Lavandula Species, Their Bioactive Phytochemicals, and Their Biosynthetic Regulation
Abstract
1. Introduction
2. Lavender Genetic Diversity
3. Lavender Distribution and Occurrence
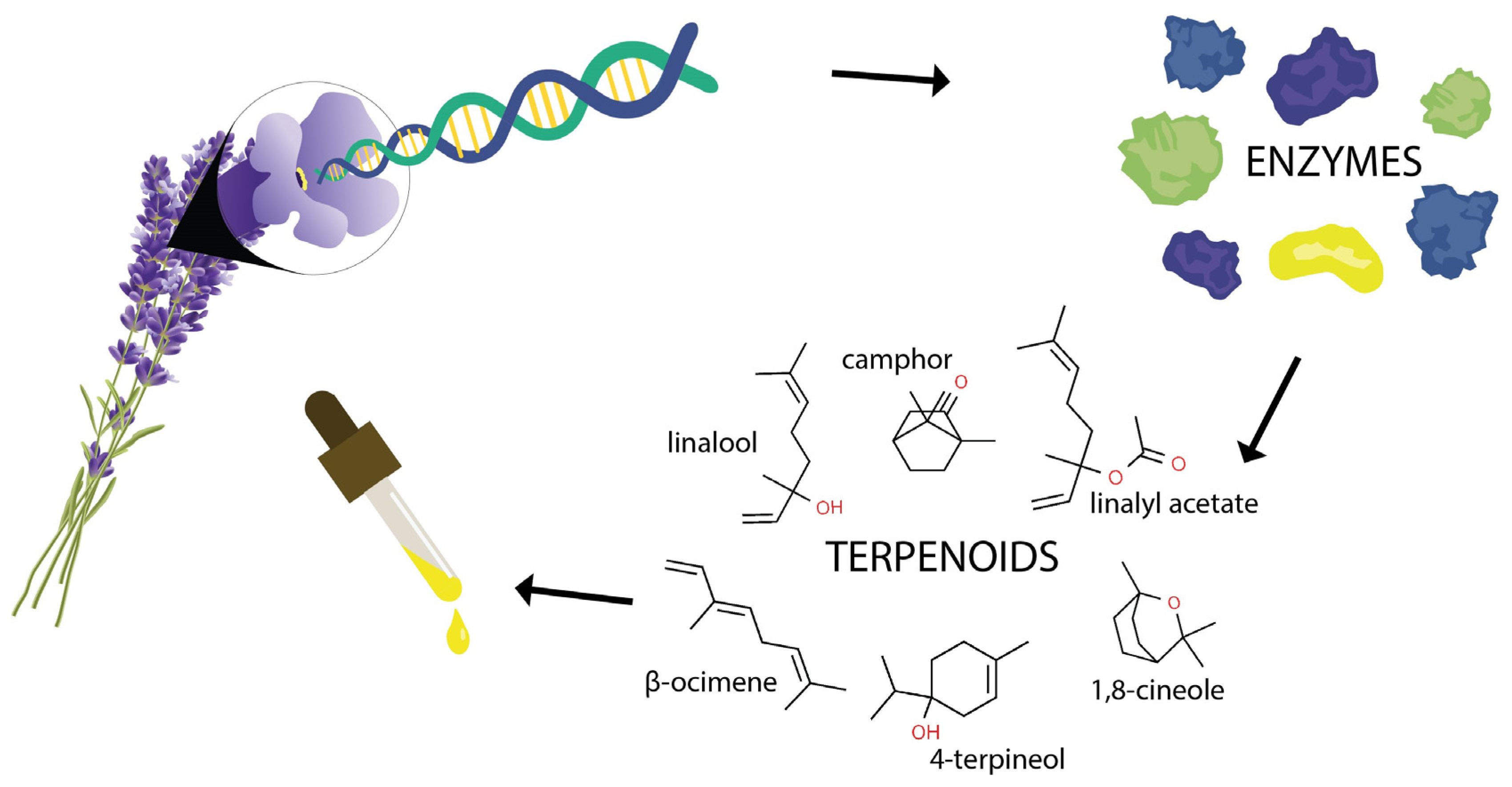
4. Lavender Bioactive Phytochemicals and Their Effects
5. Biosynthesis Regulation of Lavender Bioactive Phytochemicals
5.1. Biosynthesis of Secondary Metabolites in Lavender
5.2. Gene-Based Regulation of Lamiaceae Species Secondary Metabolites
5.3. The Role of Transcription Factors and microRNAs in the Biosynthesis of Secondary Metabolites
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Upson, T.; Andrews, S.; Harriott, G. The Genus Lavandula; Royal Botanic Gardens Kew: Richmond, UK, 2004; 442p, ISBN 1842460102. [Google Scholar]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Dinis, A.C.; Canhoto, J.M.; Salgueiro, L.R. Chemical composition and antifungal activity of the essential oils of Lavandula pedunculata (Miller) Cav. Chem. Biodivers. 2009, 6, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Hassanpouraghdam, M.B.; Hassani, A.; Vojodi, L.; Asl, B.H.; Rostami, A. Essential oil constituents of Lavandula officinalis Chaix from Northwest Iran. Chemija 2011, 22, 167–171. [Google Scholar]
- Herraiz-Peñalver, D.; Cases, M.Á.; Varela, F.; Navarrete, P. Chemical characterization of Lavandula latifolia Medik. essential oil from Spanish wild populations. Biochem. Syst. Ecol. 2013, 46, 59–68. [Google Scholar] [CrossRef]
- Cavanagh, H.M.A.; Wilkinson, J.M. Biological Activities of Lavender Essential Oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Woronuk, G.; Demissie, Z.; Rheault, M.; Mahmoud, S. Biosynthesis and therapeutic properties of Lavandula essential oil constituents. Planta Med. 2011, 77, 7–15. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Cella, M.A.; Sarker, L.S.; Thompson, T.J.; Rheault, M.R.; Mahmoud, S.S. Cloning, functional characterization and genomic organization of 1, 8-cineole synthases from Lavandula. Plant Mol. Biol. 2012, 79, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.; Boecklemann, A.; Woronuk, G.N.; Sarker, L.; Mahmoud, S.S. A genomics resource for investigating regulation of essential oil production in Lavandula angustifolia. Planta 2010, 231, 835–845. [Google Scholar] [CrossRef]
- Muñoz-Bertomeu, J.; Arrillaga, I.; Segura, J. Essential oil variation within and among natural populations of Lavandula latifolia and its relation to their ecological areas. Biochem. Syst. Ecol. 2007, 35, 479–488. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A Review of the Bioactive Components and Pharmacological Properties of Lavandula Species. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef]
- Gismondi, A.; Di Marco, G.; Redi, E.L.; Ferrucci, L.; Cantonetti, M.; Canini, A. The Antimicrobial Activity of Lavandula Angustifolia Mill. Essential Oil against Staphylococcus Species in a Hospital Environment. J. Herb. Med. 2021, 26, 100426. [Google Scholar] [CrossRef]
- Bénédicte, H.; Stierlin, E.; Fernandez, X.; Michel, T. Phytochemicals from the Genus Lavandula: A Review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- Dupont, F.; Guignard, J.L. Botanique: Les Familes de Plantes; Elsevier Masson: Paris, France, 2015. [Google Scholar]
- Simpson, M.G. Plant Systematics. Syst. Bot. 2020, 45, 409. [Google Scholar] [CrossRef]
