL-6 | interleukin 6 |
| IL-8 | interleukin 8 |
| IQR | interquartile range |
| IMA | ischemia-modified albumin |
| LDL | low density lipoprotein |
| LVEF | left ventricular ejection fraction |
| Min SaO2 | minimum nocturnal oxygen saturation |
| mDBP | median diastolic blood pressure |
| mSBP | median systolic blood pressure |
| N-terminal | amino terminal region |
| NYHA | New York Heart Association |
| OSA | obstructive sleep apnea |
| ODI | oxygen desaturation index |
| SaO2 | oxygen saturation |
| SD | standard deviation |
| SEM | standard error of the mean |
| ROS | reactive oxygen species |
Appendix A
| Parameters | No/Mild OSA | Moderate OSA | Severe OSA | p-Value |
|---|---|---|---|---|
| mSBP, mm Hg | 121.93 ± 17.26 | 122.85 ± 16.41 | 124.73 ± 18.36 | 0.122 |
| mDBP, mm Hg | 72.01 ± 11.31 | 71.76 ± 10.55 | 72.72 ± 10.60 | 0.491 |
| Heart rate, bpm | 72.25 ± 12.08 | 72.27 ± 12.53 | 72.64 ± 13.34 | 0.907 |
| Hematocrit, % | 42.40 ± 5.60 | 41.91 ± 4.43 | 42.27 ± 4.27 | 0.431 |
| Hemoglobin, g/L | 144.92 ± 16.34 | 142.20 ± 15.83 | 143.22 ± 15.72 | 0.133 |
| Erythrocytes, ×1012/L | 4.72 ± 0.55 | 4.67 ± 0.53 | 4.70 ± 0.52 | 0.568 |
| Leukocytes, ×103/µL | 10.11 ± 3.17 | 10.12 ± 2.91 | 9.97 ± 2.78 | 0.774 |
| Platelets, ×103/µL | 221.72 ± 61.15 | 215.69 ± 55.21 | 217.52 ± 63.12 | 0.489 |
| Glucose, mg/dL | 120.80 ± 50.32 | 125.05 ± 51.69 | 131.06 ± 54.39 | 0.052 |
| Creatinine, mg/dL | 1.13 ± 3.95 | 1.84 ± 8.49 | 2.96 ± 26.66 | 0.426 |
| Uric acid, mg/dL | 5.59 ± 2.54 | 5.96 ± 1.56 | 6.46 ± 5.16 | 0.047 |
| Cholesterol, mg/dL | 175.62 ± 41.75 | 178.57 ± 42.20 | 179.65 ± 43.87 | 0.523 |
| HDL, mg/dL | 41.66 ± 11.74 | 41.07 ± 11.22 | 40.79 ± 18.13 | 0.780 |
| LDL, mg/dL | 106.94 ± 37.60 | 107.74 ± 34.90 | 110.36 ± 35.79 | 0.487 |
| Triglycerides, mg/dL | 138.47 ± 79.56 | 149.04 ± 81.31 | 164.74 ± 117.05 | 0.005 * |
| AST, U/L | 60.83 ± 64.05 | 78.17 ± 93.07 | 68.89 ± 86.43 | 0.082 |
| ALT, U/L | 35.73 ± 29.25 | 43.65 ± 130.86 | 38.74 ± 30.95 | 0.502 |
Appendix B
References
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Mediano, O.; González-Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International Consensus Document on Obstructive Sleep Apnea. Arch. Bronconeumol. 2022, 58, 52–68. [Google Scholar] [CrossRef]
- Suen, C.; Wong, J.; Ryan, C.M.; Goh, S.; Got, T.; Chaudhry, R.; Lee, D.S.; Chung, F. Prevalence of Undiagnosed Obstructive Sleep Apnea among Patients Hospitalized for Cardiovascular Disease and Associated In-Hospital Outcomes: A Scoping Review. J. Clin. Med. 2020, 9, 989. [Google Scholar] [CrossRef] [PubMed]
- Shahar, E.; Whitney, C.W.; Redline, S.; Lee, E.T.; Newman, A.B.; Nieto, F.J.; O’Connor, G.T.; Boland, L.L.; Schwartz, J.E.; Samet, J.M. Sleep-disordered breathing and cardiovascular disease: Cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001, 163, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Patel, N.K.; O’Hearn, D.J.; Khan, S. Resistant hypertension and obstructive sleep apnea. Int. J. Hypertens. 2013, 2013, 193010. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, D.J.; Yenokyan, G.; Newman, A.B.; O’Connor, G.T.; Punjabi, N.M.; Quan, S.F.; Redline, S.; Resnick, H.E.; Tong, E.K.; Diener-West, M.; et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The sleep heart health study. Circulation 2010, 122, 352–360. [Google Scholar] [CrossRef]
