Abstract
Biomechanical forces are of fundamental importance in biology, diseases, and medicine. Mechanobiology is an emerging interdisciplinary field that studies how biological mechanisms are regulated by biomechanical forces and how physical principles can be leveraged to innovate new therapeutic strategies. This article reviews state-of-the-art mechanobiology knowledge about the yes-associated protein (YAP), a key mechanosensitive protein, and its roles in the development of drug resistance in human cancer. Specifically, the article discusses three topics: how YAP is mechanically regulated in living cells; the molecular mechanobiology mechanisms by which YAP, along with other functional pathways, influences drug resistance of cancer cells (particularly lung cancer cells); and finally, how the mechanical regulation of YAP can influence drug resistance and vice versa. By integrating these topics, we present a unified framework that has the potential to bring theoretical insights into the design of novel mechanomedicines and advance next-generation cancer therapies to suppress tumor progression and metastasis.
1. Introduction
One of the most important lessons from cell biology in the past decades is that biomechanical forces, both endogenous and exogenous, are crucial in regulating the physiology and pathology of the human body. Living cells sense, transduce, and respond to specific biomechanical forces and other physical stimuli in their microenvironments [1,2,3,4,5,6], such as the geometry, dimensionality, porosity, and viscoelastic properties of the extracellular matrix. In turn, these biomechanical factors can fundamentally regulate and be altered by many diseases, including cancer, arthrosclerosis, heart disease, muscular dystrophy, and neurodegeneration [7,8,9,10,11]. Hence, understanding what the universal principles are that underpin these mechanobiological processes and how to mechanically modulate cell, tissue, and organ functions is a critical issue in science today.
In this review article, we will present a unified framework (Figure 1) to discuss the previously underappreciated interplay between a key mechanosensitive protein, YAP (yes-associated protein), and the development of drug resistance in human cancer cells (as detailed in Section 5). This new framework holds the potential to facilitate the creation of novel cellular engineering and therapies through the lens of biophysics and engineering. A deeper understanding of YAP-associated mechanotransduction processes in health and diseases will likely facilitate the next-generation of biomaterials, soft bio-robotics, wearable devices, and implants.
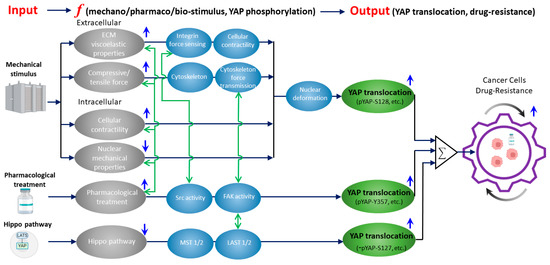
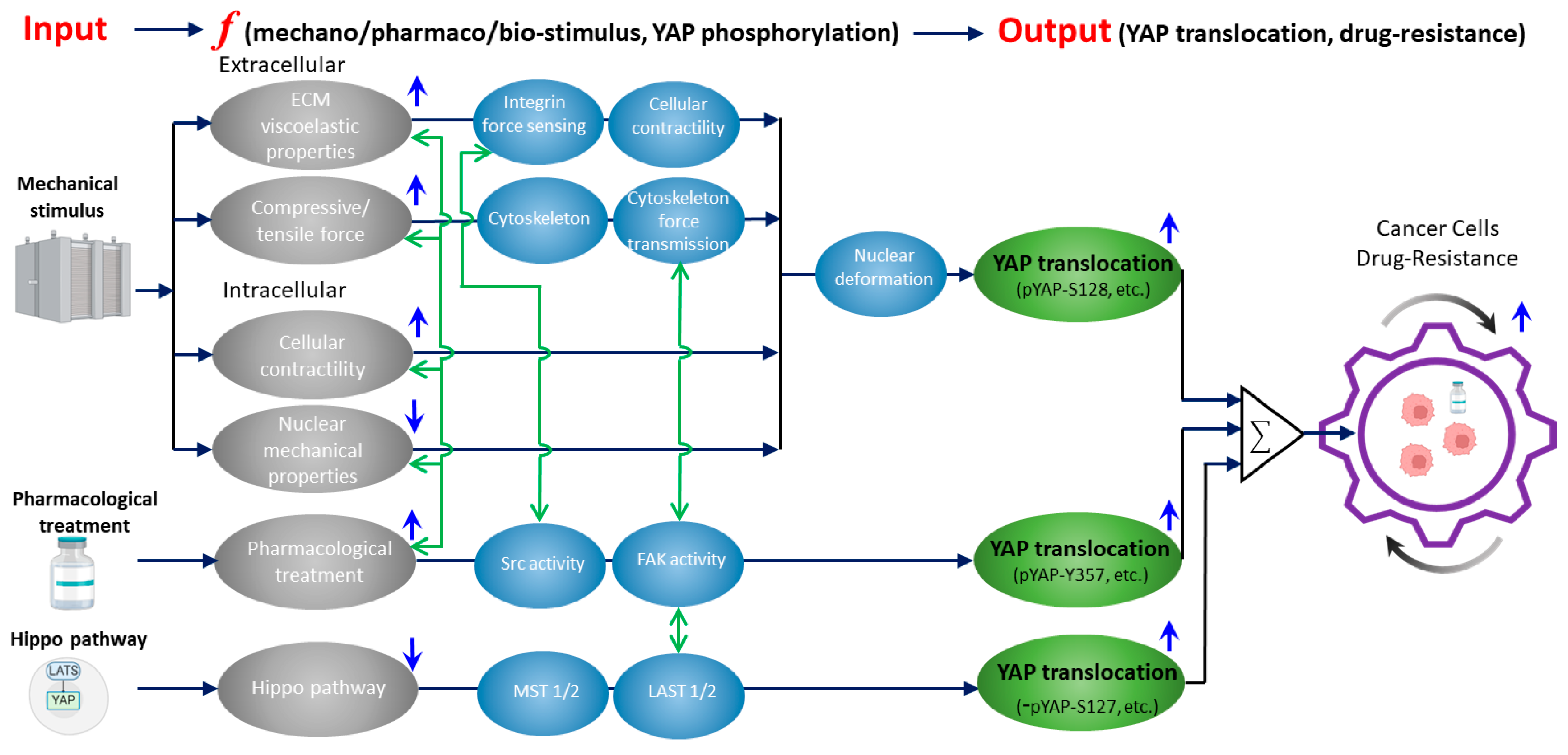
Figure 1.
