Step-by-Step Regeneration of Tentacles after Injury in Anemonia viridis—Morphological and Structural Cell Analyses
Abstract
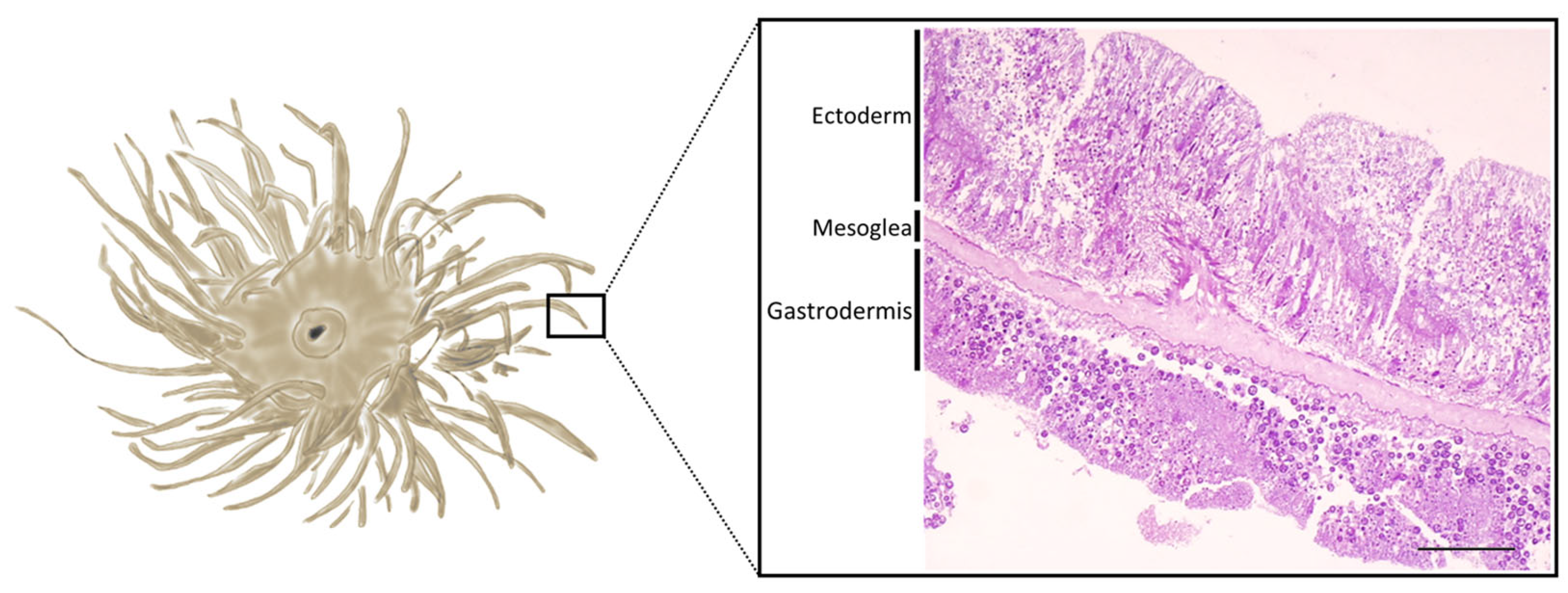
1. Introduction
2. Results
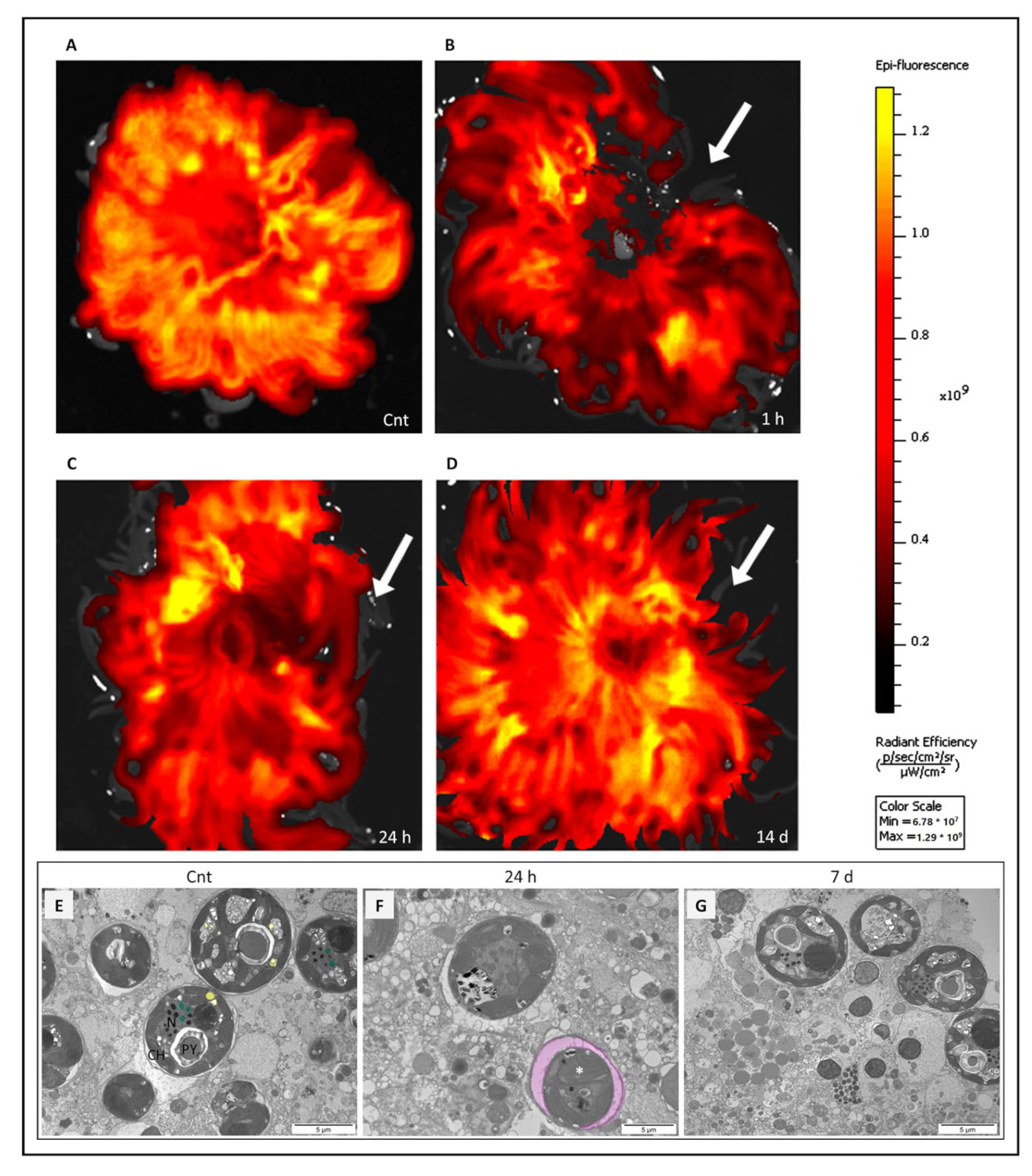
2.1. Positron Emission Tomography (PET) Analysis
2.2. Cell Measurements
2.3. Morphological Analyses and Characterisation of Cells Involved in Tentacle Regrowth in Injured A. viridis
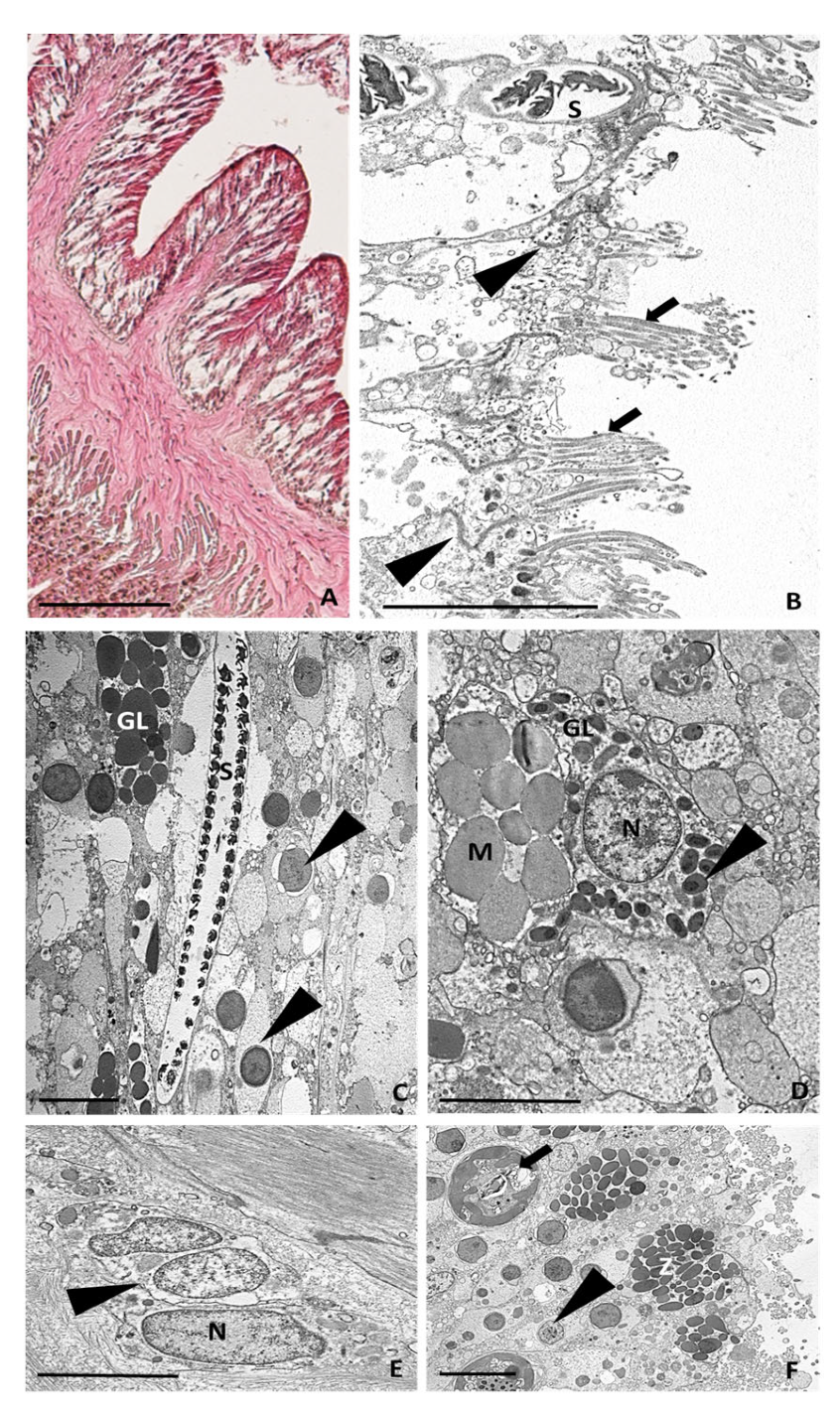
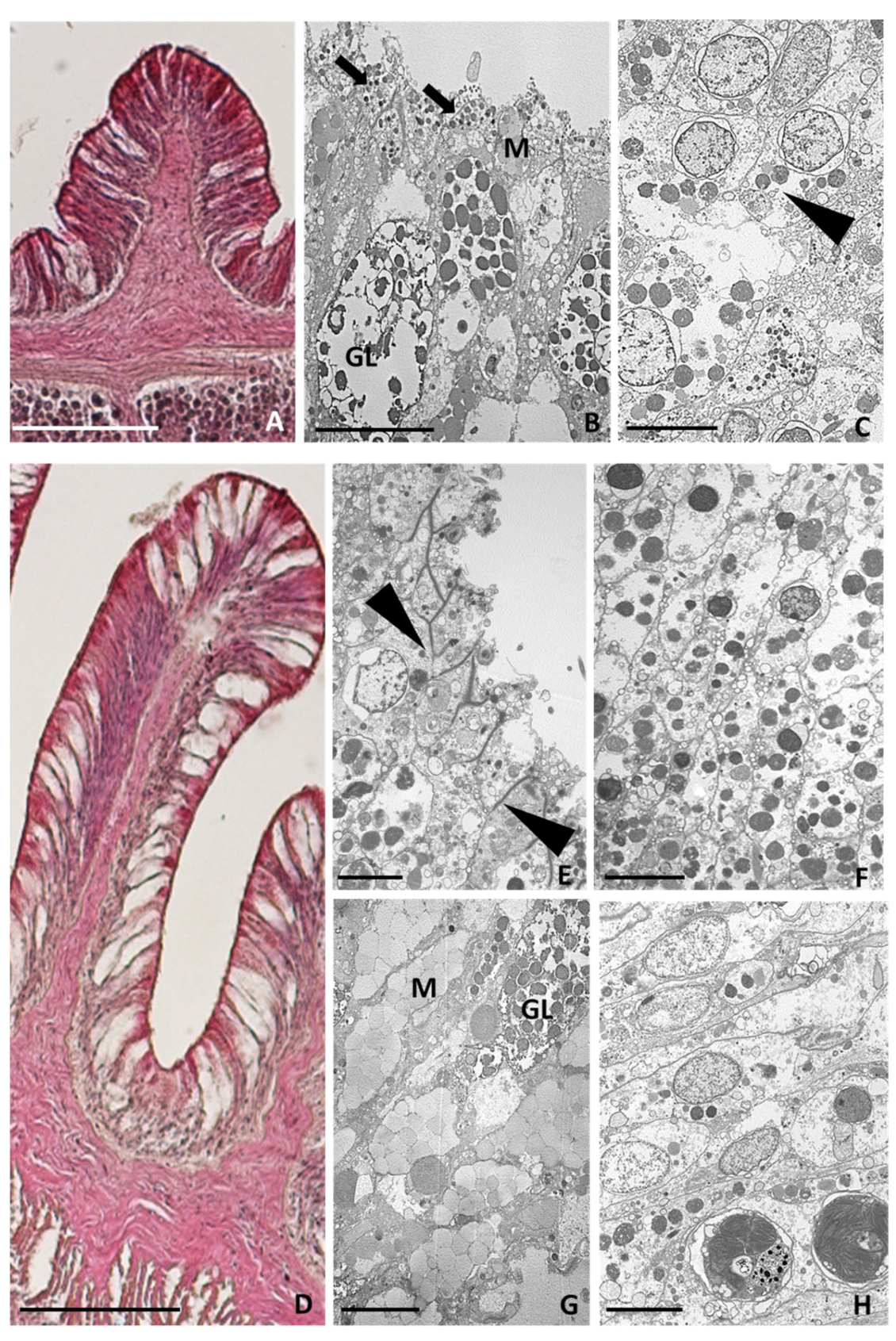
2.3.1. Healthy Tentacles
2.3.2. Tentacle Amputation at Time Zero
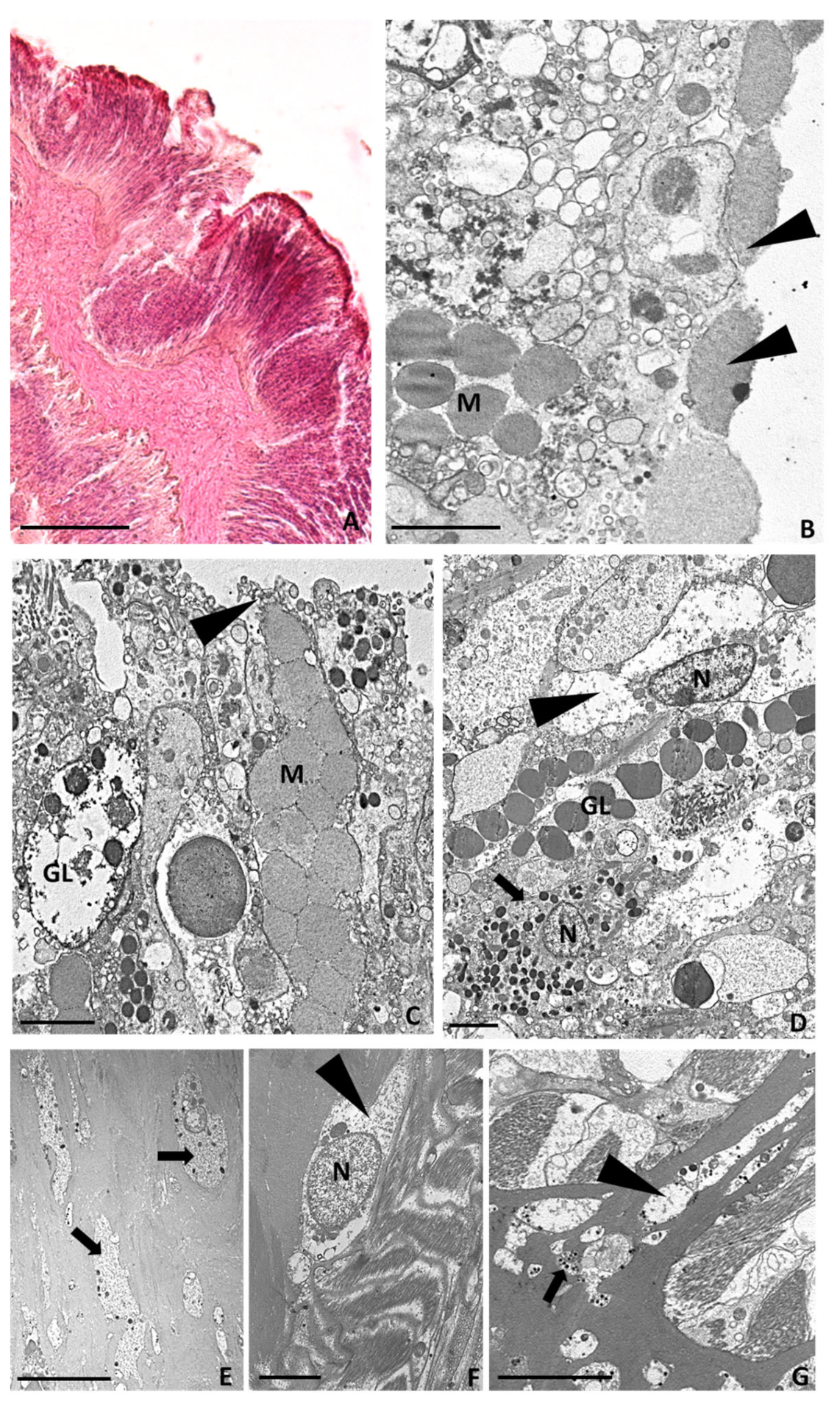
2.3.3. Six Hours after Tentacle Amputation
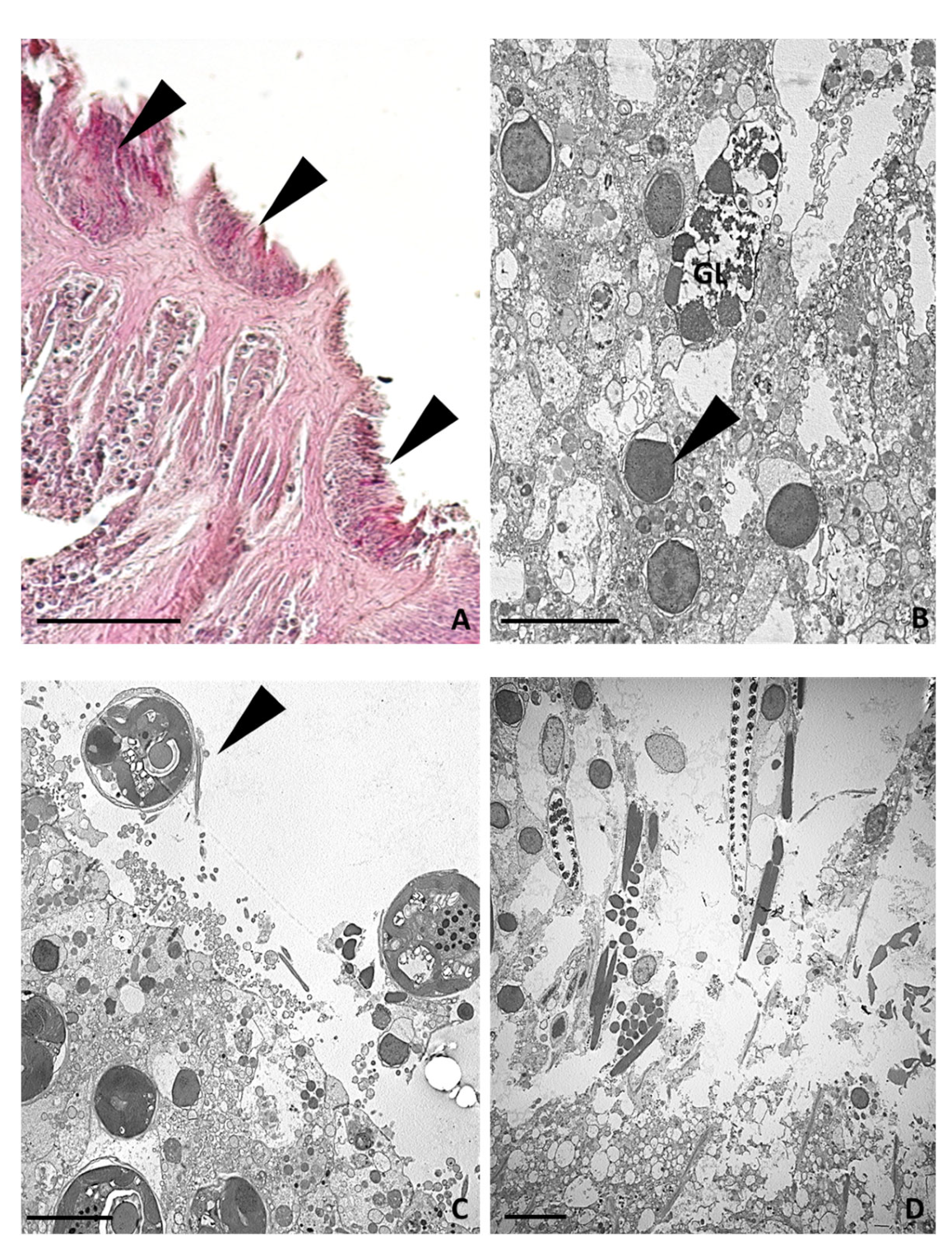
2.3.4. Twenty-Four Hours after Tentacle Amputation
2.3.5. Seven Days after Tentacle Amputation
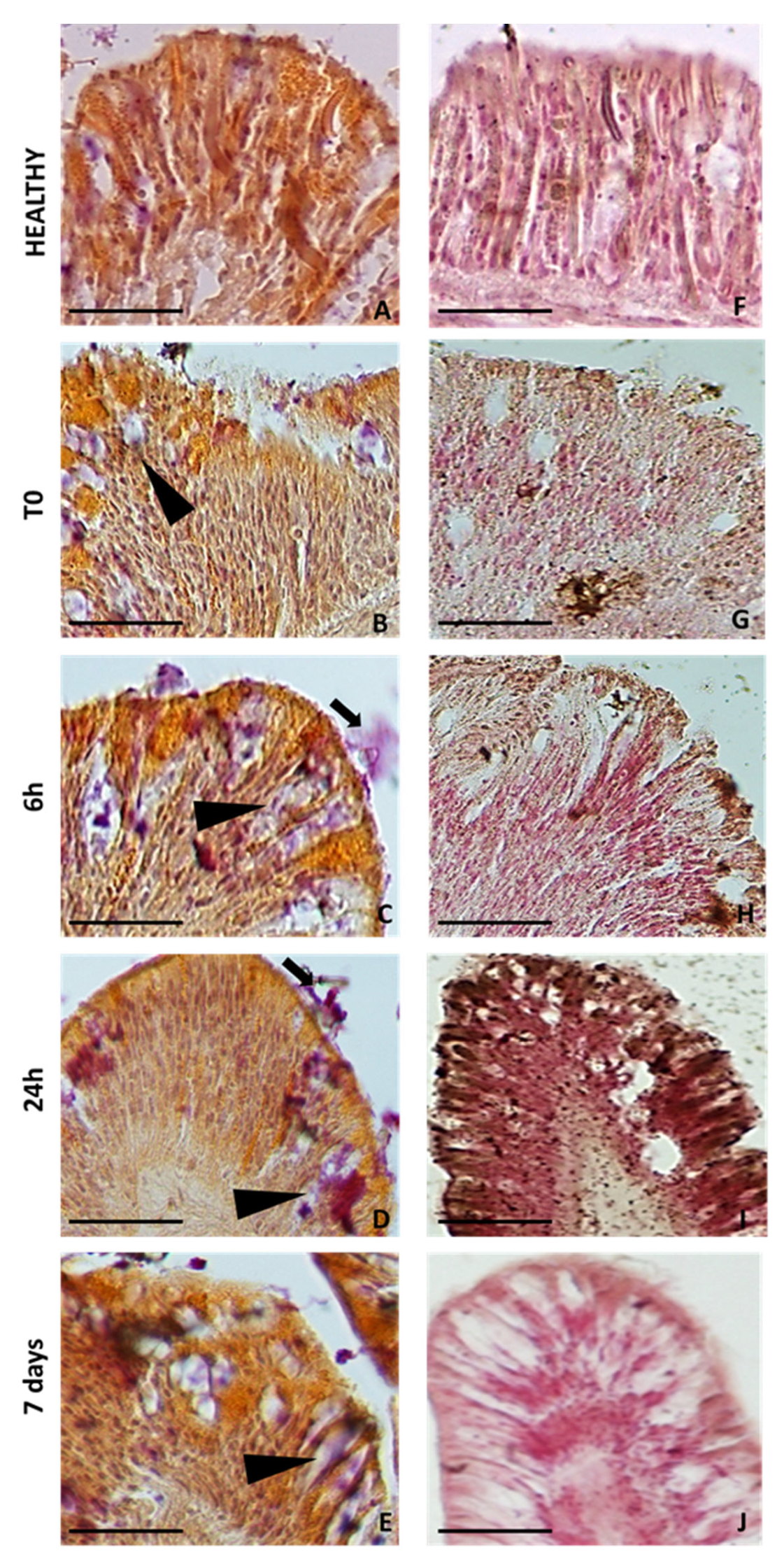
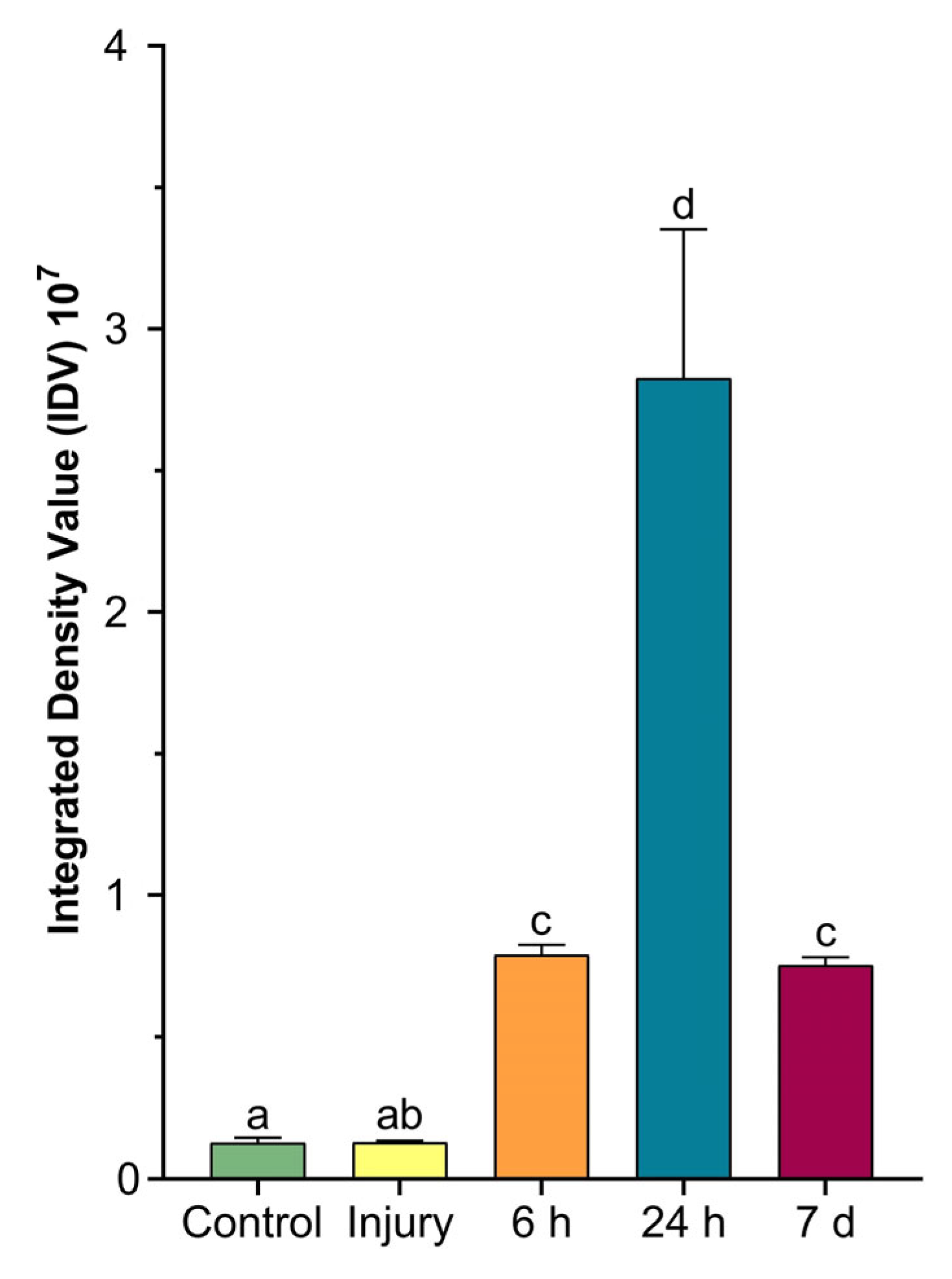
2.4. Colorimetric Analyses of Cells Involved in Wound Healing
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Positron Emission Tomography (PET)
4.3. Light and Transmission Electron Microscopy (TEM)
4.3.1. Embedding Tissue in Paraffin and Staining
4.3.2. Embedding Tissue in Epoxy Resin for TEM
4.4. Biometric Measures
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisanti, L.; de Sabata, E.; Visconti, G.; Chemello, R. Towards a Local Mass Mortality of the Mediterranean Orange Coral Astroides calycularis (Pallas, 1766) in the Pelagie Islands Marine Protected Area (Italy). Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 551–557. [Google Scholar] [CrossRef]