- Peker, Y.; Carlson, J.; Hedner, J. Increased incidence of coronary artery disease in sleep apnoea: A long-term follow-up. Eur. Respir. J. 2006, 28, 596–602. [Google Scholar] [CrossRef]
- Kuniyoshi, F.H.; Garcia-Touchard, A.; Gami, A.S.; Romero-Corral, A.; Van der Walt, C.; Pusalavidyasagar, S.; Kara, T.; Caples, S.M.; Pressman, G.S.; Vasquez, E.C.; et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J. Am. Coll. Cardiol. 2008, 52, 343–346. [Google Scholar] [CrossRef]
- Lee, C.H.; Sethi, R.; Li, R.; Ho, H.H.; Hein, T.; Jim, M.H.; Loo, G.; Koo, C.Y.; Gao, X.F.; Chandra, S.; et al. Obstructive Sleep Apnea and Cardiovascular Events after Percutaneous Coronary Intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef]
- Nakashima, H.; Kurobe, M.; Minami, K.; Furudono, S.; Uchida, Y.; Amenomori, K.; Nunohiro, T.; Takeshita, S.; Maemura, K. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 75–84. [Google Scholar] [CrossRef]
- Qu, H.; Guo, M.; Zhang, Y.; Shi, D.Z. Obstructive sleep apnea increases the risk of cardiac events after percutaneous coronary intervention: A meta-analysis of prospective cohort studies. Sleep Breath. 2018, 22, 33–40. [Google Scholar] [CrossRef]
- Jia, S.; Zhou, Y.J.; Yu, Y.; Wu, S.J.; Sun, Y.; Wang, Z.J.; Liu, X.L.; King, B.E.; Zhao, Y.X.; Shi, D.M.; et al. Obstructive sleep apnea is associated with severity and long-term prognosis of acute coronary syndrome. J. Geriatr. Cardiol. 2018, 15, 146–152. [Google Scholar] [CrossRef]
- Barbé, F.; Durán-Cantolla, J.; Sánchez-de-la-Torre, M.; Martínez-Alonso, M.; Carmona, C.; Barceló, A.; Chiner, E.; Masa, J.F.; Gonzalez, M.; Marín, J.M.; et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: A randomized controlled trial. JAMA 2012, 307, 2161–2168. [Google Scholar] [CrossRef]
- Peker, Y.; Glantz, H.; Eulenburg, C.; Wegscheider, K.; Herlitz, J.; Thunström, E. Effect of Positive Airway Pressure on Cardiovascular Outcomes in Coronary Artery Disease Patients with Nonsleepy Obstructive Sleep Apnea. The RICCADSA Randomized Controlled Trial. Am. J. Respir. Crit. Care Med. 2016, 194, 613–620. [Google Scholar] [CrossRef]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Sánchez-de-la-Torre, A.; Bertran, S.; Abad, J.; Duran-Cantolla, J.; Cabriada, V.; Mediano, O.; Masdeu, M.J.; Alonso, M.L.; Masa, J.F.; et al. Effect of obstructive sleep apnoea and its treatment with continuous positive airway pressure on the prevalence of cardiovascular events in patients with acute coronary syndrome (ISAACC study): A randomised controlled trial. Lancet Respir. Med. 2020, 8, 359–367. [Google Scholar] [CrossRef]
