Synthesis and Degradation of Poly(ADP-ribose) in Zebrafish Brain Exposed to Aluminum
Abstract
1. Introduction
2. Results
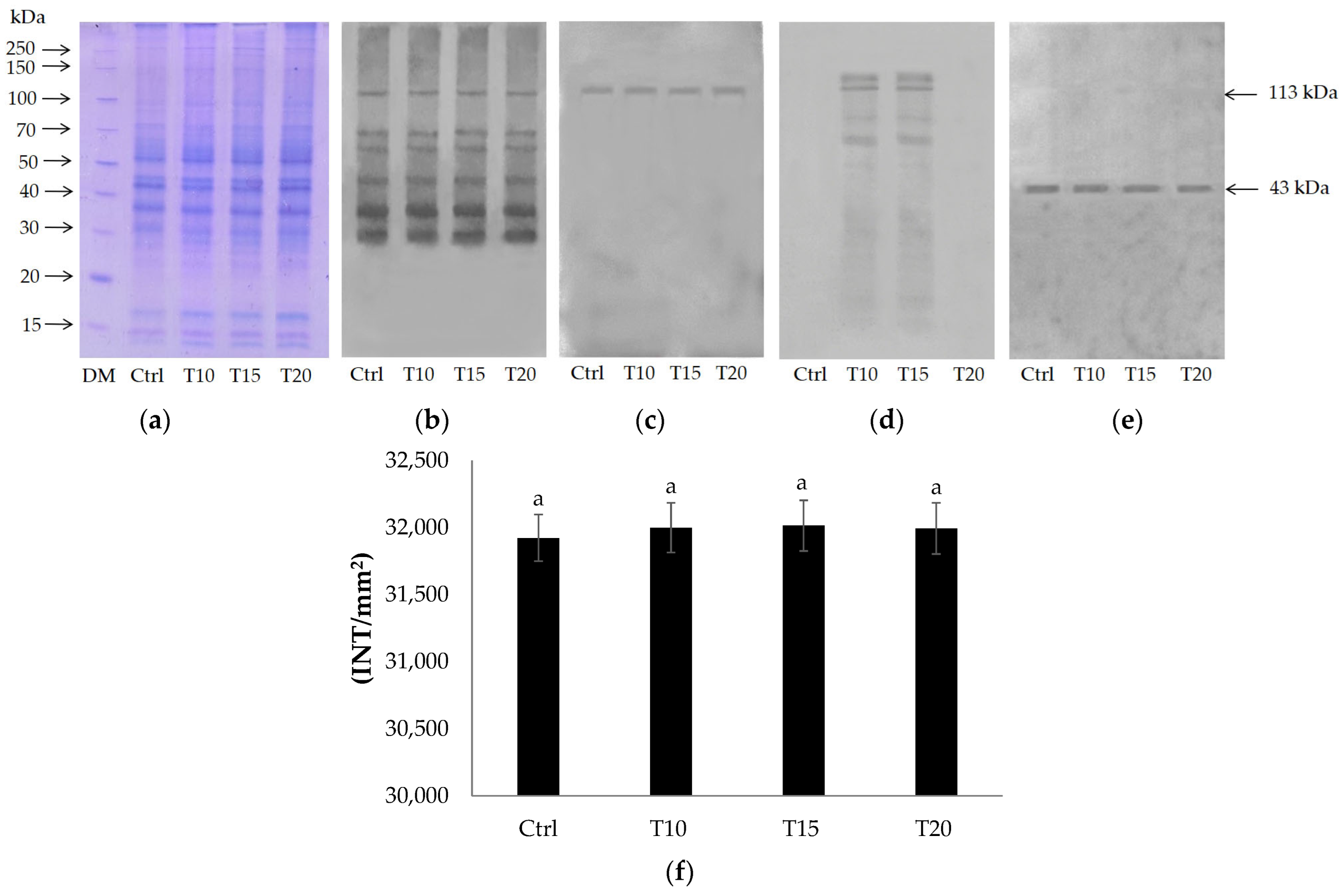
2.1. Identification of PARP Enzymes by Immunochemical Analysis
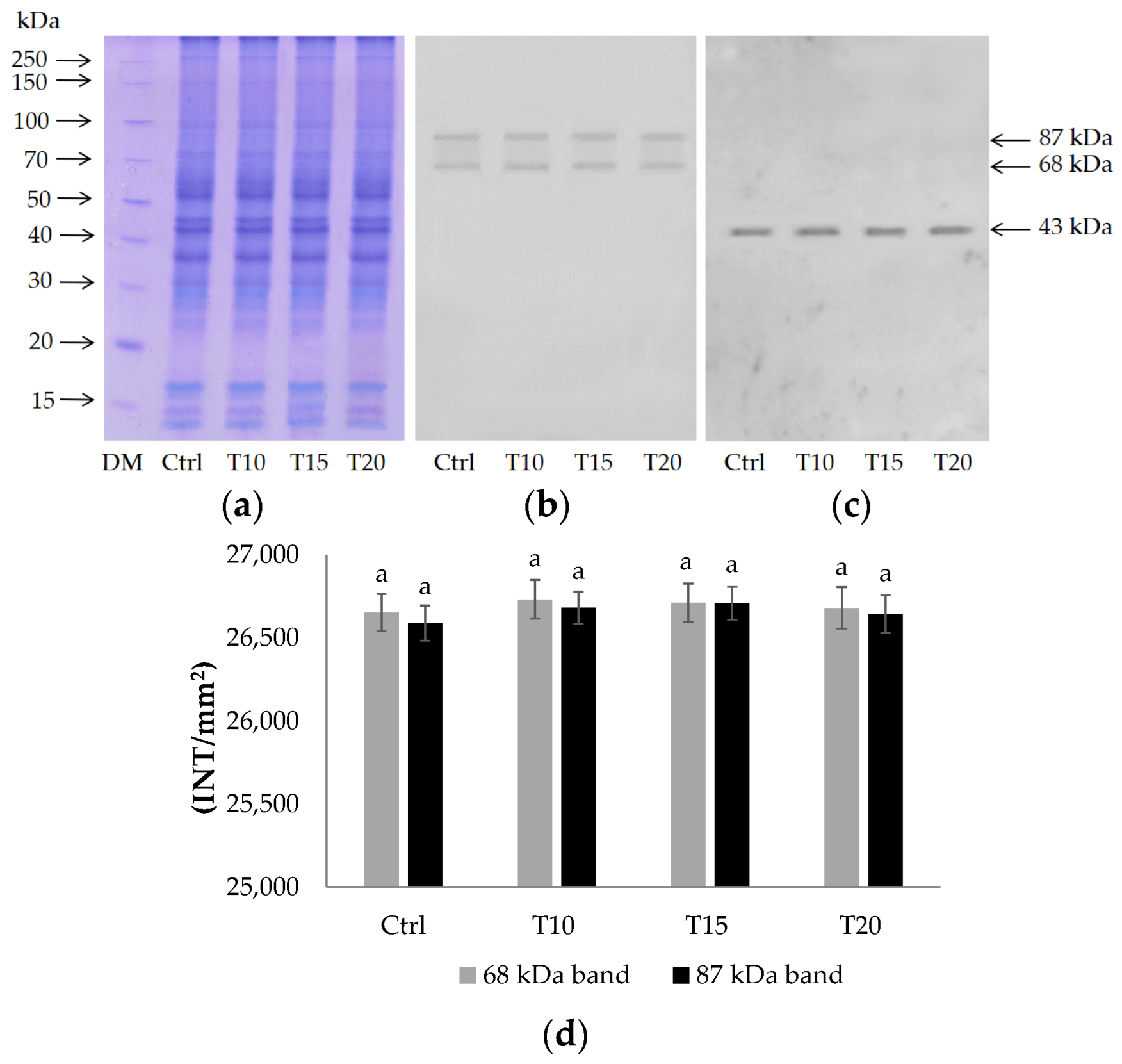
2.2. PARG Expression
2.3. PARG Assay
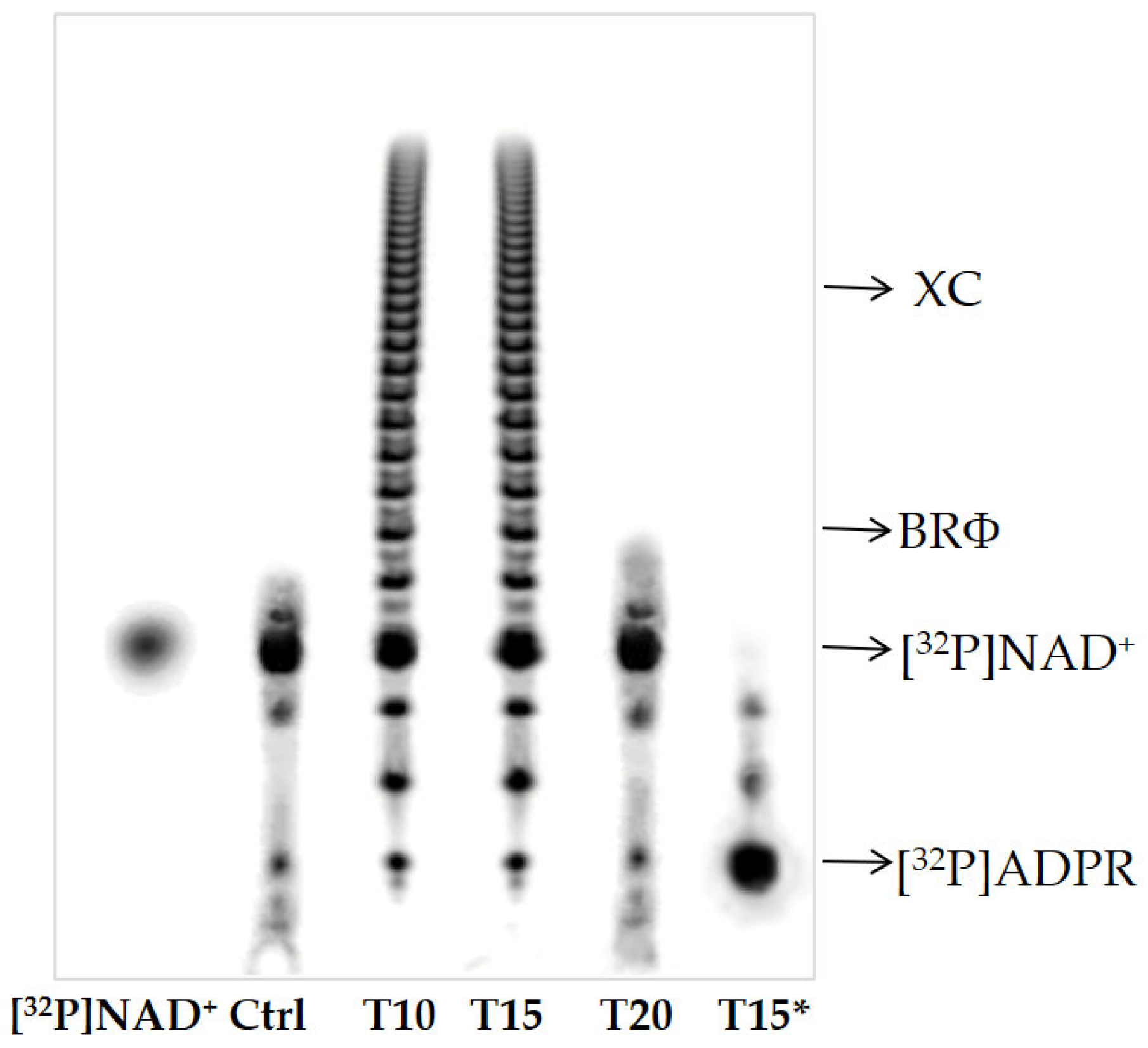
2.4. TLC and PAGE of poly(ADPR) Synthesis and Degradation
3. Discussion
4. Materials and Methods
4.1. Zebrafish Housing
4.2. Treatment Solution
4.3. Assessment of Al Concentration
4.4. Homogenates Preparation
4.5. SDS-PAGE and Immunoblotting
4.6. PAGE and TLC of Poly(ADPR) Synthesis
4.7. Poly(ADP-ribose) Glycohydrolase Activity
4.8. PAGE and TLC of Poly(ADP-ribose) Degradation
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hakmé, A.; Wong, H.K.; Dantzer, F.; Schreiber, V. The Expanding Field of Poly(ADP-Ribosyl)Ation Reactions. “Protein Modifications: Beyond the Usual Suspects” Review Series. EMBO Rep. 2008, 9, 1094–1100. [Google Scholar] [CrossRef]
- Altmeyer, M.; Messner, S.; Hassa, P.O.; Fey, M.; Hottiger, M.O. Molecular Mechanism of Poly(ADP-Ribosyl)Ation by PARP1 and Identification of Lysine Residues as ADP-Ribose Acceptor Sites. Nucleic Acids Res. 2009, 37, 3723–3738. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, J.; Bürkle, A. Introduction to Poly(ADP-Ribose) Metabolism. Cell. Mol. Life Sci. 2005, 62, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Hassa, P.O.; Hottiger, M.O. The Diverse Biological Roles of Mammalian PARPS, a Small but Powerful Family of Poly-ADP-Ribose Polymerases. Front. Biosci. 2008, 13, 3046–3082. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, V.; Dantzer, F.; Amé, J.C.; De Murcia, G. Poly(ADP-Ribose): Novel Functions for an Old Molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef]
- Amé, J.C.; Spenlehauer, C.; De Murcia, G. The PARP Superfamily. Bioessays 2004, 26, 882–893. [Google Scholar] [CrossRef]
- Kleine, H.; Poreba, E.; Lesniewicz, K.; Hassa, P.O.; Hottiger, M.O.; Litchfield, D.W.; Shilton, B.H.; Lüscher, B. Substrate-Assisted Catalysis by PARP10 Limits Its Activity to Mono-ADP-Ribosylation. Mol. Cell 2008, 32, 57–69. [Google Scholar] [CrossRef]
- Hottiger, M.O.; Hassa, P.O.; Lüscher, B.; Schüler, H.; Koch-Nolte, F. Toward a Unified Nomenclature for Mammalian ADP-Ribosyltransferases. Trends Biochem. Sci. 2010, 35, 208–219. [Google Scholar] [CrossRef]