- Serrato-Valenti, G.; Bisio, A.; Cornara, L.; Ciarallo, G. Structural and Histochemical Investigation of the Glandular Trichomes of Salvia aurea L. Leaves, and Chemical Analysis of the Essential Oil. Ann. Bot. 1997, 79, 329–336. [Google Scholar] [CrossRef]
- Crișan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current Trends for Lavender (Lavandula angustifolia Mill.) Crops and Products with Emphasis on Essential Oil Quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.L.; Ding, P. Production of Essential Oil in Plants: Ontogeny, Secretory Structures and Seasonal Variations. Pertanika J. Sch. Res. Rev. 2016, 2, 1–10. [Google Scholar]
- Maurya, S.; Chandra, M.; Yadav, R.K.; Narnoliya, L.K.; Sangwan, R.S.; Bansal, S.; Sandhu, P.; Singh, U.; Kumar, D.; Sangwan, N.S. Interspecies Comparative Features of Trichomes in Ocimum Reveal Insights for Biosynthesis of Specialized Essential Oil Metabolites. Protoplasma 2019, 256, 893–907. [Google Scholar] [CrossRef]
- Peter, K.V. Handbook of Herbs and Spices: Volume 2; Elsevier Science: Amsterdam, The Netherlands, 2004; ISBN 978-1-85573-721-1. [Google Scholar]
- Passalacqua, N.G.; Tundis, R.; Upson, T.M. A New Species of Lavandula Sect. Lavandula (Lamiaceae) and Review of Species Boundaries in Lavandula Angustifolia. Phytotaxa 2017, 292, 161. [Google Scholar] [CrossRef]
- Guitton, Y.; Nicolè, F.; Moja, S.; Valot, N.; Legrand, S.; Jullien, F.; Legendre, L. Differential Accumulation of Volatile Terpene and Terpene Synthase MRNAs during Lavender (Lavandula Angustifolia and L. x Intermedia) Inflorescence Development. Physiol. Plant. 2010, 138, 150–163. [Google Scholar] [CrossRef]
- Moja, S.; Guitton, Y.; Nicolè, F.; Legendre, L.; Pasquier, B.; Upson, T.; Jullien, F. Genome Size and Plastid TrnK-MatK Markers Give New Insights into the Evolutionary History of the Genus Lavandula L. Plant Biosyst.-Int. J. Deal. Asp. Plant Biol. 2016, 150, 1216–1224. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical Composition of the Essential Oil of the New Cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-0-203-21652-1. [Google Scholar]
- Jianu, C.; Pop, G.; Lukinich-Gruia, A.; Horhat, F. Chemical Composition and Antimicrobial Activity of Essential Oils of Lavender (Lavandula angustifolia) and Lavandin (Lavandula x intermedia) Grown in Western Romania. Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Demasi, S.; Caser, M.; Lonati, M.; Cioni, P.L.; Pistelli, L.; Najar, B.; Scariot, V. Latitude and Altitude Influence Secondary Metabolite Production in Peripheral Alpine Populations of the Mediterranean Species Lavandula angustifolia Mill. Front. Plant Sci. 2018, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Stanev, S.; Zagorcheva, T.; Atanassov, I. Lavender Cultivation in Bulgaria—21st Century Developments, Breeding Challenges and Opportunities. Bulg. J. Agric. Sci. 2016, 22, 584–590. [Google Scholar]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, S.V.; Miron, A. Essential Oils of Lavandula Genus: A Systematic Review of Their Chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Govaerts, R.; Paton, A.; Harvey, Y.; Navarro, T.; Del Rosario García Peña, M. World Checklist of Lamiaceae; The Royal Botanic Gardens, Kew: Richmond, UK, 2020; Available online: http://wcsp.science.kew.org/ (accessed on 30 April 2023).