- Boström-Einarsson, L.; Babcock, R.C.; Bayraktarov, E.; Ceccarelli, D.; Cook, N.; Ferse, S.C.A.; Hancock, B.; Harrison, P.; Hein, M.; Shaver, E.; et al. Coral Restoration—A Systematic Review of Current Methods, Successes, Failures and Future Directions. PLoS ONE 2020, 15, e0226631. [Google Scholar] [CrossRef]
- Raymundo, L.J.; Licuanan, W.L.; Kerr, A.M. Adding Insult to Injury: Ship Groundings Are Associated with Coral Disease in a Pristine Reef. PLoS ONE 2018, 13, e0202939. [Google Scholar] [CrossRef] [PubMed]
- Young, J.A. The Nature of Tissue Regeneration after Wounding in the Sea Anemone Calliactis parasitica (Couch). J. Mar. Biol. Assoc. 1974, 54, 599–617. [Google Scholar] [CrossRef]
- Parisi, M.G.; Grimaldi, A.; Baranzini, N.; La Corte, C.; Dara, M.; Parrinello, D.; Cammarata, M. Mesoglea Extracellular Matrix Reorganization during Regenerative Process in Anemonia viridis (Forskål, 1775). Int. J. Mol. Sci. 2021, 22, 5971. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, A.; Bigger, C. Qualitative and Quantitative Study of Wound Healing Processes in the Coelenterate, Plexaurella fusifera: Spatial, Temporal, and Environmental (Light Attenuation) Influences. J. Invertebr. Pathol. 