- Briggs, A.G.; Bent, A.F. Poly(ADP-Ribosyl)Ation in Plants. Trends Plant Sci. 2011, 16, 372–380. [Google Scholar] [CrossRef]
- Thomas, C.; Tulin, A.V. Poly-ADP-Ribose Polymerase: Machinery for Nuclear Processes. Mol. Asp. Med. 2013, 34, 1124–1137. [Google Scholar] [CrossRef]
- Bürkle, A. Poly(ADP-Ribose). The Most Elaborate Metabolite of NAD+. FEBS J. 2005, 272, 4576–4589. [Google Scholar] [CrossRef]
- Aubin, R.J.; Fréchette, A.; de Murcia, G.; Mandel, P.; Lord, A.; Grondin, G.; Poirier, G.G. Correlation between Endogenous Nucleosomal Hyper(ADP-Ribosyl)Ation of Histone H1 and the Induction of Chromatin Relaxation. EMBO J. 1983, 2, 1685–1693. [Google Scholar] [CrossRef]
- Krietsch, J.; Rouleau, M.; Pic, É.; Ethier, C.; Dawson, T.M.; Dawson, V.L.; Masson, J.Y.; Poirier, G.G.; Gagné, J.P. Reprogramming Cellular Events by Poly(ADP-Ribose)-Binding Proteins. Mol. Asp. Med. 2013, 34, 1066–1087. [Google Scholar] [CrossRef] [PubMed]
- Ahel, D.; Hořejší, Z.; Wiechens, N.; Polo, S.E.; Garcia-Wilson, E.; Ahel, I.; Flynn, H.; Skehel, M.; West, S.C.; Jackson, S.P.; et al. Poly(ADP-Ribose)-Dependent Regulation of DNA Repair by the Chromatin Remodeling Enzyme ALC1. Science 2009, 325, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Viña, J. Moderate Exercise Is an Antioxidant: Upregulation of Antioxidant Genes by Training. Free Radic. Biol. Med. 2008, 44, 126–131. [Google Scholar] [CrossRef]
- D’Amours, D.; Desnoyers, S.; D’Silva, I.; Poirier, G.G. Poly(ADP-Ribosyl)Ation Reactions in the Regulation of Nuclear Functions. Biochem. J. 1999, 342 Pt 2, 249–268. [Google Scholar] [CrossRef]
- Virág, L.; Szabó, C. The Therapeutic Potential of Poly(ADP-Ribose) Polymerase Inhibitors. Pharmacol. Rev. 2002, 54, 375–429. [Google Scholar] [CrossRef] [PubMed]
- Vitale, L.; Vitale, E.; Costanzo, G.; De Maio, A.; Arena, C. Photo-Protective Mechanisms and the Role of Poly (ADP-Ribose) Polymerase Activity in a Facultative CAM Plant Exposed to Long-Term Water Deprivation. Plants 2020, 9, 1192. [Google Scholar] [CrossRef]
- D’Errico, G.; Vitiello, G.; De Tommaso, G.; Abdel-Gawad, F.K.; Brundo, M.V.; Ferrante, M.; De Maio, A.; Trocchia, S.; Bianchi, A.R.; Ciarcia, G.; et al. Electron Spin Resonance (ESR) for the Study of Reactive Oxygen Species (ROS) on the Isolated Frog Skin (Pelophylax Bergeri): A Non-Invasive Method for Environmental Monitoring. Environ. Res. 2018, 165, 11–18. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; De Maio, A. Growth Alteration and Leaf Biochemical Responses in Phaseolus Vulgaris Exposed to Different Doses of Ionising Radiation. Plant Biol. 2014, 16, 194–202. [Google Scholar] [CrossRef]
