Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer’s Disease: Dietary Plant Polyphenols
Abstract
1. Introduction
2. Polyphenols and Oxidative Stress
2.1. The Oxidative Stress in Alzheimer’s Disease
2.2. Polyphenols Alleviate AD Symptoms by Reducing Oxidative Stress
3. Polyphenols and Neuroinflammatory Mechanisms
3.1. Neuroinflammation in Alzheimer’s Disease
3.2. Polyphenols Can Alleviate AD Symptoms by Inhibiting Glial Inflammatory Activation, Affecting Monocyte/Macrophage System, and Inhibiting Neuroinflammation
4. Polyphenols and Amyloid Toxicity Mechanism
4.1. Amyloid Neurotoxicity in Alzheimer’s Disease
4.2. Regulatory Role of Polyphenols in the Context of Amyloid Neurotoxicity
5. Polyphenols and Abnormal Tau Protein Phosphorylation
5.1. Abnormal Phosphorylation of Tau Protein in Alzheimer’s Disease
5.2. Regulatory Role of Polyphenols in the Context of Abnormal Tau Protein Phosphorylation
6. Influence of Polyphenol on Cholinergic Injury
6.1. Cholinergic Injury in Alzheimer’s Disease
6.2. Regulatory Role of Polyphenols in the Context of Cholinergic Injury
7. Polyphenols and ApoE in Alzheimer’s Disease
7.1. ApoE Gene in Alzheimer’s Disease
7.2. Regulatory Role of Polyphenols in the Context of ApoE Gene
8. Polyphenols and Other Mechanisms in Alzheimer’s Disease Development
8.1. Polyphenols and Insulin Resistance
8.2. Polyphenols and Mitochondrial Dysfunction
8.3. Polyphenols and Faulty Autolysosome Acidification
8.4. Polyphenols and Disruption of the Intestinal Flora
9. Potential Regulation Effect of Polyphenols on Recently Identified Targets of AD
10. The Potential and Mechanisms of Action of Other Common Plant Polyphenols for AD Treatment
11. Limitations in the Application and Improvements in Preparation of Polyphenols
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodson, R. Alzheimer’s disease. Nature 2018, 559, S1. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Feulner, T.M.; Laws, S.M.; Friedrich, P.; Wagenpfeil, S.; Wurst, S.H.; Riehle, C.; Kuhn, K.A.; Krawczak, M.; Schreiber, S.; Nikolaus, S.; et al. Examination of the current top candidate genes for AD in a genome-wide association study. Mol. Psychiatry 2010, 15, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Johannsen, P. Long-term cholinesterase inhibitor treatment of Alzheimer’s disease. CNS Drugs 2004, 18, 757–768. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s disease: Advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021, 20, 68–80. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.; Lemere, C.; Atri, A.; Sabbagh, M.; Salloway, S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimers Res. Ther. 2021, 13, 98. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Del, V.A.S.; Prieto, M.J. Combined Therapy for Alzheimer’s Disease: Tacrine and PAMAM Dendrimers Co-Administration Reduces the Side Effects of the Drug without Modifying its Activity. AAPS PharmSciTech 2020, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, J.L.; Artalejo, F.R. Methodology, results and quality of clinical trials of tacrine in the treatment of Alzheimer’s disease: A systematic review of the literature. Age Ageing 1998, 27, 161–179. [Google Scholar] [CrossRef]
- Moss, D.E.; Perez, R.G.; Kobayashi, H. Cholinesterase Inhibitor Therapy in Alzheimer’s Disease: The Limits and Tolerability of Irreversible CNS-Selective Acetylcholinesterase Inhibition in Primates. J. Alzheimers Dis. 2017, 55, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Atri, A. Current and Future Treatments in Alzheimer’s Disease. Semin. Neurol. 2019, 39, 227–240. [Google Scholar] [CrossRef]
- Imbimbo, B.P. Pharmacodynamic-tolerability relationships of cholinesterase inhibitors for Alzheimer’s disease. CNS Drugs 2001, 15, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Dhikav, V.; Anand, K.S. Acute dystonic reaction with rivastigmine. Int. Psychogeriatr. 2013, 25, 1385–1386. [Google Scholar] [CrossRef]
- Hansen, R.A.; Gartlehner, G.; Webb, A.P.; Morgan, L.C.; Moore, C.G.; Jonas, D.E. Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: A systematic review and meta-analysis. Clin. Interv. Aging 2008, 3, 211–225. [Google Scholar]
- Pariente, A.; Sanctussy, D.J.; Miremont-Salamé, G.; Moore, N.; Haramburu, F.; Fourrier-Réglat, A. Factors associated with serious adverse reactions to cholinesterase inhibitors: A study of spontaneous reporting. CNS Drugs 2010, 24, 55–63. [Google Scholar] [CrossRef]
- Pérez-García, M.P.; Sánchez-Motillas, J.M.; Mateu-Puchades, A.; Díaz-Corpas, T. Acute generalized exanthematous pustulosis induced by galantamine. Actas Dermosifiliogr. 2013, 104, 930–931. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- Howes, L.G. Cardiovascular effects of drugs used to treat Alzheimer’s disease. Drug Saf. 2014, 37, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Salloway, S.; Chalkias, S.; Barkhof, F.; Burkett, P.; Barakos, J.; Purcell, D.; Suhy, J.; Forrestal, F.; Tian, Y.; Umans, K.; et al. Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients with Early Alzheimer Disease. JAMA Neurol. 2022, 79, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Klose, J.; Griehl, C.; Roßner, S.; Schilling, S. Natural Products from Plants and Algae for Treatment of Alzheimer’s Disease: A Review. Biomolecules 2022, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- LI, L.; Wang, X.; Peng, Y. Pharmacological research of natural products in the treatment of Alzheimer’s disease. Chin. Pharmacol. Bull. 2016, 12, 149–154, 155. [Google Scholar]
- Sun, Z.; Ma, P.; Chen, H.; Zhu, X.; Fu, Z. Research progress of anti-Alzheimer’s Disease drugs. Mod. Salt Chem. Ind. 2019, 46, 45–46. (In Chinese) [Google Scholar] [CrossRef]
- Hu, N.; Yu, J.T.; Tan, L.; Wang, Y.L.; Sun, L.; Tan, L. Nutrition and the risk of Alzheimer’s disease. Biomed. Res. Int. 2013, 2013, 524820. [Google Scholar] [CrossRef]
- Jayatunga, D.P.W.; Hone, E.; Fernando, W.; Garg, M.L.; Verdile, G.; Martins, R.N. Mitoprotective Effects of a Synergistic Nutraceutical Combination: Basis for a Prevention Strategy Against Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 781468. [Google Scholar] [CrossRef]
- Bukhari, S.N.A. Dietary Polyphenols as Therapeutic Intervention for Alzheimer’s Disease: A Mechanistic Insight. Antioxidants 2022, 11, 554. [Google Scholar] [CrossRef]
- Kępka, A.; Ochocińska, A.; Borzym-Kluczyk, M.; Chojnowska, S.; Skorupa, E.; Przychodzeń, M.; Waszkiewicz, N. Healthy Food Pyramid as Well as Physical and Mental Activity in the Prevention of Alzheimer’s Disease. Nutrients 2022, 14, 1534. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Hernandez-Baixauli, J.; Puiggrós, F.; Arola, L.; Caimari, A.; Del Bas, J.M.; Baselga, L. Mediterranean natural extracts improved cognitive behavior in zebrafish and healthy rats and ameliorated lps-induced cognitive impairment in a sex dependent manner. Behav. Brain Funct. 2022, 18, 5. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Bouska, L. Optimization of the ultrasound-assisted extraction of polyphenols from Aronia and grapes. Food Chem. 2022, 386, 132703. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R.S.; Mu, S.; Feresin, R.G. Blueberry Polyphenols Increase Nitric Oxide and Attenuate Angiotensin II-Induced Oxidative Stress and Inflammatory Signaling in Human Aortic Endothelial Cells. Antioxidants 2022, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, D.; Liu, F.; Wang, X.; Cui, Y.; Li, S.; Li, X. The Ameliorating Effects of Apple Polyphenol Extract on High-Fat-Diet-Induced Hepatic Steatosis Are SIRT1-Dependent: Evidence from Hepatic-Specific SIRT1 Heterozygous Mutant C57BL/6 Mice. J. Agric. Food Chem. 2022, 70, 5579–5594. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhao, S.X.; Zhang, M.Y.; Ji, P.Y.; Chao, S.; Li, L.J.; Yin, S.; Zhao, L.; Zhao, H.; Sun, Q.Y.; et al. Tea polyphenols alleviate the adverse effects of diabetes on oocyte quality. Food Funct. 2022, 13, 5396–5405. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, A.; Cueva, C.; Silva, M.; Molinero, N.; Miralles, B.; Bartolomé, B.; Moreno-Arribas, M.V. Gastrointestinal co-digestion of wine polyphenols with glucose/whey proteins affects their bioaccessibility and impact on colonic microbiota. Food Res. Int. 2022, 155, 111010. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Yan, L.; Guo, M.S.; Zhang, Y.; Yu, L.; Wu, J.M.; Tang, Y.; Ai, W.; Zhu, F.D.; Law, B.Y.; Chen, Q.; et al. Dietary Plant Polyphenols as the Potential Drugs in Neurodegenerative Diseases: Current Evidence, Advances, and Opportunities. Oxid. Med. Cell. Longev. 2022, 2022, 5288698. [Google Scholar] [CrossRef]
- Meshginfar, N.; Tavakoli, H.; Dornan, K.; Hosseinian, F. Phenolic lipids as unique bioactive compounds: A comprehensive review on their multifunctional activity toward the prevention of Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 2021, 61, 1394–1403. [Google Scholar] [CrossRef]
- Pluta, R.; Furmaga-Jabłońska, W.; Januszewski, S.; Czuczwar, S.J. Post-Ischemic Brain Neurodegeneration in the Form of Alzheimer’s Disease Proteinopathy: Possible Therapeutic Role of Curcumin. Nutrients 2022, 14, 248. [Google Scholar] [CrossRef]
- Ontario, M.L.; Siracusa, R.; Modafferi, S.; Scuto, M.; Sciuto, S.; Greco, V.; Bertuccio, M.P.; Trovato Salinaro, A.; Crea, R.; Calabrese, E.J.; et al. Potential prevention and treatment of neurodegenerative disorders by olive polyphenols and hidrox. Mech. Ageing Dev. 2022, 203, 111637. [Google Scholar] [CrossRef]
