Abstract
Despite significant progress in modern medicine and pharmacology, damage to the nervous system with various etiologies still poses a challenge to doctors and scientists. Injuries lead to neuroimmunological changes in the central nervous system (CNS), which may result in both secondary damage and the development of tactile and thermal hypersensitivity. In our review, based on the analysis of many experimental and clinical studies, we indicate that the mechanisms occurring both at the level of the brain after direct damage and at the level of the spinal cord after peripheral nerve damage have a common immunological basis. This suggests that there are opportunities for similar pharmacological therapeutic interventions in the damage of various etiologies. Experimental data indicate that after CNS/PNS damage, the levels of 16 among the 28 CC-family chemokines, i.e., CCL1, CCL2, CCL3, CCL4, CCL5, CCL6, CCL7, CCL8, CCL9, CCL11, CCL12, CCL17, CCL19, CCL20, CCL21, and CCL22, increase in the brain and/or spinal cord and have strong proinflammatory and/or pronociceptive effects. According to the available literature data, further investigation is still needed for understanding the role of the remaining chemokines, especially six of them which were found in humans but not in mice/rats, i.e., CCL13, CCL14, CCL15, CCL16, CCL18, and CCL23. Over the past several years, the results of studies in which available pharmacological tools were used indicated that blocking individual receptors, e.g., CCR1 (J113863 and BX513), CCR2 (RS504393, CCX872, INCB3344, and AZ889), CCR3 (SB328437), CCR4 (C021 and AZD-2098), and CCR5 (maraviroc, AZD-5672, and TAK-220), has beneficial effects after damage to both the CNS and PNS. Recently, experimental data have proved that blockades exerted by double antagonists CCR1/3 (UCB 35625) and CCR2/5 (cenicriviroc) have very good anti-inflammatory and antinociceptive effects. In addition, both single (J113863, RS504393, SB328437, C021, and maraviroc) and dual (cenicriviroc) chemokine receptor antagonists enhanced the analgesic effect of opioid drugs. This review will display the evidence that a multidirectional strategy based on the modulation of neuronal–glial–immune interactions can significantly improve the health of patients after CNS and PNS damage by changing the activity of chemokines belonging to the CC family. Moreover, in the case of pain, the combined administration of such antagonists with opioid drugs could reduce therapeutic doses and minimize the risk of complications.
1. Introduction
Recent research has shown that comparisons of changes occurring after brain damage, as well as after injury to peripheral nerves, are important because often these changes have a common immunological basis. Importantly, this background may contribute to identifying drugs effective for treating injuries to the central and peripheral nervous systems. This is especially important since traumatic brain injury is one of the leading causes of death and permanent disability worldwide, and its annual global incidence is variable, estimated at 27 to 69 million injured people [1,2]. After such injury, there is direct damage to the brain structures at the site of the impact, which may include contusions and hemorrhages and thus cause loss of consciousness, motor and sensory deficits, and other temporary or permanent neurological symptoms [3]. However, secondary processes are what cause CNS injuries to have various etiologies and are complex issues. These secondary processes include, among others, hypoxic-ischemic injury, altered metabolic and vascular permeability, cerebral edema, diffuse axonal damage, hydrocephalus, and increased intracranial pressure. Ongoing neurodegeneration involves both the gradual loss of neurons and the activation of many cells and contributes to the disruption of neuroplasticity, neuronal networks, signal transmission, and communication between various areas of the brain [3]. However, the activation of glial and immune cells is still not fully understood [4,5]; moreover, apoptosis, necrosis, and axonal degeneration occur, as does the formation of amyloid plaques around neurons [6,7,8]. The abovementioned processes lead to long-term neurodegenerative changes and cognitive disorders, such as memory loss and dementia [9,10,11,12,13]. Because post-traumatic brain pathophysiology develops within a few days after injury, there is a therapeutic window that allows for the possibility of pharmacological intervention. Literature data clearly show that brain damage results in the development of central pain (changes in the second or third sensory neuron). The acute pain sensations that arise are related to a specific dysfunction and usually last up to several weeks [14]. Most of the research on pain hypersensitivity resulting from brain injury concerns the head, which is the most common site of discomfort in patients [15,16]. Other commonly reported sites of pain are the musculoskeletal tract, including the neck, shoulders, back, and limbs [17]. Additionally, many patients with moderate-to-severe CNS damage experience painful spasticity associated with limb stiffness, uncontrolled muscle movements, and poor coordination [18]. Some patients with brain or spinal cord injury develop late-onset pain syndrome with symptoms appearing six or more months after the injury [19]. Finally, all reports clearly show that dysfunctions associated with CNS damage are very heterogeneous in nature and are difficult to treat. Persistent inflammation contributes to neuronal apoptosis and the activation of glial and immune cells, which intensifies pain processes [14,20,21]. Unfortunately, therapeutic strategies for central pain are limited because the pathomechanism is not fully understood.
It is worth noting that treating disorders resulting from damage to peripheral nerves seems to be equally difficult. The published data indicate that peripheral nerve injuries constitute 2 to 3% of all diseases and most often occur during traffic accidents [22] and warfare [23]. Iatrogenic injuries caused by medical procedures account for 17.4% of treated nerve damage [24]. The most common injuries include lacerations, which cause a partial or complete loss of nerve continuity, and compression injuries, which may result in a loss of sensory nerve function despite maintaining complete continuity. This difference is believed to be caused by both ischemia and mechanical deformation as a direct result of compression. Other less common mechanisms include thermal injury or ischemia caused by vascular damage [22,25,26,27]. This damage may lead to a painful response, even in response to nonpainful stimuli [28]. This type of pain is called neuropathic pain and is particularly difficult to treat. Sensitization to sensory neuron stimulation contributes to the release of numerous nociceptive factors, both by neuronal and immune cells at the site of injury [29]. Literature data indicate that among immune cells, neutrophils are the first to react to nerve damage, followed by macrophages and lymphocytes both at the site of damage and in the dorsal root ganglia. However, numerous studies suggest that neutrophils, macrophages, and microglia are significantly involved in the development of central sensitization at the spinal cord level [30,31,32,33,34,35]. Neuropathic pain has a very diverse etiology; therefore, it is a condition that requires multidirectional therapeutic treatment. However, the mechanism of its formation still leaves many questions, and further in-depth research is needed. A growing number of scientific reports suggest that chemokines are key pain mediators [36], which we will discuss in the following sections.
We would like to emphasize that after damage to the CNS and PNS occurs, a number of changes in immunological factors that play important roles in repair and regulatory and inflammatory processes occur. The activation of secondary molecular cascades occurs within a few days after the primary injury, which creates opportunities for pharmacological therapeutic interventions. However, it is necessary to understand the factors underlying these changes. In this review, we will focus on the role of selected CC chemokines and their receptors (Table 1). To date, most of the knowledge about these chemokines came from animal studies. Especially, rodents like mice and rats serve as well-established animal models in research because of their physiological, anatomical, and genetic similarity to humans. Other advantages of using these rodents in science include their size, strain diversity, easy breeding, and, most importantly, the availability of models that reflect CNS and PNS damage occurring in patients. Animal models enable the study of neuroimmune changes in the CNS at both the molecular and cellular levels in controlled conditions and also of using new compounds, which is not possible in clinical trials.
The CC group is the largest one among chemokines and is characterized by the presence of the first two conserved cysteine residues adjacent to each other (Scheme 1). Its members have a broad spectrum of activity, including attracting monocytes, eosinophils, basophils, lymphocytes, natural killer, and dendritic cells [37,38]. They are secreted mainly by neurons, immune, and glial cells [39,40,41,42].

Scheme 1.
Cells releasing chemokines, chemical structure of chemokines, and intracellular cascades activated by binding of chemokines to their receptors. Abbreviations: GTP, Guanosine triphosphate; GDP, Guanosine diphosphate; Gα, G protein α subunit; Gαi, G protein αi subunit; Gβγ, G protein βγ subunits; PI3K, Phosphoinositide 3-kinase; Akt, Protein kinase B; Ras, Rat sarcoma virus; Raf, Rapidly accelerated fibrosarcoma; Erk1/2, Extracellular signal-regulated kinases; MAPK, Mitogen-activated protein kinase; JAK/STAT, Janus kinase/signal transducers and activators of transcription; Src, Proto-oncogene tyrosine-protein kinase; Rho A, Transforming protein RhoA; AC, adenylate cyclase; PLC-β, Phospholipase C β; PKA, protein kinase A. Scheme created based on data from the following literature [39,41,42,43,44,45,46,47].
Chemokines belonging to CC-group play important roles in the development of, among the others, autoimmune and neurodegenerative diseases, which has already been well documented [48,49,50]. Due to the huge number of members of this family, we have prepared Table 1, in which 28 chemokines and 10 of their receptors are presented. Then, in the next 10 chapters, based on data from existing studies, the role of individual receptors and their ligands after CNS and PNS damage are discussed.

Table 1.
Changes in the mRNA and/or protein levels of chemokines from the CC family in the brain or spinal cord after central and peripheral nervous system injury: evidence from mouse/rat studies.
Table 1.
Changes in the mRNA and/or protein levels of chemokines from the CC family in the brain or spinal cord after central and peripheral nervous system injury: evidence from mouse/rat studies.
| Chemokine | Chemokine Receptors | Central Nervous System Injury | Peripheral Nervous System Injury |
|---|---|---|---|
| CCL1 | CCR8 | ↑ IRBI [51] ↑ ICH [52] | ↑ PSNL [53] ↑ CCI [54] |
| CCL2 | CCR1, CCR2, CCR4 | ↑ TBI [55,56,57,58] ↑ CIBI [59,60] ↑ AIBI [61] ↑ ICH [62] ↑ SCI [63] ↑ CPSP [64] | ↑ CCI [48,54,65,66,67] |
| CCL3 | CCR1, CCR5 | ↑TBI [58,68,69] ↑ AIBI [61] | ↑ CCI [66,70,71,72] ↑ PSNL [73] |
| CCL4 | CCR1, CCR5 | ↑ TBI [68] | ↑ CCI [65,66,72] |
| CCL5 | CCR1, CCR3, CCR5 | ↑ TBI [74] ↑ IRBI [51] ↑ ICH [75] ↑ MCAO [76] | ↑ CCI [65,77] |
| CCL6 | CCR1 | ND | ↑ CCI [65,66,78] |
| CCL7 | CCR1, CCR2, CCR3, CCR5 | ↑ TBI [55] | ↑ CCI [65,66,78,79,80] ↑ SNL [81] |
| CCL8 | CCR1, CCR2, CCR3, CCR5 | ND | ↑ CCI [65] |
| CCL9/10 | CCR1 | ↑ TBI [68] ↑ IRBI [51] | ↑ CCI [65,66,82] |
| CCL11 | CCR3, CCR5 | ↑ TBI [58] ↑ NHI [83] | ↑ CCI [80] |
| CCL12 | CCR2 | ↑ TBI [55] ↑ ICH [84] | ↑ CCI [85,86] |
| CCL13 | CCR1, CCR2, CCR3, CCR5 | NOT PRESENT IN MICE/RATS | |
| CCL14 | CCR1 | NOT PRESENT IN MICE/RATS | |
| CCL15 | CCR1, CCR3 | NOT PRESENT IN MICE/RATS | |
| CCL16 | CCR1 | NOT PRESENT IN MICE/RATS | |
| CCL17 | CCR4 | ↑ TBI [87,88] ↑ IRBI [51] ↑ ICH [89,90,91] | ↑ CCI [92] |
| CCL18 | CCR8 | NOT PRESENT IN MICE/RATS | |
| CCL19 | CCR7 | ↑ TBI [56] | ND |
| CCL20 | CCR6 | ↑ TBI [93,94,95,96] ↑ ICH [97] ↑ MCAO [98] ↑ SCI [99] | ND |
| CCL21 | CCR7 | ND | ↑ CCI [100] ↑ SNL [101] |
| CCL22 | CCR4 | ↑ TBI [56,87,102] ↑ CPSP [103] ↑ NHI [104] | ↑ CCI [92] |
| CCL23 | CCR1 | NOT PRESENT IN MICE/RATS | |
| CCL24 | CCR3 | ND | – CCI [65,80] |
| CCL25 | CCR9, CCR10 | ND | ND |
| CCL26 | CCR3, CCR10 | ND | – CCI [80] |
| CCL27 | CCR10 | ND | ND |
| CCL28 | CCR3, CCR10 | ND | – CCI [80] |
Abbreviations: CNS injury model abbreviations: AIBI, acute ischemic brain injury; CIBI, cerebral ischemia–reperfusion brain injury model; CPSP, central poststroke pain; ICH, intracerebral hemorrhage; IRBI, neonatal mouse ischemia–reperfusion brain injury model; MCAO, middle cerebral artery occlusion; NHI, neonatal hypoxia-ischemia; MCAO, cerebral artery occlusion model; SCI, spinal cord injury; TBI, traumatic brain injury; PNS injury model abbreviations: CCI, chronic constriction injury to the sciatic nerve; PSNL, partial sciatic nerve ligation; SNL, spinal nerve ligation. Others: ↑, enhanced chemokine level; –, lack of change in chemokine level; ND, no data available.
Generally, the CC chemokine receptors are coupled with Gαi/o. The effect of binding chemokines to their receptors inhibits AC, which negatively affects the level of intracellular cAMP and the activation of PKA [46], as a consequence. However, through the Gβγ subunit, which activates PLC-β [105], among the others, ERK1/2 is phosphorylated [106,107] and Ca2+ flow is increased [46,108,109]. Moreover, Gβγ affects chemokine-induced immune cell migration [110,111] through the phosphoinositide-3 kinases (PI3K), and also through the generation of phosphatidylinositol (3–5)-trisphosphate (PIP3) [112].
This review focus on documenting the changes in CC chemokine receptors and their role after damage to the nervous system in patients with various etiologies, and we have discussed whether and how blocking of CC chemokine receptors may contribute to improving therapy.
2. CCR1—Ligands and Pharmacological Modulation
CCR1 is a receptor that is involved in neuroimmunological changes after PNS and CNS injury. Experimental studies have confirmed the presence of CCR1 in many brain structures [68] and in the spinal cord [72] in mice. Notably, CCR1 is localized on many cells, including neurons [113,114], glia (astroglia and microglia) [113,114,115], and immune cells (macrophages, lymphocytes, basophils, neutrophils, and eosinophils) [116,117,118,119]. The important role of this receptor results from the fact that, in rats/mice, it is a target of many chemokines, such as CCL2, CCL3, CCL4, CCL5, CCL6, CCL7, CCL8, and CCL9, whose levels increase after brain, spinal cord and nerve injury (please see Table 1), and they are important for nociceptive transmission (Scheme 2). Notably, following NHI in rats, CCR1 expression increases in the cortex, thalamus, striatum, hippocampus, and cerebral endothelium [119]. Similarly, CCR1 upregulation in the brain and spinal cord was described 22–24 h after MCAO [76] and ICH [120] in mice. Moreover, CCR1 contributes to inflammatory processes in Alzheimer’s disease [121] and autoimmune encephalomyelitis [122]. Furthermore, the importance of CCR1 was confirmed in SNL [123] and CCI [65,66] mouse/rat models of neuropathic pain. The published data support the hypothesis that pharmacological modulation of CCR1 may represent a new strategy for effective treatment of PNS and CNS injury—however, further research, especially clinical studies, is still necessary.
2.1. CCR1—Endogenous Ligands
CCL2 is a pleiotropic factor that acts as a ligand for CCR1, CCR2, and CCR4. Many studies have shown that CCL2 is one of the key immune factors upregulated in the brain after TBI [55,57,58,124,125] and in CIBI [59,60], CPSP [64], AIBI [61], ICH [62], and in the spinal cord after CCI [65,66,126,127,128] and SCI [63]. CCL2 is released by neurons, glial cells (microglia and astroglia), and immune cells (macrophages and neutrophils) [86,126,129,130,131,132,133,134]. After TBI, CCL2 expression has been shown to increase rapidly in patient cerebrospinal fluid [57] and plasma, which seems to correlate with unfavorable outcomes [135]. Experimental studies have revealed high levels of CCL2 after TBI in mouse brain structures (cortex, striatum, and thalamus), particularly in glial cells [57,124,125,136]. Importantly, several studies have shown that intrathecal administration of CCL2 induces long-lasting pain-related behavior in naive mice [86,132]. Moreover, CCL2 neutralization by antibodies or knockout by siRNA diminished hypersensitivity after CCI [86,137] and prevented glial activation [126]. Recent studies have shown that CCL2 directly contributes to the initiation and maintenance of central sensitization in CPSP model [64]; however, its pronociceptive role after brain injury has not been well studied. Moreover, CCL2 knockout mice exhibited reduced lesions in the cortex and thalamus after TBI [57]. Additionally, these animals manifested partial improvements in long-term neurological outcomes [57,138]. Furthermore, also in humans, shortly after TBI, the level of CCL2 is elevated in the CSF for up to 9 days [57]. Unfortunately, in patients after TBI, it was not possible to determine the CCL2 level in brain structures. Importantly the results from animal studies indicated that CCL2 is particularly important in the brain shortly after its injury—CCL2 upregulation in mRNA/protein level was demonstrated after 24 h not only in the cortex but also in the striatum and thalamus [55]. Moreover, research showed that CCL2 acts in the CNS not only through CCR1 but also through CCR2 and CCR4 [66,82]. However, the role of these three receptors in CCL2 effects remains to be determined. In light of the published data, the key role of CCL2 in the changes caused by PNS and CNS damage is unquestionable and suggests that it is an attractive target for new pharmacotherapies, especially in the early phase when immunological changes are initiated in the CNS.
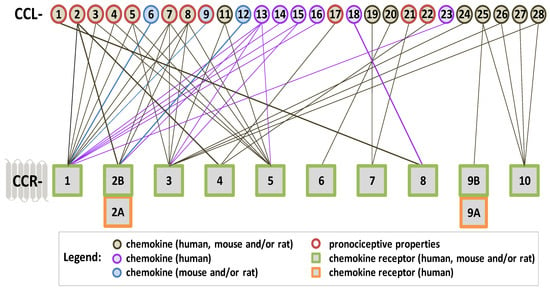
Scheme 2.
The affinity of CC-family chemokines for classic CC chemokine receptors coupled with G proteins. The algesic properties of chemokines that have been proven in behavioral studies on mice/rats are marked with a red border. Chemokines have pleiotropic properties, which we have marked in the diagram, and the lines indicate through which receptor a given chemokine acts—the purple color indicates chemokines occurring only in humans, and the blue color indicates chemokines which occur in mice/rats. Scheme created based on data from the following literature [39,40,41,53,65,73,86,100,132,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153].
CCL3 acts as a ligand for CCR1 and CCR5 and is strongly upregulated in the brain after TBI [58,68,69] and AIBI [61] and in the spinal cord after CCI [66,70,71,72]. Furthermore, an increase in CCL3 was observed in model of temporal lobe epilepsy in the brain of rats [154] and lipopolysaccharide-induced brain injury [155]. Importantly, CCL3 expression was elevated in the white matter from the pericontusional area of patients shortly after post-traumatic brain contusions [156]. Notably, blocking CCL3 prevented the development of recurrent symptoms of experimental autoimmune encephalomyelitis and mononuclear cell infiltration into the CNS [157,158,159]. Studies indicated that CCL3 is produced not only by microglia and macrophages [155] but also by other cells, such as neurons, neutrophils, and lymphocytes [41,160,161,162,163]. Recently, it has been shown that the intrathecal administration of CCL3 induces pain-related behavior in naive mice [65,164], which can be reduced by the selective CCR1 antagonist BX513 [164]. Subsequent animal research has proven that CCL3 neutralization by antibodies reduces hypersensitivity evoked by CCI [72] and PSNL [73]. Recently, it has been proven that CCL3 regulates synaptic plasticity mechanisms involved in learning processes and memory in mice [165], and changes in CCL3 have also been demonstrated in rat temporal lobe epilepsy [166]. Moreover, CCL3 was found to be upregulated in the brains of Alzheimer’s patients [167,168]. Growing evidence suggests that CCL3 is upregulated in neuroinflammatory processes initiated by CNS [58,61,68,69,155] and PNS [66,70,71,72] injury. However, the exact role and regulatory mechanism of CCL3 remains unclear, and additional research should be conducted.
CCL4 acts as a ligand of two chemokine receptors, CCR1 and CCR5, and it can be produced by neurons [169], neutrophils [170,171], microglia [41], and astroglia [172]. A strong increase in the level of CCL4 after TBI has been demonstrated in a mouse model [68], as well as in the white matter from the pericontusional area of patients after post-traumatic brain contusions [156]. In contrast, no changes in CCL4 were detected in the CCI model in the spinal cord of mice [72], likewise in streptozotocin- and chemotherapy-induced neuropathy in animals [41,173]. The results of the mentioned studies provide some evidence for the involvement of this chemokine in nociceptive processes. However, CCL4 acts as a strong chemoattractant for neutrophils [174] and is one of the main chemokines secreted by the microvascular endothelium [174]. Therefore, it may contribute to leakage through the blood–brain barrier. Moreover, the level of CCL4 is known to increase in patients affected by Alzheimer’s disease [168], osteoarthritis [175], diabetes [176], and bronchitis [177]. Although the involvement of CCL4 in nociceptive processes seems to be not crucial, contrarily, its role after CNS and PNS injury of various etiologies seems to be significant, and that is why further research is needed.
CCL5 is the major ligand of CCR5; however, it acts also through CCR1 and CCR3. CCL5 is strongly upregulated in the brain after TBI [74], IRBI [51], MCAO [76], and ICH [75] and in the spinal cord after CCI [65,77]. CCL5 can be produced by microglial and astroglial cells [71] and is a strong chemoattractant for macrophages and lymphocytes [178,179]. However, the role of CCL5 after brain injury is generally unclear. After TBI, in the cortex of mice [180] and in the brain white matter of patients [156], the mRNA level of CCL5 increases. Moreover, the high level of CCL5 in the plasma of TBI patients correlates with severe brain injury [181,182]. However, some studies suggest that CCL5 has protective effects in mice, contributing to dendritic spine and synapse formation and improving learning and memory [183]. Similarly, the study in patients after ischemic stroke gave evidence that CCL5 has the potential to play a neuroprotective role [184]. However, published data indicated also that an increase in the expression of CCL5 in the spinal cord after PNS injury is detrimental in mice and rats [65,77,185,186]. Moreover, the pronociceptive properties of CCL5 had been proven by its intrathecal administration to naive mice [65]. Moreover, CCL5 knockout mice developed diminished pain-like behavior after PSNL [187]. In addition the neutralization of CCL5 by antibodies, there was decreased hypersensitivity development in different neuropathic pain models [77,188,189]. However, there was relatively small and short-lasting increase in CCL5 levels observed in the spinal cord after PNS and CCI, suggested that its contribution to central sensitization is less important than that of other chemokines, especially CCL2, CCL7, and CCL8. Taken together, the results obtained after PNS and CNS injury suggest that CCL5 might be an important factor for modulating inflammatory processes. However, further research is needed to understand the role of this chemokine. It is unclear whether CCL5 contributes to the development of hypersensitivity after CNS injury and whether it may have neuroprotective effects after PNS injury.
CCL6 is a selective CCR1 ligand [190,191], and although it does not exist in humans, it is considered to have the human orthologs CCL15 and CCL23 [192]. Further investigation is needed to determine the role of CCL6 after PNS and CNS injury, and it would also be worthwhile to investigate the role of CCL15 and CCL23 in patients.
CCL7 shares 60–71% homology with CCL2 and has been shown to trigger immune cell trafficking to sites of injury [193]. CCL7 is a pleiotropic factor that can bind to CCR1, CCR2, CCR3, and CCR5. Initially, CCL7 appeared to be involved in the development of neurodegenerative diseases, and changes in its level were associated with increased leukocyte infiltration [126,193]. Later, it was shown that CCL7 is strongly increased in the brain after TBI in mice [55]. Similarly, nerve injury strongly increases CCL7 levels in the spinal cord [65,66,78,79,80] and DRG [80], which was shown in rat and mice studies. In the CNS, CCL7 is released mostly by astroglia [194,195,196], and it is able to activate other cells [197]. However, CCL7 is also produced by activated microglia, macrophages, and neurons [197,198,199,200]. Importantly, this chemokine can evoke chemotaxis in many cells, including microglia, macrophages, and neutrophils [79,201,202], which are known to be important in neuroinflammation and nociception. It was hypothesized, based on animal studies, that the long-lasting upregulation of CCL7 in the cortex, striatum, thalamus, and hippocampus after TBI is associated with intense multidirectional neuron–glia interactions, which lead to secondary injury [55,203,204,205]. Recently, CCL7 was found to be increased after cerebral microdialysis in patients with severe TBI [206] and correlated with aging [203]. An increasing amount of research indicated that this chemokine has strong pronociceptive effects. The newest pharmacological studies provided evidence that the intrathecal injection of CCL7 evoked severe and long-term hypersensitivity in naive mice [65,86]. This finding is consistent with studies showing that CCL7 knockout mice experienced less pain than control mice [198]. Importantly, the blockade of CCL7 is more effective in relieving pain in SNL [81] and CCI [86] models than the blockade of CCL2. The above results have suggested that CCL7 is a key factor involved in the secondary changes induced by CNS and PNS injury and is a new, promising target for effective pharmacotherapy.
CCL8 is a pleiotropic factor that can bind to CCR1, CCR2, CCR3, and CCR5 and is mostly secreted in the CNS by neurons, microglia, and macrophages [207,208]. To our knowledge, CCL8 has not been previously studied in animal models of TBI. However, in patients, it was recently found to be upregulated in the CSF after severe brain injury [206] and during neuropathy [209]. Its role after brain injury is generally unknown; however, its pronociceptive properties have already been proved in animal studies. It has been shown that intrathecal administration of CCL8 evoked a strong increase in hypersensitivity to thermal and mechanical stimuli [65]. Importantly, the latest literature data indicate that CCL8 is one of the most elevated chemokines in the spinal cord of mice after CCI, occurring from the early to late phase of neuropathic pain [65]. Based on the aforementioned data, we suggest that CCL8 may be one of the most important chemokines involved in secondary injury and nociceptive transmission. However, additional experimental and clinical studies are needed to determine its exact role in the CNS and PNS.
CCL9 is a selective ligand of CCR1. It is not present in humans but has an ortholog—CCL23. An increase in CCL9 in the brain of mice was shown in IRBI [51] and TBI [68] models. However, among the CCR1 ligands, CCL2 [55], CCL3 [68], CCL4 [68], and CCL7 [55], they exhibited greater changes than CCL9 in the mouse model of TBI [68]. These weak and slow time-course changes of CCL9 may be related to its neuronal localization. As indicated by immunohistochemical studies, CCL9 colocalizes with NeuN [41,51] but not with IBA-1 [41] or GFAP [41,210]. The role of CCL9 still needs to be defined, and the latest research indicates that its intrathecal administration in naive mice causes tactile and thermal hypersensitivity [41,65]. Changes in the CCL9 level were also observed after CCI, and it was shown both in rats [66] and mice [65]. The mRNA level increases in the DRG and/or spinal cord at many time points; however, the protein level increases only up to seventh day [66,72]. Therefore, it was suggested that CCL9 is responsible for the initiation of neuropathic pain. Moreover, CCL9 neutralization by antibodies diminishes pain-related symptoms’ development in mouse neuropathy [41]. Based on the aforementioned data, it can be concluded that CCL9 contributes to the development of secondary damage to the CNS and to the disruption of nociceptive transmission; however, additional studies are needed to determine the exact role of CCL9 in the CNS and PNS.
