Recent Developments in ZnS-Based Nanostructures Photocatalysts for Wastewater Treatment
Abstract
1. Introduction
2. ZnS Nanostructures Photocatalysts for Organic Pollutants Degradation
2.1. 0D ZnS-Based Nanostructures
2.2. 1D ZnS-Based Nanostructures
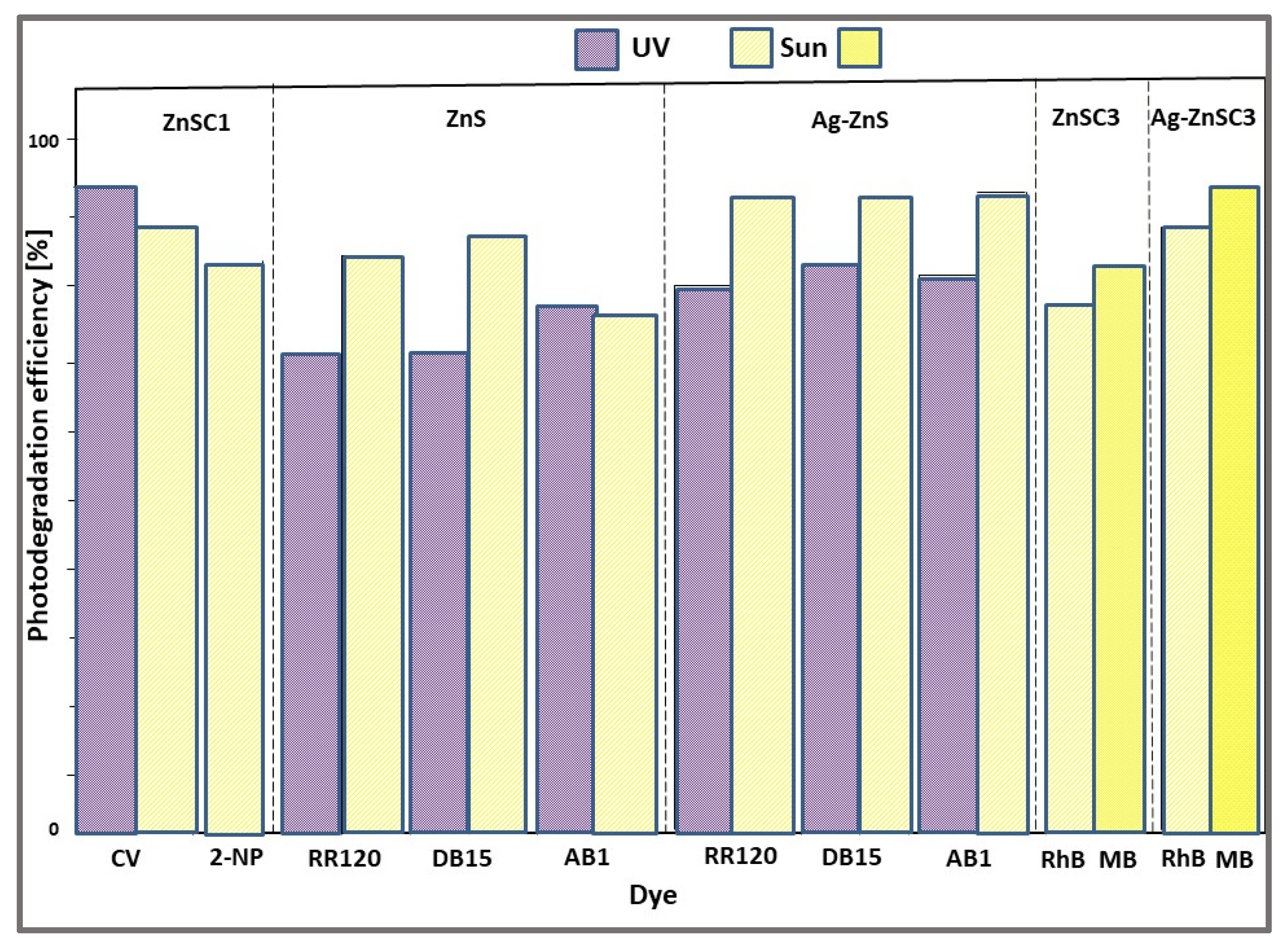
- 77% for AB1 and 87% for DB15 dyes using ZnS catalyst;
- 83% for AB1 and 94% for DB15 dyes using ZnS-Ag catalyst.
2.3. 3D ZnS-Based Nanostructures
3. Zinc Sulfide-Based Heterostructures Photocatalysts for Organic Pollutant Degradation
3.1. ZnS-Based Photocatalysts with n-n Heterojunction Structure
3.2. ZnS-Based Photocatalysts with n-p Heterojunction Structure
3.3. ZnS-Based Photocatalysts with Z-Scheme Heterojunction Structure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- El-Hout, S.I.; El-Sheikh, S.M.; Gaber, A.; Shawky, A.; Ahmed, A.I. Highly efficient sunlight-driven photocatalytic degradation of malachite green dye over reduced graphene oxide-supported CuS nanoparticles. J. Alloys Compd. 2020, 849, 156573. [Google Scholar] [CrossRef]
- Ikram, M.; Khan, M.I.; Raza, A.; Imran, M.; Ul-Hamid, A.; Ali, S. Outstanding performance of silver-decorated MoS2 nan petals used as nanocatalyst for synthetic dye degradation. Phys. E 2020, 124, 114246. [Google Scholar] [CrossRef]
- Velázquez, J.L.; Xin, Y.; Su, Y.-F.; Quiles-Vélez, C.I.; Cruz-Romero, S.S.; Torres-Mejías, G.E.; De Jesús, J.R.; Bailón-Ruiz, S.J. Synthesis, characterization, and photocatalytic activity of ZnS and Mn-doped ZnS nanostructures. MRS Adv. 2021, 6, 252–258. [Google Scholar] [CrossRef]
- Vyas, Y.; Chundawat, P.; Dharmendra, D.; Jain, A.; Punjabi, P.B.; Ameta, C. Biosynthesis and characterization of carbon quantum Dots@CuS composite using water hyacinth leaves and its usage in photocatalytic dilapidation of Brilliant Green dye. Mater. Chem. Phys. 2022, 281, 125921. [Google Scholar] [CrossRef]
- Dumbrava, A.; Daniela Berger, D.; Prodan, G.; Matei, C.; Moscalu, F.; Diacon, A. The influence of Triton X-100 surfactant on the morphology and properties of zinc sulfide nanoparticles for applications in azo dyes degradation. Mater. Chem. Phys. 2017, 193, 316–328. [Google Scholar] [CrossRef]
- You, J.; Liu, C.; Feng, X.; Lu, B.; Xia, L.; Zhuang, X. In situ synthesis of ZnS nanoparticles onto cellulose/chitosan sponge for adsorption–photocatalytic removal of Congo red. Carbohydr. Polym. 2022, 288, 119332. [Google Scholar] [CrossRef]
- Sivaranjani, P.R.; Janani, B.; Thomas, A.M.; Raju, L.L.; Khan, S.S. Recent development in MoS2-based nano-photocatalyst for the degradation of pharmaceutically active compounds. J. Clean. Prod. 2022, 352, 131506. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, R.; Zhou, L.; Li, Z.; Tian, L.; Cao, R. Phase engineering-defective 1 T @ 2 H MoS2 nanoflowers as excellent full spectrum photocatalyst. J. Alloys Compd. 2022, 910, 164898. [Google Scholar] [CrossRef]
