Rational Design of Bifunctional Microporous Organic Polymers Containing Anthracene and Triphenylamine Units for Energy Storage and Biological Applications
Abstract
1. Introduction
2. Results and Discussion
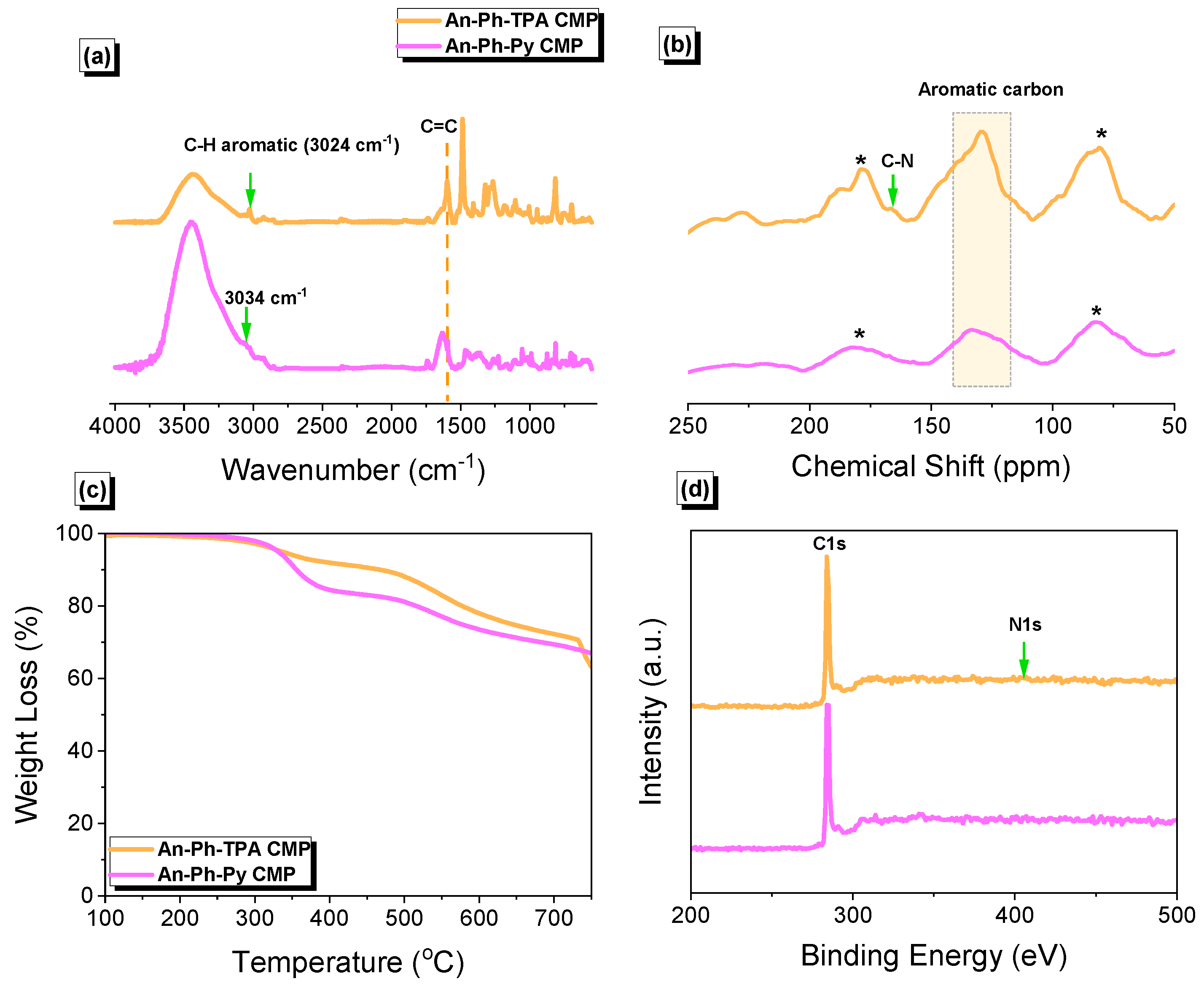
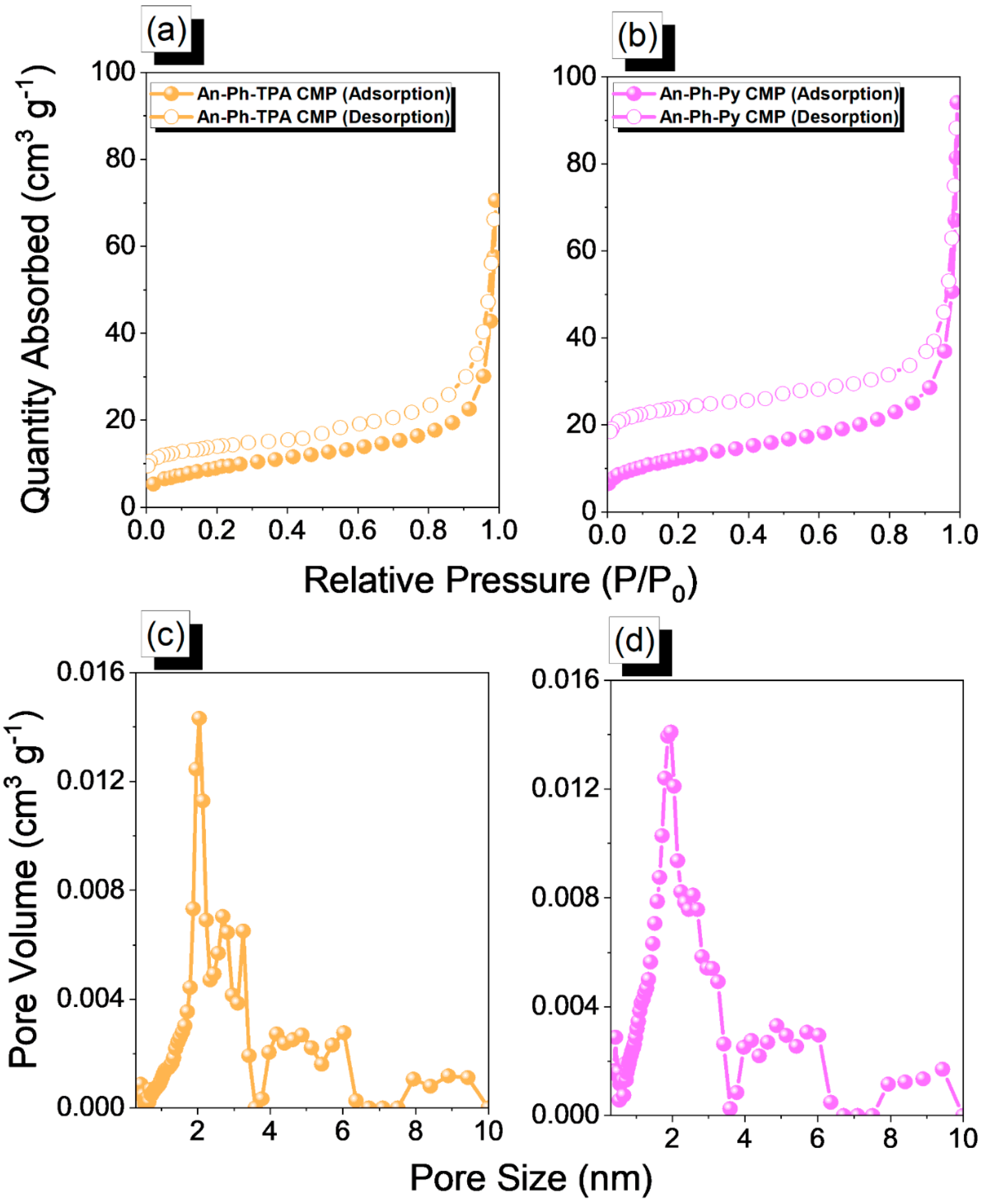
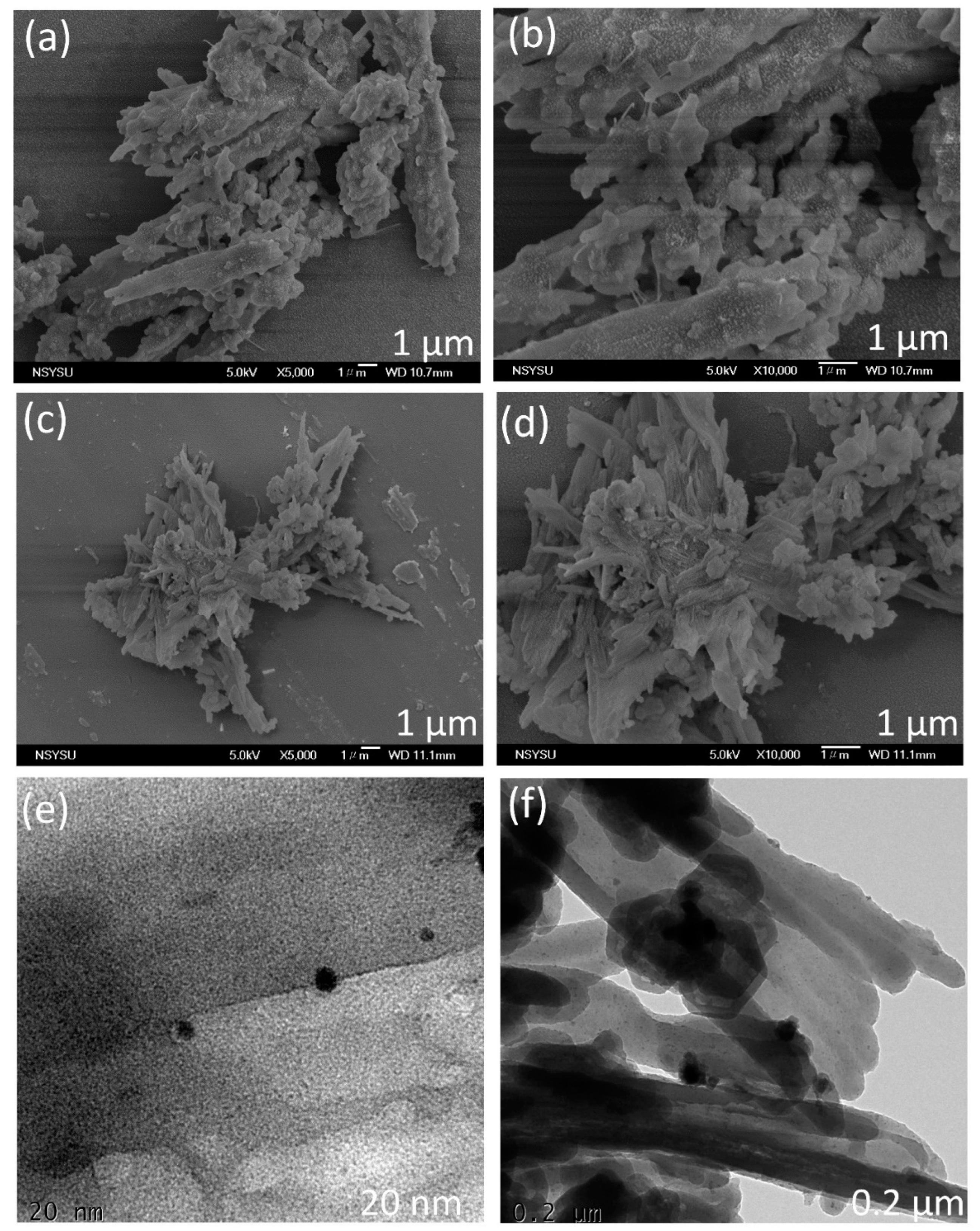
2.1. Synthesis and Molecular Characterization of An-Linked CMPs (An-Ph-TPA and An-Ph-Py CMPs)
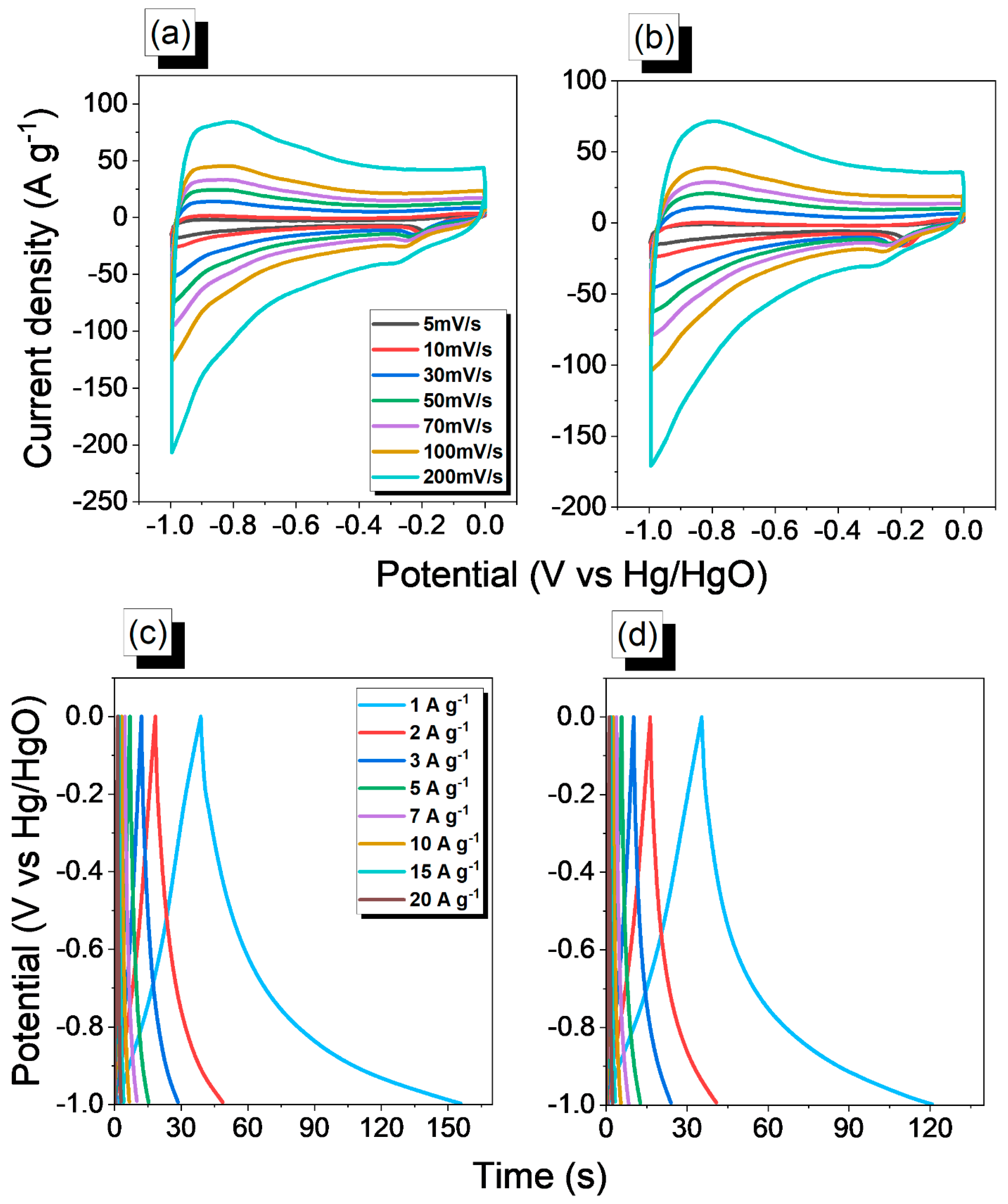
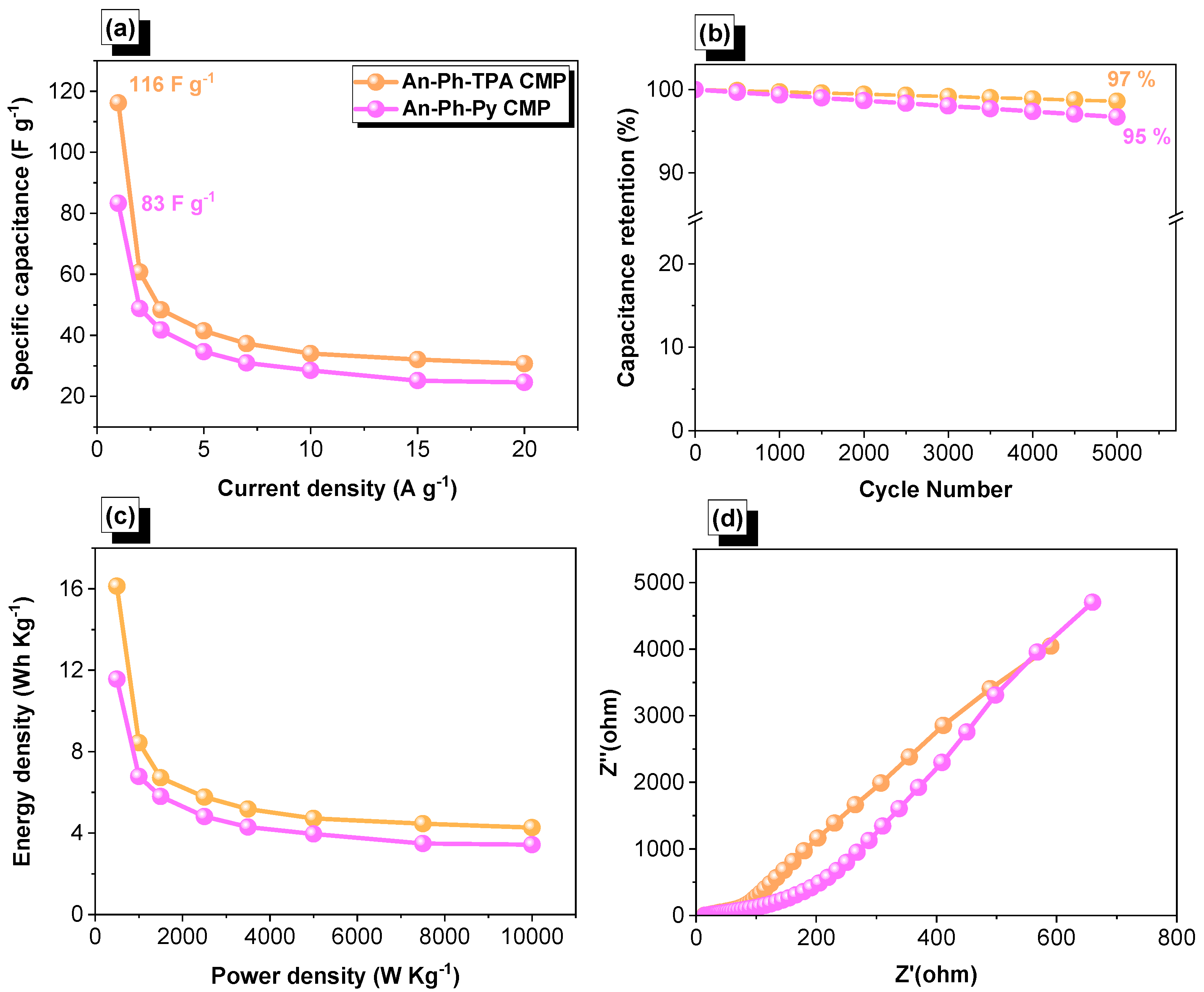
2.2. Electrochemical Performance of An-Ph-TPA CMP and An-Ph-Py CMP
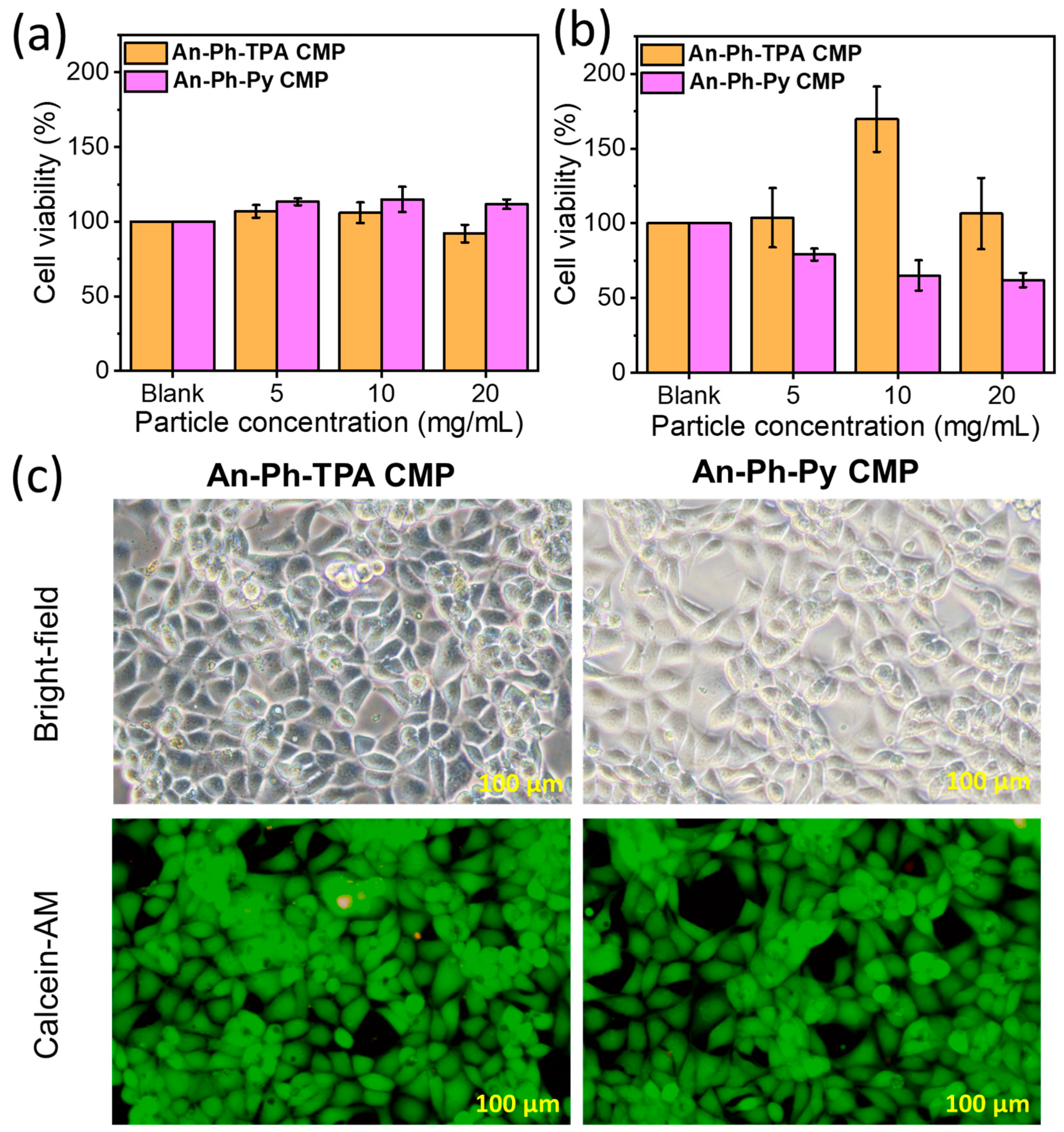
2.3. Cytotoxicity Assessment of An-Ph-TPA CMP and An-Ph-Py CMP
3. Experimental Part
3.1. Materials
3.2. Synthesis of 2,3,6,7,9,10-Hexabromoanthracene (An-Br6)
3.3. Synthesis of Tris(4-bromophenyl)amine (TPA-Br3)
3.4. Synthesis of 1,3,6,8-Tetrabromopyrene (Py-Br4)
3.5. Preparation of An-Ph-TPA and An-Ph-Py CMPs
3.6. Cell Viability via an MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyu, W.; Yan, C.; Chen, Z.; Chen, J.; Zuo, H.; Teng, L.; Liu, H.; Wang, L.; Liao, Y. Spirobifluorene-based conjugated microporous polymer-grafted carbon nanotubes for efficient supercapacitive energy storage. ACS Appl. Energy Mater. 2022, 5, 3706–3714. [Google Scholar] [CrossRef]
- Ejaz, M.; Samy, M.M.; Ye, Y.; Kuo, S.-W.; Mohamed, M.G. Design Hybrid Porous Organic/Inorganic Polymers Containing Polyhedral Oligomeric Silsesquioxane/Pyrene/Anthracene Moieties as a High-Performance Electrode for Supercapacitor. Int. J. Mol. Sci. 2023, 24, 2501. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J.; Adegoke, K.A. Advances in supercapacitor development: Materials, processes, and applications. J. Electron. Mater. 2023, 52, 96–129. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Sharma, S.U.; Chaganti, S.V.; Mansoure, T.H.; Lee, J.-T.; Chen, T.; Kuo, S.-W. Constructing conjugated microporous polymers containing triphenylamine moieties for high-performance capacitive energy storage. Polymer 2023, 264, 125541. [Google Scholar] [CrossRef]
- Weng, T.-H.; Mohamed, M.G.; Sharma, S.U.; Chaganti, S.V.; Samy, M.M.; Lee, J.-T.; Kuo, S.-W. Ultrastable three-dimensional triptycene-and tetraphenylethene-conjugated microporous polymers for energy storage. ACS Appl. Energy Mater. 2022, 5, 14239–14249. [Google Scholar] [CrossRef]
- Ye, Y.S.; Mohamed, M.G.; Chen, W.C.; Kuo, S.W. Integrating the multiple functionalities in metalloporphyrin porous organic polymers enabling strong polysulfide anchoring and rapid electrochemical kinetics in Li–S batteries. J. Mater. Chem. A 2023, 11, 9112–9124. [Google Scholar] [CrossRef]