- Ez Zoubi, Y.; Bousta, D.; Farah, A. A Phytopharmacological Review of a Mediterranean Plant: Lavandula stoechas L. Clin. Phytosci 2020, 6, 9. [Google Scholar] [CrossRef]
- Van Oost, E.; Leus, L.; De Rybel, B.; Van Laere, K. Determination of Genetic Distance, Genome Size and Chromosome Numbers to Support Breeding in Ornamental Lavandula Species. Agronomy 2021, 11, 2173. [Google Scholar] [CrossRef]
- Stefanaki, A.; Andel, T. Mediterranean Aromatic Herbs and Their Culinary Use. In Aromatic Herbs in Food; Academic Press: Cambridge, MA, USA, 2021; pp. 93–121. ISBN 978-0-12-822716-9. [Google Scholar]
- Pistelli, L.; Najar, B.; Giovanelli, S.; Lorenzini, L.; Tavarini, S.; Angelini, L.G. Agronomic and Phytochemical Evaluation of Lavandin and Lavender Cultivars Cultivated in the Tyrrhenian Area of Tuscany (Italy). Ind. Crops Prod. 2017, 109, 37–44. [Google Scholar] [CrossRef]
- Georgieva, R.; Kirchev, H.; Delibaltova, V.; Chavdarov, P.; Uhr, Z. Investigation of Some Agricultural Performances of Lavender Varieties. Üzüncü Üniversitesi J. Agric. Sci. 2021, 31, 170–178. [Google Scholar] [CrossRef]
- Piskernik, S.; Jeršek, M.; Klančnik, A.; Smole Možina, S.; Bucar, F.; Jeršek, B. Chemical composition and antimicrobial activity of essential oils made from Lavandula x intermedia from Hvar (Croatia). Nat. Prod. Res. 2022, 1–5. [Google Scholar] [CrossRef]
- Andrzejewska, J.; Pisulewska, E. Cultivation of Herbal Plants (Uprawa roślin zielarskich); Wydawnictwo Uniwersytetu Technologiczno-Przyrodniczego w Bydgoszczy: Bydgoszcz, Poland, 2019; p. 346. ISBN 978-83-65603-92-0. [Google Scholar]
- Seidler-Łożykowska, K.; Mordalski, R.; Kucharski, W.; Kędzia, B.; Bocianowski, J. Yielding and quality of lavender flowers (Lavandula angustifolia Mill.) from organic cultivation. Acta Sci. Pol. 2014, 13, 173–183. [Google Scholar]
- Habán, M. Medicinal, aromatic and spice plants (4). Their acquisition, collection, processing and use in the Slovak Republic. Liečivé Rastl.–Léčivé Rostl. 2022, 59, 158–160. [Google Scholar]
- Kocourková, B.; Pluháčková, H.; Minařík, J.; Šmirous, P. Pěstování léčivých, aromatických a kořeninových rostlin v České republice. Medicinal, aromatic and spice plants. In Proceedings of Peer-Reviewed Scientific Papers and Abstracts, ISCMASP; Comenius University Bratislava: Bratislava, Slovakia, 2022; pp. 29–33. ISBN 978-80-223-3595-3. [Google Scholar]
- Détár, E.; Zámbori-Németh, É.; Gosztola, B.; Harmath, A.; Ladányi, M.; Pluhár, Z. Ontogenesis and Harvest Time Are Crucial for High Quality Lavender—Role of the Flower Development in Essential Oil Properties. Ind. Crops Prod. 2021, 163, 113334. [Google Scholar] [CrossRef]
- Hernandez-Leon, A.; Moreno-Pérez, G.F.; Martínez-Gordillo, M.; Aguirre-Hernández, E.; Valle-Dorado, M.G.; Díaz-Reval, M.I.; González-Trujano, M.E.; Pellicer, F. Lamiaceae in Mexican Species, a Great but Scarcely Explored Source of Secondary Metabolites with Potential Pharmacological Effects in Pain Relief. Molecules 2021, 26, 7632. [Google Scholar] [CrossRef] [PubMed]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S.; et al. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef]
- Vârban, R.; Vidican, R.; Ona, A.D.; Vârban, D.; Stoie, A.; Gâdea, Ș.; Vâtcă, S.; Stoian, V.; Crișan, I.; Stoian, V. Modelling Plant Morphometric Parameters as Predictors for Successful Cultivation of Some Medicinal Agastache Species. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12638. [Google Scholar] [CrossRef]
- Bader, S.B. The Lavender Lover’s Handbook: The 100 Most Beautiful and Fragrant Varieties for Growing, Crafting, and Cooking; Timber Press: Portland, ON, USA, 2012; ISBN 978-1-60469-399-7. [Google Scholar]
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender Essential Oil: A Review. Aust. Infect. Control 2005, 10, 35–37. [Google Scholar] [CrossRef]
- Lupoae, D.; Alexe, P.; Stănciuc, N. Overview on the Potential Role of Phytochemicals from Lavender as Functional Ingredients. Ann. Univ. Dunarea Galati. Fascicle VI-Food Technol. 2020, 44, 173–188. [Google Scholar] [CrossRef]