- Vatanpour, V.; Karatas, O.; Amiri, S.; Rajabi, H.R.; Koyuncu, I.; Khataee, A. Different metal-doped ZnS quantum dots photocatalysts for enhancing the permeability and antifouling performances of polysulfone membranes with and without UV irradiation. Chemosphere 2022, 294, 133705. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef]
- Ni, L.; Wang, T.; Wang, K.; Ma, J.; Wang, Y. Novel Control Strategy for Membrane Biofouling by Surface Loading of Aerobically and Anaerobically Applicable Photocatalytic Optical Fibers Based on a Z-Scheme Heterostructure Zr-MOFs/rGO/Ag3PO4 Photocatalyst. Environ. Sci. Technol. 2022, 56, 6608–6620. [Google Scholar] [CrossRef] [PubMed]
- Völker, J.; Stapf, M.; Miehe, U.; Wagner, M. Systematic review of toxicity removal by advanced wastewater treatment technologies via ozonation and activated carbon. Environ. Sci. Technol. 2019, 53, 7215–7233. [Google Scholar] [CrossRef] [PubMed]
- Enesca, A.; Enache, C.; Duta, A.; Schoonman, J. High crystalline tungsten trioxide thin layer obtained by SPD technique. J. Eur. Ceram. Soc. 2006, 26, 571–576. [Google Scholar] [CrossRef]
- Jabbar, J.H.; Graimed, B.H. Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study. J. Water Process. Eng. 2022, 47, 102671. [Google Scholar] [CrossRef]
- Enesca, A.; Duta, A. Tailoring WO3 thin layers using spray pyrolysis technique. Phys. Status Solidi B 2008, 5, 3499–3502. [Google Scholar] [CrossRef]
- Adhikari, A.; Sarkar, D.; Madras, G. Hierarchical design of CuS architectures for visible light photocatalysis of 4-chlorophenol. ACS Omega 2017, 2, 4009–4021. [Google Scholar] [CrossRef]
- Mouchaal, Y.; Enesca, A.; Mihoreanu, C.; Khelil, A.; Duta, A. Tuning the opto-electrical properties of SnO2 thin films by Ag+1 and In+3 co-doping. Mater. Sci. Eng. B 2015, 199, 22–29. [Google Scholar] [CrossRef]
- Lee, G.J.; Wu, J.J. Recent developments in ZnS photocatalysts from synthesis to photocatalytic applications—A review. Powder Technol. 2017, 318, 8–22. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, C.; Ma, S.; Zhao, S.; Yang, J. Synthesis of nano-ZnS by lyotropic liquid crystal template method for enhanced photodegradation of methylene blue. Inorg. Chem. Commun. 2022, 135, 109089. [Google Scholar] [CrossRef]
- Yang, M.; Ren, D.; Sun, S.; Cui, J.; Yang, Q.; Luo, Y.; Liang, S. One-pot construction of unprecedented direct Z-scheme ZnS/GaOOH heterojunction for photodegradation of antibiotics. Appl. Surf. Sci. 2022, 576, 151742. [Google Scholar] [CrossRef]
- Jiang, G.; Zhu, B.; Sun, J.; Liu, F.; Wang, Y.; Zhao, C. Enhanced activity of ZnS (111) by N/Cu co-doping: Accelerated degradation of organic pollutants under visible light. Res. J. Environ. Sci. 2022, 125, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Ayodhya, D.; Veerabhadram, G. Review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today Energy. 2018, 9, 83–113. [Google Scholar] [CrossRef]
- Sofronov, D.S.; Sofronova, E.M.; Baumer, V.N.; Kudin, K.A.; Mateichenko, P.V.; Vovk, O.M.; Bryleva, E.Y.; Belikov, K.N. Formation of ZnS nano- and microparticles from thiourea solutions. Adv. Powder Technol. 2013, 24, 1017–1022. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Y.; Kong, J.; Jia, H. Tunable synthesis, characterization and photocatalytic properties of various ZnS nanostructures. Ceram. Int. 2016, 42, 2854–2860. [Google Scholar] [CrossRef]