- Zapater, A.; Sánchez-de-la-Torre, M.; Benítez, I.D.; Targa, A.; Bertran, S.; Torres, G.; Aldomà, A.; De Batlle, J.; Abad, J.; Duran-Cantolla, J.; et al. The Effect of Sleep Apnea on Cardiovascular Events in Different Acute Coronary Syndrome Phenotypes. Am. J. Respir. Crit. Care Med. 2020, 202, 1698–1706. [Google Scholar] [CrossRef]
- Sánchez-de-la-Torre, M.; Campos-Rodriguez, F.; Barbé, F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013, 1, 61–72. [Google Scholar] [CrossRef]
- Resano-Barrio, M.P.; Arroyo-Espliguero, R.; Viana-Llamas, M.C.; Mediano, O. Obstructive Sleep Apnoea Syndrome: Continuous Positive Airway Pressure Therapy for Prevention of Cardiovascular Risk. Eur. Cardiol. 2020, 29, e65. [Google Scholar] [CrossRef]
- Canto Gde, L.; Pacheco-Pereira, C.; Aydinoz, S.; Major, P.W.; Flores-Mir, C.; Gozal, D. Biomarkers associated with obstructive sleep apnea: A scoping review. Sleep Med. Rev. 2015, 23, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wei, P.; Qin, Y.; Wei, Y. Is C-reactive protein a marker of obstructive sleep apnea?: A meta-analysis. Medicine 2017, 96, e6850. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Gámez, B.; Fernandez-Marin, M.C.; Gómez-Chaparro, J.L.; Muñoz-Cabrera, L.; Lopez-Barea, J.; Perez-Jimenez, F.; Lopez-Miranda, J. Relationship of oxidative stress and endothelial dysfunction in sleep apnoea. Eur. Respir. J. 2010, 37, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.F.; Chen, Y.H.; Chen, H.C.; Huang, W.C. Interactions among Obstructive Sleep Apnea Syndrome Severity, Sex, and Obesity on Circulatory Inflammatory Biomarkers in Patients with Suspected Obstructive Sleep Apnea Syndrome: A Retrospective, Cross-Sectional Study. Int. J. Environ. Res. Public Health 2020, 17, 4701. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Chen, C.L.; Yu, C.C.; Chen, T.T.; Tseng, S.T.; Ho, C.H. Association of inflammation and oxidative stress with obstructive sleep apnea in ischemic stroke patients. Sleep Med. 2015, 16, 113–118. [Google Scholar] [CrossRef]
- Düger, M.; Seyhan, E.C.; Günlüoğlu, M.Z.; Bolatkale, M.; Özgül, M.A.; Turan, D.; Uğur, E.; Ülfer, G. Does ischemia-modified albumin level predict severity of obstructive sleep apnea? Sleep Breath. 2021, 25, 65–73. [Google Scholar] [CrossRef]
- Mothes, E.; Faller, P. Evidence that the principal CoII-binding site in human serum albumin is not at the N-terminus: Implication on the albumin cobalt binding test for detecting myocardial ischemia. Biochemistry 2007, 46, 2267–2274. [Google Scholar] [CrossRef]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Lai, E.M.; Rios, P.A.; Yang, J.; Ortega-Lopez, A.M.; Shinoda, H.; Honda, S.A.; Rios, C.N.; Sugiyama, C.E.; Ha, C.E. Evaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin. Chem. 2003, 49, 581–585. [Google Scholar] [CrossRef]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef]
- Gaze, D.C. Ischemia modified albumin: A novel biomarker for the detection of cardiac ischemia. Drug Metab. Pharmacokinet. 2009, 24, 333–341. [Google Scholar] [CrossRef]
