Pentacyclic Triterpenoid Phytochemicals with Anticancer Activity: Updated Studies on Mechanisms and Targeted Delivery
Abstract
:1. Introduction
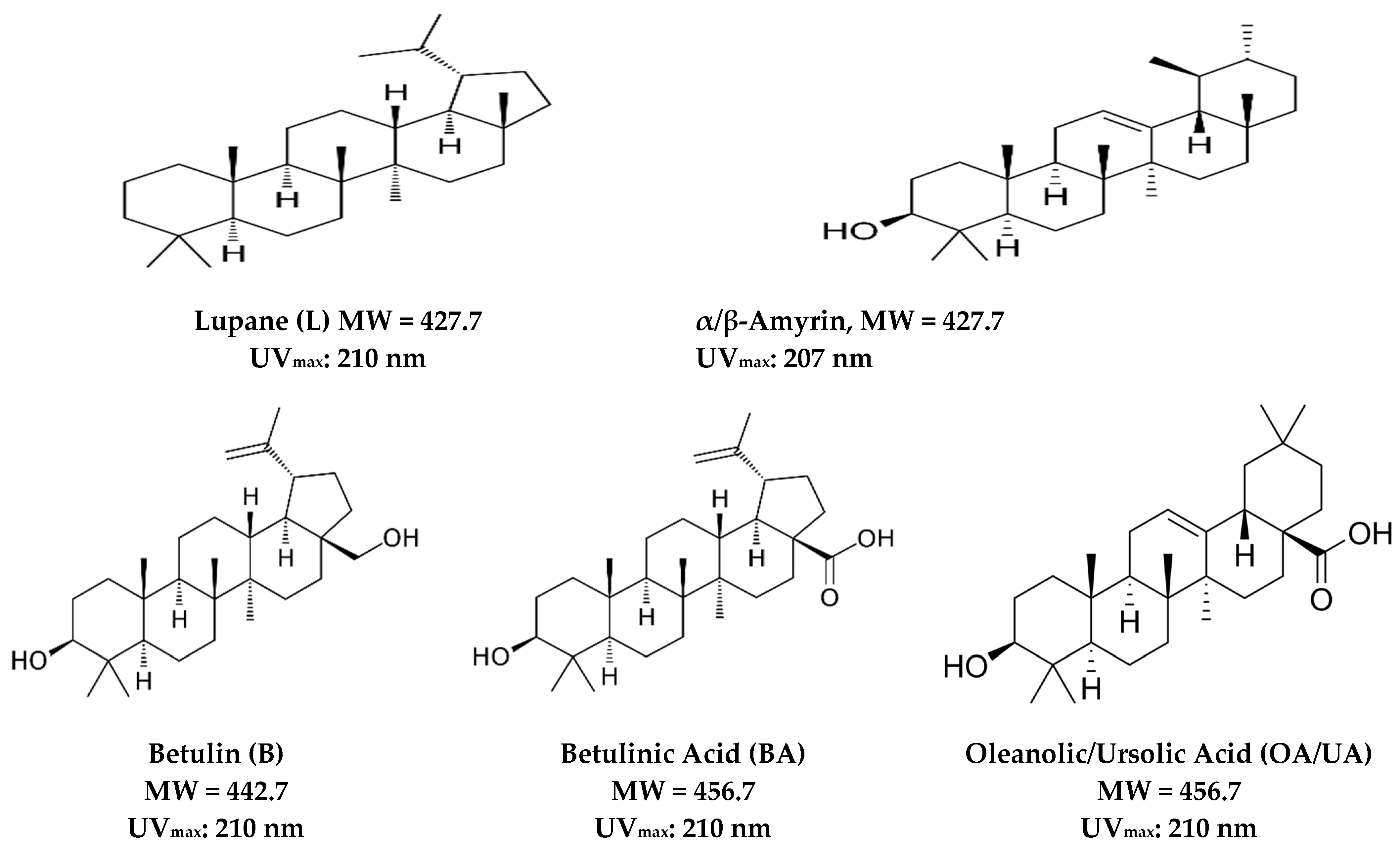
2. Isolation, Physical, and Chemical Characteristics
3. Biological and Pharmacological Activity
3.1. Antiviral, Immunotropic, and Antimicrobial Activity
3.2. Anticancer Activity
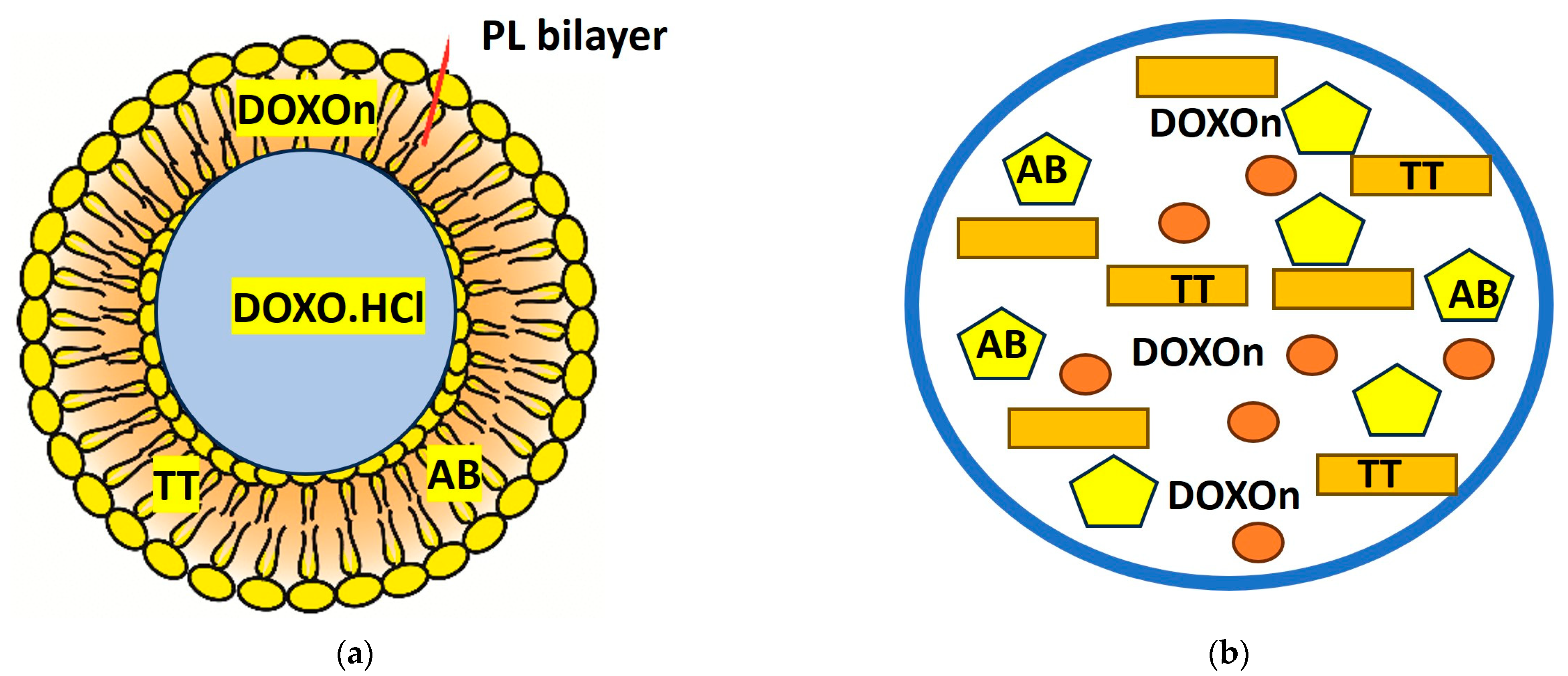
4. Nano-Delivery Vehicles (Liposomes, Nanolipids, and Other Complexes)
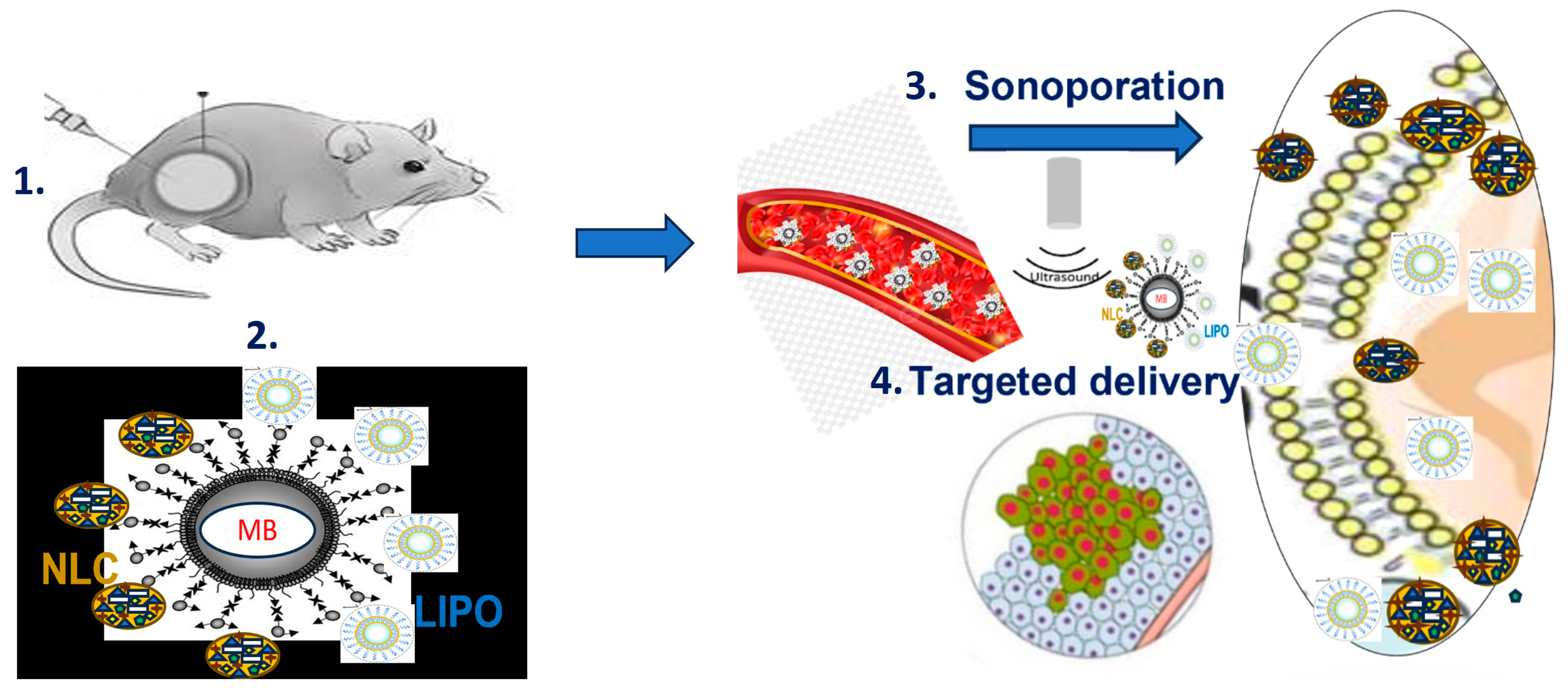
5. New Vehicles for Anticancer Theranostics: Microbubbles and Sonoporation-Assisted Delivery
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, A.C.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Preat, V.; Larondelle, Y.; André, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Sohag, A.A.; Hossain, T.; Rahaman, A.; Rahman, P.; Hasan, M.S.; Das, R.C.; Khan, K.; Sikder, M.H.; Alam, M.; Uddin, J.; et al. Molecular pharmacology and therapeutic advances of the pentacyclic triterpene lupeol. Phytomedicine Int. J. Phytother. Phytopharm. 2022, 99, 154012. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2003, 24, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulinic acid, a natural compound with potent anticancer effects. Anticancer. Drugs 2010, 21, 215–227. [Google Scholar] [CrossRef]
- Saneja, A.; Arora, D.; Kumar, R.; Dubey, R.D.; Panda, A.K.; Gupta, P.N. Therapeutic applications of betulinic acid nanoformulations. Ann. N. Y. Acad. Sci. 2018, 1421, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Demets, O.V.; Takibayeva, A.T.; Kassenov, R.Z.; Aliyeva, M.R. Methods of Betulin Extraction from Birch Bark. Molecules 2022, 27, 3621. [Google Scholar] [CrossRef]
- Moghaddam, M.G.; Ahmad, F.B.H.; Kermani, A.S. Biological activity of betulinic acid: A review. Pharmacol. Pharm. 2012, 3, 119–123. [Google Scholar] [CrossRef]
- Zhang, D.-M.; Xu, H.-G.; Wang, L.; Li, Y.-J.; Sun, P.-H.; Wu, X.-M.; Wang, G.J.; Chen, W.M.; Ye, W.-C. Betulinic Acid and its Derivatives as Potential Antitumor Agents. Med. Res. Rev. 2015, 35, 1127–1155. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, N.; Liu, Y.; Cheng, M.-S. Recent progress on betulinic acid and its derivatives as antitumor agents: A mini review. Chin. J. Nat. Med. 2021, 19, 641–647. [Google Scholar] [CrossRef]
- Zhang, J.; Yamada, S.; Ogihara, E.; Kurita, M.; Banno, N.; Qu, W.; Akihisa, T. Biological Activities of Triterpenoids and Phenolic Compounds from Myrica cerifera Bark. Chem Biodivers. 2016, 13, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Król, S.K.; Kiełbus, M.; Rivero-Muller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. BioMed Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef] [PubMed]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Zhang, L.; Ma, C.; Abd El-Aty, A.M. Edible pentacyclic triterpenes: A review of their sources, bioactivities, bioavailability, self-assembly behavior, and emerging applications as functional delivery vehicles. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal plants of the genus Betula—Traditional uses and a phytochemical–pharmacological review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Holonec, L.; Ranga, F.; Crainic, D.; Truţa, A.; Socaciu, C. Evaluation of Betulin and Betulinic Acid Content in Birch Bark from Different Forestry Areas of Western Carpathians. Not. Bot. Horti Agrobo. 2012, 40, 99–105. [Google Scholar] [CrossRef]
- Mishra, T.; Chandra, P.; Kumar, B.T.; Baleshwar, M.; Joshi, P.; Rana, T.S.; Upreti, D.K.; Pa, M. Phytochemical profiling of the stem bark of Betula utilis from different geographical regions of India using UHPLC-ESI-MS/MS. Anal. Sci. Adv. 2021, 2, 497–504. [Google Scholar] [CrossRef]
- Šiman, P.; Filipová, A.; Tichá, A.; Niang, M.; Bezrouk, A.; Havelek, R. Effective Method of Purification of Betulin from Birch Bark: The Importance of Its Purity for Scientific and Medicinal Use. PLoS ONE 2016, 11, e0154933. [Google Scholar] [CrossRef]
- Rusmawati, W.M.W.; Ahmad, F.B.H.; Anuar, K.; Hamdan, S. Solubility of betulinic acid in the microemulsion system of methyl acetate/Tween 80: BRIJ30/H2O. Orient. J. Chem. 2001, 16, 393–398. [Google Scholar]
- Ali-Seyed, M.; Jantan, I.; Vijayaraghavan, K.; Bukhari, S.N.A. Betulinic Acid: Recent Advances in Chemical Modifications, Effective Delivery, and Molecular Mechanisms of a Promising Anticancer Therapy. Chem. Biol. Drug Des. 2016, 87, 517–536. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrooj, H.; Aghanoori, M.R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef]
- Jäger, S.; Laszczyk, M.N.; Scheffler, A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef] [PubMed]
- Udeani, G.O.; Zhao, G.-M.; Shin, Y.G.; Cooke, B.P.; Graham, J.; Beecher, C.W.W.; Kinghorn, A.D.; Pezzuto, J.M. Pharmacokinetics and tissue distribution of betulinic acid in CD-1 mice. Biopharm. Drug Dispos. 1999, 20, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Zdzisiñska, B.; Rzeski, W.; Paduch, R.; Szuster-Ciesielska, A.; Kaczor, J.; Wejksza, K.; Kandefer-Szerszeñ, M. Differential effect of betulin and betulinic acid on cytokine production in human whole blood cell cultures. Pol. J. Pharmacol. 2003, 55, 235–238. [Google Scholar]
- Tang, Z.-Y.; Li, Y.; Tang, Y.-T.; Ma, X.-D.; Tang, Z.-Y. Anticancer activity of oleanolic acid and its derivatives: Recent advances in evidence, target profiling and mechanisms of action. Biomed. Pharmacother. 2022, 145, 112397. [Google Scholar] [CrossRef]
- Reyes, C.P.; Núñez, M.J.; Jiménez, I.A.; Busserolles, J.; Alcaraz, M.J.; Bazzocchi, I.L. Activity of lupane triterpenoids from Maytenus species as inhibitors of nitric oxide and prostaglandin E2. Bioorg. Med. Chem. 2006, 14, 1573–1579. [Google Scholar] [CrossRef]
- Yamashita, K.; Lu, H.; Lu, J.; Chen, G.; Yokoyama, T.; Sagara, Y.; Manabe, M.; Kodama, H. Effect of three triterpenoids, lupeol, betulin, and betulinic acid on the stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils. Clin. Chim. Acta 2002, 325, 91–96. [Google Scholar] [CrossRef]
- Csuk, R. Betulinic acid and its derivatives: A patent review (2008–2013). Expert Opin. Ther. Pat. 2014, 24, 913–923. [Google Scholar] [CrossRef]