A unified framework that overviews the current knowledge of how mechanically, biochemically (Hippo), and pharmacologically regulated YAP activities contribute to drug resistance in cancer cells. Gray ellipses represent the input signals from mechanical (as detailed in Section 2), biochemical (Hippo), and pharmacological (as detailed in Section 3) upstream regulators of YAP. Blue arrows indicate whether the activation/inhibition of the upstream regulators will induce YAP nuclear translocation. Activation of mechanical stimuli (except nuclear mechanical properties) and pharmacological treatment upregulates YAP nuclear translocation. While inhibition of Hippo pathway upregulates YAP nuclear translocation. Black arrows show the corresponding YAP-regulatory pathways with the key molecular/cellular mediators listed in blue ellipses. Green ellipses represent the resulting YAP nuclear translocation for each pathway with the corresponding phosphorylation sites of YAP. In the Hippo pathway, YAP-S127 dephosphorylation is denoted as “−” (i.e., dephosphorylation). Green arrows show the current knowledge of the crosstalk between the inputs and key mediators (as detailed in Section 4 and Section 5). The crosstalk network indicates the potential targets of (1) inhibiting the mechanical regulators of YAP or (2) suppressing the drug-treatment-induced activation of mechanotransduction events to eventually reduce drug resistance (as detailed in Section 5).
YAP and transcriptional co-activator with PDZ-binding motif (TAZ) are transcriptional co-activators that critically facilitate the signal transduction from biomechanical stimuli into gene expression [12,13,14]. In general, YAP is expressed in both the cell nucleus and cytoplasm. However, only when it is in the nucleus does YAP perform biological functions, mainly by binding to transcription factors including transcriptional enhancer activator domain (TEAD) to regulate cell proliferation, oncogenic transformation, epithelial-to-mesenchymal transition (EMT), and drug resistance [15,16]. Conventionally, YAP is considered the downstream of the Hippo signaling pathway, which is an evolutionarily conserved pathway to control organ size, development, and regeneration. In the human Hippo pathway, activation of Mst1/2 kinases sequentially phosphorylates Lats1/2 kinases and YAP. Activated Lats kinases phosphorylate YAP mainly at Ser127 site (other phosphorylation sites include S61, S109, S164, S381, T63, S138, S289, S351, and S384), promoting retention of YAP in the cytoplasm by binding to 14-3-3 proteins. Hence, pYAP-S127 level is widely used as an indicator of Hippo pathway activity [17,18]. However, pYAP-S127 alone does not determine the nuclear/cytoplasmic translocation of YAP and the downstream function. The detailed mechanism of YAP nuclear translocation will be discussed in Section 2.4.
In tumors, YAP plays important roles in regulating neoplasm initiation, growth, metastasis, and drug resistance. Importantly, overexpression and nuclear translocation of YAP occur in multiple tumor types [19] (Figure 2A). For example, in lung adenocarcinomas, 76% of the cases show overexpression of YAP and TAZ [19]. However, increasing evidence suggests that the genetic dysregulation of components within the Hippo pathway is rare (reviewed in ref. [12,20]). These data indicate that the abnormal YAP activities discovered in tumors are likely regulated by Hippo-independent mechanisms. Moreover, a dysregulated Hippo pathway may only induce dysregulated pYAP-S127. The combination of the Hippo pathway, Src-FAK pathway, and mechanical stimulus regulates the nuclear translocation of YAP. The overall nuclear translocation of YAP determines the change in the downstream gene expression and cellular function (Figure 2B,C).