- Talwalkar, S.S.; Bon-Homme, M.; Miller, J.J.; Elin, R.J. Ischemia modified albumin, a marker of acute ischemic events: A pilot study. Ann. Clin. Lab. Sci. 2008, 38, 132–137. [Google Scholar] [PubMed]
- Zhong, Y.; Wang, N.; Xu, H.; Hou, X.; Xu, P.; Zhou, Z. Ischemia-modified albumin in stable coronary atherosclerotic heart disease: Clinical diagnosis and risk stratification. Coron. Artery Dis. 2012, 23, 538–541. [Google Scholar] [CrossRef]
- Van Belle, E.; Dallongeville, J.; Vicaut, E.; Degrandsart, A.; Baulac, C.; Montalescot, G. Ischemia-modified albumin levels predict long-term outcome in patients with acute myocardial infarction. The French Nationwide OPERA study. Am. Heart J. 2010, 159, 570–576. [Google Scholar] [CrossRef]
- Kazanis, K.; Dalamaga, M.; Nounopoulos, C.; Manolis, A.S.; Sakellaris, N.; Jullien, G.; Dionyssiou-Asteriou, A. Ischemia modified albumin, high-sensitivity c-reactive protein and natriuretic peptide in patients with coronary atherosclerosis. Clin. Chim. Acta 2009, 408, 65–69. [Google Scholar] [CrossRef]
- Sinha, M.K.; Roy, D.; Gaze, D.C.; Collinson, P.O.; Kaski, J.C. Role of “Ischemia modified albumin”, a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg. Med. J. 2004, 21, 29–34. [Google Scholar] [CrossRef]
- Gurumurthy, P.; Borra, S.K.; Yeruva, R.K.; Victor, D.; Babu, S.; Cherian, K.M. Estimation of Ischemia Modified Albumin (IMA) Levels in Patients with Acute Coronary Syndrome. Indian J. Clin. Biochem. 2014, 29, 367–371. [Google Scholar] [CrossRef]
- Mehta, M.D.; Marwah, S.A.; Ghosh, S.; Shah, H.N.; Trivedi, A.P.; Haridas, N. A synergistic role of ischemia modified albumin and high-sensitivity troponin T in the early diagnosis of acute coronary syndrome. J. Family Med. Prim. Care 2015, 4, 570–575. [Google Scholar] [CrossRef]
- Shin, H.; Kim, J.G.; Jang, B.H.; Lim, T.H.; Kim, W.; Cho, Y.; Choi, K.S.; Na, M.K.; Ahn, C.; Lee, J. Diagnostic Accuracy of Ischemia-Modified Albumin for Acute Coronary Syndrome: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 614. [Google Scholar] [CrossRef]
- Ozben, S.; Huseyinoglu, N.; Hanikoglu, F.; Guvenc, T.S.; Yildirim, B.Z.; Cort, A.; Ozdem, S.; Ozben, T. Advanced oxidation protein products and ischaemia-modified albumin in obstructive sleep apnea. Eur. J. Clin. Invest. 2014, 44, 1045–1052. [Google Scholar] [CrossRef]
- Yang, L.X.; Ma, S.G.; Liu, H.; Xu, W. Influence of obstructive sleep apnea on serum butyrylcholinesterase activity and ischemia-modified albumin levels. Clinics 2013, 68, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Dogan, D.; Ocal, N.; Aydogan, M.; Tasci, C.; Arslan, Y.; Tapan, S.; Yetkin, S.; Bilgic, H. Assessment of the role of serum ischemia-modified albumin in obstructive sleep apnea in comparison with interleukin-6. Postgrad. Med. 2016, 128, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Uygur, F.; Tanriverdi, H.; Can, M.; Ornek, T.; Erboy, F.; Altinsoy, B.; Atalay, F.; Damar, M.; Kokturk, F.; Tor, M. The impact of obstructive sleep Apnoea and nasal continuous positive airway pressure on circulating ischaemia-modified albumin concentrations. Mediators Inflamm. 2016, 2016, 8907314. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Du, J.; Ling, X.; Lu, Y. Evaluation of MIh Scoring System in Diagnosis of Obstructive Sleep Apnea Syndrome. Med. Sci. Monit. 2017, 23, 4715–4722. [Google Scholar] [CrossRef]