- Subramanian, A.; Bhutada, M.; Bhutada, M.; Phan, A.R.; Ra, S.; Endrino, J.L. Environmentally sustainable visible photocatalytic nanostructured ZnS doped with CuS for chemical effluent treatment applications. Mater. Today Proc. 2017, 4, 1660–11670. [Google Scholar] [CrossRef]
- Xu, X.; Li, S.; Chen, J.; Cai, S.; Long, Z.; Fang, X. Design principles and material engineering of ZnS for optoelectronic devices and catalysis. Adv. Funct. Mater. 2018, 28, 1802029. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Li, Z.; Lou, Z.; Wang, Z.; Dai, Y.; Whangbo, M.H. Synthesis and characterization of ZnS with controlled amount of S vacancies for photocatalytic H2 production under visible light. Sci. Rep. 2015, 5, 8544. [Google Scholar] [CrossRef]
- Kozhevnikova, N.S.; Melkozerova, M.A.; Enyashin, A.N.; Tyutyunnik, A.P.; Pasechnik, L.A.; Baklanova, I.V.; Suntsov, A.Y.; Yushkov, A.A.; Buldakova, L.Y.; Yanchenko, M.Y. Janus ZnS nanoparticles: Synthesis and photocatalytic properties. J. Phys. Chem. Solids 2022, 161, 110459. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Ren, X.; Sun, X.; Tian, D.; Wei, Q.; Wu, D. Defect-rich ZnS nanoparticles supported on reduced graphene oxide for high-efficiency ambient N2—To-NH3 conversion. Appl. Catal. B Environ. 2021, 284, 119746. [Google Scholar] [CrossRef]
- Talebi, S.; Chaibakhsh, N.; Moradi-Shoeili, Z. Application of nanoscale ZnS/TiO2 composite for optimized photocatalytic decolorization of a textile dye. J. Appl. Res. Technol. 2017, 15, 378–385. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Zhu, B.; Wang, J.; Lan, H.; Chen, X. Synthesis of porous ZnS, ZnO and ZnS/ZnO nanosheets and their photocatalytic properties. RSC Adv. 2017, 7, 30956. [Google Scholar] [CrossRef]
- Xue, S.; Huang, W.; Lin, W.; Xing, W.; Shen, M.; Ye, X.; Liang, X.; Yang, C.; Hou, Y.; Yu, Z.; et al. Interfacial engineering of lattice coherency at ZnO-ZnS photocatalytic heterojunctions. Chem. Catal. 2022, 2, 125–139. [Google Scholar] [CrossRef]
- Murillo-Sierra, J.C.; Maya-Treviño, M.D.L.; Nuñez-Salas, R.E.; Pino-Sandoval, D.A.; Hernández-Ramírez, A. Facile synthesis of ZnS/WO3 coupled photocatalyst and its application on sulfamethoxazole degradation. Ceram. Int. 2022, 48, 13761–13769. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.V.; Alhassan, S.M. Facile and scalable production of heterostructured ZnS-ZnO/Graphene nano-photocatalysts for environmental remediation. Sci. Rep. 2018, 8, 13401. [Google Scholar] [CrossRef]
- Huang, J.; Yang, C.; Song, Q.; Liu, D.; Li, L. Photocatalytic performance of Ag2S/ZnO/ZnS nanocomposites with high visible light response prepared via microwave-assisted hydrothermal two-step method. Water Sci. Technol. 2018, 78, 1802–1811. [Google Scholar] [CrossRef]