1999, 73, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Van De Water, J.A.J.M.; Ainsworth, T.D.; Leggat, W.; Bourne, D.G.; Willis, B.L.; Van Oppen, M.J.H. The Coral Immune Response Facilitates Protection against Microbes during Tissue Regeneration. Mol. Ecol. 2015, 24, 3390–3404. [Google Scholar] [CrossRef]
- Work, T.M.; Aeby, G.S. Wound Repair in Montipora capitata. J. Invertebr. Pathol. 2010, 105, 116–119. [Google Scholar] [CrossRef]
- La Corte, C.; Baranzini, N.; Grimaldi, A.; Parisi, M.G. Invertebrate Models in Innate Immunity and Tissue Remodeling Research. Int. J. Mol. Sci. 2022, 23, 6843. [Google Scholar] [CrossRef]
- Dinsmore, C.E. A History of Regeneration Research: Milestones in the Evolution of a Science; Cambridge University Press: Cambridge, UK, 2007; ISBN 052104796X. [Google Scholar]
- Bely, A.E.; Nyberg, K.G. Evolution of Animal Regeneration: Re-Emergence of a Field. Trends Ecol. Evol. 2010, 25, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Alvarado, A.; Tsonis, P.A. Bridging the Regeneration Gap: Genetic Insights from Diverse Animal Models. Nat. Rev. Genet. 2006, 7, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Arenas Gómez, C.M.; Sabin, K.Z.; Echeverri, K. Wound Healing across the Animal Kingdom: Crosstalk between the Immune System and the Extracellular Matrix. Dev. Dyn. 2020, 249, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound Healing: Biologic Features and Approaches to Maximize Healing Trajectories. Curr. Probl. Surg. 2001, 38, A1. [Google Scholar] [CrossRef]