- Eldeghedy, H.I.; El-Gendy, A.E.-N.G.; Nassrallah, A.A.; Aboul-Enein, A.M.; Omer, E.A. Comparative Chemical Profiles of Lavandula Species Essential Oils Grown in Egypt and Others from France and Australia: Evidence from Chemometric Analysis. J. Essent. Oil Bear. Plants 2022, 25, 52–63. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P.; Bouloumpasi, E.; Biliaderis, C.G. Phenolic Extracts from Solid Wastes of the Aromatic Plant Essential Oil Industry: Potential Uses in Food Applications. Food Chem. Adv. 2022, 1, 100065. [Google Scholar] [CrossRef]
- Vasileva, I.; Denkova, R.; Chochkov, R.; Teneva, D.; Denkova, Z.; Dessev, T.; Denev, P.; Slavov, A. Effect of Lavender (Lavandula angustifolia) and Melissa (Melissa officinalis) Waste on Quality and Shelf Life of Bread. Food Chem. 2018, 253, 13–21. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the Physical Properties, Antioxidant and Antimicrobial Activity of Ternary Potato Starch-Furcellaran-Gelatin Films Incorporated with Lavender Essential Oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.; Truong, F.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. Lavandula Essential Oils: A Current Review of Applications in Medicinal, Food, and Cosmetic Industries of Lavender. Nat. Prod. Commun. 2018, 13, 1934578X1801301038. [Google Scholar] [CrossRef]
- Basch, E.; Foppa, I.; Liebowitz, R.; Nelson, J.; Smith, M.; Sollars, D.; Ulbricht, C. Lavender (Lavandula angustifolia Miller). J. Herb. Pharmacother. 2009, 4, 63–78. [Google Scholar] [CrossRef]
- Kara, N.; Baydar, H. Determination of Lavender and Lavandin Cultivars (Lavandula sp.) Containing High Quality Essential Oil in Isparta, Turkey. Turk. J. Field Crops 2013, 18, 58–65. [Google Scholar]
- Marovska, G.; Vasileva, I.; Petkova, N.; Ognyanov, M.; Gandova, V.; Stoyanova, A.; Merdzhanov, P.; Simitchiev, A.; Slavov, A. Lavender (Lavandula angustifolia Mill.) Industrial by-Products as a Source of Polysaccharides. Ind. Crops Prod. 2022, 188, 115678. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.; Stobiecka, A.; Kunicka-Styczyńska, A. Hydrolates from Lavender (Lavandula angustifolia)—Their Chemical Composition as Well as Aromatic, Antimicrobial and Antioxidant Properties. Nat. Prod. Res. 2016, 30, 386–393. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Prusinowska, R.; Krosowiak, K.; Sikora, M. Comparison of Qualitative and Quantitative Chemical Composition of Hydrolate and Essential Oils of Lavender (Lavandula angustifolia). J. Essent. Oil Res. 2013, 25, 291–299. [Google Scholar] [CrossRef]
- Rathore, S.; Kumar, R. Essential Oil Content and Compositional Variability of Lavandula Species Cultivated in the Mid Hill Conditions of the Western Himalaya. Molecules 2022, 27, 3391. [Google Scholar] [CrossRef]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The Effects of Lavender Essential Oil on Wound Healing: A Review of the Current Evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef]
- El Abdali, Y.; Agour, A.; Allali, A.; Bourhia, M.; El Moussaoui, A.; Eloutassi, N.; Salamatullah, A.M.; Alzahrani, A.; Ouahmane, L.; Aboul-Soud, M.A.M.; et al. Lavandula dentata L.: Phytochemical Analysis, Antioxidant, Antifungal and Insecticidal Activities of Its Essential Oil. Plants 2022, 11, 311. [Google Scholar] [CrossRef]
- Prusinowska, R.; Śmigielski, K.B. Composition, Biological Properties and Therapeutic Effects of Lavender (Lavandula Angustifolia L). A Review. Herba Pol. 2014, 60, 56–66. [Google Scholar] [CrossRef]
- Ghanimi, R.; Ouhammou, A.; Atki, Y.E.; Cherkaoui, M. Antioxidant and Antibacterial Activities of Essential Oils from Three Moroccan Species (Lavandula Mairei Humbert, Lavandula dentata L. and, Lavandula stoechas L.). J. Pharm. Res. Int. 2021, 33, 64–71. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula Angustifolia and Lavandula x Intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef]