- Ejaz, M.; Mohamed, M.G.; Kuo, S.W. Solid state chemical transformation provides a fully benzoxazine-linked porous organic polymer displaying enhanced CO2 capture and supercapacitor performance. Polym. Chem. 2023. [Google Scholar] [CrossRef]
- Samy, M.M.; Sharma, S.U.; Mohamed, M.G.; Mohammed, A.A.; Chaganti, S.V.; Lee, J.-T.; Kuo, S.-W. Conjugated microporous polymers containing ferrocene units for high carbon dioxide uptake and energy storage. Mater. Chem. Phys. 2022, 287, 126177. [Google Scholar] [CrossRef]
- Yadlapalli, R.T.; Alla, R.R.; Kandipati, R.; Kotapati, A. Super capacitors for energy storage: Progress, applications and challenges. J. Energy Storage 2022, 49, 104194. [Google Scholar] [CrossRef]
- Chen, J.; Lee, P.S. Electrochemical supercapacitors: From mechanism understanding to multifunctional applications. Adv. Energy Mater. 2021, 11, 2003311. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Wang, H. Review on reliability of supercapacitors in energy storage applications. Appl. Energy 2020, 278, 115436. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Raghavendra, K.V.G.; Vinoth, R.; Zeb, K.; Gopi, C.V.M.; Sambasivam, S.; Kummara, M.R.; Obaidat, I.M.; Kim, H.J. An intuitive review of supercapacitors with recent progress and novel device applications. J. Energy Storage 2020, 31, 101652. [Google Scholar] [CrossRef]
- Minakshi, M.; Wickramaarachchi, K. Electrochemical aspects of supercapacitors in perspective: From electrochemical configurations to electrode materials processing. Prog. Solid State Chem. 2023, 69, 100390. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode materials for supercapacitors: A review of recent advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Mousa, A.O.; Mohamed, M.G.; Chuang, C.-H.; Kuo, S.-W. Carbonized Aminal-Linked Porous Organic Polymers Containing Pyrene and Triazine Units for Gas Uptake and Energy Storage. Polymers 2023, 15, 1891. [Google Scholar] [CrossRef]
- Lou, R.; Cao, Q.; Niu, T.; Zhang, Y.; Zhang, Y.; Wang, Z.; Zhang, X. Metal–Organic-Framework-Mediated Fast Self-Assembly 3D Interconnected Lignin-Based Cryogels in Deep Eutectic Solvent for Supercapacitor Applications. Polymers 2023, 15, 1824. [Google Scholar] [CrossRef]