- Li, X.; Zhang, M.; Feng, J.; Bai, C.; Ren, Y. Electrostatic self-assembly to form unique LiNbO3/ZnS core-shell structure for photocatalytic nitrate reduction enhancement. J. Colloid Interface Sci. 2022, 607, 1323–1332. [Google Scholar] [CrossRef]
- Riazian, M.; Yousefpoor, M. Photocatalytic activity, nanostructure and optical properties of 3D ZnS urchin-like via hydrothermal method. Int. J. Smart Nano Mater. 2020, 11, 47–64. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Liu, Y.; Liu, M.; Miao, X.; Wang, Y. Influence of ZnS crystal morphology on adsorption-photocatalytic efficiency of pseudocrystal ZnS nanomaterials for methylene blue degradation. J. Mol. Struct. 2022, 1256, 132514. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Xia, W.; Deng, B.Y.; Liu, J.; Wang, F. Host-guest assemblies of anchoring molecular catalysts of CO2 reduction onto CuInS2/ZnS quantum dots for robust photocatalytic syngas production in water. Mol. Catal. 2022, 520, 112168. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, Y.; Du, L.; Wang, Q.; Liu, X.; Li, L.; Tian, G. Fabrication of size-controlled hierarchical ZnS@ZnIn2S4 heterostructured cages for enhanced gas-phase CO2 photoreduction. J. Colloid Interface Sci. 2022, 605, 253–262. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, G.; Shao, Y.; Wang, J.; Zhou, S.; Su, Y. Step-scheme ZnO@ZnS hollow microspheres for improved photocatalytic H2 production performance. Chin. J. Catal. 2022, 43, 329–338. [Google Scholar] [CrossRef]
- Tian, S.; Ren, S.; Liu, Z.; Miao, Z.; Tian, L.; Li, J.; Liu, Y.; Wei, S.; Wang, P. ZnS/g-C3N4 heterojunction with Zn-vacancy for efficient hydrogen evolution in water splitting driven by visible light. Catal. Commun. 2022, 164, 106422. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Samanta, D.; Basnet, P.; Jha, S.; Chatterjee, S. Proficient route in synthesis of glucose stabilized Ag modified ZnS nanospheres for mechanistic understandings of commercially used dyes degradation. Inorg. Chem. Commun. 2022, 141, 109498. [Google Scholar] [CrossRef]
- Yu, D.; Fang, H.; Qiu, F.; Meng, F.; Liu, H.; Wang, S.; Lv, P.; Cong, X.; Niu, Q.; Li, T. Improving the Performance of ZnS Photocatalyst in Degrading Organic Pollutants by Constructing Composites with Ag2O. Nanomaterials 2021, 11, 1451. [Google Scholar] [CrossRef]
- Rodríguez, S.; Zandalazini, C.; Navarro, J.; Vadiraj, K.T.; Albanesi, E.A. First principles calculations and experimental study of the optical properties of Ni-doped ZnS. Mater. Res. Express 2020, 7, 016303. [Google Scholar] [CrossRef]
- Vaiano, V.; Sacco, O.; Barba, D.; Palma, V. Zinc Sulfide Prepared through ZnO Sulfuration: Characterization and Photocatalytic Activity. Chem. Eng. Trans. 2019, 74, 1159–1164. [Google Scholar] [CrossRef]
- Madkour, M.; Al Sagheer, F. Au/ZnS and Ag/ZnS nanoheterostructures as regenerated nanophotocatalysts for photocatalytic degradation of organic dyes. Opt. Mater. Express 2017, 7, 158–169. [Google Scholar] [CrossRef]
