Synthesis of Cellulose–Poly(Acrylic Acid) Using Sugarcane Bagasse Extracted Cellulose Fibres for the Removal of Heavy Metal Ions
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation
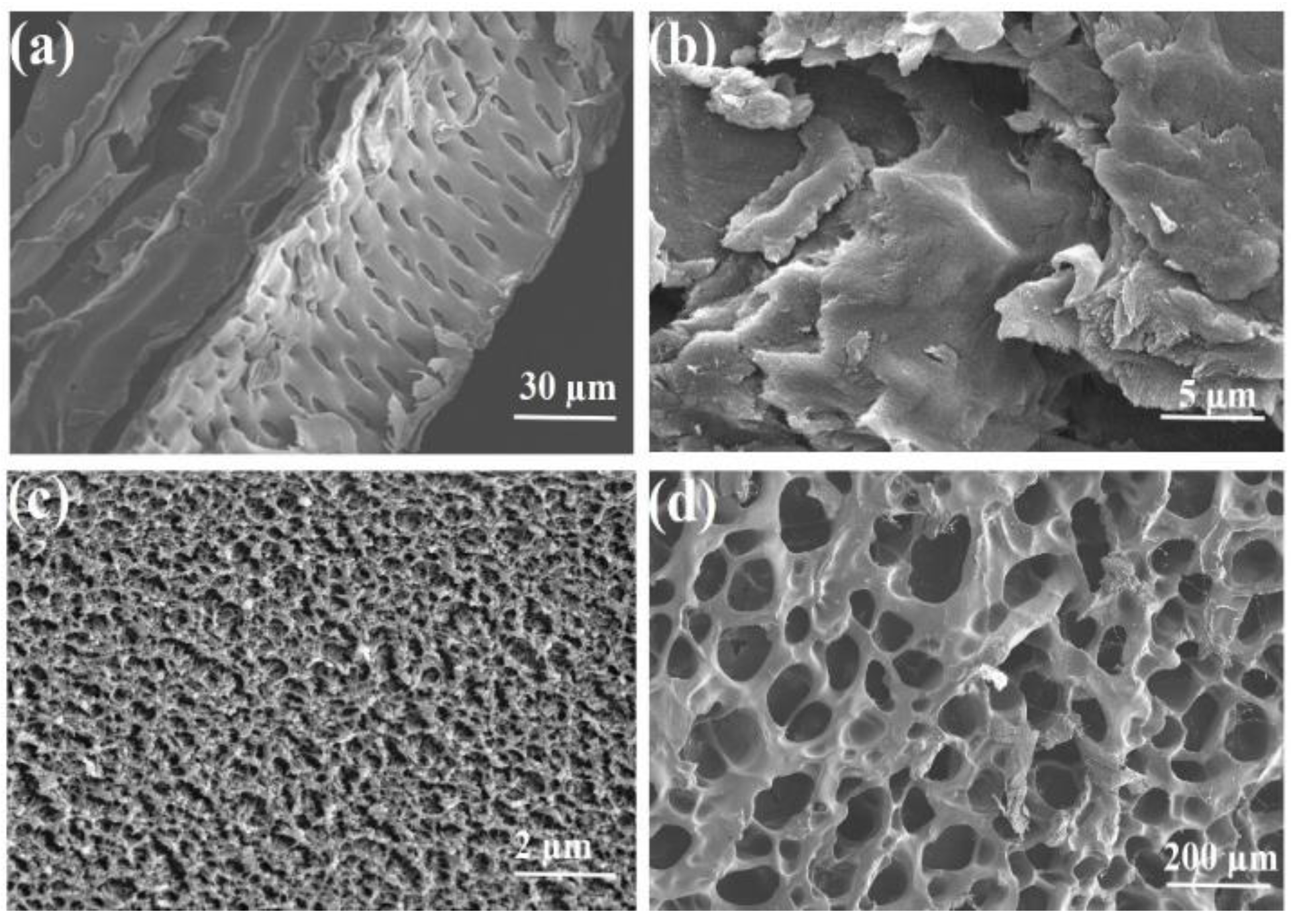
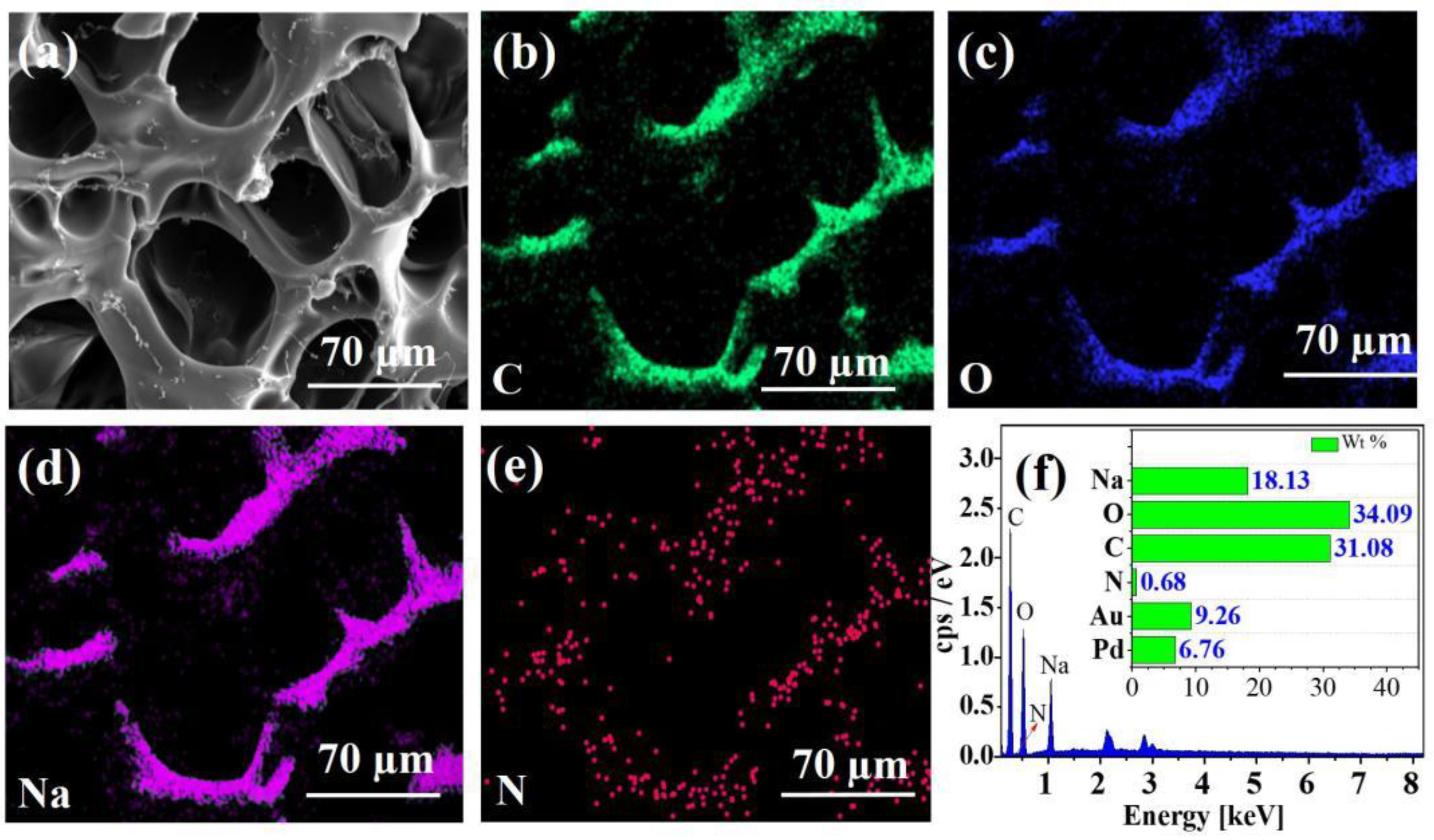
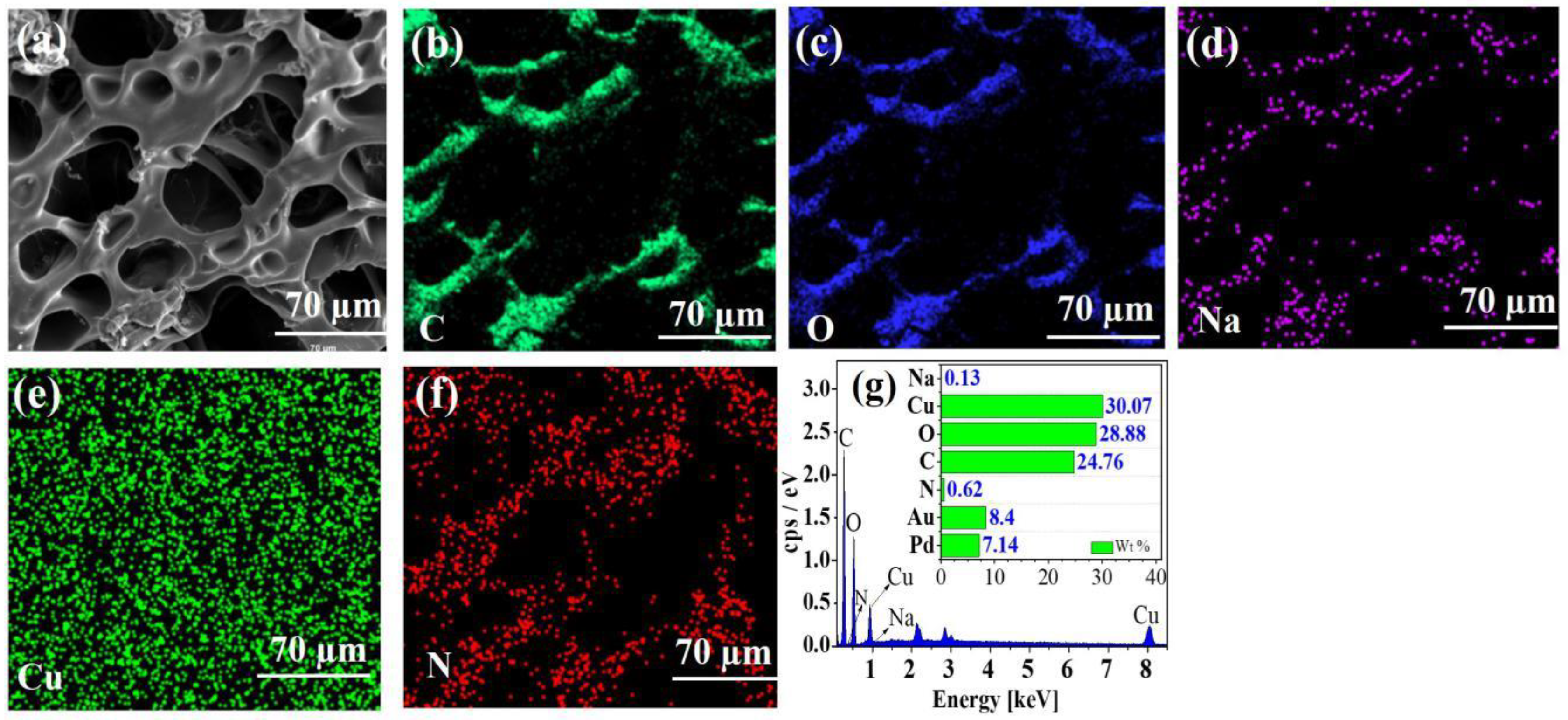
2.1.1. Surface Morphology Analysis
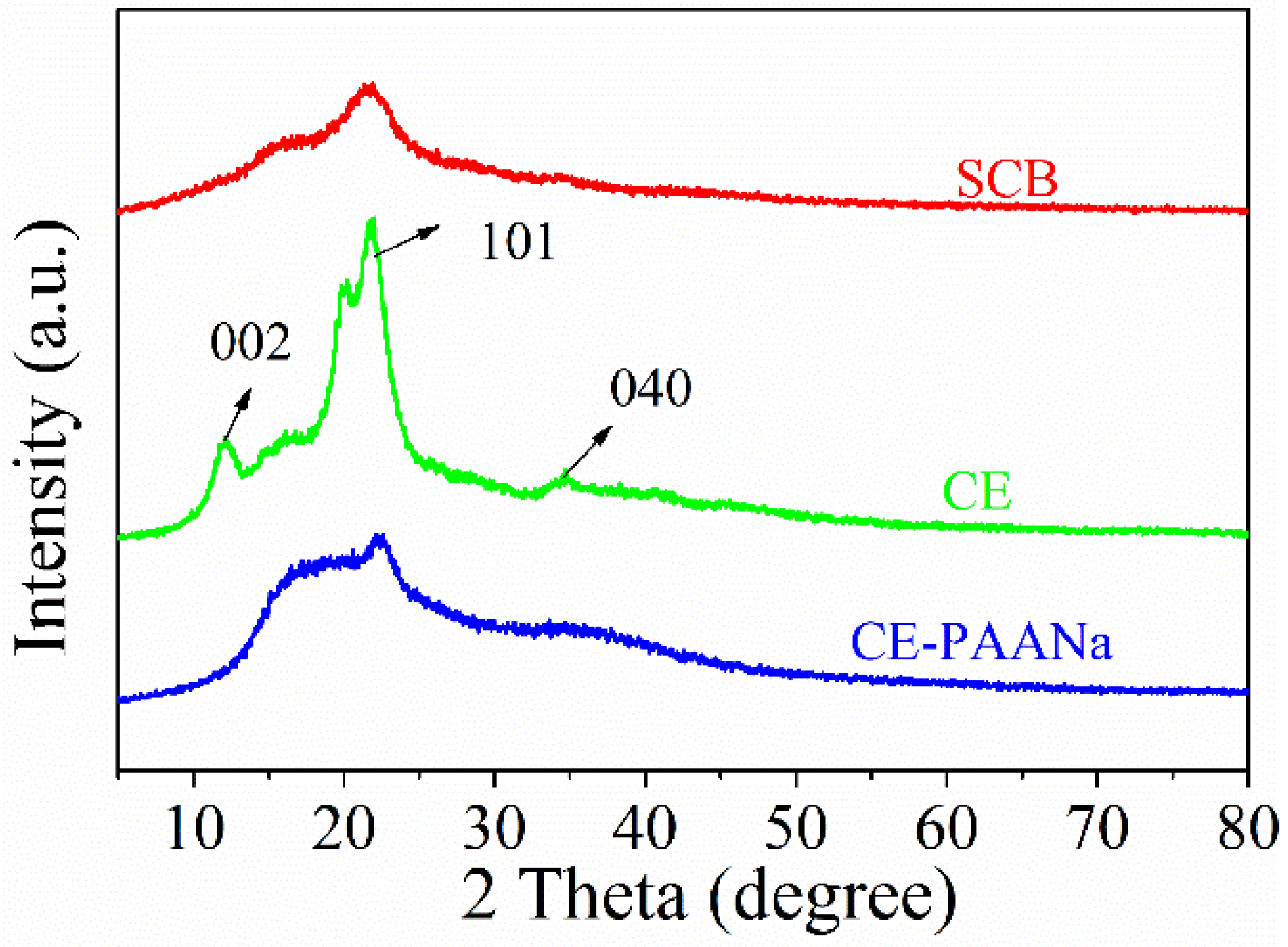
2.1.2. X-ray Diffraction (XRD) Analysis
2.1.3. FTIR Analysis
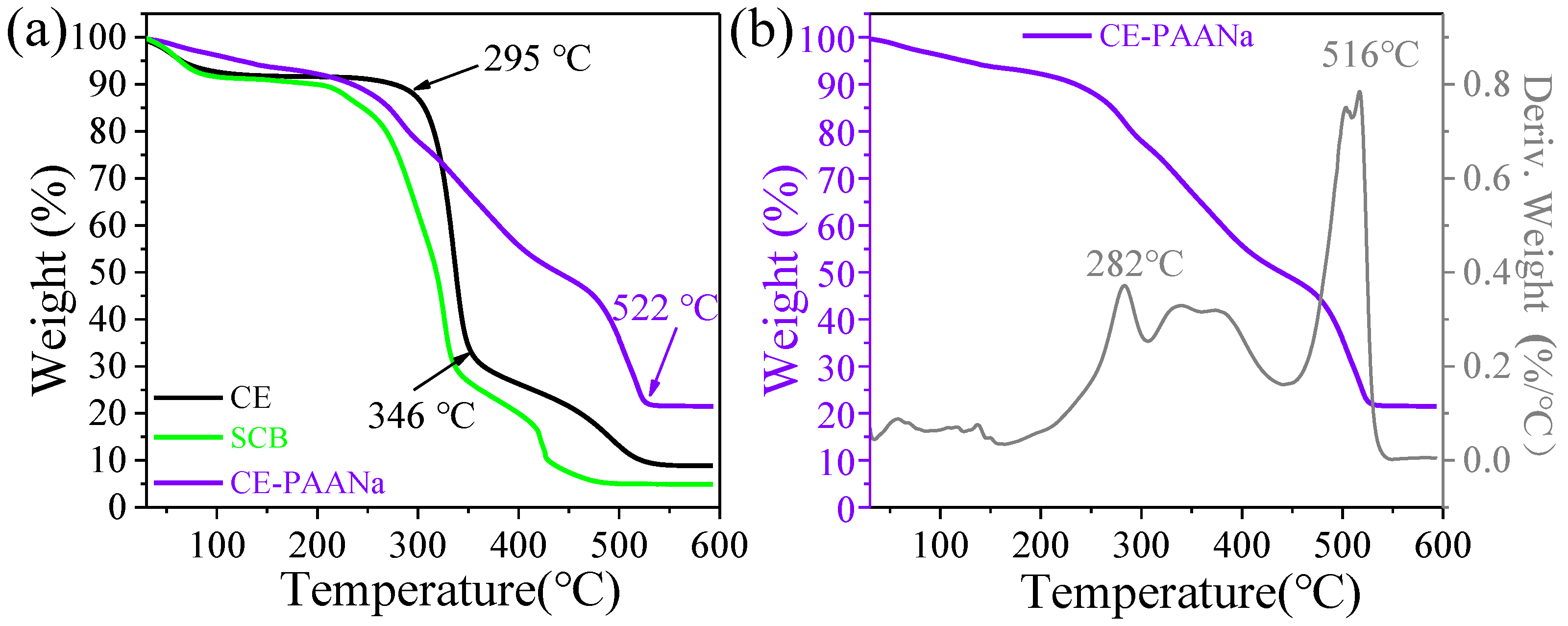
2.1.4. Thermogravimetric Analysis
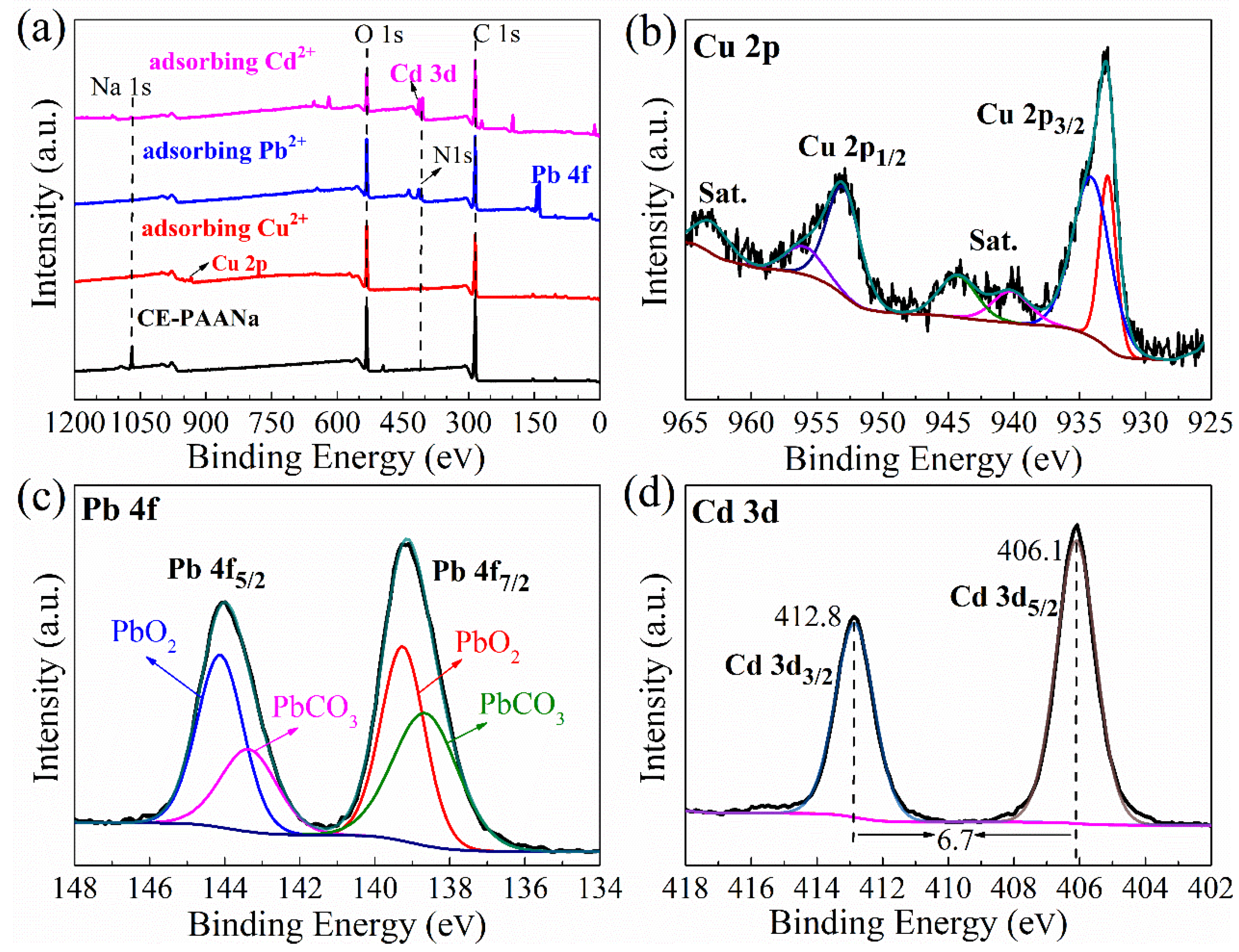
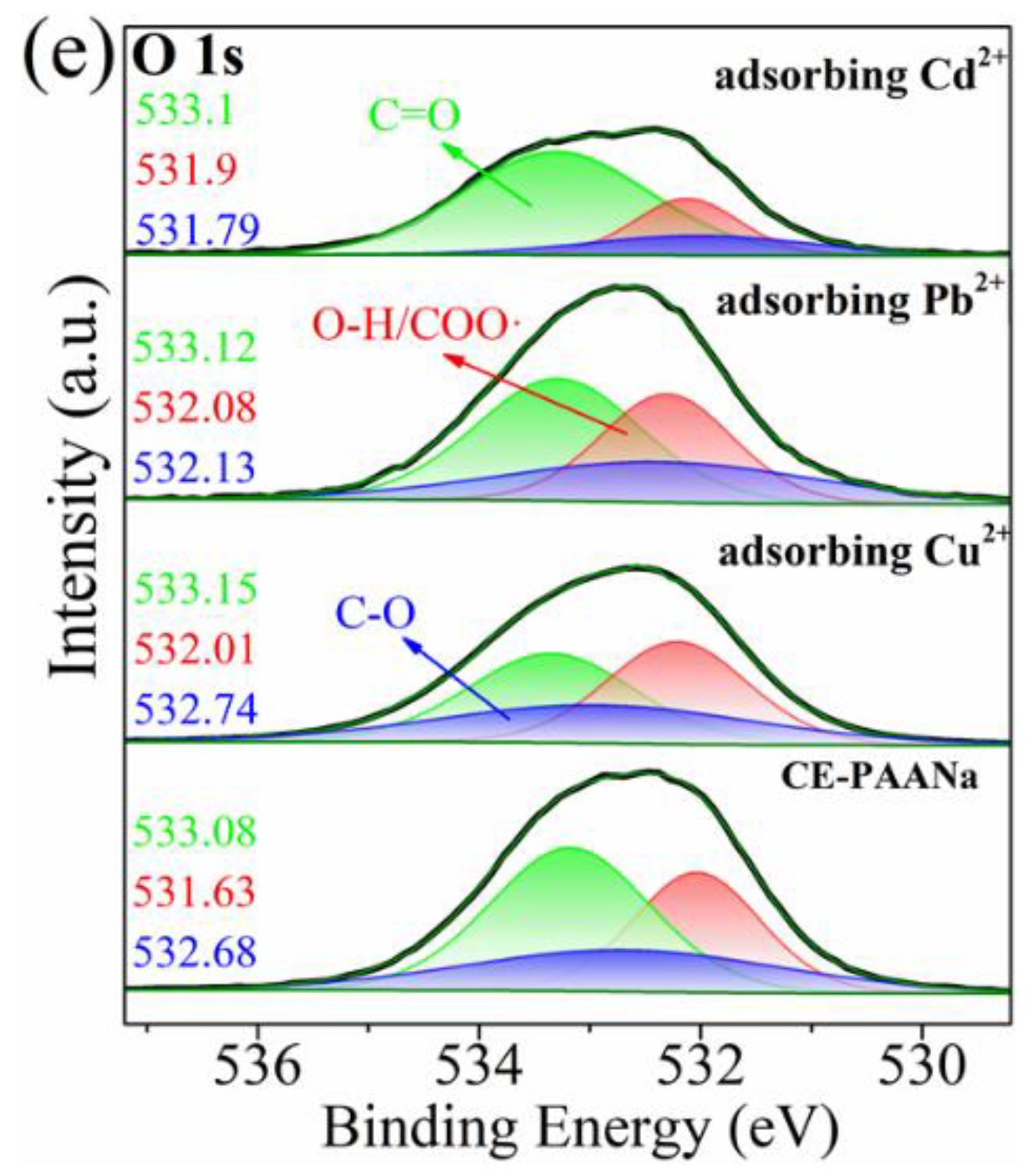
2.1.5. Adsorption Mechanism
2.2. Adsorption Studies
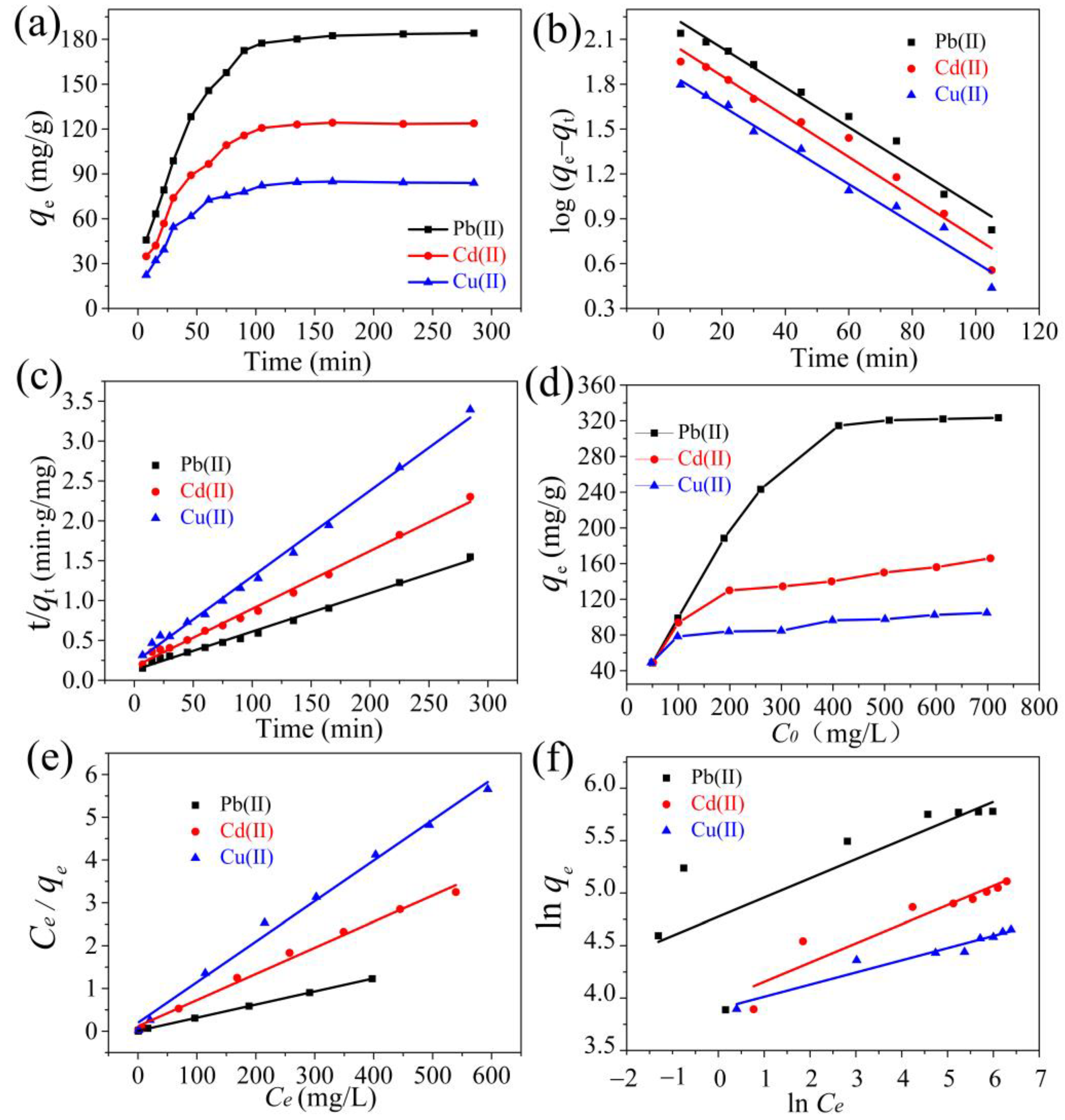
2.2.1. Adsorption Kinetics
2.2.2. Adsorption Isotherm
2.2.3. Effect of pH on Adsorption
2.2.4. Exploring Synthetic Ratios
2.2.5. Stability and Reusability
3. Materials and Methods
3.1. Materials
3.2. Extraction of CE
3.3. Preparation of CE–PAANa
3.4. Characterisation
3.5. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: A critical review. Chemosphere 2020, 241, 125043. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liu, L.; Xiang, H.; Wang, Y.; Sun, X. Biomass-based carbon microspheres for removing heavy metals from the environment: A review. Mater. Today Sustain. 2022, 18, 100136. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhao, M.; Yu, Z.; Rong, H.; Zhang, C. Utilization of nanomaterials for in-situ remediation of heavy metal(loid) contaminated sediments: A review. Sci. Total Environ. 2019, 662, 205–217. [Google Scholar] [CrossRef]
- Gokmen, F.O.; Yaman, E.; Temel, S. Eco-friendly polyacrylic acid based porous hydrogel for heavy metal ions adsorption: Characterization, adsorption behavior, thermodynamic and reusability studies. Microchem. J. 2021, 168, 106357. [Google Scholar] [CrossRef]
- Sharma, R.K.; Kumar, R.; Singh, A.P. Metal ions and organic dyes sorption applications of cellulose grafted with binary vinyl monomers. Sep. Purif. Technol. 2019, 209, 684–697. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Pugazhendhi, A.; Sindhu, R.; Sirohi, R.; Awasthi, M.K.; Pandey, A.; Binod, P. Nanocellulose as green material for remediation of hazardous heavy metal contaminants. J. Hazard. Mater. 2022, 424, 127516. [Google Scholar] [CrossRef]
- Qiu, B.; Tao, X.; Wang, H.; Li, W.; Ding, X.; Chu, H. Biochar as a low-cost adsorbent for aqueous heavy metal removal: A review. J. Anal. Appl. Pyrolysis 2021, 155, 105081. [Google Scholar] [CrossRef]