- Loof, T.G.; Schmidt, O.; Herwald, H.; Theopold, U. Coagulation Systems of Invertebrates and Vertebrates and Their Roles in Innate Immunity: The Same Side of Two Coins? J. Innate Immun. 2011, 3, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Traylor-Knowles, N. Heat Stress Compromises Epithelial Integrity in the Coral, Acropora hyacinthus. PeerJ 2019, 2019, e6510. [Google Scholar] [CrossRef]
- Baranzini, N.; Weiss-Gayet, M.; Chazaud, B.; Monti, L.; de Eguileor, M.; Tettamanti, G.; Acquati, F.; Grimaldi, A. Recombinant HvRNASET2 Protein Induces Marked Connective Tissue Remodelling in the Invertebrate Model Hirudo verbana. Cell Tissue Res. 2020, 380, 565–579. [Google Scholar] [CrossRef]
- Tettamanti, G.; Grimaldi, A.; Rinaldi, L.; Arnaboldi, F.; Congiu, T.; Valvassori, R.; de Eguileor, M. The Multifunctional Role of Fibroblasts during Wound Healing in Hirudo medicinalis (Annelida, Hirudinea). Biol. Cell 2004, 96, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.G.; Baranzini, N.; Dara, M.; Corte, C.L.; Vizioli, J.; Cammarata, M. AIF-1 and RNASET2 Are Involved in the Inflammatory Response in the Mediterranean Mussel Mytilus galloprovincialis Following Vibrio Infection. Fish Shellfish Immunol. 2022, 127, 109–118. [Google Scholar] [CrossRef]