- De Maio, A.; Trocchia, S.; Guerriero, G. The Amphibian Pelophylax Bergeri (Günther, 1986) Testis Poly(ADP-Ribose)Polymerases: Relationship to Endocrine Disruptors during Spermatogenesis. Ital. J. Zool. 2014, 81, 256–263. [Google Scholar] [CrossRef]
- Guerriero, G.; Brundo, M.V.; Labar, S.; Bianchi, A.R.; Trocchia, S.; Rabbito, D.; Palumbo, G.; Abdel-Gawad, F.K.; De Maio, A. Frog (Pelophylax Bergeri, Günther 1986) Endocrine Disruption Assessment: Characterization and Role of Skin Poly(ADP-Ribose) Polymerases. Environ. Sci. Pollut. Res. 2018, 25, 18303–18313. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, L.; Bianchi, A.; Mistretta, C.; Vitale, E.; Parisi, C.; Guerriero, G.; Magliulo, V.; De Maio, A. The Ageing Process Affects the Antioxidant Defences and the Poly (ADPribosyl)Ation Activity in Cistus Incanus L. Leaves. Antioxidants 2019, 8, 528. [Google Scholar] [CrossRef]
- Park, H.; Kam, T.I.; Dawson, T.M.; Dawson, V.L. Poly (ADP-Ribose) (PAR)-Dependent Cell Death in Neurodegenerative Diseases. Int. Rev. Cell Mol. Biol. 2020, 353, 1–29. [Google Scholar] [CrossRef]
- Henning, R.J.; Bourgeois, M.; Harbison, R.D. Poly(ADP-Ribose) Polymerase (PARP) and PARP Inhibitors: Mechanisms of Action and Role in Cardiovascular Disorders. Cardiovasc. Toxicol. 2018, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.N.; Gómez de las Heras, M.M.; Mittelbrunn, M. Nicotinamide Adenine Dinucleotide Metabolism in the Immune Response, Autoimmunity and Inflammageing. Br. J. Pharmacol. 2022, 179, 1839–1856. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, Y.; Liu, X.; Liu, B.; Wang, Z.-Y.; Xie, F.; Qiao, W.; Liang, E.S.; Lu, Q.H.; Zhang, M.X. Loss of PARP-1 Attenuates Diabetic Arteriosclerotic Calcification via Stat1/Runx2 Axis. Cell Death Dis. 2020, 11, 22. [Google Scholar] [CrossRef]
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Chad Brenner, J.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual Roles of PARP-1 Promote Cancer Growth and Progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef]