- Raj, V.; Chauhan, M.S.; Pal, S.L. Potential of sugarcane bagasse in remediation of heavy metals: A review. Chemosphere 2022, 307, 135825. [Google Scholar] [CrossRef]
- Licona-Aguilar, Á.I.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Palma-Ramírez, D.; Conde-Barajas, E.; Negrete-Rodríguez, M.X.L.; Rodríguez-Salazar, A.E.; García-Zaleta, D.S. Reutilization of waste biomass from sugarcane bagasse and orange peel to obtain carbon foams: Applications in the metal ions removal. Sci. Total Environ. 2022, 831, 154883. [Google Scholar] [CrossRef]
- Fan, S.; Zhou, J.; Zhang, Y.; Feng, Z.; Hu, H.; Huang, Z.; Qin, Y. Preparation of sugarcane bagasse succinate/alginate porous gel beads via a self-assembly strategy: Improving the structural stability and adsorption efficiency for heavy metal ions. Bioresour. Technol. 2020, 306, 123128. [Google Scholar] [CrossRef] [PubMed]
- Palamae, S.; Dechatiwongse, P.; Choorit, W.; Chisti, Y.; Prasertsan, P. Cellulose and hemicellulose recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carbohydr. Polym. 2017, 155, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jiang, H.; Lin, Z.; Xu, S.; Xie, J.; Zhang, A. Preparation of acrylamide/acrylic acid cellulose hydrogels for the adsorption of heavy metal ions. Carbohydr. Polym. 2019, 224, 115022. [Google Scholar] [CrossRef]
- Bai, H.; Li, Z.; Zhang, S.; Wang, W.; Dong, W. Interpenetrating polymer networks in polyvinyl alcohol/cellulose nanocrystals hydrogels to develop absorbent materials. Carbohydr. Polym. 2018, 200, 468–476. [Google Scholar] [CrossRef]
- Dong, C.; Zhang, F.; Pang, Z.; Yang, G. Efficient and selective adsorption of multi-metal ions using sulfonated cellulose as adsorbent. Carbohydr. Polym. 2016, 151, 230–236. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, Q.; Hu, X.; Zhang, Q.; Ma, T. Heavy metal ions and organic dyes removal from water by cellulose modified with maleic anhydride. J. Mater. Sci. 2012, 47, 5019–5029. [Google Scholar] [CrossRef]
- Guleria, A.; Kumari, G.; Lima, E.C. Cellulose-g-poly-(acrylamide-co-acrylic acid) polymeric bioadsorbent for the removal of toxic inorganic pollutants from wastewaters. Carbohydr. Polym. 2020, 228, 115396. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, J.; Liu, Y.; Liu, L.; Yu, J.; Fan, Y. Controlled delivery of aspirin from nanocellulose-sodium alginate interpenetrating network hydrogels. Ind. Crops Prod. 2023, 192, 116081. [Google Scholar] [CrossRef]