- Aulakh, M.K.; Dua, J.; Pal, B. Influence of capping agents on morphology and photocatalytic response of ZnS nanostructures towards crystal violet degradation under UV and sunlight. Sep. Purif. Technol. 2022, 281, 119869. [Google Scholar] [CrossRef]
- Ravikumar, S.; Mani, D.; Khan, M.R.; Ahmad, N.; Gajalakshmi, P.; Surya, C.; Durairaj, S.; Pandiyan, V.; Ahn, Y.-H. Effect of silver incorporation on the photocatalytic degradation of Reactive Red 120 using ZnS nanoparticles under UV and solar light irradiation. Environ. Res. 2022, 209, 112819. [Google Scholar] [CrossRef]
- Amani-Ghadim, A.R.; Arefi-Oskoui, S.; Mahmoudi, R.; Sareshkeh, A.T.; Khataee, A.; Khodam, F.; Dorraji, M.S.S. Improving photocatalytic activity of the ZnS QDs via lanthanide doping and photosensitizing with GO and g-C3N4 for degradation of an azo dye and bisphenol-A under visible light irradiation. Chemosphere 2022, 295, 133917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yang, Q.; Du, J.; Yin, P.; Yi, J.; Liu, Y.; Wu, X.; Zhang, Z. A Z-scheme CuO–ZnO–ZnS–CuS quaternary nanocomposite for solar-light-driven photocatalytic performance. Curr. Appl. Phys. 2022, 39, 113–121. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- Paydary, P.; Larese-Casanova, P. Water chemistry influences on long-term dissolution kinetics of CdSe/ZnS quantum dots. J. Environ. Sci. 2020, 90, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Wunderlich, W.; Kuchmizhak, A.A.; Kulinich, S.A. Preparation and Photocatalytic Properties of CdS and ZnS Nanomaterials Derived from Metal Xanthate. Materials 2019, 12, 3313. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Karthik, K.; Kuntalue, B.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Refuxing synthesis and characterization of ZnS nanoparticles and their photocatalytic properties. Chalcogenide Lett. 2019, 16, 387–393. [Google Scholar]
- Janani, B.; Okla, M.K.; Abdel-Maksoud, M.A.; AbdElgawad, H.; Thomas, A.M.; Raju, L.L.; Al-Qahtani, W.H.; Khan, S.S. CuO loaded ZnS nanoflower entrapped on PVA-chitosan matrix for boosted visible light photocatalysis for tetracycline degradation and anti-bacterial application. J. Environ. Manag. 2022, 306, 114396. [Google Scholar] [CrossRef]
- Wang, C.C.; Luo, J.; Liu, Z.Z.; Sun, S.H.; Zhu, H.; Hu, Y.M. High photocatalytic activity of ZnS@Ag8SnS6 nanocomposites: Preparation and investigation. Mater. Lett. 2022, 318, 132213. [Google Scholar] [CrossRef]
- Amuthan, T.; Sanjeevi, R.; Kannan, G.R.; Sridevi, A. A novel p-CuFe2O4/n-ZnS heterojunction photocatalyst: Co-precipitation synthesis, characterization and improved visible-light driven photocatalytic activity for removal of MB and CV dyes. Phys. B 2022, 638, 413842. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Optical and structural studies of ZnS nanoparticles synthesized via chemical in situ technique. Chem. Phys. Lett. 2016, 646, 69–74. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Kumar, K.Y. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Vaccaria, M.; Prasad, S.; van Hullebusch, E.D.; Rtimi, S. Preparation and applications of chitosan and cellulose composite materials. J. Environ. Manag. 2022, 301, 113850. [Google Scholar] [CrossRef] [PubMed]