- Motataianu, A.; Serban, G.; Barcutean, L.; Balasa, R. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int. J. Mol. Sci. 2022, 23, 9339. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Rodrigues, C.M.P. Oxidative Stress and Regulated Cell Death in Parkinson’s Disease. Ageing Res. Rev. 2021, 67, 101263. [Google Scholar] [CrossRef]
- Ionescu-Tucker, A.; Cotman, C.W. Emerging Roles of Oxidative Stress in Brain Aging and Alzheimer’s Disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.J.; Cappai, R.; Volitakis, I.; Cherny, R.A.; White, A.R.; Beyreuther, K.; Masters, C.L.; Bush, A.I.; Li, Q.X. Overexpression of Alzheimer’s Disease Amyloid-Beta Opposes the Age-Dependent Elevations of Brain Copper and Iron. J. Biol. Chem. 2002, 277, 44670–44676. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.I. The Metallobiology of Alzheimer’s Disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Yang, E.Y.; Tsai-Turton, M.; Bondy, S.C. Pro-Inflammatory Effects of Aluminum in Human Glioblastoma Cells. Brain Res. 2002, 933, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pogue, A.; Jaber, V.; Zhao, Y.; Lukiw, W. Systemic Inflammation in C57BL/6J Mice Receiving Dietary Aluminum Sulfate; Up-Regulation of the Pro-Inflammatory Cytokines IL-6 and TNFα, C-Reactive Protein (CRP) and MiRNA-146a in Blood Serum. J. Alzheimers Dis. Park. 2017, 7, 403. [Google Scholar] [CrossRef]
- Garza-Lombó, C.; Posadas, Y.; Quintanar, L.; Gonsebatt, M.E.; Franco, R. Neurotoxicity Linked to Dysfunctional Metal Ion Homeostasis and Xenobiotic Metal Exposure: Redox Signaling and Oxidative Stress. Antioxid. Redox Signal. 2018, 28, 1669–1703. [Google Scholar] [CrossRef]
- Song, Y.; Xue, Y.; Liu, X.; Wang, P.; Liu, L. Effects of Acute Exposure to Aluminum on Blood-Brain Barrier and the Protection of Zinc. Neurosci. Lett. 2008, 445, 42–46. [Google Scholar] [CrossRef]
- Kawahara, M.; Kato-Negishi, M. Link between Aluminum and the Pathogenesis of Alzheimer’s Disease: The Integration of the Aluminum and Amyloid Cascade Hypotheses. Int. J. Alzheimers Dis. 2011, 2011, 276393. [Google Scholar] [CrossRef]
- Jones, K.; Linhart, C.; Hawkins, C.; Exley, C. Urinary Excretion of Aluminium and Silicon in Secondary Progressive Multiple Sclerosis. EBioMedicine 2017, 26, 60–67. [Google Scholar] [CrossRef]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s Disease: Mechanisms and Biochemical Processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef]