- Li, C.; Duan, L.; Cheng, X. Facile method to synthesize fluorescent chitosan hydrogels for selective detection and adsorption of Hg2+/Hg+. Carbohydr. Polym. 2022, 288, 119417. [Google Scholar] [CrossRef]
- Hasanpour, M.; Hatami, M. Application of three dimensional porous aerogels as adsorbent for removal of heavy metal ions from water/wastewater: A review study. Adv. Colloid Interface Sci. 2020, 284, 102247. [Google Scholar] [CrossRef]
- Li, D.; Liu, N.; Yao, X.; Gou, Q.; Yue, J.; Yang, D.; Chen, X.; Xiao, M. Characterization of semi-interpenetrating hydrogel based on Artemisia sphaerocephala Krasch Polysaccharide and cellulose nanocrystals crosslinked by ferric ions. Food Hydrocoll. 2023, 139, 108596. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, Y.; Ma, L.; Zhang, H.; Huang, H. Synthesis and response of pineapple peel carboxymethyl cellulose-g-poly (acrylic acid-co-acrylamide)/graphene oxide hydrogels. Carbohydr. Polym. 2019, 215, 366–376. [Google Scholar] [CrossRef]
- Yu, F.; Yang, P.; Yang, Z.; Zhang, X.; Ma, J. Double-network hydrogel adsorbents for environmental applications. Chem. Eng. J. 2021, 426, 131900. [Google Scholar] [CrossRef]
- Pereira, A.G.B.; Rodrigues, F.H.A.; Paulino, A.T.; Martins, A.F.; Fajardo, A.R. Recent advances on composite hydrogels designed for the remediation of dye-contaminated water and wastewater: A review. J. Clean. Prod. 2021, 284, 124703. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, F.; Yuan, Q.; Zhu, L.; Wang, C.; Yang, S. Hydrogen-bonded thin films of cellulose ethers and poly(acrylic acid). Carbohydr. Polym. 2019, 215, 58–62. [Google Scholar] [CrossRef]
- Chen, Y.-G.; Liao, R.-P.; Yu, C.; Yu, X. Sorption of Pb(II) on sodium polyacrylate modified bentonite. Adv. Powder Technol. 2020, 31, 3274–3286. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, G.; Goledzinowski, M.; Comeau, F.J.E.; Li, K.; Cumberland, T.; Lenos, J.; Xu, P.; Li, M.; Yu, A.; et al. Green Solid Electrolyte with Cofunctionalized Nanocellulose/Graphene Oxide Interpenetrating Network for Electrochemical Gas Sensors. Small Methods 2017, 1, 1700237. [Google Scholar] [CrossRef]
- Hu, L.-Q.; Dai, L.; Liu, R.; Si, C.-L. Lignin-graft-poly(acrylic acid) for enhancement of heavy metal ion biosorption. J. Mater. Sci. 2017, 52, 13689–13699. [Google Scholar] [CrossRef]
- Thien, D.V.H.; Lam, D.-N.; Diem, H.N.; Pham, T.Y.N.; Bui, N.Q.; Truc, T.N.T.; Van-Pham, D.-T. Synthesis of cellulose-g-poly(acrylic acid) with high water absorbency using pineapple-leaf extracted cellulose fibers. Carbohydr. Polym. 2022, 288, 119421. [Google Scholar] [CrossRef]