- Sayout, A.; Ouarhach, A.; Dilagui, I.; Soraa, N.; Romane, A. Antibacterial Activity and Chemical Composition of Essential Oil from Lavandula Tenuisecta Coss.Ex Ball. an Endemic Species from Morocco. Eur. J. Integr. Med. 2020, 33, 101017. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula Luisieri and Lavandula Pedunculata Essential Oils. Plants 2022, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Lesage-Meessen, L.; Bou, M.; Sigoillot, J.-C.; Faulds, C.B.; Lomascolo, A. Essential Oils and Distilled Straws of Lavender and Lavandin: A Review of Current Use and Potential Application in White Biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef]
- Pardede, J.; Simanjuntak, G.V.; Manalu, N. Effectiveness of Deep Breath Relaxation and Lavender Aromatherapy against Preoperative Patent Anxiety. Divers. Equal. Health Care 2020, 17, 168–173. [Google Scholar]
- Malli, R.P.N.; Adal, A.M.; Sarker, L.S.; Liang, P.; Mahmoud, S.S. De Novo Sequencing of the Lavandula Angustifolia Genome Reveals Highly Duplicated and Optimized Features for Essential Oil Production. Planta 2019, 249, 251–256. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Dong, Y.; Zhang, W.; Wang, D.; Bai, H.; Li, K.; Li, H.; Shi, L. The Chromosome-Based Lavender Genome Provides New Insights into Lamiaceae Evolution and Terpenoid Biosynthesis. Hortic. Res. 2021, 8, 53. [Google Scholar] [CrossRef]
- Guillén, M.D.; Cabo, N. Burillo, and Jesus Characterisation of the Essential Oils of Some Cultivated Aromatic Plants of Industrial Interest. J. Sci. Food Agric. 1996, 70, 359–363. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M.; Boronat, A. Elucidation of the Methylerythritol Phosphate Pathway for Isoprenoid Biosynthesis in Bacteria and Plastids. A Metabolic Milestone Achieved through Genomics. Plant Physiol. 2002, 130, 1079–1089. [Google Scholar] [CrossRef]
- Rodríguez-Concepción, M. Early Steps in Isoprenoid Biosynthesis: Multilevel Regulation of the Supply of Common Precursors in Plant Cells. Phytochem. Rev. 2006, 5, 1–15. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef]
- Guo, D.; Kang, K.; Wang, P.; Li, M.; Huang, X. Transcriptome Profiling of Spike Provides Expression Features of Genes Related to Terpene Biosynthesis in Lavender. Sci. Rep. 2020, 10, 6933. [Google Scholar] [CrossRef]
- Schuhr, C.A.; Radykewicz, T.; Sagner, S.; Latzel, C.; Zenk, M.H.; Arigoni, D.; Bacher, A.; Rohdich, F.; Eisenreich, W. Quantitative Assessment of Crosstalk between the Two Isoprenoid Biosynthesis Pathways in Plants by NMR Spectroscopy. Phytochem. Rev. 2003, 2, 3–16. [Google Scholar] [CrossRef]
- Dudareva, N.; Andersson, S.; Orlova, I.; Gatto, N.; Reichelt, M.; Rhodes, D.; Boland, W.; Gershenzon, J. The Nonmevalonate Pathway Supports Both Monoterpene and Sesquiterpene Formation in Snapdragon Flowers. Proc. Natl. Acad. Sci. USA 2005, 102, 933–938. [Google Scholar] [CrossRef]
- Davis, E.M.; Croteau, R. Cyclization Enzymes in the Biosynthesis of Monoterpenes, Sesquiterpenes, and Diterpenes. In Biosynthesis: Aromatic Polyketides, Isoprenoids, Alkaloids; Leeper, F.J., Vederas, J.C., Eds.; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2000; pp. 53–95. ISBN 978-3-540-48146-1. [Google Scholar]
- Lupien, S.; Karp, F.; Wildung, M.; Croteau, R. Regiospecific Cytochrome P450 Limonene Hydroxylases from Mint (Mentha) Species: CDNA Isolation, Characterization, and Functional Expression of (-)-4S-Limonene-3-Hydroxylase and (-)-4S-Limonene-6-Hydroxylase. Arch. Biochem. Biophys. 1999, 368, 181–192. [Google Scholar] [CrossRef]
- Lange, B.M.; Wildung, M.R.; Stauber, E.J.; Sanchez, C.; Pouchnik, D.; Croteau, R. Probing Essential Oil Biosynthesis and Secretion by Functional Evaluation of Expressed Sequence Tags from Mint Glandular Trichomes. Proc. Natl. Acad. Sci. USA 2000, 97, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W. Unearthing the Roots of the Terpenome. Curr. Opin. Chem. Biol. 2008, 12, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Bathe, U.; Tissier, A. Cytochrome P450 Enzymes: A Driving Force of Plant Diterpene Diversity. Phytochemistry 2019, 161, 149–162. [Google Scholar] [CrossRef]
- Panchy, N.; Lehti-Shiu, M.; Shiu, S.-H. Evolution of Gene Duplication in Plants. Plant Physiol. 2016, 171, 2294–2316. [Google Scholar] [CrossRef]