- del Valle, M.A.; Gacitúa, M.A.; Hernández, F.; Luengo, M.; Hernández, L.A. Nanostructured Conducting Polymers and Their Applications in Energy Storage Devices. Polymers 2023, 15, 1450. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, R.; Joanni, E.; Singh, R.K.; Shim, J.S. Advances in pseudocapacitive and battery-like electrode materials for high performance supercapacitors. J. Mater. Chem. A 2022, 10, 13190–13240. [Google Scholar] [CrossRef]
- Loganathan, N.N.; Perumal, V.; Pandian, B.R.; Atchudan, R.; Edison, T.N.J.I.; Ovinis, M. Recent studies on polymeric materials for supercapacitor development. J. Energy Storage 2022, 49, 104149. [Google Scholar] [CrossRef]
- Xiong, S.; Liu, J.; Wang, Y.; Wang, X.; Chu, J.; Zhang, R.; Gong, M.; Wu, B. Solvothermal synthesis of triphenylamine-based covalent organic framework nanofibers with excellent cycle stability for supercapacitor electrodes. J. Appl. Polym. Sci. 2022, 139, 51510. [Google Scholar] [CrossRef]
- Ahmad, Z.; Kim, W.B.; Kumar, S.; Yoon, T.H.; Lee, J.S. Redox-active supercapacitor electrode from two-monomer-connected precursor (Pyrrole: Anthraquinonedisulfonic acid: Pyrrole) and sulfonated multi-walled carbon nanotube. Electrochim. Acta 2022, 415, 140243. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.-F.; Xu, S.; Cheng, T.; Wang, S.; Lai, W.-Y.; Huang, W. Porous organic polymers for high-performance supercapacitors. Chem. Soc. Rev. 2022, 51, 3181–3225. [Google Scholar] [CrossRef]
- Gao, Y.; Zhi, C.; Cui, P.; Zhang, K.A.; Lv, L.-P.; Wang, Y. Halogen-functionalized triazine-based organic frameworks towards high performance supercapacitors. Chem. Eng. J. 2020, 400, 125967. [Google Scholar] [CrossRef]
- Wang, M.; Guo, H.; Xue, R.; Li, Q.; Liu, H.; Wu, N.; Yao, W.; Yang, W. Covalent organic frameworks: A new class of porous organic frameworks for supercapacitor electrodes. ChemElectroChem 2019, 6, 2984–2997. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Z.; Liu, X.; Mei, P.; Yang, Y. Redox-active polymers as organic electrode materials for sustainable supercapacitors. Renew. Sustain. Energy Rev. 2021, 147, 111247. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Sharma, S.U.; Liu, N.-Y.; Mansoure, T.H.; Samy, M.M.; Chaganti, S.V.; Chang, Y.-L.; Lee, J.-T.; Kuo, S.-W. Ultrastable covalent triazine organic framework based on anthracene moiety as platform for high-performance carbon dioxide adsorption and supercapacitors. Int. J. Mol. Sci. 2022, 23, 3174. [Google Scholar] [CrossRef]
- Ejaz, M.; Mohamed, M.G.; Sharma, S.U.; Lee, J.-T.; Huang, C.-F.; Chen, T.; Kuo, S.-W. An Ultrastable Porous Polyhedral Oligomeric Silsesquioxane/Tetraphenylthiophene Hybrid as a High-Performance Electrode for Supercapacitors. Molecules 2022, 27, 6238. [Google Scholar] [CrossRef]
