Knockout of sws2a and sws2b in Medaka (Oryzias latipes) Reveals Their Roles in Regulating Vision-Guided Behavior and Eye Development
Abstract
1. Introduction
2. Results
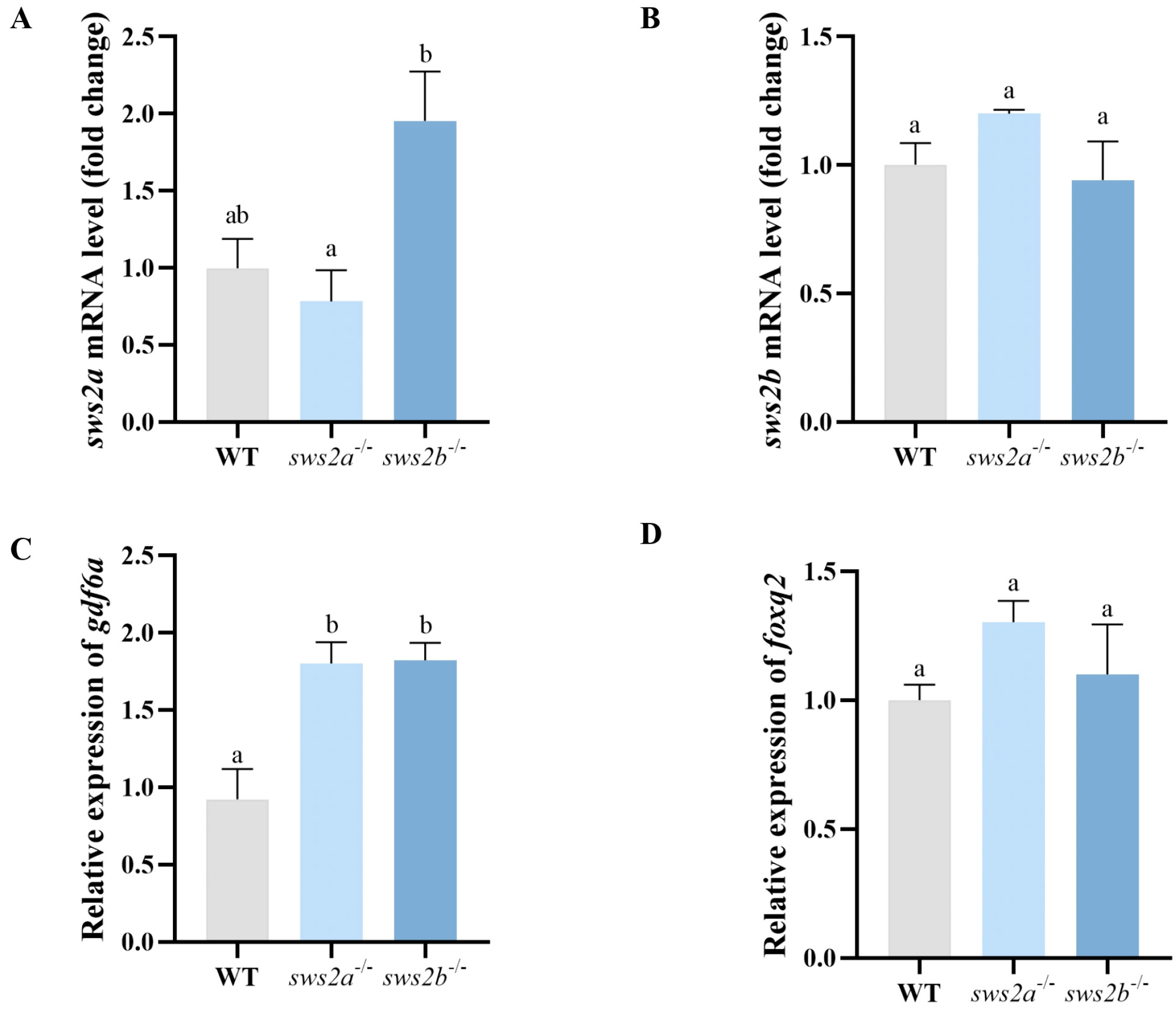
2.1. Expression of sws2a and sws2b in Medaka
2.2. Establishment of sws2a and sws2b Mutant Medaka
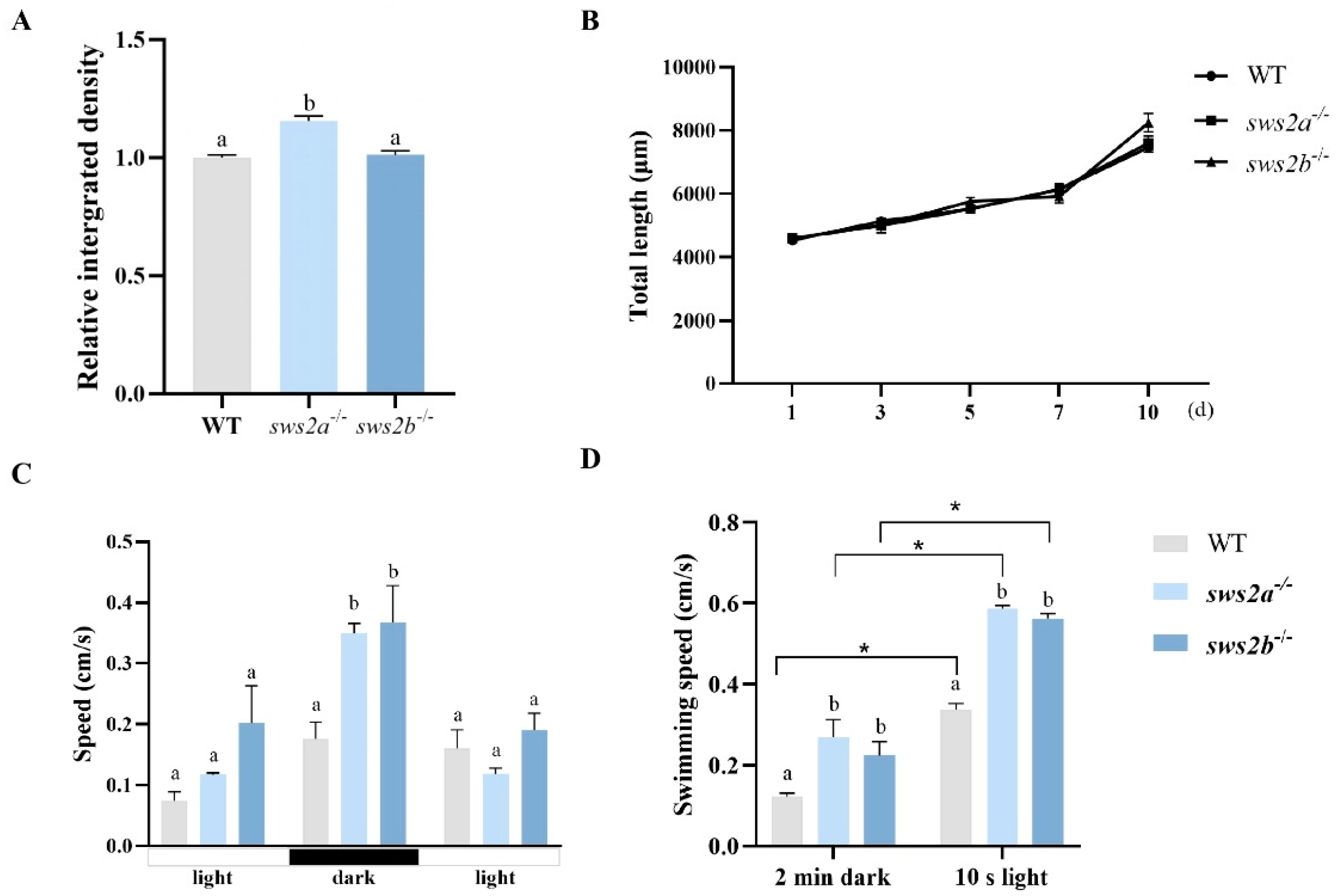
2.3. Feeding and the Behavioral Tests
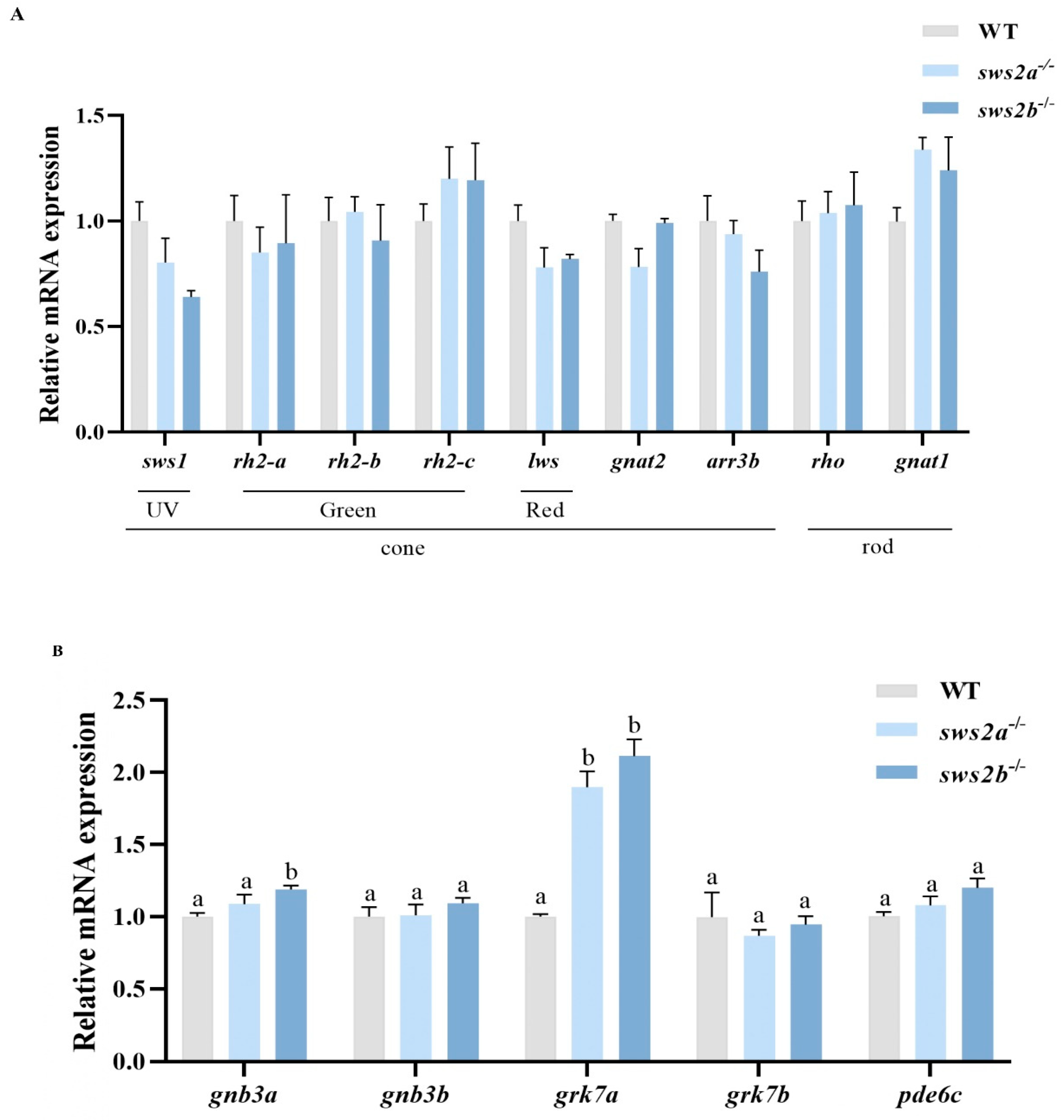
2.4. Transcript Levels of Phototransduction-Related Genes in Larvae of sws2a−/− and sws2b−/− Mutants
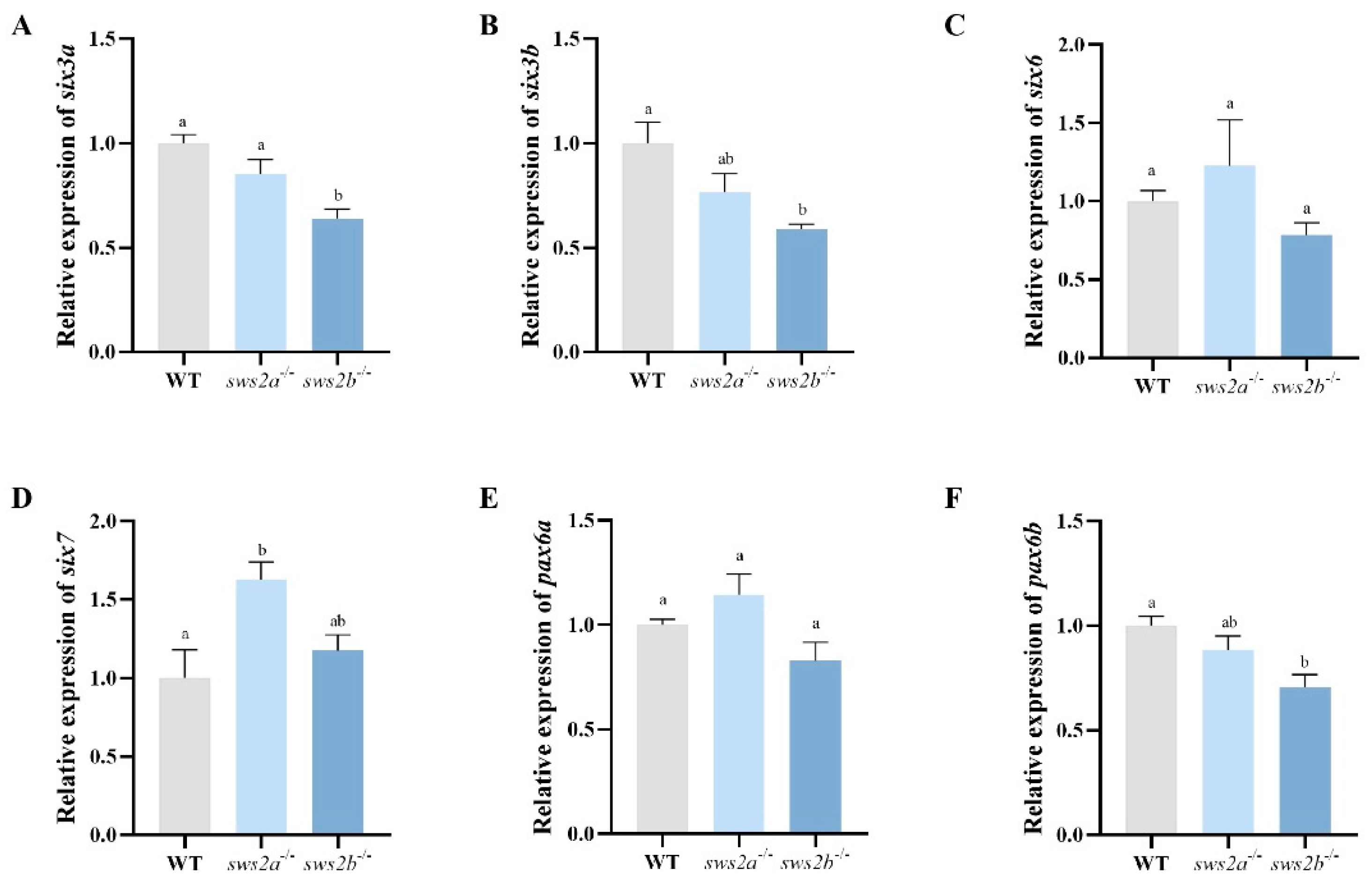
2.5. Transcript Levels of Eye Development Gene in Larval sws2a and sws2b Knockout Medaka
3. Discussion
4. Materials and Methods
4.1. Medaka Lines and Maintenance
4.2. Generating sws2a−/− and sws2b−/− Mutants by CRISPR/Cas9 Technology
4.3. Larvae Feeding Assays
4.4. Growth Performance and Survival Rate
4.5. Behavioral Tests
4.6. Hematoxylin-Eosin (H&E) Staining
4.7. RNA Isolation and Quantitative RT-PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archer, S. Light and photoreception: Visual pigments and photoreception. In Adaptive Mechanisms in the Ecology of Vision; Springer: Berlin/Heidelberg, Germany, 1999; pp. 25–42. [Google Scholar]
- Palczewski, K.; Kiser, P.D. Shedding new light on the generation of the visual chromophore. Proc. Natl. Acad. Sci. USA 2020, 117, 19629–19638. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S. Molecular evolution of vertebrate visual pigments. Prog. Retin. Eye Res. 2000, 19, 385–419. [Google Scholar] [CrossRef] [PubMed]
- Carleton, K. Cichlid fish visual systems: Mechanisms of spectral tuning. Integr. Zool. 2009, 4, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Flamarique, I.N. Opsin switch reveals function of the ultraviolet cone in fish foraging. Proc. Biol. Sci. 2013, 280, 20122490. [Google Scholar]
- Stieb, S.M.; Cortesi, F.; Queiroz, L.J.D.; Carleton, K.L.; Seehausen, O.; Marshall, N.J. Long-wavelength-sensitive (lws) opsin gene expression, foraging and visual communication in coral reef fishes. Mol. Ecol. 2022, 32, 1656–1672. [Google Scholar] [CrossRef]