- Dos Santos, M.G.; Schimith, L.E.; André-Miral, C.; Muccillo-Baisch, A.L.; Arbo, B.D.; Hort, M.A. Neuroprotective Effects of Resveratrol in In vivo and In vitro Experimental Models of Parkinson’s Disease: A Systematic Review. Neurotox. Res. 2022, 40, 319–345. [Google Scholar] [CrossRef]
- Ding, J.; Huang, J.; Yin, D.; Liu, T.; Ren, Z.; Hu, S.; Ye, Y.; Le, C.; Zhao, N.; Zhou, H.; et al. Trilobatin Alleviates Cognitive Deficits and Pathologies in an Alzheimer’s Disease Mouse Model. Oxid. Med. Cell. Longev. 2021, 2021, 3298400. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cui, K.; Li, X.; Zhao, J.; Zeng, Z.; Song, R.; Qi, X.; Xu, W. Effect of Polyphenols on Cognitive Function: Evidence from Population-Based Studies and Clinical Trials. J. Nutr. Health Aging 2021, 25, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Molino, S.; Dossena, M.; Buonocore, D.; Ferrari, F.; Venturini, L.; Ricevuti, G.; Verri, M. Polyphenols in dementia: From molecular basis to clinical trials. Life Sci. 2016, 161, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef]
- Battaglini, M.; Marino, A.; Carmignani, A.; Tapeinos, C.; Cauda, V.; Ancona, A.; Garino, N.; Vighetto, V.; La Rosa, G.; Sinibaldi, E.; et al. Polydopamine Nanoparticles as an Organic and Biodegradable Multitasking Tool for Neuroprotection and Remote Neuronal Stimulation. ACS Appl. Mater. Interfaces 2020, 12, 35782–35798. [Google Scholar] [CrossRef]
- Abramov, A.Y.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Dolgacheva, L.P. Interaction of misfolded proteins and mitochondria in neurodegenerative disorders. Biochem. Soc. Trans. 2017, 45, 1025–1033. [Google Scholar] [CrossRef]
- Owen, J.B.; Sultana, R.; Aluise, C.D.; Erickson, M.A.; Price, T.O.; Bu, G.; Banks, W.A.; Butterfield, D.A. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer disease: Implications for Aβ accumulation in AD brain. Free Radic. Biol. Med. 2010, 49, 1798–1803. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Casado, A.; Encarnación López-Fernández, M.; Concepción Casado, M.; de La Torre, R. Lipid peroxidation and antioxidant enzyme activities in vascular and Alzheimer dementias. Neurochem. Res. 2008, 33, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Wang, Y.; Zhang, Y.; Zhou, H.; Song, Y.; Cao, Z.; Kou, J.; Yu, B. Cocktail of Four Active Components Derived from Sheng Mai San Inhibits Hydrogen Peroxide-Induced PC12 Cell Apoptosis Linked with the Caspase-3/ROCK1/MLC Pathway. Rejuvenation Res. 2015, 18, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mei, Q.; Wang, L.; Feng, X.; Tao, X.; Qiu, C.; Zhu, J. TIGAR suppresses seizures induced by kainic acid through inhibiting oxidative stress and neuronal apoptosis. Biochem. Biophys. Res. Commun. 2019, 515, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pu, Z.; Li, M.; Wang, K.; Deng, L.; Chen, W. Antioxidative and antiapoptosis: Neuroprotective effects of dauricine in Alzheimer’s disease models. Life Sci. 2020, 243, 117237. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Aminzadeh, A. Protective effect of tropisetron on high glucose induced apoptosis and oxidative stress in PC12 cells: Roles of JNK, P38 MAPKs, and mitochondria pathway. Metab. Brain Dis. 2017, 32, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K.; Mishra, P.K. Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 2010, 30, 818–860. [Google Scholar] [CrossRef]
- Trujillo, J.; Chirino, Y.I.; Molina-Jijón, E.; Andérica-Romero, A.C.; Tapia, E.; Pedraza-Chaverrí, J. Renoprotective effect of the antioxidant curcumin: Recent findings. Redox Biol. 2013, 1, 448–456. [Google Scholar] [CrossRef]
- Yin, H.; Guo, Q.; Li, X.; Tang, T.; Li, C.; Wang, H.; Sun, Y.; Feng, Q.; Ma, C.; Gao, C.; et al. Curcumin Suppresses IL-1β Secretion and Prevents Inflammation through Inhibition of the NLRP3 Inflammasome. J. Immunol. 2018, 200, 2835–2846. [Google Scholar] [CrossRef]
- Zheng, D.; Huang, C.; Huang, H.; Zhao, Y.; Khan, M.R.U.; Zhao, H.; Huang, L. Antibacterial Mechanism of Curcumin: A Review. Chem. Biodivers. 2020, 17, e2000171. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, J.R.; Bettadaiah, B.K.; Negi, P.S.; Srinivas, P. Synthesis of amino acid conjugates of tetrahydrocurcumin and evaluation of their antibacterial and anti-mutagenic properties. Food Chem. 2013, 139, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021, 1291, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Bao, W.; Li, K.; Rong, S.; Yao, P.; Hao, L.; Ying, C.; Zhang, X.; Nussler, A.; Liu, L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J. Ethnopharmacol. 2010, 128, 549–553. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Kanninen, K.; Malm, T.M.; Jyrkkänen, H.K.; Goldsteins, G.; Keksa-Goldsteine, V.; Tanila, H.; Yamamoto, M.; Ylä-Herttuala, S.; Levonen, A.L.; Koistinaho, J. Nuclear factor erythroid 2-related factor 2 protects against beta amyloid. Mol. Cell. Neurosci. 2008, 39, 302–313. [Google Scholar] [CrossRef]
- Xiang, B.; Li, D.; Chen, Y.; Li, M.; Zhang, Y.; Sun, T.; Tang, S. Curcumin Ameliorates Copper-Induced Neurotoxicity Through Inhibiting Oxidative Stress and Mitochondrial Apoptosis in SH-SY5Y Cells. Neurochem. Res. 2021, 46, 367–378. [Google Scholar] [CrossRef]
- Eun, C.S.; Lim, J.S.; Lee, J.; Lee, S.P.; Yang, S.A. The protective effect of fermented Curcuma longa L. on memory dysfunction in oxidative stress-induced C6 gliomal cells, proinflammatory-activated BV2 microglial cells, and scopolamine-induced amnesia model in mice. BMC Complement. Altern. Med. 2017, 17, 367. [Google Scholar] [CrossRef]
- Yue, Y.K.; Mo, B.; Zhao, J.; Yu, Y.J.; Liu, L.; Yue, C.L.; Liu, W. Neuroprotective effect of curcumin against oxidative damage in BV-2 microglia and high intraocular pressure animal model. J. Ocul. Pharmacol. Ther. 2014, 30, 657–664. [Google Scholar] [CrossRef] [PubMed]
- da Silva Marques, J.G.; Antunes, F.T.T.; da Silva Brum, L.F.; Pedron, C.; de Oliveira, I.B.; de Barros Falcão Ferraz, A.; Martins, M.I.M.; Dallegrave, E.; de Souza, A.H. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav. Brain Res. 2021, 399, 113002. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W.; Kori, R.K.; Jain, J.; Yadav, R.S.; Jat, D. Neuroprotective effects of mitochondria-targeted curcumin against rotenone-induced oxidative damage in cerebellum of mice. J. Biochem. Mol. Toxicol. 2020, 34, e22416. [Google Scholar] [CrossRef] [PubMed]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Takaoka, M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. Nippon Kagaku Kaishi 1939, 60, 1090–1100. [Google Scholar] [CrossRef]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Cardile, V.; Chillemi, R.; Lombardo, L.; Sciuto, S.; Spatafora, C.; Tringali, C. Antiproliferative activity of methylated analogues of E- and Z-resveratrol. Z. Naturforsch. C J. Biosci. 2007, 62, 189–195. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Mastromarino, P.; Sessa, R. Therapeutic potential of resveratrol against emerging respiratory viral infections. Pharmacol. Ther. 2020, 214, 107613. [Google Scholar] [CrossRef]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Butt, M.S.; Nadeem, M.; Peters, D.G.; Mubarak, M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1428–1447. [Google Scholar] [CrossRef] [PubMed]
- Tellone, E.; Galtieri, A.; Russo, A.; Giardina, B.; Ficarra, S. Resveratrol: A Focus on Several Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 392169. [Google Scholar] [CrossRef] [PubMed]
- Castellani, R.J.; Rolston, R.K.; Smith, M.A. Alzheimer disease. Dis. Mon. 2010, 56, 484–546. [Google Scholar] [CrossRef] [PubMed]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [PubMed]
- Sovak, M. Grape Extract, Resveratrol, and Its Analogs: A Review. J. Med. Food 2001, 4, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shen, Y.; Xiao, H.; Sun, W. Resveratrol attenuates rotenone-induced inflammation and oxidative stress via STAT1 and Nrf2/Keap1/SLC7A11 pathway in a microglia cell line. Pathol. Res. Pract. 2021, 225, 153576. [Google Scholar] [CrossRef]
- Cong, L.; Lei, M.Y.; Liu, Z.Q.; Liu, Z.F.; Ma, Z.; Liu, K.; Li, J.; Deng, Y.; Liu, W.; Xu, B. Resveratrol attenuates manganese-induced oxidative stress and neuroinflammation through SIRT1 signaling in mice. Food Chem. Toxicol. 2021, 153, 112283. [Google Scholar] [CrossRef]
- Silva, J.M.R.D.; Rigaud, J.; Cheynier, V.; Cheminat, A.; Moutounet, M. Procyanidin dimers and trimers from grape seeds. Phytochemistry 1991, 30, 1259–1264. [Google Scholar] [CrossRef]
- Spranger, I.; Sun, B.; Mateus, A.M.; Freitas, V.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Garg, A.; Krohn, R.L.; Bagchi, M.; Tran, M.X.; Stohs, S.J. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res. Commun. Mol. Pathol. Pharmacol. 1997, 95, 179–189. [Google Scholar] [PubMed]
- Mendoza-Wilson, A.M.; Castro-Arredondo, S.I.; Balandrán-Quintana, R.R. Computational study of the structure-free radical scavenging relationship of procyanidins. Food Chem. 2014, 161, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Bak, M.J.; Jun, M.; Kong, A.N.; Ho, C.T.; Jeong, W.S. Antioxidant defense and hepatoprotection by procyanidins from almond (Prunus amygdalus) skins. J. Agric. Food Chem. 2014, 62, 8668–8678. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardèvol, A.; Pinent, M.; Blay, M.T. Procyanidins and inflammation: Molecular targets and health implications. Biofactors 2012, 38, 257–265. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Pinent, M.; Casanova-Marti, A.; Arola, L.; Blay, M.; Ardevol, A. Procyanidins and their healthy protective effects against type 2 diabetes. Curr. Med. Chem. 2015, 22, 39–50. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Li, S.; Chen, M.; Xiao, J.; Xie, B.; Sun, Z. Oligomer Procyanidins from Lotus Seedpod Regulate Lipid Homeostasis Partially by Modifying Fat Emulsification and Digestion. J. Agric. Food Chem. 2019, 67, 4524–4534. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, C.; Niu, Y.; Xia, J.; Fan, L.; Wu, Y.; Gao, W. Procyanidins mediates antineoplastic effects against non-small cell lung cancer via the JAK2/STAT3 pathway. Transl. Cancer Res. 2021, 10, 2023–2035. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J. Relationship between Neuroprotective Effects and Structure of Procyanidins. Molecules 2022, 27, 2308. [Google Scholar] [CrossRef]