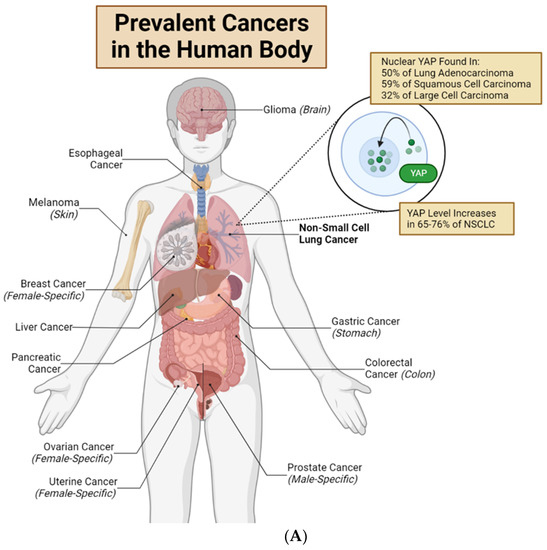
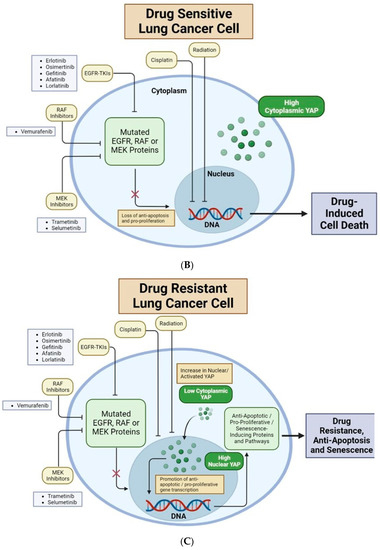
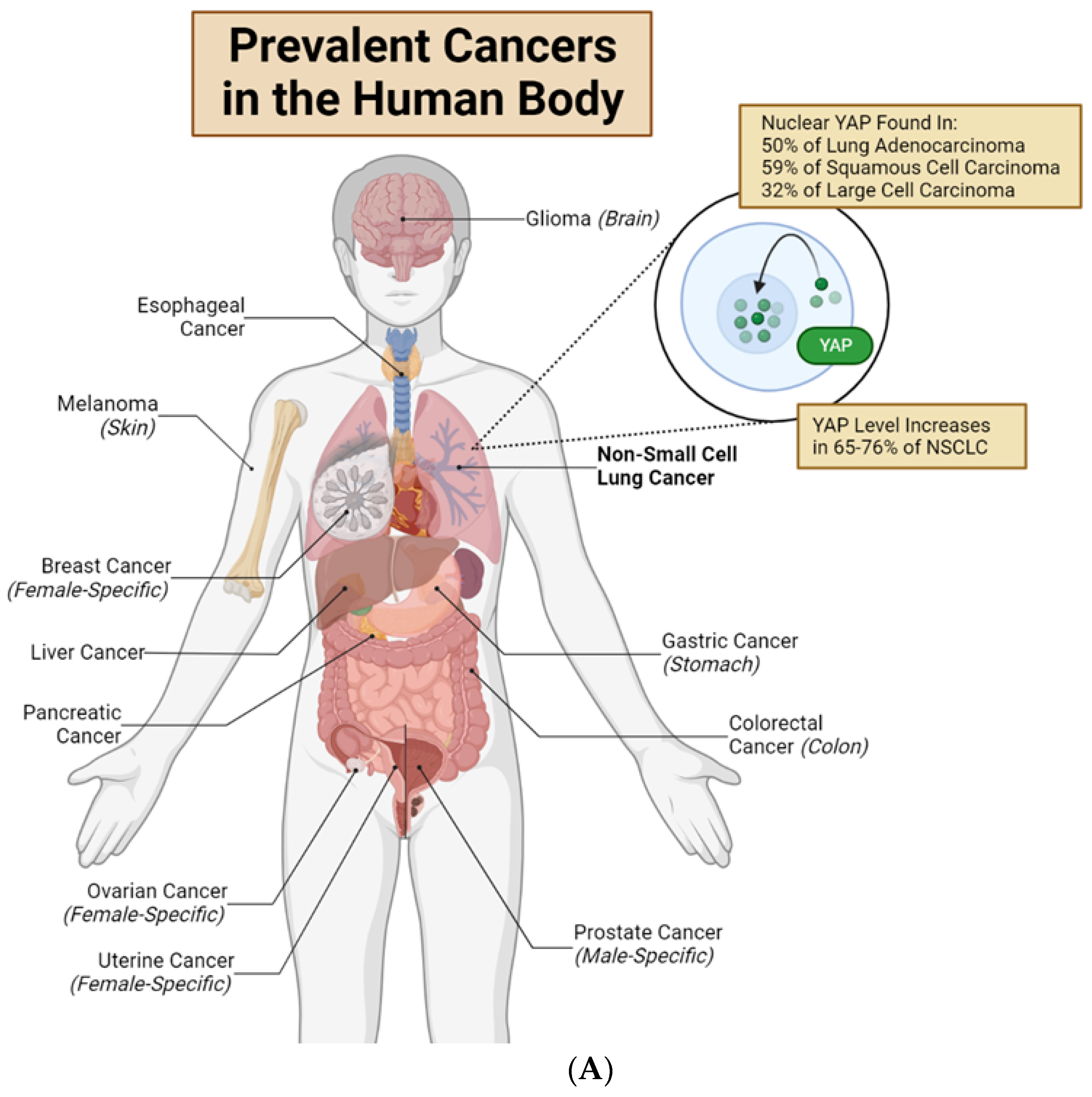
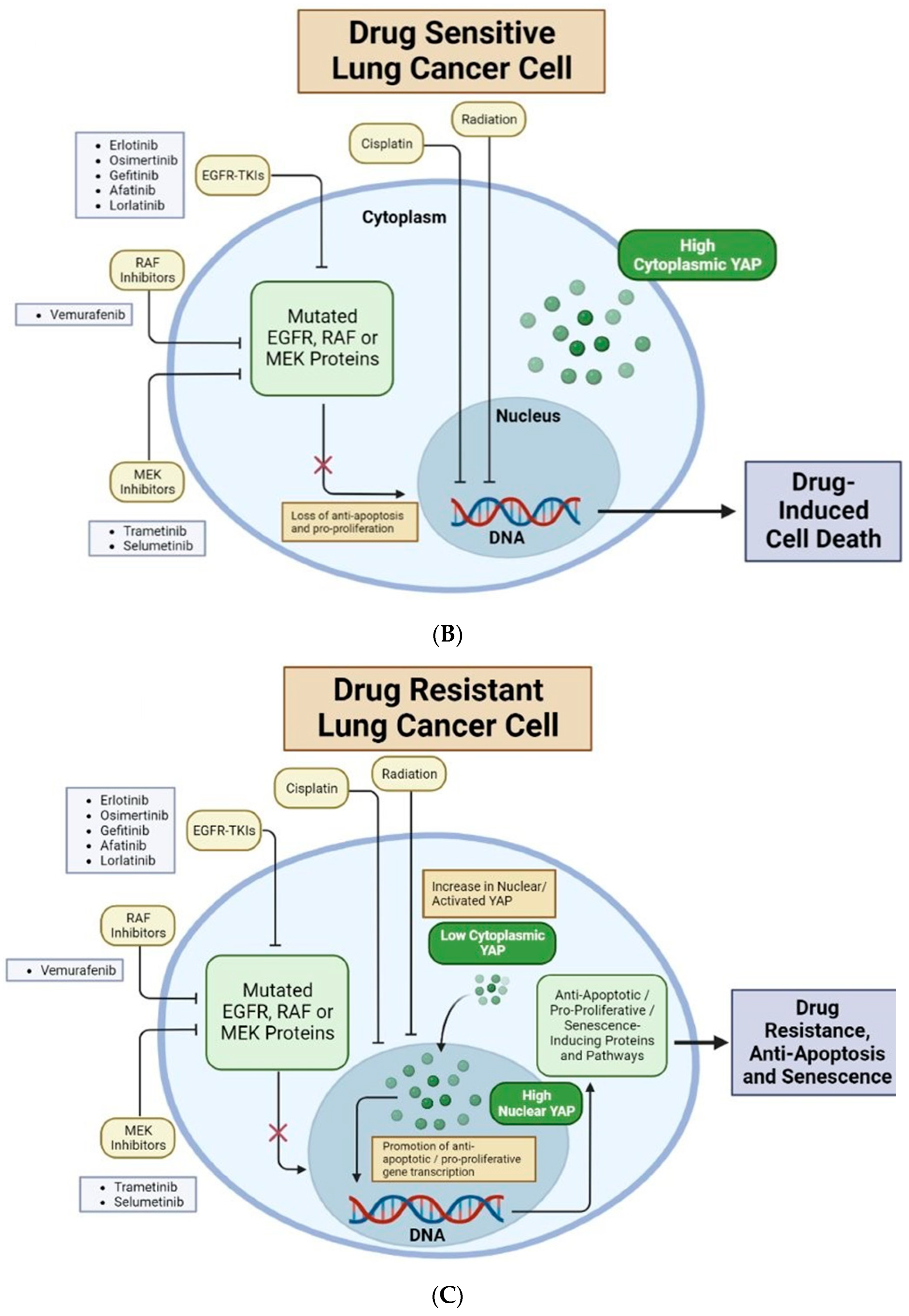
Figure 2.
Nuclear YAP contributes to drug resistance of human cancer cells. (A) Major organs and associated common cancers in the human body. Non-small cell lung cancer (NSCLC) is bolded to highlight this review’s unique focus on YAP in lung adenocarcinomas and non-small cell lung cancer [12,21]. (B) Lung cancer cells with high cytoplasmic (low nuclear) YAP are drug-sensitive. EGFR-TKIs, RAF inhibitors, and MEK inhibitors target mutated EGFR, RAF or MEK proteins, respectively. This causes loss of anti-apoptotic and pro-proliferative downstream effects, resulting in drug-induced cell death. Cisplatin and radiation directly target cancer cell DNA, inducing cell death. (C) Lung cancer cells with high nuclear (low cytoplasmic) YAP are often drug-resistant. YAP in the nucleus will bind to TEAD transcription factors to promote transcription of genes which produce anti-apoptotic/pro-proliferative/senescence-inducing proteins and pathways. These downstream mechanisms lead to drug resistance, anti-apoptosis, and senescence. New research shows that several Hippo-independent pathways can regulate YAP activity (Figure 3). First, biophysical stimulus, such as tissue stiffness, regulates the nuclear translocation of YAP and serves as an “on/off” switch of the Hippo pathway to regulate YAP in parallel [22]. The mechanism of this mechanically regulated YAP dynamics will be explained in detail in Section 2. Second, a few biochemical signals can regulate YAP, independent of the Hippo pathway, through focal adhesion kinase (FAK) [23,24,25] and Aurora A kinase [26]. However, FAK activity is downstream of cells’ integrin-mediated sensing of biophysical signals [27,28,29,30,31]; therefore, whether the FAK-YAP pathway is part of the mechano-regulated YAP pathway is currently under active investigation. T-bar arrows indicate drug-induced inhibition; black arrows indicate pathway activation, stimulation or promotion; and a red ‘X’ over an arrow denotes pathway loss. Created with Biorender.com.