- Sankhla, S.; Sardar, H.H.; Neogi, S. Greener extraction of highly crystalline and thermally stable cellulose micro-fibers from sugarcane bagasse for cellulose nano-fibrils preparation. Carbohydr. Polym. 2021, 251, 117030. [Google Scholar] [CrossRef] [PubMed]
- Thiangtham, S.; Runt, J.; Manuspiya, H. Sulfonation of dialdehyde cellulose extracted from sugarcane bagasse for synergistically enhanced water solubility. Carbohydr. Polym. 2019, 208, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.L.; Auyoong, Y.L.; Hassan, K.; Farivar, F.; Tran, D.N.H.; Ma, J.; Losic, D. Multithiol functionalized graphene bio-sponge via photoinitiated thiol-ene click chemistry for efficient heavy metal ions adsorption. Chem. Eng. J. 2020, 395, 124965. [Google Scholar] [CrossRef]
- Khan, S.A.; Khan, T.A. Clay-hydrogel nanocomposites for adsorptive amputation of environmental contaminants from aqueous phase: A review. J. Environ. Chem. Eng. 2021, 9, 105575. [Google Scholar] [CrossRef]
- Du, P.; Xu, L.; Ke, Z.; Liu, J.; Wang, T.; Chen, S.; Mei, M.; Li, J.; Zhu, S. A highly efficient biomass-based adsorbent fabricated by graft copolymerization: Kinetics, isotherms, mechanism and coadsorption investigations for cationic dye and heavy metal. J. Colloid Interface Sci. 2022, 616, 12–22. [Google Scholar] [CrossRef]
- Bernardinelli, O.D.; Lima, M.A.; Rezende, C.A.; Polikarpov, I.; deAzevedo, E.R. Quantitative 13C MultiCP solid-state NMR as a tool for evaluation of cellulose crystallinity index measured directly inside sugarcane biomass. Biotechnol. Biofuels 2015, 8, 110. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Zheng, L.; Dang, Z.; Zhang, L. Classical theory and electron-scale view of exceptional Cd(II) adsorption onto mesoporous cellulose biochar via experimental analysis coupled with DFT calculations. Chem. Eng. J. 2018, 350, 1000–1009. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; Wu, W.; Jing, Y.; Dai, H.; Fang, G. Nanocellulose/Poly(2-(dimethylamino)ethyl methacrylate)Interpenetrating polymer network hydrogels for removal of Pb(II) and Cu(II) ions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 474–480. [Google Scholar] [CrossRef]
- Huang, S.; Wang, X.; Shen, J.; Wu, R.; Zhao, H.; Wang, Y.; Wang, Y.; Xia, Y. Surface functionalization of cellulose nanocrystals with polymeric ionic liquids during phase transfer. Carbohydr. Polym. 2017, 157, 1426–1433. [Google Scholar] [CrossRef]