The chemokines CCL13, CCL14, CCL15, CCL16, and CCL23 are also agonists of CCR1; however, they are not present in mice or rats. To our knowledge, there are no published data concerning the roles of CCL13, CCL14, CCL15, and CCL16 after PNS or CNS injury in humans. Importantly, CCL23 seems to be strongly involved in the inflammatory response after brain injury and may even serve as a potential biomarker for predicting patients’ prognosis after stroke and ischemia [211,212,213]. Moreover, CCL23 is known to be upregulated in the CSF of patients with neuropathic pain [209].
Taken together, the above literature data have suggested that the levels of eight CCR1 agonists—CCL2, CCL3, CCL4, CCL5, CCL6, CCL7, CCL8, and CCL9—increase in the CNS of mice/rats after PNS/CNS injury. Therefore, these agonists appear to play a proinflammatory role, especially in secondary damage. CCL6 and CCL9 are not present in humans; therefore, CCL2/3/4/5/7/8/23-CCR1 axes can play a role after PNS/CNS injury, but clinical studies are still needed. Very strong algesic properties of CCL2, CCL3, CCL5, CCL7, CCL8, and CCL9 have already been demonstrated, particularly in animals. Importantly, it is known that all these chemokines are responsible for the development of hypersensitivity to thermal and tactile stimuli, and three of them (CCL2, CCL7, and CCL8) are also responsible for the persistence of pain.
2.2. CCR1—Pharmacological Modulation
Considering the strong involvement of CCR1 ligands in neuroimmune processes after brain injury of different etiologies (Table 1), we could expect numerous pharmacological studies in animal models concerning their role. However, to our knowledge, there are no data showing the effect of selective CCR1 antagonists on the development of secondary brain damage or findings from CCR1 knockout mice after CNS injury; therefore, such experiments are undoubtedly necessary. However, it has already been shown that CCR1 knockout mice exhibit reduced hypersensitivity after irritation with acetone [214] and SNL [123]. In our opinion, the above-described changes in the levels of CCR1 and its ligands, as well as the results obtained in genetically modified animals, encourage pharmacological studies in models of CNS and PNS injury. Thus far, intrathecal administration of the CCR1 antagonist J113863 has been shown to diminish mechanical/thermal hypersensitivity in mouse [65,72] and rat [66] CCI models (Table 2).
Similarly, pain relief was observed after the intrathecal administration of BX513 in SSNL rats [164] and J113863 in diabetic mice [41]. Importantly, repeated intrathecal administration of J113863 in rats reduces the activation/infiltration of microglia, macrophages, neutrophils, and lymphocytes into the spinal cord and/or DRG and in parallel with the pronociceptive interleukins IL-1β, IL-6, and IL-18 [66]. Currently, it is known that those pronociceptive factors’ spinal release from activated immune cells might attenuate opioid analgesia during neuropathy [37,48,215,216,217].
Importantly, opioids are drugs often used for the treatment of moderate-to-severe pain [218,219]; however, in neuropathic pain, their effectiveness is lower [37]. The literature clearly showed that CCR1 blockade increased morphine/buprenorphine-induced analgesia in rats exposed to CCI, probably by silencing immune factors [66]. In addition to the abovementioned interleukins, chemokines, which are CCR1 ligands, also play important roles in opioid effectiveness. Recently, it was revealed that a single administration of neutralizing antibodies against CCL2, CCL3, CCL7, and CCL9 can increase the effectiveness of opioid drugs [41,86]. Importantly, several CCR1 antagonists, such as MLN3897 for rheumatoid arthritis [220], BX471 for multiple sclerosis [220], and BAY86-5047 for endometriosis [221], are under investigation in clinical studies. These findings suggest that these antagonists can also be used in other diseases. Unfortunately, there is still a lack of clinical studies on PNS and CNS damage. In our opinion, pharmacological modulation via CCR1 may represent a novel strategy for effective therapy for neuroimmune disorders and simultaneously enhance opioid efficacy. Considering the strong proinflammatory and pronociceptive properties of CCR1 ligands, further research is undoubtedly necessary. This is especially so when taking into account how little is known about the ligands that are present in humans but not in mice or rats, e.g., CCL13, CCL14, CCL15, CCL16, and CCL23.

Table 2.
Analgesic potential of blocking CC chemokine receptors activated by the binding of one or more chemokines after central and peripheral nervous system injury—evidence from mouse/rat studies.
Table 2.
Analgesic potential of blocking CC chemokine receptors activated by the binding of one or more chemokines after central and peripheral nervous system injury—evidence from mouse/rat studies.
| Receptor(s) | Antagonist | Beneficial Effect of Antagonist after CNS Injury | Beneficial Effect of Antagonist after PNS Injury |
|---|---|---|---|
| SINGLE ANTAGONISTS OF CC-FAMILY RECEPTORS | |||
| CCR1 | J113863 | ND | CCI [65,66] |
| BX513 | ND | SSNL [164] | |
| CCR2 | RS504393 | TBI [222,223]; LDH [197] | CCI [48,224]; LAMNT [225] |
| CCX872 | TBI [226] | ND | |
| INCB3344 | CIBI [227] | CCI [228] | |
| AZ889 | ND | CCI [229] | |
| CCR3 | SB328437 | ND | CCI [65,80] |
| SB 297006 | ND | ND | |
| CCR4 | C021 | ICH [89,90] | CCI [92,230] |
| AZD-2098 | SAH [231] | ND | |
| CCR5 | Maraviroc | TBI [232,233]; FCS [234]; CIBI [235]; ICH [236] | CCI [71,85,237] |
| AZD-5672 | ND | CCI [72] | |
| TAK-220 | FCI [238] | CCI [72] | |
| CCR6 | PF-07054894 | ND | ND |
| CCR7 | - | ND | ND |
| CCR8 | AZ084 | ND | ND |
| R243 | ND | ND | |
| CCR9 | Vercirnon | ND | ND |
| MLN3126 | ND | ND | |
| CCR10 | BI-6901 | ND | ND |
| DOUBLE ANTAGONISTS OF CC-FAMILY RECEPTORS | |||
| CCR1/CCR3 | UCB 35625 | ND | CCI [65] |
| CCR2/CCR5 | Cenicriviroc | TBI [203] | CCI [70,85] |
| BMS-813160 | ND | ND | |
| PF-04634817 | ND | ND | |
Abbreviations: CNS injury model abbreviations: CIBI, cerebral ischemia–reperfusion brain injury model; FCS, focal cortical stroke; FCI, focal cerebral ischemia; ICH, intracerebral hemorrhage; LDH, lumbar disc herniation; NHI, neonatal hypoxia-ischemia; SAH, subarachnoid hemorrhage; TBI, traumatic brain injury; PNS injury model abbreviations: CCI, chronic constriction injury to the sciatic nerve; SSNL, segmental spinal nerve ligation model; IAMNT, inferior alveolar nerve and mental nerve transection model. Others: ND—no data available.
3. CCR2—Ligands and Pharmacological Modulation
CCR2 is also a receptor known to be strongly involved in neuroimmunological changes after PNS and CNS injury. The important role of CCR2 was demonstrated for the first time in animal models of degenerative and autoimmune diseases [239]. Subsequently, CCR2 was shown to be expressed on macrophages and leukocytes after brain injury. It is now recognized that in the CNS, CCR2 is present in microglia/macrophages, as well as in astroglia, leukocytes, and neurons in humans and mice. Although, its levels are highest in microglia/macrophages [57,132,240,241,242,243,244]. CCR2 is preferentially bound by CCL2 [245]; however, several other chemokines, CCL7, CCL8, CCL12, and CCL13 (please see Table 1), can also bind to this receptor [37,239,245,246]. Importantly, CCR2 activation promotes angiogenesis [247] and fracture healing [248] and decreases amyloid plaque formation in Alzheimer’s disease models through macrophage-mediated phagocytosis [249,250]. On the other hand, CCR2 plays an unfavorable role and has been documented in many animal models, e.g., TBI [55,56,57,58,251], CIBI [59,60,252], AIBI [61], ICH [62], SCI [63], CPSP [64], EAE [253], diabetic retinopathy [254], cancer [255], arthritis [256], and neuropathy [48,70,257]. After brain injury, CCR2 knockout mice exhibit reduced macrophage infiltration, improved hippocampus-dependent cognitive outcomes, and preserved hippocampal neurons viability [57,226,258]. Similarly, after CCI, CCR2 knockout mice develop diminished hypersensitivity, which is associated with decreased infiltration of CCR2-positive macrophages [259]. The recruitment and activation of those cells in the CNS are known to significantly contribute to the development of both inflammation and neuropathic pain. Therefore, CCR2 receptor blockade may provide a new therapeutic method for the treatment of CNS and PNS disorders.
Importantly, the CCR2 gene encodes two isoforms, CCR2A and CCR2B, which feature different amino acid sequences in their C-terminal intracellular loops due to alternative splicing [260,261]. Most biochemical and behavioral studies on CCR2-related responses have focused on CCR2B, with few reports focused on CCR2A. A study by Bartoli et al. showed that CCR2 isoforms are cell-specific in humans [262]. CCR2A is the major isoform expressed by mononuclear cells, and CCR2B is expressed by satellite cells [262]. CCR2B is the most abundant form; however, both CCR2 isoforms are expressed on different immune (monocytes, lymphocytes, and dendritic cells) [239] and endothelial [263,264,265] cells. Importantly, CCR2A, but not CCR2B, is expressed in solid cancer-derived cells, indicating that CCR2A may play an essential role in tumor progression. The results revealed that the C-terminal region of CCR2A is unfavorable for GRK phosphorylation, which is necessary for β-arrestin interaction and subsequent receptor internalization [266]. Furthermore, CCR2A is expressed in vessel walls and by some mononuclear cells, especially in cells involved in partial invasion in polymyositis and inclusion body myositis [262]. According to the Ensembl genome analysis database, the splicing process used to produce CCR2A may be unique in some primate species, and both of these transcripts have been identified in humans [266]. Since the rodent CCR2 gene does not undergo alternative splicing and thus does not generate CCR2A, mice/rats are not good models for exploring the tissue expression and biological functions of CCR2A [266]. Therefore, the presence and role of CCR2A and CCR2B in the CNS/PNS in humans need to be studied.
3.1. CCR2—Endogenous Ligands
As described above, many studies have shown that chemokine CCL2, CCL7, and CCL8 (which are known to bind to several receptors; Scheme 2) levels are increased after PNS/CNS injury [37,245]; therefore, they appear to play a role in neuroimmune disorders, especially secondary damage and nociceptive transmission. The newest data indicate that CCL2, CCL7, and CCL8 are multidirectional factors responsible for communication between neurons, microglia, and astroglia; thus, they are essential for the functioning of the nervous system. However, the possible involvement of CCL13 in neuroimmune processes remains to be studied since this chemokine does not exist in mice/rats, making its role more difficult to clarify. We know relatively little about the role of CCL12—the selective ligand of CCR2. However, its upregulation was already described in various animal models, such as in TBI [55,68,203], ICH [84], CCI [85,86,267], and osteoarthritis [268,269]. Importantly, in contrast to CCL2, CCL7, and CCL8, CCL12 is devoid of pronociceptive properties [65,86]; however, after CNS injury, it exerts potent proinflammatory effects by inducing leukocyte, macrophage, and T-cell chemotaxis [84,270,271]. The intrathecal injection of CCL12-neutralizing antibodies is neuroprotective after brain injury [84]. Interestingly, the changes in CCL12 in the hippocampus seem to be age-dependent [203]. Therefore, the blockade of CCL12 may constitute a new therapeutic approach for the treatment of CNS damage, e.g., occurring along with the aging process, but further in-depth research is needed.
3.2. CCR2—Pharmacological Modulation
Increasing amounts of published data indicate that the pharmacological modulation of CCR2 may be beneficial in the treatment of neuroimmune disorders. Results from CCR2 knockout mice have shown both significantly improved cognitive function after TBI [258] and reduced hypersensitivity after CCI [259], which encourages pharmacological studies. Literature data indicate that the selective CCR2 antagonist RS504393 reduces apoptosis and improves performance in the Morris water maze after TBI, suggesting again that this receptor has deleterious effects on neuronal survival and learning [222,223]. Subsequent research has shown that after stroke, monocyte numbers increase significantly in both the blood and brain (even up to 10-fold), and the new CCR2 antagonist INCB3344 selectively prevents these changes. Additionally, INCB3344 administration limits brain damage and improves functional deficits, which are associated with promoting M2 macrophage polarization [227,228]. Importantly, recent studies have shown that blocking CCR2 with the new antagonist CCX872 immediately after TBI is highly beneficial because it reduces the type I IFN response [272] and blocks the influx of CCR2+ macrophages [226] in acutely injured brain tissue in mice. Notably, this antagonist is currently used in clinical trials for the treatment of patients diagnosed with pancreatic cancer [273], and if it passes clinical trials successfully, it could be used to treat CNS damage in the future. Importantly, extensive literature data clearly suggest that CCR2 also plays an extremely important role after PNS injury. It has been shown that repeated intrathecal injection of RS504393 decreases CCI-evoked hypersensitivity in mice and rats [48,85]. Moreover, RS504393 administration weakens microglial cell activation [224] and prevents the upregulation of interleukins (IL-1β, IL-18, and IL-6) in the spinal cord [48] and chemokines (CCL2, CCL3, CCL4, CCL5, CCL7, and CCL11) in the DRG in rats [85]. Moreover, the intrathecal administration of other CCR2 antagonists, INCB3344 [228] and AZ889 [229], also attenuated neuropathic pain symptoms in rats. Similar analgesic effects of intracisternal injections of RS504393 were described after inferior alveolar nerve transection [225], mental nerve transection [225], and lumbar disc herniation [274]. However, a single intraperitoneal injection of RS504393 in mice did not diminish already well-established neuropathic pain-related behavior, which may indicate poor passage of this compound through the BBB [85]. The effectiveness of CCX872 would undoubtedly be worth checking due to its beneficial effect on brain damage, but for now, there are no such data in the literature concerning its efficacy after nerve damage. This approach would also be valuable because it has been shown that repeated intrathecal injection of RS504393 enhances the analgesic effects of opioid drugs (morphine and buprenorphine) [48]. Further research is needed to determine why this is possible, but it is already known that excessive production of interleukins, such as IL-1β and IL-18, might be key for reducing opioid analgesia in neuropathy [215,216,275]. Several studies have indicated that opioid signaling is connected not only to interleukins but also to chemokines [37,246]. CCR2 is preferentially bound by CCL2 [245], but repeated administration of morphine increases the level of CCL2 in the spinal cord; however, its neutralization by an antibody diminishes the development of morphine tolerance [276]. Furthermore, an agonist of the μ opioid receptor (DAMGO) was shown to enhance the expression of CCL2 in human peripheral blood mononuclear cells [277]. CCL2 is known to interfere with the analgesic effects induced by opioids through heterologous desensitization between CCR2 and opioid receptors [278]. These effects correspond well with the reductions in CCI-induced elevation of CCL2, IL-1β, IL-18, and IL-6 [48,224]. Therefore, intrathecally administered RS504393 seems to inhibit heterologous desensitization between opioid and chemokine receptors by preventing the CCI-induced increase in the expression of these anti-opioid factors, although this still needs to be proven. In summary, the available data suggest that the pharmacological blockade of CCR2 beneficially influences immunological changes and, therefore, it may constitute a new strategy for effective therapy in patients suffering from CNS and PNS damage. However, additional studies are needed, possibly with the use of new pharmacological tools.
In summary, the abovementioned literature data suggest that in mice/rats treated with CCR2 agonists, CCL2, CCL7, CCL8, and CCL12 levels are increased after PNS/CNS injury; therefore, these cytokines appear to play proinflammatory roles, especially in secondary CNS damage. However, in humans, CCL12 is not present. After injury to PNS/CNS in patients, the CCL2/7/8-CCR2 axis likely plays proinflammatory and pronociceptive roles.
4. CCR3—Ligands and Pharmacological Modulation
CCR3 seems to be involved in neuroimmunological changes after PNS and CNS injury [65,83,279,280] because it is a target of numerous chemokines, including CCL5, CCL7, CCL8, CCL11, CCL13, CCL15, CCL24, CCL26, and CCL28 (please see Table 1). Moreover, in the nervous system, CCR3 is expressed in neuronal, glial (microglia, astroglia, and satellite cells), and immune (basophils, eosinophils, neutrophils, and lymphocytes) cells [80,281,282,283,284,285,286,287,288]. To date, data in the literature indicate the important role of CCR3 in disorders such as inflammation, asthma, allergies, and cancer [287,289,290,291,292,293] and, recently, in neuropathy [65,80,294]. In 2013, for the first time, after ischemic injury in a mouse model was shown, the expression of CCR3 was induced in neurons around the peri-infarct areas [184]. In vitro studies have shown that CCR3 knockout and blockade protect neurons from oxygen/glucose deprivation-induced cytotoxicity in primary cortical cultures [282]. Moreover, after ischemic injury, CCR3 knockout mice exhibited a decrease in infarct volume in the brain [282], which provided the first evidence that this receptor is involved in neuronal death. All these findings confirm the need for additional research on the role of CCR3 in the nervous system to determine whether blocking this receptor may contribute to more effective treatment of PNS or CNS damage.
4.1. CCR3—Endogenous Ligands
The proinflammatory properties of CCL5, CCL7, and CCL8 are already well documented due to their pleiotropic effects and occurrence in various mammalian species, while the roles of CCL13 and CCL15, which are absent in mice/rats, are poorly understood. Importantly, little is known about the remaining CCR3 ligands (CCL11, CCL24, CCL26, and CCL28), although they are present in rodents and humans. Notably, three CCR3 agonists (CCL5, CCL7, and CCL8, which are described in detail above) are important pronociceptive factors (Scheme 2).
CCL11 acts via CCR3, CCR5, and CXCR3. This chemokine is secreted in the CNS by microglia and astroglia [114,295] and is a strong chemoattractant for eosinophils, basophils, neutrophils, and macrophages [296,297]. Recently, it was shown that CCL11 is upregulated in TBI [58] and NHI [83] animal models; however, its role needs to be clarified. Similarly, in the CCI model, the level of mRNA increases in the spinal cord in rats [80] but not in mice [65]. However, CCL11 protein levels are increased in the DRG but not in the spinal cord after CCI in rats [80]. Importantly, it was recently shown that CCL11 expression increases in the CSF of patients with neuropathic pain, suggesting its potential role in nociception [209]. In particular, in patients with fibromyalgia [298] and osteoarthrosis [299], CCL11 levels are enhanced. Recently, clinical trials of the anti-CCL11 monoclonal antibody (bertilimumab) have been underway, and the preliminary results indicate that this antibody has some beneficial effects on the treatment of severe allergic disorders, skin diseases, and inflammatory bowel disease [300,301]. Considering the promising results of pharmacotherapy with bertilimumab, further research is undoubtedly necessary in patients after PNS/CNS damage.
To date, the other three CCR3 ligands, CCL24, CCL26, and CCL28, have been poorly investigated in the CNS and preliminary results vary among species. It is known that in rats after CCI, CCL28 mRNA is undetectable in the spinal cord [80]. However, in mice, the mRNA levels of CCL24 [65] and CCL28 [65] are detectable; however, they remain unchanged after injury. Moreover, in rats, CCL26 expression is slightly spinally elevated after CCI, but it remains unchanged in mice [65,80]. That is why the role of these chemokines after PNS/CNS injury still needs to be investigated.
4.2. CCR3—Pharmacological Modulation
Studies on the impact of pharmacological modulation of CCR3 on the development of neuroimmune changes in animal models of CNS damage should be conducted soon in light of the published data suggesting the role of its ligands (CCL5, CCL7, CCL8, and CCL11). Additionally, little is known about the extent to which modulation of this receptor influences neuroimmunological changes after PNS damage. In 2021, the first paper proving the key role of CCR3 in nociceptive transmission was published [80]. These studies demonstrated for the first time that blocking CCR3 through repeated intrathecal injections of SB328437 attenuates the development of hypersensitivity in a rat model of CCI [80]. In other studies, the authors showed that SB328437 administered in this way prevents the CCI-induced activation of lymphocytes, neutrophils, and satellite cells in the spinal cord and DRG and consequently results in a reduction in the level of proinflammatory cytokines (IL-6, CCL7, and CCL11), which are known to have anti-opioid properties [80]. Further studies in mice and rats have shown that even single administrations of SB328437 have analgesic effects [65,80] and, importantly, increase the effectiveness of morphine and buprenorphine in a neuropathic pain model [80]. Further research is needed to determine whether this beneficial effect on opioid efficacy is caused by the silencing of immunological factors or whether heterologous desensitization of CCR3-MOR is possible (as already been demonstrated in the case of CCR5-MOR [302,303,304]). Additionally, SB328437 has been shown to reduce hypersensitivity in the mice pIONT neuropathy model [294]. Moreover, it was shown that CCR3 plays an important role in the pathogenesis of osteoarthritis [280], allergic asthma [305], and cancer [306] in patients. In summary, the available literature data support the theory that the pharmacological blockade of CCR3 may be a new strategy for effective pharmacotherapy of PNS and CNS disorders; however, further research using newly synthesized antagonists is undoubtedly necessary.
Taken together, the above literature data suggest that among CCR3 agonists, the levels of CCL5, CCL7, CCL8, and CCL11 increase after PNS/CNS damage. Therefore, the CCL5/7/8/11-CCR3 axis appears to play a proinflammatory and pronociceptive role, especially in secondary CNS damage; however, further studies are needed.
5. CCR4—Ligands and Pharmacological Modulation
CCR4 is a target of two selective agonists, CCL17 and CCL22, and one nonselective agonist, described above, CCL2 (please see Table 1) [82,108,307]. The receptor is expressed on numerous immune cells (including lymphocytes, platelets, natural killers, and macrophages) [108,307,308] and on neurons [166], microglia [286], and astroglia [108,286]. Recently, the upregulation of CCR4 expression was described in peripheral blood monocytes stimulated with serum collected from TBI patients [309]. Moreover, CCR4 expressed on immune cells was shown to play a role in cell migration and polarization [310,311,312,313]. The role of this receptor in the CNS was suggested in many animal studies after CNS injury (in a mouse MCAO model [312]), after PNS injury (in mouse/rat CCI models [92,230]), and in diabetes in monkeys [314] and mice [315]. Furthermore, CCR4 has been shown to play an important role in the pathogenesis of many diseases in patients, such as TBI [309], asthma [316,317], dermatitis [318], and cancer [319]. The presence of CCR4 in the CNS [320,321] and PNS [186] suggests that CCR4 plays a role after injury; however, further studies are needed.
5.1. CCR4—Endogenous Ligands
The proinflammatory properties of CCL2 are quite well documented, as described above. Importantly, due to the pleiotropic properties of this chemokine, it is one of the best studied. Recent pharmacological studies have proven that intrathecally administered CCL2 evoked pain-related behavior in naive mice more effectively through CCR4 than through CCR2 [230]. However, both the CCL2/CCR2 [48,224,245] and CCL2/CCR4 [230] axes seem to be important in the nervous system, and further in-depth research is needed. Moreover, there is still insufficient information in the current literature regarding the role of CCR4 and its selective ligands (CCL17 and CCL22) after CNS and PNS injury.
CCL17 is a selective ligand of CCR4 and is produced mainly by thymic cells and is also secreted by lymphocytes, macrophages, microglia, dendritic cells, and neurons [82,322,323]. Recently, CCL17 was shown to be upregulated in brain structures in several animal models of TBI [87,88], IRBI [51], and ICH [89,90,91]. Moreover, although CCL17 levels in the spinal cord remain unchanged after nerve damage [66,230], its levels in the rat DRGs significantly increased [92]. Furthermore, pharmacological studies have confirmed that intrathecally administered CCL17 evoked strong pain-related behavior in naive mice through CCR4 [230]. Targeting this chemokine has not been extensively tested in clinics, but recent reports have shown that CCL17 is important for patients suffering from ICH [91], diabetic retinopathy [324], and fibromyalgia [298]. Therefore, the role of CCL17 after PNS/CNS injury urgently needs to be studied.
CCL22 is also a selective ligand of CCR4 that can be produced by immune cells, mainly macrophages, and is able to mediate monocyte, lymphocyte, and natural killer chemotaxis [82,325,326,327]. Both CCL17 and CCL22 bind to CCR4, and these chemokines have 37% identity at the amino acid sequence level [328]. However, CCL17 and CCL22 are not equivalent in receptor binding, and CCL22 acts as the dominant cross-desensitizing ligand toward CCR4 [329]. The role of CCL22 is still unclear, but it seems to be dualistic since chemokines exhibit both pro- and anti-inflammatory effects depending on the circumstances [330]. Downregulation of CCL22 was shown to be correlated with the attenuation of neurological changes after injury in rats [56]; however, others have proposed that CCL22 is an anti-inflammatory factor responsible for M2 microglial polarization [102,330]. Our unpublished results demonstrated time-dependent changes in the mRNA and protein expression of CCL22 within the cortex, striatum, and thalamus after TBI in mice, and the data are similar to those of other studies [56,87]. Moreover, it was shown that the level of CCL22 is increased in the cortex after concussion in rats [102], ischemic stroke in mice [103], and also in patients with fibromyalgia [298]. Moreover, CCL22 was shown to play an important role in a mouse neonatal hypoxic-ischemic brain injury model [104]. These data strongly suggest that CCL22 plays a role in the pathology of brain injury. The situation seems to be different after nerve injury, since the spinal level of CCL22 remains unchanged, unlike the level of CCL2, which was highly increased [230]. However, after CCI, the level of CCL22 is strongly increased in rats, similar to CCL2 [230]. In summary, the role of the CCL22/CCR4 axis at the PNS and CNS levels still needs to be clarified, but its involvement in pathological changes is unquestionable.
5.2. CCR4—Pharmacological Modulation
Recently, neutralizing molecules for CCL17 and CCL22 (GPN279 and GPN136) were created, and they have been shown to have anti-inflammatory effects in a mouse model of asthma [331]. Moreover, neutralization of CCL17 using antibodies has been shown to reduce the proliferation of HeLa and SiHa tumor cells [332], and CCL22 delays the development of experimental autoimmune encephalomyelitis [333]. Due to the favorable properties of neutralizing molecules CCL2 (described in Section 2.1), CCL17, and CCL22, these chemokines appear to be particularly important targets for future therapies.