- Isac, L.; Cazan, C.; Andronic, L.; Enesca, A. CuS-Based Nanostructures as Catalysts for Organic Pollutants Photodegradation. Catalysts 2022, 12, 1135. [Google Scholar] [CrossRef]
- Isac, L.; Cazan, C.; Enesca, A.; Andronic, L. Copper Sulfide Based Heterojunctions as Photocatalysts for Dyes Photodegradation. Front. Chem. 2019, 7, 694. [Google Scholar] [CrossRef]
- Kaur, S. A brief review on photocatalytic activity of ZnS and CQDs nanoparticles. Int. J. Eng. Appl. Sci. Technol. 2017, 2, 75–82. [Google Scholar]
- Yao, Y.; Sang, D.; Zou, L.; Wang, Q.; Liu, C. A Review on the Properties and Applications of WO3 Nanostructure-Based Optical and Electronic Devices. Nanomaterials 2021, 11, 2136. [Google Scholar] [CrossRef]
- Aslam, M.; Qamar, M.T.; Ali, S.; Rehman, A.U.; Soomro, M.T.; Ahmed, I.; Ismail, I.M.I.; Hameed, A. Evaluation of SnO2 for sunlight photocatalytic decontamination of water. J. Environ. Manag. 2018, 217, 805–814. [Google Scholar] [CrossRef]
- Tuckute, S.; Varnagiris, S.; Urbonavicius, M.; Lelis, M.; Sakalauskaite, S. Tailoring of TiO2 film crystal texture for higher photocatalysis efficiency. Appl. Surf. Sci. 2019, 489, 576–583. [Google Scholar] [CrossRef]
- Davis, K.; Yarbrough, R.; Froeschle, M.; Whitea, J.; Rathnayake, H. Band gap engineered zinc oxide nanostructures via a sol–gel synthesis of solvent driven shape-controlled crystal growth. RSC Adv. 2019, 9, 14638–14648. [Google Scholar] [CrossRef]
- Musa, I.; Qamhieh, N. Study of optical energy gap and quantum confinement effects in Zinc Oxide nanoparticles and nanorods. Dig. J. Nanomater. Biostructures. 2019, 14, 119–125. [Google Scholar]
- Galante, M.T.; Sotelo, P.; Hossain, M.K.; Vali, A.; Raamann, A.; Longo, C.; Macaluso, R.T.; Rajeshwar, K. Silver Oxide-Based Semiconductors for Solar Fuels Production and Environmental Remediation: A Solid-State Chemistry Approach. ChemElectroChem 2019, 6, 87–96. [Google Scholar] [CrossRef]
- Bhaduri, A.; Kajal, G. Facile synthesis and characterization of cupric oxide (CuO) nanoparticles: Inexpensive and abundant candidate for light harvesting. AIP Conf. Proc. 2019, 2093, 020047. [Google Scholar] [CrossRef]
- Zhoua, H.; Wang, S.; Jiang, J.; Shao, L.; Li, D.; Yuan, J.; Xu, F. Magnetic Fe3S4/MoS2 with visible-light response as an efficient photo-Fenton-like catalyst: Validation in degrading tetracycline hydrochloride under mild pH conditions. J. Alloys Compd. 2022, 921, 166023. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.; Chen, Y.; Luo, Y. Effective removal of tetracycline antibiotics from wastewater using practically applicable iron(III)-loaded cellulose nanofibres. R. Soc. Open Sci. 2021, 8, 210336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cai, W.; Rong, S.; Qu, H.; Xie, H. Hollow CuFe2O4/MgFe2O4 Heterojunction Boost Photocatalytic Oxidation Activity for Organic Pollutants. Catalysts 2022, 12, 910. [Google Scholar] [CrossRef]