- Ferrandino, I.; Capriello, T.; Félix, L.M.; Di Meglio, G.; Santos, D.; Monteiro, S.M. Histological Alterations and Oxidative Stress in Adult Zebrafish Muscle after Aluminium Exposure. Environ. Toxicol. Pharmacol. 2022, 94, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C. Low Levels of Aluminum Can Lead to Behavioral and Morphological Changes Associated with Alzheimer’s Disease and Age-Related Neurodegeneration. Neurotoxicology 2016, 52, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, L.; Sadir, S.; Batool, Z.; Tabassum, S.; Shahzad, S.; Afzal, A.; Haider, S. Acute Aluminum Chloride Toxicity Revisited: Study on DNA Damage and Histopathological, Biochemical and Neurochemical Alterations in Rat Brain. Life Sci. 2019, 217, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Bondy, S.C. Aluminum Induced Oxidative Events and Its Relation to Inflammation: A Role for the Metal in Alzheimer’s Disease. Cell. Mol. Biol. 2000, 46, 721–730. [Google Scholar] [PubMed]
- Capriello, T.; Monteiro, S.M.; Félix, L.M.; Donizetti, A.; Aliperti, V.; Ferrandino, I. Apoptosis, Oxidative Stress and Genotoxicity in Developing Zebrafish after Aluminium Exposure. Aquat. Toxicol. 2021, 236, 105872. [Google Scholar] [CrossRef]
- Capriello, T.; Félix, L.M.; Monteiro, S.M.; Santos, D.; Cofone, R.; Ferrandino, I. Exposure to Aluminium Causes Behavioural Alterations and Oxidative Stress in the Brain of Adult Zebrafish. Environ. Toxicol. Pharmacol. 2021, 85, 103636. [Google Scholar] [CrossRef]
- Capriello, T.; Di Meglio, G.; De Maio, A.; Scudiero, R.; Bianchi, A.R.; Trifuoggi, M.; Toscanesi, M.; Giarra, A.; Ferrandino, I. Aluminium Exposure Leads to Neurodegeneration and Alters the Expression of Marker Genes Involved to Parkinsonism in Zebrafish Brain. Chemosphere 2022, 307, 135752. [Google Scholar] [CrossRef]
- Son, M.; Kim, D.Y.; Kim, C.H. Disease Modeling of Rare Neurological Disorders in Zebrafish. Int. J. Mol. Sci. 2022, 23, 3946. [Google Scholar] [CrossRef]
- Dai, Y.J.; Jia, Y.F.; Chen, N.; Bian, W.P.; Li, Q.K.; Ma, Y.B.; Chen, Y.L.; Pei, D.S. Zebrafish as a Model System to Study Toxicology. Environ. Toxicol. Chem. 2014, 33, 11–17. [Google Scholar] [CrossRef]
- Monaco, A.; Capriello, T.; Grimaldi, M.C.; Schiano, V.; Ferrandino, I. Neurodegeneration in Zebrafish Embryos and Adults after Cadmium Exposure. Eur. J. Histochem. 2017, 61, 276–279. [Google Scholar] [CrossRef]