- Bowes, D.; Corrin, B. Ultrastructural Immunocytochemical Localisation of Lysozyme in Human Bronchial Glands. Thorax 1977, 32, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Fautin, D.G.; Mariscal, R.N. Cnidaria: Anthozoa. In Microscopic Anatomy of Invertebrates: Placozoa, Porifera, Cnidaria, and Ctenophora; Harrison, F.W., Westfall, J.A., Eds.; Microscopic Anatomy of Invertebrates; Wiley-Liss: New York, NY, USA, 1991; pp. 267–358. ISBN 9780471561132. [Google Scholar]
- Palmer, C.V.; Bythell, J.C.; Willis, B.L. Levels of Immunity Parameters Underpin Bleaching and Disease Susceptibility of Reef Corals. FASEB J. 2010, 24, 1935–1946. [Google Scholar] [CrossRef]
- Palmer, C.V.; Traylor-Knowles, N.G.; Willis, B.L.; Bythell, J.C. Corals Use Similar Immune Cells and Wound-Healing Processes as Those of Higher Organisms. PLoS ONE 2011, 6, e23992. [Google Scholar] [CrossRef] [PubMed]
- Parisi, M.G.; Trapani, M.R.; Cammarata, M. Granulocytes of Sea Anemone Actinia equina (Linnaeus, 1758) Body Fluid Contain and Release Cytolysins Forming Plaques of Lysis. Invertebr. Surviv. J. 2014, 11, 39–46. [Google Scholar]
- Mydlarz, L.D.; Holthouse, S.F.; Peters, E.C.; Harvell, C.D. Cellular Responses in Sea Fan Corals: Granular Amoebocytes React to Pathogen and Climate Stressors. PLoS ONE 2008, 3, e1811. [Google Scholar] [CrossRef]