Several pharmacological inhibitions of YAP or YAP-TEAD interaction show repression effects on tumor progression, although all current inhibitors are not clinically viable [19,42,43,44]. For example, Verteporfin binds with YAP and abrogates the YAP-TEAD interaction to reduce both tumor growth and drug resistance in multiple tumor types [42,45,46]. However, Verteporfin requires a high cellular concentration to effectively bind to YAP, which makes it currently not viable in clinical practices [42,43]. Poor target selectivity towards drug-resistant tumor cells is a major limitation of the pharmacological inhibition of YAP [19,43]. For example, the non-cell-specific inhibition of YAP worsens the fibrosis and vascular leakage of lung tissue in mice. However, targeting selectively expressed genes upstream of YAP inhibits lung fibrosis; Ref. [47] shows potential to partially overcome this challenge. Mechanistically, the DRD1 gene is selectively expressed in lung fibroblast and regulates YAP nuclear translocation. DRD1 agonism selectively inhibits YAP activity only in the DRD1-expressed fibroblast without affecting the viability of surrounding epithelial and endothelial cells, and thus reduces fibrosis [47]. These results indicate that pharmacological YAP inhibition will simultaneously reduce the survival of both drug-resistant cells and healthy normal cells. Instead, leveraging the unique characteristics of drug-resistant cells (e.g., selectively expressed genes or unique mechano-sensing properties) may overcome the limitation of selectivity. A similar strategy is also applicable in the inhibition of mechano-regulated YAP. Emerging evidence shows that tumor cells receive abnormal mechanical stimulus from surrounding tumor and tissue microenvironments, including elevated tissue stiffness, solid stress, and interstitial fluid shear forces. In addition, tumor cells show differential mechano-sensing compared to normal cells [48,49,50]. If drug-resistant cells (1) receive unique mechanical stimulus that can regulate YAP, and (2) show unique mechanism in mechano-regulated YAP different from that in the normal cells, then selectively targeting the unique stimulus or mechano-regulation mechanism in drug-resistant cells may reduce drug resistance without affecting normal cells.