- Kang, H.R.; Eom, H.J.; Lee, S.R.; Choi, S.U.; Kang, K.S.; Lee, K.R.; Kim, K.H. Bioassay-guided isolation of antiproliferative triterpenoids from Euonymus alatus twigs. Nat. Prod. Commun. 2015, 10, 1929–1932. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Park, H.-J.; Yoo, H.M.; Cho, N. Betulin Protects HT-22 Hippocampal Cells against ER Stress through Induction of Heme Oxygenase-1 and Inhibition of ROS Production. Nat. Prod. Commun. 2019, 14, 1934578X19896684. [Google Scholar] [CrossRef]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, K.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not normal cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Ahmadu, A.A.; Delehouzé, C.; Haruna, A.; Mustapha, L.; Lawal, B.A.; Udobre, A.; Baratte, B.; Triscornia, C.; Autret, A.; Robert, T.; et al. Betulin, a Newly Characterized Compound in Acacia auriculiformis Bark, Is a Multi-Target Protein Kinase Inhibitor. Molecules 2021, 26, 4599. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.-Z.; Yu, Y.; Wang, J.-J.; Yin, P.-H.; Xu, K. Triterpenoids Extracted from Rhus chinensis Mill Act Against Colorectal Cancer by Inhibiting Enzymes in Glycolysis and Glutaminolysis: Network Analysis and Experimental Validation. Nutr. Cancer 2019, 72, 293–319. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.J.; Fang, H.C.; Chung, H.M.; Cheng, J.S.; Lee, K.C.; Tseng, L.L.; Tang, K.Y.; Jan, C.R. Effect of betulinic acid on intracellular-free Ca2þ levels in Madin Darby canine kidney cells. Eur. J. Pharmacol. 2000, 408, 99–106. [Google Scholar] [CrossRef]
- Tubek, B.; Mituła, P.; Niezgoda, N.; Kempińska, K.; Wietrzyk, J.; Wawrzeńczyk, C. Synthesis and cytotoxic activity of new betulin and betulinic acid esters with conjugated linoleic acid (CLA). Nat. Prod. Commun. 2013, 8, 435–438. [Google Scholar] [CrossRef]
- Mizerska-Kowalska, M.; Sławińska-Brych, A.; Kaławaj, K.; Żurek, A.; Pawińska, B.; Rzeski, W.; Zdzisińska, B. Betulin Promotes Differentiation of Human Osteoblasts In Vitro and Exerts an Osteoinductive Effect on the hFOB 1.19 Cell Line Through Activation of JNK, ERK1/2, and mTOR Kinases. Molecules 2019, 24, 2637. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Kumar, A.; Gupta, K.B.; Dhiman, M.; Arora, S.; Jaitak, V. New pentacyclic triterpene from Potentilla atrosanguinea Lodd. as anticancer agent for breast cancer targeting estrogen receptor-α. Nat. Prod. Res. 2022, 36, 4352–4357. [Google Scholar] [CrossRef]
- Fujioka, T.; Kashiwada, Y.; Kilkuskie, R.E.; Cosentino, L.M.; Ballas, L.M.; Jiang, J.B.; Janzen, W.P.; Chen, I.S.; Lee, K.H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994, 57, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Darshani, P.; Sen Sarma, S.; Srivastava, A.K. Anti-viral triterpenes: A review. Phytochem. Rev. 2022, 21, 1761–1842. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Morris-Natschke, S.L. Recent advances in the discovery and development of plant-derived natural products and their analogs as anti-HIV agents. Pure Appl. Chem. 1999, 71, 1045–1051. [Google Scholar] [CrossRef]
- Akihisa, T.; Ogihara, J.; Kato, J.; Yasukawa, K.; Ukiya, M.; Yamanouchi, S.; Oishi, K. Inhibitory effects of triterpenoids and sterols on human immunodeficiency virus-1 reverse transcriptase. Lipids 2001, 36, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Baglin, I.; Mitaine-Offer, A.C.; Nour, M.; Tan, K.; Cave, C.; Lacaille-Dubois, M.A. A review of natural and modified betulinic, ursolic and echinocystic acid derivatives as potential antitumor and anti-HIV agents. Mini Rev. Med. Chem. 2003, 3, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Drąg-Zalesińska, M.; Borska, S. Betulin and its derivatives—Precursors of new drugs. World Sci. News 2019, 127, 123–138. [Google Scholar]
- Kirstgen, M.; Lowjaga, K.A.A.T.; Müller, S.F.; Goldmann, N.; Lehmann, F.; Alakurtti, S.; Geyer, J. Selective hepatitis B and D virus entry inhibitors from the group of pentacyclic lupane-type betulin-derived triterpenoids. Sci. Rep. 2020, 10, 21772. [Google Scholar] [CrossRef]
- Ríos, J.L. Effects of triterpenes on the immune system. J. Ethnopharmacol. 2010, 128, 1–14. [Google Scholar] [CrossRef]
- Anikina, L.V.; Tolmacheva, I.A.; Vikharev, Y.B.; Grishko, V.V. The immunotropic activity of lupane and oleanane 2,3-seco-triterpenoids. Russ. J. Bioorg. Chem. 2010, 36, 240–244. [Google Scholar] [CrossRef]
- Safayhi, H.; Sailer, E.-R. Anti-inflammatory actions of pentacyclic triterpenes. Planta Med. 1997, 63, 487–493. [Google Scholar] [CrossRef]
- Duric, K.; Kovac-Besovic, E.; Niksic, H.; Sofic, E. Antibacterial activity of methanolic extracts, decoction and isolated triterpene products from different parts of birch, Betula pendula, Roth. J. Plant Stud. 2013, 2, 61–70. [Google Scholar] [CrossRef]
- Huang, L.R.; Luo, H.; Yang, X.S.; Chen, L.; Zhang, J.X.; Wang, D.P.; Hao, X.J. Enhancement of anti-bacterial and antitumor activities of pentacyclic triterpenes by introducing exocyclic α,β-unsaturated ketone moiety in ring A. Med. Chem. Res. 2014, 23, 4631–4641. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chen, H.-T.; Wu, Z.-Y.; Jhan, Y.-L.; Shyu, C.-L.; Chou, C.-H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef]
- Sousa, M.C.; Varandas, R.; Santos, R.C.; Santos-Rosa, M.; Alves, V.; Salvador, J.A.R. Antileishmanial Activity of Semisynthetic Lupane Triterpenoids Betulin and Betulinic Acid Derivatives: Synergistic Effects with Miltefosine. PLoS ONE 2014, 9, e89939. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Racoviceanu, R.; Petrova, A.; Mioc, M.; Militaru, A.; Udrescu, L.; Udrescu, M.; Voicu, A.; Cummings, J.; Robertson, G.; et al. New Investigations with Lupane Type A-Ring Azepane Triterpenoids for Antimycobacterial Drug Candidate Design. Int. J. Mol. Sci. 2021, 22, 12542. [Google Scholar] [CrossRef]
- Nick, A.; Wright, A.D.; Rali, T.; Sticher, O. Antibacterial triterpenoids from Dillenia papuana and their structure–activity relationships. Phytochemistry 1995, 40, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, J.M.R.; Rao, C.V. Triterpenoids for cancer prevention and treatment: Current status and future prospects. Curr. Pharm. Biotechnol. 2012, 13, 147–155. [Google Scholar] [CrossRef]