Currently, four groups of CCR4 antagonists, distinguished based on their chemical properties, are available for experimental research: aryl sulfonamides, substituted heterocyclic amines, thiazolidinones, and lactams [334,335]. However, of these antagonists, only indazole arylsulfonamide (GSK 2239633) was tested in phase I clinical trials, and further studies were not performed [108,336]. Recently, a CCR4 antagonist called C021 was experimentally tested, and it is known that it is able to inhibit the chemotaxis of immune cells in mouse and human in vitro studies [337,338]. C021 has also been shown to reduce microglial activation in animal models of hepatic encephalopathy [339] and neuropathic pain [92,230]. Importantly, it was shown that repeated intrathecal and intraperitoneal injections of C021 diminish pain and, in parallel, spinal levels of macrophage/microglia activation [92,230] and, consequently, the levels of the proinflammatory cytokines IL-1β and IL-18 in rats [92]. Moreover, in CCI-exposed rats and mice, C021 administration improved morphine and buprenorphine analgesia [92,230] and reduced the development of morphine tolerance [230]. Finally, we would like to emphasize that clinical trials of mogamulizumab (a humanized CCR4 antibody) are currently ongoing in Japan. Mogamulizumab is being tested for the treatment of T-cell lymphomas and leukemia [340,341] and advanced solid tumors [341]. Moreover, the use of mogamulizumab has also been tested in allergic diseases, such as asthma and atopic dermatitis [307]. However, this drug remains in the early stages of clinical studies, and further research is needed. In summary, published research has indicated that targeting CCR4 and its ligands is a promising strategy for treating nervous system damage with various etiologies in animal models.
In summary, the abovementioned literature data suggest that in humans and also in rodents like mice/rats, the expression of all CCR4 agonists is increased after PNS/CNS injury. Therefore, the CCL2/17/22-CCR4 axis appears to play a proinflammatory and pronociceptive role, especially in secondary CNS damage. However, further clinical studies are needed.
6. CCR5—Ligands and Pharmacological Modulation
CCR5 is known to be strongly involved in neuroimmunological changes after PNS and CNS injury, which is not surprising since it is a target of several chemokines, CCL3, CCL4, CCL5, CCL7, CCL8, CCL11, and CCL13 (please see Table 1). CCR5 expression is well documented in a variety of immune cells (macrophages, lymphocytes, and granulocytes) and glia (microglia and astroglia) but was recently found on neuronal cells [342,343]. Importantly, stroke induces CCR5 expression in neurons, and this expression persists throughout recovery. The downregulation of CCR5 in microglia at similar time points when CCR5 expression is increased in neurons reflects complex cell-specific changes in CCR5 signaling after stroke. Recently, CCR5 was shown to be involved in learning, memory, and plasticity processes in hippocampal and cortical circuits [344]. In a TBI model, CCR5 knockout mice exhibited reduced learning deficits and improved cognitive function [345]. Importantly, after stroke, patients who were carriers of a naturally occurring loss-of-function mutation in CCR5 (CCR5-Δ32) exhibited improvements in cognitive and neurological disorders [345]. Moreover, CCR5 knockout mice developed hypersensitivity to a lesser extent than control mice [304]. Therefore, the CCR5 receptor appears to be a therapeutic target for the treatment of pain-related diseases, including neuropathy [237,346,347,348]. In summary, CCR5 is proposed to be a pharmacological target for regeneration and/or pain relief after CNS and PNS injury [237,345,346,347,348].
6.1. CCR5—Endogenous Ligands
Notably, CCR5 agonists, namely, CCL3, CCL4, CCL5, CCL7, CCL8, and CCL11, exhibit pleiotropic properties and have potent and well-documented proinflammatory and/or pronociceptive properties in the CNS and/or PNS. However, there is a lack of information about the role of CCL13, a chemokine found in humans but not in mice/rats, after PNS or CNS damage. Due to the well-documented role of the abovementioned six CCR5 ligands, this receptor seems to be a particularly valuable drug target.
6.2. CCR5—Pharmacological Modulation
Literature data clearly indicate that CCR5 plays an important role in recovery after brain injury of different etiologies (please see Table 1) [75,232,233,234,235,236,238,345]. Poststroke neuronal knockdown of CCR5 in the motor cortex led to the early recovery of motor control in mice [345]. It was proven that this recovery is associated with the preservation of dendritic spines and novel patterns of cortical projections to the contralateral premotor cortex [345]. Importantly, it has been proved that stroke induces strong CCR5 expression in neurons. This process is accompanied by a simultaneous reduction in the level of CCR5 in microglia, which indicates how complex the neuroimmunological changes after CNS damage are [345]. Notably, the administration of maraviroc, a CCR5 antagonist clinically used for HIV infection treatment, has been shown to produce similar effects on motor recovery after stroke and cognitive decline after TBI in mice [345]. The beneficial effects of maraviroc have also been shown by others in various animal models, such as TBI [192,193], stroke [194], and CIBI [195] models. Additionally, TAK-220, a potent and selective antagonist of CCR5, was shown to be protective against focal cerebral ischemia in mice [197]. Importantly, it has recently been shown that after stroke, patients who carry a naturally occurring loss-of-function mutation in CCR5 (CCR5-Δ32) show better improvement in neurological and cognitive disorders [345]. Therefore, CCR5 can be considered an important pharmacological target after CNS injury. Furthermore, CCR5 is the first human chemokine receptor known to play a role in improving recovery from stroke and ICH [75].
Importantly, after PNS injury, CCR5 is upregulated in the spinal cord, which was also shown in a rat CCI model [71]. Therefore, the blockade of CCR5 after PNS injury has also beneficial effects. In a mouse CCI model, the administration of the CCR5 antagonists maraviroc [85,237], AZD-5672 [72], and TAK-220 [72] was shown to diminish hypersensitivity to tactile and thermal stimuli. Moreover, in rats, intrathecal administration of maraviroc was shown to diminish pain symptoms in a CCI model and, in parallel, spinal microglia/astroglia activation, which are important for pain development. These activated cells are also responsible for the release of many anti-opioid immune factors, such as IL-1β [216], IL-18 [215], CCL3 [41,86,349], and CCL5 [77,188,189]. In addition, morphine administration enhances the level of CCR5 in lymphocytes [348]. Therefore, blocking CCR5 with maraviroc [71,85], AZD-5672 [72], and TAK-220 [72] not only diminished pain but also enhanced opioid effectiveness in mouse and rat neuropathic pain models. Moreover, the latest research indicates that heterologous desensitization of opioid and chemokine receptors may occur at the CNS level, as it has been shown that CCR5 and mu and kappa opioid receptors are present on glial and neuronal cells [187,348,350,351]. Considering the abovementioned literature, we believe that CCR5 is a good target for drug development in the treatment of CNS and PNS injury and that maraviroc seems to be a drug that should be subject to clinical trials. In particular, maraviroc has received approval from the Food and Drug Administration and is used as a treatment for patients infected with human immunodeficiency virus R5 type 1 [352,353]. The clinical use of maraviroc may weaken the development of secondary changes after damage to the CNS and PNS, reduce the development of pain hypersensitivity, and enhance the analgesic effects of opioid drugs in patients treated for neuropathy.
In summary, the abovementioned literature data suggest that in mice/rats and also in humans, the expression of the CCR5 agonists CCL3, CCL4, CCL5, CCL7, CCL8, and CCL11 increases after PNS/CNS injury. Therefore, the CCL3/4/5/7/8/11-CCR5 axis appears to play a proinflammatory and pronociceptive role, especially in secondary CNS damage; however, additional clinical studies are needed.
7. CCR6—Ligands and Pharmacological Modulation
CCR6 and CCL20 are unique in that they interact only with each other (please see Table 1). The receptor is expressed on immature dendritic cells, B and T cells, and neutrophils [354]. There are limited data in the literature about the role of the CCL20/CCR6 axis after PNS and CNS injury; therefore, further studies are necessary.
7.1. CCR6—Endogenous Ligands
CCL20 is expressed by endothelial and epithelial cells, monocytes, and Th17 lymphocytes [355] and attracts immature dendritic cells and T and B cells [354]. In brain tissue, CCL20 is localized mainly to neurons and microglia, while little CCL20 was found in astroglia [356]. Studies have shown that CCL20 expression was increased after TBI [93,94,95], ICH [97], cerebral ischemia [98], and spinal cord injury [99] in rodents. CCL20 levels have also been shown to be elevated in human plasma after severe TBI [181]. Available data showed that the binding of CCL20 to CCR6-positive T cells is crucial for their infiltration into the CNS [357]. Recently, after injury or inflammation, activated astroglia that form the blood–brain barrier produce CCL20, which makes it easier for CCR6-positive cells to infiltrate the CNS [358]. Moreover, activation of the CCL20-CCR6 axis seems to be important in the development of dry eye disease, which affects approximately 37% of TBI patients [359]. Interestingly, a recent study reported that CCL20 expression is upregulated after ICH in mice [356] and rats [360] and that chemokine was suggested as a potential target for pharmacological intervention. The algesic properties of CCL20 have not yet been documented. However, CCL20 levels were shown to increase after breast tumor surgery in patients who developed persistent postoperative neuropathic pain [361]. Similarly, an increase in the plasma CCL20 concentration was shown to be associated with hypersensitivity in patients after nerve damage [362]. However, further investigation is needed to determine the underlying molecular mechanisms through which CCL20 influences neuroimmunological changes.
7.2. CCR6—Pharmacological Modulation
A selective CCR6 antagonist (PF-07054894) can be used in experiments, but to our knowledge, such studies have not yet been carried out after damage to the CNS and PNS. However, treatment with shCCL20-CCR6 nanodendriplexes improved pathology in mice after TBI [96]. Importantly, CCR6 knockout mice exhibited significant protection against retinal damage, which is often induced by TBI [93]. After spinal cord injury, CCL20-neutralizing antibodies helped to restore motor functions and inhibited upregulation of TNF-α, IL-1β, and IL-6 [356]. The expression of CCL20 and CCR6 was found to be upregulated in ischemic brain injury, while a CCL20-neutralizing antibody reduced the volume of cerebral infarction in mice [356]. However, the contributions of CCL20 to the pathology of early brain injury after SAH and its underlying mechanisms are still unclear. In conclusion, further research is necessary to elucidate the role of the CCL20-CCR6 axis after CNS and PNS injury.
In summary, the abovementioned literature data suggest that in mice, rats, and humans, the levels of the CCR6 agonist CCL20 are increased after PNS/CNS injury. Therefore, the CCL20-CCR6 axis seems to play a proinflammatory role in patients after PNS/CNS injury. However, additional advanced studies on this issue are still needed.
8. CCR7—Ligands and Pharmacological Modulation
There are two known ligands for CCR7, CCL19 and CCL21 (please see Table 1) [363]. In 2010, it was shown that CCR7 is upregulated in astroglia activated by LPS, which suggested that this receptor is important during CNS inflammation [364]. CCR7 levels are diminished in hippocampal neurons during pilocarpine-induced status epilepticus in mice [365]. CCR7 is expressed in the nervous system on several immune cells, including T and B cells, lymphocytes, and dendritic cells [363]. The role of CCR7 is not sufficiently understood, but CCR7 stimulation causes dendritic cell maturation [363]; moreover, CCR7 knockout mice more frequently develop autoimmune disorders. In the light of the available literature, the roles of the CCL19/CCR7 and CCL21/CCR7 axes in CNS and PNS damage are unclear, and further in-depth research is needed.
8.1. CCR7—Endogenous Ligands
CCL19 is known as a strong chemoattractant for leukocytes and B/T cells in the brain after injury [56]. Moreover, the level of CCL19 is increased in the cerebrospinal fluid of patients with neuropathic pain [209,366]. Additionally, it was shown that this chemokine plays a key role in rat orofacial pain development [367]. In light of the scarce literature, the role of CCL19 in the neuroimmune changes caused by damage to the PNS and CNS should be determined.
CCL21 is expressed exclusively in damaged neurons of the CNS, and CCL21 rapidly induces inflammation because it strongly activates microglia [101,368]. Importantly, CCL21 exerts proinflammatory effects through two receptors: CCR7 and CXCR3 [369]. It has been shown that in a spinal cord injury model, mice with low CCL21 gene expression exhibit weakened classic microglial activation, which is associated with the proinflammatory phase [27]. Importantly, in the CCI model, an increase in the level of CCL21 in the spinal cord was also demonstrated, and intrathecal administration of CCL21-neutralizing antibodies inhibited the development of hypersensitivity to thermal and tactile stimuli [100]. Moreover, the presence of CCL21 in the serum of patients is an independent risk factor for cognitive impairment after spinal cord injury [370]. Therefore, CCL21 may be an important target for pharmacological intervention in neuroinflammation after spinal cord injury. Undoubtedly, CCL21 plays an important role in hypersensitivity development [368,371]. However, the CCL21/CXCR3 axis is likely more important than the CCL21/CCR7 axis for neuropathic pain [372] and needs to be studied further.
8.2. CCR7—Pharmacological Modulation
CCR7 consists of 353 amino acids [373], and recently, it was shown that its crystal structure contains an allosterically bound antagonist termed Cmp2105 [374,375]. Therefore, additional studies of CCR7 are necessary to determine the therapeutic potential of its intracellular allosteric pocket. Currently, some known substances are weak inhibitors of CCR7 [376]. N-truncated CCL19 was shown to bind to but not activate CCR7 by binding to the extracellularly exposed part of the receptor and preventing the binding of CCL19 and CCL21 [376,377,378,379]. Moreover, cosalane, a cholesterol derivative designed as a therapeutic for human immunodeficiency virus, can inhibit CCR7 in response to both CCL19 and CCL21 agonists in humans and mice [376]. To our knowledge, there are no published data showing the effects of CCR7 blockade or deficiency after damage to the nervous system. To date, the published data revealed that the deletion of CCR7 is not lethal in mice. Moreover, CCR7−/− mice exhibit impaired early production of specific IgG isotype antibodies in response to the antigen, failure to initiate fast primary B- and T-cell responses, and impaired homing of immune cells [380,381,382]. Interestingly, mice with CCR7 deletion exhibit an enhanced immune response to a variety of stimuli, including contact hypersensitivity reactions. B cells from these mutant strains are partially capable of migrating to the lymph nodes and splenic white pulp, which only proves that CCR7 is not the only regulator of lymphocyte mobilization [380,382]. Undoubtedly, research is necessary to determine the impact of CCR7 modulation on nervous system function after injury.
In summary, the above literature data suggest that in mice/rats and also in humans, CCR7 agonists, i.e., CCL19 and CCL21, may be important after PNS/CNS injuries. However, confirmation of these findings in further basic and clinical studies is needed. For nociceptive transmission, the CCL21/CXCR3 axis is likely more important than the CCL21/CCR7 axis is.
9. CCR8—Ligands and Pharmacological Modulation
Although CCR8 was discovered relatively long ago, in 1997, its role in the CNS and PNS still remains poorly understood [383]. CCR8 is known to have two selective ligands, CCL1, which was described in 1989 [384], and CCL18, which was discovered in 2013 [385] (please see Table 1). However, due to false reports of the absence of CCR8 in human peripheral immune cells and due to the lack of phenotypic abnormalities in CCR8 knockout mice, intensive research on this receptor was not conducted after its discovery [386]. Nevertheless, it was recently shown that this receptor is present on various types of lymphocytes, mainly in the skin but also in peripheral blood [386]. CCR8 is already known to play an important role in immune-mediated disorders, both in patients with atopic dermatitis [387] and in mouse models of allergic enteritis [388] and diabetes [153,389]. Importantly, recent immunofluorescence staining of the CNS demonstrated that CCR8 is highly expressed in neurons [153], suggesting that the initial assumptions about its nonessential role were most likely incorrect and that further studies are needed to determine its participation in changes after damage to the CNS and PNS. Importantly, the first published data suggested that in the mouse PSNL model, an increase in the CCR8 levels in the spinal cord is observed in both neuronal and glial cells [53], which indicates a possible important role for this receptor in the CNS.
9.1. CCR8—Endogenous Ligands
CCL1 was initially thought to be a chemokine produced primarily by lymphocytes, but recently, it was shown that it can also be secreted by many other cells, including neurons, monocytes, mast cells, and epithelial/endothelial cells [153,390]. The currently available literature indicates that CCL1 most likely plays an important role in the CNS and PNS as a mediator of neuron–glia interactions, which is why we believe that it may contribute to the progression of secondary disorders of the nervous system, including the development of neuropathic pain [391]. Importantly, an increase in CCL1 levels in mice was observed in the brain after ICH [52] and CIBI [51] and also in the spinal cord after CCI and PSNL. Importantly, the CCL1 changes occur after nerve injury in parallel with the development of neuropathic pain [53,153,392]. Notably, subsequent studies have indicated that pain symptoms are reduced after the intrathecal injection of a CCL1-neutralizing antibody [53,153] and that hypersensitivity developed to a lesser extent in CCR8 knockout mice [53]. Other studies have provided direct evidence for the pronociceptive effects of CCL1—as recombinant CCL1 administered intrathecally to naive mice induces thermal and tactile hypersensitivity [53,153] and parallel activation of spinal glia, resulting in the release of proinflammatory cytokines with pronociceptive effects [53]. Considering the available literature data from experimental studies, it can be concluded that the CCL1/CCR8 signaling pathway plays an important role in both CNS and PNS damage; however, further clinical studies are necessary.
Notably, the role of CCL18, the second endogenous CCR8 ligand present only in humans, after both CNS and PNS damage has not been determined, and clinical research on the role of the CCL18/CCR8 axis is needed.
9.2. CCR8—Pharmacological Modulation
Studies on the impact of the pharmacological CCR8 blockade on changes following CNS and PNS damage have yet to be conducted. These studies have only recently become possible because the first pharmacological tools, for example, AZ084 and R243, have only now been synthesized. Importantly, the results obtained after intrathecal administration of CCR8 siRNA are promising, and it has been shown that such a receptor blockade attenuates CCI-induced hypersensitivity [53]. However, in the case of damage both to the CNS and PNS, pharmacological studies with newly synthesized antagonists are still needed.
In summary, the above literature data suggest that in mice/rats/humans, the level of the CCR8 agonist CCL1 increases after PNS/CNS injury. Therefore, the CCL1-CCR8 axis seems to play an important role in neuroimmune disorders and may cause secondary CNS damage and/or the development of neuropathic pain. Clinical studies in particular are needed.
10. CCR9—Ligands and Pharmacological Modulation
CCR9 has only one ligand, CCL25 (please see Table 1) [393,394]. The receptor is expressed on dendritic cells, neutrophils, lymphocytes, monocyte macrophages, and vascular endothelial cells [395,396,397]. CCR9 levels are diminished in hippocampal neurons during pilocarpine-induced status epilepticus in mice [365]. The observed changes in the level of CCR9 during epilepsy suggested that this receptor may play an important, although still unknown, role in its pathomechanism, as well as in changes following CNS and PNS damage with various etiologies, and further research is certainly needed. Recently, it was found that the CCR9 gene encodes two isoforms: CCR9A and CCR9B. CCR9A most likely contains 12 additional AAs at its N-terminus. The longer N-terminal region of CCR9A may produce a receptor with enhanced affinity for CCL25. CCR9A and B are not functionally equivalent to each other. CCR9A signaling is suppressed, and CCR9A is desensitized at lower concentrations of CCL25 compared to CCR9B [398]. Yu et al. explained this phenomenon by the possibility that the active conformation of the receptor is the target of kinases that inactivate G protein-coupled receptors [399]. The decreased affinity of CCR9B for this ligand may also explain the relative insensitivity of this receptor subtype to homologous desensitization. The authors observed that, in the examined lymphocytes, there was a ∼10:1 ratio of CCR9A:CCR9B mRNA [399]. Additionally, with the use of calcium flux assays, the authors indicated that CCR9A is the most efficient receptor. To date, CCR9A and CCR9B—two CCR9 gene products resulting from differential mRNA splicing and different translation starting points—have been described in humans but not in mice [400]. Importantly, the CCR9 antagonist CCX282-B is an equally potent antagonist of mouse, rat, and human CCR9 [400]; however, the importance of the two isoforms, CCR9A and CCR9B, in the CNS and PNS needs to be studied in patients.
10.1. CCR9—Endogenous Ligands
CCL25 was discovered in 1999 [393,394]; therefore, the role of the CCL25/CCR9 signaling pathway in neuroimmune changes caused by damage to the PNS and CNS should be studied. The murine recombinant CCL25 protein exhibited chemotactic effects on activated macrophages, dendritic cells, and thymocytes [401]. Literature data thus far indicate that the CCR9/CCL25 axis participates in several inflammatory diseases, including cardiovascular diseases, autoimmune diseases, hepatitis, rheumatoid arthritis, inflammatory bowel disease, and asthma [401,402], but the mechanism of action still needs to be elucidated. Moreover, it was first published in 2022 that the level of CCL25 is increased in the cerebrospinal fluid of patients who developed neuropathic pain after breast cancer surgery, which suggests that CCL25 has pronociceptive effects [361]. However, the proinflammatory and pronociceptive roles of the CCL25/CCR9 axis need to be investigated.
10.2. CCR9—Pharmacological Modulation
CCX282-B is a selective CCR9 antagonist and it is bioavailable in the circulation after oral administration, as assessed in mouse models of IBD and in clinical trials [401].
In summary, further research is needed to investigate the possible proinflammatory and pronociceptive effects of the CCL25-CCR9 axis after PNS/CNS injury.
11. CCR10—Ligands and Pharmacological Modulation
CCR10 is a target of three chemokines, CCL26, CCL27, and CCL28 (please see Table 1). To date, little is known about the role of this receptor after CNS/PNS injury. However, the presence of CCR10 in mouse CNS tissue at both the protein and mRNA levels has already been documented [365]. In the spinal cord (but not in the thalamus) of diabetic monkeys, astroglia and microglia cells were shown to be activated. In parallel, an increased level of CCR10 and a decrease in opioid receptors (MOP, KOP, and DOP) was observed [314]. CCR10 is also expressed in the hippocampus by neurons, in the CA1-3 region by interneurons, and in the dentate gyrus by hilar cells [365]. Moreover, GCA+ immune cells derived from bone marrow were shown to migrate to the mouse brain through the CCR10-CCL28 axis. This pathway seems to be critical for the progression of Alzheimer’s disease [403]. Importantly, CCR10 levels are reduced in hippocampal neurons during pilocarpine-induced status epilepticus in mice [365]. Moreover, CCR10 is highly expressed in human glioblastoma brain tissue [404]; therefore, this receptor seems to be essential for glioma proliferation and invasion [404]. Furthermore, an enhanced level of CCR10 has recently been associated with poor survival in glioblastoma patients [404]. In the case of hepatocarcinogenesis, the expression of CCR10 seems to act as a link between TNFα stimulation and the activation of the PI3K/Akt pathway [405]. However, the significance of CCR10 for neuroinflammation and nociceptive transmission remains to be elucidated.
11.1. CCR10—Endogenous Ligands
To our knowledge, there is no available information showing CCL26, CCL27, and CCL28’s potential role after PNS/CNS damage; therefore, both experimental and clinical studies are needed. Overall, it is only known that the CCL27/CCR10 axis can mediate glioma cell proliferation and invasion [404] and is involved in T-cell-mediated skin inflammation [406].
11.2. CCR10—Pharmacological Modulation
A key role of CCR10 in carcinogenesis has been demonstrated. Therefore, in the future, the blocking of this receptor may become the basis of pharmacotherapy for malignant glioma in the brain. However, there is no information about the involvement of this receptor in changes following brain, spinal, or peripheral nerve injury [404]. Recently, it was suggested that blocking CCR10 and CCR4 simultaneously is beneficial in the treatment of T-cell-mediated skin diseases [407].
In summary, the possible proinflammatory and pronociceptive roles of the CCL26/27/28-CCR10 axis after PNS/CNS injury still need to be studied.
12. Analgesic Potential of Dual Chemokine CC Receptor Antagonists
The results of the latest research confirmed the advantage of compounds targeting more than one molecular target over the physical combination of individual antagonists in terms of their anti-inflammatory properties, which is also the case with double CC chemokine receptor antagonists, although this type of research is still insufficient.
A small-molecule chemokine receptor antagonist, UCB 35625, which is a potent, selective blocker of CCR1 and CCR3, is an interesting double CC chemokine receptor antagonist [408]. Importantly, among the described chemokines that play important roles in secondary changes occurring after CNS and PNS damage in patients, seven CCR1 ligands (CCL2, CCL3, CCL4, CCL5, CCL7, CCL8, and CCL23) and four CCR3 ligands (CCL5, CCL7, CCL8, and CCL11) occur in humans. Notably, the abovementioned receptors share three common ligands in humans (CCL5, CCL7, and CCL8) and four common ligands in rodents (CCL5, CCL7, CCL8, and CCL9). In 2022, UCB35625 was shown to diminish hypersensitivity to thermal and mechanical stimuli in a mouse CCI model [65]. However, the analgesic effect of this dual antagonist was similar to that obtained after administrations of a single CCR1 (J113863) and CCR3 (SB328437) antagonist [65]. These results are intriguing, and further in-depth research and possibly the creation of new dual CCR1/3 antagonists are needed. As the authors suggest, the effect may depend on the strength of the substance’s binding to the receptor, but it may also depend on effectiveness, which reflects the ability of the substance to activate the receptor and generate a cellular response [65]. The efficacy of these drugs may also depend on the pharmacokinetics and pharmacodynamics of these particular compounds and not necessarily on the spectrum of their action, which is why further study on the phenomenon of the double blockade of chemokine receptors is necessary. Additionally, it is important to understand the chemical structure of antagonists and their receptors, as they may affect the obtained pharmacological response [65]. However, the effect of UCB 35625 or new dual antagonists on secondary damage after CNS damage has yet to be determined in various animal models.
Another very interesting substance is cenicriviroc, a potent selective CCR2 and CCR5 receptor antagonist. Chemokines acting through these receptors play important roles in secondary changes occurring after CNS and PNS damage in patients. There are four CCR2 ligands (CCL2, CCL7, CCL8, and CCL12) and six CCR5 ligands (CCL3, CCL4, CCL5, CCL7, CCL8, and CCL11). Two of these chemokines (CCL7 and CCL8) are common ligands of CCR2 and CCR5 in humans and rodents; for example, CCR1 and CCR3 may or may not be beneficial for the effects of dual CCR2/CCR5 antagonists. Furthermore, importantly, CCR2 and CCR5 have been shown to heterodimerize after costimulation with their ligands [409]. There are currently three dual CCR2/CCR5 antagonists available: cenicriviroc [70,85], BMS-813160 [410], and PF-04634817 [411]. However, to our knowledge, only cenicriviroc has been used in experimental animal studies thus far. In 2016, it was shown that the response of the aging brain to TBI is the increased expression of chemokines (CCL2, CCL7, CCL8, and CCL5) involved in the recruitment of peripheral macrophages to the damaged parenchyma. Importantly, the administration of cenicriviroc significantly attenuated the influx of these cells while reducing inflammatory and neurotoxic symptoms [203]. Similar beneficial effects were obtained after repeated administrations of cenicriviroc in animal models of neuropathy [70,85]. Studies after PNS damage have shown that repeated intrathecal administrations of both selective CCR2 (RS504393) [48] and CCR5 (maraviroc) [71] antagonists and a double CCR2/CCR5 antagonist (cenicriviroc) [70,85] provide pain relief. However, cenicriviroc was the most effective compound for silencing immunological changes in a rat model of CCI. After administration of cenicriviroc, the levels of six chemokines (CCL2, CCL3, CCL4, CCL5, CCL7, and CCL12), which are CCR2 and/or CCR5 ligands, were reduced in the spinal cord and/or DRG [85]. Importantly, a single intrathecal and intraperitoneal administration of cenicriviroc had been shown to have greater analgesic effects than RS504393 or maraviroc in CCI-exposed mice. Moreover, a single intraperitoneal administration of cenicriviroc enhanced the analgesic effects evoked by opioid drugs (morphine and buprenorphine) [85], which may be crucial in the context of potential clinical use. It is still unclear what underlies this beneficial phenomenon. It may be the previously described heterologous desensitization occurring between CCR2 and CCR5 [409], the already proved suppression of immunological changes caused by injury to the nervous system [85], or perhaps both. Given that cenicriviroc is presently in the final phase of clinical trials for adult HIV infection and nonalcoholic steatohepatitis treatment [412], its anti-inflammatory and analgesic properties seem to be very promising.