In summary, YAP activity is critical in the drug resistance of cancer cells. Mechanical stimulus shows great potential in regulating YAP in cancer cells and reducing drug resistance (Figure 1). In the following sections, we will discuss three intertwined topics: (1) molecular mechanisms that underpin the mechanical regulation of YAP; (2) the contribution of YAP to drug resistance in cancer; and (3) the current understanding of reducing drug resistance using mechanically regulated YAP dynamics.
2. Mechanical Regulation of YAP Dynamics
In living cells, YAP is sensitive to diverse types of mechanical signals, reflected by its translocation between nucleus and cytoplasm (Figure 1). Cells constantly experience mechanical signals from the extracellular matrix (ECM), interstitial fluid, and neighboring cells [51,52,53,54]. Intracellular mechanosensors such as integrin, GPCR, mechanosensitive ion channels (Piezo ½ and Transient Receptor Potential (TRP) channels, and the cell nucleus can sense the extracellular mechanical signals to induce nuclear/cytoplasmic translocation of YAP [54,55,56,57]. Specifically, YAP can be influenced by three types of mechanical signals: (1) Mechanical properties of the ECM; (2) Actively applied extracellular force; and (3) Endogenous contractility and geometric attributes of the cell. These mechanical stimuli regulate YAP via different molecular mechanisms but most of the mechanisms involve nuclear mechano-sensing as the direct regulator of YAP. Next, we will discuss these three types of mechanical signals and their influences on YAP.
2.1. Viscoelastic Mechanics of ECM Regulates YAP
In general, ECM stiffness (Young’s modulus) is sensed by transmembrane integrins on the cell surface, which sequentially induce activation of Src, FAK at focal adhesion sites and intracellular Rho-ROCK machinery to increase cytoskeleton contractility, which is mechanical tension in cells generated by the sliding of actin and myosin filaments along each other [58,59,60,61]. As demonstrated by our own CRISPR/Cas9 imaging of real-time YAP dynamics and by other independent research groups, increased cytoskeleton contractility triggers nuclear translocation of YAP, while reduced contractility induces cytoplasmic retention of YAP [19,22,34,57,62,63,64]. For example, in human mammary epithelial cells, 80% of the cells cultured on stiff plastic (~10 MPa; 1 Pa = 1 Newton/m2) substrate show nuclear YAP translocation, while the rest 20% of the cells show homogenous intracellular YAP distribution. In contrast, the cells of the same type cultured on soft hydrogel (0.7 kPa) substrate do not show nuclear YAP translocation [22]. In adipose tissue-derived mesenchymal stem cells, the YAP nuclear/cytoplasm (N/C) ratio on 28 kPa substrate is 2-fold higher than the YAP N/C ratio on 1.5 kPa substrate [65]. Cytoskeleton contractility is necessary for substrate stiffness to regulate YAP; however, the exact mechanism of action is unknown [22,57].
Current understanding is that nucleus receives mechanical force from cytoskeletal contractility and induces conformational change in nuclear pores. Such conformational change regulates the active transport rate (both import and export) of YAP through nuclear pores to tune the overall dynamics of YAP nuclear/cytoplasmic translocation [57]. If cytoskeleton structure is disrupted or cytoskeletal contractility is inhibited, substrate stiffness sensed by integrin cannot be transmitted to the cell nucleus through tensed cytoskeletal filaments. Thus, nuclear pores do not change their conformation to regulate YAP active transport rate. However, exogeneous force applied on the cell nucleus can bypass the cytoskeletal force transmission between substrate and cell nucleus [57] and directly alter nuclear pores, which will be detailed in Section 2.2 and Section 2.3.
Viscoelasticity of ECM enhances nuclear translocation of YAP. For example, polysaccharide alginate gels with higher viscoelasticity (relaxation time = 30 s) induce nuclear YAP translocation in 10–50% of the cells, while gels with lower viscoelasticity (relaxation time = 350 s) induce nuclear YAP translocation in 0% of the cells. Recent evidence suggests that viscoelasticity regulates YAP through a FAK-Arp2/3-complex-dependent mechanism [66].
2.2. Geometric Attributes and Contractility of the Cell Regulate YAP
Cell spreading area (in 2D culture), i.e., area covered by a cell as it adheres on a 2D substrate, and cell volume (in 3D culture) positively regulate nuclear YAP translocation [22,57,65,67,68,69]. To differentiate the regulation of YAP by cell spreading area and focal adhesion area, recent studies have modulated focal adhesion area without changing cell spreading area. The data show that modulation of focal adhesion area results in no noticable regulation on YAP nuclear translocation [22]. However, inhibition of FAK activity induces decrease in YAP nuclear translocation [24]. These results indicate that, FAK activity rather than focal adhesion area regulates YAP nuclear translocation.
Cell contractility, cell spreading area and substrate stiffness often functionally intertwine with each other to influence dynamics of YAP and other cell signaling effectors [70,71]. positively regulates YAP nuclear translocation and shows temporal correlation with YAP, while inhibition of contractility suppresses nuclear translocation of YAP [22,57,71]. Contractility, however, is not absolutely necessary for YAP nuclear translocation if the force is directly applied to the nucleus [57]. These data indicate that contractility serves as a key bridge for the force transmission from cell membrane into the nucleus to mechanically regulate YAP.