- Samy, M.M.; Mohamed, M.G.; Kuo, S.-W. Conjugated Microporous Polymers Based on Ferrocene Units as Highly Efficient Electrodes for Energy Storage. Polymers 2023, 15, 1095. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Zhang, X.; Mansoure, T.H.; El-Mahdy, A.F.M.; Huang, C.F.; Danko, M.; Xin, Z.; Kuo, S.W. Hypercrosslinked porous organic polymers based on tetraphenylanthraquinone for CO2 uptake and high-performance supercapacitor. Polymer 2020, 205, 122857. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.-F.; Lai, W.-Y.; Huang, W. Porous Organic Polymers as Promising Electrode Materials for Energy Storage Devices. Adv. Mater. Technol. 2020, 5, 2000154. [Google Scholar] [CrossRef]
- Luo, L.-W.; Zhang, C.; Xiong, P.X.; Zhao, Y.B.; Ma, W.Y.; Chen, Y.; Zeng, J.H.; Xu, Y.; Jiang, J.-X. A redox-active conjugated microporous polymer cathode for high-performance lithium/potassium-organic batteries. Sci. China Ser. B Chem. 2021, 64, 72–81. [Google Scholar] [CrossRef]
- He, Y.; Cheng, Z.H.; Zuo, H.Y.; Yan, C.N.; Liao, Y.Z. Green Synthesis of Pyridyl Conjugated Microporous Polymers as Precursors for Porous Carbon Microspheres for Efficient Electrochemical Energy Storage. ChemElectroChem 2019, 7, 959–966. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, C.W.; Lee, S.M.; Kim, H.J.; Ko, Y.; Choi, J.; Son, S.U. Nanoparticulate Conjugated Microporous Polymer with Post-Modified Benzils for Enhanced Pseudocapacitor Performance. Chem. Eur. J. 2020, 26, 12343–12348. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Cui, X.; Duan, Q.; Li, Y.; Lv, X.; Wang, H.-g. Metal phthalocyanine-linked conjugated microporous polymer hybridized with carbon nanotubes as a high-performance flexible electrode for supercapacitors. Int. J. Hydrogen Energy 2020, 45, 22950–22958. [Google Scholar] [CrossRef]
- Luo, B.; Chen, Y.; Zhang, Y.; Huo, J. Nitrogen-rich anthraquinone–triazine conjugated microporous polymer networks as high-performance supercapacitor. New J. Chem. 2021, 45, 17278–17286. [Google Scholar] [CrossRef]
- Li, X.-C.; Zhang, Y.; Wang, C.-Y.; Wan, Y.; Lai, W.-Y.; Pang, H.; Huang, W. Redox-active triazatruxene-based conjugated microporous polymers for high-performance supercapacitors. Chem. Sci. 2017, 8, 2959–2965. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chaganti, S.V.; Sharma, S.U.; Samy, M.M.; Ejaz, M.; Lee, J.-T.; Zhang, K.; Kuo, S.-W. Constructing Conjugated Microporous Polymers Containing the Pyrene-4, 5, 9, 10-Tetraone Unit for Energy Storage. ACS Appl. Energy Mater. 