- Calisi, A.; Lionetto, M.G.; Caricato, R.; Giordano, M.E.; Schettino, T. Morphometric Alterations In Mytilus galloprovincialis Granulocytes: A New Biomarker. Environ. Toxicol. Chem. 2008, 27, 1435–1441. [Google Scholar] [CrossRef]
- Di Falco, F.; Cammarata, M.; Vizzini, A. Molecular Characterisation, Evolution and Expression Analysis of g-Type Lysozymes in Ciona intestinalis. Dev. Comp. Immunol. 2017, 67, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Coaglio, A.L.; Ferreira, M.A.N.D.; Dos Santos Lima, W.; De Jesus Pereira, C.A. Identification of a Phenoloxidase- and Melanin-Dependent Defence Mechanism in Achatina fulica Infected with Angiostrongylus vasorum. Parasites Vectors 2018, 11, 113. [Google Scholar] [CrossRef]
- Vargas-Ángel, B.; Peters, E.C.; Kramarsky-Winter, E.; Gilliam, D.S.; Dodge, R.E. Cellular Reactions to Sedimentation and Temperature Stress in the Caribbean Coral Montastraea cavernosa. J. Invertebr. Pathol. 2007, 95, 140–145. [Google Scholar] [CrossRef]
- de Lima, F.M.R.; Abrahão, I.; Pentagna, N.; Carneiro, K. Gradual Specialization of Phagocytic Ameboid Cells May Have Impaired Regenerative Capacities in Metazoan Lineages. Dev. Dyn. 2023, 252, 343–362. [Google Scholar] [CrossRef]