- Leong, B.J.; Last, R.L. Promiscuity, Impersonation and Accommodation: Evolution of Plant Specialized Metabolism. Curr. Opin. Struct. Biol. 2017, 47, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Nützmann, H.-W.; Huang, A.; Osbourn, A. Plant Metabolic Clusters—From Genetics to Genomics. New Phytol. 2016, 211, 771–789. [Google Scholar] [CrossRef]
- Schläpfer, P.; Zhang, P.; Wang, C.; Kim, T.; Banf, M.; Chae, L.; Dreher, K.; Chavali, A.K.; Nilo-Poyanco, R.; Bernard, T.; et al. Genome-Wide Prediction of Metabolic Enzymes, Pathways, and Gene Clusters in Plants. Plant Physiol. 2017, 173, 2041–2059. [Google Scholar] [CrossRef]
- Sakamoto, T.; Miura, K.; Itoh, H.; Tatsumi, T.; Ueguchi-Tanaka, M.; Ishiyama, K.; Kobayashi, M.; Agrawal, G.K.; Takeda, S.; Abe, K.; et al. An Overview of Gibberellin Metabolism Enzyme Genes and Their Related Mutants in Rice. Plant Physiol. 2004, 134, 1642–1653. [Google Scholar] [CrossRef]
- Matsuba, Y.; Nguyen, T.T.H.; Wiegert, K.; Falara, V.; Gonzales-Vigil, E.; Leong, B.; Schäfer, P.; Kudrna, D.; Wing, R.A.; Bolger, A.M.; et al. Evolution of a Complex Locus for Terpene Biosynthesis in Solanum. Plant Cell 2013, 25, 2022–2036. [Google Scholar] [CrossRef] [PubMed]
- Bryson, A.E.; Lanier, E.R.; Lau, K.H.; Hamilton, J.P.; Vaillancourt, B.; Mathieu, D.; Yocca, A.E.; Miller, G.P.; Edger, P.P.; Buell, C.R.; et al. Uncovering a Miltiradiene Biosynthetic Gene Cluster in the Lamiaceae Reveals a Dynamic Evolutionary Trajectory. Nat. Commun. 2023, 14, 343. [Google Scholar] [CrossRef] [PubMed]
- Godden, G.T.; Kinser, T.J.; Soltis, P.S.; Soltis, D.E. Phylotranscriptomic Analyses Reveal Asymmetrical Gene Duplication Dynamics and Signatures of Ancient Polyploidy in Mints. Genome Biol. Evol. 2019, 11, 3393–3408. [Google Scholar] [CrossRef]
- Landmann, C.; Fink, B.; Festner, M.; Dregus, M.; Engel, K.-H.; Schwab, W. Cloning and Functional Characterization of Three Terpene Synthases from Lavender (Lavandula angustifolia). Arch. Biochem. Biophys. 2007, 465, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.C.; Croteau, R.B. Genomic Organization of Plant Terpene Synthases and Molecular Evolutionary Implications. Genetics 2001, 158, 811–832. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Sarker, L.S.; Mahmoud, S.S. Cloning and Functional Characterization of β-Phellandrene Synthase from Lavandula Angustifolia. Planta 2011, 233, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Sarker, L.S.; Galata, M.; Demissie, Z.A.; Mahmoud, S.S. Molecular Cloning and Functional Characterization of Borneol Dehydrogenase from the Glandular Trichomes of Lavandula x intermedia. Arch. Biochem. Biophys. 2012, 528, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Adal, A.M.; Sarker, L.S.; Lemke, A.D.; Mahmoud, S.S. Isolation and Functional Characterization of a Methyl Jasmonate-Responsive 3-Carene Synthase from Lavandula x intermedia. Plant Mol. Biol. 2017, 93, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Sarker, L.S.; Demissie, Z.A.; Mahmoud, S.S. Cloning of a Sesquiterpene Synthase from Lavandula x intermedia Glandular Trichomes. Planta 2013, 238, 983–989. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Erland, L.E.; Rheault, M.R.; Mahmoud, S.S. The Biosynthetic Origin of Irregular Monoterpenes in Lavandula. J. Biol. Chem. 2013, 288, 6333–6341. [Google Scholar] [CrossRef]
- Sarker, L.S.; Mahmoud, S.S. Cloning and Functional Characterization of Two Monoterpene Acetyltransferases from Glandular Trichomes of L. x intermedia. Planta 2015, 242, 709–719. [Google Scholar] [CrossRef]
- Adal, A.M.; Najafianashrafi, E.; Sarker, L.S.; Mahmoud, S.S. Cloning, Functional Characterization and Evaluating Potential in Metabolic Engineering for Lavender (+)-Bornyl Diphosphate Synthase. Plant Mol. Biol. 2022, 111, 117–130. [Google Scholar] [CrossRef]
- Despinasse, Y.; Fiorucci, S.; Antonczak, S.; Moja, S.; Bony, A.; Nicolè, F.; Baudino, S.; Magnard, J.-L.; Jullien, F. Bornyl-Diphosphate Synthase from Lavandula Angustifolia: A Major Monoterpene Synthase Involved in Essential Oil Quality. Phytochemistry 2017, 137, 24–33. [Google Scholar] [CrossRef]
- Landmann, C.; Hücherig, S.; Fink, B.; Hoffmann, T.; Dittlein, D.; Coiner, H.A.; Schwab, W. Substrate Promiscuity of a Rosmarinic Acid Synthase from Lavender (Lavandula angustifolia L.). Planta 2011, 234, 305–320. [Google Scholar] [CrossRef]