In summary, increasing evidence confirms the excellent analgesic efficacy of multifunctional compounds in animal models and indicates the advantage of compounds targeting more than one molecular target. The benefits for future therapy of this approach may result from simultaneous access to two or more receptors at the same dose of a pharmacologically active compound. However, further pharmacological studies are necessary.
13. Conclusions
Despite significant progress in modern medicine and pharmacology, nervous system diseases still pose a challenge to doctors and scientists. Numerous studies indicate that CNS/PNS damage leads to neuroimmune changes that may ultimately result in both secondary damage and pain hypersensitivity. Importantly, the analysis of the results of various studies indicates that the mechanisms occurring both at the level of the brain after direct injury and at the level of the spinal cord after nerve damage largely have a common immunological basis. Notably, chemokines and their receptors are very conserved between species, which permits the use of rodent models to conduct pharmacological experiments and draw conclusions. Understanding the role of individual chemokines from the CC family may lead to the development of innovative and effective pharmacotherapies.
Experimental data indicate that after both CNS and PNS damage, the levels of 12 of 28 chemokines from the CC family, i.e., CCL1, CCL2, CCL3, CCL4, CCL5, CCL7, CCL8, CCL9, CCL11, CCL12, CCL17, CCL20, and CCL22, increase in the brain and/or spinal cord (Table 1), and their strong proinflammatory and/or pronociceptive properties have been proven (Scheme 3).
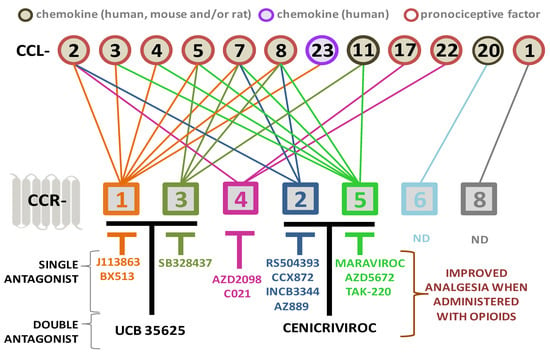
Scheme 3.
The beneficial effects of blocking the action of CC family chemokine receptors with antagonists after CNS and/or PNS damage. Additionally, the chemokines whose algesic effects have been confirmed in behavioral studies in mice/rats are marked with a red border. Scheme created based on data from the following literature [39,40,41,48,53,65,66,70,71,72,73,80,85,86,89,90,92,100,132,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,164,203,222,223,224,225,226,227,229,230,231,232,233,234,235,236,237,238,315].
To understand the role of the next four chemokines, i.e., CCL6, CCL8, CCL19, and CCL21, experimental research is still needed; however, preliminary results indicate changes in these cytokines after CNS and/or PNS damage (Table 1). We would like to point out that little is known about the changes and roles of the remaining five chemokines common in rodents and humans (CCL24, CCL25, CCL26, CCL27, and CCL28), as well as another six chemokines found exclusively in humans, e.g., CCL13, CCL14, CCL15, CCL16, CCL18, and CCL23 (Table 1). In the diagram, we have summarized the data about chemokines that exist in humans and their receptors that are known to play important roles in the brain and/or spinal cord after CNS and/or PNS damage (Scheme 3). Numerous behavioral studies have indicated that chemokine receptor antagonists may play an important role in therapy in the future. Available pharmacological tools indicate that blocking individual receptors, e.g., CCR1 (J113863 and BX513), CCR2 (RS504393, CCX872, INCB3344, and AZ889), CCR3 (SB328437), CCR4 (C021 and AZD-2098), and CCR5 (Maraviroc, AZD-5672, and TAK-220), has beneficial effects after both CNS and PNS damage (Scheme 3). However, it is still necessary to test whether and how blocking CCR6 and CCR8 affects the activities of their endogenous ligands, which are known to be upregulated, as is well documented for CCL1 and CCL20 after nerve and brain injury, respectively (Table 1).
Importantly, several chemokines, e.g., CCL1 [5], CCL2 [12], CCL3 [17], CCL7 [12], and CCL9 [17], decreased opioid analgesic properties in chronic pain, and the use of neutralizing antibodies restores the effectiveness of morphine and/or buprenorphine. Moreover, single (J113863 [41,66], RS504393 [48], SB328437 [80], C021 [92,230,315], and maraviroc [71,85]) and dual (cenicriviroc [70,85]) antagonists also enhance opioid analgesia. A multidirectional strategy based on the modulation of neuronal–glial–immune interactions by changing the activity of the chemokine family can significantly improve the quality of life of patients suffering from CNS and PNS damage.
Literature data indicated that novel CC-family receptor antagonists are currently being tested in patients for the management of various diseases. For example, several CCR1 antagonists have entered clinical trials, including CP-481,715 (Pfizer, New York, NY, USA) and MLN3897 (Millennium, New York, NY, USA) in rheumatoid arthritis trials, BX471 (Berlex/Scherring AG, Bothell, WA, USA) in multiple sclerosis, AZD-4818 (Astra-Zeneca, Cambridge, UK) in chronic obstructive disease spit, and BAY86-5047 for endometriosis [220,221]. The lack of beneficial effects observed in the studies in the case of some of them may be caused by their pharmacokinetic properties. For example, the CP481,715 is a competitive CCR1 antagonist; therefore, the IC50 varies depending on the concentration of several ligands and it can be hypothesized that their potentially high levels exceed those achieved by the drug. In this case, non-competitive antagonists would have the advantage of having activity regardless of the concentration of ligands present [220]. Another possibility of CCR1 antagonist failure can be the pleiotropic properties of its ligands, as we have shown in Scheme 3. However, the effectiveness of the next discussed compound gave far more promising results in clinical studies. The CCR5 antagonist maraviroc is an antiviral drug. By selectively binding to the CCR5 receptor, maraviroc prevents CCR5-tropic entering the cell by HIV-1 viruses [413]. However, in animal studies after CNS injury, it has been shown that maraviroc produces neuroprotective effects in TBI [232,233], FCS [234], CIBI [235], and ICH [236] models. Moreover in neuropathy models, it is able not only to diminish pain but enhance opioid effectiveness [71,85,237]. Importantly, maraviroc has received approval from the Food and Drug Administration and is used as a treatment for patients infected with human immunodeficiency virus R5 type 1 [352,353]. The fact that it is a drug used in clinics strongly suggests that it is worth trying to use it in the treatment of various diseases. Considering the good results obtained in animals, we hope that clinical trials will be conducted in patients after CNS and PNS injuries.
However, the blocking of one type of CC receptor may not be sufficient because of the overall pleiotropy and redundancy in the chemokine family. First, most of the receptors bind more than one chemokine, and second, most of the chemokines bind more than one receptor (Scheme 3), which makes planning pharmacological treatment quite a challenge. The results of the latest experimental studies confirmed the advantage of compounds targeting more than one CC chemokine receptor. It has already been proven that double antagonists, like UCB 35625 (blocker of CCR1/CCR3) [408] and cenicriviroc (blocker of CCR2/CCR5) [70,85], provide effective pain relief (Scheme 3, Table 2). Moreover, taking into account that cenicriviroc is currently in the final phase of clinical trials for adult HIV infection and nonalcoholic steatohepatitis treatment [412], its anti-inflammatory and analgesic properties seem to be very promising. Perhaps the use of a substance that would be able to block five chemokine receptors, CCR1-5 (Scheme 3), would have beneficial effects on therapy, but such an antagonist is currently unavailable.
Another approach to blocking chemokine receptor binding is the use of vMIP-II, also called viral master KEYmokine 2, vCCL2, or herpesvirus-8 macrophage inflammatory protein-II [414]. This is the only chemokine identified so far that is able to bind to six chemokine receptors from CC group (CCR1, CCR2, CCR3, CCR5, CCR8, and CCR10) but also receptors belonging to other groups (CXCR3, CXCR4, XCR1, and CX3CR1) [414,415]. vMIP-II is a chemokine encoded by human herpes virus-8 and was created by the virus to disrupt the homeostasis of the chemokine receptor network in the host and promote its survival. To date, most data on vMIP-II effects originate from in vitro studies. However, recently, it was shown that vMIP-II diminishes hypersensitivity in CCI-exposed mice [40] and improves the lymphocyte decrease in COVID-19 patients [416]. Computer tomography after a week of vMIP-II treatment showed that the lung lesions were significantly alleviated and the clinical symptoms were diminished [416]. In the light of the obtained results, it seems that the use of vMIP-II, a molecule with a broad spectrum of action, could also bring benefits after damage to the PNS and CNS, but this requires further pharmacological studies, first in animal models, and then, if good results are achieved, in clinical trials.
To sum up, in the future, the CC receptor blockers may also be used after CNS/PNS damage, but additional experimental and clinical studies are needed since the knowledge about the role of the CC family in neuroimmune changes is still insufficient. Considering the pleiotropic properties of chemokines, the pharmacological blockade of several chemokine receptors through the use of a multidirectional antagonist may be an innovative and effective method for treating CNS and PNS damage. Moreover, in the case of pain, the combined administration of multitarget CC chemokine antagonists with opioid drugs could allow for a decrease in therapeutic doses and thus minimize the risk of complications.
Author Contributions
Conceptualization, A.C. and J.M.; writing—original draft preparation, A.C. and J.M.; writing—review and editing, A.C. and J.M.; visualization, A.C. and J.M.; supervision, J.M.; project administration, J.M.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland grants, OPUS 22 2021/43/B/NZ7/00230, and statutory funds from the Maj Institute of Pharmacology Polish Academy of Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- James, S.L.; Bannick, M.S.; Montjoy-Venning, W.C.; Lucchesi, L.R.; Dandona, L.; Dandona, R.; Hawley, C.; Hay, S.I.; Jakovljevic, M.; Khalil, I.; et al. Global, Regional, and National Burden of Traumatic Brain Injury and Spinal Cord Injury, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the Global Incidence of Traumatic Brain Injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- NIH (National Institutes of Health). Traumatic Brain Injury Information. Available online: https://www.nih.gov/about-nih/what-we-do/nih-turning-discovery-into-health/healthy-mind/traumatic-brain-injury-tbi (accessed on 16 November 2023).
- Bouras, M.; Asehnoune, K.; Roquilly, A. Immune Modulation after Traumatic Brain Injury. Front. Med. 2022, 9, 995044. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Lira, M.; Cerpa, W. Traumatic Brain Injury: Mechanisms of Glial Response. Front. Physiol. 2021, 12, 740939. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, W.; Smith, D.H. Widespread Tau and Amyloid-Beta Pathology Many Years after a Single Traumatic Brain Injury in Humans. Brain Pathol. 2012, 22, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, S.M.; Nash, M.J.; Sweeting, C.J.; Graham, D.I.; Roberts, G.W. β-Amyloid Precursor Protein (ΒAPP) as a Marker for Axonal Injury after Head Injury. Neurosci. Lett. 1993, 160, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.S.N.; Sharp, D.J. Understanding Neurodegeneration after Traumatic Brain Injury: From Mechanisms to Clinical Trials in Dementia. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Scheid, R.; Walther, K.; Guthke, T.; Preul, C.; Von Cramon, D.Y. Cognitive Sequelae of Diffuse Axonal Injury. Arch. Neurol. 2006, 63, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Sariaslan, A.; Sharp, D.J.; D’Onofrio, B.M.; Larsson, H.; Fazel, S. Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes. PLoS Med. 2016, 13, 15–19. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Li, X.; Zhang, S.; Zhao, J.; Zhu, X.; Tian, G. Head Injury as a Risk Factor for Dementia and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of 32 Observational Studies. PLoS ONE 2017, 12, e0169650. [Google Scholar] [CrossRef]
- Nordström, A.; Nordström, P. Traumatic Brain Injury and the Risk of Dementia Diagnosis: A Nationwide Cohort Study. PLoS Med. 2018, 15, e1002496. [Google Scholar] [CrossRef] [PubMed]
- Fann, J.R.; Ribe, A.R.; Pedersen, H.S.; Fenger-Grøn, M.; Christensen, J.; Benros, M.E.; Vestergaard, M. Long-Term Risk of Dementia among People with Traumatic Brain Injury in Denmark: A Population-Based Observational Cohort Study. Lancet Psychiatry 2018, 5, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.A.; David Clark, J. Chronic Pain after Traumatic Brain Injury: Pathophysiology and Pain Mechanisms. Pain Med. 2018, 19, 1315–1333. [Google Scholar] [CrossRef] [PubMed]
- Smith-Seemiller, L.; Fow, N.R.; Kant, R.; Franzen, M.D. Presence of Post-Concussion Syndrome Symptoms in Patients with Chronic Pain vs Mild Traumatic Brain Injury. Brain Inj. 2003, 17, 199–206. [Google Scholar] [CrossRef]
- Dobscha, S.K.; Clark, M.E.; Morasco, B.J.; Freeman, M.; Campbell, R.; Helfand, M. Systematic Review of the Literature on Pain in Patients with Polytrauma Including Traumatic Brain Injury. Pain Med. 2009, 10, 1200–1217. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S. Posttraumatic Headache: Clinical Characterization and Management. Curr. Pain Headache Rep. 2015, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Hou, J.; Nelson, R.; Nissim, N.; Parmer, R.; Keener, J.; Wacnik, P.W.; Thompson, F.J. Effects of Acute Intrathecal Baclofen in an Animal Model of Tbi-Induced Spasticity, Cognitive, and Balance Disabilities. J. Neurotrauma 2013, 30, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Ofek, H.; Defrin, R. The Characteristics of Chronic Central Pain after Traumatic Brain Injury. Pain 2007, 131, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, H.L.; Pu, H.J.; Shi, Y.J.; Zhang, J.; Zhang, W.T.; Wang, G.H.; Hu, X.M.; Leak, R.K.; Chen, J.; et al. Ethyl Pyruvate Protects against Blood-Brain Barrier Damage and Improves Long-Term Neurological Outcomes in a Rat Model of Traumatic Brain Injury. CNS Neurosci. Ther. 2015, 21, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Costantini, T.W.; D’Mello, R.; Eliceiri, B.P.; Coimbra, R.; Bansal, V. Altering Leukocyte Recruitment Following Traumatic Brain Injury with Ghrelin Therapy. J. Trauma Acute Care Surg. 2014, 77, 709–715. [Google Scholar] [CrossRef]
- Noble, J.; Munro, C.A.; Prasad, V.S.S.V.; Midha, R. Analysis of Upper and Lower Extremity Peripheral Nerve Injuries in a Population of Patients with Multiple Injuries. J. Trauma-Inj. Infect. Crit. Care 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W. Evaluation and Management of Peripheral Nerve Injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, T.; Antoniadis, G.; Braun, V.; Rath, S.A.; Richter, H.P. Evaluation of Iatrogenic Lesions in 722 Surgically Treated Cases of Peripheral Nerve Trauma. J. Neurosurg. 2001, 94, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Althagafi, A.; Nadi, M. Acute Nerve Injury; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Selecki, B.; Ring, I.; Simpson, D.; Vanderfield, G.; Sewell, M. Trauma to the Central and Peripheral Nervous Systems. Part II: A Statistical Profile of Surgical Treatment New South Wales 1977. Aust. N. Z. J. Surg. 1982, 52, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.; Keenan, M.A. Peripheral Nerve Injuries in the Adult with Traumatic Brain Injury. Clin. Orthop. Relat. Res. 1988, 233, 136–144. [Google Scholar] [CrossRef]
- Wordliczek, J.; Dobrogowski, J. Leczenie Bólu; PZWL: Warszawa, Poland, 2007. [Google Scholar]
- Latremoliere, A.; Woolf, C.J. Central Sensitization: A Generator of Pain Hypersensitivity by Central Neural Plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Muley, M.M.; Krustev, E.; Reid, A.R.; McDougall, J.J. Prophylactic Inhibition of Neutrophil Elastase Prevents the Development of Chronic Neuropathic Pain in Osteoarthritic Mice. J. Neuroinflamm. 2017, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Lopes, A.H.; Talbot, J.; Cecilio, N.T.; Rossato, M.F.; Silva, R.L.; Souza, G.R.; Silva, C.R.; Lucas, G.; Fonseca, B.A.; et al. Neuroimmune–Glia Interactions in the Sensory Ganglia Account for the Development of Acute Herpetic Neuralgia. J. Neurosci. 2017, 37, 6408–6422. [Google Scholar] [CrossRef]
- Lim, H.; Lee, H.; Noh, K.; Lee, S.J. IKK/NF-B-Dependent Satellite Glia Activation Induces Spinal Cord Microglia Activation and Neuropathic Pain after Nerve Injury. Pain 2017, 158, 1666–1677. [Google Scholar] [CrossRef]
- Purwata, T.E. High TNF-Alpha Plasma Levels and Macrophages INOS and TNF-Alpha Expression as Risk Factors for Painful Diabetic Neuropathy. J. Pain Res. 2011, 4, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kambrun, C.; Roca-Lapirot, O.; Salio, C.; Landry, M.; Moqrich, A.; Le Feuvre, Y. TAFA4 Reverses Mechanical Allodynia through Activation of GABAergic Transmission and Microglial Process Retraction. Cell Rep. 2018, 22, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- White, F.A.; Jung, H.; Miller, R.J. Chemokines and the Pathophysiology of Neuropathic Pain. Proc. Natl. Acad. Sci. USA 2007, 104, 20151–20158. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Mika, J. The Importance of Chemokines in Neuropathic Pain Development and Opioid Analgesic Potency. Pharmacol. Rep. 2018, 70, 821–830. [Google Scholar] [CrossRef]
- Savarin-Vuaillat, C.; Ransohoff, R.M. Chemokines and Chemokine Receptors in Neurological Disease: Raise, Retain, or Reduce? Neurotherapeutics 2007, 4, 590–601. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowska, A.; Rojewska, E.; Piotrowska, A.; Barut, J.; Pawlik, K.; Ciapała, K.; Kreiner, G.; Mika, J. New Insights into the Analgesic Properties of the XCL1/XCR1 and XCL1/ITGA9 Axes Modulation under Neuropathic Pain Conditions-Evidence from Animal Studies. Front. Immunol. 2022, 13, 1058204. [Google Scholar] [CrossRef] [PubMed]
- Rojewska, E.; Zychowska, M.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front. Immunol. 2018, 9, 494. [Google Scholar] [CrossRef]
- Gleissner, C.A.; Von Hundelshausen, P.; Ley, K. Platelet Chemokines in Vascular Disease. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1920–1927. [Google Scholar] [CrossRef]
- Steen, A.; Larsen, O.; Thiele, S.; Rosenkilde, M.M. Biased and g Protein-Independent Signaling of Chemokine Receptors. Front. Immunol. 2014, 5, 277. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, R.; Cong, Y.; Liu, Z. Critical Roles of G Protein-Coupled Receptors in Regulating Intestinal Homeostasis and Inflammatory Bowel Disease. Mucosal Immunol. 2022, 15, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Poluri, K.M. Molecular Insights into Kinase Mediated Signaling Pathways of Chemokines and Their Cognate G Protein Coupled Receptors. Front. Biosci. 2020, 25, 1361–1385. [Google Scholar] [CrossRef]
- Henrot, P.; Prevel, R.; Berger, P.; Dupin, I. Chemokines in COPD: From Implication to Therapeutic Use. Int. J. Mol. Sci. 2019, 20, 2785. [Google Scholar] [CrossRef] [PubMed]
- Rizeq, B.; Malki, M.I. The Role of CCL21/CCR7 Chemokine Axis in Breast Cancer Progression. Cancers 2020, 12, 1036. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Mika, J. The RS504393 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in Neuropathic Rats. J. Neuroimmune Pharmacol. 2017, 12, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1 Alpha, and MIP-1 Beta as the Major HIV-Suppressive Factors Produced by CD8+ T Cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Menten, P.; Wuyts, A.; Van Damme, J. Macrophage Inflammatory Protein-1. Cytokine Growth Factor Rev. 2002, 13, 455–481. [Google Scholar] [CrossRef] [PubMed]
- Baba, N.; Wang, F.; Iizuka, M.; Shen, Y.; Yamashita, T.; Takaishi, K.; Tsuru, E.; Matsushima, S.; Miyamura, M.; Fujieda, M.; et al. Induction of Regional Chemokine Expression in Response to Human Umbilical Cord Blood Cell Infusion in the Neonatal Mouse Ischemia-Reperfusion Brain Injury Model. PLoS ONE 2019, 14, e0221111. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Kai, T.; Shimizu, Y.; Yano, Y.; Urabe, Y.; Tasaka, S.; Akagi, M.; Yamaguchi, Y.; Inoue, A. Gadolinium Causes M1 and M2 Microglial Apoptosis after Intracerebral Haemorrhage and Exerts Acute Neuroprotective Effects. J. Pharm. Pharmacol. 2020, 72, 709–718. [Google Scholar] [CrossRef]
- Akimoto, N.; Honda, K.; Uta, D.; Beppu, K.; Ushijima, Y.; Matsuzaki, Y.; Nakashima, S.; Kido, M.A.; Imoto, K.; Takano, Y.; et al. CCL-1 in the Spinal Cord Contributes to Neuropathic Pain Induced by Nerve Injury. Cell Death Dis. 2013, 4, e679. [Google Scholar] [CrossRef]
- Zajaczkowska, R.; Kwiatkowski, K.; Pawlik, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Wordliczek, J.; Mika, J. Metamizole Relieves Pain by Influencing Cytokine Levels in Dorsal Root Ganglia in a Rat Model of Neuropathic Pain. Pharmacol. Rep. 2020, 72, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Barczyk, K.; Ciechanowska, A.; Ciapała, K.; Pawlik, K.; Oggioni, M.; Mercurio, D.; De Simoni, M.G.; Mika, J. The CCL2/CCL7/CCL12/CCR2 Pathway Is Substantially and Persistently Upregulated in Mice after Traumatic Brain Injury, and CCL2 Modulates the Complement System in Microglia. Mol. Cell. Probes 2020, 54, 101671. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lin, W.; Su, Y.C.; Cheng-Yo Hsuan, Y.; Chen, Y.C.; Chang, C.P.; Chou, W.; Lin, K.C. Modulation of Parietal Cytokine and Chemokine Gene Profiles by Mesenchymal Stem Cell as a Basis for Neurotrauma Recovery. J. Formos. Med. Assoc. 2019, 118, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Bye, N.; Rancan, M.; Ziebell, J.M.; Morganti-Kossmann, M.C. Role of CCL2 (MCP-1) in Traumatic Brain Injury (TBI): Evidence from Severe TBI Patients and CCL2−/− Mice. J. Cereb. Blood Flow Metab. 2010, 30, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Clausen, F.; Marklund, N.; Hillered, L. Acute Inflammatory Biomarker Responses to Diffuse Traumatic Brain Injury in the Rat Monitored by a Novel Microdialysis Technique. J. Neurotrauma 2019, 36, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Chen, L.; Tang, J.; Chen, Y.; Wang, L. The Role of CCL2/CCR2 Axis in Cerebral Ischemia-Reperfusion Injury and Treatment: From Animal Experiments to Clinical Trials. Int. J. Mol. Sci. 2022, 23, 3485. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, X.; Ma, C.; Ji, J.; Xu, W.; Shao, Q.; Liao, X.; Li, Y.; Cheng, F.; Wang, Q. Identification of Potential Regulating Effect of Baicalin on NFκB/CCL2/CCR2 Signaling Pathway in Rats with Cerebral Ischemia by Antibody-Based Array and Bioinformatics Analysis. J. Ethnopharmacol. 2022, 284, 114773. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ma, Q.; Li, Y.; Li, B.; Zhang, L. Inhibition of MicroRNA-210 Suppresses pro-Inflammatory Response and Reduces Acute Brain Injury of Ischemic Stroke in Mice. Exp. Neurol. 2018, 300, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Tsirka, S.E. The CCL2-CCR2 System Affects the Progression and Clearance of Intracerebral Hemorrhage. Glia 2012, 60, 908–918. [Google Scholar] [CrossRef]