- Stieb, S.M.; Cortesi, F.; Sueess, L.; Carleton, K.L.; Salzburger, W.; Marshall, N.J. Why UV vision and red vision are important for damselfish (Pomacentridae): Structural and expression variation in opsin genes. Mol. Ecol. 2017, 26, 1323–1342. [Google Scholar] [CrossRef] [PubMed]
- Shimmura, T.; Nakayama, T.; Shinomiya, A.; Fukamachi, S.; Yasugi, M.; Watanabe, E.; Shimo, T.; Senga, T.; Nishimura, T.; Tanaka, M.; et al. Dynamic plasticity in phototransduction regulates seasonal changes in color perception. Nat. Commun. 2017, 8, 412. [Google Scholar] [CrossRef]
- Lin, J.-J.; Wang, F.-Y.; Li, W.-H.; Wang, T.-Z. The rises and falls of opsin genes in 59 ray-finned fish genomes and their implications for environmental adaptation. Sci. Rep. 2017, 7, 15568. [Google Scholar] [CrossRef]
- Trezise, A.E.; Collin, S.P. Opsins: Evolution in waiting. Curr. Biol. 2005, 15, R794–R796. [Google Scholar] [CrossRef]
- Musilova, Z.; Cortesi, F. Multiple ancestral and a plethora of recent gene duplications during the evolution of the green sensitive opsin genes (RH2) in teleost fishes. BioRxiv 2021. [Google Scholar] [CrossRef]
- Cortesi, F.; Musilová, Z.; Stieb, S.M.; Salzburger, W. Ancestral duplications and highly dynamic opsin gene evolution in percomorph fishes. Proc. Natl. Acad. Sci. USA 2015, 112, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Härer, A.; Torres-Dowdall, J.; Meyer, A. Rapid adaptation to a novel light environment: The importance of ontogeny and phenotypic plasticity in shaping the visual system of Nicaraguan Midas cichlid fish (Amphilophus citrinellus spp.). Mol. Ecol. 2017, 26, 5582–5593. [Google Scholar] [CrossRef]
- Marques, D.A.; Taylor, J.S.; Jones, F.C.; Palma, F.D.; Kingsley, D.M.; Reimchen, T.E. Convergent evolution of SWS2 opsin facilitates adaptive radiation of threespine stickleback into different light environments. PLoS Biol. 2017, 15, e2001627. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-L.; Liang, X.-F.; Li, L.; Wu, J.; Lu, K. Genome-wide identification and expression patterns of opsin genes during larval development in Chinese perch (Siniperca chuatsi). Gene 2022, 825, 146434. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Gan, K.J.; Flamarique, I.N. The ultraviolet opsin is the first opsin expressed during retinal development of salmonid fishes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 866–873. [Google Scholar] [CrossRef]