- Katz, D.L.; Doughty, K.; Ali, A. Cocoa and chocolate in human health and disease. Antioxid. Redox Signal. 2011, 15, 2779–2811. [Google Scholar] [CrossRef]
- Xu, J.; Rong, S.; Xie, B.; Sun, Z.; Zhang, L.; Wu, H.; Yao, P.; Zhang, X.; Zhang, Y.; Liu, L. Rejuvenation of antioxidant and cholinergic systems contributes to the effect of procyanidins extracted from the lotus seedpod ameliorating memory impairment in cognitively impaired aged rats. Eur. Neuropsychopharmacol. 2009, 19, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, M.; Duan, Y.; Duan, W.; Zhang, H.; He, Y.; Yin, C.; Sun, G.; Sun, X. Chemoprotective action of lotus seedpod procyanidins on oxidative stress in mice induced by extremely low-frequency electromagnetic field exposure. Biomed. Pharmacother. 2016, 82, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.R.; Kim, K.J.; Lee, S.G.; Cho, H.S.; Cho, Y.S.; Kim, D.O. Phenolic Profiles of Hardy Kiwifruits and Their Neuroprotective Effects on PC-12 and SH-SY5Y Cells against Oxidative Stress. J. Microbiol. Biotechnol. 2020, 30, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Xu, L.; Fang, X.; Zhao, X.; Du, Y.; Wu, H.; Qian, Y.; Ma, Z.; Xia, T.; Gu, X. Protective effects of grape seed procyanidin on isoflurane-induced cognitive impairment in mice. Pharm. Biol. 2020, 58, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabbà, C.; Pilotto, A.; Logroscino, G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.S.; Liu, W.H.; Fang, Y.Y.; Xu, S.B.; Li, J.; Luo, X.; Pan, D.J.; Wang, M.H.; Wang, W. Alcohol, coffee and tea intake and the risk of cognitive deficits: A dose-response meta-analysis. Epidemiol. Psychiatr. Sci. 2021, 30, e13. [Google Scholar] [CrossRef]
- Ide, K.; Matsuoka, N.; Yamada, H.; Furushima, D.; Kawakami, K. Effects of Tea Catechins on Alzheimer’s Disease: Recent Updates and Perspectives. Molecules 2018, 23, 2357. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Y.; Zhu, S.; Lu, Y.; Zhu, L.; Wang, Y.; Wang, X. Inhibition of Aβ aggregates in Alzheimer’s disease by epigallocatechin and epicatechin-3-gallate from green tea. Bioorg. Chem. 2020, 105, 104382. [Google Scholar] [CrossRef]
- Amirpour, M.; Mirshekar, M.A.; Sedaghat, G.; Montazerifar, F.; Shourestani, S.; Arabmoazzen, S.; Naghizadeh, M. The effects of green tea on cognitive impairments in the rat model of Alzheimer’s disease: Protection against inflammatory and oxidative damage. Nutr. Neurosci. 2022, 25, 2659–2667. [Google Scholar] [CrossRef]
- Liu, B.; Yan, W. Lipophilization of EGCG and effects on antioxidant activities. Food Chem. 2019, 272, 663–669. [Google Scholar] [CrossRef]
- Wang, M.; Zhong, H.; Zhang, X.; Huang, X.; Wang, J.; Li, Z.; Chen, M.; Xiao, Z. EGCG promotes PRKCA expression to alleviate LPS-induced acute lung injury and inflammatory response. Sci. Rep. 2021, 11, 11014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Bi, K.; He, Y.; Yan, W.; Yang, C.S.; Zhang, J. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci. Technol. 2021, 114, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Eng, Q.Y.; Thanikachalam, P.V.; Ramamurthy, S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J. Ethnopharmacol. 2018, 210, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, W.; Chopra, S.; Kaur, D.; Wang, H.; Li, M.; Chen, P.; Zhang, W. The Epigenetic Modification of Epigallocatechin Gallate (EGCG) on Cancer. Curr. Drug Targets 2020, 21, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Xia, J.; Cheng, B.H.; Gao, H.C.; Fu, L.W.; Luo, X.L. Tea polyphenol EGCG inhibited colorectal-cancer-cell proliferation and migration via downregulation of STAT3. Gastroenterol. Rep. 2021, 9, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2016, 15, 60. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.J.; Xiong, L.; Zhang, L.; Sun, D.; Liu, H. Green tea polyphenols inhibit cognitive impairment induced by chronic cerebral hypoperfusion via modulating oxidative stress. J. Nutr. Biochem. 2010, 21, 741–748. [Google Scholar] [CrossRef]
- Biasibetti, R.; Tramontina, A.C.; Costa, A.P.; Dutra, M.F.; Quincozes-Santos, A.; Nardin, P.; Bernardi, C.L.; Wartchow, K.M.; Lunardi, P.S.; Gonçalves, C.A. Green tea (-)epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav. Brain Res. 2013, 236, 186–193. [Google Scholar] [CrossRef]
- Reznichenko, L.; Amit, T.; Zheng, H.; Avramovich-Tirosh, Y.; Youdim, M.B.; Weinreb, O.; Mandel, S. Reduction of iron-regulated amyloid precursor protein and beta-amyloid peptide by (-)-epigallocatechin-3-gallate in cell cultures: Implications for iron chelation in Alzheimer’s disease. J. Neurochem. 2006, 97, 527–536. [Google Scholar] [CrossRef]
- Hensley, K. Neuroinflammation in Alzheimer’s disease: Mechanisms, pathologic consequences, and potential for therapeutic manipulation. J. Alzheimers Dis. 2010, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Schulzer, M.; McGeer, E.G. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: A review of 17 epidemiologic studies. Neurology 1996, 47, 425–432. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; McGeer, E.G. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neurobiol. Aging 2007, 28, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Frischer, J.M.; Bramow, S.; Dal-Bianco, A.; Lucchinetti, C.F.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Lassmann, H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 2009, 132, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Thawkar, B.S.; Kaur, G. Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer’s disease. J. Neuroimmunol. 2019, 326, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhao, Z.; Zhang, R.; Chen, P.; Zhang, X.; Cheng, F.; Gou, X. Monocytes in the Peripheral Clearance of Amyloid-β and Alzheimer’s Disease. J. Alzheimers Dis. 2019, 68, 1391–1400. [Google Scholar] [CrossRef]
- Lawson, L.J.; Perry, V.H.; Gordon, S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience 1992, 48, 405–415. [Google Scholar] [CrossRef]
- Wang, D.; Liu, F.; Zhu, L.; Lin, P.; Han, F.; Wang, X.; Tan, X.; Lin, L.; Xiong, Y. FGF21 alleviates neuroinflammation following ischemic stroke by modulating the temporal and spatial dynamics of microglia/macrophages. J. Neuroinflammation 2020, 17, 257. [Google Scholar] [CrossRef]
- Fu, A.K.; Hung, K.W.; Yuen, M.Y.; Zhou, X.; Mak, D.S.; Chan, I.C.; Cheung, T.H.; Zhang, B.; Fu, W.Y.; Liew, F.Y.; et al. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc. Natl. Acad. Sci. USA 2016, 113, E2705–E2713. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Marsh, S.E.; Stevens, B. Microglia and Astrocytes in Disease: Dynamic Duo or Partners in Crime? Trends Immunol. 2020, 41, 820–835. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Sharma, V.; Deshmukh, R. Activation of microglia and astrocytes: A roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology 2019, 27, 663–677. [Google Scholar] [CrossRef]
- Liu, C.; Cui, G.; Zhu, M.; Kang, X.; Guo, H. Neuroinflammation in Alzheimer’s disease: Chemokines produced by astrocytes and chemokine receptors. Int. J. Clin. Exp. Pathol. 2014, 7, 8342–8355. [Google Scholar]
- Zuroff, L.; Daley, D.; Black, K.L.; Koronyo-Hamaoui, M. Clearance of cerebral Aβ in Alzheimer’s disease: Reassessing the role of microglia and monocytes. Cell. Mol. Life Sci. 2017, 74, 2167–2201. [Google Scholar] [CrossRef]
- Saresella, M.; Marventano, I.; Calabrese, E.; Piancone, F.; Rainone, V.; Gatti, A.; Alberoni, M.; Nemni, R.; Clerici, M. A complex proinflammatory role for peripheral monocytes in Alzheimer’s disease. J. Alzheimers Dis. 2014, 38, 403–413. [Google Scholar] [CrossRef]
- Gu, B.J.; Huang, X.; Ou, A.; Rembach, A.; Fowler, C.; Avula, P.K.; Horton, A.; Doecke, J.D.; Villemagne, V.L.; Macaulay, S.L.; et al. Innate phagocytosis by peripheral blood monocytes is altered in Alzheimer’s disease. Acta Neuropathol. 2016, 132, 377–389. [Google Scholar] [CrossRef]
- Fiala, M.; Lin, J.; Ringman, J.; Kermani-Arab, V.; Tsao, G.; Patel, A.; Lossinsky, A.S.; Graves, M.C.; Gustavson, A.; Sayre, J.; et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2005, 7, 221–232; discussion 255–262. [Google Scholar] [CrossRef]
- Yuan, J.; Amin, P.; Ofengeim, D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat. Rev. Neurosci. 2019, 20, 19–33. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Seyedzadeh, M.H.; Safari, Z.; Zare, A.; Gholizadeh Navashenaq, J.; Razavi, S.A.; Kardar, G.A.; Khorramizadeh, M.R. Study of curcumin immunomodulatory effects on reactive astrocyte cell function. Int. Immunopharmacol. 2014, 22, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ran, Y.; Huang, S.; Wen, S.; Zhang, W.; Liu, X.; Ji, Z.; Geng, X.; Ji, X.; Du, H.; et al. Curcumin Protects against Ischemic Stroke by Titrating Microglia/Macrophage Polarization. Front. Aging Neurosci. 2017, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Liang, H.; Niedermayer, G.; Münch, G.; Gyengesi, E. Evaluation of Phytosomal Curcumin as an Anti-inflammatory Agent for Chronic Glial Activation in the GFAP-IL6 Mouse Model. Front. Neurosci. 2020, 14, 170. [Google Scholar] [CrossRef]