- Abdelwahab, N.A.; Ammar, N.S.; Ibrahim, H.S. Graft copolymerization of cellulose acetate for removal and recovery of lead ions from wastewater. Int. J. Biol. Macromol. 2015, 79, 913–922. [Google Scholar] [CrossRef]
- Aflaki Jalali, M.; Dadvand Koohi, A.; Sheykhan, M. Experimental study of the removal of copper ions using hydrogels of xanthan, 2-acrylamido-2-methyl-1-propane sulfonic acid, montmorillonite: Kinetic and equilibrium study. Carbohydr. Polym. 2016, 142, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Chang, M.; Zhang, C.; Liu, X.; He, B.; Ren, J. Preparation of Xylan-g-/P(AA-co-AM)/GO Nanocomposite Hydrogel and its Adsorption for Heavy Metal Ions. Polymers 2019, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Gharekhani, H.; Olad, A.; Mirmohseni, A.; Bybordi, A. Superabsorbent hydrogel made of NaAlg-g-poly(AA-co-AAm) and rice husk ash: Synthesis, characterization, and swelling kinetic studies. Carbohydr. Polym. 2017, 168, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.; Tang, J.; Yang, Z.; Zhang, L.; Huang, X. New insights into the interactions between Pb(II) and fruit waste biosorbent. Chemosphere 2022, 303, 135048. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Feng, L.; Wang, Z.; Zhang, X. Surface analysis of 4-(bis(5-bromo-1H-indol-3-yl)methyl)phenol adsorbed on copper by spectroscopic experiments. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117752. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, Y.; Xiao, T.; Wang, J.; Chen, Y.; Xu, X.; Kang, Z.; Fan, L.; Yu, H. Comparative studies on Pb(II) biosorption with three spongy microbe-based biosorbents: High performance, selectivity and application. J. Hazard. Mater. 2019, 373, 39–49. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Z.; Chen, Z.; Sun, J.; Gao, Y.; Wu, E. Resource utilization of swine sludge to prepare modified biochar adsorbent for the efficient removal of Pb(II) from water. J. Clean. Prod. 2020, 257, 120322. [Google Scholar] [CrossRef]
- Li, S.; Li, S.; Wen, N.; Wei, D.; Zhang, Y. Highly effective removal of lead and cadmium ions from wastewater by bifunctional magnetic mesoporous silica. Sep. Purif. Technol. 2021, 265, 118341. [Google Scholar] [CrossRef]
- Cid, H.A.; Flores, M.I.; Pizarro, J.F.; Castillo, X.A.; Barros, D.E.; Moreno-Piraján, J.C.; Ortiz, C.A. Mechanisms of Cu2+ biosorption on Lessonia nigrescens dead biomass: Functional groups interactions and morphological characterization. J. Environ. Chem. Eng. 2018, 6, 2696–2704. [Google Scholar] [CrossRef]