- Benabdelkader, T.; Guitton, Y.; Pasquier, B.; Magnard, J.L.; Jullien, F.; Kameli, A.; Legendre, L. Functional Characterization of Terpene Synthases and Chemotypic Variation in Three Lavender Species of Section Stoechas. Physiol. Plant 2015, 153, 43–57. [Google Scholar] [CrossRef]
- Adal, A.M.; Sarker, L.S.; Malli, R.P.N.; Liang, P.; Mahmoud, S.S. RNA-Seq in the Discovery of a Sparsely Expressed Scent-Determining Monoterpene Synthase in Lavender (Lavandula). Planta 2019, 249, 271–290. [Google Scholar] [CrossRef]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The Family of Terpene Synthases in Plants: A Mid-Size Family of Genes for Specialized Metabolism That Is Highly Diversified throughout the Kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Sarker, L.S.; Adal, A.M.; Mahmoud, S.S. Diverse Transcription Factors Control Monoterpene Synthase Expression in Lavender (Lavandula). Planta 2019, 251, 5. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Srivastava, S.; Shasany, A.K.; Sharma, A. Identification of MiRNAs and Their Targets Involved in the Secondary Metabolic Pathways of Mentha spp. Comput. Biol. Chem. 2016, 64, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kumar, P.; Sanyal, R.; Mane, A.B.; Arvind Prasanth, D.; Patil, M.; Dey, A. Unravelling the Regulatory Role of MiRNAs in Secondary Metabolite Production in Medicinal Crops. Plant Gene 2021, 27, 100303. [Google Scholar] [CrossRef]
- Sun, M.; Shi, M.; Wang, Y.; Huang, Q.; Yuan, T.; Wang, Q.; Wang, C.; Zhou, W.; Kai, G. The Biosynthesis of Phenolic Acids Is Positively Regulated by the JA-Responsive Transcription Factor ERF115 in Salvia Miltiorrhiza. J. Exp. Bot. 2019, 70, 243–254. [Google Scholar] [CrossRef]
- Pani, A.; Mahapatra, R.K. Computational Identification of MicroRNAs and Their Targets in Catharanthus Roseus Expressed Sequence Tags. Genom. Data 2013, 1, 2–6. [Google Scholar] [CrossRef]
- Fan, R.; Li, Y.; Li, C.; Zhang, Y. Differential MicroRNA Analysis of Glandular Trichomes and Young Leaves in Xanthium Strumarium L. Reveals Their Putative Roles in Regulating Terpenoid Biosynthesis. PLoS ONE 2015, 10, e0139002. [Google Scholar] [CrossRef]
- Biswas, S.; Hazra, S.; Chattopadhyay, S. Identification of Conserved MiRNAs and Their Putative Target Genes in Podophyllum Hexandrum (Himalayan mayapple). Plant Gene 2016, 6, 82–89. [Google Scholar] [CrossRef]
- Ding, K.; Pei, T.; Bai, Z.; Jia, Y.; Ma, P.; Liang, Z. SmMYB36, a Novel R2R3-MYB Transcription Factor, Enhances Tanshinone Accumulation and Decreases Phenolic Acid Content in Salvia Miltiorrhiza Hairy Roots. Sci. Rep. 2017, 7, 5104. [Google Scholar] [CrossRef]
- Lv, M.; Sun, X.; Li, D.; Wei, G.; Liu, L.; Chen, F.; Cai, Y.; Fan, H. Terpenoid Biosynthesis in Dendrobium Officinale: Identification of (E)-β-Caryophyllene Synthase and the Regulatory MYB Genes. Ind. Crops Prod. 2022, 182, 114875. [Google Scholar] [CrossRef]
- Singh, N.; Sharma, A. In-Silico Identification of MiRNAs and Their Regulating Target Functions in Ocimum Basilicum. Gene 2014, 552, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.X.; Wang, L.J.; Zhao, B.; Shan, C.M.; Zhang, Y.H.; Chen, D.F.; Chen, X.Y. Progressive Regulation of Sesquiterpene Biosynthesis in Arabidopsis and Patchouli (Pogostemon Cablin) by the MiR156-Targeted SPL Transcription Factors. Mol. Plant 2015, 8, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wilderman, P.R.; Peters, R.J. Following Evolution’s Lead to a Single Residue Switch for Diterpene Synthase Product Outcome. Proc. Natl. Acad. Sci. USA 2007, 104, 7397–7401. [Google Scholar] [CrossRef]
- Legrand, S.; Valot, N.; Nicolé, F.; Moja, S.; Baudino, S.; Jullien, F.; Magnard, J.-L.; Caissard, J.-C.; Legendre, L. One-Step Identification of Conserved MiRNAs, Their Targets, Potential Transcription Factors and Effector Genes of Complete Secondary Metabolism Pathways after 454 Pyrosequencing of Calyx CDNAs from the Labiate Salvia sclarea L. Gene 2010, 450, 55–62. [Google Scholar] [CrossRef]