- Zhang, L.; Fiala, M.; Cashman, J.; Sayre, J.; Espinosa, A.; Mahanian, M.; Zaghi, J.; Badmaev, V.; Graves, M.C.; Bernard, G.; et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2006, 10, 1–7. [Google Scholar] [CrossRef]
- Mohammadi, A.; Blesso, C.N.; Barreto, G.E.; Banach, M.; Majeed, M.; Sahebkar, A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J. Nutr. Biochem. 2019, 66, 1–16. [Google Scholar] [CrossRef]
- Yang, X.; Xu, S.; Qian, Y.; Xiao, Q. Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 2017, 64, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Bellaver, B.; Souza, D.G.; Bobermin, L.D.; Souza, D.O.; Gonçalves, C.A.; Quincozes-Santos, A. Resveratrol Protects Hippocampal Astrocytes Against LPS-Induced Neurotoxicity Through HO-1, p38 and ERK Pathways. Neurochem. Res. 2015, 40, 1600–1608. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Q.; Cheng, X.; Li, X.; Li, N.; Liu, T.; Li, J.; Yang, Q.; Dong, R.; Zhang, Y.; et al. Inhibitive Effect of Resveratrol on the Inflammation in Cultured Astrocytes and Microglia Induced by Aβ(1-42). Neuroscience 2018, 379, 390–404. [Google Scholar] [CrossRef]
- Lee, E.O.; Park, H.J.; Kang, J.L.; Kim, H.S.; Chong, Y.H. Resveratrol reduces glutamate-mediated monocyte chemotactic protein-1 expression via inhibition of extracellular signal-regulated kinase 1/2 pathway in rat hippocampal slice cultures. J. Neurochem. 2010, 112, 1477–1487. [Google Scholar] [CrossRef]
- Han, S.; Gao, H.; Chen, S.; Wang, Q.; Li, X.; Du, L.J.; Li, J.; Luo, Y.Y.; Li, J.X.; Zhao, L.C.; et al. Procyanidin A1 Alleviates Inflammatory Response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 Pathways in RAW264.7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yang, C.; Yao, Q.; Qian, L.; Liu, J.; Xie, X.; Ma, W.; Nie, X.; Lai, B.; Xiao, L.; et al. Procyanidin B2 Activates PPARγ to Induce M2 Polarization in Mouse Macrophages. Front. Immunol. 2019, 10, 1895. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Palozza, P.; Fernandez-Larrea, J.; Ardevol, A.; Blade, C.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M.T. Procyanidin dimer B1 and trimer C1 impair inflammatory response signalling in human monocytes. Free Radic. Res. 2011, 45, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.B.; Sung, N.Y.; Byun, E.H.; Song, D.S.; Kim, J.K.; Park, J.H.; Song, B.S.; Park, S.H.; Lee, J.W.; Byun, M.W.; et al. The procyanidin trimer C1 inhibits LPS-induced MAPK and NF-κB signaling through TLR4 in macrophages. Int. Immunopharmacol. 2013, 15, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xiong, R.; Wu, A.G.; Yu, C.L.; Zhao, Y.; Qiu, W.Q.; Wang, X.L.; Teng, J.F.; Liu, J.; Chen, H.X.; et al. Polyphenols Derived from Lychee Seed Suppress Aβ (1-42)-Induced Neuroinflammation. Int. J. Mol. Sci. 2018, 19, 2109. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wang, C.; Zhang, L.; Song, L.; Chen, Y.; Liu, B.; Liu, W.T.; Hu, L.; Pan, Y. Procyanidins attenuate neuropathic pain by suppressing matrix metalloproteinase-9/2. J. Neuroinflamm. 2018, 15, 187. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Kong, H.; Pan, Y.B.; Jiang, L.; Pan, X.X.; Hu, L.; Qian, Y.N.; Jiang, C.Y.; Liu, W.T. Procyanidins alleviates morphine tolerance by inhibiting activation of NLRP3 inflammasome in microglia. J. Neuroinflamm. 2016, 13, 53. [Google Scholar] [CrossRef]
- Thal, D.R.; Walter, J.; Saido, T.C.; Fändrich, M. Neuropathology and biochemistry of Aβ and its aggregates in Alzheimer’s disease. Acta Neuropathol. 2015, 129, 167–182. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Rajmohan, R.; Reddy, P.H. Amyloid-Beta and Phosphorylated Tau Accumulations Cause Abnormalities at Synapses of Alzheimer’s disease Neurons. J. Alzheimers Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef]
- Patterson, C.; Feightner, J.W.; Garcia, A.; Hsiung, G.Y.; MacKnight, C.; Sadovnick, A.D. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. CMAJ 2008, 178, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Sadleir, K.R.; Kandalepas, P.C.; Buggia-Prévot, V.; Nicholson, D.A.; Thinakaran, G.; Vassar, R. Presynaptic dystrophic neurites surrounding amyloid plaques are sites of microtubule disruption, BACE1 elevation, and increased Aβ generation in Alzheimer’s disease. Acta Neuropathol. 2016, 132, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.R.; Bangen, K.J.; Weigand, A.J.; Edmonds, E.C.; Wong, C.G.; Cooper, S.; Delano-Wood, L.; Bondi, M.W. Objective subtle cognitive difficulties predict future amyloid accumulation and neurodegeneration. Neurology 2020, 94, e397–e406. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, F.J.; Inestrosa, N.C. Interactions of AChE with Aβ Aggregates in Alzheimer’s Brain: Therapeutic Relevance of IDN 5706. Front. Mol. Neurosci. 2011, 4, 19. [Google Scholar] [CrossRef]
- Swerdlow, R.H. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 62, 1403–1416. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-β and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef]
- Scheltens, P.; Blennow, K.; Breteler, M.M.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Zheng, Q.; Kebede, M.T.; Kemeh, M.M.; Islam, S.; Lee, B.; Bleck, S.D.; Wurfl, L.A.; Lazo, N.D. Inhibition of the Self-Assembly of Aβ and of Tau by Polyphenols: Mechanistic Studies. Molecules 2019, 24, 2316. [Google Scholar] [CrossRef]
- So, M.; Kimura, Y.; Yamaguchi, K.; Sugiki, T.; Fujiwara, T.; Aguirre, C.; Ikenaka, K.; Mochizuki, H.; Kawata, Y.; Goto, Y. Polyphenol-solubility alters amyloid fibril formation of α-synuclein. Protein Sci. 2021, 30, 1701–1713. [Google Scholar] [CrossRef]
- Choi, C.W.; Choi, Y.H.; Cha, M.R.; Kim, Y.S.; Yon, G.H.; Hong, K.S.; Park, W.K.; Kim, Y.H.; Ryu, S.Y. In vitro BACE-1 inhibitory activity of resveratrol oligomers from the seed extract of Paeonia lactiflora. Planta Med. 2011, 77, 374–376. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yoo, M.Y.; Choi, C.W.; Cha, M.R.; Yon, G.H.; Kwon, D.Y.; Kim, Y.S.; Park, W.K.; Ryu, S.Y. A new specific BACE-1 inhibitor from the stembark extract of Vitis vinifera. Planta Med. 2009, 75, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Kim, S.; Jang, B.G.; Kim, M.J. Piceatannol, a natural analogue of resveratrol, effectively reduces beta-amyloid levels via activation of alpha-secretase and matrix metalloproteinase-9. J. Funct. Foods 2016, 23, 124–134. [Google Scholar] [CrossRef]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [PubMed]
- Miners, J.S.; Barua, N.; Kehoe, P.G.; Gill, S.; Love, S. Aβ-degrading enzymes: Potential for treatment of Alzheimer disease. J. Neuropathol. Exp. Neurol. 2011, 70, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Melchor, J.P.; Pawlak, R.; Strickland, S. The tissue plasminogen activator-plasminogen proteolytic cascade accelerates amyloid-beta (Abeta) degradation and inhibits Abeta-induced neurodegeneration. J. Neurosci. 2003, 23, 8867–8871. [Google Scholar] [CrossRef]
- Santos, L.M.; Rodrigues, D.; Alemi, M.; Silva, S.C.; Ribeiro, C.A.; Cardoso, I. Resveratrol administration increases Transthyretin protein levels ameliorating AD features- importance of transthyretin tetrameric stability. Mol. Med. 2016, 22, 597–607. [Google Scholar] [CrossRef]
- Qiang, W.; Yau, W.M.; Lu, J.X.; Collinge, J.; Tycko, R. Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature 2017, 541, 217–221. [Google Scholar] [CrossRef]
- Tanaka, T.; Betkekar, V.V.; Ohmori, K.; Suzuki, K.; Shigemori, H. Evaluation of Amyloid Polypeptide Aggregation Inhibition and Disaggregation Activity of A-Type Procyanidins. Pharmaceuticals 2021, 14, 1118. [Google Scholar] [CrossRef] [PubMed]
- Toda, T.; Sunagawa, T.; Kanda, T.; Tagashira, M.; Shirasawa, T.; Shimizu, T. Apple Procyanidins Suppress Amyloid β-Protein Aggregation. Biochem. Res. Int. 2011, 2011, 784698. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Mori, T.; Tanaka, T.; Mizuno, D.; Yamaji, R.; Kumazawa, S.; Nakayama, T.; Akagawa, M. Covalent modification of proteins by green tea polyphenol (-)-epigallocatechin-3-gallate through autoxidation. Free Radic. Biol. Med. 2008, 45, 1384–1394. [Google Scholar] [CrossRef]
- Sato, M.; Murakami, K.; Uno, M.; Nakagawa, Y.; Katayama, S.; Akagi, K.; Masuda, Y.; Takegoshi, K.; Irie, K. Site-specific inhibitory mechanism for amyloid β42 aggregation by catechol-type flavonoids targeting the Lys residues. J. Biol. Chem. 2013, 288, 23212–23224. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S. When quinones meet amino acids: Chemical, physical and biological consequences. Amino Acids 2006, 30, 205–224. [Google Scholar] [CrossRef] [PubMed]
- Shinde, M.N.; Barooah, N.; Bhasikuttan, A.C.; Mohanty, J. Inhibition and disintegration of insulin amyloid fibrils: A facile supramolecular strategy with p-sulfonatocalixarenes. Chem. Commun. 2016, 52, 2992–2995. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, T.; Huang, X.; Wang, Y.; Yin, G.; Du, W. Procyanidine resists the fibril formation of human islet amyloid polypeptide. Int. J. Biol. Macromol. 2021, 183, 1067–1078. [Google Scholar] [CrossRef]
- Park, G.; Xue, C.; Wang, H.; Guo, Z. Distinguishing the Effect on the Rate and Yield of Aβ42 Aggregation by Green Tea Polyphenol EGCG. ACS Omega 2020, 5, 21497–21505. [Google Scholar] [CrossRef]
- Chan, S.; Kantham, S.; Rao, V.M.; Palanivelu, M.K.; Pham, H.L.; Shaw, P.N.; McGeary, R.P.; Ross, B.P. Metal chelation, radical scavenging and inhibition of Aβ₄₂ fibrillation by food constituents in relation to Alzheimer’s disease. Food Chem. 2016, 199, 185–194. [Google Scholar] [CrossRef]