- Sureda, A.; Martorell, M.; Capó, X.; Monserrat-Mesquida, M.; Quetglas-Llabrés, M.M.; Rasekhian, M.; Nabavi, S.M.; Tejada, S. Antitumor Effects of Triterpenes in Hepatocellular Carcinoma. Curr. Med. Chem. 2021, 28, 2465–2484. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Prendecka-Wróbel, M.; Kurach, Ł.; Horecka, A.; Olszewska, A.; Pigoń-Zając, D.; Małecka-Massalska, T.; Kurzepa, J. Antiproliferative Properties of Triterpenoids by ECIS Method—A New Promising Approach in Anticancer Studies? Molecules 2022, 27, 3150. [Google Scholar] [CrossRef]
- Bozek, J.; Tomala, J.; Wójcik, S.; Kaminska, B.; Brand, I.; Pochec, E.; Szostak, E. Effects of Piptoporus betulinus Ethanolic Extract on the Proliferation and Viability of Melanoma Cells and Models of Their Cell Membranes. Int. J. Mol. Sci. 2022, 23, 13907. [Google Scholar] [CrossRef]
- Ghante, M.H.; Jamkhande, P.G. Role of Pentacyclic Triterpenoids in Chemoprevention and Anticancer Treatment: An Overview on Targets and Underling Mechanisms. J. Pharmacopunct. 2019, 22, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Jhimli, B.; Sovan, S.; Rubai, A.; Sandeep, D.K. Bioactive Pentacyclic Triterpenes Trigger Multiple Signalling Pathways for Selective Apoptosis Leading to Anticancer Efficacy: Recent Updates and Future Perspectives. Curr. Protein Peptide Sci. 2023, 24. [Google Scholar] [CrossRef]
- Shi, L.-S.; Wu, C.-H.; Yang, T.-C.; Yao, C.-W.; Lin, H.-C.; Chang, W.-L. Cytotoxic effect of triterpenoids from the root bark of Hibiscus syriacus. Fitoterapia 2014, 97, 184–191. [Google Scholar] [CrossRef]
- Schmidt, M.L.; Kuzmanoff, K.L.; Ling-Indeck, L.; Pezzuto, J.M. Betulinic acid induces apoptosis in human neuroblastoma cell lines. Eur. J. Cancer 1997, 33, 2007–2010. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef]
- Xu, Y.; Li, J.; Li, Q.-J.; Feng, Y.-L.; Pan, F. Betulinic acid promotes TRAIL function on liver cancer progression inhibition through p53/Caspase-3 signaling activation. Biomed. Pharmacother. 2017, 88, 349–358. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Turgambayeva, A.; Tashenova, G.; Tulebayeva, A.; Bazarbayeva, A.; Kapanova, G.; Abzaliyeva, S. Multifunctional Roles of Betulinic Acid in Cancer Chemoprevention: Spotlight on JAK/STAT, VEGF, EGF/EGFR, TRAIL/TRAIL-R, AKT/mTOR and Non-Coding RNAs in the Inhibition of Carcinogenesis and Metastasis. Molecules 2023, 28, 67. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, S.A.; Shakhtshneider, T.P.; Mikhailenko, M.A.; Malyar, Y.N.; Kichkailo, A.S.; Drebushchak, V.A.; Kuznetsov, B.N. Preparation and Antitumor Activity of Betulin Dipropionate and its Composites. Biointerface Res. Int. Appl. Chem. 2022, 12, 6873–6894. [Google Scholar]
- Kwon, H.J.; Shim, J.S.; Kim, J.H.; Cho, H.Y.; Yum, Y.N.; Kim, S.H.; Yu, J. Betulinic acid inhibits growth factor-induced in vitro angiogenesis via the modulation of mitochondrial function in endothelial cells. Jpn. J. Cancer Res. 2002, 93, 417–425. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Feflea, S.; Molnár, J.; Zupko, I.; Soica, C. Betulin as an antitumor agent tested in vitro on A431, HeLa and MCF7, and as an angiogenic inhibitor in vivo in the CAM assay. Nat. Prod. Commun. 2012, 7, 981–985. [Google Scholar]
- Shen, M.; Wang, D.; Sennari, Y.; Zeng, Z.; Baba, Z.R.; Morimoto, H.; Kitamura, N.; Nakanishi, T.; Tsukada, J.; Ueno, M.; et al. Pentacyclic triterpenoid ursolic acid induces apoptosis with mitochondrial dysfunction in adult T-cell leukemia MT-4 cells to promote surrounding cell growth. Med. Oncol. 2022, 39, 118. [Google Scholar] [CrossRef] [PubMed]
- Brandes, B.; Hoenke, S.; Fischer, L.; Csuk, R. Design, synthesis and cytotoxicity of BODIPY FL labelled triterpenoids. Eur. J. Med. Chem. 2020, 185, 111858. [Google Scholar] [CrossRef] [PubMed]
- Kouzi, S.A.; Chatterjee, P.; Pezzuto, J.M.; Hamann, M.T. Microbial transformations of the antimelanoma agent betulinic acid. J. Nat. Prod. 2000, 63, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sheng, L.; Zhang, J.; Zhang, L.; Liu, J.; Wen, X.; Liu, Y.; Si, Y.; Cheng, K. Synthesis and in vitro/in vivo anticancer evaluation of pentacyclic triterpenoid derivatives linked with L-phenylalanine or L-proline. Bioorg. Chem. 2022, 126, 105865. [Google Scholar] [CrossRef]
- Hata, K.; Hori, K.; Takahashi, S. Differentiation and apoptosis-inducing activities by pentacyclic triterpenes on a mouse melanoma cell line. J. Nat. Prod. 2002, 65, 645–648. [Google Scholar] [CrossRef]
- Dzhemileva, L.U.; Tuktarova, R.A.; Dzhemilev, U.M.; D’yakonov, V.A. Pentacyclic Triterpenoids-Based Ionic Compounds: Synthesis, Study of Structure–Antitumor Activity Relationship, Effects on Mitochondria and Activation of Signaling Pathways of Proliferation, Genome Reparation and Early Apoptosis. Cancers 2023, 15, 756. [Google Scholar] [CrossRef]
- Sharma, N.; Deepak, C.; Sandeep, K. Nanoparticles: Fundamental and Prospectives. Res. J. Pharmaceut. Biol. Chem. Sci. 2018, 9, 152–164. [Google Scholar]
- Kaps, A.; Gwiazdoń, P.; Chodurek, E. Nanoformulations for Delivery of Pentacyclic Triterpenoids in Anticancer Therapies. Molecules 2021, 26, 1764. [Google Scholar] [CrossRef]
- Sesarman, A.; Tefas, L.; Sylvester, B.; Licarete, E.; Rauca, V.; Luput, L.; Patras, L.; Banciu, M.; Porfire, A. Anti-angiogenic and anti-inflammatory effects of long-circulating liposomes coencapsulating curcumin and doxorubicin on C26 murine colon cancer cells. Pharm. Rep. 2010, 70, 331–339. [Google Scholar] [CrossRef]
- Socaciu, M.; Diaconeasa, Z.; Socaciu, C. Simple and fast procedure to incorporate doxorubicine in small unilamellar liposomes: Effects on liposome size and zeta potential. Studia UBB Chemia, LXIV 2019, 3, 181–192. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Alexiev, U.; Sinambela, P.; Keck, C.M. Nanostructured Lipid Carriers (NLC): The Second Generation of Solid Lipid Nanoparticles. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2016; pp. 161–185. [Google Scholar]
- Sreedhar, R.; Kumar, V.S.; Bhaskaran Pillai, A.K.; Mangalathillam, S. Omega-3 Fatty Acid Based Nanolipid Formulation of Atorvastatin for Treating Hyperlipidemia. Adv. Pharmaceut. Bull. 2019, 9, 271–280. [Google Scholar] [CrossRef]