- Cheng, C.L.; Flamarique, I.N. Chromatic organization of cone photoreceptors in the retina of rainbow trout: Single cones irreversibly switch from UV (SWS1) to blue (SWS2) light sensitive opsin during natural development. J. Exp. Biol. 2007, 210, 4123–4135. [Google Scholar] [CrossRef]
- Valen, R.; Edvardsen, R.B.; Søviknes, A.M.; Drivenes, Ø.; Helvik, J.V. Molecular evidence that only two opsin subfamilies, the blue light-(SWS2) and green light-sensitive (RH2), drive color vision in Atlantic cod (Gadus morhua). PLoS ONE 2014, 9, e115436. [Google Scholar] [CrossRef]
- Liu, D.W.; Lu, Y.; Yan, H.Y.; Zakon, H.H. South American Weakly Electric Fish (Gymnotiformes) Are Long-Wavelength-Sensitive Cone Monochromats. Brain Behav. Evol. 2016, 88, 204–212. [Google Scholar] [CrossRef]
- Musilova, Z.; Salzburger, W.; Cortesi, F. The visual opsin gene repertoires of teleost fishes: Evolution, ecology, and function. Annu. Rev. Cell Dev. Bi. 2021, 37, 441–468. [Google Scholar] [CrossRef]
- Duval, M.G.; Oel, A.P.; Allison, W.T. gdf6a is required for cone photoreceptor subtype differentiation and for the actions of tbx2b in determining rod versus cone photoreceptor fate. PLoS ONE 2014, 9, e92991. [Google Scholar] [CrossRef]
- Ogawa, Y.; Shiraki, T.; Fukada, Y.; Kojima, D. Foxq2 determines blue cone identity in zebrafish. Sci. Adv. 2021, 7, eabi9784. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Shiraki, T.; Asano, Y.; Fukada, Y. Six6 and Six7 coordinately regulate expression of middle-wavelength opsins in zebrafish. Proc. Natl. Acad. Sci. USA 2019, 116, 4651–4660. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Popowitz, J.; Delbridge-Perry, M.; Rowe, C.J.; Connaughton, V.P. The role of estrogen and thyroid hormones in zebrafish visual system function. Front. Pharmacol. 2022, 13, 837687. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Shoji Fukamachi, S.; Mitani, H.; Kawamura, S. Functional characterization of visual opsin repertoire in Medaka (Oryzias latipes). Gene 2006, 371, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Kitambi, S.S.; Malicki, J.J. Spatiotemporal features of neurogenesis in the retina of medaka, Oryzias Latipes. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2008, 237, 3870–3881. [Google Scholar] [CrossRef]
- Tohya, S.; Mochizuki, A.; Iwasa, Y. Difference in the retinal cone mosaic pattern between zebrafish and medaka: Cell-rearrangement model. J. Theor. Biol. 2003, 221, 289–300. [Google Scholar] [CrossRef]
- Harada, Y.; Matsuo, M.; Kamei, Y.; Goto, M.; Fukamachi, S. Evolutionary history of the medaka long-wavelength sensitive genes and effects of artificial regression by gene loss on behavioural photosensitivity. Sci. Rep. 2019, 9, 2726. [Google Scholar] [CrossRef]
- Matsuo, M.; Matsuyama, M.; Kobayashi, T.; Kanda, S.; Ansai, S.; Kawakami, T.; Hosokawa, E.; Daido, Y.; Kusakabe, T.G.; Naruse, K.; et al. Retinal Cone Mosaic in sws1-Mutant Medaka (Oryzias latipes), A Teleost. Investig. Ophthalmol. Vis. Sci. 2022, 63, 21. [Google Scholar] [CrossRef]
- Rennison, D.J.; Owens, G.L.; Taylor, J.S. Opsin gene duplication and divergence in ray-finned fish. Mol. Phylogenet Evol. 2012, 62, 986–1008. [Google Scholar] [CrossRef]
- Musser, J.M.; Arendt, D. Loss and gain of cone types in vertebrate ciliary photoreceptor evolution. Dev. Biol. 2017, 431, 26–35. [Google Scholar] [CrossRef]
- Yokoyama, S. Evolution of dim-light and color vision pigments. Annu. Rev. Genom. Hum. Genet. 2008, 9, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Wang, Y.; Li, Z.; Zhou, L.; Gui, J.-F. Sequential, divergent, and cooperative requirements of Foxl2a and Foxl2b in ovary development and maintenance of zebrafish. Genetics 2017, 205, 1551–1572. [Google Scholar] [CrossRef]
- Chang, C.-H.; Catchen, J.; Moran, R.L.; Rivera-Colón, A.G.; Wang, Y.-C.; Fuller, R.C. Sequence analysis and ontogenetic expression patterns of cone opsin genes in the bluefin killifish (Lucania goodei). J. Hered. 2021, 112, 357–366. [Google Scholar] [CrossRef]