- Ahmed, R.; VanSchouwen, B.; Jafari, N.; Ni, X.; Ortega, J.; Melacini, G. Molecular Mechanism for the (-)-Epigallocatechin Gallate-Induced Toxic to Nontoxic Remodeling of Aβ Oligomers. J. Am. Chem. Soc. 2017, 139, 13720–13734. [Google Scholar] [CrossRef]
- Lopez del Amo, J.M.; Fink, U.; Dasari, M.; Grelle, G.; Wanker, E.E.; Bieschke, J.; Reif, B. Structural properties of EGCG-induced, nontoxic Alzheimer’s disease Aβ oligomers. J. Mol. Biol. 2012, 421, 517–524. [Google Scholar] [CrossRef]
- Bao, J.; Liu, W.; Zhou, H.Y.; Gui, Y.R.; Yang, Y.H.; Wu, M.J.; Xiao, Y.F.; Shang, J.T.; Long, G.F.; Shu, X.J. Epigallocatechin-3-gallate Alleviates Cognitive Deficits in APP/PS1 Mice. Curr. Med. Sci. 2020, 40, 18–27. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Shytle, D.; Sun, N.; Mori, T.; Hou, H.; Jeanniton, D.; Ehrhart, J.; Townsend, K.; Zeng, J.; Morgan, D.; et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005, 25, 8807–8814. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, Y.K.; Ban, J.O.; Ha, T.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 2009, 139, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in Alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1159. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Xia, Y.Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal hyperphosphorylation of tau: Sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimers Dis. 2013, 33 (Suppl. 1), S123–S139. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Emeršič, A.; Vrillon, A.; Lantero-Rodriguez, J.; Ashton, N.J.; Kramberger, M.G.; Dumurgier, J.; Hourregue, C.; Čučnik, S.; Brinkmalm, G.; et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimers Dement. 2021, 17, 755–767. [Google Scholar] [CrossRef]
- Hoskin, J.L.; Sabbagh, M.N.; Al-Hasan, Y.; Decourt, B. Tau immunotherapies for Alzheimer’s disease. Expert Opin. Investig. Drugs 2019, 28, 545–554. [Google Scholar] [CrossRef]
- Ruthirakuhan, M.; Herrmann, N.; Suridjan, I.; Abraham, E.H.; Farber, I.; Lanctôt, K.L. Beyond immunotherapy: New approaches for disease modifying treatments for early Alzheimer’s disease. Expert Opin. Pharmacother. 2016, 17, 2417–2429. [Google Scholar] [CrossRef]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76 Pt A, 27–50. [Google Scholar] [CrossRef]
- Sivanantharajah, L.; Mudher, A. Curcumin as a Holistic Treatment for Tau Pathology. Front. Pharmacol. 2022, 13, 903119. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zuo, X.; Yang, F.; Ubeda, O.J.; Gant, D.J.; Alaverdyan, M.; Teng, E.; Hu, S.; Chen, P.P.; Maiti, P.; et al. Curcumin suppresses soluble tau dimers and corrects molecular chaperone, synaptic, and behavioral deficits in aged human tau transgenic mice. J. Biol. Chem. 2013, 288, 4056–4065. [Google Scholar] [CrossRef]
- Rane, J.S.; Bhaumik, P.; Panda, D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. J. Alzheimers Dis. 2017, 60, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, T.; Xie, C.; Yoshimura, S.; Shinzaki, Y.; Yoshina, S.; Kage-Nakadai, E.; Mitani, S.; Ihara, Y. Curcumin improves tau-induced neuronal dysfunction of nematodes. Neurobiol. Aging 2016, 39, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Chidambaram, H.; Boral, D.; Gorantla, N.V.; Balmik, A.A.; Dangi, A.; Ramasamy, S.; Marelli, U.K.; Chinnathambi, S. EGCG impedes human Tau aggregation and interacts with Tau. Sci. Rep. 2020, 10, 12579. [Google Scholar] [CrossRef]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chesser, A.S.; Ganeshan, V.; Yang, J.; Johnson, G.V. Epigallocatechin-3-gallate enhances clearance of phosphorylated tau in primary neurons. Nutr. Neurosci. 2016, 19, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Arendash, G.W.; Hou, H.; Fernandez, F.; Jensen, M.; Runfeldt, M.; Shytle, R.D.; Tan, J. Green tea epigallocatechin-3-gallate (EGCG) reduces beta-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008, 1214, 177–187. [Google Scholar] [CrossRef]
- Akıncıoğlu, H.; Gülçin, İ. Potent Acetylcholinesterase Inhibitors: Potential Drugs for Alzheimer’s Disease. Mini Rev. Med. Chem. 2020, 20, 703–715. [Google Scholar] [CrossRef]
- Shekari, A.; Fahnestock, M. Retrograde axonal transport of BDNF and proNGF diminishes with age in basal forebrain cholinergic neurons. Neurobiol. Aging 2019, 84, 131–140. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Kukreja, H.; Chugh, R.; Silakari, O.; Singh, D. Acetylcholinesterase inhibitors as Alzheimer therapy: From nerve toxins to neuroprotection. Eur. J. Med. Chem. 2013, 70, 165–188. [Google Scholar] [CrossRef]
- Korabecny, J.; Spilovska, K.; Mezeiova, E.; Benek, O.; Juza, R.; Kaping, D.; Soukup, O. A Systematic Review on Donepezil-based Derivatives as Potential Cholinesterase Inhibitors for Alzheimer’s Disease. Curr. Med. Chem. 2019, 26, 5625–5648. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Oboh, G.; Fadaka, A.O.; Olatunji, B.P.; Akomolafe, S. Curcumin administration suppress acetylcholinesterase gene expression in cadmium treated rats. Neurotoxicology 2017, 62, 75–79. [Google Scholar] [CrossRef]
- Agrawal, R.; Mishra, B.; Tyagi, E.; Nath, C.; Shukla, R. Effect of curcumin on brain insulin receptors and memory functions in STZ (ICV) induced dementia model of rat. Pharmacol. Res. 2010, 61, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Okello, E.J.; Mather, J. Comparative Kinetics of Acetyl- and Butyryl-Cholinesterase Inhibition by Green Tea Catechins|Relevance to the Symptomatic Treatment of Alzheimer’s Disease. Nutrients 2020, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Srividhya, R.; Gayathri, R.; Kalaiselvi, P. Impact of epigallo catechin-3-gallate on acetylcholine-acetylcholine esterase cycle in aged rat brain. Neurochem. Int. 2012, 60, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Jamal, Q.M.; Shams, S.; Al-Wabel, N.A.; Siddiqui, M.U.; Alzohairy, M.A.; Al Karaawi, M.A.; Kesari, K.K.; Mushtaq, G.; Kamal, M.A. In Silico Analysis of Green Tea Polyphenols as Inhibitors of AChE and BChE Enzymes in Alzheimer’s Disease Treatment. CNS Neurol Disord. Drug Targets 2016, 15, 624–628. [Google Scholar] [CrossRef]
- Utermann, G. Isolation and partial characterization of an arginine-rich apolipoprotein from human plasma very-low-density lipoproteins: Apolipoprotein E. Hoppe Seylers Z. Physiol. Chem. 1975, 356, 1113–1121. [Google Scholar] [CrossRef]
- Shore, V.G.; Shore, B. Heterogeneity of human plasma very low density lipoproteins. Separation of species differing in protein components. Biochemistry 1973, 12, 502–507. [Google Scholar] [CrossRef]
- Wernette-Hammond, M.E.; Lauer, S.J.; Corsini, A.; Walker, D.; Taylor, J.M.; Rall, S.C., Jr. Glycosylation of human apolipoprotein E. The carbohydrate attachment site is threonine 194. J. Biol. Chem. 1989, 264, 9094–9101. [Google Scholar] [CrossRef]
- Wang, T.; Dong, Y.; Yao, L.; Lu, F.; Wen, C.; Wan, Z.; Fan, L.; Li, Z.; Bu, T.; Wei, M.; et al. Adoptive transfer of metabolically reprogrammed macrophages for atherosclerosis treatment in diabetic ApoE (-/-) mice. Bioact. Mater. 2022, 16, 82–94. [Google Scholar] [CrossRef]
- Iwaki, T.; Arakawa, T.; Sandoval-Cooper, M.J.; Smith, D.L.; Donahue, D.; Ploplis, V.A.; Umemura, K.; Castellino, F.J. Plasminogen Deficiency Significantly Reduces Vascular Wall Disease in a Murine Model of Type IIa Hypercholesterolemia. Biomedicines 2021, 9, 1832. [Google Scholar] [CrossRef]
- Chen, Y.; Strickland, M.R.; Soranno, A.; Holtzman, D.M. Apolipoprotein E: Structural Insights and Links to Alzheimer Disease Pathogenesis. Neuron 2021, 109, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Lindner, K.; Beckenbauer, K.; van Ek, L.C.; Titeca, K.; de Leeuw, S.M.; Awwad, K.; Hanke, F.; Korepanova, A.V.; Rybin, V.; van der Kam, E.L.; et al. Isoform- and cell-state-specific lipidation of ApoE in astrocytes. Cell Rep. 2022, 38, 110435. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Carnwath, T.P.; Wang, X.; Allen, M.; Lincoln, S.J.; Lewis-Tuffin, L.J.; Quicksall, Z.S.; Lin, S.; Tutor-New, F.Q.; Ho, C.C.G.; et al. Transcriptional landscape of human microglia implicates age, sex, and APOE-related immunometabolic pathway perturbations. Aging Cell 2022, 21, e13606. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Gmeiner, B.; Hüttinger, M. Implication of apoE isoforms in cholesterol metabolism by primary rat hippocampal neurons and astrocytes. Biochimie 2006, 88, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Pagnon de la Vega, M.; Näslund, C.; Brundin, R.; Lannfelt, L.; Löwenmark, M.; Kilander, L.; Ingelsson, M.; Giedraitis, V. Mutation analysis of disease causing genes in patients with early onset or familial forms of Alzheimer’s disease and frontotemporal dementia. BMC Genom. 2022, 23, 99. [Google Scholar] [CrossRef]
- Jin, Y.; Li, F.; Sonoustoun, B.; Kondru, N.C.; Martens, Y.A.; Qiao, W.; Heckman, M.G.; Ikezu, T.C.; Li, Z.; Burgess, J.D.; et al. APOE4 exacerbates α-synuclein seeding activity and contributes to neurotoxicity in Alzheimer’s disease with Lewy body pathology. Acta Neuropathol. 2022, 143, 641–662. [Google Scholar] [CrossRef]
- Han, S.H.; Noh, D.H.; Jo, E.J.; Kam, K.Y. Effects of Apolipoprotein E ɛ4 and Risk Factors on Domains of Cognition in Mild Cognitive Impairment and Dementia. J. Alzheimers Dis. 2022, 87, 1181–1188. [Google Scholar] [CrossRef]
- Baek, M.S.; Cho, H.; Lee, H.S.; Lee, J.H.; Ryu, Y.H.; Lyoo, C.H. Effect of APOE ε4 genotype on amyloid-β and tau accumulation in Alzheimer’s disease. Alzheimers Res. Ther. 2020, 12, 140. [Google Scholar] [CrossRef]