- Mahmoud, K.; Swidan, S.; El-Nabarawi, M.; Teaima, M. Lipid based nanoparticles as a novel treatment modality for hepatocellular carcinoma: A comprehensive review on targeting and recent advances. J. Nanobiotechnol. 2022, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, T.G.; Huang, Y.H.; Xia, X.; Cole, S.P.C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Muntean, P.; Socaciu, C.; Socaciu, M. Lipid nanostructured particles as emerging carriers for targeted delivery of bioactive molecules: Applications in food and biomedical sciences (an overview). Bull. UASVM Food Sci. Technol. 2020, 77, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P.; Campardelli, R. Production of solid lipid nanoparticles with a supercritical fluid assisted process. J. Supercrit. Fluids 2019, 143, 16–23. [Google Scholar] [CrossRef]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Mierina, I.; Vilskersts, R.; Turks, M. Delivery Systems for Birch-bark Triterpenoids and their Derivatives in Anticancer Research. Curr. Med. Chem. 2020, 27, 1308–1336. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 20, 193. [Google Scholar] [CrossRef]
- Falamas, A.; Dehelean, C.A.; Cinta Pinzaru, S. Monitoring of betulin nanoemulsion treatment and molecular changes in mouse skin cancer using surface enhanced Raman spectroscopy. Vib. Spectrosc. 2018, 95, 44–50. [Google Scholar] [CrossRef]
- Bag, B.G.; Majumdar, R. Self-assembly of Renewable Nano-sized Triterpenoids. Chem. Rec. 2017, 17, 841–873. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Dash, S.S. Hierarchical Self-Assembly of a Renewable Nanosized Pentacyclic Dihydroxy-triterpenoid Betulin Yielding Flower-Like Architectures. Langmuir 2015, 31, 13664–13672. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-K.; Ho, J.C.K.; Cheung, F.W.K.; Liu, B.P.L.; Ye, W.-C.; Che, C.-T. Apoptotic activity of betulinic acid derivatives on murine melanoma B16 cell line. Eur. J. Pharmacol. 2004, 498, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Małaczewska, J.; Kaczorek-Łukowska, E.; Kazuń, B. High cytotoxicity of betulin towards fish and murine fibroblasts: Is betulin safe for nonneoplastic cells? BMC Vet. Res. 2021, 17, 2–40. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.V.; Semenova, A.A.; Ilzorkina, A.I.; Mikheeva, I.B.; Yashin, V.A.; Penkov, N.V.; Vydrina, V.A.; Yu, G. Effect of betulin and betulonic acid on isolated rat liver mitochondria and liposomes. BBA—Biomembranes 2020, 1862, 183383. [Google Scholar] [CrossRef] [PubMed]
- Habib, L.; Jraij, A.; Khreich, N.; Charcosset, C.; Greige-Gerges, H. Effect of Erythrodiol, A Natural Pentacyclic Triterpene from Olive Oil, on the Lipid Membrane Properties. J. Membr. Biol. 2015, 248, 1079–1087. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Liu, M.; Zhang, Y.; Yang, T.; Li, D.; Huang, Y.; Li, Q.; Bai, G.; Shi, L. Betulinic acid induces apoptosis and suppresses metastasis in hepatocellular carcinoma cell lines in vitro and in vivo. J. Cell. Mol. Med. 2019, 23, 586–595. [Google Scholar] [CrossRef]
- Zhao, J.; Li, R.; Pawlak, A.; Henklewska, M.; Sysak, A.; Wen, L.; Yi, J.E.; Obmińska-Mrukowicz, B. Antitumor Activity of Betulinic Acid and Betulin in Canine Cancer Cell Lines. Vivo 2018, 32, 1081–1088. [Google Scholar] [CrossRef]
- Zehra, B.; Ahmed, A.; Sarwar, R.; Khan, A.; Farooq, U.; Abid Ali, S.; Al-Harrasi, A. Apoptotic and antimetastatic activities of betulin isolated from Quercus incana against non-small cell lung cancer cells. Cancer Manag. Res. 2019, 11, 1667–1683. [Google Scholar] [CrossRef]
- Hsu, R.-J.; Hsu, Y.-C.; Chen, S.-P.; Fu, C.-L.; Yu, J.-C.; Chang, F.-W.; Yu, C.-P. The triterpenoids of Hibiscus syriacus induce apoptosis and inhibit cell migration in breast cancer cells. BMC Complement. Altern. Med. 2015, 15, 16. [Google Scholar] [CrossRef]
- Dutta, D.; Paul, B.; Mukherjee, B.; Mondal, L.; Sen, S.; Chowdhury, C.; Debnath, M.C. Nanoencapsulated betulinic acid analogue distinctively improves colorectal carcinoma in vitro and in vivo. Sci. Rep. 2019, 9, 11506. [Google Scholar] [CrossRef] [PubMed]
- Csuk, R.; Barthel, A.; Sczepek, R.; Siewert, B.; Schwarz, S. Synthesis, Encapsulation and Antitumor Activity of New Betulin Derivatives. Archiv. Der. Pharmazie 2010, 344, 37–49. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Wang, Y.S.; Li, G.L.; Zhu, S.B.; Jing, F.-C.; Liu, R.-D.; Li, S.S.; He, J.; Lei, J.D. A Self-assembled Nanoparticle Platform Based on Amphiphilic Oleanolic Acid Polyprodrug for Cancer Therapy. Chin. J. Polym. Sci. 2020, 38, 819–829. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, D.; Zhang, X.; Liu, Z.; Dai, K.; Ji, B.; Wang, Q.; Luo, L. Antitumor drug effect of betulinic acid mediated by polyethylene glycol modified liposomes. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Mullauer, F.B.; van Bloois, L.; Daalhuisen, J.B.; Ten Brink, M.S.; Storm, G.; Medema, J.P.; Schiffelers, R.M.; Kessler, J.H. Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity. Anticancer Drugs 2011, 22, 223–233. [Google Scholar] [CrossRef]
- Ribeiro Rocha, T.G.; Lopes, S.C.; Cassali, G.D.; Ferreira, Ê.; Veloso, E.S.; Leite, E.A.; Braga, F.C.; Ferreira, L.A.; Balvay, D.; Garofalakis, A.; et al. Evaluation of Antitumor Activity of Long-Circulating and pH-Sensitive Liposomes Containing Ursolic Acid in Animal Models of Breast Tumor and Gliosarcoma. Integr. Cancer Ther. 2016, 15, 512–524. [Google Scholar] [CrossRef]
- De Araújo Lopes, C.S.; Melo Novais, M.V.; Teixeira, L.C.; Honorato-Sampaio, K.; Tadeu Pereira, M.; Ferreira, L.A.; Braga, F.C.; Cristina Oliveira, M. Preparation, physicochemical characterization, and cell viability evaluation of long-circulating and pH-sensitive liposomes containing ursolic acid. Biomed. Res. Int. 2013, 2013, 467147. [Google Scholar] [CrossRef]
- Wu, J.; Niu, Y.; Jiao, Y.; Chen, Q. Fungal chitosan from Agaricus bisporus (Lange) Sing. Chaidam increased the stability and antioxidant activity of liposomes modified with biosurfactants and loading betulinic acid. Int. J. Biol. Macromol. 2019, 193, 291–299. [Google Scholar] [CrossRef]
- Chen, X.; Lu, S.; Gong, F.; Sui, X.; Liu, T.; Wang, T. Research on the synthesis of nanoparticles of betulinic acid and their targeting antitumor activity. J. Biomed. Mater. Res. 2022, 110, 1789–1795. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Luo, L.; Li, L.; He, Y.; An, J.; Gao, D. Self-assembly of Stimuli-Responsive Au-Pd Bimetallic Nanoflowers Based on Betulinic Acid Liposomes for Synergistic Chemo-Photothermal Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 2911–2921. [Google Scholar] [CrossRef]