2022, 5, 10130–10140. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Mansoure, T.H.; Samy, M.M.; Takashi, Y.; Mohammed, A.A.; Ahamad, T.; Alshehri, S.M.; Kim, J.; Matsagar, B.M.; Wu, K.C.-W. Ultrastable Conjugated Microporous Polymers Containing Benzobisthiadiazole and Pyrene Building Blocks for Energy Storage Applications. Molecules 2022, 27, 2025. [Google Scholar] [CrossRef]
- Amin, K.; Ashraf, N.; Mao, L.; Faul, C.F.; Wei, Z. Conjugated microporous polymers for energy storage: Recent progress and challenges. Nano Energy 2021, 85, 105958. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Wang, H.; Xiong, W.; Song, B.; Zhou, C.; Duan, A.; Tan, X.; He, Q.; Zeng, G. Recent progress in conjugated microporous polymers for clean energy: Synthesis, modification, computer simulations, and applications. Prog. Polym. Sci. 2021, 115, 101374. [Google Scholar] [CrossRef]
- Mohamed, M.G.; EL-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.-W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; El Jery, A.; Al-Zaydi, K.M.; Raza, S.; Ali, H.; Al-Hadeethi, Y.; Taha, T.; Din, I.U.; Khan, M.A. Recent advances in ground-breaking conjugated microporous polymers-based materials, their synthesis, modification and potential applications. Mater. Today. 2023, 64, 180–208. [Google Scholar] [CrossRef]
- Lee, J.-S.M.; Cooper, A.I. Advances in conjugated microporous polymers. Chem. Rev. 2020, 120, 2171–2214. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Ahmed, M.M.M.; Du, W.-T.; Kuo, S.-W. Meso/Microporous Carbons from Conjugated Hyper-Crosslinked Polymers Based on Tetraphenylethene for High-Performance CO2 Capture and Supercapacitor. Molecules 2021, 26, 738. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chang, W.C.; Kuo, S.W. Crown Ether- and Benzoxazine-Linked Porous Organic Polymers Displaying Enhanced Metal Ion and CO2 Capture through Solid-State Chemical Transformation. Macromolecules 2022, 55, 7879–7892. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hu, H.-Y.; Madhu, M.; Ejaz, M.; Sharma, S.U.; Tseng, W.-L.; Samy, M.M.; Huang, C.-W.; Lee, J.-T.; Kuo, S.-W. Construction of Ultrastable Conjugated Microporous Polymers Containing Thiophene and Fluorene for Metal Ion Sensing and Energy Storage. Micromachines 2022, 13, 1466. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Hu, H.-Y.; Madhu, M.; Samy, M.M.; Mekhemer, I.M.; Tseng, W.-L.; Chou, H.-H.; Kuo, S.-W. Ultrastable Two-Dimensional Fluorescent Conjugated Microporous Polymers Containing Pyrene and Fluorene Units for Metal Ion Sensing and Energy Storage. Eur. Polym. J. 2023, 189, 111980. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Elsayed, M.H.; Elewa, A.M.; El-Mahdy, A.F.M.; Yang, C.-H.; Mohammed, A.A.K.; Chou, H.-H.; Kuo, S.-W. Pyrene-containing conjugated organic microporous polymers for photocatalytic hydrogen evolution from water. Catal. Sci. Technol. 2021, 11, 2229–2241. [Google Scholar] [CrossRef]
- Wang, T.-X.; Liang, H.-P.; Anito, D.A.; Ding, X.; Han, B.-H. Emerging applications of porous organic polymers in visible-light photocatalysis. J. Mater. Chem. A 2020, 8, 7003–7034. [Google Scholar] [CrossRef]
- Dai, C.; Zhong, L.; Gong, X.; Zeng, L.; Xue, C.; Li, S.; Liu, B. Triphenylamine based conjugated microporous polymers for selective photoreduction of CO2 to CO under visible light. Green Chem. 2019, 21, 6606–6610. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, P.; Zhang, X.; Wu, D. Emerging porous organic polymers for biomedical applications. Chem. Soc. Rev. 2022, 51, 1377–1414. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.G.; Chen, T.-C.; Kuo, S.-W. Solid-state chemical transformations to enhance gas capture in benzoxazine-linked conjugated microporous polymers. Macromolecules 2021, 54, 5866–5877. [Google Scholar] [CrossRef]
- Zhang, W.; Zuo, H.; Cheng, Z.; Shi, Y.; Guo, Z.; Meng, N.; Thomas, A.; Liao, Y. Macroscale conjugated microporous polymers: Controlling versatile functionalities over several dimensions. Adv. Mater. 2022, 34, 2104952. [Google Scholar] [CrossRef]
- Dai, Y.; Li, W.; Chen, Z.; Zhu, X.; Liu, J.; Zhao, R.; Wright, D.S.; Noori, A.; Mousavi, M.F.; Zhang, C. An air-stable electrochromic conjugated microporous polymer as an emerging electrode material for hybrid energy storage systems. J. Mater. Chem. A 2019, 7, 16397–16405. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Liu, Y.; Meng, X.; Ye, Y.; Song, X.; Liang, Z. Novel D-π-A conjugated microporous polymer as visible light-driven oxidase mimic for efficient colorimetric detection of glutathione. Sens. Actuators B Chem. 2021, 326, 128808. [Google Scholar] [CrossRef]
- Li, L.; Zhang, B.; Liu, F.; Xue, Z.; Lu, X.; Liu, X. Direct sensing of peroxynitrite anion at sensitive hollow tubular organic conjugated microporous polymers modified electrode. Application to selective analysis of ROS and RNS in cells. Sens. Actuators B Chem. 2020, 306, 127560. [Google Scholar] [CrossRef]
- Tan, J.; Chen, W.-J.; Guo, J. Conjugated microporous polymers with distinctive π-electronic properties exhibiting enhanced optical applications. Chin. Chem. Lett. 2016, 27, 1405–1411. [Google Scholar] [CrossRef]