- Boon-on, P.; Aragaw, A.A.; Lee, C.-Y.; Shican, J.B.; Lee, M.-W. Ag8SnS6: A new IR solar absorber material with a near optimal bandgap. RSC Adv. 2018, 8, 39470. [Google Scholar] [CrossRef]
- Upadhyay, D.; Joshi, N.; Jha, P.K. Two dimensional hexagonal GaOOH: A promising ultrawide bandgap semiconductor for smart optoelectronic applications. Chem. Phys. Lett. 2021, 765, 138310. [Google Scholar] [CrossRef]
- Zhou, L.; Zou, G.; Deng, H. The Roles of Graphene and Ag in the Hybrid Ag@Ag2O-Graphene for Sulfamethoxazole Degradation. Catalysts 2018, 8, 272. [Google Scholar] [CrossRef]
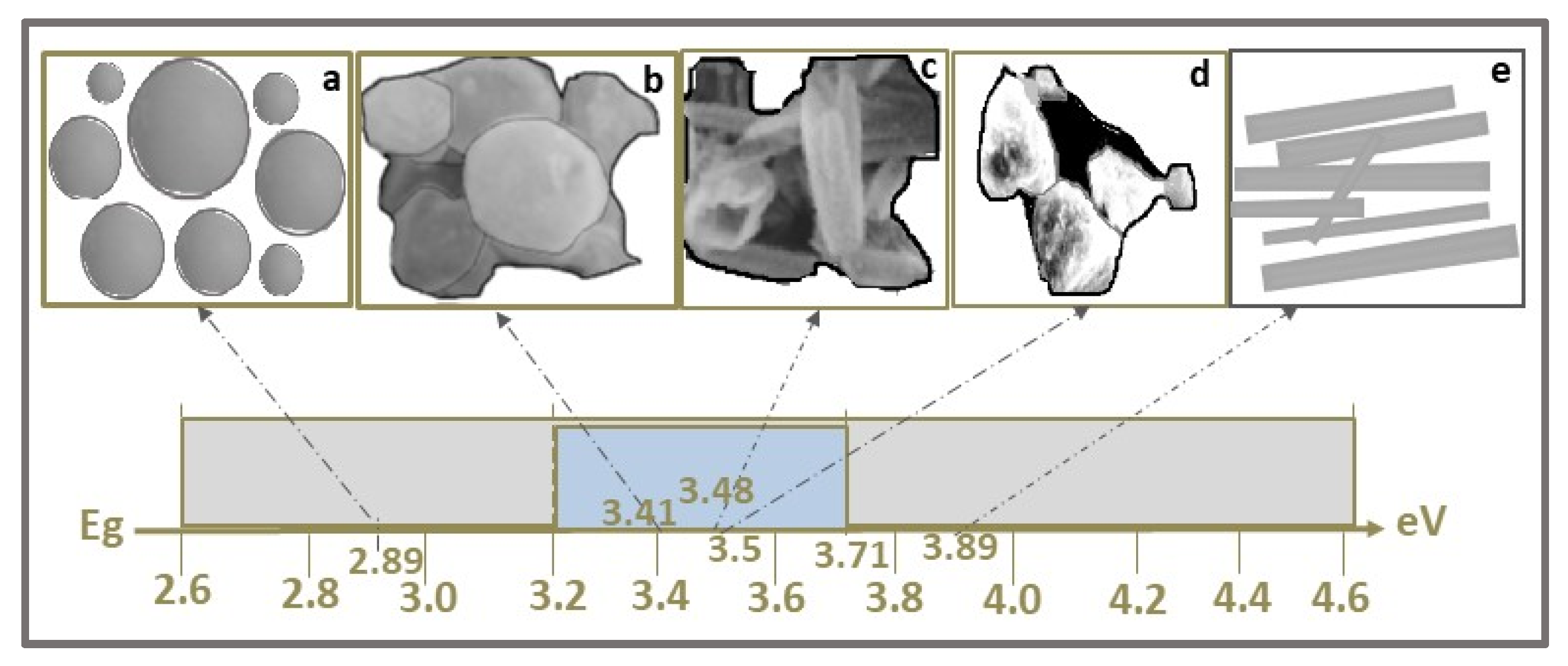
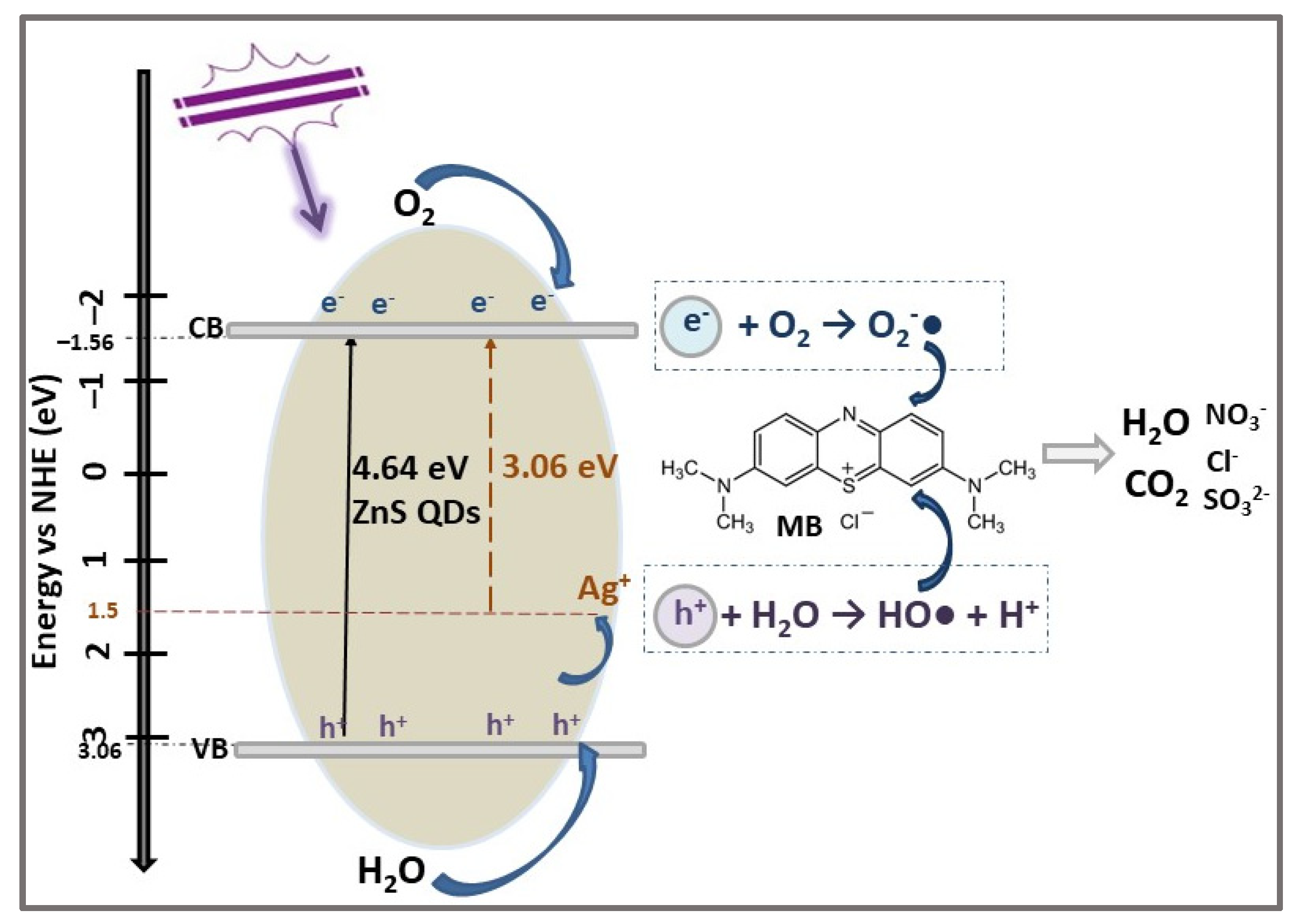
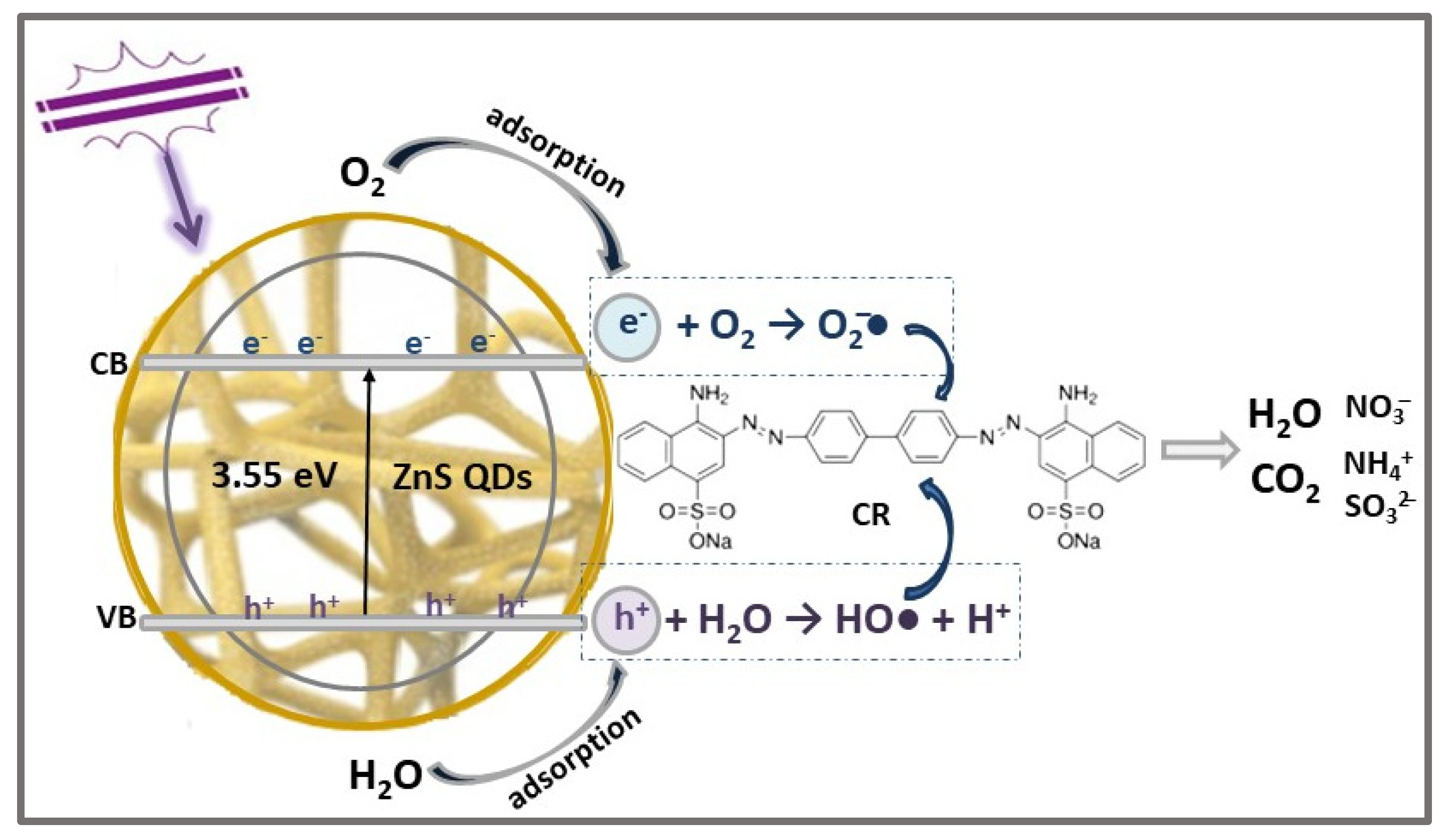
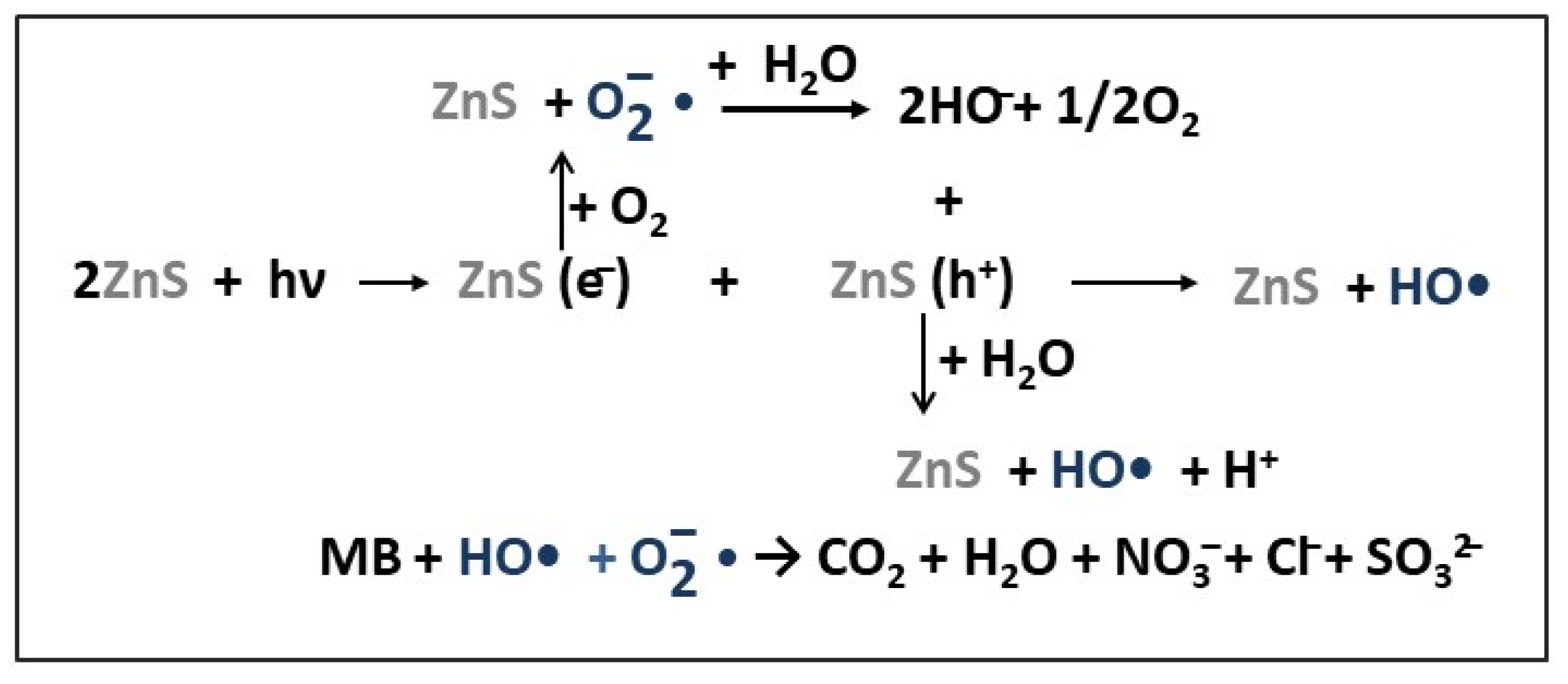

| Photocatalyst | Structure | Synthesis Method | Pollutant Conc. (mg/L) | Catalyst Dosage (g/L) | Light Source | η* (%) | t (min) | Ref. |
|---|---|---|---|---|---|---|---|---|
| ZnS Au-ZnS Ag-ZnS | 0D NsS QDs | HT | MB (30) | 0.05 | UV (6 W lamp) | 72.5 96.7 92.6 | 180 | [48] |
| ZnS Mn-ZnS | 0D NsS QDs | Refluxing | TO (12.65) | 0.5 g | UV (8 W lamp) | 92.6 94 | 30 90 | [3] |
| ZnS Gd-ZnS | 0D NsS QDs | Refluxing | AR14 (20) | 0.2 | UV | 85.4 91.1 | 180 | [51] |
| ZnCCSs | ZnS QDs | HT | CR (50) | 0.2 | UV (350 W Hg lamp) | 95.94 | 120 | [6] |
| ZnS | 0D NsS QDs | PP at mild temperatures (60–80 °C) | MB (2.6) | 0.1 | VIS (1000 W halogen lamp) | 61 | 180 | [55] |
| ZnS | 1D NSs nanospheres | Capping free HT | MB (12) | 0.1 | UV | 96 | 240 | [24] |
| ZnS | 1D NSs nanospheres | Refluxing | MB (3.2) MO (3.27) | 1 | UV (three black light lamps) | 96.73 94.68 | 120 | [56] |
| ZnS | 1D NSs nanosheets short nanotubes nanotubes | Substitution reaction using ZnO as templates | MB (10) | 0.2 | UV (30 W lamp) | 96 92 88 | 300 | [38] |
| ZnS | 1D NSs | Sulfuration of ZnO NPs obtained by PP | PNP (10) | 1.5 | UV (12 W/m2 LEDs) | 51 | 240 | [47] |
| ZnS | 1D NSs nanorods nanospheres | Refluxing using different capping agents + calcination | CV (10.2) 2-NP(10.2) | 2 | UV (Hg arc, 125 W) Solar Solar | 54–93 28–88 83 | 180 | [49] |
| ZnS Ag-ZnS | 1D NSs spherical balls | HT | RR120 (50) DB15 (50) AB1 (50) | 0.1 | UV-A Solar UV-A Solar | 71–77 75–87 80–83 94 | 120 | [50] |
| ZnS Ag-ZnS | 1D NSs nanospheres | HT | RhB (2.4) MB (1.6) | 1 | Solar | 77 86 84 93 | 120 | [44] |
| ZnS | 1D NSs ZnS NPs | Lyotropic liquid crystal template | MB (20) | 0.4 | VIS light (300 W Xe lamp) | 99.76 | 150 | [19] |
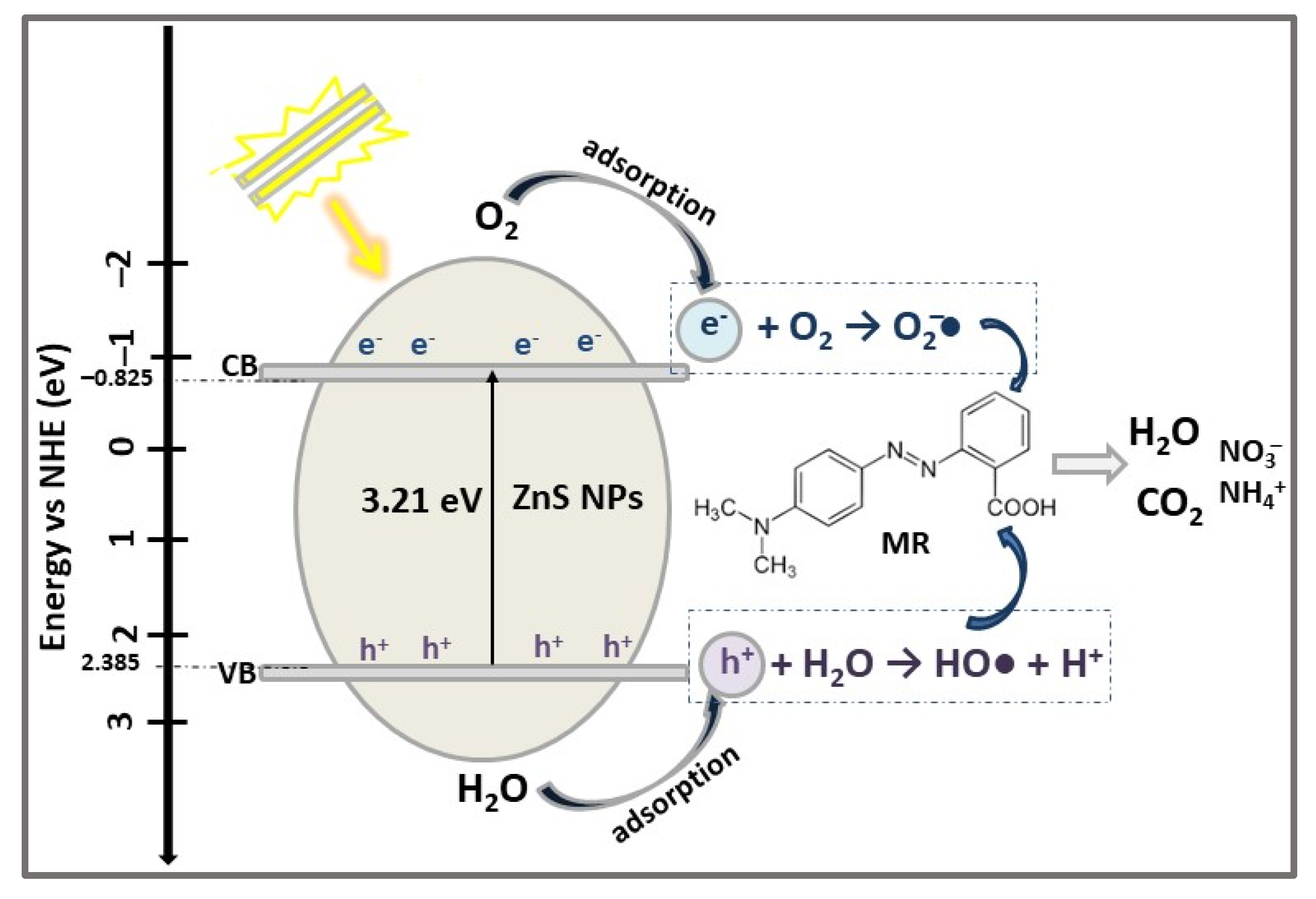
| ZnS | 1D NSs ZnS NPs | PP | MB (50) XO (50) MO (50) MR (50) | 0.05 | VIS (18 W lamp) | 78.41 81.22 90.90 95.10 | 120 | [53] |
| ZnS | 1D NSs ZnS NPs | PP | CR (30) | 0.2 | Vis (45 W halogen lamp) | 98.78 | 90 | [5] |
| ZnS | 3D NSS ZnS urchin-like particles | HT | MO (3.75) | 0.7 | UV-C (Philips lamps) | 80 | 60 | [37] |
| ZnS/TiO2 | HNSs n-n heterojunction | Chemical deposition | AB113 (25) | 0.185 | UV-A (400 W Kr UV lamp) | 99.2 | 27 | [30] |
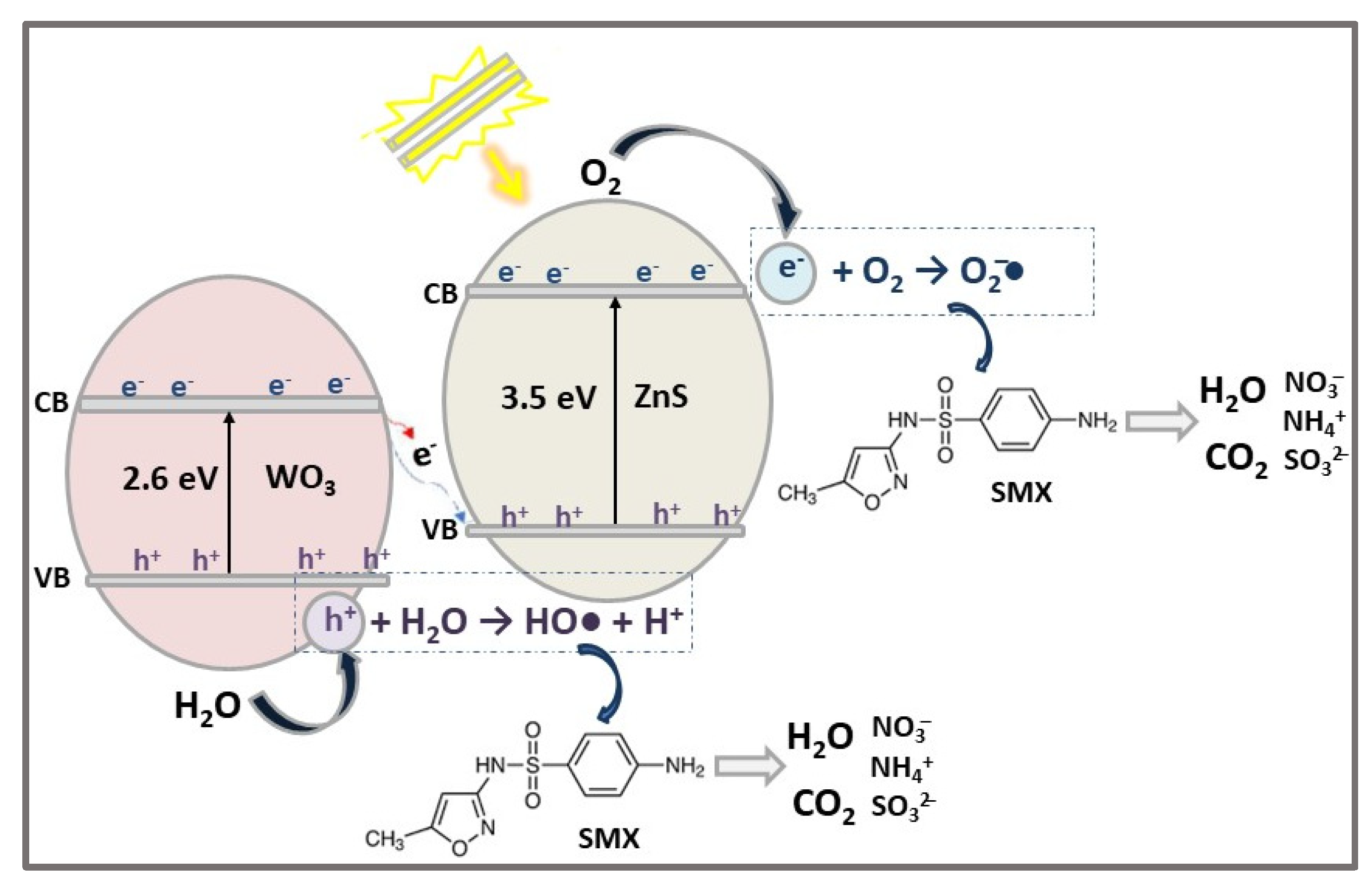
| ZnS/WO3 | HNSs n-n heterojunction | CoPP | SMX (20) | 0.5 | VIS (Sunset XLS + solar simulator) | 95 | 60 | [33] |