- Shi, D.; Xie, S.; Li, A.; Wang, Q.; Guo, H.; Han, Y.; Xu, H.; Gan, W.B.; Zhang, L.; Guo, T. APOE-ε4 modulates the association among plasma Aβ(42)/Aβ(40), vascular diseases, neurodegeneration and cognitive decline in non-demented elderly adults. Transl. Psychiatry 2022, 12, 128. [Google Scholar] [CrossRef]
- Huang, Y.A.; Zhou, B.; Wernig, M.; Südhof, T.C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell 2017, 168, 427–441.e421. [Google Scholar] [CrossRef]
- Dafnis, I.; Argyri, L.; Chroni, A. Amyloid-peptide β 42 Enhances the Oligomerization and Neurotoxicity of apoE4: The C-terminal Residues Leu279, Lys282 and Gln284 Modulate the Structural and Functional Properties of apoE4. Neuroscience 2018, 394, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.V.; Davis, A.A.; Ulrich, J.D.; Holtzman, D.M. Apolipoprotein E and Alzheimer’s disease: The influence of apolipoprotein E on amyloid-β and other amyloidogenic proteins. J. Lipid Res. 2017, 58, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhao, Z.; Sagare, A.P.; Wu, Y.; Wang, M.; Owens, N.C.; Verghese, P.B.; Herz, J.; Holtzman, D.M.; Zlokovic, B.V. Blood-brain barrier-associated pericytes internalize and clear aggregated amyloid-β42 by LRP1-dependent apolipoprotein E isoform-specific mechanism. Mol. Neurodegener. 2018, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Holtzman, D.M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 2018, 18, 759–772. [Google Scholar] [CrossRef]
- Farfel, J.M.; Yu, L.; De Jager, P.L.; Schneider, J.A.; Bennett, D.A. Association of APOE with tau-tangle pathology with and without β-amyloid. Neurobiol. Aging 2016, 37, 19–25. [Google Scholar] [CrossRef]
- Gale, S.C.; Gao, L.; Mikacenic, C.; Coyle, S.M.; Rafaels, N.; Murray Dudenkov, T.; Madenspacher, J.H.; Draper, D.W.; Ge, W.; Aloor, J.J.; et al. APOε4 is associated with enhanced in vivo innate immune responses in human subjects. J. Allergy Clin. Immunol. 2014, 134, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Cullen, A.E.; Centner, A.M.; Deitado, R.; Salazar, J.F.A. The Impact of Dietary Supplementation of Whole Foods and Polyphenols on Atherosclerosis. Nutrients 2020, 12, 2069. [Google Scholar] [CrossRef]
- Ghura, S.; Tai, L.; Zhao, M.; Collins, N.; Che, C.T.; Warpeha, K.M.; LaDu, M.J. Arabidopsis thaliana extracts optimized for polyphenols production as potential therapeutics for the APOE-modulated neuroinflammation characteristic of Alzheimer’s disease in vitro. Sci. Rep. 2016, 6, 29364. [Google Scholar] [CrossRef]
- Mountaki, C.; Dafnis, I.; Panagopoulou, E.A.; Vasilakopoulou, P.B.; Karvelas, M.; Chiou, A.; Karathanos, V.T.; Chroni, A. Mechanistic insight into the capacity of natural polar phenolic compounds to abolish Alzheimer’s disease-associated pathogenic effects of apoE4 forms. Free Radic. Biol. Med. 2021, 171, 284–301. [Google Scholar] [CrossRef]
- Aubert, L.; Pichierri, S.; Hommet, C.; Camus, V.; Berrut, G.; de Decker, L. Association between comorbidity burden and rapid cognitive decline in individuals with mild to moderate Alzheimer’s disease. J. Am. Geriatr. Soc. 2015, 63, 543–547. [Google Scholar] [CrossRef]
- Cooper, C.; Sommerlad, A.; Lyketsos, C.G.; Livingston, G. Modifiable predictors of dementia in mild cognitive impairment: A systematic review and meta-analysis. Am. J. Psychiatry 2015, 172, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Burillo, J.; Marqués, P.; Jiménez, B.; González-Blanco, C.; Benito, M.; Guillén, C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef]
- Moller, D.E.; Flier, J.S. Insulin resistance--mechanisms, syndromes, and implications. N. Engl. J. Med. 1991, 325, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.L.; Dang, H.Z.; Fan, H.; Chen, X.P.; Rao, Y.X.; Ren, Y.; Yang, J.D.; Shi, J.; Wang, P.W.; Tian, J.Z. Curcumin ameliorates insulin signalling pathway in brain of Alzheimer’s disease transgenic mice. Int. J. Immunopathol. Pharmacol. 2016, 29, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Kebede, M.T.; Lee, B.; Krasinski, C.A.; Islam, S.; Wurfl, L.A.; Kemeh, M.M.; Ivancic, V.A.; Jakobsche, C.E.; Spratt, D.E.; et al. Differential Effects of Polyphenols on Insulin Proteolysis by the Insulin-Degrading Enzyme. Antioxidants 2021, 10, 1342. [Google Scholar] [CrossRef]
- Krasinski, C.A.; Ivancic, V.A.; Zheng, Q.; Spratt, D.E.; Lazo, N.D. Resveratrol Sustains Insulin-Degrading Enzyme Activity toward Aβ42. ACS Omega 2018, 3, 13275–13282. [Google Scholar] [CrossRef] [PubMed]
- Kapogiannis, D.; Mattson, M.P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011, 10, 187–198. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Ramesh, S.; Neel, L.; Fabbrini, M.; Buabeid, M.; Fujihashi, A.; Dwyer, D.; Lynd, T.; Shah, K.; Mohanakumar, K.P.; et al. Nutraceutical based SIRT3 activators as therapeutic targets in Alzheimer’s disease. Neurochem. Int. 2021, 144, 104958. [Google Scholar] [CrossRef]
- Chen, C.M.; Liu, S.H.; Lin-Shiau, S.Y. Honokiol, a neuroprotectant against mouse cerebral ischaemia, mediated by preserving Na+, K+-ATPase activity and mitochondrial functions. Basic Clin. Pharmacol. Toxicol. 2007, 101, 108–116. [Google Scholar] [CrossRef]
- Esselun, C.; Theyssen, E.; Eckert, G.P. Effects of Urolithin A on Mitochondrial Parameters in a Cellular Model of Early Alzheimer Disease. Int. J. Mol. Sci. 2021, 22, 8333. [Google Scholar] [CrossRef]
- Lee, J.H.; Yang, D.S.; Goulbourne, C.N.; Im, E.; Stavrides, P.; Pensalfini, A.; Chan, H.; Bouchet-Marquis, C.; Bleiwas, C.; Berg, M.J.; et al. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques. Nat. Neurosci. 2022, 25, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Arotcarena, M.L.; Soria, F.N.; Cunha, A.; Doudnikoff, E.; Prévot, G.; Daniel, J.; Blanchard-Desce, M.; Barthélémy, P.; Bezard, E.; Crauste-Manciet, S.; et al. Acidic nanoparticles protect against α-synuclein-induced neurodegeneration through the restoration of lysosomal function. Aging Cell 2022, 21, e13584. [Google Scholar] [CrossRef] [PubMed]
- Armour, S.M.; Baur, J.A.; Hsieh, S.N.; Land-Bracha, A.; Thomas, S.M.; Sinclair, D.A. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging 2009, 1, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Maiuri, M.C.; Markaki, M.; Megalou, E.; Pasparaki, A.; Palikaras, K.; Criollo, A.; Galluzzi, L.; Malik, S.A.; Vitale, I.; et al. The life span-prolonging effect of sirtuin-1 is mediated by autophagy. Autophagy 2010, 6, 186–188. [Google Scholar] [CrossRef]
- Borra, M.T.; Smith, B.C.; Denu, J.M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005, 280, 17187–17195. [Google Scholar] [CrossRef]
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients 2020, 12, 1344. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, J.; Shu, W.; Gao, B.; Xiong, S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015, 6, e1770. [Google Scholar] [CrossRef]
- Li, D.; Cui, Y.; Wang, X.; Liu, F.; Li, X. Apple polyphenol extract alleviates lipid accumulation in free-fatty-acid-exposed HepG2 cells via activating autophagy mediated by SIRT1/AMPK signaling. Phytother. Res. 2021, 35, 1416–1431. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Bartolomé, B.; Peñalvo, J.L.; Pérez-Matute, P.; Motilva, M.J. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients 2020, 12, 3082. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Z.; Li, X.Y.; Wang, S.; Shen, L.; Ji, H.F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, N.; Zheng, G.; Yang, L. Oral Administration of Resveratrol-Selenium-Peptide Nanocomposites Alleviates Alzheimer’s Disease-like Pathogenesis by Inhibiting Aβ Aggregation and Regulating Gut Microbiota. ACS Appl. Mater. Interfaces 2021, 13, 46406–46420. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, E.; O’Driscoll, N.A.; Matallanas, D. The MST/Hippo Pathway and Cell Death: A Non-Canonical Affair. Genes 2016, 7, 28. [Google Scholar] [CrossRef]
- Khan, M.; Rutten, B.P.F.; Kim, M.O. MST1 Regulates Neuronal Cell Death via JNK/Casp3 Signaling Pathway in HFD Mouse Brain and HT22 Cells. Int. J. Mol. Sci. 2019, 20, 2504. [Google Scholar] [CrossRef]
- Wang, H.; Shang, Y.; Wang, E.; Xu, X.; Zhang, Q.; Qian, C.; Yang, Z.; Wu, S.; Zhang, T. MST1 mediates neuronal loss and cognitive deficits: A novel therapeutic target for Alzheimer’s disease. Prog. Neurobiol. 2022, 214, 102280. [Google Scholar] [CrossRef]
- Li, Q.; Qiu, Z.; Wang, Y.; Guo, C.; Cai, X.; Zhang, Y.; Liu, L.; Xue, H.; Tang, J. Tea polyphenols alleviate hydrogen peroxide-induced oxidative stress damage through the Mst/Nrf2 axis and the Keap1/Nrf2/HO-1 pathway in murine RAW264.7 cells. Exp. Ther. Med. 2021, 22, 1473. [Google Scholar] [CrossRef]
- Stanger, B.Z.; Leder, P.; Lee, T.H.; Kim, E.; Seed, B. RIP: A novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell 1995, 81, 513–523. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Strilic, B.; Yang, L.; Albarrán-Juárez, J.; Wachsmuth, L.; Han, K.; Müller, U.C.; Pasparakis, M.; Offermanns, S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 2016, 536, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Ofengeim, D.; Mazzitelli, S.; Ito, Y.; DeWitt, J.P.; Mifflin, L.; Zou, C.; Das, S.; Adiconis, X.; Chen, H.; Zhu, H.; et al. RIPK1 mediates a disease-associated microglial response in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, e8788–e8797. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Lee, D.K.; Shin, J.; Lee, S.; Baek, S.; Kim, J.; Jung, H.; Hah, J.M.; Kim, Y. Nec-1 alleviates cognitive impairment with reduction of Aβ and tau abnormalities in APP/PS1 mice. EMBO Mol. Med. 2017, 9, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, R.; Xia, S.; Wei, G.; Ishfaq, M.; Zhang, Y.; Zhang, X. Protective role of curcumin on aflatoxin B1-induced TLR4/RIPK pathway mediated-necroptosis and inflammation in chicken liver. Ecotoxicol. Environ. Saf. 2022, 233, 113319. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Pan, H.; Peng, J.; He, J.; Tang, M.; Yan, S.; Rong, J.; Li, J.; Zheng, Z.; Wang, H.; et al. Resveratrol inhibits necroptosis by mediating the TNF-α/RIP1/RIP3/MLKL pathway in myocardial hypoxia/reoxygenation injury. Acta Biochim. Biophys. Sin. 2021, 53, 430–437. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, anti-amyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef]