- Stammers, A.T.; Liu, J.; Kwon, B.K. Expression of Inflammatory Cytokines Following Acute Spinal Cord Injury in a Rodent Model. J. Neurosci. Res. 2012, 90, 782–790. [Google Scholar] [CrossRef]
- Yang, F.; Jing, J.J.; Fu, S.Y.; Su, X.Z.; Zhong, Y.L.; Chen, D.S.; Wu, X.Z.; Zou, Y.Q. Spinal MCP-1 Contributes to Central Post-Stroke Pain by Inducing Central Sensitization in Rats. Mol. Neurobiol. 2023, 60, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Kwiatkowski, K.; Mika, J. Pharmacological Evidence of the Important Roles of CCR1 and CCR3 and Their Endogenous Ligands CCL2/7/8 in Hypersensitivity Based on a Murine Model of Neuropathic Pain. Cells 2023, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Piotrowska, A.; Kwiatkowski, K.; Ciapała, K.; Popiolek-Barczyk, K.; Makuch, W.; Mika, J. The Blockade of CC Chemokine Receptor Type 1 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in a Rat Model of Neuropathic Pain. Immunology 2020, 159, 413–428. [Google Scholar] [CrossRef] [PubMed]
- Ciapała, K.; Rojewska, E.; Pawlik, K.; Ciechanowska, A.; Mika, J. Analgesic Effects of Fisetin, Peimine, Astaxanthin, Artemisinin, Bardoxolone Methyl and 740 Y-P and Their Influence on Opioid Analgesia in a Mouse Model of Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 9000. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowska, A.; Popiolek-Barczyk, K.; Pawlik, K.; Ciapała, K.; Oggioni, M.; Mercurio, D.; De Simoni, M.G.; Mika, J. Changes in Macrophage Inflammatory Protein-1 (MIP-1) Family Members Expression Induced by Traumatic Brain Injury in Mice. Immunobiology 2020, 225, 151911. [Google Scholar] [CrossRef] [PubMed]
- Förstner, P.; Rehman, R.; Anastasiadou, S.; Haffner-Luntzer, M.; Sinske, D.; Ignatius, A.; Roselli, F.; Knöll, B. Neuroinflammation after Traumatic Brain Injury Is Enhanced in Activating Transcription Factor 3 Mutant Mice. J. Neurotrauma 2018, 35, 2317–2329. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Pawlik, K.; Ciapała, K.; Piotrowska, A.; Makuch, W.; Mika, J. Bidirectional Action of Cenicriviroc, a CCR2/CCR5 Antagonist, Results in Alleviation of Pain-Related Behaviors and Potentiation of Opioid Analgesia in Rats With Peripheral Neuropathy. Front. Immunol. 2020, 11, 615327. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Jurga, A.; Slusarczyk, J.; Trojan, E.; Basta-Kaim, A.; Mika, J. Beneficial Properties of Maraviroc on Neuropathic Pain Development and Opioid Effectiveness in Rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 68–78. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Pawlik, K.; Ciapała, K.; Mika, J. Pharmacological Modulation of the MIP-1 Family and Their Receptors Reduces Neuropathic Pain Symptoms and Influences Morphine Analgesia: Evidence from a Mouse Model. Brain Sci. 2023, 13, 579. [Google Scholar] [CrossRef]
- Kiguchi, N.; Kobayashi, Y.; Maeda, T.; Saika, F.; Kishioka, S. CC-Chemokine MIP-1α in the Spinal Cord Contributes to Nerve Injury-Induced Neuropathic Pain. Neurosci. Lett. 2010, 484, 17–21. [Google Scholar] [CrossRef]
- Sewell, D.L.; Nacewicz, B.; Liu, F.; Macvilay, S.; Erdei, A.; Lambris, J.D.; Sandor, M.; Fabry, Z. Complement C3 and C5 Play Critical Roles in Traumatic Brain Cryoinjury: Blocking Effects on Neutrophil Extravasation by C5a Receptor Antagonist. J. Neuroimmunol. 2004, 155, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xu, Y.; Guo, P.; Chen, Y.J.; Zhou, J.; Xia, M.; Tan, B.; Liu, X.; Feng, H.; Chen, Y. CCL5/CCR5-Mediated Peripheral Inflammation Exacerbates Blood–brain Barrier Disruption after Intracerebral Hemorrhage in Mice. J. Transl. Med. 2023, 21, 196. [Google Scholar] [CrossRef] [PubMed]
- Offner, H.; Subramanian, S.; Parker, S.M.; Afentoulis, M.E.; Vandenbark, A.A.; Hurn, P.D. Experimental Stroke Induces Massive, Rapid Activation of the Peripheral Immune System. J. Cereb. Blood Flow Metab. 2006, 26, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Fan, Q.; Zhao, Y.; Cheng, M.-Y.; Liu, H.; Li, J.; Lu, F.-F.; Jia, J.-T.; Cheng, W.; Yan, C.-D. Spinal NF-ΚB and Chemokine Ligand 5 Expression during Spinal Glial Cell Activation in a Neuropathic Pain Model. PLoS ONE 2015, 10, e0115120. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Rojewska, E.; Mika, J. Comparison of the Effects of Chemokine Receptors CXCR2 and CXCR3 Pharmacological Modulation in Neuropathic Pain Model—In Vivo and In Vitro Study. Int. J. Mol. Sci. 2021, 22, 11074. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Narita, M.M.; Ikegami, D.; Yamashita, A.; Shimizu, T.; Narita, M.M.; Niikura, K.; Furuya, M.; Kobayashi, Y.; Miyashita, K.; et al. Epigenetic Transcriptional Activation of Monocyte Chemotactic Protein 3 Contributes to Long-Lasting Neuropathic Pain. Brain 2013, 136, 828–843. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Ciechanowska, A.; Ciapała, K.; Rojewska, E. Blockade of CC Chemokine Receptor Type 3 Diminishes Pain and Enhances Opioid Analgesic Potency in a Model of Neuropathic Pain. Front. Immunol 2021, 12, 781310. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, G.; Wang, H.; Yang, M.; Yang, R.; Li, X.; Zhang, X.; Yuan, H. Interleukin-1β Pre-Treated Bone Marrow Stromal Cells Alleviate Neuropathic Pain through CCL7-Mediated Inhibition of Microglial Activation in the Spinal Cord. Sci. Rep. 2017, 7, 42260. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Mika, J. CC Chemokine Receptor 4 (CCR4) as a Possible New Target for Therapy. Int. J. Mol. Sci. 2022, 23, 15638. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Baba, N.; Shen, Y.; Yamashita, T.; Tsuru, E.; Tsuda, M.; Maeda, N.; Sagara, Y. CCL11 Promotes Migration and Proliferation of Mouse Neural Progenitor Cells. Stem Cell Res. Ther. 2017, 8, 26. [Google Scholar] [CrossRef]
- Huang, J.; Yang, G.; Xiong, X.; Wang, M.; Yuan, J.; Zhang, Q.; Gong, C.; Qiu, Z.; Meng, Z.; Xu, R.; et al. Age-Related CCL12 Aggravates Intracerebral Hemorrhage-Induced Brain Injury via Recruitment of Macrophages and T Lymphocytes. Aging Dis. 2020, 11, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Ciapała, K.; Rojewska, E.; Makuch, W.; Mika, J. Comparison of the Beneficial Effects of RS504393, Maraviroc and Cenicriviroc on Neuropathic Pain-Related Symptoms in Rodents: Behavioral and Biochemical Analyses. Int. Immunopharmacol. 2020, 84, 106540. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Popiolek-Barczyk, K.; Piotrowska, A.; Rojewska, E.; Ciapała, K.; Makuch, W.; Mika, J. Chemokines CCL2 and CCL7, but Not CCL12, Play a Significant Role in the Development of Pain-Related Behavior and Opioid-Induced Analgesia. Cytokine 2019, 119, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Chio, C.C.; Lin, H.J.; Tian, Y.F.; Chen, Y.C.; Lin, M.T.; Lin, C.H.; Chang, C.P.; Hsu, C.C. Exercise Attenuates Neurological Deficits by Stimulating a Critical HSP70/NF-ΚB/IL-6/Synapsin I Axis in Traumatic Brain Injury Rats. J. Neuroinflamm. 2017, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Hylin, M.J.; Kobori, N.; Hood, K.N.; Moore, A.N.; Dash, P.K. Post-Injury Administration of Galantamine Reduces Traumatic Brain Injury Pathology and Improves Outcome. J. Neurotrauma 2018, 35, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Jin, P.; Sherchan, P.; Liu, S.; Cui, Y.; Huang, L.; Zhang, J.H.; Gong, Y.; Tang, J. Recombinant CCL17-Dependent CCR4 Activation Alleviates Neuroinflammation and Neuronal Apoptosis through the PI3K/AKT/Foxo1 Signaling Pathway after ICH in Mice. J. Neuroinflamm. 2021, 18, 62. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Sherchan, P.; Jin, P.; Huang, L.; Travis, Z.; Zhang, J.H.; Gong, Y.; Tang, J. Recombinant CCL17 Enhances Hematoma Resolution and Activation of CCR4/ERK/Nrf2/CD163 Signaling Pathway After Intracerebral Hemorrhage in Mice. Neurotherapeutics 2020, 17, 1940–1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bian, L.; Du, Y.; Wang, D.; Jiang, R.; Lu, J.; Zhao, X. The Roles of Chemokines Following Intracerebral Hemorrhage in Animal Models and Humans. Front. Mol. Neurosci. 2023, 15, 1091498. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Popiolek-Barczyk, K.; Pawlik, K.; Ciechanowska, A.; Makuch, W.; Rojewska, E.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Influences the Level of Nociceptive Factors and Enhances the Analgesic Potency of Morphine in a Rat Model of Neuropathic Pain. Eur. J. Pharmacol. 2020, 880, 173166. [Google Scholar] [CrossRef]
- Das, M.; Tang, X.; Han, J.Y.; Mayilsamy, K.; Foran, E.; Biswal, M.R.; Tzekov, R.; Mohapatra, S.S.; Mohapatra, S. CCL20-CCR6 Axis Modulated Traumatic Brain Injury-Induced Visual Pathologies. J. Neuroinflamm. 2019, 16, 115. [Google Scholar] [CrossRef]
- Dalgard, C.L.; Cole, J.T.; Kean, W.S.; Lucky, J.J.; Sukumar, G.; McMullen, D.C.; Pollard, H.B.; Watson, W.D. The Cytokine Temporal Profile in Rat Cortex after Controlled Cortical Impact. Front. Mol. Neurosci. 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, C.C.; Musso, J.; Das, M.; Rowe, D.D.; Collier, L.A.; Mohapatra, S.; Pennypacker, K.R. CCL20 Is Associated with Neurodegeneration Following Experimental Traumatic Brain Injury and Promotes Cellular Toxicity In Vitro. Transl. Stroke Res. 2012, 3, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Mayilsamy, K.; Markoutsa, E.; Das, M.; Chopade, P.; Puro, D.; Kumar, A.; Gulick, D.; Willing, A.E.; Mohapatra, S.S.; Mohapatra, S. Treatment with ShCCL20-CCR6 Nanodendriplexes and Human Mesenchymal Stem Cell Therapy Improves Pathology in Mice with Repeated Traumatic Brain Injury. Nanomedicine 2020, 29, 102247. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Li, Y.; Han, J.; Wu, S.; Liu, X.; Wang, Q.; Li, Z.; Shi, F.D. Microglia-Derived CCL20 Deteriorates Neurogenesis Following Intraventricular Hemorrhage. Exp. Neurol. 2023, 370, 114561. [Google Scholar] [CrossRef] [PubMed]
- Terao, Y.; Ohta, H.; Oda, A.; Nakagaito, Y.; Kiyota, Y.; Shintani, Y. Macrophage Inflammatory Protein-3alpha Plays a Key Role in the Inflammatory Cascade in Rat Focal Cerebral Ischemia. Neurosci. Res. 2009, 64, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yang, Z.; Li, X.; Lu, H. C-C Motif Chemokine Ligand 20 Regulates Neuroinflammation Following Spinal Cord Injury via Th17 Cell Recruitment. J. Neuroinflamm. 2016, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Zychowska, M.; Mika, J. Pharmacological Blockade of CXCR3 by (±)-NBI-74330 Reduces Neuropathic Pain and Enhances Opioid Effectiveness-Evidence from in Vivo and in Vitro Studies. BBA-Mol. Basis Dis. 2018, 1864, 3418–3437. [Google Scholar] [CrossRef] [PubMed]
- Biber, K.; Tsuda, M.; Tozaki-Saitoh, H.; Tsukamoto, K.; Toyomitsu, E.; Masuda, T.; Boddeke, H.; Inoue, K. Neuronal CCL21 Up-Regulates Microglia P2X4 Expression and Initiates Neuropathic Pain Development. EMBO J. 2011, 30, 1864–1873. [Google Scholar] [CrossRef] [PubMed]
- Madathil, S.K.; Wilfred, B.S.; Urankar, S.E.; Yang, W.; Leung, L.Y.; Gilsdorf, J.S.; Shear, D.A. Early Microglial Activation Following Closed-Head Concussive Injury Is Dominated by Pro-Inflammatory M-1 Type. Front. Neurol. 2018, 9, 964. [Google Scholar] [CrossRef]
- Pu, Z.; Bao, X.; Xia, S.; Shao, P.; Xu, Y. Serpine1 Regulates Peripheral Neutrophil Recruitment and Acts as Potential Target in Ischemic Stroke. J. Inflamm. Res. 2022, 15, 2649–2663. [Google Scholar] [CrossRef]
- Zhang, B.; Ran, Y.; Wu, S.; Zhang, F.; Huang, H.; Zhu, C.; Zhang, S.; Zhang, X. Inhibition of Colony Stimulating Factor 1 Receptor Suppresses Neuroinflammation and Neonatal Hypoxic-Ischemic Brain Injury. Front. Neurol. 2021, 12, 607370. [Google Scholar] [CrossRef]
- Murdoch, C.; Finn, A. Chemokine Receptors and Their Role in Inflammation and Infectious Diseases. Blood 2000, 95, 3032–3043. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Nagira, M.; Kitaura, M.; Imagawa, N.; Imai, T.; Yoshie, O. Secondary Lymphoid-Tissue Chemokine Is a Functional Ligand for the CC Chemokine Receptor CCR7. J. Biol. Chem. 1998, 273, 7118–7122. [Google Scholar] [CrossRef] [PubMed]
- Kohout, T.A.; Nicholas, S.L.; Perry, S.J.; Reinhart, G.; Junger, S.; Struthers, R.S. Differential Desensitization, Receptor Phosphorylation, Beta-Arrestin Recruitment, and ERK1/2 Activation by the Two Endogenous Ligands for the CC Chemokine Receptor 7. J. Biol. Chem. 2004, 279, 23214–23222. [Google Scholar] [CrossRef] [PubMed]
- Scheu, S.; Ali, S.; Ruland, C.; Arolt, V.; Alferink, J. The C-C Chemokines CCL17 and CCL22 and Their Receptor CCR4 in CNS Autoimmunity. Int. J. Mol. Sci. 2017, 18, 2306. [Google Scholar] [CrossRef] [PubMed]
- N’Diaye, M.; Le Ferrec, E.; Lagadic-Gossmann, D.; Corre, S.; Gilot, D.; Lecureur, V.; Monteiro, P.; Rauch, C.; Galibert, M.D.; Fardel, O. Aryl Hydrocarbon Receptor- and Calcium-Dependent Induction of the Chemokine CCL1 by the Environmental Contaminant Benzo[a]Pyrene. J. Biol. Chem. 2006, 281, 19906–19915. [Google Scholar] [CrossRef]
- Arai, H.; Tsou, C.L.; Charo, I.F. Chemotaxis in a Lymphocyte Cell Line Transfected with C-C Chemokine Receptor 2B: Evidence That Directed Migration Is Mediated by Βγ Dimers Released by Activation of Gαi-Coupled Receptors. Proc. Natl. Acad. Sci. USA 1997, 94, 14495. [Google Scholar] [CrossRef]
- Neptune, E.R.; Bourne, H.R. Receptors Induce Chemotaxis by Releasing the Betagamma Subunit of Gi, Not by Activating Gq or Gs. Proc. Natl. Acad. Sci. USA 1997, 94, 14489–14494. [Google Scholar] [CrossRef]
- Hirsch, E.; Katanaev, V.L.; Garlanda, C.; Azzolino, O.; Pirola, L.; Silengo, L.; Sozzani, S.; Mantovani, A.; Altruda, F.; Wymann, M.P. Central Role for G Protein-Coupled Phosphoinositide 3-Kinase Gamma in Inflammation. Science 2000, 287, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, E.; Aloisi, F. Chemokines and Glial Cells: A Complex Network in the Central Nervous System. Neurochem. Res. 2004, 29, 1017–1038. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.K.; Giles, D.A.; Segal, B.M.; Irani, D.N. An Emerging Role for Eotaxins in Neurodegenerative Disease. Clin. Immunol. 2018, 189, 29–33. [Google Scholar] [CrossRef]
- Popiolek-Barczyk, K.; Mika, J. Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain. Curr. Med. Chem. 2016, 23, 2908–2928. [Google Scholar] [CrossRef]
- Futosi, K.; Fodor, S.; Mócsai, A. Neutrophil Cell Surface Receptors and Their Intracellular Signal Transduction Pathways. Int. Immunopharmacol. 2013, 17, 638–650. [Google Scholar] [CrossRef]
- Henc, I.; Bryl, E. Chemokiny Jako Ważne Mediatory Stanu Zapalnego. Forum Med. Rodz. 2013, 7, 251–262. [Google Scholar]
- Strazza, M.; Mor, A. Consider the Chemokines: A Review of the Interplay between Chemokines and T Cell Subset Function. Discov. Med. 2017, 24, 31–39. [Google Scholar] [PubMed]
- Cowell, R.M.; Xu, H.; Galasso, J.M.; Silverstein, F.S. Hypoxic-Ischemic Injury Induces Macrophage Inflammatory Protein-1alpha Expression in Immature Rat Brain. Stroke 2002, 33, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zuo, G.; Sherchan, P.; Huang, L.; Ocak, U.; Xu, W.; Travis, Z.D.; Wang, W.; Zhang, J.H.; Tang, J. CCR1 Activation Promotes Neuroinflammation Through CCR1/TPR1/ERK1/2 Signaling Pathway After Intracerebral Hemorrhage in Mice. Neurotherapeutics 2020, 17, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Halks-Miller, M.; Schroeder, M.L.; Haroutunian, V.; Moenning, U.; Rossi, M.; Achim, C.; Purohit, D.; Mahmoudi, M.; Horuk, R. CCR1 Is an Early and Specific Marker of Alzheimer’s Disease. Ann. Neurol. 2003, 54, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Sunnemark, D.; Eltayeb, S.; Wallström, E.; Appelsved, L.; Malmberg, Å.; Lassmann, H.; Ericsson-Dahlstrand, A.; Piehl, F.; Olsson, T. Differential Expression of the Chemokine Receptors CX3CR1 and CCR1 by Microglia and Macrophages in Myelin-Oligodendrocyte-Glycoprotein-Induced Experimental Autoimmune Encephalomyelitis. Brain Pathol. 2003, 13, 617–629. [Google Scholar] [CrossRef]
- Shi, C.; Jin, J.; Xu, H.; Ma, J.; Li, T.; Xie, Y.; Li, Z. CCR1 Enhances SUMOylation of DGCR8 by Up-Regulating ERK Phosphorylation to Promote Spinal Nerve Ligation-Induced Neuropathic Pain. Gene Ther. 2021, 29, 379–389. [Google Scholar] [CrossRef]
- Lloyd, E.; Somera-Molina, K.; Van Eldik, L.J.; Watterson, D.M.; Wainwright, M.S. Suppression of Acute Proinflammatory Cytokine and Chemokine Upregulation by Post-Injury Administration of a Novel Small Molecule Improves Long-Term Neurologic Outcome in a Mouse Model of Traumatic Brain Injury. J. Neuroinflamm. 2008, 5, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Israelsson, C.; Bengtsson, H.; Kylberg, A.; Kullander, K.; Lewén, A.; Hillered, L.; Ebendal, T. Distinct Cellular Patterns of Upregulated Chemokine Expression Supporting a Prominent Inflammatory Role in Traumatic Brain Injury. J. Neurotrauma 2008, 25, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Thacker, M.A.; Clark, A.K.; Bishop, T.; Grist, J.; Yip, P.K.; Moon, L.D.F.; Thompson, S.W.N.; Marchand, F.; McMahon, S.B. CCL2 Is a Key Mediator of Microglia Activation in Neuropathic Pain States. Eur. J. Pain 2009, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Shin, H.Y.; Cui, Y.; Kim, H.; Le Thi, A.H.; Choi, J.Y.; Kim, E.Y.; Hwang, D.H.; Kim, B.G. CCL2 Mediates Neuron–Macrophage Interactions to Drive Proregenerative Macrophage Activation Following Preconditioning Injury. J. Neurosci. 2015, 35, 15934–15947. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, N.; Kobayashi, Y.; Saika, F.; Kishioka, S. Epigenetic Upregulation of CCL2 and CCL3 via Histone Modifications in Infiltrating Macrophages after Peripheral Nerve Injury. Cytokine 2013, 64, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kotani, T.; Kuwabara, H.; Suzuka, T.; Kiboshi, T.; Fukui, K.; Ishida, T.; Fujiki, Y.; Shiba, H.; Hata, K.; et al. CCL2 Produced by CD68+/CD163+ Macrophages as a Promising Clinical Biomarker of Microscopic Polyangiitis-Interstitial Lung Disease. Rheumatology 2021, 60, 4643–4653. [Google Scholar] [CrossRef] [PubMed]
- Tecchio, C.; Cassatella, M.A. Neutrophil-Derived Chemokines on the Road to Immunity. Semin. Immunol. 2016, 28, 119. [Google Scholar] [CrossRef] [PubMed]
- Errede, M.; Annese, T.; Petrosino, V.; Longo, G.; Girolamo, F.; de Trizio, I.; d’Amati, A.; Uccelli, A.; Kerlero de Rosbo, N.; Virgintino, D. Microglia-Derived CCL2 Has a Prime Role in Neocortex Neuroinflammation. Fluids Barriers CNS 2022, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.-Z.Z.; Park, J.-Y.Y.; Lind, A.-L.L.; Ma, Q.; Ji, R.-R.R. JNK-Induced MCP-1 Production in Spinal Cord Astrocytes Contributes to Central Sensitization and Neuropathic Pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef]
- Ajuebor, M.N.; Flower, R.J.; Hannon, R.; Christie, M.; Bowers, K.; Verity, A.; Perretti, M. Endogenous Monocyte Chemoattractant Protein-1 Recruits Monocytes in the Zymosan Peritonitis Model. J. Leukoc. Biol. 1998, 63, 108–116. [Google Scholar] [CrossRef]
- Rotterman, T.M.; Haley-Johnson, Z.; Pottorf, T.S.; Chopra, T.; Chang, E.; Zhang, S.; McCallum, W.M.; Fisher, S.; Franklin, H.; Alvarez, M.; et al. Modulation of Central Synapse Remodeling after Remote Peripheral Injuries by the CCL2-CCR2 Axis and Microglia. Cell Rep. 2024, 43, 113776. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Rhind, S.G.; Hutchison, M.G.; Hassan, S.; Shiu, M.Y.; Inaba, K.; Topolovec-vranic, J.; Neto, A.C.; Rizoli, S.B.; Baker, A.J. Inflammatory Cytokine and Chemokine Profiles Are Associated with Patient Outcome and the Hyperadrenergic State Following Acute Brain Injury. J. Neuroinflamm. 2016, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Zuurman, M.W.; Heeroma, J.; Brouwer, N.; Boddeke, H.W.G.M.; Biber, K. LPS-Induced Expression of a Novel Chemokine Receptor (L-CCR) in Mouse Glial Cells in Vitro and in Vivo. Glia 2003, 41, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Xie, W.; Zhang, J.; Strong, J.A.; Zhang, J.M. Sympathectomy Decreases Pain Behaviors and Nerve Regeneration by Downregulating Monocyte Chemokine CCL2 in Dorsal Root Ganglia in the Rat Tibial Nerve Crush Model. Pain 2022, 163, E106–E120. [Google Scholar] [CrossRef] [PubMed]
- Semple, B.D.; Frugier, T.; Morganti-Kossmann, M.C. CCL2 Modulates Cytokine Production in Cultured Mouse Astrocytes. J. Neuroinflamm. 2010, 7, 67. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.R.; Iftinca, M.; Gomes, F.I.F.; Segal, J.P.; Smith, O.M.A.; Bannerman, C.A.; Mendes, A.S.; Defaye, M.; Robinson, M.E.C.; Gilron, I.; et al. Skin-Resident Dendritic Cells Mediate Postoperative Pain via CCR4 on Sensory Neurons. Proc. Natl. Acad. Sci. USA 2022, 119, e2118238119. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Nalepa, I.; Mika, J. Pharmacological Blockade of Spinal CXCL3/CXCR2 Signaling by NVP CXCR2 20, a Selective CXCR2 Antagonist, Reduces Neuropathic Pain Following Peripheral Nerve Injury. Front. Immunol. 2019, 10, 2198. [Google Scholar] [CrossRef] [PubMed]
- Zychowska, M.; Rojewska, E.; Pilat, D.; Mika, J. The Role of Some Chemokines from the CXC Subfamily in a Mouse Model of Diabetic Neuropathy. J. Diabetes Res. 2015, 2015, 750182. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.C.; Cao, D.L.; Zhang, X.; Zhang, Z.J.; He, L.N.; Li, C.H.; Zhang, W.W.; Wu, X.B.; Berta, T.; Ji, R.R.; et al. CXCL13 Drives Spinal Astrocyte Activation and Neuropathic Pain via CXCR5. J. Clin. Investig. 2016, 126, 745–761. [Google Scholar] [CrossRef]
- Rojewska, E.; Ciapała, K.; Mika, J. Kynurenic Acid and Zaprinast Diminished CXCL17-Evoked Pain-Related Behaviour and Enhanced Morphine Analgesia in a Mouse Neuropathic Pain Model. Pharmacol. Rep. 2019, 71, 139–148. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Mika, J. Microglial Inhibition Influences XCL1/XCR1 Expression and Causes Analgesic Effects in a Mouse Model of Diabetic Neuropathy. Anesthesiology 2016, 125, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Staniland, A.A.; Clark, A.K.; Wodarski, R.; Sasso, O.; Maione, F.; D’Acquisto, F.; Malcangio, M. Reduced Inflammatory and Neuropathic Pain and Decreased Spinal Microglial Response in Fractalkine Receptor (CX3CR1) Knockout Mice. J. Neurochem. 2010, 114, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Li, J.; Wang, J.; Zhou, L.; Li, J.; Liang, J.; Yin, G.; Li, X.; Cheng, Y.; Zhang, K. ITGA9 Inhibits Proliferation and Migration of Dermal Microvascular Endothelial Cells in Psoriasis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2795–2806. [Google Scholar] [CrossRef] [PubMed]