- Sunnetcioglu, A.; Asker, S.; Alp, H.H.; Gunbatar, H. Increased Asymmetric Dimethylarginine and Ischemia-Modified Albumin Levels in Obstructive Sleep Apnea. Respir. Care 2016, 61, 1038–1043. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Dutt, N.; Sahu, D. Obstructive sleep apnea and the effect of CPAP treatment on ischemia-modified albumin levels: A multi effect size meta-analysis with diagnostic test accuracy. Sleep Breath. 2019, 23, 179–191. [Google Scholar] [CrossRef]
- Karamanli, H.; Kiyici, A.; Arik, B.; Efe, D.; Akgedik, R. Influence of obstructive sleep apnea on ischemia-modified albumin levels and carotid intima-media thickness. J. Investig. Med. 2016, 64, 1035–1041. [Google Scholar] [CrossRef]
- Jung, E.; Ryu, H.H.; Ro, Y.S.; Cha, K.C.; Shin, S.D.; Hwang, S.O. Interactions between Sleep Apnea and Coronary Artery Disease on the Incidence of Sudden Cardiac Arrest: A Multi-Center Case-Control Study. Yonsei Med. J. 2023, 64, 48–53. [Google Scholar] [CrossRef]
- Barbé, F.; Sánchez-de-la-Torre, A.; Abad, J.; Durán-Cantolla, J.; Mediano, O.; Amilibia, J.; Masdeu, M.J.; Florés, M.; Barceló, A.; De la Peña, M.; et al. Effect of obstructive sleep apnoea on severity and short-term prognosis of acute coronary syndrome. Eur. Respir. J. 2015, 45, 419–427. [Google Scholar] [CrossRef]
- Azarbarzin, A.; Sands, S.A.; Stone, K.L.; Taranto-Montemurro, L.; Messineo, L.; Terrill, P.I.; Ancoli-Israel, S.; Ensrud, K.; Purcell, S.; White, D.P.; et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur. Heart J. 2019, 40, 1149–1157. [Google Scholar] [CrossRef]
- Bock, J.M.; Vungarala, S.; Karim, S.; Somers, V.K. Obstructive Sleep Apnea as a Cardiovascular Risk Factor-Beyond CPAP. Can. J. Cardiol. 2021, 37, 756–765. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, N.; Xu, H. Is there a dynamic change in ischemia-modified albumin in patients with obstructive sleep apnea, which often leads to ischemic diseases? Clinics 2013, 68, 1474. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Salvagno, G.L.; Guidi, G.C. Potential value for new diagnostic markers in the early recognition of acute coronary syndromes. CJEM 2006, 8, 27–31. [Google Scholar] [CrossRef]
- Esquinas, C.; Sánchez-de-la Torre, M.; Aldomá, A.; Florés, M.; Martínez, M.; Barceló, A.; Barbé, F. Rationale and methodology of the impact of continuous positive airway pressure on patients with ACS and nonsleepy OSA: The ISAACC Trial. Clin. Cardiol. 2013, 36, 495–501. [Google Scholar] [CrossRef]
- Johns, M.W. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Consenso Nacional Sobre el Síndrome de Apneas-Hipopneas del Sueño. Spanish National Consensus in Sleep Apnea-Hypopnea Syndrome (SAHS). Arch. Bronconeumol. 2005, 41, 7–9. [Google Scholar]