- Yang, S.; Yang, C.; Zhang, X.; Zheng, Z.; Bi, S.; Zhang, Y.; Zhou, H. A conjugated microporous polymer film fabricated by in situ electro-chemical deposition as a hole transporting layer in organic photovoltaics. J. Mater. Chem. C 2018, 6, 9044–9048. [Google Scholar] [CrossRef]
- Wang, M.; Wang, S.; Song, X.; Liang, Z.; Su, X. Photo-responsive oxidase mimic of conjugated microporous polymer for constructing a pH-sensitive fluorescent sensor for bio-enzyme sensing. Sens. Actuators B Chem. 2020, 316, 128157. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Y.-W. Creation and bioapplications of porous organic polymer materials. J. Mater. Chem. B 2017, 5, 9278–9290. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.M.; Mekhemer, I.M.; Mohamed, M.G.; Elsayed, M.H.; Lin, K.-H.; Chen, Y.-K.; Wu, T.-L.; Chou, H.-H.; Kuo, S.-W. Conjugated microporous polymers incorporating Thiazolo [5, 4-d] thiazole moieties for Sunlight-Driven hydrogen production from water. Chem. Eng. J. 2022, 446, 137158. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Xu, P.; Wang, H.; Huang, D.; Liu, Y.; Shao, B. Recent advances in conjugated microporous polymers for photocatalysis: Designs, applications, and prospects. J. Mater. Chem. A. 2020, 8, 6434–6470. [Google Scholar] [CrossRef]
- Jiao, R.; Zhang, W.; Sun, H.; Zhu, Z.; Yang, Z.; Liang, W.; Li, A. N- and S-doped nanoporous carbon framework derived from conjugated microporous polymers incorporation with ionic liquids for efficient oxygen reduction reaction. Mater. Today Energy 2020, 16, 100382. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, Z.; Fan, Y.; Liu, C.; Sun, H.; Liang, W.; Li, A. Calcination of Porphyrin-Based Conjugated Microporous Polymers Nanotubes As Nanoporous N-Rich Metal-Free Electrocatalysts for Efficient Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2020, 3, 5260–5268. [Google Scholar] [CrossRef]
- Chen, D.; Xing, H.; Su, Z.; Wang, C. Electrical conductivity and electroluminescence of a new anthracene-based metal–organic framework with p-conjugated zigzag chains. Chem. Commun. 2016, 52, 2019. [Google Scholar] [CrossRef]
- Hong, H.; Wu, N.; Han, M.; Guo, Z.; Zhan, H.; Du, S.; Chen, B. An anthracene based conjugated triazine framework as a luminescent probe for selective sensing of p-nitroaniline and Fe(III) ions. Mater. Chem. Front. 2021, 5, 6568–6574. [Google Scholar] [CrossRef]