- Reyes, N.; Benedetti, I.; Rebollo, J.; Correa, O.; Geliebter, J. Atypical Chemokine Receptor CCRL2 Is Overexpressed in Prostate Cancer Cells. J. Biomed. Res. 2019, 33, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; Sozio, F.; Sozzani, S.; Prete, A. Del Role of Atypical Chemokine Receptors in Microglial Activation and Polarization. Front. Aging Neurosci. 2017, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Pruenster, M.; Mudde, L.; Bombosi, P.; Dimitrova, S.; Zsak, M.; Middleton, J.; Richmond, A.; Graham, G.J.; Segerer, S.; Nibbs, R.J.B.; et al. The Duffy Antigen Receptor for Chemokines Transports Chemokines and Supports Their Promigratory Activity. Nat. Immunol. 2009, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hansell, C.A.; Maclellan, L.M.; Oldham, R.S.; Doonan, J.; Chapple, K.J.; Anderson, E.J.R.; Linington, C.; Mcinnes, I.B.; Nibbs, R.J.B.; Goodyear, C.S. The Atypical Chemokine Receptor ACKR2 Suppresses Th17 Responses to Protein Autoantigens. Immunol. Cell Biol. 2015, 93, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Banisadr, G.; Podojil, J.R.; Miller, S.D.; Miller, R.J. Pattern of CXCR7 Gene Expression in Mouse Brain Under Normal and Inflammatory Conditions. J. Neuroimmune Pharmacol. 2016, 11, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Friess, M.C.; Kritikos, I.; Schineis, P.; Medina-Sanchez, J.D.; Gkountidi, A.O.; Vallone, A.; Sigmund, E.C.; Schwitter, C.; Vranova, M.; Matti, C.; et al. Mechanosensitive ACKR4 Scavenges CCR7 Chemokines to Facilitate T Cell De-Adhesion and Passive Transport by Flow in Inflamed Afferent Lymphatics. Cell Rep. 2022, 38, 110334. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Spinal CCL1/CCR8 Signaling Interplay as a Potential Therapeutic Target–Evidence from a Mouse Diabetic Neuropathy Model. Int. Immunopharmacol. 2017, 52, 261–271. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Chen, O.; Xue, J.; Huang, S.; Zhu, W.; Wang, Y. Neuroprotective and Anti-Inflammatory Effects of Isoliquiritigenin in Kainic Acid-Induced Epileptic Rats via the TLR4/MYD88 Signaling Pathway. Inflammopharmacology 2019, 27, 1143–1153. [Google Scholar] [CrossRef]
- Zhu, X.; Wei, D.; Chen, O.; Zhang, Z.; Xue, J.; Huang, S.; Zhu, W.; Wang, Y. Upregulation of CCL3/MIP-1alpha Regulated by MAPKs and NF-KappaB Mediates Microglial Inflammatory Response in LPS-Induced Brain Injury. Acta Neurobiol. Exp. 2016, 76, 304–317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stefini, R.; Catenacci, E.; Piva, S.; Sozzani, S.; Valerio, A.; Bergomi, R.; Cenzato, M.; Mortini, P.; Latronico, N. Chemokine Detection in the Cerebral Tissue of Patients with Posttraumatic Brain Contusions. J. Neurosurg. 2008, 108, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Karpus, W.J.; Kennedy, K.J. MIP-1alpha and MCP-1 Differentially Regulate Acute and Relapsing Autoimmune Encephalomyelitis as Well as Th1/Th2 Lymphocyte Differentiation. J. Leukoc. Biol. 1997, 62, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Karpus, W.J.; Lukacs, N.W.; McRae, B.L.; Strieter, R.M.; Kunkel, S.L.; Miller, S.D. An Important Role for the Chemokine Macrophage Inflammatory Protein-1 Alpha in the Pathogenesis of the T Cell-Mediated Autoimmune Disease, Experimental Autoimmune Encephalomyelitis. J. Immunol. 1995, 155, 5003–5010. [Google Scholar] [CrossRef]
- Kennedy, K.J.; Strieter, R.M.; Kunkel, S.L.; Lukacs, N.W.; Karpus, W.J. Acute and Relapsing Experimental Autoimmune Encephalomyelitis Are Regulated by Differential Expression of the CC Chemokines Macrophage Inflammatory Protein-1α and Monocyte Chemotactic Protein-1. J. Neuroimmunol. 1998, 92, 98–108. [Google Scholar] [CrossRef] [PubMed]
- De Haas, A.H.; Van Weering, H.R.J.; De Jong, E.K.; Boddeke, H.W.G.M.; Biber, K.P.H. Neuronal Chemokines: Versatile Messengers in Central Nervous System Cell Interaction. Mol. Neurobiol. 2007, 36, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, U.K. Microglia as a Source and Target of Cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.A.; Moir, G.; Malone, D.F.G.; Duncan, L.; Devarajan, G.; Crane, I.J. Regulation of T-Lymphocyte CCL3 and CCL4 Production by Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 722–730. [Google Scholar] [CrossRef][Green Version]
- Blidberg, K.; Palmberg, L.; Dahlén, B.; Lantz, A.S.; Larsson, K. Chemokine Release by Neutrophils in Chronic Obstructive Pulmonary Disease. Innate Immun. 2012, 18, 503–510. [Google Scholar] [CrossRef]
- Matsushita, K.; Tozaki-Saitoh, H.; Kojima, C.; Masuda, T.; Tsuda, M.; Inoue, K.; Hoka, S. Chemokine (C-C Motif) Receptor 5 Is an Important Pathological Regulator in the Development and Maintenance of Neuropathic Pain. Anesthesiology 2014, 120, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, E.; Faivre, E.; Dutar, P.; Alves Pires, C.; Demeyer, D.; Caillierez, R.; Laloux, C.; Buée, L.; Blum, D.; Humez, S. The Chemokine MIP-1α/CCL3 Impairs Mouse Hippocampal Synaptic Transmission, Plasticity and Memory. Sci. Rep. 2015, 5, 15862. [Google Scholar] [CrossRef] [PubMed]
- Meucci, O.; Fatatis, A.; Simen, A.A.; Bushell, T.J.; Gray, P.W.; Miller, R.J. Chemokines Regulate Hippocampal Neuronal Signaling and Gp120 Neurotoxicity. Proc. Natl. Acad. Sci. USA 1998, 95, 14500–14505. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, G.; Zhu, M.; Kang, X.; Guo, H. Neuroinflammation in Alzheimer’s Disease: Chemokines Produced by Astrocytes and Chemokine Receptors. Int. J. Clin. Exp. Pathol. 2014, 7, 8342–8355. [Google Scholar] [PubMed]
- Xia, M.Q.; Qin, S.X.; Wu, L.J.; Mackay, C.R.; Hyman, B.T. Immunohistochemical Study of the Beta-Chemokine Receptors CCR3 and CCR5 and Their Ligands in Normal and Alzheimer’s Disease Brains. Am. J. Pathol. 1998, 153, 31–37. [Google Scholar] [CrossRef] [PubMed]
- MacNair, L.; Xiao, S.; Miletic, D.; Ghani, M.; Julien, J.-P.; Keith, J.; Zinman, L.; Rogaeva, E.; Robertson, J. MTHFSD and DDX58 Are Novel RNA-Binding Proteins Abnormally Regulated in Amyotrophic Lateral Sclerosis. Brain 2016, 139, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, S.; Bliss, S.K.; Curiel, T.J.; Denkers, E.Y. Cross-Talk in the Innate Immune System: Neutrophils Instruct Recruitment and Activation of Dendritic Cells during Microbial Infection. J. Immunol. 2003, 171, 6052–6058. [Google Scholar] [CrossRef] [PubMed]
- Charmoy, M.; Brunner-Agten, S.; Aebischer, D.; Auderset, F.; Launois, P.; Milon, G.; Proudfoot, A.E.I.; Tacchini-Cottier, F. Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Leishmania Major Inoculation in Resistant Mice. PLoS Pathog. 2010, 6, e1000755. [Google Scholar] [CrossRef] [PubMed]
- Ghirnikar, R.S.; Lee, Y.L.; He, T.R.; Eng, L.F. Chemokine Expression in Rat Stab Wound Brain Injury. J. Neurosci. Res. 1996, 46, 727–733. [Google Scholar] [CrossRef]
- Makker, P.G.S.; Duffy, S.S.; Lees, J.G.; Perera, C.J.; Tonkin, R.S.; Butovsky, O.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy. PLoS ONE 2017, 12, e0170814. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.W.; Zhao, L.; Wang, H.; Yang, N.; Dai, S.S.; He, F. Metabotropic Glutamate Receptor 5 Deficiency Inhibits Neutrophil Infiltration after Traumatic Brain Injury in Mice. Sci. Rep. 2017, 7, 9998. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.E.; Kunkel, S.L.; Shah, M.R.; Fu, R.; Mazarakis, D.D.; Haines, G.K.; Burdick, M.D.; Pope, R.M.; Strieter, R.M. Macrophage Inflammatory Protein-Iβ: A C-C Chemokine in Osteoarthritis. Clin. Immunol. Immunopathol. 1995, 77, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Hanifi-Moghaddam, P.; Kappler, S.; Seissler, J.; Müller-Scholze, S.; Martin, S.; Roep, B.O.; Strassburger, K.; Kolb, H.; Schloot, N.C. Altered Chemokine Levels in Individuals at Risk of Type 1 Diabetes Mellitus. Diabet. Med. 2006, 23, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Capelli, A.; Di Stefano, A.; Gnemmi, I.; Balbo, P.; Cerutti, C.G.; Balbi, B.; Lusuardi, M.; Donner, C.F. Increased MCP-1 and MIP-1β in Bronchoalveolar Lavage Fluid of Chronic Bronchitics. Eur. Respir. J. 1999, 14, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ajuebor, M.N.; Hogaboam, C.M.; Kunkel, S.L.; Proudfoot, A.E.I.; Wallace, J.L. The Chemokine RANTES Is a Crucial Mediator of the Progression from Acute to Chronic Colitis in the Rat. J. Immunol. 2001, 166, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Bacon, K.; Toy, K.J.; Goeddel, D.V. Selective Attraction of Monocytes and T Lymphocytes of the Memory Phenotype by Cytokine RANTES. Nature 1990, 347, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Puri, V.; Klein, R.M.; Berman, N.E.J. Differential Expression of Cytokines and Chemokines during Secondary Neuron Death Following Brain Injury in Old and Young Mice. Neurosci. Lett. 2004, 369, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Helmy, A.; Carpenter, K.L.H.; Menon, D.K.; Pickard, J.D.; Hutchinson, P.J.A. The Cytokine Response to Human Traumatic Brain Injury: Temporal Profiles and Evidence for Cerebral Parenchymal Production. J. Cereb. Blood Flow Metab. 2011, 31, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Lumpkins, K.; Bochicchio, G.V.; Zagol, B.; Ulloa, K.; Simard, J.M.; Schaub, S.; Meyer, W.; Scalea, T. Plasma Levels of the Beta Chemokine Regulated upon Activation, Normal T Cell Expressed, and Secreted (RANTES) Correlate with Severe Brain Injury. J. Trauma-Inj. Infect. Crit. Care 2008, 64, 358–361. [Google Scholar] [CrossRef]
- Ajoy, R.; Lo, Y.C.; Ho, M.H.; Chen, Y.Y.; Wang, Y.; Chen, Y.H.; Jing-Yuan, C.; Changou, C.A.; Hsiung, Y.C.; Chen, H.M.; et al. CCL5 Promotion of Bioenergy Metabolism Is Crucial for Hippocampal Synapse Complex and Memory Formation. Mol. Psychiatry 2021, 26, 6451–6468. [Google Scholar] [CrossRef]
- Tokami, H.; Ago, T.; Sugimori, H.; Kuroda, J.; Awano, H.; Suzuki, K.; Kiyohara, Y.; Kamouchi, M.; Kitazono, T. RANTES Has a Potential to Play a Neuroprotective Role in an Autocrine/Paracrine Manner after Ischemic Stroke. Brain Res. 2013, 1517, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.; Mao, C.-C.; Ching-Wah Sum, D.; Liu, F.-C.; Lai, Y.-S.; Li, J.-C.; Day, Y.-J. Peritoneal Administration of Met-RANTES Attenuates Inflammatory and Nociceptive Responses in a Murine Neuropathic Pain Model. J. Pain 2013, 14, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.B.; Tran, P.B.; Gillard, S.E.; Hurley, R.W.; Hammond, D.L.; Miller, R.J. Chemokines and Glycoprotein 120 Produce Pain Hypersensitivity by Directly Exciting Primary Nociceptive Neurons. J. Neurosci. 2001, 21, 5027–5035. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.T.; Yuan, H.B.; Mao, C.C.; Lai, Y.S.; Day, Y.J. Absence of C-C Motif Chemokine Ligand 5 in Mice Leads to Decreased Local Macrophage Recruitment and Behavioral Hypersensitivity in a Murine Neuropathic Pain Model. Pain 2012, 153, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Malon, J.T.; Cao, L. Calcitonin Gene-Related Peptide Contributes to Peripheral Nerve Injury-Induced Mechanical Hypersensitivity through CCL5 and P38 Pathways. J. Neuroimmunol. 2016, 297, 68–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hang, L.; Shao, D.-H.; Chen, Z.; Chen, Y.-F.; Shu, W.-W.; Zhao, Z.-G. Involvement of Spinal CC Chemokine Ligand 5 in the Development of Bone Cancer Pain in Rats. Basic Clin. Pharmacol. Toxicol. 2013, 113, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Du, X.; Lan, F.; Li, N.; Zhang, C.; Zhu, C.; Wang, X.; He, Y.; Shao, Z.; Chen, H.; et al. Eosinophilic Inflammation Promotes CCL6-Dependent Metastatic Tumor Growth. Sci. Adv. 2021, 7, 5943–5969. [Google Scholar] [CrossRef] [PubMed]
- Kanno, M.; Suzuki, S.; Fujiwara, T.; Yokoyama, A.; Sakamoto, A.; Takahashi, H.; Imai, Y.; Tanaka, J. Functional Expression of CCL6 by Rat Microglia: A Possible Role of CCL6 in Cell–Cell Communication. J. Neuroimmunol. 2005, 167, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, F.; Zhang, C.; Li, N.; Huang, H.; Shao, Z.; Zhang, M.; Zhan, X.; He, Y.; Ju, Z.; et al. Eosinophil-Derived Chemokine (HCCL15/23, MCCL6) Interacts with CCR1 to Promote Eosinophilic Airway Inflammation. Signal Transduct. Target. Ther. 2021 61 2021, 6, 91. [Google Scholar] [CrossRef]
- Kim, K.-S.; Rajarathnam, K.; Clark-Lewis, I.; Sykes, B.D. Structural Characterization of a Monomeric Chemokine: Monocyte Chemoattractant Protein-3. FEBS Lett. 1996, 395, 24–25. [Google Scholar] [CrossRef]
- Renner, N.A.; Ivey, N.S.; Redmann, R.K.; Lackner, A.A.; MacLean, A.G. MCP-3/CCL7 Production by Astrocytes: Implications for SIV Neuroinvasion and AIDS Encephalitis. J. Neurovirol. 2011, 17, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.L.; Van Eldik, L.J. Inflammatory Cytokines Stimulate the Chemokines CCL2/MCP-1 and CCL7/MCP-7 through NFκB and MAPK Dependent Pathways in Rat Astrocytes. Brain Res. 2009, 1287, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.A.P.; Kim, B.S. The Scope and Activation Mechanisms of Chemokine Gene Expression in Primary Astrocytes Following Infection with Theiler’s Virus. J. Neuroimmunol. 2004, 149, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W.; Sadeghi, Z.; Levine, A.D.; Penn, M.S.; von Recum, H.A.; Caplan, A.I.; Hijaz, A. The Role of CXCL12 and CCL7 Chemokines in Immune Regulation, Embryonic Development, and Tissue Regeneration. Cytokine 2014, 69, 277–283. [Google Scholar] [CrossRef]
- Ke, B.; Huang, X.X.; Li, Y.; Li, L.Y.; Xu, Q.X.; Gao, Y.; Liu, Y.; Luo, J. Neuronal-Derived Ccl7 Drives Neuropathic Pain by Promoting Astrocyte Proliferation. Neuroreport 2016, 27, 849–857. [Google Scholar] [CrossRef]
- Thirion, S.; Nys, G.; Fiten, P.; Masure, S.; Van Damme, J.; Opdenakker, G. Mouse Macrophage Derived Monocyte Chemotactic Protein-3: CDNA Cloning and Identification as MARC/FIC. Biochem. Biophys. Res. Commun. 1994, 201, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhang, Y.; Zhang, J.; Zhu, Z.; Lv, Q.; Su, J. Astrocyte-Derived CCL7 Promotes Microglia-Mediated Inflammation Following Traumatic Brain Injury. Int. Immunopharmacol. 2021, 99, 107975. [Google Scholar] [CrossRef]
- Ali, S.; Robertson, H.; Wain, J.H.; Isaacs, J.D.; Malik, G.; Kirby, J.A. A Non-Glycosaminoglycan-Binding Variant of CC Chemokine Ligand 7 (Monocyte Chemoattractant Protein-3) Antagonizes Chemokine-Mediated Inflammation. J. Immunol. 2005, 175, 1257–1266. [Google Scholar] [CrossRef]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.-H. The Chemotaxis of M1 and M2 Macrophages Is Regulated by Different Chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Morganti, J.M.; Riparip, L.K.; Chou, A.; Liu, S.; Gupta, N.; Rosi, S. Age Exacerbates the CCR2/5-Mediated Neuroinflammatory Response to Traumatic Brain Injury. J. Neuroinflamm. 2016, 13, 80. [Google Scholar] [CrossRef]
- Chou, A.; Krukowski, K.; Morganti, J.M.; Riparip, L.K.; Rosi, S. Persistent Infiltration and Impaired Response of Peripherally-Derived Monocytes after Traumatic Brain Injury in the Aged Brain. Int. J. Mol. Sci. 2018, 19, 1616. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Suhett, C.P.; Li, Z.; Marini, A.M.; Braga, M.F.M.; Eiden, L.E. Temporal Course of Changes in Gene Expression Suggests a Cytokine-Related Mechanism for Long-Term Hippocampal Alteration after Controlled Cortical Impact. J. Neurotrauma 2014, 31, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Dyhrfort, P.; Shen, Q.; Clausen, F.; Thulin, M.; Enblad, P.; Kamali-Moghaddam, M.; Lewén, A.; Hillered, L. Monitoring of Protein Biomarkers of Inflammation in Human Traumatic Brain Injury Using Microdialysis and Proximity Extension Assay Technology in Neurointensive Care. J. Neurotrauma 2019, 36, 2872–2885. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, B.C.; Cao, D.L.; Zhao, L.X.; Zhang, Y.L. Chemokine CCL8 and Its Receptor CCR5 in the Spinal Cord Are Involved in Visceral Pain Induced by Experimental Colitis in Mice. Brain Res. Bull. 2017, 135, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, W.; Xu, H.; Yang, J.; Jiang, J.; Jiang, Y.; Xu, G. Correlation of CCL8 Expression with Immune Cell Infiltration of Skin Cutaneous Melanoma: Potential as a Prognostic Indicator and Therapeutic Pathway. Cancer Cell Int. 2021, 21, 635. [Google Scholar] [CrossRef] [PubMed]
- Bäckryd, E.; Lind, A.-L.; Thulin, M.; Larsson, A.; Gerdle, B.; Gordh, T. High Levels of Cerebrospinal Fluid Chemokines Point to the Presence of Neuroinflammation in Peripheral Neuropathic Pain: A Cross-Sectional Study of 2 Cohorts of Patients Compared with Healthy Controls. Pain 2017, 158, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Sadik, C.D.; Kim, N.D.; Luster, A.D. Neutrophils Cascading Their Way to Inflammation. Trends Immunol. 2011, 32, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Simats, A.; García-Berrocoso, T.; Penalba, A.; Giralt, D.; Llovera, G.; Jiang, Y.; Ramiro, L.; Bustamante, A.; Martinez-Saez, E.; Canals, F.; et al. CCL23: A New CC Chemokine Involved in Human Brain Damage. J. Intern. Med. 2018, 283, 461–475. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Montecucco, F. CCL23 Is a Promising Biomarker of Injury in Patients with Ischaemic Stroke. J. Intern. Med. 2018, 283, 476–478. [Google Scholar] [CrossRef]
- Lin, H.; Shen, J.; Zhu, Y.; Zhou, L.; Zhang, S.; Liu, Z.; Wu, F.; Zhan, R. Serum CCL23 Emerges as a Biomarker for Poor Prognosis in Patients with Intracerebral Hemorrhage. Clin. Chim. Acta 2022, 537, 188–193. [Google Scholar] [CrossRef]
- Rottman, J.; Slavin, A.; Silva, R.; Weiner, H.; Gerard, C.; Hancock, W. Leukocyte Recruitment during Onset of Experimental Allergic Encephalomyelitis Is CCR1 Dependent. Eur. J. Immunol. 2000, 30, 2372–2377. [Google Scholar] [CrossRef]
- Pilat, D.; Piotrowska, A.; Rojewska, E.; Jurga, A.; Ślusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Blockade of IL-18 Signaling Diminished Neuropathic Pain and Enhanced the Efficacy of Morphine and Buprenorphine. Mol. Cell. Neurosci. 2016, 71, 114–124. [Google Scholar] [CrossRef]
- Pilat, D.; Rojewska, E.; Jurga, A.M.; Piotrowska, A.; Makuch, W.; Przewlocka, B.; Mika, J. IL-1 Receptor Antagonist Improves Morphine and Buprenorphine Efficacy in a Rat Neuropathic Pain Model. Eur. J. Pharmacol. 2015, 764, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of Glial Activation in Neuropathic Pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Wordliczek, J.; Dobrogowski, J.; Zajączkowska, R. Zastosowanie Leków Opioidowych w Leczeniu Bólu. Wszechświat 2013, 114, 10–12. [Google Scholar]
- Szczudlik, A.; Dobrogowski, J.; Wordliczek, J.; Stępień, A.; Krajnik, M.; Leppert, W.; Woroń, J.; Przeklasa-Muszyńska, A.; Kocot-Kępska, M.; Zajączkowska, R.; et al. Diagnosis and Management of Neuropathic Pain: Review of Literature and Recommendations of the Polish Society for the Study of Pain and the Neurological Society-Part One. Ból 2014, 15, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Gladue, R.P.; Brown, M.F.; Zwillich, S.H. CCR1 Antagonists: What Have We Learned from Clinical Trials. Curr. Top. Med. Chem. 2010, 10, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Trummer, D.; Walzer, A.; Groettrup-Wolfers, E.; Schmitz, H. Efficacy, Safety and Tolerability of the CCR1 Antagonist BAY 86-5047 for the Treatment of Endometriosis-Associated Pelvic Pain: A Randomized Controlled Trial. Acta Obstet. Gynecol. Scand. 2017, 96, 694–701. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Wu, Q.; Wang, T. Chemokine CCL2 Induces Apoptosis in Cortex Following Traumatic Brain Injury. J. Mol. Neurosci. 2013, 51, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dong, H.; Qian, Q.; Zhang, X.; Wang, Y.; Jin, W.; Qian, Y. Astrocyte-Derived CCL2 Participates in Surgery-Induced Cognitive Dysfunction and Neuroinflammation via Evoking Microglia Activation. Behav. Brain Res. 2017, 332, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Slusarczyk, J.; Makuch, W.; Basta-Kaim, A.; Przewlocka, B.; Mika, J. Direct and Indirect Pharmacological Modulation of CCL2/CCR2 Pathway Results in Attenuation of Neuropathic Pain-In Vivo and in Vitro Evidence. J. Neuroimmunol. 2016, 297, 9–19. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Dong, Y.L.; Lu, Y.; Cao, S.; Zhao, Z.Q.; Gao, Y.J. Chemokine CCL2 and Its Receptor CCR2 in the Medullary Dorsal Horn Are Involved in Trigeminal Neuropathic Pain. J. Neuroinflamm. 2012, 9, 136. [Google Scholar] [CrossRef]
- Morganti, J.M.; Jopson, T.D.; Liu, S.; Riparip, L.-K.; Guandique, C.K.; Gupta, N.; Ferguson, A.R.; Rosi, S. CCR2 Antagonism Alters Brain Macrophage Polarization and Ameliorates Cognitive Dysfunction Induced by Traumatic Brain Injury. J. Neurosci. 2015, 35, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.X.; Broughton, B.R.S.; Ah Kim, H.; Lee, S.; Drummond, G.R.; Sobey, C.G. Evidence That Ly6C(Hi) Monocytes Are Protective in Acute Ischemic Stroke by Promoting M2 Macrophage Polarization. Stroke 2015, 46, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Van Steenwinckel, J.; Goazigo, A.R.L.; Pommier, B.; Mauborgne, A.; Dansereau, M.A.; Kitabgi, P.; Sarret, P.; Pohl, M.; Parsadaniantz, S.M. CCL2 Released from Neuronal Synaptic Vesicles in the Spinal Cord Is a Major Mediator of Local Inflammation and Pain after Peripheral Nerve Injury. J. Neurosci. 2011, 31, 5865–5875. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Paré, M.; Mcintosh, F.; Jr Elmes, S.; Martino, G.; Jomphe, C.; Lessard, E.; Lembo, P.M.; Vaillancourt, F.; Perkins, M.N.; et al. Blocking Spinal CCR2 with AZ889 Reversed Hyperalgesia in a Model of Neuropathic Pain. Mol. Pain 2010, 6, 1744–8069. [Google Scholar] [CrossRef]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Administration Diminishes Hypersensitivity and Enhances the Analgesic Potency of Morphine and Buprenorphine in a Mouse Model of Neuropathic Pain. Front. Immunol. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Xu, H.; Zhang, Z.; Wang, X.; Yuan, L.; Lenahan, C.; Zhang, C.; Jiang, J.; Fang, C.; et al. CCL17 Exerts Neuroprotection through Activation of CCR4/MTORC2 Axis in Microglia after Subarachnoid Haemorrhage in Rats. Stroke Vasc. Neurol. 2022, 8, 4–16. [Google Scholar] [CrossRef]
- Liu, X.L.; Sun, D.D.; Zheng, M.T.; Li, X.T.; Niu, H.H.; Zhang, L.; Zhou, Z.W.; Rong, H.T.; Wang, Y.; Wang, J.W.; et al. Maraviroc Promotes Recovery from Traumatic Brain Injury in Mice by Suppression of Neuroinflammation and Activation of Neurotoxic Reactive Astrocytes. Neural Regen. Res. 2023, 18, 141–149. [Google Scholar] [CrossRef]
- Friedman-Levi, Y.; Liraz-Zaltsman, S.; Shemesh, C.; Rosenblatt, K.; Kesner, E.L.; Gincberg, G.; Carmichael, S.T.; Silva, A.J.; Shohami, E. Pharmacological Blockers of CCR5 and CXCR4 Improve Recovery after Traumatic Brain Injury. Exp. Neurol. 2021, 338, 113604. [Google Scholar] [CrossRef]