- Barbazuk, W.B.; Korf, I.; Kadavi, C.; Heyen, J.; Tate, S.; Wun, E.; Bedell, J.A.; McPherson, J.D.; Johnson, S.L. The Syntenic Relationship of the Zebrafish and Human Genomes. Genome Res. 2000, 10, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.A.; Peterson, R.T. Zebrafish as Tools for Drug Discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Langelier, M.F.; Pascal, J.M. PARP-1 Mechanism for Coupling DNA Damage Detection to Poly(ADP-Ribose) Synthesis. Curr. Opin. Struct. Biol. 2013, 23, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Karvandi, M.S.; Sheikhzadeh Hesari, F.; Aref, A.R.; Mahdavi, M. The Neuroprotective Effects of Targeting Key Factors of Neuronal Cell Death in Neurodegenerative Diseases: The Role of ER Stress, Oxidative Stress, and Neuroinflammation. Front. Cell. Neurosci. 2023, 17, 1105247. [Google Scholar] [CrossRef]
- Liu, C.; Fang, Y. New Insights of Poly(ADP-Ribosylation) in Neurodegenerative Diseases: A Focus on Protein Phase Separation and Pathologic Aggregation. Biochem. Pharmacol. 2019, 167, 58–63. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Kim, N.S.; Yu, S.-W.; Wang, H.; Koh, D.W.; Sasaki, M.; Klaus, J.A.; Otsuka, T.; Zhang, Z.; Koehler, R.C.; et al. Poly(ADP-Ribose) (PAR) Polymer Is a Death Signal. Proc. Natl. Acad. Sci. USA 2006, 103, 18308–18313. [Google Scholar] [CrossRef]
- Klotz, K.; Weistenhöfer, W.; Neff, F.; Hartwig, A.; Van Thriel, C.; Drexler, H. The Health Effects of Aluminum Exposure. Dtsch. Arztebl. Int. 2017, 114, 653–659. [Google Scholar] [CrossRef]
- Walton, J.R. Aluminum Disruption of Calcium Homeostasis and Signal Transduction Resembles Change That Occurs in Aging and Alzheimer’s Disease. J. Alzheimers Dis. 2012, 29, 255–273. [Google Scholar] [CrossRef]
- Bondy, S.C.; Campbell, A. Aluminum and Neurodegenerative Diseases. In Advances in Neurotoxicology; Aschner, M., Costa, L.G., Eds.; Elsevier: Atlanta, GA, USA, 2017; Volume 1, pp. 131–156. ISBN 9780128127643. [Google Scholar]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Jubin, T.; Kadam, A.; Jariwala, M.; Bhatt, S.; Sutariya, S.; Gani, A.R.; Gautam, S.; Begum, R. The PARP Family: Insights into Functional Aspects of Poly (ADP-Ribose) Polymerase-1 in Cell Growth and Survival. Cell Prolif. 2016, 49, 421–437. [Google Scholar] [CrossRef]