- He, S.; Zhang, F.; Cheng, S.; Wang, W. Synthesis of Sodium Acrylate and Acrylamide Copolymer/GO Hydrogels and Their Effective Adsorption for Pb2+ and Cd2+. ACS Sustain. Chem. Eng. 2016, 4, 3948–3959. [Google Scholar] [CrossRef]
- Kemik, Ö.F.; Ngwabebhoh, F.A.; Yildiz, U. A response surface modelling study for sorption of Cu2+, Ni2+, Zn2+ and Cd2+ using chemically modified poly(vinylpyrrolidone) and poly(vinylpyrrolidone-co-methylacrylate) hydrogels. Adsorpt. Sci. Technol. 2016, 35, 263–283. [Google Scholar] [CrossRef]
- Corami, A.; Mignardi, S.; Ferrini, V. Copper and zinc decontamination from single- and binary-metal solutions using hydroxyapatite. J. Hazard. Mater. 2007, 146, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, K.; Xing, H.; Chen, J.; Lu, R. Effective removal of Cu2+ from aqueous solution by synthetic abalone shell hydroxyapatite microspheres adsorbent. Environ. Technol. Innov. 2021, 23, 101663. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, X.; Xin, J.; Liu, G.; Chen, J.; Wu, G.; Liu, T.; Zhang, J.; Kong, Z. Thiol–Ene Synthesis of Cysteine-Functionalized Lignin for the Enhanced Adsorption of Cu(II) and Pb(II). Ind. Eng. Chem. Res. 2018, 57, 7872–7880. [Google Scholar] [CrossRef]
- Raganati, F.; Alfe, M.; Gargiulo, V.; Chirone, R.; Ammendola, P. Kinetic study and breakthrough analysis of the hybrid physical/chemical CO2 adsorption/desorption behavior of a magnetite-based sorbent. Chem. Eng. J. 2019, 372, 526–535. [Google Scholar] [CrossRef]
- Geng, J.; Gu, F.; Chang, J. Fabrication of magnetic lignosulfonate using ultrasonic-assisted in situ synthesis for efficient removal of Cr(Ⅵ) and Rhodamine B from wastewater. J. Hazard. Mater. 2019, 375, 174–181. [Google Scholar] [CrossRef]
- Yuan, B.; Li, H.; Hong, H.; Wang, Q.; Tian, Y.; Lu, H.; Liu, J.; Lin, L.; Wu, G.; Yan, C. Immobilization of lead(Ⅱ) and zinc(Ⅱ) onto glomalin-related soil protein (GRSP): Adsorption properties and interaction mechanisms. Ecotoxicol. Environ. Saf. 2022, 236, 113489. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S.; Wilson, L.D. Re-evaluation of the century-old Langmuir isotherm for modeling adsorption phenomena in solution. Chem. Phys. 2018, 513, 99–104. [Google Scholar] [CrossRef]
- Leonov, D.V.; Dzuba, S.A.; Surovtsev, N.V. Membrane–Sugar Interactions Probed by Low-Frequency Raman Spectroscopy: The Monolayer Adsorption Model. Langmuir 2020, 36, 11655–11660. [Google Scholar] [CrossRef]