- Qu, Z.; Yimer, T.A.; Xie, S.; Wong, F.; Yu, S.; Liu, X.; Han, S.; Ma, J.; Lu, Z.; Hu, X.; et al. Knocking out lca5 in zebrafish causes cone-rod dystrophy due to impaired outer segment protein trafficking. BBA-Mol. Basis Dis. 2019, 1865, 2694–2705. [Google Scholar] [CrossRef] [PubMed]
- Takechi, M.; Seno, S.; Kawamura, S. Identification of cis-acting elements repressing blue opsin expression in zebrafish UV cones and pineal cells. J. Biol. Chem. 2008, 283, 31625–31632. [Google Scholar] [CrossRef]
- Gosse, N.J.; Baier, H. An essential role for Radar (Gdf6a) in inducing dorsal fate in the zebrafish retina. Proc. Nat. Acad. Sci. USA 2009, 106, 2236–2241. [Google Scholar] [CrossRef] [PubMed]
- Deveau, C.; Jiao, X.; Suzuki, S.C.; Krishnakumar, A.; Yoshimatsu, T.; Hejtmancik, J.F.; Nelson, R.F. Thyroid hormone receptor beta mutations alter photoreceptor development and function in Danio rerio (Zebrafish). PLoS Genet. 2020, 16, e1008869. [Google Scholar] [CrossRef]
- Novales Flamarique, I.; Ahmed, A.S.; Cheng, C.L.; Molday, R.S.; Devlin, R.H. Growth hormone regulates opsin expression in the retina of a salmonid fish. J. Neuroendocrinol. 2019, 31, e12804. [Google Scholar] [CrossRef]
- Uhlenhaut, N.H.; Treier, M. Foxl2 function in ovarian development. Mol. Genet. Metab. 2006, 88, 225–234. [Google Scholar] [CrossRef]
- Flamarique, N.I. Diminished foraging performance of a mutant zebrafish with reduced population of ultraviolet cones. Proc. Biol. Sci. 2016, 283, 20160058. [Google Scholar]
- Viken, E.N. Expression of Visual Opsins in the Retina of Atlantic salmon (Salmo salar L.) during smoltification. Master’s Thesis, The University of Bergen, Bergen, Norway, 2020. [Google Scholar]
- Downing, G. Impact of spectral composition on larval haddock, Melanogrammus aeglefinus L., growth and survival. Aquac. Res. 2002, 33, 251–259. [Google Scholar] [CrossRef]
- Villamizar, N.; Blanco-Vives, B.; Migaud, H.; Davie, A.; Davie, A.; Carboni, S.; Sánchez-Vázquez, F.J. Effects of light during early larval development of some aquacultured teleosts: A review. Aquaculture 2011, 315, 86–94. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Chen, M.; He, S.; Fang, C.; Chen, M.; Li, D.; Wu, D.; Chernick, M.; Hinton, D.E.; Bo, J.; et al. Microplastics decrease the toxicity of triphenyl phosphate (TPhP) in the marine medaka (Oryzias melastigma) larvae. Sci. Total Environ. 