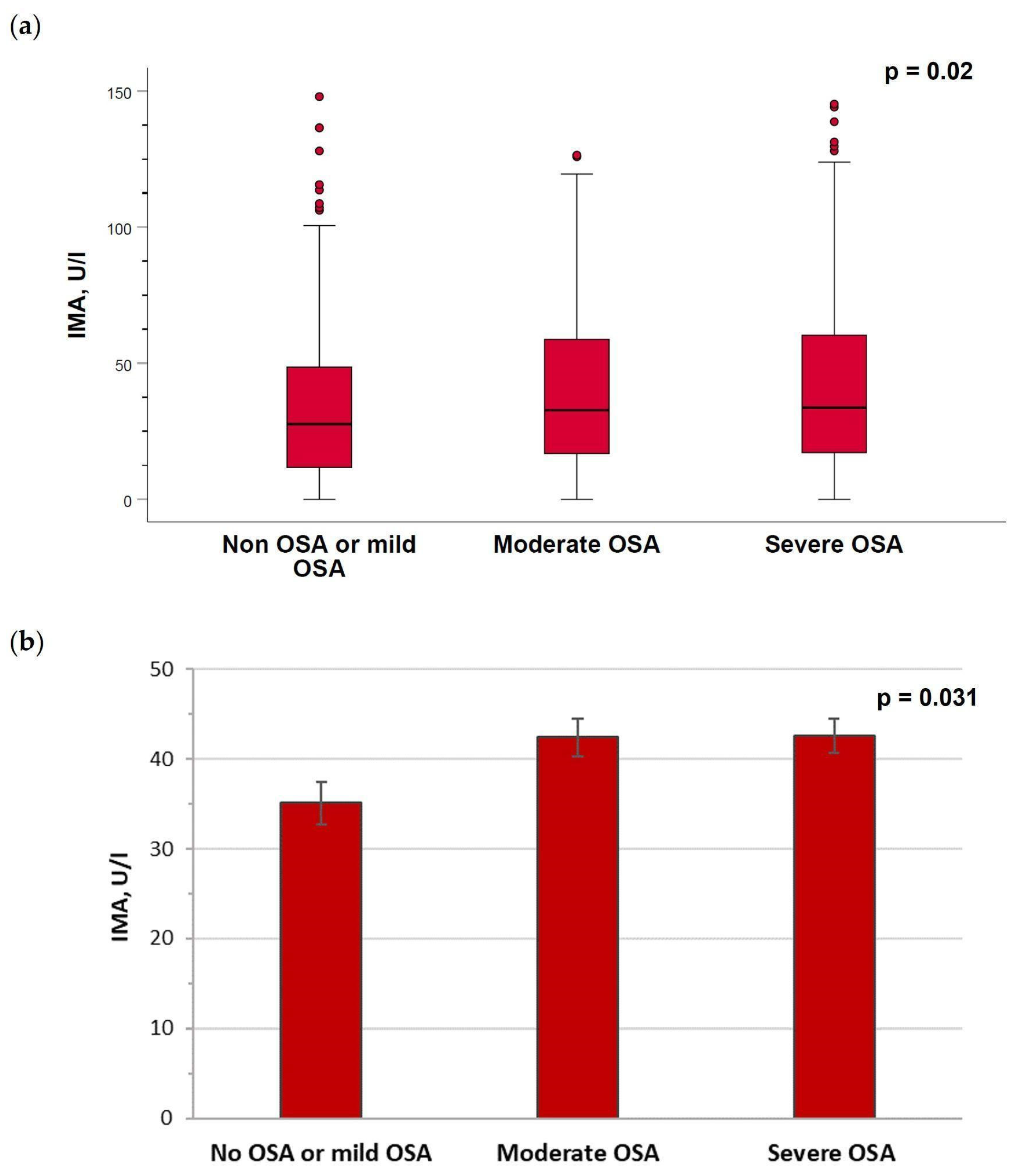
| Author (Year) | Number of Participants (OSA/Controls) | Age (Years) and Sex (% Males) (OSA/Controls) | Diagnostic Criteria for OSA Severity | IMA Values (OSA/Controls) | Main Results | |
|---|---|---|---|---|---|---|
| Yang LX et al. (2013) [41] | 30/32 | 40/40 69/70% | AHI ≥ 5 events/h | 65.10 ± 9.84/ 47.50 ± 9.16 U/L | IMA levels were significantly higher in the OSA group compared with the control group. The CPAP treatment decreased the IMA levels. There was a positive correlation between IMA values and AHI (r = 0.462, p = 0.008) at baseline. | |
| Ozben S et al. (2014) [40] | 59/25 | 51/49 56/64% | AHI ≥ 5 events/h | 30.30 ± 29.02/ 31.50 ± 48.43 | Plasma IMA levels were not statistically different between the OSA patients and controls. | |
| Dogan D et al. (2016) [42] | 39/12 | 41/40 100/100% | AHI ≥ 5 events/h | 0.36 ± 0.04/ 0.89 ± 0.14 U/mL | IMA levels were significantly higher in the OSA group compared with the control group (r = 0.600, p < 0.05) Mean AHI and IMA values were higher in obese + OSA group. | |
| Uygur F et al. (2016) [43] | 97/30 | 49/52 70/71% | AHI ≥ 5 events/h associated with symptoms or AHI ≥ 15 events/h | 0.518 ± 0.09/ 0.415 ± 0.07 ABSU | OSA is associated with elevated concentrations of IMA, which can be reversed by effective CPAP treatment. IMA concentrations correlated significantly with the AHI (r = 0.471, p < 0.001). | |
| Sunnetcioglu A et al. (2016) [45] | Mild/moderate OSA Severe OSA No OSA | 29 28 22 | 46/76% 47/75% 36/82% | AHI ≥ 5 < 30/h AHI ≥ 30/h AHI < 5/h | 1.98 ± 1.43 U/L 2.28 ± 1.00 U/L 0.84 ± 0.49 U/L | IMA levels were higher in the OSA group compared with controls. |
| Karamanli H et al. (2016) [47] | 61/24 | 51/49 62/63% | 1.23 ± 0.10/1.09 ± 0.16 ABSU | Serum IMA levels were significantly higher in individuals with OSA compared with the control group. IMA levels were positively correlated with the AHI (r = 0.32, p = 0.004) at baseline. | ||
| Xu et al. (2017) [44] | 33/30 | 52/49 70/70% | AHI ≥ 5 events/h associated with symptoms or AHI ≥ 15 events/h | 0.58 ± 0.11/0.43 ± 0.09 ABSU | The levels of IMA were significantly higher in patients with OSA than in the control group. These values can be reversed by CPAP treatment. A significant correlation was noted between IMA and AHI (r = 0.614; p < 0.001). | |
| Varikasuvu S.R et al. (2019) [46] Meta-analysis | 411/216 | AHI ≥ 5 events/h associated with symptoms or AHI ≥ 15 events/h | OSA patients showed a significantly increased IMA level compared with the control group. These IMA values decreased with CPAP treatment Meta-analysis of correlations showed significant associations of IMA with AHI (r = 0.448, p < 0.001). | |||
| Düger M et al. (2021) [26] | 86/83 | 45/43 63/59% | AHI ≥ 5 events/h | 0.46 ± 0.1/0.31 ± 0.09 ABSU | IMA levels were significantly higher in patients with OSA than controls. The serum IMA levels increased significantly as OSA severity increased and was positively correlated with the AHI (r = 0.41, p < 0.001). | |