- Wang, B.; Wen, J.-L.; Sun, S.-L.; Wang, H.-M.; Wang, S.-F.; Liu, Q.-Y.; Charlton, A.; Sun, R.-C. Chemosynthesis and structural characterization of a novel lignin-based bio-sorbent and its strong adsorption for Pb (II). Ind. Crops Prod. 2017, 108, 72–80. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, X.; Qian, Y.; Xiong, W.; Yang, D. pH-responsive lignin-based complex micelles: Preparation, characterization and application in oral drug delivery. Chem. Eng. J. 2017, 327, 1176–1183. [Google Scholar] [CrossRef]
- Kong, W.; Li, Q.; Li, X.; Su, Y.; Yue, Q.; Zhou, W.; Gao, B. Removal of copper ions from aqueous solutions by adsorption onto wheat straw cellulose-based polymeric composites. J. Appl. Polym. Sci. 2018, 135, 46680. [Google Scholar] [CrossRef]
- Chu, L.; Liu, C.; Zhou, G.; Xu, R.; Tang, Y.; Zeng, Z.; Luo, S. A double network gel as low cost and easy recycle adsorbent: Highly efficient removal of Cd(II) and Pb(II) pollutants from wastewater. J. Hazard. Mater. 2015, 300, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Ma, J.; Tang, Y.; Zeng, Z.; Luo, S. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent. Water Res. 2016, 89, 151–160. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Li, Y.; Zhang, Q.; Huang, J.; Wu, Q.; Wang, S. Fabrication of Cellulose Nanocrystal-g-Poly(Acrylic Acid-Co-Acrylamide) Aerogels for Efficient Pb(II) Removal. Polymers 2020, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Jafarigol, E.; Afshar Ghotli, R.; Hajipour, A.; Pahlevani, H.; Baghban Salehi, M. Tough dual-network GAMAAX hydrogel for the efficient removal of cadmium and nickle ions in wastewater treatment applications. J. Ind. Eng. Chem. 2021, 94, 352–360. [Google Scholar] [CrossRef]
- Sultan, M.; Nagieb, Z.A.; El-Masry, H.M.; Taha, G.M. Physically-crosslinked hydroxyethyl cellulose-g-poly (acrylic acid-co-acrylamide)-Fe3+/silver nanoparticles for water disinfection and enhanced adsorption of basic methylene blue dye. International J. Biol. Macromol. 2022, 196, 180–193. [Google Scholar] [CrossRef]
- Song, J.; Zhao, H.; Zhao, G.; Xiang, Y.; Liu, Y. Novel Semi-IPN Nanocomposites with Functions of both Nutrient Slow-Release and Water Retention. 1. Microscopic Structure, Water Absorbency, and Degradation Performance. J. Agric. Food. Chem. 2019, 67, 7587–7597. [Google Scholar] [CrossRef]



| Adsorbent | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | qe (mg/g) | R2 | K2 (min−1) | qe (g⋅mg/min) | R2 | |
| Pb (Ⅱ) | 0.03058 | 203.4 | 0.9767 | 1.813 × 10−4 | 207.0 | 0.9938 |