2.3. Actively Applied Extracellular Forces Regulate YAP
Cells constantly receive mechanical tension and compression forces from the surrounding tissues and cells, especially in the lung and heart tissues. Both static and cyclic stretching of the cells induce nuclear YAP translocation [22,64,72,73]. Cytoskeleton structure rather than contractility is necessary for the stretching-induced YAP translocation [72,73]. Vertical compression (24 Pa) on HeLa and MCF-10A cells by polydimethylsiloxane (PDMS) causes F-actin depolymerization and suppresses YAP nuclear translocation [51]. In contrast, vertical compression (1.5 nN) by atomic force microscopy (AFM) induces YAP nuclear translocation with disrupted cytoskeleton [57]. These results indicate that, independent from the existence of cytoskeletal contractility, nuclear-force-sensing alone is sufficient to regulate YAP nuclear translocation.
2.4. Mechanism of Mechanically Regulated YAP Nuclear Translocation
In a high percentage of solid tumors, YAP/TAZ activities of tumor cells are deregulated, while the conventional Hippo pathway is not [12,19,20]. Because YAP/TAZ activities are mechano-sensitive and solid tumors present aberrant mechanical microenvironment to their constituent cells, it is natural to hypothesize that the deregulated YAP/TAZ activities observed in these tumors are due to the altered mechanical microenvironments in tumors but not the Hippo pathway. Hence, how mechanical stimulus regulates YAP nuclear translocation is under active investigations today.
Several research groups show that mechanical force regulates YAP nuclear translocation through (1) inducing unique phosphorylation of YAP, and (2) altering conformation of nuclear pores, competing with conventional Hippo-pathway-induced phosphorylation. For example, osmotic pressure increases pYAP-S127 indicating activation of Hippo pathway, and simultaneously induces Hippo-independent pYAP-S128 to disrupt the binding between pYAP-S127 and 14-3-3, eventually resulting in increased nuclear translocation of YAP [74]. Recent research shows that, independent from Hippo pathway, activation of FAK phosphorylates S397 site of YAP in mice (S381 in human) and Y357 site in human to promote YAP nuclear translocation [23,75,76]. In this study, mechanical force is not directly investigated in the FAK-induced YAP nuclear translocation. However, FAK activity is known to be sensitive to mechanical stimulus [27,28,29,30,31], indicating that mechanical stimulus potentially regulates both phosphorylation and nuclear translocation of YAP through enhanced FAK activity.
Besides phosphorylation of YAP, mechanical stimulus regulates YAP nuclear translocation through regulating nuclear pore conformation. Because YAP lacks nuclear localization sequence, it needs to bind with importin to enter the cell nucleus by active transport through the nuclear pore. Compressing of nucleus changes the conformation of nuclear pore and increases the importing rate of YAP into the nucleus [57,77]. However, phosphorylation of YAP other than pYAP-S127 is not investigated. One remaining question in the field is that whether force-induced conformational changes in nuclear pores regulate YAP purely through physical restriction, or is phosphorylation concurrently involved? Collectively, these datasets suggest that: (1) pYAP-S127 serves as an indicator of Hippo pathway activity alone; (2) phosphorylated YAP at other different sites together likely contribute to YAP nuclear translocation; and (3) Mechanical regulation of nuclear pore size simultaneously regulates YAP nuclear translocation.
In summary, YAP can be regulated by mechanical signals through nuclear mechano-sensing, either endogenous-contractility-mediated or exogenous-force-regulated. Therefore, we propose that possible mechanical strategies that aim to inhibit YAP activity may target (1) changing extracellular mechanical signals; (2) changing force transmission in cytoskeleton; and (3) changing nuclear mechano-sensing efficiency.
3. YAP and Drug Resistance
Drug resistance is one of the most prominent obstacles encountered during cancer treatment [78,79,80,81]. Today many available drugs in clinical practices can provide initial promising prevention of tumor progression. However, despite the high efficacy of these drugs, a subpopulation of tumor cells often survives the initial drug treatments, due to the pre-existing drug resistance or by developing capability of drug resistance during treatment, and re-grow into new tumors after the drug administrations. These drug-resistant tumor cells are likely the real culprit in causing cancer mortality [78,82]. Hence, to design next-generation comprehensive therapeutic strategies to eradicate cancer, elucidation of the mechanisms underpinning their drug resistance is one utmost goal in cancer research [83]. Interestingly, increasing recent evidence indicates that nuclear expression of mechano-sensitive YAP proteins contributes significantly to the increase in drug resistance in multiple types of cancer cells. In this section, we review the most current data and understanding of YAP’s contribution to drug resistance in lung cancer, as well as their mechanisms of action (Figure 2).
3.1. YAP Is More Activated in Drug-Resistant Cancer Cells
Recent studies indicate that YAP proteins tend to be more activated (i.e., YAP nuclear translocation) in drug-resistant cancer cells than in non-drug-resistant ones. The increased YAP nuclear translocation can induce anti-apoptotic and pro-proliferative phenotypes which can contribute to drug resistance and tumor relapse (Figure 2B,C). Based on the reported mechanisms that activate YAP, we have categorized these studies into two groups.