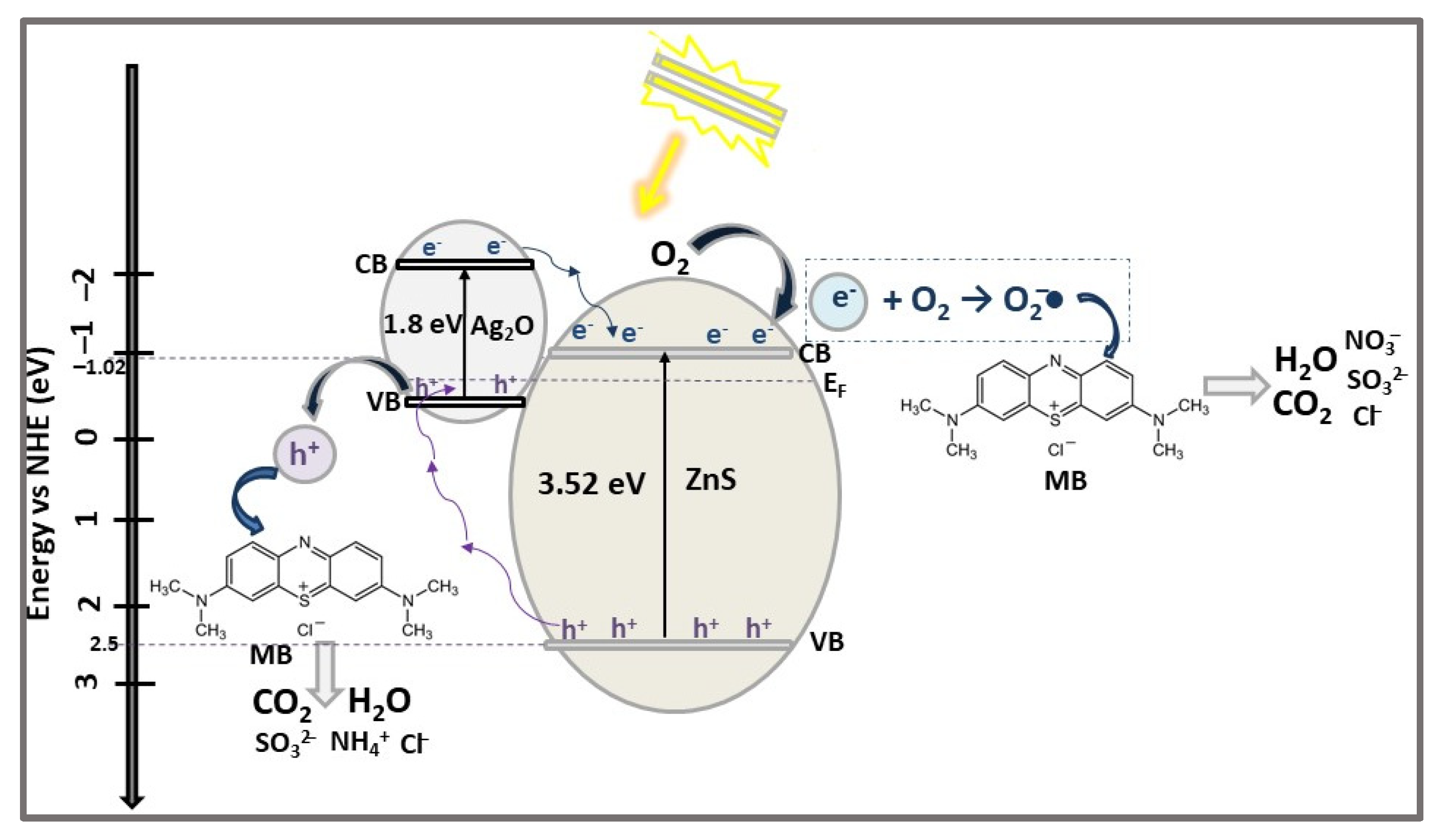
| ZnS/Ag2O | HNSs n-p heterojunction | PP | MB (10) | 0.5 | VIS (300 W Xe lamp) | 92.4 | 50 | [45] |
| ZnS-CuO/PVA/Chit | HNSs ZnS-CuO n-p heterojunction | CoPP | TC (25) | 0.5 | VIS (800 W tungsten halogen lamp) | 94.7 | 180 | [57] |
| ZnS/ATS | HNSs n-p heterojunction | One step heating | MB (5) | 1.5 | VIS (400 W metal halide lamp) | 85.5 | 300 | [58] |
| ZnS/CuS/pC | HNSs n-p heterojunction | Vacuum evaporation+ SILAR | MB (20) | 0.5 | Vis (300W UV lamp) | 98 | 60 | [11] |
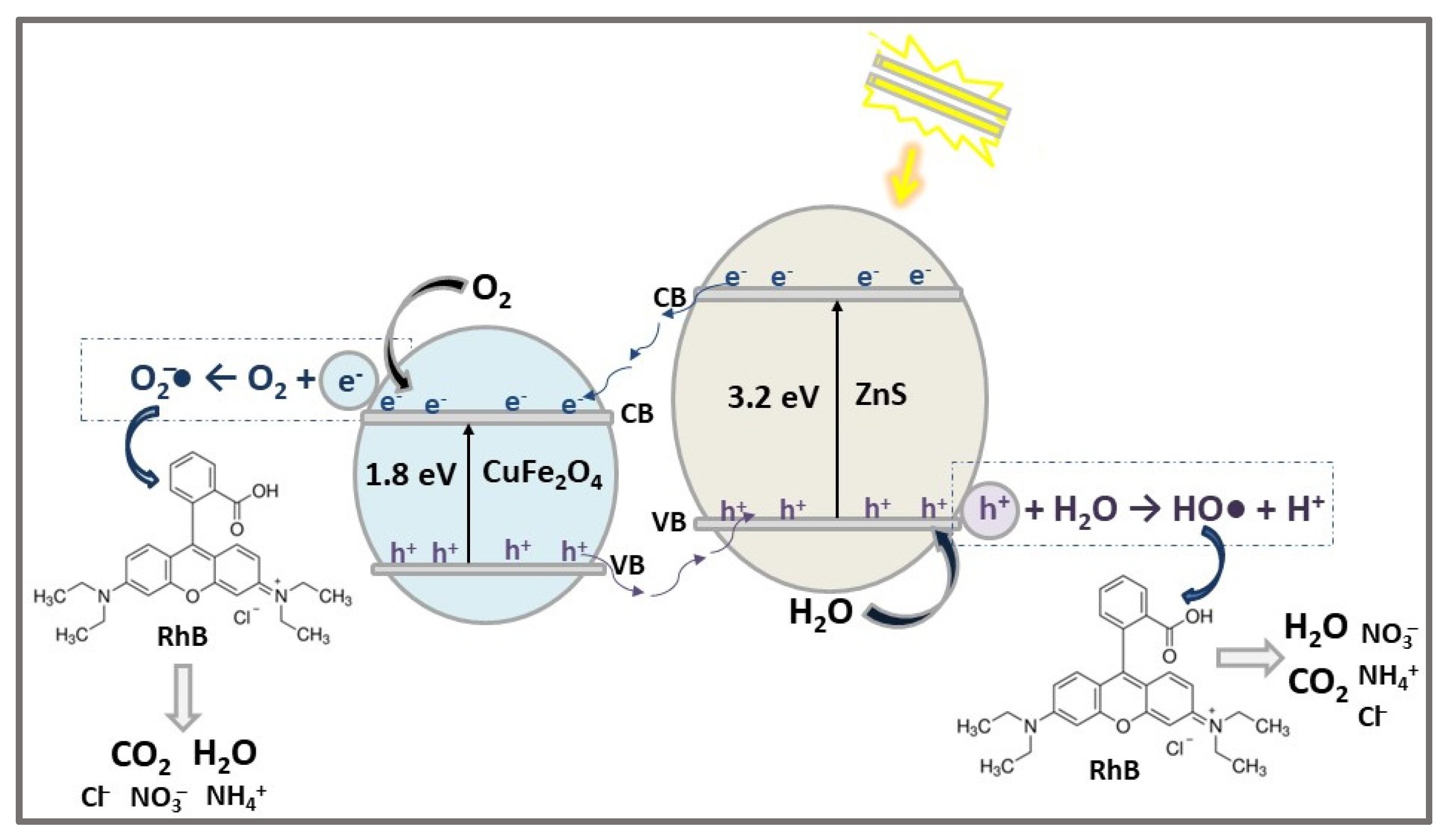
| ZnS/CuFe2O4 | HNSs n-p heterojunction | CoPP | MB (30) CV (30) | 0.01 | VIS (200 W tungsten halogen light) | 82 87 | 120 | [59] |
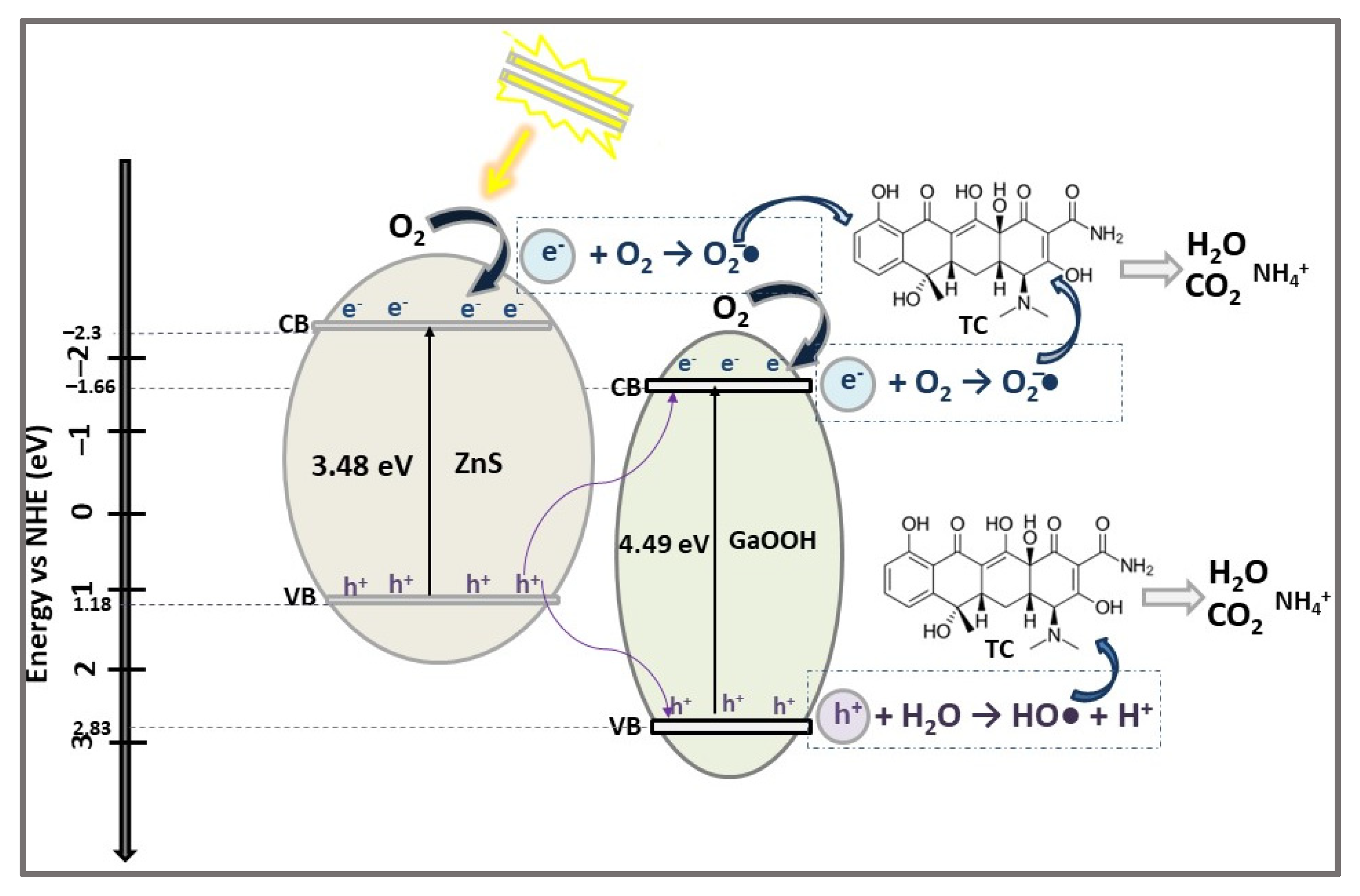
| ZnS/GaOOH | HNSs Z-scheme heterojunction | HT | TC (20) CIP (20) LF (20) RhB (5) | 0.4 0.2 | VIS light (300 W Xe lamp) | 86.4 31.6 20.3 89.8 | 150 60 5 | [20] |
| Photocatalyst | Eg (eV) | Particle Size (nm) | Photodegradation | ||||
|---|---|---|---|---|---|---|---|
| Dye | η* (%) | Time (min) | Ref. | ||||
| Chemical Formula and Structure | λmax (nm) | ||||||
| ZnS QDs | 4 | 4.2 | Methylene Blue, MB C16H18N3ClS 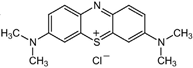 | 665 | 61 (Vis) | 180 | [55] |
| ZnS QDs | 4.64 | 4.3 | 72.5 | 180 | [48] | ||
| Ag-ZnS QDs | 3.06 | 4.7 | 92.6 | ||||
| Au-ZnS QDs | 2.4 | 5.6 | 96.7 | ||||
| ZnS QDs | 3.7 | 21–44 | Acid red 14, AR14 C20H12N2Na2O7S2  | 515 | 85.4 | 180 | [51] |
| Gd-ZnS QDs | 3.45 | 20–34 | 91.1 | ||||
| ZnS QDs | 3.7 | ~4 | Tropaeolin O, TO C12H9N2NaO5S 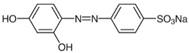 | 426 | 92.6 | 30 | [3] |
| Mn-ZnS QDs | 3.7 | ~4 | 94 | 90 | |||
| ZnCCSs | 3.55 | 15 | Congo Red, CR C32H22N6Na2O6S2  | 510 | 95.94 | 120 | [6] |
| ZnS NPs | 3.21 | 40 | Methylene Blue, MB C16H18N3ClS  | 665 | 78.4 (Vis) | 120 | [53] |
| Xylenol orange, XO C31H32N2O13S 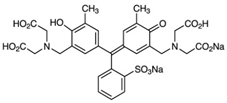 | 583 | 81.2 (Vis) | |||||
| Methyl Orange, MO C14H14N3NaO3S 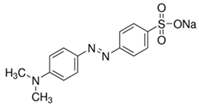 | 463 | 90.9 (Vis) | |||||
| Methyl Red (acid red 2), MR C15H15N3O2  | 425 | 95.1 (Vis) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isac, L.; Enesca, A. Recent Developments in ZnS-Based Nanostructures Photocatalysts for Wastewater Treatment. Int. J. Mol. Sci. 2022, 23, 15668. https://doi.org/10.3390/ijms232415668
Isac L, Enesca A. Recent Developments in ZnS-Based Nanostructures Photocatalysts for Wastewater Treatment. International Journal of Molecular Sciences. 2022; 23(24):15668. https://doi.org/10.3390/ijms232415668
Chicago/Turabian StyleIsac, Luminita, and Alexandru Enesca. 2022. "Recent Developments in ZnS-Based Nanostructures Photocatalysts for Wastewater Treatment" International Journal of Molecular Sciences 23, no. 24: 15668. https://doi.org/10.3390/ijms232415668
APA StyleIsac, L., & Enesca, A. (2022). Recent Developments in ZnS-Based Nanostructures Photocatalysts for Wastewater Treatment. International Journal of Molecular Sciences, 23(24), 15668. https://doi.org/10.3390/ijms232415668