| Parameters | Non-OSA/Mild OSA (n = 255) | Moderate OSA (n = 300) | Severe OSA (n = 370) | Total (n = 925) | p-Value |
|---|---|---|---|---|---|
| General characteristics | |||||
| Women | 38 (14.9%) | 56 (18.7%) | 49 (13.2%) | 143 (15.5%) | 0.149 |
| Age, years | 58 ± 12 | 59 ± 10 | 60 ± 10 | 59 ± 10 | 0.199 |
| BMI, kg/m2 | 27.0 ± 3.9 | 28.4 ± 4.0 | 30.5 ± 4.8 | 28.8 ± 4.6 | <0.001 * |
| Neck circumference, cm | 40 ± 4 | 40 ± 4 | 41 ± 3 | 41 ± 4 | <0.001 * |
| Waist/hip ratio | 0.98 ± 0.07 | 0.98 ± 0.10 | 0.99 ± 0.07 | 0.99 ± 0.08 | 0.048 * |
| Sleep parameters | |||||
| ESS | 5 ± 3 | 5 ± 2 | 5 ± 2 | 5 ± 3 | <0.001 * |
| Study time, min | 414.40 ± 58.45 | 406.78 ± 51.24 | 408.14 ± 56.42 | 409.42 ± 55.40 | 0.232 |
| AHI, events/h | 6.11 ± 3.97 | 21.38 ± 4.50 | 49.70 ± 17.42 | 28.50 ± 21.61 | <0.001 * |
| ODI 4%, events/h | 9.06 ± 15.34 | 19.99 ± 14.03 | 46.76 ± 39.83 | 27.65 ± 31.96 | <0.001 * |
| Mean SaO2, % | 92.55 ± 8.42 | 92.29 ± 7.66 | 91.92 ± 6.72 | 92.22 ± 7.52 | 0.586 |
| Lowest SaO2, % | 85.05 ± 10.61 | 82.93 ± 8.55 | 80.14 ± 9.34 | 82.40 ± 9.66 | <0.001 * |
| Medical history | |||||
| HBP | 107 (42.0%) | 160 (53.3%) | 217 (58.6%) | 484 (52.3%) | <0.001 * |
| Diabetes | 55 (21.6%) | 73 (24.3%) | 91 (24.6%) | 219 (23.7%) | 0.647 |
| Dyslipidemia | 134 (52.5%) | 179 (59.7%) | 213 (57.6%) | 526 (56.9%) | 0.226 |
| CV disease | 56 (22.0%) | 59 (19.7%) | 71 (19.2%) | 186 (20.1%) | 0.679 |
| Cerebrovascular disease | 9 (3.5%) | 8 (2.7%) | 10 (2.7%) | 27 (2.9%) | 0.793 |
| Respiratory disease | 17 (6.7%) | 12 (4.0%) | 25 (6.8%) | 54 (5.8%) | 0.255 |
| Neurological disease | 18 (7.1%) | 14 (4.7%) | 13 (3.5%) | 45 (4.9%) | 0.126 |
| Medication | |||||
| ARAII | 32 (12.6%) | 45 (15.0%) | 69 (18.6%) | 148 (15.8%) | 0.113 |
| ACEI | 56 (22.0%) | 60 (20.0%) | 82 (22.2%) | 198 (21.4%) | 0.764 |
| Hypolipidemic | 96 (37.6%) | 117 (39.0%) | 140 (37.8%) | 353 (38.2%) | 0.935 |
| Oral antidiabetic | 47 (18.4%) | 53 (17.7%) | 75 (20.3%) | 175 (18.9%) | 0.675 |
| Insulin | 13 (5.1%) | 16 (5.3%) | 24 (6.5%) | 53 (5.7%) | 0.716 |
| Antiplatelet agents | 56 (22.0%) | 76 (25.3%) | 79 (21.4%) | 211 (22.8%) | 0.441 |
| Anticoagulants | 13 (5.1%) | 12 (4.0%) | 17 (4.6%) | 42 (4.5%) | 0.824 |
| Beta blockers | 59 (23.1%) | 69 (23.0%) | 58 (15.7%) | 186 (20.1%) | 0.023 * |
| Calcium antagonists | 19 (7.5%) | 39 (13.0%) | 60 (16.2%) | 118 (12.8%) | 0.005 * |
| Diuretics | 36 (14.1%) | 49 (16.3%) | 76 (20.5%) | 161 (17.4%) | 0.096 |
| Antacids | 65 (25.5%) | 74 (24.7%) | 96 (25.9%) | 235 (25.4%) | 0.930 |
| Parameters | Non-OSA/Mild OSA (n = 255) | Moderate OSA (n = 300) | Severe OSA (n = 370) | Total (n = 925) | p-Value |
|---|---|---|---|---|---|
| ACS parameters | |||||
| LVEF, % | 57.51 ± 9.36 | 53.21 ± 10.81 | 54.59 ± 9.70 | 54.94 ± 10.11 | <0.001 * |
| Number of coronary vessels, mean | 1.66 ± 0.91 | 1.77 ± 0.87 | 1.79 ± 0.95 | 1.75 ± 0.92 | 0.201 |
| Vessel obstruction, % | 86.78 ± 20.90 | 86.48 ± 18.41 | 87.77 ± 20.80 | 87.10 ± 20.17 | 0.753 |
| Stent, % of patients | 215 (84.3%) | 261 (87.0%) | 311 (84.1%) | 787 (85.1%) | 0.523 |