2021, 763, 143040. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Chen, Y.; Shang, Y.; Cui, J.; Li, Z.; Li, Y. Knockout of zebrafish interleukin 7 receptor (IL7R) by the CRISPR/Cas9 system delays retinal neurodevelopment. Cell Death Dis. 2018, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Li, F.; Lu, M.; Ge, K.; Gan, L.; Shang, D. LIM Homeobox 9 knockdown by morpholino does not affect zebrafish retinal development. Biol. Open 2021, 10, bio056382. [Google Scholar] [CrossRef]
- Rinner, O.; Makhankov, Y.V.; Biehlmaier, O.; Neuhauss, S.C.F. Knockdown of cone-specific kinase GRK7 in larval zebrafish leads to impaired cone response recovery and delayed dark adaptation. Neuron 2005, 47, 231–242. [Google Scholar] [CrossRef]
- Chrispell, J.D.; Dong, E.; Osawa, S.; Liu, J.; Cameron, D.J.; Weiss, E.R. Grk1b and Grk7a both contribute to the recovery of the isolated cone photoresponse in larval zebrafish. Investig. Ophth. Vis. Sci. 2018, 59, 5116–5124. [Google Scholar] [CrossRef]
- Nikon, S.S.; Lyubarsky, A.; Fina, M.E.; Nikonova, E.S.; Sengupta, A.; Chinniah, C.; Ding, X.-J.; Smith, R.G.; Pugh Jr, E.N.; Vardi, N.; et al. Cones respond to light in the absence of transducin β subunit. J. Neurosci. 2013, 33, 5182–5194. [Google Scholar] [CrossRef]
- Berry, M.H.; Holt, A.; Salari, A.; Veit, J.; Visel, M.; Levitz, J.; Aghi, K.; Gaub, B.M.; Sivyer, B.; Flannery, J.G.; et al. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat. Commun. 2019, 10, 1221. [Google Scholar] [CrossRef]
- Kanazawa, N.; Goto, M.; Harada, Y.; Takimoto, C.; Sasaki, Y.; Uchikawa, T.; Kamei, Y.; Matsuo, M.; Fukamachi, S. Changes in a cone opsin repertoire affect color-dependent social behavior in medaka but not behavioral photosensitivity. Front Genet. 2020, 11, 801. [Google Scholar] [CrossRef]