- Pinzaru, I.; Sarau, C.; Coricovac, D.; Marcovici, I.; Utescu, C.; Tofan, S.; Popovici, R.A.; Manea, H.C.; Pavel, I.E.; Soica, C.; et al. Silver Nanocolloids Loaded with Betulinic Acid with Enhanced Antitumor Potential: Physicochemical Characterization and In Vitro Evaluation. Nanomaterials 2021, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Wu, J.; Chen, Q. Synthesis, Characterization of Liposomes Modified with Biosurfactant MEL-A Loading Betulinic Acid and Its Anticancer Effect in HepG2 Cell. Molecules 2019, 24, 3939. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, R.; Wang, M.; Xing, S.; Li, L.; He, Y.; Cao, W.; Gao, D. Targeted therapy of octreotide-modified oleanolic acid liposomes to somatostatin receptor overexpressing tumor cells. Nanomedicine 2017, 12, 927–940. [Google Scholar] [CrossRef]
- Lis, M.; Barycza, B.; Sysak, A.; Pawlak, A.; Suszko-Pawłowska, A.; Szczypka, M.; Wawrzeńczyk, C.; Obmińska-Mrukowicz, B. Modulating effect of a new ester, 28-O-phosphatidylbetulin (DAPB), obtained from hen egg yolk lecithin and betulin on lymphocyte subsets and humoral immune response in mice. Immunopharmacol. Immunotoxicol. 2019, 41, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chu, F.; Zhang, Y.; Wang, X.; Li, Q.; Liu, W.; Xu, X.; Xing, Y.; Chen, J.; Wang, P.; et al. A Series of New Ligustrazine-Triterpenes Derivatives as Anti-Tumor Agents: Design, Synthesis, and Biological Evaluation. Int. J. Mol. Sci. 2015, 16, 21035–21055. [Google Scholar] [CrossRef]
- Strzemski, M.; Wojnicki, K.; Sowa, I.; Wojas-Krawczyk, K.; Krawczyk, P.; Kocjan, R.; Such, J.; Latalski, M.; Wnorowski, A.; Wójciak-Kosior, M. In Vitro Antiproliferative Activity of Extracts of Carlina acaulis subsp. caulescens and Carlina acanthifolia subsp. utzka. Front. Pharmacol. 2017, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Zhou, K.; Zhao, Y.; Xia, X.; Guo, Y.; Gao, Y.; Peng, G.; Liu, Y. Betulinic acid, a major therapeutic triterpene of Celastrus orbiculatus Thunb., acts as a chemosensitizer of gemcitabine by promoting Chk1 degradation. J. Ethnopharmacol. 2023, 309, 116295. [Google Scholar] [CrossRef] [PubMed]
- Şoica, C.M.; Dehelean, C.A.; Peev, C.; Aluas, M.; Zupkó, I.; Kása, P., Jr.; Alexa, E. Physico-chemical comparison of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431),breast carcinoma(MCF7)and cervix adenocarcinoma(HeLa) cell lines. Nat. Prod. Res. 2012, 26, 968–974. [Google Scholar] [CrossRef]
- Şoica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic acid in complex with a gamma-cyclodextrin derivative decreases proliferation and in vivo tumor development of non-metastatic and metastatic B164A5 cells. Int. J. Mol. Sci. 2014, 15, 8235. [Google Scholar] [CrossRef]
- Şoica, C.; Dehelean, C.; Danciu, C.; Wang, H.M.; Wenz, G.; Ambrus, R.; Bojin, F.; Anghel, M. Betulin complex in gamma-cyclodextrin derivatives: Properties and antineoplasic activities in vitro and in vivo tumor models. Int. J. Mol. Sci. 2012, 13, 14992. [Google Scholar] [CrossRef]
- Coricovac, D.O.; Soica, C.; Muntean, D.; Popovici, R.A.; Dehelean, C.; Hogea, E. Assessment of the effects induced by two triterpenoids on liver mitochondria respiratory function isolated from aged rats. Rev. Chim. 2015, 66, 1707–1710. [Google Scholar]
- Hertrampf, A.; Gründemann, C.; Jäger, S.; Laszczyk, M.; Giesemann, T.; Huber, R. In Vitro Cytotoxicity of Cyclodextrin-bonded Birch Bark Extract. Planta Medica 2012, 78, 881–889. [Google Scholar] [CrossRef]
- Cheng, B.; Chu, X.; Liu, R.; Ma, X.; Wang, M.; Zhang, J.; Jiao, P.; Gao, Q.; Ma, W.; Zhang, Y.; et al. Synthesis of Novel Pentacyclic Triterpenoid Derivatives that Induce Apoptosis in Cancer Cells through a ROS-dependent, Mitochondrial-Mediated Pathway. Mol. Pharm. 2023, 20, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Ji, H.; Wang, W.; Han, X.; Zhang, X.; Xia, L.; Guo, L.; Huang, L.; Gao, W. Mitochondria-targeted pentacyclic triterpenoid carbon dots for selective cancer cell destruction via inducing autophagy, apoptosis, as well as ferroptosis. Bioorganic Chem. 2023, 130, 106259. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, G.; Pellegretti, P.; Trucco, A. (Eds.) Ultrasound Contrast Agents, Targeting and Processing Methods for Theranostics; Springer: Milano, Italy, 2010. [Google Scholar]
- Stride, E.; Saffari, N. Microbubble ultrasound contrast agents: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2003, 217, 429–447. [Google Scholar] [CrossRef]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.G.; Kleven, R.T.; Dewitte, H.; O’reilly, M.A.; Escoffre, J.-M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-responsive cavitation nuclei for therapy and drug delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef] [PubMed]
- Kooiman, K.; Vos, H.J.; Versluis, M.; De Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef]
- Mørch, Y.; Hansen, R.; Berg, S.; Åslund, A.K.O.; Glomm, W.R.; Eggen, S.; Schmid, R.; Johnsen, H.; Kubowicz, S.; Snipstad, S.; et al. Nanoparticle stabilized microbubbles for multimodal imaging and drug delivery. Contrast Media Mol. Imaging 2015, 10, 356–366. [Google Scholar] [CrossRef]
- Kurup, N.; Naik, P. Microbubbles: A Novel Delivery System, a review. Asian J. Pharm. Res. Health Care 2008, 2, 228–234. [Google Scholar]
- Yildirim, A.; Blum, N.T.; Goodwin, A.P. Colloids, nanoparticles, and materials for imaging, delivery, ablation, and theranostics by focused ultrasound (FUS). Theranostics 2019, 9, 2572–2594. [Google Scholar] [CrossRef]
- Snipstad, S.; Hanstad, S.; Bjørkøy, A.; Mørch, Ý.; de Lange Davies, C. Sonoporation Using Nanoparticle-Loaded Microbubbles Increases Cellular Uptake of Nanoparticles Compared to Co-Incubation of Nanoparticles and Microbubbles. Pharmaceutics 2021, 13, 640. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Pancholi, K.; Melzer, A. Microbubble-liposome conjugate: Payload evaluation of potential theranostic vehicle. Nanobiomedicine 2016, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L. Ligand carrying gas filled microbubble: Ultrasound contrast agents for targeted molecular imaging. Bioconjug. Chem. 2005, 16, 9–17. [Google Scholar] [CrossRef]