- Meyer-Ficca, M.L.; Lonchar, J.D.; Ihara, M.; Meistrich, M.L.; Austin, C.A.; Meyer, R.G. Poly(ADP-Ribose) Polymerases PARP1 and PARP2 Modulate Topoisomerase II Beta (TOP2B) Function during Chromatin Condensation in Mouse Spermiogenesis. Biol. Reprod. 2011, 84, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kassab, M.A.; Dantzer, F.; Yu, X. PARP2 Mediates Branched Poly ADP-Ribosylation in Response to DNA Damage. Nat. Commun. 2018, 9, 3233. [Google Scholar] [CrossRef]
- Porzio, L.; Buia, M.C.; Lorenti, M.; De Maio, A.; Arena, C. Physiological Responses of a Population of Sargassum vulgare (Phaeophyceae) to High PCO2/Low PH: Implications for Its Long-Term Distribution. Sci. Total Environ. 2017, 576, 917–925. [Google Scholar] [CrossRef]
- Vitale, L.; Vitale, E.; Bianchi, A.R.; De Maio, A.; Arena, C. Role of Poly(ADP-Ribose) Polymerase (PARP) Enzyme in the Systemic Acquired Acclimation Induced by Light Stress in Phaseolus Vulgaris L. Plants. Plants 2022, 11, 1870. [Google Scholar] [CrossRef]
- Arena, C.; Mistretta, C.; Di Natale, E.; Faraone Mennella, M.R.; De Santo, A.V.; De Maio, A. Characterization and Role of Poly(ADP-Ribosyl)Ation in the Mediterranean Species Cistus Incanus L. under Different Temperature Conditions. Plant Physiol. Biochem. 2011, 49, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Giorio, P.; Guida, G.; Mistretta, C.; Sellami, M.H.; Oliva, M.; Punzo, P.; Iovieno, P.; Arena, C.; De Maio, A.; Grillo, S.; et al. Physiological, Biochemical and Molecular Responses to Water Stress and Rehydration in Mediterranean Adapted Tomato Landraces. Plant Biol. 2018, 20, 995–1004. [Google Scholar] [CrossRef]
- Boehler, C.; Gauthier, L.R.; Mortusewicz, O.; Biard, D.S.; Saliou, J.M.; Bresson, A.; Sanglier-Cianferani, S.; Smith, S.; Schreiber, V.; Boussin, F.; et al. Poly(ADP-Ribose) Polymerase 3 (PARP3), a Newcomer in Cellular Response to DNA Damage and Mitotic Progression. Proc. Natl. Acad. Sci. USA 2011, 108, 2783–2788. [Google Scholar] [CrossRef]
- Cantó, C.; Menzies, K.J.; Auwerx, J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell. Metab. 2015, 22, 31–53. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish, 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Liguori, G.; Paino, S.; Mirabella, N.; Squillacioti, C.; De Luca, A.; Vittoria, A. Expression of Orexin A and Its Receptor 1 in the Epididymis of the South American Camelid Alpaca (Vicugna Pacos). Anat. Histol. Embryol. 2014, 43, 42–47. [Google Scholar] [CrossRef]