| Cd (Ⅱ) | 0.03125 | 134.1 | 0.9680 | 3.061 × 10−4 | 137.9 | 0.9939 |
| Cu (Ⅱ) | 0.03015 | 82.55 | 0.9794 | 5.178 × 10−4 | 92.76 | 0.9948 |
| Adsorbent | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| KL (L/g) | qmax (mg/g) | R2 | KF (L/g) | 1/n | R2 | |
| Pb (Ⅱ) | 0.2971 | 333.3 | 0.9997 | 118.6 | 0.1825 | 0.5632 |
| Cd (Ⅱ) | 0.05209 | 163.9 | 0.9912 | 53.14 | 0.1827 | 0.8743 |
| Cu (Ⅱ) | 0.04731 | 106.3 | 0.9925 | 49.24 | 0.1160 | 0.9373 |
| Types | Cellulose % | Hemicelluloses % | Lignin % | Yield % |
|---|---|---|---|---|
| SCB | 33.75 | 30.77 | 13.16 | - |
| CE | 77.83 | 6.11 | 2.18 | 30.12 |
| Adsorbent | qm (mg/g) | pH | T(k) | Refs | ||
|---|---|---|---|---|---|---|
| Cu2+ | Pb2+ | Cd2+ | ||||
| PVA/PAA gel | - | 195.0 | 115.9 | 5 | 313 | [64] |
| Polyampholyte hydrogel | - | 216.1 | 153.8 | Pb 5.0 Cd 6.0 | 313 | [65] |
| Cellulose nanocrystal-g-poly(acrylic acid-co-acrylamide) aerogels | - | 366.3 | - | 6 | 293 | [66] |
| GAMAAX | - | - | 312.1 | 7 | 298 | [67] |
| P(AANa-co-AM)/GO hydrogel | - | 452.3 | 196.4 | 4.5 | 298 | [50] |
| MCC-g-(AA-co-AM) | 157.5 | 393.2 | 289.9 | 5 | 300.1 | [13] |
| Cellulose-g-poly-(acrylamide-co -acrylic acid) polymeric | 61.8 | 209.6 | 101.7 | Cu Pb 5.5 Cd 6.0 | 298 | [17] |
| CE–PAANa | 106.3 | 333.3 | 163.9 | 5 | 296.6 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Xie, Z.; Wen, J.; Tang, T.; Jiang, L.; Hu, G.; Li, M. Synthesis of Cellulose–Poly(Acrylic Acid) Using Sugarcane Bagasse Extracted Cellulose Fibres for the Removal of Heavy Metal Ions. Int. J. Mol. Sci. 2023, 24, 8922. https://doi.org/10.3390/ijms24108922
Li F, Xie Z, Wen J, Tang T, Jiang L, Hu G, Li M. Synthesis of Cellulose–Poly(Acrylic Acid) Using Sugarcane Bagasse Extracted Cellulose Fibres for the Removal of Heavy Metal Ions. International Journal of Molecular Sciences. 2023; 24(10):8922. https://doi.org/10.3390/ijms24108922
Chicago/Turabian StyleLi, Fuchao, Zhemin Xie, Jianfeng Wen, Tao Tang, Li Jiang, Guanghui Hu, and Ming Li. 2023. "Synthesis of Cellulose–Poly(Acrylic Acid) Using Sugarcane Bagasse Extracted Cellulose Fibres for the Removal of Heavy Metal Ions" International Journal of Molecular Sciences 24, no. 10: 8922. https://doi.org/10.3390/ijms24108922
APA StyleLi, F., Xie, Z., Wen, J., Tang, T., Jiang, L., Hu, G., & Li, M. (2023). Synthesis of Cellulose–Poly(Acrylic Acid) Using Sugarcane Bagasse Extracted Cellulose Fibres for the Removal of Heavy Metal Ions. International Journal of Molecular Sciences, 24(10), 8922. https://doi.org/10.3390/ijms24108922