- Klibanov, A.L. Targeted delivery of gas filled microspheres, contrast agents for ultrasound imaging. Adv. Drug Deliv. Rev. 1999, 37, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.M.; Bouakaz, A. (Eds.) Therapeutic Ultrasound: From biophysics concepts to clinical applications. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 880, pp. 35–78. [Google Scholar]
- Abou-Saleh, R.H.; Peyman, S.A.; Johnson, B.R.G.; Marston, G.; Ingram, N.; Bushby, R.; Coletta, P.L.; Markham, A.F.; Evans, S.D. The Influence of Intercalating Perfluorohexane into Lipid Shells on Nano and Microbubble Stability. Soft Matter 2016, 12, 7223–7230. [Google Scholar] [CrossRef]
- Jablonowski, L.J.; Cochran, M.C.; Eisenbrey, J.R.; Teraphongphom, N.T.; Wheatley, M.A. Shell Effects on Acoustic Performance of A Drug-Delivery System Activated by Ultrasound. J. Biomed. Mater Res. Part A 2017, 105, 3189–3196. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; Zhou, M.; Tortora, M.; Lucilla, B.; Ashokkumar, M. Methods of Preparation of Multifunctional Microbubbles and their In Vitro / In Vivo Assessment of Stability, Functional and Structural Properties. Curr. Pharm. Des. 2012, 18, 2135–2151. [Google Scholar] [CrossRef]
- Turánek, J.; Miller, A.D.; Kauerová, Z.; Lukáč, R.; Mašek, J.; Koudelka, Š.; Raška, M. Lipid-Based Nanoparticles and Microbubbles–Multifunctional Lipid-Based Biocompatible Particles for In vivo Imaging and Theranostics. In Advances in Bioengineering; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Al-Jawadi, S.; Thakur, S.S. Ultrasound-responsive lipid microbubbles for drug delivery: A review of preparation techniques to optimize formulation size, stability and drug loading. Int. J. Pharm. 2020, 585, 119559. [Google Scholar] [CrossRef]
- Qin, J.; Wang, T.-Y.; Willmann, J.K. Sonoporation: Applications for Cancer Therapy, in Therapeutic Ultrasound. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 880, pp. 263–292. [Google Scholar]
- Bouakaz, A.; Zeghimi, A.; Doinikov, A.A. Sonoporation: Concept and Mechanisms, in Therapeutic Ultrasound. In Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; Volume 880, pp. 165–191. [Google Scholar]
- Segers, T.; De Jong, N.; Lohse, D.; Versluis, M. Microbubbles for Medical Applications in Microfluidics for Medical Applications; van den Berg, A., Segerink, L., Eds.; RSC Nanoscience & Nanotechnology: London, UK, 2015; Volume 36, pp. 80–101. [Google Scholar]
- Roovers, S.; Lajoinie, G.; De Cock, I.; Brans, T.; Dewitte, H.; Braeckmans, K.; Versuis, M.; De Smedt, S.C.; Lentacker, I. Sonoprinting of nanoparticle-loaded microbubbles: Unraveling the multi-timescale mechanism. Biomaterials 2019, 217, 119250. [Google Scholar] [CrossRef]
- Nie, X.-B.; Wang, Y.; Ran, X.; Wu, J.-C.; Wei, R.; Yan, W.-C. Preparation of Nanoparticle-Loaded Microbubbles via an Electrohydrodynamic Atomization Process. Appl. Sci. 2022, 12, 3621. [Google Scholar] [CrossRef]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S. Lentacker, Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, Y.; Jiang, P.; Li, F.; Yu, B.; Yan, F. Lipid-PLGA hybrid microbubbles as a versatile platform for non-invasive image-guided targeted drug delivery. ACS Appl. Mater. Interfaces 2019, 11, 41842–41852. [Google Scholar] [CrossRef] [PubMed]
- Socaciu, M.A.; Rugină, D.; Nistor, M.; Diaconeasa, Z.; Socaciu, C. In vitro delivery of betulinic acid, birch bark standardized extract comparative to DOXO by sonoporation of MB-liposome and MB-NLC conjugates in tumor cells. 2023; Unpublished data. [Google Scholar]
| Molecules | Effects: Targets and Mechanisms | References |
|---|---|---|
| BA | Inhibition of cyclic AMP-dependent protein kinase, sulfonylureas, stromelysin, and collagenase | [3] |
| B and BA | Differential effects on cytokine production in human blood cell cultures | [26] |
| OA and derivatives | Anticancer activity, target profiling, and mechanisms of action | [27] |
| L and derivatives | Inhibitors of nitric oxide and prostaglandin E2 | [28] |
| B, BA, and L | Stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils | [29] |
| TTs | Induction of the mitochondrial pathway of apoptosis, especially in melanoma cells | [30] |
| TTs (Euonymus alatus twigs) | Bioassay-guided isolation and antiproliferative effects | [31] |
| B | Protects HT-22 hippocampal cells against ER stress by induction of heme oxygenase-1 and inhibition of ROS production | [32] |
| BA | Cytotoxic against tumor cells and reduced cytotoxicity against normal dermal fibroblasts and blood lymphocytes | [33] |
| B extract (Acacia auricularis) | Multi-target inhibitor of protein kinases, a modulator of mitogen-activated protein, affects the kinase pathway (ABL1 inhibitor) | [34] |
| TTs from Rhus chinensis Mill | Inhibition of enzymes in glycolytic enzymes involved in glutaminolysis | [35] |
| BA | In vitro: significant increase of intracellular-free calcium and a slight decrease in cell viability (low cytotoxicity at relatively high therapeutic doses) | [1,36] |
| B and BA esters of conjugated linoleic acid | Low cytotoxic activity in vitro and in vivo | [37] |
| B | Promotes differentiation of human osteoblasts in vitro. Osteoinductive effect on the hFOB Cell Line by Activation of JNK, ERK1/2, and mTOR Kinases | [38] |
| B, BA, L, OA, and UA | Effects on glucose absorption and uptake, insulin secretion, diabetic vascular dysfunction, retinopathy, and nephropathy | [39] |
| UA and Dev-UA Euscaphic acid extracts of Potentilla atr. | In vitro antiproliferative activity and antiestrogenic activity (high binding affinity towards estrogen receptor-α and decreased cell growth) by downregulating the expression of mRNA | [40] |
| Experiments | Results | Ref. |
|---|---|---|
| BA action on melanoma cells in vitro and in vivo against TPA-induced tumors and ovarian and melanoma xenografts in mice | Induction of p53 upregulation in metastatic melanoma cells results in surface blebbing and cytoplasmic shrinking and the formation of characteristic DNA fragments, which are indicative of apoptosis. Without toxic effects at a concentration of 500 mg/kg but significant inhibition of tumor development at 5 mg/kg | [1] |