- Zheng, S.; Shao, F.; Tao, W.; Liu, Z.; Long, J.; Wang, X.; Zhang, S.; Zhao, Q.; Carleton, K.L.; Kocher, T.D.; et al. Chromosome-level assembly of southern catfish (Silurus meridionalis) provides insights into visual adaptation to nocturnal and benthic lifestyles. Mol. Ecol. Resour. 2021, 21, 1575–1592. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, D.A.; Krejca, J.K.; Martinez, J.M.R. Mexican blindcats genus Prietella (Siluriformes: Ictaluridae): An overview of recent explorations. Environ. Biol. Fish. 2001, 62, 315–337. [Google Scholar] [CrossRef]
- Schluessel, V.; Rick, I.P.; Seifert, F.T.; Baumann, C.; Davies, W.I.L. Not just shades of grey: Life is full of colour for the ocellate river stingray (Potamotrygon motoro). J. Exp. Biol. 2021, 224, 9. [Google Scholar] [CrossRef] [PubMed]
- Delroisse, J.; Duchatelet, L.; Flammang, P.; Mallefet, J. De novo transcriptome analyses provide insights into opsin-based photoreception in the lanternshark Etmopterus spinax. PLoS ONE 2018, 13, e0209767. [Google Scholar] [CrossRef]
- Fasick, J.I.; Algrain, H.; Serba, K.M.; Robinson, P.R. The retinal pigments of the whale shark (Rhincodon typus) and their role in visual foraging ecology. Vis. Neurosci. 2019, 36, E011. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, Y.; Ji, W.; Li, X.; Shi, Z.; Hou, J.; Li, W.; Fu, Y. Thyroid Hormone Signaling Is Required for Dynamic Variation in Opsins in the Retina during Metamorphosis of the Japanese Flounder (Paralichthys olivaceus). Biology. 2023, 12, 397. [Google Scholar] [CrossRef]
- Rapicavoli, N.A.; Poth, E.M.; Zhu, H.; Blackshaw, S. The long noncoding RNA Six3OS acts in trans to regulate retinal development by modulating Six3 activity. Neural. Dev. 2011, 6, 32. [Google Scholar] [CrossRef]
- Inbal, A.; Kim, S.; Shin, J.; Solnica-Krezel, L. Six3 represses nodal activity to establish early brain asymmetry in zebrafish. Neuron 2007, 55, 407–415. [Google Scholar] [CrossRef]
- Li, X.; Perissi, V.; Liu, F.; Rose, D.W.; Rosenfeld, M.G. Tissue-specific regulation of retinal and pituitary precursor cell proliferation. Science 2002, 297, 1180–1183. [Google Scholar] [CrossRef]
- Chen, Y.; Slack, J. Identifying the precursor zone of muscle satellite cells in Xenopus laevis embryos. Dev. Biol. 2008, 319, 557–558. [Google Scholar] [CrossRef]