- Dourado, N.S.; Souza, C.D.S.; de Almeida, M.M.A.; Bispo da Silva, A.; Dos Santos, B.L.; Silva, V.D.A.; De Assis, A.M.; da Silva, J.S.; Souza, D.O.; Costa, M.F.D.; et al. Neuroimmunomodulatory and Neuroprotective Effects of the Flavonoid Apigenin in in vitro Models of Neuroinflammation Associated with Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 119. [Google Scholar] [CrossRef]
- Jin, X.; Liu, M.Y.; Zhang, D.F.; Zhong, X.; Du, K.; Qian, P.; Yao, W.F.; Gao, H.; Wei, M.J. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 2019, 25, 575–590. [Google Scholar] [CrossRef]
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen. Res. 2018, 13, 1827–1832. [Google Scholar] [CrossRef]
- Kou, J.J.; Shi, J.Z.; He, Y.Y.; Hao, J.J.; Zhang, H.Y.; Luo, D.M.; Song, J.K.; Yan, Y.; Xie, X.M.; Du, G.H.; et al. Luteolin alleviates cognitive impairment in Alzheimer’s disease mouse model via inhibiting endoplasmic reticulum stress-dependent neuroinflammation. Acta Pharmacol. Sin. 2022, 43, 840–849. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, J.H.; Seo, Y.H.; Jang, J.H.; Jeong, C.H.; Lee, S.; Jeong, G.S.; Park, B. Epicatechin Prevents Methamphetamine-Induced Neuronal Cell Death via Inhibition of ER Stress. Biomol. Ther. 2019, 27, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Multifunction of myricetin on A beta: Neuroprotection via a conformational change of A beta and reduction of A beta via the interference of secretases. J. Neurosci. Res. 2008, 86, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ (25-35) and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. Biomed. Res. Int. 2020, 2020, 8210578. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Luo, T.; Li, S.; Zhou, Y.; Shen, X.Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons. PLoS ONE 2016, 11, e0152371. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Q.; Yu, Q. Quercetin enrich diet during the early-middle not middle-late stage of alzheimer’s disease ameliorates cognitive dysfunction. Am. J. Transl. Res. 2018, 10, 1237–1246. [Google Scholar]
- Kim, J.H.; Wang, Q.; Choi, J.M.; Lee, S.; Cho, E.J. Protective role of caffeic acid in an Aβ25-35-induced Alzheimer’s disease model. Nutr. Res. Pract. 2015, 9, 480–488. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Yokoo, T.; Segawa, T.; Maeda, M.; Sawmiller, D.; Tan, J.; Town, T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020, 295, 16251–16266. [Google Scholar] [CrossRef]
- Ogunlade, B.; Adelakun, S.A.; Agie, J.A. Nutritional supplementation of gallic acid ameliorates Alzheimer-type hippocampal neurodegeneration and cognitive impairment induced by aluminum chloride exposure in adult Wistar rats. Drug Chem. Toxicol. 2022, 45, 651–662. [Google Scholar] [CrossRef]
- Yu, M.; Chen, X.; Liu, J.; Ma, Q.; Zhuo, Z.; Chen, H.; Zhou, L.; Yang, S.; Zheng, L.; Ning, C.; et al. Gallic acid disruption of Aβ(1-42) aggregation rescues cognitive decline of APP/PS1 double transgenic mouse. Neurobiol. Dis. 2019, 124, 67–80. [Google Scholar] [CrossRef]
- Kim, M.J.; Seong, A.R.; Yoo, J.Y.; Jin, C.H.; Lee, Y.H.; Kim, Y.J.; Lee, J.; Jun, W.J.; Yoon, H.G. Gallic acid, a histone acetyltransferase inhibitor, suppresses β-amyloid neurotoxicity by inhibiting microglial-mediated neuroinflammation. Mol. Nutr. Food Res. 2011, 55, 1798–1808. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic Acid Alleviates Aβ(25-35)-Induced Autophagy and Cognitive Impairment via the mTOR/TFEB Signaling Pathway. Drug Des. Dev. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sun, F.; Wang, Y.; Kang, J.; Zhang, S.; Li, H. CGA restrains the apoptosis of Aβ(25-35)-induced hippocampal neurons. Int. J. Neurosci. 2020, 130, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.Y.; Li, J.N.; Liu, W.L.; Huang, Q.; Li, W.X.; Tan, Y.H.; Liu, F.; Song, Z.H.; Wang, M.Y.; Xie, N.; et al. Ferulic Acid Ameliorates Alzheimer’s Disease-like Pathology and Repairs Cognitive Decline by Preventing Capillary Hypofunction in APP/PS1 Mice. Neurotherapeutics 2021, 18, 1064–1080. [Google Scholar] [CrossRef]
- Yan, J.J.; Cho, J.Y.; Kim, H.S.; Kim, K.L.; Jung, J.S.; Huh, S.O.; Suh, H.W.; Kim, Y.H.; Song, D.K. Protection against beta-amyloid peptide toxicity in vivo with long-term administration of ferulic acid. Br. J. Pharmacol. 2001, 133, 89–96. [Google Scholar] [CrossRef]
- Sun, P.; Yin, J.B.; Liu, L.H.; Guo, J.; Wang, S.H.; Qu, C.H.; Wang, C.X. Protective role of Dihydromyricetin in Alzheimer’s disease rat model associated with activating AMPK/SIRT1 signaling pathway. Biosci. Rep. 2019, 39, BSR20180902. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Zhang, H.; Li, X.; Zhan, H.; Chen, F.; Han, L.; Xu, Y.; Cao, X. Ampelopsin attenuates lipopolysaccharide-induced inflammatory response through the inhibition of the NF-κB and JAK2/STAT3 signaling pathways in microglia. Int. Immunopharmacol. 2017, 44, 1–8. [Google Scholar] [CrossRef]
- Petry, F.D.S.; Hoppe, J.B.; Klein, C.P.; Dos Santos, B.G.; Hözer, R.M.; Bifi, F.; Matté, C.; Salbego, C.G.; Trindade, V.M.T. Genistein attenuates amyloid-beta-induced cognitive impairment in rats by modulation of hippocampal synaptotoxicity and hyperphosphorylation of Tau. J. Nutr. Biochem. 2021, 87, 108525. [Google Scholar] [CrossRef] [PubMed]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Majkutewicz, I.; Mantej, J.; Węgrzyn, A.; Osiadły, M.; Myślińska, D.; Węgrzyn, G. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacology 2019, 148, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.D.S.; Coelho, B.P.; Gaelzer, M.M.; Kreutz, F.; Guma, F.; Salbego, C.G.; Trindade, V.M.T. Genistein protects against amyloid-beta-induced toxicity in SH-SY5Y cells by regulation of Akt and Tau phosphorylation. Phytother. Res. 2020, 34, 796–807. [Google Scholar] [CrossRef]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-κB Signaling in an Aβ Mouse Model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef]
- Kim, Y.E.; Hwang, C.J.; Lee, H.P.; Kim, C.S.; Son, D.J.; Ham, Y.W.; Hellström, M.; Han, S.B.; Kim, H.S.; Park, E.K.; et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB. Neuropharmacology 2017, 117, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Bahn, G.; Park, J.S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Baek, S.H.; Choi, B.Y.; Cho, Y.S.; Kim, H.K.; et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Ohya, R.; Kita, M.; Taniguchi, Y.; Kondo, K. Theaflavins Improve Memory Impairment and Depression-Like Behavior by Regulating Microglial Activation. Molecules 2019, 24, 467. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [PubMed]
- Macready, A.L.; Kennedy, O.B.; Ellis, J.A.; Williams, C.M.; Spencer, J.P.; Butler, L.T. Flavonoids and cognitive function: A review of human randomized controlled trial studies and recommendations for future studies. Genes Nutr. 2009, 4, 227–242. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z. Comparison in antioxidant and antitumor activities of pine polyphenols and its seven biotransformation extracts by fungi. PeerJ 2017, 5, e3264. [Google Scholar] [CrossRef]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by curcumin: A review on brain delivery strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef]
- Tsai, Y.M.; Chien, C.F.; Lin, L.C.; Tsai, T.H. Curcumin and its nano-formulation: The kinetics of tissue distribution and blood-brain barrier penetration. Int. J. Pharm. 2011, 416, 331–338. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Neves, A.R.; van der Putten, L.; Queiroz, J.F.; Pinheiro, M.; Reis, S. Transferrin-functionalized lipid nanoparticles for curcumin brain delivery. J. Biotechnol. 2021, 331, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Y.; Liu, Z.; Cai, F.; Niu, W.; Song, L.; Liang, H.; Su, Z.; Yu, B.; Yan, F. Brain Delivery of Curcumin Through Low-Intensity Ultrasound-Induced Blood-Brain Barrier Opening via Lipid-PLGA Nanobubbles. Int. J. Nanomed. 2021, 16, 7433–7447. [Google Scholar] [CrossRef] [PubMed]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Ghersi-Egea, J.F.; Mönkkönen, K.S.; Schmitt, C.; Honnorat, J.; Fèvre-Montange, M.; Strazielle, N. Blood-brain interfaces and cerebral drug bioavailability. Rev. Neurol. 2009, 165, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Pereira, M.D.C.; Loureiro, J.A. Resveratrol Brain Delivery for Neurological Disorders Prevention and Treatment. Front. Pharmacol. 2018, 9, 1261. [Google Scholar] [CrossRef] [PubMed]