- Van der Fits, L.; Memelink, J. The Jasmonate-Inducible AP2/ERF-Domain Transcription Factor ORCA3 Activates Gene Expression via Interaction with a Jasmonate-Responsive Promoter Element. Plant J. 2001, 25, 43–53. [Google Scholar] [CrossRef]
- Patel, M.; Mangukia, N.; Jha, N.; Gadhavi, H.; Shah, K.; Patel, S.; Mankad, A.; Pandya, H.; Rawal, R. Computational Identification of MiRNA and Their Cross Kingdom Targets from Expressed Sequence Tags of Ocimum Basilicum. Mol. Biol. Rep. 2019, 46, 2979–2995. [Google Scholar] [CrossRef]


| Country | Locality | References |
|---|---|---|
| France | Provence (Valensole), Lourmarin, Grass, Sisteron | [29] |
| Italy | Piemont (Demont), Andonno, Civitella Marittima, Borgo, Mozzano | [29,33] |
| UK | Catswold, Hitchin (Hertfordshire, Bedfordshire), Banstead (Woodmansterne–Surrey Hampshire), Lordington (West Sussex), Heacham, Terrington (York) | [24] |
| Canada | Vancouver, Campbellville, Windham Centre, Campbellcroft–Port Hope, Niagara on The Lake, Seagrave, Dundas, Waterford, Greenbank, Ayr (Brant) | [32] |
| USA | Oregon, Matanzas Creek Winery, Mt. Shasta Lavender Farms, Montague (California), Blanco (Texas), Soleado (Maryland), Lavender by the Bay (New York), Sequim, (Washington) | [24] |
| Australia | Nabowla TAS, Wandin North VIC, Laggan NSW, Mount Alford QLD, Lyndoch SA, Carlotta WA, Pemberton WA | [24] |
| Japan | Furano, Tomita (Hokkaido) | [24,32] |
| Bulgaria | Szumen, Dobricz, Karlovo | [34] |
| Croatia | Hvar (Velo Grablje) | [35] |
| Poland | Kujawy (Grebocin, Złotniki), Podlasie (Dworzysk, Dzięciołowo), Wielkopolska (Pakszyn, Kicin, Krzemieniewo, Kotuń, Jutrosin, Murzynowo, Ląd, Sławno), Malopolska (Masłomiąca, Ostrów-Kraków), Łózkie (Wieluń, Uniejów, Zelów), Dolnośląskie (Świdnica, Oborniki, Gryfów), Lubelskie (Siedliszcze, Kiełczewice, Końskowola), Lubuskie (Silna), Mazowieckie (Ryczołek, Korabiewice, Borkowice, Borowiczki, Siwianka), Opolskie (Grodków, Zdziechowice, Biadacz), Warmińsko-Mazurskie (Jonkowo), Pomorskie (Rotmanka, Przywidz, Górzyca) | [36,37] |
| Slovakia | Šaľa (Šaľa), Malé Leváre (Malacky), Modra (Pezinok), Oščadnica (Čadca), Tomášikovo (Galanta), Východná (Liptovský Mikuláš), Branovo (Nové Zámky), Stankovce (Trebišov), Kapoňa–Leles (Trebišov) | [38] |
| Czechia | Starovičky (Morava), Ostrava-Poruba, Vysočina (Třebič), Bezděkov (Klatovy), Židovice (Litoměřice), Strání (Uherské Hradiště), Chodouň (Beroun) | [39] |
| Hungary | Tihany (Balaton) | [40] |
| miRNA | Target | Plant Species | Reference |
|---|---|---|---|
| miR156 | deoxy-D-xylulose 5-phosphate synthase SPL transcription factor | Mentha spp. Salvia sclarea L. | [104] [115] |
| miR414 | terpene synthase 21 | Mentha spp. | [104] |
| miR5021 | deoxy-D-xylulose 5-phosphate synthase isopenteyl diphosphate isomerase geranylgeranyl pyrophosphate synthase | Mentha spp. | [104] |
| miR5072 | acetyl-CoA C-acetyl transferase | Salvia sclarea L. Salvia miltiorrhiza Bunge | [115] [114] |
| miR172 | AP2 domain-containing transcription factor | Salvia sclarea L. | [115] |
| miR828 | MYB12 | Salvia sclarea L. | [115] |
| miR858 | MYB family transcription factor | Salvia miltiorrhiza Bunge | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habán, M.; Korczyk-Szabó, J.; Čerteková, S.; Ražná, K. Lavandula Species, Their Bioactive Phytochemicals, and Their Biosynthetic Regulation. Int. J. Mol. Sci. 2023, 24, 8831. https://doi.org/10.3390/ijms24108831
Habán M, Korczyk-Szabó J, Čerteková S, Ražná K. Lavandula Species, Their Bioactive Phytochemicals, and Their Biosynthetic Regulation. International Journal of Molecular Sciences. 2023; 24(10):8831. https://doi.org/10.3390/ijms24108831
Chicago/Turabian StyleHabán, Miroslav, Joanna Korczyk-Szabó, Simona Čerteková, and Katarína Ražná. 2023. "Lavandula Species, Their Bioactive Phytochemicals, and Their Biosynthetic Regulation" International Journal of Molecular Sciences 24, no. 10: 8831. https://doi.org/10.3390/ijms24108831
APA StyleHabán, M., Korczyk-Szabó, J., Čerteková, S., & Ražná, K. (2023). Lavandula Species, Their Bioactive Phytochemicals, and Their Biosynthetic Regulation. International Journal of Molecular Sciences, 24(10), 8831. https://doi.org/10.3390/ijms24108831