- Mei, J.; Diao, Y.; Appleton, A.L.; Fang, L.; Bao, Z. Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. J. Am. Chem. Soc. 2013, 135, 6724–6746. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, H.; Meng, Q.; Gong, X.; Hu, W. Organic photoresponse materials and devices. Chem. Soc. Rev. 2012, 41, 1754–1808. [Google Scholar] [CrossRef]
- Ejaz, M.; Mohamed, M.G.; Chang, W.C.; Kuo, S.-W. Synthesis and design of hypercrosslinked porous organic frameworks containing tetraphenylpyrazine unit for high-performance supercapacitor. J. Polym. Sci. 2023. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chang, S.-Y.; Ejaz, M.; Samy, M.M.; Mousa, A.O.; Kuo, S.-W. Design and Synthesis of Bisulfone-Linked Two-Dimensional Conjugated Microporous Polymers for CO2 adsorption and Energy Storage. Molecules 2023, 28, 3234. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.M.; Mohamed, M.G.; El-Mahdy, A.F.M.; Mansoure, T.H.; Wu, K.C.-W.; Kuo, S.-W. High-performance supercapacitor electrodes prepared from dispersions of tetrabenzonaphthalene-based conjugated microporous polymers and carbon nanotubes. ACS Appl. Mater. Interfaces 2021, 13, 51906–51916. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, A.O.; Lin, Z.-I.; Chuang, C.-H.; Chen, C.-K.; Kuo, S.-W.; Mohamed, M.G. Rational Design of Bifunctional Microporous Organic Polymers Containing Anthracene and Triphenylamine Units for Energy Storage and Biological Applications. Int. J. Mol. Sci. 2023, 24, 8966. https://doi.org/10.3390/ijms24108966
Mousa AO, Lin Z-I, Chuang C-H, Chen C-K, Kuo S-W, Mohamed MG. Rational Design of Bifunctional Microporous Organic Polymers Containing Anthracene and Triphenylamine Units for Energy Storage and Biological Applications. International Journal of Molecular Sciences. 2023; 24(10):8966. https://doi.org/10.3390/ijms24108966
Chicago/Turabian StyleMousa, Aya Osama, Zheng-Ian Lin, Cheng-Hsin Chuang, Chih-Kuang Chen, Shiao-Wei Kuo, and Mohamed Gamal Mohamed. 2023. "Rational Design of Bifunctional Microporous Organic Polymers Containing Anthracene and Triphenylamine Units for Energy Storage and Biological Applications" International Journal of Molecular Sciences 24, no. 10: 8966. https://doi.org/10.3390/ijms24108966
APA StyleMousa, A. O., Lin, Z.-I., Chuang, C.-H., Chen, C.-K., Kuo, S.-W., & Mohamed, M. G. (2023). Rational Design of Bifunctional Microporous Organic Polymers Containing Anthracene and Triphenylamine Units for Energy Storage and Biological Applications. International Journal of Molecular Sciences, 24(10), 8966. https://doi.org/10.3390/ijms24108966