| BA action on melanoma cells via the mitochondrial pathway of apoptosis, according to former experimental observations [1] and patent review | Anti-proliferative effect via topoisomerase I and II inhibition in tumor cells (1), mitochondrial apoptosis (2), and anti-angiogenesis (3) in cancer cells | [30] |
| BA action on melanoma cells in vitro | Induction of changes in the potential of the mitochondrial membrane, activation of caspases, and production of reactive oxygen species | [61,62] |
| BA distribution across various tissues was administered with a single intraperitoneal dose of 500 mg/kg, as determined by LC–ESI-/MS. | Accumulation of BA (452.2 µg/g) in the tumor and 50% in the liver at a significantly higher concentration but not detectable in the blood | [63] |
| BA action against different cancer cell types, e.g., acting through induction of apoptosis independent of the cell’s p53 status. | Selective induction of apoptosis in a human neuroblastoma cell line is independent of the cell’s p53 status without affecting normal cells | [33,64] |
| BA action on cells in vitro | Direct induction of mitochondrial damage, leading to Bax/Bak-independent release of cytochrome C, overcoming the resistance of tumor cell mitochondria | [65] |
| BA action on liver cancer progression | Inhibition through p53/Caspase-3 signaling activation | [66] |
| BA’s multifunctional action as an anticancer agent | Effects on JAK/STAT, VEGF, EGF/EGFR, TRAIL/TRAIL-R, and AKT/mTOR | [67] |
| Water-soluble composites of B dipropionate tested in vitro against ascites carcinoma cells and human lung adenocarcinoma cells | Improvement of antitumor and proapoptotic effects compared with B | [68] |
| BA effects in vitro | Inhibition of growth factor-induced angiogenesis via the modulation of mitochondrial function also occurs in endothelial cells through an antioxidant mechanism. | [69] |
| B effects in vitro and in vivo (chicken embryo) | Superior specificity for cervical cancer cells, followed by skin cancer cells. Reduction of newly formed capillaries, especially in the mesenchyme, is possible by targeting the normal function of endothelial cells. | [70] |
| Compounds | In Vitro or In Vivo Systems | Results | Ref. |
|---|---|---|---|
| OA and its derivatives | In vitro: Tumor cell lines MCF-7 and MCF-7/ADR, 1321N1 astrocytoma, Hepatocellular carcinoma, colorectal HCT-116 cells | Inhibition of cell proliferation, tumor cell apoptosis, autophagy, regulating cell cycle proteins, inhibiting vascular endothelial growth, tumor cell migration and invasion, and anti-angiogenesis | [27] |
| UA, OA, and BA free and modified [exocyclic 2-methylene-3-oxo] | In vitro: K562, A549, and MCF-7 Cell lines Cytotoxicity: MTT | Derivatization intensifies activity. Cytotoxicity and DNA-damaging activity in cancer cell lines in vitro | [52] |
| BA | Murine melanoma B16 cell line | Apoptotic activity | [93] |
| B | Fish and murine fibroblasts | High cytotoxicity | [94] |
| B | In vitro: A431, HeLa, and MCF7 cells In vivo: mouse, CAM assay | Angiogenic inhibitor | [77] |
| B and Betulonic acid | In vitro: isolated Wistar rat liver mitochondria and liposomes | Activate mitochondrial aggregation Inhibit ADP and DNP synthesis. No effect on membrane permeability. | [95] |
| Erythrodiol (E) | In vitro: effects on liposomal membrane properties | Interacts with the polar head groups of phospholipids E-Liposomes: spherical and smaller than control | [96] |
| BA | In vivo: HCC tumor cells, including HepG2, LM3, and MHCC97H In vivo: pulmonary metastasis in mice | Inhibition of HCC growth in vivo Blocked pulmonary metastasis-related proteins, including MMP-2, MMP-9, and TIMP2, without obvious toxicity Increase of the pro-apoptotic protein Bax and cleaved caspase-3; decrease of the anti-apoptotic protein Bcl-2. Reduced the reactive oxygen species (ROS) level | [97] |
| BA and B | In vitro: canine T-cell and B-cell lymphoma and osteosarcoma cell lines | Anti-proliferative and pro-apoptotic effects (concentration- and time-dependent), stronger for BA compared to B | [98] |
| B isolated from Quercus incana | In vitro: non-small cell lung cancer cells, murine melanoma B16 cell line | Apoptotic and antimetastatic activities | [99] |
| TTs isolated from Hibiscus syriacus | In vitro: breast cancer cells | Apoptosis and inhibition of cell migration | [100] |
| Lead-BA derivative in a polymeric nanocarrier | In vitro and in vivo colon carcinoma therapeutic efficacy by MTT assay, cell cycle | Apoptosis/antiproliferative activity are significantly increased by the nanocarrier system compared to a free-drug, effective therapeutic agent | [101] |
| B derivatives with ethynyl side chain | In vitro: Liposomes (100 nm) on human cancer cell lines Cytotoxicity: sulforhodamine-B-assay. Apoptosis test with trypan blue dye on A431 and A2780 | Encapsulation efficiency of 60% Cytotoxicity Antitumor activity by triggering apoptosis. | [102] |
| OA nanoparticles poly[oligo[ethylene glycol] methyl/methacrylate]-b-poly[oleanolic acid methacrylate] | Encapsulated 10-hydroxycamptothecin to achieve efficient cancer therapy In vitro: breast tumor cells In vivo high antitumor efficiency with low adverse effects on 4T1 mouse breast tumor xenograft | Nanostructures of 100 nm size with good drug stability, loading capacity, and efficiency Drug release up to 132 h Anti-inflammatory and anti-cancer activities. good potential as a platform for drug delivery applications | [103] |
| BA encapsulated in PEGylated Liposomes (LIPO) | Liposomes obtained by ethanol injection are characterized by TEM, AFM, DLS, and FTIR Cell lines: HepG2, HeLa, and U14 In vivo: Female Kunming mice | Liposomes of 142 nm, encapsulation efficiency: 64–95%. Good drug release on PEG-LIPO > LIPO Pegylated-LIPO: better inhibitory effect compared to BA-LIPO or free BA, both in vitro and in vivo Rat liver mitochondrial aggregation; no effect on membrane permeability | [104] |
| BA-LIPO and LIPO | In vitro: Cancer cell lines A549, sw480 fluorescent marker: rhodamine-phosphatidylethanolamine in the lipid bilayer In vivo: Nude mice xenografted with human colon and lung cancer adm. LIPO/BA-LIPO (5 mg/)mL iv. 3×/week i.v. 50 mg/kg | Liposomes of 1–1.5 microns No toxicity is caused by LIPO Reduced growth of human colon and lung tumors in mice (>50%) Oral administration also led to slow tumor growth | [105] |