- Ferrario, C.; Ben Khadra, Y.; Czarkwiani, A.; Zakrzewski, A.; Martinez, P.; Colombo, G.; Bonasoro, F.; Candia Carnevali, M.D.; Oliveri, P.; Sugni, M. Fundamental Aspects of Arm Repair Phase in Two Echinoderm Models. Dev. Biol. 2018, 433, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Menzel, L.P.; Tondo, C.; Stein, B.; Bigger, C.H. Histology and Ultrastructure of the Coenenchyme of the Octocoral Swiftia eserta, a Model Organism for Innate Immunity/Graft Rejection. Zoology 2015, 118, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Jahnel, S.M.; Walzl, M.; Technau, U. Development and Epithelial Organisation of Muscle Cells in the Sea Anemone Nematostella vectensis. Front. Zool. 2014, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Cirino, L.; Tsai, S.; Wang, L.H.; Hsieh, W.C.; Huang, C.L.; Wen, Z.H.; Lin, C. Effects of Cryopreservation on the Ultrastructure of Coral Larvae. Coral Reefs 2022, 41, 131–147. [Google Scholar] [CrossRef]
- Raskin, C.A. Apoptosis and Cutaneous Biology. J. Am. Acad. Dermatol. 1997, 36, 885–896. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. The Role of Apoptosis in Wound Healing. Int. J. Biochem. Cell Biol. 1998, 30, 1019–1030. [Google Scholar] [CrossRef]
- Fransolet, D.; Roberty, S.; Herman, A.C.; Tonk, L.; Hoegh-Guldberg, O.; Plumier, J.C. Increased Cell Proliferation and Mucocyte Density in the Sea Anemone Aiptasia pallida Recovering from Bleaching. PLoS ONE 2013, 8, e65015. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Corte, C.; Baranzini, N.; Dara, M.; Bon, C.; Grimaldi, A.; Parisi, M.G.; Zizzo, M.G.; Cammarata, M. Step-by-Step Regeneration of Tentacles after Injury in Anemonia viridis—Morphological and Structural Cell Analyses. Int. J. Mol. Sci. 2023, 24, 8860. https://doi.org/10.3390/ijms24108860
La Corte C, Baranzini N, Dara M, Bon C, Grimaldi A, Parisi MG, Zizzo MG, Cammarata M. Step-by-Step Regeneration of Tentacles after Injury in Anemonia viridis—Morphological and Structural Cell Analyses. International Journal of Molecular Sciences. 2023; 24(10):8860. https://doi.org/10.3390/ijms24108860
Chicago/Turabian StyleLa Corte, Claudia, Nicolò Baranzini, Mariano Dara, Camilla Bon, Annalisa Grimaldi, Maria Giovanna Parisi, Maria Grazia Zizzo, and Matteo Cammarata. 2023. "Step-by-Step Regeneration of Tentacles after Injury in Anemonia viridis—Morphological and Structural Cell Analyses" International Journal of Molecular Sciences 24, no. 10: 8860. https://doi.org/10.3390/ijms24108860
APA StyleLa Corte, C., Baranzini, N., Dara, M., Bon, C., Grimaldi, A., Parisi, M. G., Zizzo, M. G., & Cammarata, M. (2023). Step-by-Step Regeneration of Tentacles after Injury in Anemonia viridis—Morphological and Structural Cell Analyses. International Journal of Molecular Sciences, 24(10), 8860. https://doi.org/10.3390/ijms24108860