- De Maio, A.; Porzio, E.; Angelo, R.D.; Rotondo, S.; Bianchi, A.R.; Confalone, E.; Raucci, R.; Natale, E.; Faraone-Mennella, M.R. A Glycosyltransferase from Sulfolobus Solfataricus MT-4 Exhibits Poly(ADP-Ribose) Glycohydrolase Activity. Curr. Proteom. 2015, 12, 253–263. [Google Scholar] [CrossRef]
- Panzeter, P.; Zweifel, B.; Malanga, M.; Waser, S.; Richard, M.; Althaus, F. Targeting of Histone Tails by Poly(ADP-Ribose). J. Biol. Chem. 1993, 268, 17662–17664. [Google Scholar] [CrossRef] [PubMed]
- De Maio, A.; Natale, E.; Rotondo, S.; Di Cosmo, A.; Faraone-Mennella, M.R. Vault-Poly-ADP-Ribose Polymerase in the Octopus Vulgaris Brain: A Regulatory Factor of Actin Polymerization Dynamic. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 166, 40–47. [Google Scholar] [CrossRef] [PubMed]

| Zebrafish Brain | PARP Activity (mU/mg) | PARG Activity (mU/mg) | Poly(ADPR) Degradation (%) |
|---|---|---|---|
| Control group | 53.3 ± 4.88 a | 0.6 ± 0.02 a | 99 |
| Exposed to Al for 10 days | 1086 ± 27.6 b | 48.3 ± 4.27 b | 96 |
| Exposed to Al for 15 days | 1071 ± 25.4 b | 24.4 ± 1.56 c | 98 |
| Exposed to Al for 20 days | 56.5 ± 10.69 a | 1.5 ± 0.36 a | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, A.R.; La Pietra, A.; Guerretti, V.; De Maio, A.; Capriello, T.; Ferrandino, I. Synthesis and Degradation of Poly(ADP-ribose) in Zebrafish Brain Exposed to Aluminum. Int. J. Mol. Sci. 2023, 24, 8766. https://doi.org/10.3390/ijms24108766
Bianchi AR, La Pietra A, Guerretti V, De Maio A, Capriello T, Ferrandino I. Synthesis and Degradation of Poly(ADP-ribose) in Zebrafish Brain Exposed to Aluminum. International Journal of Molecular Sciences. 2023; 24(10):8766. https://doi.org/10.3390/ijms24108766
Chicago/Turabian StyleBianchi, Anna Rita, Alessandra La Pietra, Valeria Guerretti, Anna De Maio, Teresa Capriello, and Ida Ferrandino. 2023. "Synthesis and Degradation of Poly(ADP-ribose) in Zebrafish Brain Exposed to Aluminum" International Journal of Molecular Sciences 24, no. 10: 8766. https://doi.org/10.3390/ijms24108766
APA StyleBianchi, A. R., La Pietra, A., Guerretti, V., De Maio, A., Capriello, T., & Ferrandino, I. (2023). Synthesis and Degradation of Poly(ADP-ribose) in Zebrafish Brain Exposed to Aluminum. International Journal of Molecular Sciences, 24(10), 8766. https://doi.org/10.3390/ijms24108766