| Number of stents, mean | 1.35 ± 1.12 | 1.40 ± 1.08 | 1.45 ± 1.21 | 1.41 ± 1.14 | 0.563 |
| Surgery, % of patients | 6 (2.4%) | 16 (5.4%) | 21 (5.8%) | 43 (4.7%) | 0.111 |
| Hospital stay, days | 5.98 ± 2.87 | 7.01 ± 5.17 | 6.66 ± 4.29 | 6.59 ± 4.29 | 0.017 * |
| ICU stay, days | 2.06 ± 1.32 | 2.36 ± 2.00 | 2.19 ± 1.81 | 2.21 ± 1.76 | 0.127 |
| First episode ACS | 215 (84.6%) | 255 (85.0%) | 320 (86.5%) | 790 (85.5%) | 0.779 |
| Complications, % of patients | 18 (7.1%) | 38 (12.7%) | 41 (11.1%) | 97 (10.5%) | 0.088 |
| NYHA scale | |||||
| n | 190 | 130 | 179 | 499 | 0.417 |
| 0 | 179 (94.2%) | 125 (96.2%) | 164 (91.6%) | 468 (93.8%) | |
| 1 | 9 (4.7%) | 4 (3.1%) | 12 (6.7%) | 25 (5.0%) | |
| 2 | 2 (1.1%) | 1 (0.8%) | 1 (0.6%) | 4 (0.8%) | |
| 3 | 0 (0.0%) | 0 (0.0%) | 2 (1.1%) | 2 (0.4%) | |
| Killip | |||||
| n | 242 | 287 | 354 | 883 | 0.095 |
| I | 226 (93.4%) | 262 (91.3%) | 312 (88.1%) | 800 (90.6%) | |
| II | 15 (6.2%) | 24 (8.4%) | 34 (9.6%) | 73 (8.3%) | |
| III | 0 (0.0%) | 0 (0.0%) | 5 (1.4%) | 5 (0.6%) | |
| IV | 1 (0.4%) | 1 (0.3%) | 3 (0.8%) | 5 (0.6%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resano-Barrio, P.; Alfaro, E.; Solano-Pérez, E.; Coso, C.; Cubillos-Zapata, C.; Díaz-García, E.; Romero-Peralta, S.; Izquierdo-Alonso, J.L.; Barbé, F.; García-Rio, F.; et al. Analysis of the Ischemia-Modified Albumin as a Potential Biomarker for Cardiovascular Damage in Obstructive Sleep Apnea Patients with Acute Coronary Syndrome. Int. J. Mol. Sci. 2023, 24, 9019. https://doi.org/10.3390/ijms24109019
Resano-Barrio P, Alfaro E, Solano-Pérez E, Coso C, Cubillos-Zapata C, Díaz-García E, Romero-Peralta S, Izquierdo-Alonso JL, Barbé F, García-Rio F, et al. Analysis of the Ischemia-Modified Albumin as a Potential Biomarker for Cardiovascular Damage in Obstructive Sleep Apnea Patients with Acute Coronary Syndrome. International Journal of Molecular Sciences. 2023; 24(10):9019. https://doi.org/10.3390/ijms24109019
Chicago/Turabian StyleResano-Barrio, Pilar, Enrique Alfaro, Esther Solano-Pérez, Carlota Coso, Carolina Cubillos-Zapata, Elena Díaz-García, Sofía Romero-Peralta, Jose Luis Izquierdo-Alonso, Ferran Barbé, Francisco García-Rio, and et al. 2023. "Analysis of the Ischemia-Modified Albumin as a Potential Biomarker for Cardiovascular Damage in Obstructive Sleep Apnea Patients with Acute Coronary Syndrome" International Journal of Molecular Sciences 24, no. 10: 9019. https://doi.org/10.3390/ijms24109019
APA StyleResano-Barrio, P., Alfaro, E., Solano-Pérez, E., Coso, C., Cubillos-Zapata, C., Díaz-García, E., Romero-Peralta, S., Izquierdo-Alonso, J. L., Barbé, F., García-Rio, F., Sánchez-de-la-Torre, M., Mediano, O., & on behalf of the Spanish Sleep Network. (2023). Analysis of the Ischemia-Modified Albumin as a Potential Biomarker for Cardiovascular Damage in Obstructive Sleep Apnea Patients with Acute Coronary Syndrome. International Journal of Molecular Sciences, 24(10), 9019. https://doi.org/10.3390/ijms24109019