- Favor, J.; Gloeckner, C.J.; Neuhäuser-Klaus, A.; Pretsch, W.; Sandulache, R.; Saule, S.; Zaus, I. Relationship of Pax6 activity levels to the extent of eye development in the mouse, Mus musculus. Genet. 2008, 179, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Shiraki, T.; Kojima, D.; Fukada, Y. Homeobox transcription factor Six7 governs expression of green opsin genes in zebrafish. Proc. Biol. Sci. 2015, 282, 20150659. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Hong, N.; Hong, Y. Derivation and characterization of haploid embryonic stem cell cultures in medaka fish. Nat. Protoc. 2010, 5, 1418–1430. [Google Scholar] [CrossRef]
- Shimada, Y.; Hirano, M.; Nishimura, Y.; Tanaka, T. A high-throughput fluorescence-based assay system for appetite-regulating gene and drug screening. PLoS ONE 2012, 7, e52549. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Huang, C.; Wang, L.; Ye, X.; Bai, C.; Simonich, M.T.; Tanguay, R.L.; Dong, X. Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat. Toxicol. 2010, 98, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, K.; Wu, J.; Tang, S.; Jia, X.; Liang, X.-F. Knockout of sws2a and sws2b in Medaka (Oryzias latipes) Reveals Their Roles in Regulating Vision-Guided Behavior and Eye Development. Int. J. Mol. Sci. 2023, 24, 8786. https://doi.org/10.3390/ijms24108786
Lu K, Wu J, Tang S, Jia X, Liang X-F. Knockout of sws2a and sws2b in Medaka (Oryzias latipes) Reveals Their Roles in Regulating Vision-Guided Behavior and Eye Development. International Journal of Molecular Sciences. 2023; 24(10):8786. https://doi.org/10.3390/ijms24108786
Chicago/Turabian StyleLu, Ke, Jiaqi Wu, Shulin Tang, Xiaodan Jia, and Xu-Fang Liang. 2023. "Knockout of sws2a and sws2b in Medaka (Oryzias latipes) Reveals Their Roles in Regulating Vision-Guided Behavior and Eye Development" International Journal of Molecular Sciences 24, no. 10: 8786. https://doi.org/10.3390/ijms24108786
APA StyleLu, K., Wu, J., Tang, S., Jia, X., & Liang, X.-F. (2023). Knockout of sws2a and sws2b in Medaka (Oryzias latipes) Reveals Their Roles in Regulating Vision-Guided Behavior and Eye Development. International Journal of Molecular Sciences, 24(10), 8786. https://doi.org/10.3390/ijms24108786