- Wu, Q.L.; Cui, L.Y.; Ma, W.Y.; Wang, S.S.; Zhang, Z.; Feng, Z.P.; Sun, H.S.; Chu, S.F.; He, W.B.; Chen, N.H. A Novel Small-Molecular CCR5 Antagonist Promotes Neural Repair after Stroke. Acta Pharmacol. Sin. 2023, 44, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cao, P.; Guo, X.; Yin, M.; Li, X.; Jiang, L.; Shao, J.; Chen, X.; Jiang, C.; Tao, L.; et al. Maraviroc, an Inhibitor of Chemokine Receptor Type 5, Alleviates Neuroinflammatory Response after Cerebral Ischemia/Reperfusion Injury via Regulating MAPK/NF-ΚB Signaling. Int. Immunopharmacol. 2022, 108, 108755. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Jin, F.; Hao, Y.; Zhao, T.; Zheng, W.; He, Z. Inflammatory Regulation by Restraining M2 Microglial Polarization: Neurodestructive Effects of Kallikrein-Related Peptidase 8 Activation in Intracerebral Hemorrhage. Int. Immunopharmacol. 2023, 124, 110855. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Makuch, W.; Mika, J. Maraviroc Reduces Neuropathic Pain through Polarization of Microglia and Astroglia—Evidence from in Vivo and in Vitro Studies. Neuropharmacology 2016, 108, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Takami, S.; Minami, M.; Katayama, T.; Nagata, I.; Namura, S.; Satoh, M. TAK-779, a Nonpeptide CC Chemokine Receptor Antagonist, Protects the Brain against Focal Cerebral Ischemia in Mice. J. Cereb. Blood Flow Metab. 2002, 22, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte Chemoattractant Protein-1 (MCP-1): An Overview. J. Interf. Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, T.; Bravo, R. Cloning and Functional Expression of MCCR2, a Murine Receptor for the C-C Chemokines JE and FIC. J. Biol. Chem. 1996, 271, 11603–11606. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Bhangoo, S.; Banisadr, G.; Freitag, C.; Ren, D.; White, F.A.; Miller, R.J. Visualization of Chemokine Receptor Activation in Transgenic Mice Reveals Peripheral Activation of CCR2 Receptors in States of Neuropathic Pain. J. Neurosci. 2009, 29, 8051–8062. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiang, Q.S.; Echeverry, S.; Mogil, J.S.; De Koninck, Y.; Rivest, S. Expression of CCR2 in Both Resident and Bone Marrow-Derived Microglia Plays a Critical Role in Neuropathic Pain. J. Neurosci. 2007, 27, 12396–12406. [Google Scholar] [CrossRef]
- Mizutani, M.; Pino, P.A.; Saederup, N.; Charo, I.F.; Ransohoff, R.M.; Cardona, A.E. The Fractalkine Receptor but Not CCR2 Is Present on Microglia from Embryonic Development throughout Adulthood. J. Immunol. 2012, 188, 29–36. [Google Scholar] [CrossRef]
- Saederup, N.; Cardona, A.E.; Croft, K.; Mizutani, M.; Cotleur, A.C.; Tsou, C.L.; Ransohoff, R.M.; Charo, I.F. Selective Chemokine Receptor Usage by Central Nervous System Myeloid Cells in CCR2-Red Fluorescent Protein Knock-in Mice. PLoS ONE 2010, 5, e13693. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Ji, R.R. Chemokines, Neuronal-Glial Interactions, and Central Processing of Neuropathic Pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, K.; Mika, J. Targeting Members of the Chemokine Family as a Novel Approach to Treating Neuropathic Pain. Molecules 2023, 28, 5766. [Google Scholar] [CrossRef] [PubMed]
- Willenborg, S.; Lucas, T.; Van Loo, G.; Knipper, J.A.; Krieg, T.; Haase, I.; Brachvogel, B.; Hammerschmidt, M.; Nagy, A.; Ferrara, N.; et al. CCR2 Recruits an Inflammatory Macrophage Subpopulation Critical for Angiogenesis in Tissue Repair. Blood 2012, 120, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Lu, C.; Hu, D.; Yu, Y.Y.; Wang, X.; Colnot, C.; Nakamura, M.; Wu, Y.; Miclau, T.; Marcucio, R.S. Multiple Roles for CCR2 during Fracture Healing. DMM Dis. Model. Mech. 2010, 3, 451–458. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, J.; Toft, M.; Hickman, S.E.; Means, T.K.; Terada, K.; Geula, C.; Luster, A.D. Ccr2 Deficiency Impairs Microglial Accumulation and Accelerates Progression of Alzheimer-like Disease. Nat. Med. 2007, 13, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Naert, G.; Rivest, S. CC Chemokine Receptor 2 Deficiency Aggravates Cognitive Impairments and Amyloid Pathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Neurosci. 2011, 31, 6208–6220. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Kim, C.C.; Ryba, B.E.; Niemi, E.C.; Bando, J.K.; Locksley, R.M.; Liu, J.; Nakamura, M.C.; Seaman, W.E. Traumatic Brain Injury Induces Macrophage Subsets in the Brain. Eur. J. Immunol. 2013, 43, 2010–2022. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, O.B.; Stamatovic, S.M.; Keep, R.F.; Andjelkovic, A.V. Absence of the Chemokine Receptor CCR2 Protects against Cerebral Ischemia/Reperfusion Injury in Mice. Stroke 2007, 38, 1345–1353. [Google Scholar] [CrossRef]
- Mahad, D.J.; Ransohoff, R.M. The Role of MCP-1 (CCL2) and CCR2 in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis (EAE). Semin. Immunol. 2003, 15, 23–32. [Google Scholar] [CrossRef]
- Saadane, A.; Veenstra, A.A.; Minns, M.S.; Tang, J.; Du, Y.; Abubakr Elghazali, F.; Lessieur, E.M.; Pearlman, E.; Kern, T.S. CCR2-Positive Monocytes Contribute to the Pathogenesis of Early Diabetic Retinopathy in Mice. Diabetologia 2023, 66, 590–602. [Google Scholar] [CrossRef]
- Belarbi, K.; Jopson, T.; Arellano, C.; Fike, J.R.; Rosi, S. CCR2 Deficiency Prevents Neuronal Dysfunction and Cognitive Impairments Induced by Cranial Irradiation. Cancer Res. 2013, 73, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Quinones, M.P.; Estrada, C.A.; Kalkonde, Y.; Ahuja, S.K.; Kuziel, W.A.; Mack, M.; Ahuja, S.S. The Complex Role of the Chemokine Receptor CCR2 in Collagen-Induced Arthritis: Implications for Therapeutic Targeting of CCR2 in Rheumatoid Arthritis. J. Mol. Med. 2005, 83, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Mika, J. Chemokines under Neuropathic Pain. Ból 2014, 15, 19–35. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Niemi, E.C.; Wang, S.H.; Lee, C.C.; Bingham, D.; Zhang, J.; Cozen, M.L.; Charo, I.; Huang, E.J.; Liu, J.; et al. CCR2 Deficiency Impairs Macrophage Infiltration and Improves Cognitive Function after Traumatic Brain Injury. J. Neurotrauma 2014, 31, 1677–1688. [Google Scholar] [CrossRef]
- Abbadie, C.; Lindia, J.A.; Cumiskey, A.M.; Peterson, L.B.; Mudgett, J.S.; Bayne, E.K.; DeMartino, J.A.; MacIntyre, D.E.; Forrest, M.J. Impaired Neuropathic Pain Responses in Mice Lacking the Chemokine Receptor CCR2. Proc. Natl. Acad. Sci. USA 2003, 100, 7947–7952. [Google Scholar] [CrossRef] [PubMed]
- Charo, I.F.; Myers, S.J.; Herman, A.; Franci, C.; Connolly, A.J.; Coughlin, S.R. Molecular Cloning and Functional Expression of Two Monocyte Chemoattractant Protein 1 Receptors Reveals Alternative Splicing of the Carboxyl-Terminal Tails. Proc. Natl. Acad. Sci. USA 1994, 91, 2752–2756. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.M.; Myers, S.J.; Tsou, C.L.; Gosling, J.; Arai, H.; Charo, I.F. Organization and Differential Expression of the Human Monocyte Chemoattractant Protein 1 Receptor Gene. Evidence for the Role of the Carboxyl-Terminal Tail in Receptor Trafficking. J. Biol. Chem. 1997, 272, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, C.; Civatte, M.; Pellissier, J.; Figarella-Branger, D. CCR2A and CCR2B, the Two Isoforms of the Monocyte Chemoattractant Protein-1 Receptor Are up-Regulated and Expressed by Different Cell Subsets in Idiopathic Inflammatory Myopathies. Acta Neuropathol. 2001, 102, 385–392. [Google Scholar] [CrossRef]
- Weber, K.S.C.; Nelson, P.J.; Gröne, H.J.; Weber, C. Expression of CCR2 by Endothelial Cells: Implications for MCP-1 Mediated Wound Injury Repair and In Vivo Inflammatory Activation of Endothelium. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2085–2093. [Google Scholar] [CrossRef]
- Spinetti, G.; Wang, M.; Monticone, R.; Zhang, J.; Zhao, D.; Lakatta, E.G. Rat Aortic MCP-1 and Its Receptor CCR2 Increase with Age and Alter Vascular Smooth Muscle Cell Function. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Stamatovic, S.M.; Keep, R.F.; Kunkel, S.L.; Andjelkovic, A.V. Potential Role of MCP-1 in Endothelial Cell Tight Junction “Opening”: Signaling via Rho and Rho Kinase. J. Cell Sci. 2003, 116, 4615–4628. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Na, Y.H.; Nguyen, H.T.; Nguyen, L.P.; Hurh, S.; Seong, J.Y.; Lee, C.S.; Ham, B.J.; Hwang, J.I. Analysis of CCR2 Splice Variant Expression Patterns and Functional Properties. Cell Biosci. 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Denk, F.; Crow, M.; Didangelos, A.; Lopes, D.M.; McMahon, S.B. Persistent Alterations in Microglial Enhancers in a Model of Chronic Pain. Cell Rep. 2016, 15, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Dawes, J.M.; Kiesewetter, H.; Perkins, J.R.; Bennett, D.L.H.; McMahon, S.B. Chemokine Expression in Peripheral Tissues from the Monosodium Iodoacetate Model of Chronic Joint Pain. Mol. Pain 2013, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Longobardi, L.; Temple, J.D.; Tagliafierro, L.; Willcockson, H.; Esposito, A.; D’Onofrio, N.; Stein, E.; Li, T.; Myers, T.J.; Ozkan, H.; et al. Role of the C-C Chemokine Receptor-2 in a Murine Model of Injury-Induced Osteoarthritis. Osteoarthr. Cartil. 2017, 25, 914–925. [Google Scholar] [CrossRef]
- Yamagami, S.; Tamura, M.; Hayashi, M.; Endo, N.; Tanabe, H.; Katsuura, Y.; Komoriya, K. Differential Production of MCP-1 and Cytokine-Induced Neutrophil Chemoattractant in the Ischemic Brain after Transient Focal Ischemia in Rats. J. Leukoc. Biol. 1999, 65, 744–749. [Google Scholar] [CrossRef]
- Singhal, G.; Baune, B.T. Microglia: An Interface between the Loss of Neuroplasticity and Depression. Front. Cell. Neurosci. 2017, 11, 270. [Google Scholar] [CrossRef]
- Somebang, K.; Rudolph, J.; Imhof, I.; Li, L.; Niemi, E.C.; Shigenaga, J.; Tran, H.; Gill, T.M.; Lo, I.; Zabel, B.A.; et al. CCR2 Deficiency Alters Activation of Microglia Subsets in Traumatic Brain Injury. Cell Rep. 2021, 36, 109727. [Google Scholar] [CrossRef]
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 Inhibition Reduces Tumor Myeloid Cells and Unmasks a Checkpoint Inhibitor Effect to Slow Progression of Resistant Murine Gliomas. Proc. Natl. Acad. Sci. USA 2020, 117, 1129–1138. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, S.; Zhu, M.D.; Liu, J.Q.; Chen, J.J.; Gao, Y.J. Contribution of Chemokine CCL2/CCR2 Signaling in the Dorsal Root Ganglion and Spinal Cord to the Maintenance of Neuropathic Pain in a Rat Model of Lumbar Disc Herniation. J. Pain 2014, 15, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.; Milligan, E.; Wieseler-Frank, J.; Frank, M.G.; Zapata, V.; Campisi, J.; Langer, S.; Martin, D.; Green, P.; Fleshner, M.; et al. A Role for Proinflammatory Cytokines and Fractalkine in Analgesia, Tolerance, and Subsequent Pain Facilitation Induced by Chronic Intrathecal Morphine. J. Neurosci. 2004, 24, 7353–7365. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, R.; Hu, F.; Chen, P.; Cui, Y.; Feng, J.; Meng, J.; Mo, L.; Liao, X. Spinal MCP-1 Contributes to the Development of Morphine Antinociceptive Tolerance in Rats. Am. J. Med. Sci. 2012, 344, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, M.A.; Steele, A.D.; Eisenstein, T.K.; Adler, M.W.; Henderson, E.E.; Rogers, T.J. Mu-Opioid Induction of Monocyte Chemoattractant Protein-1, RANTES, and IFN-Gamma-Inducible Protein-10 Expression in Human Peripheral Blood Mononuclear Cells. J. Immunol. 2000, 165, 6519–6524. [Google Scholar] [CrossRef]
- Szabo, I.; Chen, X.H.; Xin, L.; Adler, M.W.; Howard, O.M.Z.; Oppenheim, J.J.; Rogers, T.J. Heterologous Desensitization of Opioid Receptors by Chemokines Inhibits Chemotaxis and Enhances the Perception of Pain. Proc. Natl. Acad. Sci. USA 2002, 99, 10276–10281. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Jun, Y.C.; Choi, J.K.; Kim, J.I.; Carp, R.I.; Kim, Y.S. The Expression of RANTES and Chemokine Receptors in the Brains of Scrapie-Infected Mice. J. Neuroimmunol. 2005, 158, 26–33. [Google Scholar] [CrossRef]
- Chang, X.; Shen, J.; Yang, H.; Xu, Y.; Gao, W.; Wang, J.; Zhang, H.; He, S. Upregulated Expression of CCR3 in Osteoarthritis and CCR3 Mediated Activation of Fibroblast-like Synoviocytes. Cytokine 2016, 77, 211–219. [Google Scholar] [CrossRef]
- Van der Meer, P.; Ulrich, A.M.; Gonalez-Scarano, F.; Lavi, E. Immunohistochemical Analysis of CCR2, CCR3, CCR5, and CXCR4 in the Human Brain: Potential Mechanisms for HIV Dementia. Exp. Mol. Pathol. 2000, 69, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Sherbini, O.; Pai, E.L.L.; Kang, S.U.; Kwon, J.S.; Yang, J.; He, W.; Wang, H.; Eacker, S.M.; et al. High-Content Genome-Wide RNAi Screen Reveals CCR3 as a Key Mediator of Neuronal Cell Death. eNeuro 2016, 3, ENEURO.0185-16.2016. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Delarasse, C. Complex Role of Chemokine Mediators in Animal Models of Alzheimer’s Disease. Biomed. J. 2018, 41, 34–40. [Google Scholar] [CrossRef]
- Albright, A.V.; Shieh, J.T.C.; Itoh, T.; Lee, B.; Pleasure, D.; O’Connor, M.J.; Doms, R.W.; González-Scarano, F. Microglia Express CCR5, CXCR4, and CCR3, but of These, CCR5 Is the Principal Coreceptor for Human Immunodeficiency Virus Type 1 Dementia Isolates. J. Virol. 1999, 73, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.N.; Lloyd, C.M.; Sabroe, I.; Durham, S.R.; Till, S.J. T Lymphocytes Expressing CCR3 Are Increased in Allergic Rhinitis Compared with Non-Allergic Controls and Following Allergen Immunotherapy. Allergy Eur. J. Allergy Clin. Immunol. 2007, 62, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.; Maru, S.; Loughlin, J.; Romero, I.A.; Male, D. Regulation of Chemokine Receptor Expression in Human Microglia and Astrocytes. J. Neuroimmunol. 2003, 136, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Huaux, F.; Gharaee-Kermani, M.; Liu, T.; Morel, V.; McGarry, B.; Ullenbruch, M.; Kunkel, S.L.; Wang, J.; Xing, Z.; Phan, S.H. Role of Eotaxin-1 (CCL11) and CC Chemokine Receptor 3 (CCR3) in Bleomycin-Induced Lung Injury and Fibrosis. Am. J. Pathol. 2005, 167, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Humbles, A.A.; Lu, B.; Friend, D.S.; Okinaga, S.; Lora, J.; Al-garawi, A.; Martin, T.R.; Gerard, N.P.; Gerard, C. The Murine CCR3 Receptor Regulates Both the Role of Eosinophils and Mast Cells in Allergen-Induced Airway Inflammation and Hyperresponsiveness. Proc. Natl. Acad. Sci. USA 2002, 99, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.P.; Ponath, P.D. CCR3 Blockade as a New Therapy for Asthma. Expert Opin. Investig. Drugs 2000, 9, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Kim, S.Y.; Song, S.J.; Hong, H.K.; Lee, Y.; Oh, B.Y.; Lee, W.Y.; Cho, Y.B. Crosstalk between CCL7 and CCR3 Promotes Metastasis of Colon Cancer Cells via ERK-JNK Signaling Pathways. Oncotarget 2016, 7, 36842–36853. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, K.; Kukova, G.; Bunemann, E.; Buhren, B.A.; Sonkoly, E.; Szollosi, A.G.; Muller, A.; Savinko, T.; Lauerma, A.I.; Alenius, H.; et al. The Chemokine Receptor CCR3 Participates in Tissue Remodeling during Atopic Skin Inflammation. J. Dermatol. Sci. 2013, 71, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, H.; Honda, Y.; Tanaka, S.; Miyagawa, T.; Honda, M.; Honda, K.; Tokunaga, K.; Kodama, T. Narcolepsy Susceptibility Gene CCR3 Modulates Sleep-Wake Patterns in Mice. PLoS ONE 2017, 12, e0187888. [Google Scholar] [CrossRef]
- Kindstedt, E.; Holm, C.K.; Sulniute, R.; Martinez-Carrasco, I.; Lundmark, R.; Lundberg, P. CCL11, a Novel Mediator of Inflammatory Bone Resorption. Sci. Rep. 2017, 7, 5334. [Google Scholar] [CrossRef]
- Zhu, L.-P.; Xu, M.-L.; Yuan, B.-T.; Ma, L.-J.; Gao, Y.-J. Chemokine CCL7 Mediates Trigeminal Neuropathic Pain via CCR2/CCR3-ERK Pathway in the Trigeminal Ganglion of Mice. Mol. Pain 2023, 19, 174480692311693. [Google Scholar] [CrossRef] [PubMed]
- Nazarinia, D.; Behzadifard, M.; Gholampour, J.; Karimi, R.; Gholampour, M. Eotaxin-1 (CCL11) in Neuroinflammatory Disorders and Possible Role in COVID-19 Neurologic Complications. Acta Neurol. Belg. 2022, 122, 865–869. [Google Scholar] [CrossRef]
- Provost, V.; Larose, M.-C.; Langlois, A.; Rola-Pleszczynski, M.; Flamand, N.; Laviolette, M. CCL26/Eotaxin-3 Is More Effective to Induce the Migration of Eosinophils of Asthmatics than CCL11/Eotaxin-1 and CCL24/Eotaxin-2. J. Leukoc. Biol. 2013, 94, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, A.; Ying, S.; Sabroe, I.; Stubbs, V.L.; Soler, D.; Williams, T.J.; Kay, A.B. Eotaxin (CCL11) and Eotaxin-2 (CCL24) Induce Recruitment of Eosinophils, Basophils, Neutrophils, and Macrophages as Well as Features of Early- and Late-Phase Allergic Reactions Following Cutaneous Injection in Human Atopic and Nonatopic Volunteers. J. Immunol. 2002, 169, 2712–2718. [Google Scholar] [CrossRef]
- García, J.J.; Cidoncha, A.; Bote, M.E.; Hinchado, M.D.; Ortega, E. Altered Profile of Chemokines in Fibromyalgia Patients. Ann. Clin. Biochem. 2014, 51, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Y.L.; Yu, S.Y. Synovial Fluid Eotaxin-1 Levels May Reflect Disease Progression in Primary Knee Osteoarthritis Among Elderly Han Chinese: A Cross-Sectional Study. Cartilage 2019, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Bieber, K.; Ludwig, R.J. Current Clinical Trials in Pemphigus and Pemphigoid. Front. Immunol. 2019, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.R.; Mukhtar, F. Passive Monoclonal and Polyclonal Antibody Therapies. Immunol. Concepts Transfus. Med. 2020, 251–348. [Google Scholar] [CrossRef]
- Ugur, M.; Derouiche, L.; Massotte, D. Heteromerization Modulates Mu Opioid Receptor Functional Properties in Vivo. Front. Pharmacol. 2018, 9, 1240. [Google Scholar] [CrossRef]
- Rogers, T.J. Bidirectional Regulation of Opioid and Chemokine Function. Front. Immunol. 2020, 11, 94. [Google Scholar] [CrossRef]
- Lee, Y.K.; Choi, D.Y.; Jung, Y.Y.; Yun, Y.W.; Lee, B.J.; Han, S.B.; Hong, J.T. Decreased Pain Responses of C-C Chemokine Receptor 5 Knockout Mice to Chemical or Inflammatory Stimuli. Neuropharmacology 2013, 67, 57–65. [Google Scholar] [CrossRef]
- Shah, S.A.; Kanabar, V.; Riffo-Vasquez, Y.; Mohamed, Z.; Cleary, S.J.; Corrigan, C.; James, A.L.; Elliot, J.G.; Shute, J.K.; Page, C.P.; et al. Platelets Independently Recruit into Asthmatic Lungs and Models of Allergic Inflammation via CCR3. Am. J. Respir. Cell Mol. Biol. 2021, 64, 557–568. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Hong, T.; Cheng, B.; Gan, S.; Chen, L.; Zhang, J.; Zuo, L.; Li, J.; Cui, X. Blocking the Autocrine Regulatory Loop of Gankyrin/STAT3/CCL24/CCR3 Impairs the Progression and Pazopanib Resistance of Clear Cell Renal Cell Carcinoma. Cell Death Dis. 2020, 11, 117. [Google Scholar] [CrossRef]
- Yoshie, O.; Matsushima, K. CCR4 and Its Ligands: From Bench to Bedside. Int. Immunol. 2015, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Salanga, C.L.; Handel, T.M. Chemokine Oligomerization and Interactions with Receptors and Glycosaminoglycans: The Role of Structural Dynamics in Function. Exp. Cell Res. 2011, 317, 590–601. [Google Scholar] [CrossRef]
- Cadosch, D.; Al-Mushaiqri, M.S.; Gautschi, O.P.; Chan, E.; Jung, F.J.; Skirving, A.P.; Filgueira, L. Immune Response Deviation and Enhanced Expression of Chemokine Receptor CCR4 in TBI Patients Due to Unknown Serum Factors. Injury 2010, 41, e4–e9. [Google Scholar] [CrossRef] [PubMed]
- Yoshie, O. CCR4 as a Therapeutic Target for Cancer Immunotherapy. Cancers 2021, 13, 5542. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, J.; Huang, M.; Kosonen, R.; Lee, J.E. CCR4 and CCR5 Involvement in Monocyte-Derived Macrophage Migration in Neuroinflammation. Front. Immunol. 2022, 13, 876033. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chu, S.F.; Ai, Q.D.; Zhang, Z.; Guan, F.F.; Wang, S.S.; Dong, Y.X.; Zhu, J.; Jian, W.X.; Chen, N.H. CKLF1 Aggravates Focal Cerebral Ischemia Injury at Early Stage Partly by Modulating Microglia/Macrophage Toward M1 Polarization Through CCR4. Cell. Mol. Neurobiol. 2019, 39, 651–669. [Google Scholar] [CrossRef]
- Bai, M.; Sun, R.; Cao, B.; Feng, J.; Wang, J. Monocyte-Related Cytokines/Chemokines in Cerebral Ischemic Stroke. CNS Neurosci. Ther. 2023, 29, 3693–3712. [Google Scholar] [CrossRef]
- Kiguchi, N.; Ding, H.; Peters, C.M.; Kock, N.D.; Kishioka, S.; Cline, J.M.; Wagner, J.D.; Ko, M.C. Altered Expression of Glial Markers, Chemokines, and Opioid Receptors in the Spinal Cord of Type 2 Diabetic Monkeys. Biochim. Biophys. Acta 2017, 1863, 274. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. Blockade of CCR4 Diminishes Hypersensitivity and Enhances Opioid Analgesia–Evidence from a Mouse Model of Diabetic Neuropathy. Neuroscience 2020, 441, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, K.; Fujimura, M.; Myou, S.; Ishiura, Y.; Onai, N.; Matsushima, K. Asthma Severity Is Associated with an Increase in Both Blood CXCR3+ and CCR4+ T Cells. Respirology 2006, 11, 152–157. [Google Scholar] [CrossRef] [PubMed]
- El-Husseini, Z.W.; Vonk, J.M.; van den Berge, M.; Gosens, R.; Koppelman, G.H. Association of Asthma Genetic Variants with Asthma-Associated Traits Reveals Molecular Pathways of Eosinophilic Asthma. Clin. Transl. Allergy 2023, 13, e12239. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, T.; Nakamura, K.; Wakugawa, M.; Mitsui, H.; Tada, Y.; Saeki, H.; Torii, H.; Asahina, A.; Onai, N.; Matsushima, K.; et al. Thymus and Activation-Regulated Chemokine in Atopic Dermatitis: Serum Thymus and Activation-Regulated Chemokine Level Is Closely Related with Disease Activity. J. Allergy Clin. Immunol. 2001, 107, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wu, H.; Jin, Z.J.; Ma, D.; Yuen, S.; Jing, X.Q.; Shi, M.M.; Shen, B.Y.; Peng, C.H.; Zhao, R.; et al. Up-Regulation of Chemokine Receptor CCR4 Is Associated with Human Hepatocellular Carcinoma Malignant Behavior. Sci. Rep. 2017, 7, 12362. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Arabi, Z.; Ahangar-Parvin, R.; Mohammadi-Kordkhayli, M.; Nemati, M. Ginger Extract Modulates the Expression of Chemokines CCL20 and CCL22 and Their Receptors (CCR6 and CCR4) in the Central Nervous System of Mice with Experimental Autoimmune Encephalomyelitis. Drug Res. 2017, 67, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Bajetto, A.; Bonavia, R.; Barbero, S.; Schettini, G. Characterization of Chemokines and Their Receptors in the Central Nervous System: Physiopathological Implications. J. Neurochem. 2002, 82, 1311–1329. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, H.; Imai, T.; Kusuda, J.; Miura, R.; Callen, D.F.; Yoshie, O. Assignment of the Human CC Chemokine Gene TARC (SCYA17) to Chromosome 16q13. Genomics 1997, 40, 211–213. [Google Scholar] [CrossRef]
- Fülle, L.; Offermann, N.; Niklas, J.; Belen, A.; Oliver, E.; Luca, S.; Fabian, R.; Knöpper, K.; Alferink, J.; Abdullah, Z.; et al. CCL17 Exerts a Neuroimmune Modulatory Function and Is Expressed in Hippocampal Neurons. Glia 2018, 66, 2246–2261. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, Z.; Wang, F.; Zhang, Z.; Yu, M. Identification of Chemokines and Growth Factors in Proliferative Diabetic Retinopathy Vitreous. BioMed. Res. Int. 2014, 2014, 486386. [Google Scholar] [CrossRef] [PubMed]