- Frozza, R.L.; Bernardi, A.; Hoppe, J.B.; Meneghetti, A.B.; Battastini, A.M.; Pohlmann, A.R.; Guterres, S.S.; Salbego, C. Lipid-core nanocapsules improve the effects of resveratrol against Abeta-induced neuroinflammation. J. Biomed. Nanotechnol. 2013, 9, 2086–2104. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ji, C.; Xu, H.; Li, X.; Ding, H.; Ye, M.; Zhu, Z.; Ding, D.; Jiang, X.; Ding, X.; et al. Resveratrol-loaded polymeric micelles protect cells from Abeta-induced oxidative stress. Int. J. Pharm. 2009, 375, 89–96. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Stoupi, S.; Williamson, G.; Viton, F.; Barron, D.; King, L.J.; Brown, J.E.; Clifford, M.N. In vivo bioavailability, absorption, excretion, and pharmacokinetics of [14C]procyanidin B2 in male rats. Drug Metab. Dispos. 2010, 38, 287–291. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, Z.; Yin, Z.; Zhou, Y.; Liu, T.; Zhou, X.; Chang, D. Profiling and Distribution of Metabolites of Procyanidin B2 in Mice by UPLC-DAD-ESI-IT-TOF-MS(n) Technique. Front. Pharmacol. 2017, 8, 231. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Deng, H.; Lin, H. Research progress of methods to improve the stability of procyanidins. J. Guangdong Pharm. Univ. 2014, 30, 245–248. [Google Scholar]
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Farag, M.A.; Zhong, Z.; Zhang, C.; Yang, Y.; Wang, S.; Wang, Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021, 176, 113870. [Google Scholar] [CrossRef] [PubMed]
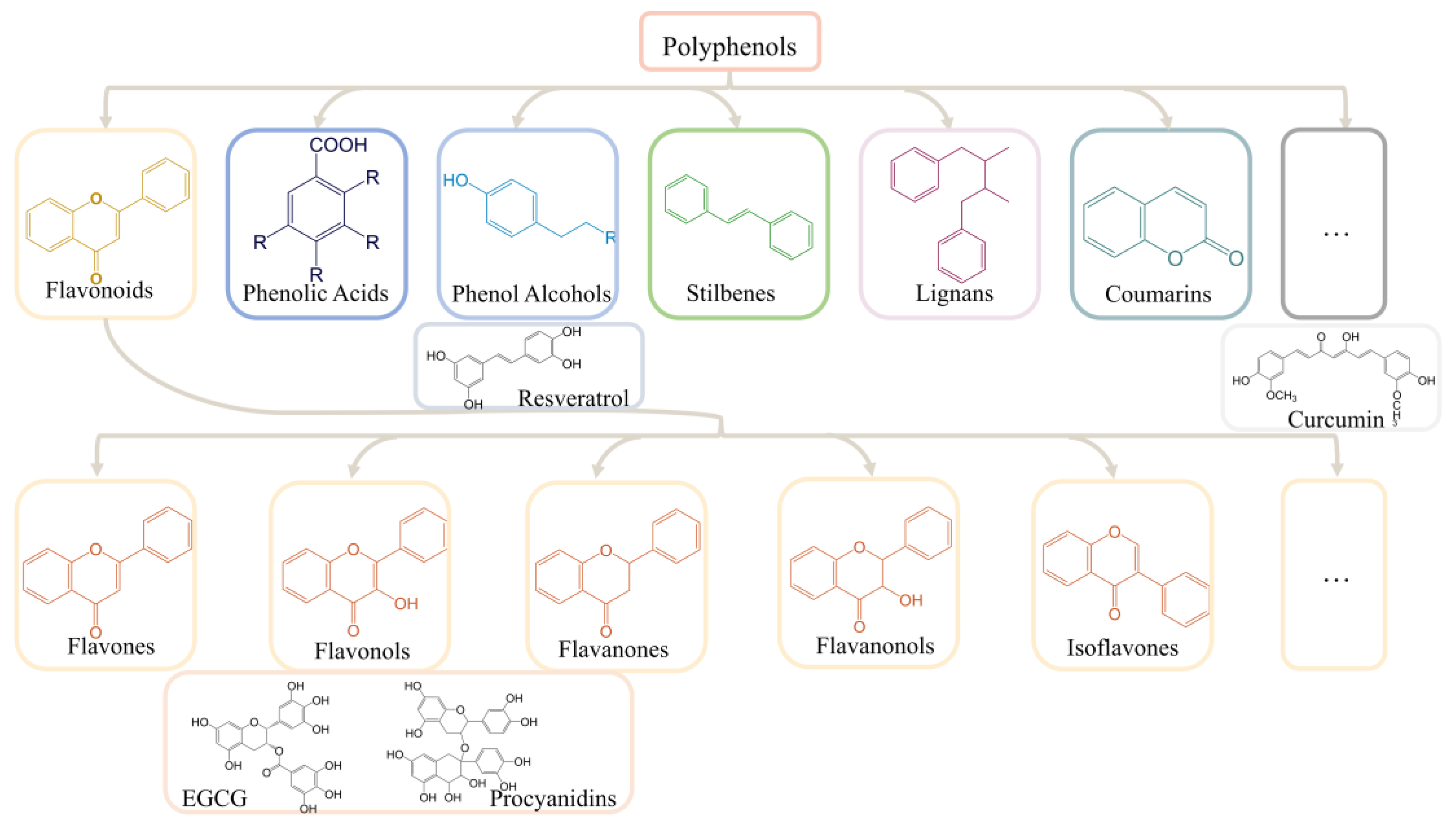

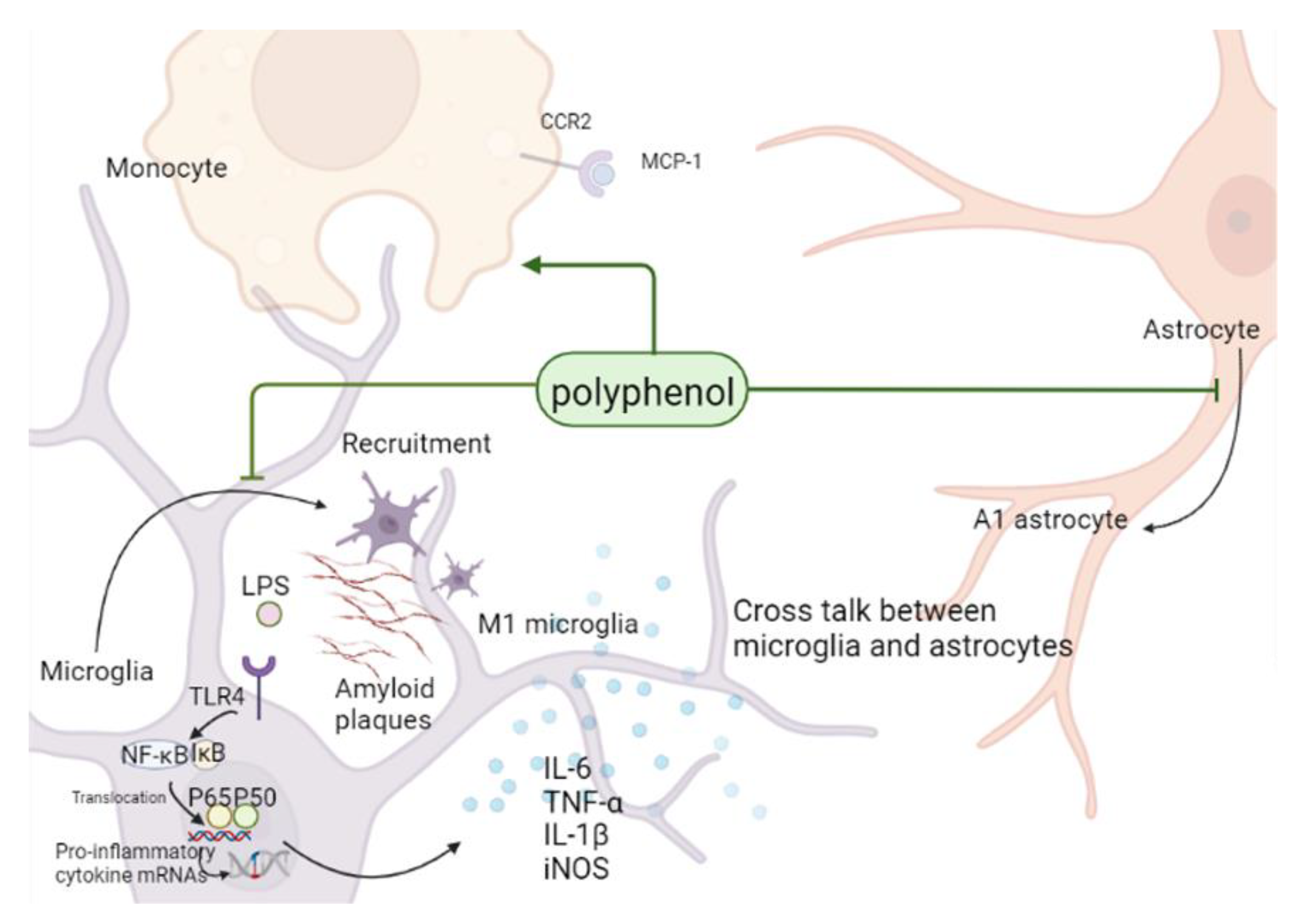
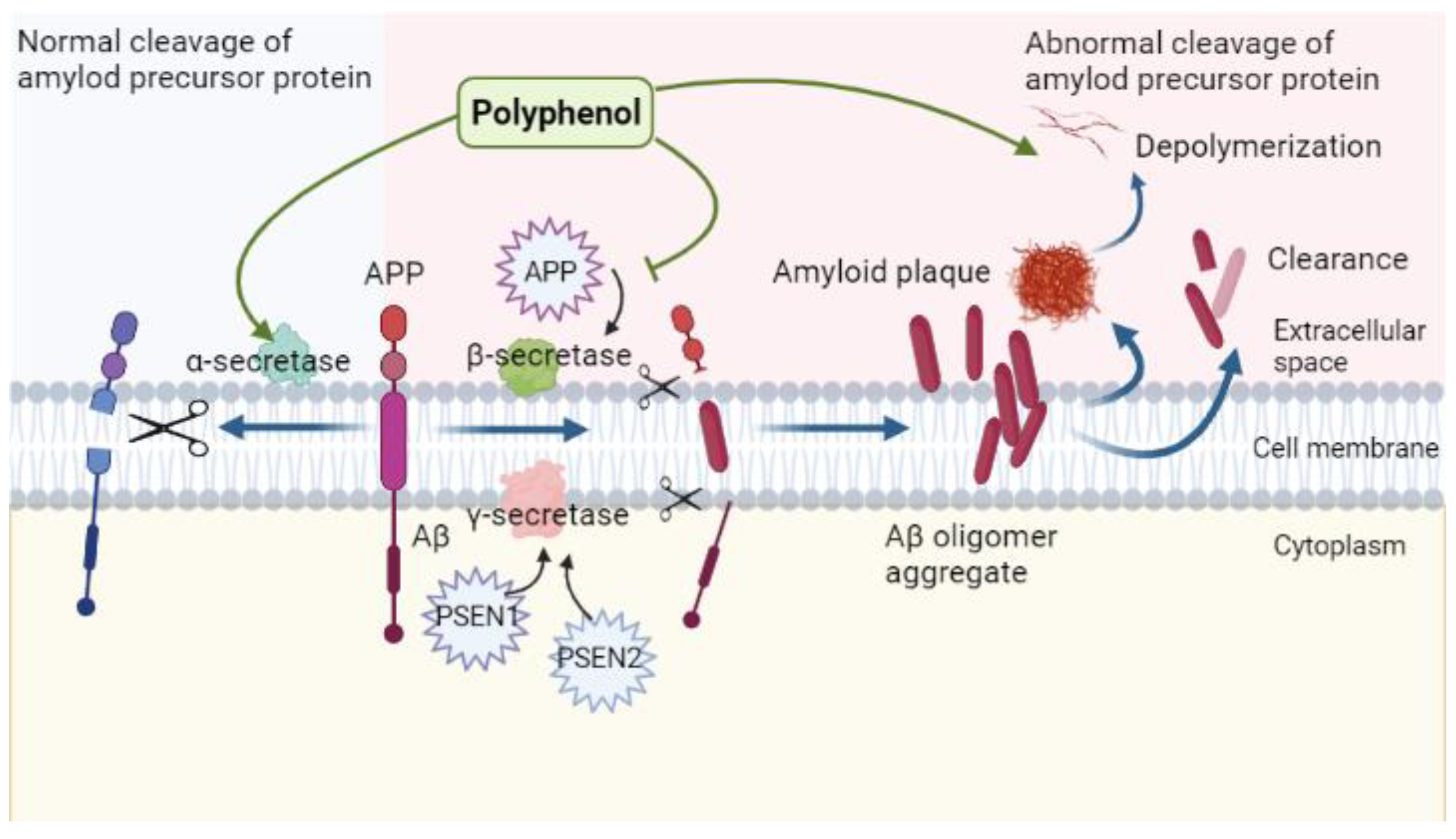
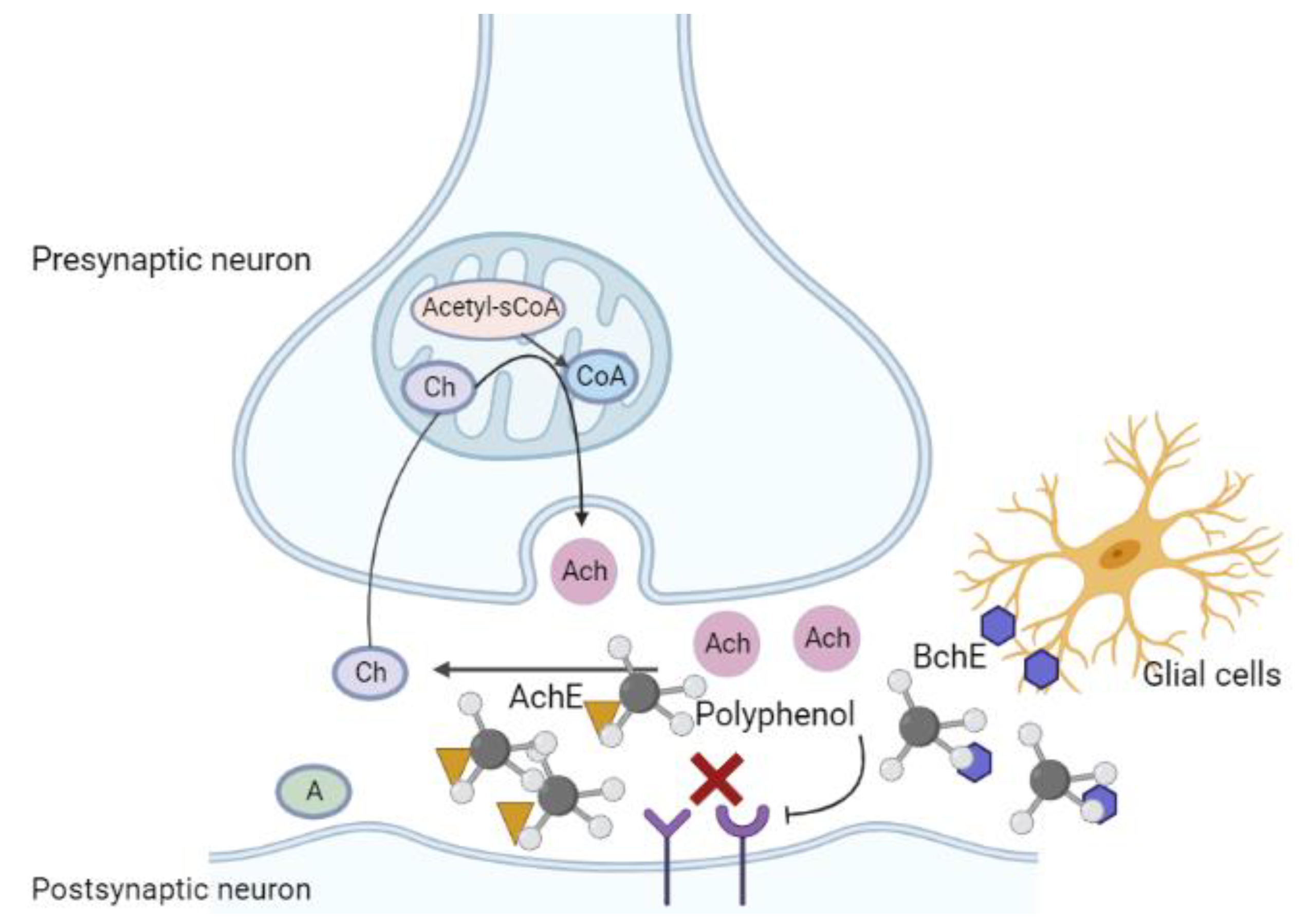
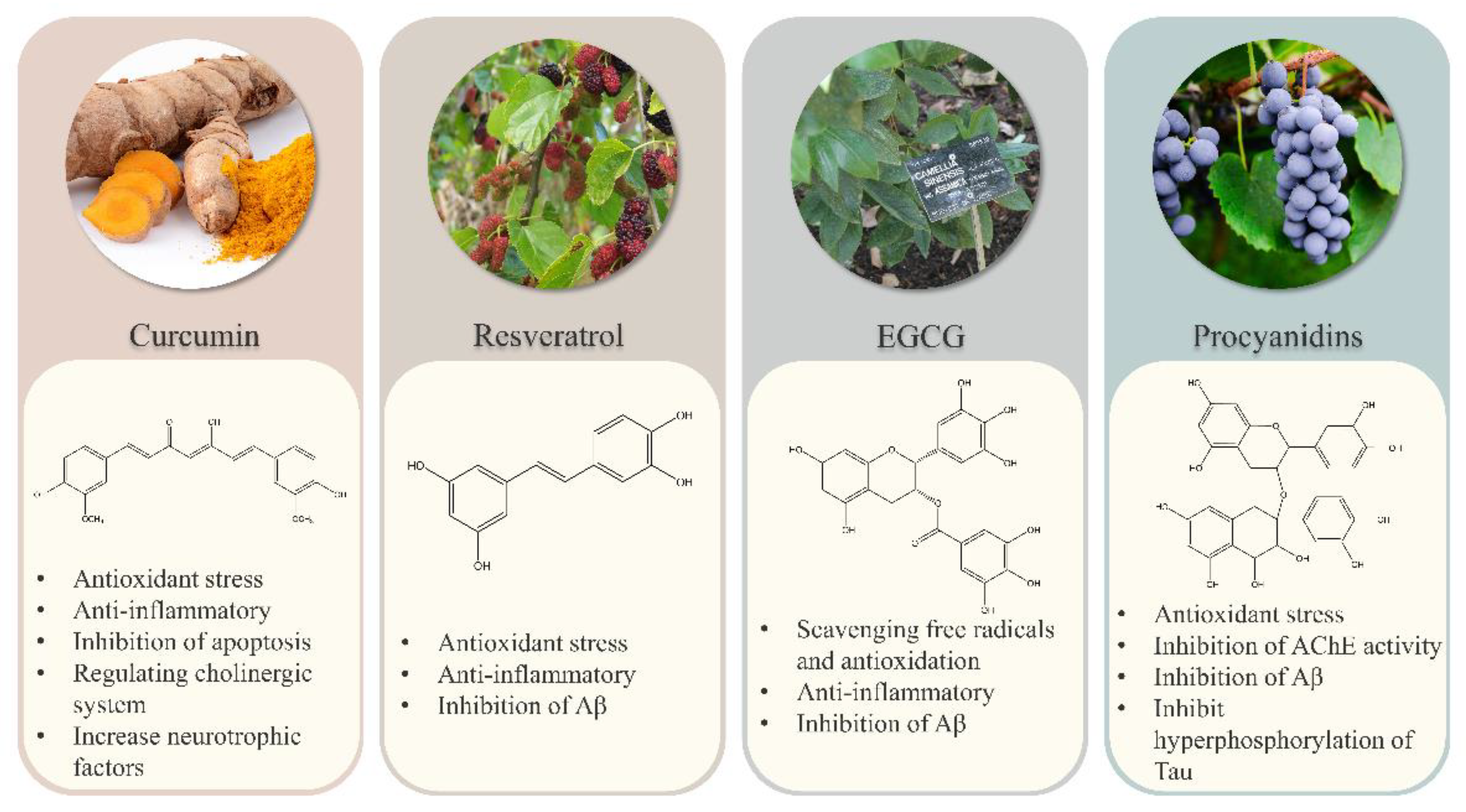
| Drugs | Mechanisms of Action | Main Limitations | Reference |
|---|---|---|---|
| Tacrine | Inhibition of acetylcholinesterase activity and increase in acetylcholine level | Oral administration leads to strong hepatotoxicity and gastrointestinal adverse reactions, which quickly leads to increase in transaminase activity | [11,12] |
| Donepezil | Inhibition of acetylcholinesterase activity and increase in acetylcholine level. Inhibition of aberrant glia cell activation to alleviate neuroinflammation | Low CNS selectivity, gastrointestinal toxicity (nausea, vomiting, anorexia, flatulence, loose stool, diarrhea, salivation, and abdominal colic) | [13,14,15] |
| Rivastigmine | Selective enhancement of Ach activity in the cerebral cortex and hippocampus. Improvements in cognitive function and deceleration of APP formation | Adverse reactions such as acute dystonia, nausea, vomiting, diarrhea, dizziness, and weight loss | [16,17,18] |
| Galantamine | Inhibition of acetylcholinesterase activity and increase in acetylcholine level. Regulation of nicotinic acid receptors outside the brain to increase Ach release | Severe cutaneous adverse drug reactions | [19] |
| Memantine | Antagonizing effect on NMDAR | Bradycardia | [20,21] |
| Aducanumab | Recognition of an epitope of Aβ, reduction of aggregated soluble and insoluble forms of Aβ. | ARIA, effusion, minor hemorrhage, and hemosiderosis | [22] |
| Classification | Compounds | Main Sources | AD Model | Dose and Time | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Flavone | Apigenin | Apium graveolens, Petroselinum crispum, Citrus sinensis, Vitis vinifera, Allium sativum, Marchantia polymorpha | APP/PS1 mice | 40 mg/kg, 12 weeks | Antioxidant stress and inhibition of Aβ production | [276] |
| Aβ1–42 or LPS-induced coculture of cortical neurons and glial cells in neonatal Wistar rats | 1 μM, 24 h | Anti-inflammatory | [277] | |||
| Baicalin | Scutellaria baicalensis Georgi | APP/PS1 mice; Aβ1–42-induced BV2 and SH-SY5Y cells | 100 mg/kg, 33 days; 0, 10, 20, 40 µM, 24 h | Inhibiting NLRP3 inflammatory corpuscle activation by TLR4/NF-κB pathway | [278] | |
| Kaempferol | Allium tuberosum, Allium cepa, Vigna radiata, Cucurbita moschata, Solanum tuberosum, Solanum lycopersicum, Fragaria ananassa, Forsythia suspensa, Rosmarinus officinalis, Acacia farnesiana, Ginkgo, Mimosa pudica, Cinnamomum tamala | Female ovariectomized (OVX) Wistar rats induced by ICV of STZ | 10 mg/kg, 21 days | Antioxidative stress, anti-neuroinflammation | [279] | |
| Lipoprotein particles containing ApoE4-induced SK-N-SH cells | 20 μM, 24 h | Changing the conformation and function of ApoE4 | [239] | |||
| Luteolin | Arachis hypogaea, Brassica napus, Olea europaea, Fagopyrum esculentum, Theobroma, Capsicum annuum, Apium graveolens, Daucus carota, Cucumis sativus, Lactuca sativus, Punica granatum | 3 × Tg-AD mice | 20, 40 mg/kg, 3 weeks | inhibiting endoplasmic reticulum stress-dependent neuroinflammation | [280] | |
| Flavanol | Epicatechin | Tea-leaves, cocoa, grape seeds, Fagopyrum dibotrys, Amygdalus persica, Malus pumila | Methamphetamine (METH) induced HT22 cells | 10, 20 μM, 1 h | Antioxidant | [281] |
| Myricetin | Fragaria ananassa, Malus pumila, Spinacia oleracea, Aloe vera, Daucus carota, Fructus Mori, red wine | Primary rat cortical neurons induced by Aβ1–42 | 10μM, 48 h | Inhibiting the activity of BACE-1; inhibiting Aβ | [282] | |
| Quercetin | Citrus reticulata, Momordica charantia, Malus pumila, Allium cepa, Vitis vinifera, Vaccinium spp, Rubus idaeus | Aβ25–35-induced PC-12 cells | 10, 20, 40, 80 μM, 48 h | Antioxidant | [283] | |
| Okadaic acid (OA)-induced HT22 cells | 5, 10 μM, 12 h | Antioxidant, inhibiting tau hyperphosphorylation | [284] | |||
| APP/PS1 mice | 2 mg/kg, 7–8 months | inhibition of Aβ production | [285] | |||
| Phenolic acid | Caffeic acid | Solanum lycopersicum, Daucus carota, Fragaria ananassa, Semen Trigonellae, wheat wine, tea, coffee, apple juice | Mice induced by ICV of Aβ25–35 | 10, 50 mg/kg, 14 days | Antioxidant | [286] |
| Gallic acid | Vitis vinifera, Lilium brownii viridulum, Punica granatum, Rosa rugosa, Toxicodendron vernicifluum, Quercus palustris, Hamamelis mollis, Rhus chinensis, Terminalia chebula | APP/PS1 mice | 20 mg/kg, 6 months | Antioxidant stress, anti-inflammatory, affecting α-, β-, γ-secretase activity | [287] | |
| AlCl3-induced Wistar rats | 100 mg/kg, 60 days | Antioxidant | [288] | |||
| APP/PS1 mice | 30 mg/kg, 30 days | Inhibiting Aβ1–42 aggregation | [289] | |||
| Aβ1–42-induced primary microglia and BV2 cells | 5–50 μM, 24 h | Inhibiting NF-κB acetylation, antioxidant | [290] | |||
| Phenylpropanoid | Chlorogenic acid | coffee, Lycium chinense, Lonicera japonica, Eucommia ulmoides, Arctium lappa, Chrysanthemum indicum, Malus pumila, Prunus pseudocerasus, Camellia sinensis | APP/PS1 mice; Aβ25–35-induced SH-SY5Y cells | 40 mg/kg, 6 months; 3.125, 6.25, 12.5, 25, 50 µM, 24, 48 h | Inhibiting excessive autophagy and Aβ production by regulating mTOR/TFEB signaling pathway | [291] |
| Aβ25–35-induced primary rat hippocampal neurons | 12.5, 25, 50 μM, 2 h | Antioxidant | [292] | |||
| Ferulic acid | Triticum sativum, beer, coffee, berries, Avena sativa, Ananas comosus, Arachis hypogaea | APP/PS1 mice | 20 mg/kg, 30 days | Improving hippocampal capillary perfusion insufficiency | [293] | |
| ICR mice induced by ICV of Aβ1–42 | 14–19 mg/kg, 30 days | Antioxidant | [294] | |||
| Flavanonol | Dihydromyricetin | Vitis vinifera, Myrica rubra, Ampelopsis grossedentata, Ginkgo | SD rats induced by ICV of Aβ1–42 | 100, 200 mg/kg, 21 days | Anti-inflammatory by activating AMPK/SIRT1 signaling pathway | [295] |
| LPS-induced BV2 and primary microglias | 10, 30, 50 μM, 1 h | Anti-inflammatory by NF-κB signaling pathway | [296] | |||
| Isoflavone | Genistein | Glycine max | Wistar rats induced by bilateral ICV of Aβ1–42 | 10 mg/kg, 10 days | Attenuating synaptic toxicity, inhibiting tau hyperphosphorylation, inactivating ERK | [297] |
| Wistar rats induced by ICV of STZ | 150 mg/kg, 30, 90 days | Anti-inflammatory; Stimulating autophagy of Aβ40, 42 and p-tau | [298] | |||
| Aβ25–35-induced SH-SY5Y cells | 1, 10 nM, 24 h | Inhibiting Aβ induced apoptosis and Akt inactivation, inhibiting tau hyperphosphorylation | [299] | |||
| Flavonone | Hesperidin | Citrus reticulata Blanco | C57BL/6N induced by ICV of Aβ1–42; Aβ1–42-induced BV2 and HT22 cells | 50 mg/kg, 6 weeks; 50 μM, 24 h | Inhibiting oxidative stress by regulating Nrf2/HO-1; Inhibiting neuroinflammation by regulating TLR4/NF-κB; Inhibiting the expression of APP, BACE-1, Aβ | [300] |
| Ellagitannin | Punicalagin | Punica granatum | LPS-induced ICR; Primary rat cortical astrocytes and BV2 | 1.5 mg/kg, 4 weeks; 10, 20, 50 μM, 24 h | Anti-inflammatory | [301] |
| Isothiocyanate | Sulforaphane | Brassica oleracea, Nasturtium officinale, Brassica oleracea var. acephala DC, Brassica oleracea var. capitata, Brussels sprouts, Brassica rapa var. glabra Regel, Brassica juncea, Brassica oleracea var. botrytis Linnaeus | 5 × FAD mice | 5, 10 mg/kg, 2 months | Inhibiting Aβ, inhibiting phosphorylation tau | [302] |
| Benzotropolones | Theaflavin | Camellia sinensis | ICR mice induced by ICV of LPS; LPS, IFN-γ induced primary microglias | 10, 50 mg/kg, 3 days; 10, 30 μM, 12 h | Anti-inflammatory | [303] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, K.; Yan, J.; Zhou, Q.; Wang, X. Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer’s Disease: Dietary Plant Polyphenols. Int. J. Mol. Sci. 2022, 23, 13886. https://doi.org/10.3390/ijms232213886
Wang Y, Wang K, Yan J, Zhou Q, Wang X. Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer’s Disease: Dietary Plant Polyphenols. International Journal of Molecular Sciences. 2022; 23(22):13886. https://doi.org/10.3390/ijms232213886
Chicago/Turabian StyleWang, Yi, Kaiyue Wang, Junyuan Yan, Qian Zhou, and Xiaoying Wang. 2022. "Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer’s Disease: Dietary Plant Polyphenols" International Journal of Molecular Sciences 23, no. 22: 13886. https://doi.org/10.3390/ijms232213886
APA StyleWang, Y., Wang, K., Yan, J., Zhou, Q., & Wang, X. (2022). Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer’s Disease: Dietary Plant Polyphenols. International Journal of Molecular Sciences, 23(22), 13886. https://doi.org/10.3390/ijms232213886