| UA free UA-LIPO | In vitro: Cell culture 9L—rat gliosarcoma; MCF-7 transfected with the luciferase gene In vivo: female nude mice ip. injection, 5 days free UA—23 mg/kg Vs. LIPO | UA-LIPO (0.77 mg/)mL size: 182.7 nm UA-LIPO: Antitumor/antiangiogenic effect in the human breast tumor model but not in the gliosarcoma model | [106] |
| UA-LIPO | In vitro: breast [MDA-MB-231] and prostate [LNCaP] cancer In vivo: iv mice | UA entrapped: 0.77 mg/mL; stability: 2 months, 4 °C Cytotoxicity: significant inhibition of cancer cell proliferation | [107] |
| BA-LIPO coated with chitosan [extracted from Agaricus) vs. commercial chitosan | FTIR, XRD [X-ray diffraction], DSC [differential scanning calorimeter], DLS [EE: RP-HPLC] Antioxidant activity: DPPH, ABTS | BA-LIPO: smaller size, higher zeta potential. increased antioxidant activity compared to commercial chitosan | [108] |
| BA-LIPO ± cancer cell membranes | BA-LIPO coated with cell membranes by US compared to control LIPO Characterization: HPLC, TEM, EE, fluorescence microscopy In vitro: HeLa Cell line Viability: MTT, fluorescence | LIPO size: 120 nm (without cell membrane) and 150 nm (with cell membrane) EE: efficiency of 88% on cell toxicity | [109] |
| BA-LIPO coating of poly-branched Au-Pd bimetallic nanoflowers | NIR photothermal therapy In vitro: HeLa Cell line In vivo model: U14 tumor-bearing mice: intratumoral injection (1.4 mg/kg BA) | BA-LIPO Size: 144.4 nm Photothermal conversion efficiency: 64.6% Tumor inhibition ratio: 91.7% | [110] |
| BA in nanosized SilCo and PEG-SilCo-coated formulations | In vitro: HepG2 human hepatocellular carcinoma, A549 human lung carcinoma cell Cytotoxicity: Almar blue assay | Size: 20–80 nm SilCo and PEG_SilCo Drug carriers with improved solubility Enhanced antitumor activity using silver, SilCo Significant cytotoxic effect on cancer cell lines | [111] |
| BA-LIPO | BA 1% in LIPO modified with the biosurfactant mannosylerythritol lipid-A (MEL-A) In vitro: HepG2 cells | Size BA-LIPO: 80 nm The addition of MEL-A significantly promotes cell cytotoxicity and apoptosis, destroying mitochondrial membrane potential | [112] |
| OA-LIPO modified with octreotide (O) | LIPO is prepared by the ethanol injection method and loaded with synthetic octreotide that mimics somatostatin Size and zeta potential | OA-LIPO: 127 nm OA-LIPO-O: 130 nm ζ potentials OA-LIPO: −20.73 mV; OA-LIPO-O: −1.42 mV OA-LIPO-O: higher inhibition on cell proliferation and cell uptake (for somatostatic receptor-positive A549) | [113] |
| Ester, 28-O-phosphatidylbetulin (DAPB) | DAPB obtained from hen egg yolk lecithin and B In vitro: lymphocyte subsets In vivo: mice | Immunomodulating effect on lymphocyte subsets and humoral immune response in mice | [114] |
| Ligustrazine-TT derivatives | In vitro: Cell lines Bel-7402, HepG2, HT-29, Hela, and MCF-7, and Madin-Darby canine kidney | Anti-tumor effect and better antiproliferative actions when coupled with ligustrazine | [115] |
| TTs extract: UA, OA, BA, L, BA, from Carlina acanthifolia and C. acaulis | Individual TT and phenolics identified by LC–MS In vitro: human melanoma cell lines vs. human fibroblasts (control) | IC50 higher than 43.2 to 86% micrograms/mL Apoptotic effects | [116] |
| BA combined with gemcitabine | BA by US-assisted extraction from Celastrus orbiculatus ± gemcitabine In vitro: Human pancreatic cancer cell line, non-small-cell lung cancer cell line In vivo: BxPC-3-derived mouse xenograft model. | BA had equivalent cytotoxicity toward gemcitabine-resistant and sensitive cells Synergistic effects of BA and gemcitabine on viability, apoptosis, and DNA double-strand breaks The combination significantly delayed BxPC-3 tumor growth in vivo compared to gemcitabine alone, accompanied by reduced Chk1 expression | [117] |
| BA, B, and birch bark TTs extract | Physico-chemical comparison In vitro: skin epidermoid carcinoma (A431), breast carcinoma (MCF7), and cervix adenocarcinoma (HeLa) cell lines | Cytotoxic effects on all cell lines | [118] |
| BA complex with gamma-cyclodextrin | In vitro: mitochondria isolated from the liver of aged rats In vivo tumor development of B164A5 cells | Decrease in in vitro proliferation and in vivo inhibition of mitochondrial respiration Antineoplastic, inhibition of tumor development | [119,120,121] |
| Cyclodextrin-bonded TTs Extract | In vitro: primary liver cancer cells compared to healthy human hepatocytes | Cytotoxicity at low concentrations is induced by caspase 3/7-mediated apoptosis. Less toxic in healthy hepatocytes | [122] |
| BA and OA conjugated with per-O-methylated-β-cyclodextrin | Human cancer cell lines (MCF-7, BGC-823, and HL-60) | Two OA-derivatives decreased up to 20× the IC50 values (6.06–8.47 μM) compared to OA, Induction of the intrinsic apoptosis pathway via the ROS-mediated activation of caspase-3 signaling | [123] |
| OA, UA, and GA conjugates with cyclodextrins | In vivo (mouse biotherapy) | Induction of cell death through 3 pathways (apoptosis, ferroptosis, and autophagy) Low toxicity | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, M.; Rugina, D.; Diaconeasa, Z.; Socaciu, C.; Socaciu, M.A. Pentacyclic Triterpenoid Phytochemicals with Anticancer Activity: Updated Studies on Mechanisms and Targeted Delivery. Int. J. Mol. Sci. 2023, 24, 12923. https://doi.org/10.3390/ijms241612923
Nistor M, Rugina D, Diaconeasa Z, Socaciu C, Socaciu MA. Pentacyclic Triterpenoid Phytochemicals with Anticancer Activity: Updated Studies on Mechanisms and Targeted Delivery. International Journal of Molecular Sciences. 2023; 24(16):12923. https://doi.org/10.3390/ijms241612923
Chicago/Turabian StyleNistor, Madalina, Dumitrita Rugina, Zorita Diaconeasa, Carmen Socaciu, and Mihai Adrian Socaciu. 2023. "Pentacyclic Triterpenoid Phytochemicals with Anticancer Activity: Updated Studies on Mechanisms and Targeted Delivery" International Journal of Molecular Sciences 24, no. 16: 12923. https://doi.org/10.3390/ijms241612923
APA StyleNistor, M., Rugina, D., Diaconeasa, Z., Socaciu, C., & Socaciu, M. A. (2023). Pentacyclic Triterpenoid Phytochemicals with Anticancer Activity: Updated Studies on Mechanisms and Targeted Delivery. International Journal of Molecular Sciences, 24(16), 12923. https://doi.org/10.3390/ijms241612923