In one group, drug-resistant cancer cells, either immortalized cell lines or primary tumor cells, are found to be intrinsically YAP-rich intrinsically (i.e., high YAP expression in the cell nucleus) compared to that of non-drug-resistant cells. One hypothesis is that the intrinsic high YAP expression in these cancer cells results in their drug resistance capability. Another hypothesis is that drug treatment triggers YAP nuclear translocation in these cells thus increases the drug resistance. For instance, Lee TF et al. find that upregulated YAP expression in EGFR-mutant lung cancer cells led to the development of resistance against Epithelial Growth Factor Receptor (EGFR) tyrosine kinase inhibitors (TKIs) gefitinib, afatinib and osimertinib [84]. McGowan et al. find higher nuclear YAP concentration in an osimertinib (EGFR-TKI)-resistant H1975 lung cancer cell line in culture [85]. Song et al. find increased YAP expression and stemness in A549 lung cancer spheres compared with normal A549 adherent lung cancer cells, and note an association between increased YAP and enhanced cisplatin resistance [86]. Further, Kirsten Rat Sarcoma (KRAS) mutated tumor show resistance to Mitogen-Activated Protein Kinase Kinase (MEK) inhibitor monotherapy. Increased YAP nuclear translocation is observed in KRAS-mutated cancer cells [21,87]. For excample, Cheng H. et al. observe higher YAP1 nuclear staining in EGFR- or KRAS-mutated lung adenocarcinoma in comparison to EGFR/KRAS wild type [21]. Lin L. et al. observe increased nuclear YAP expression in v-Raf murine sarcoma viral oncogene homolog B1 (BRAF)-mutant lung cancer cells (specifically in tumors encoding BRAF V600E NSCLC and melanoma) and in KRAS-mutant NSCLC tumors [87]. Taken together, these findings suggest that the heightened activation of YAP, as observed in EGFR- or KRAS-mutated lung adenocarcinoma, as well as BRAF-mutant lung cancer cells and KRAS-mutant NSCLC tumors, might play a significant role in conferring resistance to MEK inhibition. Together, these studies reveal that drug-resistant lung cancer cells tend to have high YAP activation, suggesting an important correlation between YAP expression and drug resistance.
In the other group, drug treatment directly increases YAP nuclear translocation in a subpopulation of treated cancer cells and thus actively induce drug resistance. It is worth noting that this second group may overlap with the first one to a certain level. For instance, Kurppa KJ et al. find that YAP nuclear localization increased significantly in EGFR-mutant NSCLC after 10-day combined treatment with EGFR-TKIs osimertinib and trametinib [88]. Similarly, Yamazoe et al. find YAP nuclear localization is induced by short-term (72 h) lorlatinib treatment in ROS1-rearranged KTOR71 NSCLC cells and promote cell survival through activation of AKT. Meanwhile, the treated cells exhibit elongated morphology and increased cell-ECM adhesion signature in the proteome analysis [89].
In both groups, the studies propose various direct mechanisms by which YAP nuclear activation increases after drug treatment. For example, by monitoring LATS1 kinase, Kurppa KJ et al. find decreased LATS1 phosphorylation in response to drug treatment, suggesting YAP activation can be drug-treatment-induced by influencing Hippo pathway [88]. In exploring possible driver genes promoting YAP in drug-resistant lung cancer cell lines, Lee TF et al. detect an upregulation of Her2 mRNA expression [73]. Because knockdown of Her2 can reduce YAP expression, Her2 is hypothesized to have a role in increasing YAP activation and subsequent TKI-resistance. McGowan et al. note that erlotinib-, gefitinib- and osimertinib-resistant lung cancer cell lines have reduced expression of E-cadherin, a protein previously is found to promote YAP degradation [85]. Yamazoe et al. find that short-term (72 h) ROS1-TKI lorlatinib treatment in ROS1-rearranged NSCLC, shows no effect on Lats1 phosphorylation but increases YAP nuclear translocation [90]. These data indicate that lorlatinib regulates YAP in a Hippo-indenpend mechanism. Importantly, they also find that cellular exposure to lorlatinib upregulates proteins involved in cytoskeleton and morphology changes. Since it is known that larger cell spreading induces nuclear YAP translocation, these data suggest that lorlatinib-induced elongation in morphology induces YAP activation and nuclear localization, indicating the novel crosstalk between mechanobiological phenotypes and pharmacological actions. Future studies are needed to clarify the mechanisms that lead to increased YAP activation in various drug-resistant cell types, contributing knowledge which may be crucial in developing new therapeutic strategies.
3.2. Artificially Changed YAP Expression Directly Influences Drug Resistance
To explore a stronger causal link between YAP expression and drug resistance, several studies examine the impact of artificially overexpressing and inhibiting YAP on drug resistance in cancer cells. In general, these studies have found that increased YAP activation induces drug resistance in cancer cells. For instance, Kurppa KJ et al. find that re-expression of wild-type YAP1 in YAP1 KO EGFR-mutated NSCLC re-induces resistance to EGFR-TKIs osimertinib and trametinib [88]. Lee TF et al. find that both overexpression of wide type YAP and constitutional activated YAP-5SA promote drug resistance in HCC827 and H1975 lung cancer cells [84].
In a similar fashion, various studies find that decreased YAP activation causes drug-resistant cancer to become sensitive to drug treatment. For instance, Cheng H. et al. finds that knockdown of YAP by either shRNA or siRNA improves sensitivity to cisplatin treatment in PC9 lung cancer cells; to radiation treatment in PC9, HCC827 and H157 lung cancer cell lines; and to EGFR tyrosine kinase inhibitor (TKI) erlotinib in EGFR-mutated NSCLC PC9 and HCC827 cell lines [21]. They also find that combining YAP1 knockdown and platinum therapy induces apoptosis in PC9 lung cancer cells. Finally, they find that verteporfin, a pharmacological inhibitor of YAP-TEAD interaction, sensitizes PC9 lung cancer cells to cisplatin, radiation, and erlotinib treatment [21]. Kurppa KJ et al. find that YAP1 knockout induced accelerated apoptosis in EGFR-mutated lung cancer cells when treated with EGFR-TKIs osimertinib and trametinib [88]. Lee TF et al. find that dasatinib, a Src family kinase inhibitor, effectively reduces YAP expression levels and EGFR-TKI--resistance of HCC827 lung adenocarcinoma cells [73]. Furthermore, combined EGFR and YAP inhibition effectively reduced the viability of TKI-resistant cells. Lin L et al. find that shRNA-mediated YAP1 knockdown in HCC364 cells enhances sensitivity to vemurafenib and to MEK inhibitor trametinib [87]. McGowan et al. find significant increased sensitivity to EGFR-TKIs erlotinib and gefitinib, as well as to T790M-specific osimertinib, after YAP knockdown in drug-resistant lung cancer cell lines [85]. Song et al. find siRNA-mediated knockdown of YAP resensitized A549 cells and cell spheres to cisplatin treatment, suggesting reactivation of apoptotic cascades as a primary mechanism of resensitization [84]. Yamazoe et al. find that inhibition of YAP enhances sensitivity to ROS1-TKI lorlatinib and induces apoptosis in ROS1-rearranged KTOR71 NSCLC cells, and that combination therapy with YAP inhibitor verteporfin and lorlatinib suppresses tumor regrowth in vivo [84].