- Vulcano, M.; Albanesi, C.; Stoppacciaro, A.; Bagnati, R.; D’Amico, G.; Struyf, S.; Transidico, P.; Bonecchi, R.; Del Prete, A.; Allavena, P.; et al. Dendritic Cells as a Major Source of Macrophage-Derived Chemokine/CCL22 in Vitro and in Vivo. Eur. J. Immunol. 2001, 31, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, U.; Kuroda, E. Regulation of Macrophage-Derived Chemokine (MDC, CCL22) Production. Crit. Rev. Immunol. 2002, 22, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Rapp, M.; Wintergerst, M.W.M.; Kunz, W.G.; Vetter, V.K.; Knott, M.M.L.; Lisowski, D.; Haubner, S.; Moder, S.; Thaler, R.; Eiber, S.; et al. CCL22 Controls Immunity by Promoting Regulatory T Cell Communication with Dendritic Cells in Lymph Nodes. J. Exp. Med. 2019, 216, 1170. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Chantry, D.; Raport, C.J.; Wood, C.L.; Nishimura, M.; Godiska, R.; Yoshie, O.; Gray, P.W. Macrophage-Derived Chemokine Is a Functional Ligand for the CC Chemokine Receptor 4. J. Biol. Chem. 1998, 273, 1764–1768. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.; Lang, R.; Binda, E.; Panina-Bordignon, P.; D’Ambrosio, D. Dominance of CCL22 over CCL17 in Induction of Chemokine Receptor CCR4 Desensitization and Internalization on Human Th2 Cells. Eur. J. Immunol. 2004, 34, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Q.; Chen, Q.H.; Ran, F.Y.; Yu, L.M.; Liu, X.; Fu, Q.; Song, G.Y.; Tang, J.M.; Zhang, T. Combination of G-CSF and AMD3100 Improves the Anti-Inflammatory Effect of Mesenchymal Stem Cells on Inducing M2 Polarization of Macrophages through NF-ΚB-IL1RA Signaling Pathway. Front. Pharmacol. 2019, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Abboud, D.; Daubeuf, F.; Do, Q.T.; Utard, V.; Villa, P.; Haiech, J.; Bonnet, D.; Hibert, M.; Bernard, P.; Galzi, J.L.; et al. A Strategy to Discover Decoy Chemokine Ligands with an Anti-Inflammatory Activity. Sci. Rep. 2015, 5, 14746. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.B.; Xie, F.; Chang, K.K.; Shang, W.Q.; Meng, Y.H.; Yu, J.J.; Li, H.; Sun, Q.; Yuan, M.M.; Jin, L.P.; et al. Chemokine CCL17 Induced by Hypoxia Promotes the Proliferation of Cervical Cancer Cell. Am. J. Cancer Res. 2015, 5, 3072–3084. [Google Scholar]
- Dogan, R.-N.E.; Long, N.; Forde, E.; Dennis, K.; Kohm, A.P.; Miller, S.D.; Karpus, W.J. CCL22 Regulates Experimental Autoimmune Encephalomyelitis by Controlling Inflammatory Macrophage Accumulation and Effector Function. J. Leukoc. Biol. 2011, 89, 93. [Google Scholar] [CrossRef]
- Purandare, A.; Somerville, J. Antagonists of CCR4 as Immunomodulatory Agents. Curr. Top. Med. Chem. 2012, 6, 1335–1344. [Google Scholar] [CrossRef]
- Purandare, A.V.; Gao, A.; Wan, H.; Somerville, J.; Burke, C.; Seachord, C.; Vaccaro, W.; Wityak, J.; Poss, M.A. Identification of Chemokine Receptor CCR4 Antagonist. Bioorganic Med. Chem. Lett. 2005, 15, 2669–2672. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.; Hodgson, S.; Wilson, R.; Robertson, J.; Watson, J.; Beerahee, M.; Hughes, S.C.; Young, G.; Graves, R.; Hall, D.; et al. Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of GSK2239633, a CC-Chemokine Receptor 4 Antagonist, in Healthy Male Subjects: Results from an Open-Label and from a Randomised Study. BMC Pharmacol. Toxicol. 2013, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Ishikawa, N.; Igarashi, S.; Kawano, N.; Masuda, N.; Hattori, K.; Miyazaki, T.; Ogino, S.I.; Orita, M.; Matsumoto, Y.; et al. Potent CCR4 Antagonists: Synthesis, Evaluation, and Docking Study of 2,4-Diaminoquinazolines. Bioorganic Med. Chem. 2008, 16, 7968–7974. [Google Scholar] [CrossRef]
- Yokoyama, K.; Ishikawa, N.; Igarashi, S.; Kawano, N.; Masuda, N.; Hamaguchi, W.; Yamasaki, S.; Koganemaru, Y.; Hattori, K.; Miyazaki, T.; et al. Potent and Orally Bioavailable CCR4 Antagonists: Synthesis and Structure-Activity Relationship Study of 2-Aminoquinazolines. Bioorganic Med. Chem. 2009, 17, 64–73. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Thompson, M.; Galindo, C.; Standeford, H.; Whittington, E.; Alpini, G.; DeMorrow, S. Neuronal CCL2 Is Upregulated during Hepatic Encephalopathy and Contributes to Microglia Activation and Neurological Decline. J. Neuroinflamm. 2014, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Remer, M.; Al-Shamkhani, A.; Glennie, M.; Johnson, P. Mogamulizumab and the Treatment of CCR4-Positive T-Cell Lymphomas. Immunotherapy 2014, 6, 1187–1206. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Muro, K.; Ishii, H.; Kato, T.; Tsushima, T.; Takenoyama, M.; Oizumi, S.; Gemmoto, K.; Suna, H.; Enokitani, K.; et al. A Phase I Study of the Anti-CC Chemokine Receptor 4 Antibody, Mogamulizumab, in Combination with Nivolumab in Patients with Advanced or Metastatic Solid Tumors. Clin. Cancer Res. 2019, 25, 6614–6622. [Google Scholar] [CrossRef] [PubMed]
- Kaul, M.; Ma, Q.; Medders, K.E.; Desai, M.K.; Lipton, S.A. HIV-1 Coreceptors CCR5 and CXCR4 Both Mediate Neuronal Cell Death but CCR5 Paradoxically Can Also Contribute to Protection. Cell Death Differ. 2007, 14, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Rottman, J.B.; Ganley, K.P.; Williams, K.; Wu, L.; Mackay, C.R.; Ringler, D.J. Cellular Localization of the Chemokine Receptor CCR5: Correlation to Cellular Targets of HIV-1 Infection. Am. J. Pathol. 1997, 151, 1341–1351. [Google Scholar]
- Zhou, M.; Greenhill, S.; Huang, S.; Silva, T.K.; Sano, Y.; Wu, S.; Cai, Y.; Nagaoka, Y.; Sehgal, M.; Cai, D.J.; et al. CCR5 Is a Suppressor for Cortical Plasticity and Hippocampal Learning and Memory. Elife 2016, 5, e20985. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.T.; Ben Assayag, E.; Shabashov-Stone, D.; Liraz-Zaltsman, S.; Mazzitelli, J.; Arenas, M.; Abduljawad, N.; Kliper, E.; Korczyn, A.D.; Thareja, N.S.; et al. CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury. Cell 2019, 176, 1143–1157. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, W.S.; Murase, S.I.; Horwitz, A.F.; Mandell, J.W. Migration of Perilesional Microglia after Focal Brain Injury and Modulation by CC Chemokine Receptor 5: An in Situ Time-Lapse Confocal Imaging Study. J. Neurosci. 2005, 25, 7040–7047. [Google Scholar] [CrossRef] [PubMed]
- Marella, M.; Chabry, J. Neurons and Astrocytes Respond to Prion Infection by Inducing Microglia Recruitment. J. Neurosci. 2004, 24, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Chuang, L.F.; Doi, R.H.; Carlos, M.P.; Torres, J.V.; Chuang, R.Y. Morphine Induces Gene Expression of CCR5 in Human CEM X174 Lymphocytes. J. Biol. Chem. 2000, 275, 31305–31310. [Google Scholar] [CrossRef] [PubMed]
- Ochi-Ishi, R.; Nagata, K.; Inoue, T.; Tozaki-Saitoh, H.; Tsuda, M.; Inoue, K. Involvement of the Chemokine CCL3 and the Purinoceptor P2×7 in the Spinal Cord in Paclitaxel-Induced Mechanical Allodynia. Mol. Pain 2014, 10, 1744–8069-10-53. [Google Scholar] [CrossRef] [PubMed]
- Bidlack, J.M. Detection and Function of Opioid Receptors on Cells from the Immune System. Clin. Diagn. Lab. Immunol. 2000, 7, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Mika, J.; Popiolek-Barczyk, K.; Rojewska, E.; Makuch, W.; Starowicz, K.; Przewlocka, B. Delta-Opioid Receptor Analgesia Is Independent of Microglial Activation in a Rat Model of Neuropathic Pain. PLoS ONE 2014, 9, e104420. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Tramutola, A.; De Luca, A.; Navarra, P.; Dello Russo, C. Modulatory Effects of the CCR5 Antagonist Maraviroc on Microglial Pro-Inflammatory Activation Elicited by Gp120. J. Neurochem. 2012, 120, 106–114. [Google Scholar] [CrossRef]
- Pease, J.E.; Horuk, R. Chemokine Receptor Antagonists: Part 2. Expert Opin. Ther. Pat. 2009, 19, 199–221. [Google Scholar] [CrossRef]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC Chemokine CCL20 and Its Receptor CCR6. Cytokine Growth Factor Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef]
- Lee, A.Y.S.; Reimer, D.; Zehrer, A.; Lu, M.; Mielenz, D.; Körner, H. Expression of Membrane-Bound CC Chemokine Ligand 20 on Follicular T Helper Cells in T-B-Cell Conjugates. Front. Immunol. 2017, 8, 1871. [Google Scholar] [CrossRef]
- Liao, L.S.; Zhang, M.W.; Gu, Y.J.; Sun, X.C. Targeting CCL20 Inhibits Subarachnoid Hemorrhage-Related Neuroinflammation in Mice. Aging 2020, 12, 14849–14862. [Google Scholar] [CrossRef]
- Reboldi, A.; Coisne, C.; Baumjohann, D.; Benvenuto, F.; Bottinelli, D.; Lira, S.; Uccelli, A.; Lanzavecchia, A.; Engelhardt, B.; Sallusto, F. C-C Chemokine Receptor 6-Regulated Entry of TH-17 Cells into the CNS through the Choroid Plexus Is Required for the Initiation of EAE. Nat. Immunol. 2009, 10, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Sachi, N.; Kamiyama, N.; Saechue, B.; Ozaka, S.; Dewayani, A.; Ariki, S.; Chalalai, T.; Soga, Y.; Fukuda, C.; Kagoshima, Y.; et al. CCL20/CCR6 Chemokine Signaling Is Not Essential for Pathogenesis in an Experimental Autoimmune Encephalomyelitis Mouse Model of Multiple Sclerosis. Biochem. Biophys. Res. Commun. 2023, 641, 123–131. [Google Scholar] [CrossRef]
- Coursey, T.G.; Gandhi, N.B.; Volpe, E.A.; Pflugfelder, S.C.; De Paiva, C.S. Chemokine Receptors CCR6 and CXCR3 Are Necessary for CD4(+) T Cell Mediated Ocular Surface Disease in Experimental Dry Eye Disease. PLoS ONE 2013, 8, e78508. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Q.; Yuan, G.Q.; Zhang, G.G.; Chen, Y.T.; Nie, Q.Q.; Wang, Z. Identification of CCL20 as a Key Biomarker of Inflammatory Responses in the Pathogenesis of Intracerebral Hemorrhage. Inflammation 2023, 46, 1290–1304. [Google Scholar] [CrossRef] [PubMed]
- Lötsch, J.; Mustonen, L.; Harno, H.; Kalso, E. Machine-Learning Analysis of Serum Proteomics in Neuropathic Pain after Nerve Injury in Breast Cancer Surgery Points at Chemokine Signaling via SIRT2 Regulation. Int. J. Mol. Sci. 2022, 23, 3488. [Google Scholar] [CrossRef]
- Miclescu, A.A.; Granlund, P.; Butler, S.; Gordh, T. Association between Systemic Inflammation and Experimental Pain Sensitivity in Subjects with Pain and Painless Neuropathy after Traumatic Nerve Injuries. Scand. J. Pain 2023, 23, 184–199. [Google Scholar] [CrossRef]
- Alrumaihi, F. The Multi-Functional Roles of CCR7 in Human Immunology and as a Promising Therapeutic Target for Cancer Therapeutics. Front. Mol. Biosci. 2022, 9, 834149. [Google Scholar] [CrossRef]
- Gomez-Nicola, D.; Pallas-Bazarra, N.; Valle-Argos, B.; Nieto-Sampedro, M. CCR7 Is Expressed in Astrocytes and Upregulated after an Inflammatory Injury. J. Neuroimmunol. 2010, 227, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.X.; Cao, X.; Tang, Y.C.; Liu, Y.; Tang, F.R. CCR7, CCR8, CCR9 and CCR10 in the Mouse Hippocampal CA1 Area and the Dentate Gyrus during and after Pilocarpine-Induced Status Epilepticus. J. Neurochem. 2007, 100, 1072–1088. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.; Gerdle, B.; Ghafouri, B.; Bäckryd, E. The Inflammatory Profile of Cerebrospinal Fluid, Plasma, and Saliva from Patients with Severe Neuropathic Pain and Healthy Controls-a Pilot Study. BMC Neurosci. 2021, 22, 6. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Liu, L.; Wen, J.; Yang, H.; Zhu, Y.; Gao, M.; Liang, H.; Lai, W.; Long, H. Nerve Growth Factor Enhances Tooth Mechanical Hyperalgesia Through C-C Chemokine Ligand 19 in Rats. Front. Neurol. 2021, 12, 596. [Google Scholar] [CrossRef]
- Zhao, P.; Waxman, S.G.; Hains, B.C. Modulation of Thalamic Nociceptive Processing after Spinal Cord Injury through Remote Activation of Thalamic Microglia by Cysteine–Cysteine Chemokine Ligand 21. J. Neurosci. 2007, 27, 8893–8902. [Google Scholar] [CrossRef]
- Hauser, M.A.; Legler, D.F. Common and Biased Signaling Pathways of the Chemokine Receptor CCR7 Elicited by Its Ligands CCL19 and CCL21 in Leukocytes. J. Leukoc. Biol. 2016, 99, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, L.; Cao, S.; Hou, G.; Zhang, Q.; Ma, H.; Shi, B. Serum CCL21 as a Potential Biomarker for Cognitive Impairment in Spinal Cord Injury. BioMed Res. Int. 2020, 2020, 6692802. [Google Scholar] [CrossRef]
- Rappert, A.; Biber, K.; Nolte, C.; Lipp, M.; Schubel, A.; Lu, B.; Gerard, N.P.; Gerard, C.; Boddeke, H.W.G.M.; Kettenmann, H. Secondary Lymphoid Tissue Chemokine (CCL21) Activates CXCR3 to Trigger a Cl− Current and Chemotaxis in Murine Microglia. J. Immunol. 2002, 168, 3221–3226. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, K.; Pickert, G.; Wijnvoord, N.; Häussler, A.; Tegeder, I. Dichotomy of CCL21 and CXCR3 in Nerve Injury-Evoked and Autoimmunity-Evoked Hyperalgesia. Brain. Behav. Immun. 2013, 32, 186–200. [Google Scholar] [CrossRef]
- Schweickart, V.L.; Raport, C.J.; Godiska, R.; Byers, M.G.; Eddy, R.L.; Shows, T.B.; Gray, P.W. Cloning of Human and Mouse EBI1, a Lymphoid-Specific G-Protein-Coupled Receptor Encoded on Human Chromosome 17q12-Q21.2. Genomics 1994, 23, 643–650. [Google Scholar] [CrossRef]
- Jaeger, K.; Bruenle, S.; Weinert, T.; Guba, W.; Muehle, J.; Miyazaki, T.; Weber, M.; Furrer, A.; Haenggi, N.; Tetaz, T.; et al. Structural Basis for Allosteric Ligand Recognition in the Human CC Chemokine Receptor 7. Cell 2019, 178, 1222–1230.e10. [Google Scholar] [CrossRef]
- Saha, S.; Shukla, A.K. The Inside Story: Crystal Structure of the Chemokine Receptor CCR7 with an Intracellular Allosteric Antagonist. Biochemistry 2020, 59, 12–14. [Google Scholar] [CrossRef] [PubMed]
- Hull-Ryde, E.A.; Porter, M.A.; Fowler, K.A.; Kireev, D.; Li, K.; Simpson, C.D.; Sassano, M.F.; Suto, M.J.; Pearce, K.H.; Janzen, W.; et al. Identification of Cosalane as an Inhibitor of Human and Murine CC-Chemokine Receptor 7 Signaling via a High-Throughput Screen. SLAS Discov. Adv. Life Sci. Drug Discov. 2018, 23, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Hasegawa, H.; Kohno, M.; Inoue, A.; Ito, M.R.; Fujita, S. Antagonist of Secondary Lymphoid-Tissue Chemokine (CCR Ligand 21) Prevents the Development of Chronic Graft-versus-Host Disease in Mice. J. Immunol. 2003, 170, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, K.R.; Clark-Lewis, I.; McColl, S.R. Inhibition of Generation of Cytotoxic T Lymphocyte Activity by a CCL19/Macrophage Inflammatory Protein (MIP)-3beta Antagonist. J. Biol. Chem. 2004, 279, 40276–40282. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Alotaibi, M.; Mroueh, R.; Basheer, H.A.; Afarinkia, K. CCR7 as a Therapeutic Target in Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188499. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Müller, I.; Wolf, E.; Lipp, M. CCR7 Coordinates the Primary Immune Response by Establishing Functional Microenvironments in Secondary Lymphoid Organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Ravens, S.; Fleige, H.; Haas, J.D.; Oberdörfer, L.; Łyszkiewicz, M.; Förster, R.; Prinz, I. CCR7-Mediated Migration in the Thymus Controls Γδ T-Cell Development. Eur. J. Immunol. 2014, 44, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.A.; Meingassner, J.G.; Lipp, M.; Moore, H.D.; Rot, A. CCR7 Is Required for the in Vivo Function of CD4+ CD25+ Regulatory T Cells. J. Exp. Med. 2007, 204, 735–745. [Google Scholar] [CrossRef]
- Tiffany, H.L.; Lautens, L.L.; Gao, J.L.; Pease, J.; Locati, M.; Combadiere, C.; Modi, W.; Bonner, T.I.; Murphy, P.M. Identification of CCR8: A Human Monocyte and Thymus Receptor for the CC Chemokine I-309. J. Exp. Med. 1997, 186, 165–170. [Google Scholar] [CrossRef]
- Miller, M.D.; Hata, S.; De Waal Malefyt, R.; Krangel, M.S. A Novel Polypeptide Secreted by Activated Human T Lymphocytes. J. Immunol. 1989, 143, 2907–2916. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.A.; Ling, M.F.; Leung, J.; Shreffler, W.G.; Luster, A.D. Identification of Human CCR8 as a CCL18 Receptor. J. Exp. Med. 2013, 210, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Moser, B. Chemokine Receptor-Targeted Therapies: Special Case for CCR8. Cancers 2022, 14, 511. [Google Scholar] [CrossRef] [PubMed]
- Gombert, M.; Dieu-Nosjean, M.-C.; Winterberg, F.; Bünemann, E.; Kubitza, R.C.; Da Cunha, L.; Haahtela, A.; Lehtimäki, S.; Müller, A.; Rieker, J.; et al. CCL1-CCR8 Interactions: An Axis Mediating the Recruitment of T Cells and Langerhans-Type Dendritic Cells to Sites of Atopic Skin Inflammation. J. Immunol. 2005, 174, 5082–5091. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Pérez, F.; Kato, Y.; Gonzalez-Menendez, I.; Laiño, J.; Ohbayashi, M.; Burggraf, M.; Krause, M.; Kirberg, J.; Iwakura, Y.; Martella, M.; et al. CCR8 Leads to Eosinophil Migration and Regulates Neutrophil Migration in Murine Allergic Enteritis. Sci. Rep. 2019, 9, 9608. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhong, S.; Du, H.; Han, S.; Wei, X.; Gong, Q. MiR-184-5p Represses Neuropathic Pain by Regulating CCL1/CCR8 Signaling Interplay in the Spinal Cord in Diabetic Mice. Neurol. Res. 2024, 46, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Jun, K.J.; Lee, M.J.; Shin, D.C.; Woo, M.Y.; Kim, K.; Park, S. Identification of CCL1 as a Gene Differentially Expressed in CD4+ T Cells Expressing TIM-3. Immune Netw. 2011, 11, 203. [Google Scholar] [CrossRef]
- Akimoto, N.; Ifuku, M.; Mori, Y.; Noda, M. Effects of Chemokine (C-C Motif) Ligand 1 on Microglial Function. Biochem. Biophys. Res. Commun. 2013, 436, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xiao, W.; Wang, F.; Liu, J.; Zhi, L.J. MiR-21-5p Inhibits Neuropathic Pain Development via Directly Targeting C-C Motif Ligand 1 and Tissue Inhibitor of Metalloproteinase-3. J. Cell. Biochem. 2019, 120, 16614–16623. [Google Scholar] [CrossRef]
- Zaballos, Á.; Gutiérrez, J.; Varona, R.; Ardavín, C.; Márquez, G. Cutting Edge: Identification of the Orphan Chemokine Receptor GPR-9-6 as CCR9, the Receptor for the Chemokine TECK. J. Immunol. 1999, 162, 5671–5675. [Google Scholar] [CrossRef]
- Igaki, K.; Komoike, Y.; Nakamura, Y.; Watanabe, T.; Yamasaki, M.; Fleming, P.; Yang, L.; Soler, D.; Fedyk, E.; Tsuchimori, N. MLN3126, an Antagonist of the Chemokine Receptor CCR9, Ameliorates Inflammation in a T Cell Mediated Mouse Colitis Model. Int. Immunopharmacol. 2018, 60, 160–169. [Google Scholar] [CrossRef]
- Wermers, J.D.; McNamee, E.N.; Wurbel, M.A.; Jedlicka, P.; Riveranieves, J. The Chemokine Receptor CCR9 Is Required for the T-Cell-Mediated Regulation of Chronic Ileitis in Mice. Gastroenterology 2011, 140, 1526–1535.e3. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Doan, N.; Said, J.; Karunasiri, D.; Pullarkat, S.T. Strong Expression of Chemokine Receptor CCR9 in Diffuse Large B-Cell Lymphoma and Follicular Lymphoma Strongly Correlates with Gastrointestinal Involvement. Hum. Pathol. 2014, 45, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Xiao, R.; Xiong, J.; Tembo, K.M.; Deng, X.; Xiong, M.; Liu, P.; Wang, M.; Zhang, Q. CCR9 in Cancer: Oncogenic Role and Therapeutic Targeting. J. Hematol. Oncol. 2016, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-R.; Peden, K.W.C.; Zaitseva, M.B.; Golding, H.; Farber, J.M. CCR9A and CCR9B: Two Receptors for the Chemokine CCL25/TECK/Ck Beta-15 That Differ in Their Sensitivities to Ligand. J. Immunol. 2000, 164, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Spencer, D.M.; Wandless, T.J.; Schreiber, S.L.; Crabtree, G.R. Controlling Signal Transduction with Synthetic Ligands. Science 1993, 262, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Walters, M.J.; Wang, Y.; Lai, N.; Baumgart, T.; Zhao, B.N.; Dairaghi, D.J.; Bekker, P.; Ertl, L.S.; Penfold, M.E.T.; Jaen, J.C.; et al. Characterization of CCX282-B, an Orally Bioavailable Antagonist of the CCR9 Chemokine Receptor, for Treatment of Inflammatory Bowel Disease. J. Pharmacol. Exp. Ther. 2010, 335, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, M.; Yang, Z.; Lu, C.; Wang, Q.; Wang, H.; Deng, C.; Liu, Y.; Yang, Y. The Roles of CCR9/CCL25 in Inflammation and Inflammation-Associated Diseases. Front. Cell Dev. Biol. 2021, 9, 686548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Han, J.; Wu, M.; Xu, L.; Wang, Y.; Yuan, W.; Hua, F.; Fan, H.; Dong, F.; Qu, X.; et al. Toll-Like Receptor 4 Promotes Th17 Lymphocyte Infiltration Via CCL25/CCR9 in Pathogenesis of Experimental Autoimmune Encephalomyelitis. J. Neuroimmune Pharmacol. 2019, 14, 493–502. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, L.; Chen, L.; Feng, X.; Zhou, R.; Xiang, P.; Wen, J.; Huang, Y.; Zhou, H. Bone Marrow-Derived GCA+ Immune Cells Drive Alzheimer’s Disease Progression. Adv. Sci. 2023, 10, e2303402. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Zhang, H.Y.; Du, W.; Qin, Z.; Yao, Y.; Mao, Y.; Zhou, L. Upregulation of Chemokine Receptor CCR10 Is Essential for Glioma Proliferation, Invasion and Patient Survival. Oncotarget 2014, 5, 6576–6583. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Q.; Chen, J.X.; Chen, Y.; Cai, L.L.; Wang, X.Z.; Guo, W.H.; Zheng, J.F. The Chemokine Receptor CCR10 Promotes Inflammation-Driven Hepatocarcinogenesis via PI3K/Akt Pathway Activation. Cell Death Dis. 2018, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Homey, B.; Alenius, H.; Müller, A.; Soto, H.; Bowman, E.P.; Yuan, W.; McEvoy, L.; Lauerma, A.I.; Assmann, T.; Bünemann, E.; et al. CCL27-CCR10 Interactions Regulate T Cell-Mediated Skin Inflammation. Nat. Med. 2002, 8, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Mirshahpanah, P.; Li, Y.Y.Y.; Burkhardt, N.; Asadullah, K.; Zollner, T.M. CCR4 and CCR10 Ligands Play Additive Roles in Mouse Contact Hypersensitivity. Exp. Dermatol. 2008, 17, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Wise, E.L.; Duchesnes, C.; Da Fonseca, P.C.A.; Allen, R.A.; Williams, T.J.; Pease, J.E. Small Molecule Receptor Agonists and Antagonists of CCR3 Provide Insight into Mechanisms of Chemokine Receptor Activation. J. Biol. Chem. 2007, 282, 27935–27943. [Google Scholar] [CrossRef] [PubMed]
- Salanga, C.L.; O’Hayre, M.; Handel, T. Modulation of Chemokine Receptor Activity through Dimerization and Crosstalk. Cell. Mol. Life Sci. 2009, 66, 1370–1386. [Google Scholar] [CrossRef] [PubMed]
- Cherney, R.J.; Anjanappa, P.; Selvakumar, K.; Batt, D.G.; Brown, G.D.; Rose, A.V.; Vuppugalla, R.; Chen, J.; Pang, J.; Xu, S.; et al. BMS-813160: A Potent CCR2 and CCR5 Dual Antagonist Selected as a Clinical Candidate. ACS Med. Chem. Lett. 2021, 12, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Gale, J.D.; Gilbert, S.; Blumenthal, S.; Elliott, T.; Pergola, P.E.; Goteti, K.; Scheele, W.; Perros-Huguet, C. Effect of PF-04634817, an Oral CCR2/5 Chemokine Receptor Antagonist, on Albuminuria in Adults with Overt Diabetic Nephropathy. Kidney Int. Rep. 2018, 3, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Saag, M.; Dejesus, E.; Gathe, J.; Lalezari, J.; Landay, A.L.; Cade, J.; Enejosa, J.; Lefebvre, E.; Feinberg, J. A 48-Week Randomized Phase 2b Study Evaluating Cenicriviroc versus Efavirenz in Treatment-Naive HIV-Infected Adults with C-C Chemokine Receptor Type 5-Tropic Virus. AIDS 2016, 30, 869–878. [Google Scholar] [CrossRef]
- Fadel, H.; Temesgen, Z. Maraviroc. Drugs Today 2007, 43, 749–758. [Google Scholar] [CrossRef]
- Szpakowska, M.; Chevigné, A. VCCL2/VMIP-II, the Viral Master KEYmokine. J. Leukoc. Biol. 2016, 99, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Lindow, M.; Nansen, A.; Bartholdy, C.; Stryhn, A.; Hansen, N.J.V.; Boesen, T.P.; Wells, T.N.C.; Schwartz, T.W.; Thomsen, A.R. The Virus-Encoded Chemokine VMIP-II Inhibits Virus-Induced Tc1-Driven Inflammation. J. Virol. 2003, 77, 7393–7400. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, S.; Jiang, Z.; Feng, L.; Gao, Y.; Chen, Y.; Xu, A.; Huang, W.; Zhang, N.; Sun, H. Study on the Promotion of Lymphocytes in Patients with COVID-19 by Broad-Spectrum Chemokine Receptor Inhibitor VMIP-II and Its Mechanism of Signal Transmission in Vitro. Signal Transduct. Target. Ther. 2021, 6, 104. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).