Together, the collective evidence strongly supports a causative relationship between YAP activation and drug resistance in cancer cells (Figure 2B,C). Moreover, the findings suggest that combined YAP inhibition and drug treatment hold promise as a potential therapeutic strategy to overcome drug resistance in clinical practices.
3.3. Mechanisms of YAP-Mediated Drug Resistance
Next, we review and discuss the various proposed mechanisms of YAP-mediated drug resistance.
In the upstream of YAP activation, drug treatments potentially influence the positive/negative upstream regulators of YAP, such as NF2, Src, and FAK. For example, NF2 inhibits YAP activation through Hippo pathway [12,14]. NF2 mutation contributes to drug resistance in multiple cancer types [89,90], while molecular alterations of NF2 is found in 5% of lung adenocarcinoma patients and 15% of lung squamous cell carcinoma patients [91]. Inhibition of NF2 increases the drug resistance of NSCLC and melanoma [89]. In addition, drug treatment induces activation of Src [92,93]. Src regulates YAP to induce EGFR-TKI resistance through three main mechanisms: (1) the direct phosphorylation; (2) the activation of pathways that repress Hippo kinases; and (3) Hippo-independent mechanisms (reviewed in ref. [94]). Importantly, because Src activation is related to FAK activation in mechano-sensing [54], we propose that mechanical regulation of FAK may be one of the Hippo-independent mechanisms of Src-YAP regulation.
In the downstream of YAP activation, nuclear translocation of YAP potentially contributes to drug resistance, through (1) transcriptional regulation of anti-apoptosis genes; and (2) crosstalk with MAPK pathway and PI3K-AKT pathway. First, in multiple types of human normal and cancer cells, YAP binds to transcriptional factor and upregulates the expression of Connective tissue growth factor (CTGF) and Cyr61 to increase drug resistance [15]. Mechanistically, in breast cancer cells, overexpression of CTGF upregulates anti-apoptotic factor Bcl-xL and cellular inhibitor of apoptosis protein 1 (cIAP1) to increase drug resistance [95]. In osteosarcoma, CTGF promotes drug resistance through upregulation of surviving expression and nuclear factor kappa B (NF-κB) pathway [96]. Enhanced Cyr61 expression increases drug resistance through upregulation of NF-κB-regulated anti-apoptotic gene XIAP [97].
In parallel with these upstream and downstream events, the activated YAP coordinates with multiple intracellular cytoplasmic and nuclear molecules to collectively regulate drug resistance. For example, Kurppa KJ et al. assert that YAP mediates the evasion of apoptosis by repressing the induction of the pro-apoptotic protein BMF (Bcl2 Modifying Factor) via engagement of Epithelial-Mesenchymal-Transition (EMT) transcription factor SLUG [88]. Lin L et al. postulate that YAP and RAF-MEK signaling work in parallel to enhance expression of BCL-xL in RAF- and MEK-mutant cells, thereby inducing drug resistance [87]. Song et al. propose anti-apoptosis as a primary mechanism of cisplatin-resistance in A549 lung cancer cells and cell spheres [86]. They also suggest that YAP transcriptional regulation of ABCB1 protein, a glycoprotein involved in multi-drug resistance, may be a potential mechanism of induced drug resistance. Yamazoe et al. propose that YAP1 mediates initial ROS1-rearranged NSCLC cell survival in response to ROS1-TKI lorlatinib through AKT signaling [38].
Together, these diverse studies highlight new potential pathways, proteins, and mechanisms outside of the known causal effects of YAP which may play a role in YAP-mediated drug resistance in lung cancer. However, prior to effectively targeting YAP to reduce drug resistance, we need to address two questions that remain poorly understood to date: (1) whether mechanically regulated and biochemically regulated YAP contribute to drug resistance in the same mechanism? The phosphorylation sites of YAP due to mechanical signals are different from YAP-S127 in Hippo pathway but its exact sites are unknown (only pYAP-S128 is identified in the osmotic-pressure-induced YAP nuclear tranlocation). Such structural and chemical differences may contribute to distinct binding of YAP with downstream transcription factors and differentially regulated cellular functions. An exact identification of these sites will facilitate the innovation of precise medicine; (2) after inhibition of YAP, how the activation of compensating or redundant pathways (e.g., MAPK and PI3K-AKT pathway) reduces the drug resistance? A deeper understanding of their functional dynamics will promote the design of combinatorial strategies that may be necessary to increase the efficacy of YAP inhibition.
3.4. YAP and Immunotherapy
Emerging studies have shown that YAP (yes-associated protein) likely has a significant role in regulating the cellular response to immunotherapy in non-small cell lung cancer (NSCLC). Specifically, YAP has been found to regulate the expression of Programmed Death-Ligand 1 (PD-L1), which serves as a predictive biomarker for anti-PD-1/PD-L1 immunotherapy.
The binding of PD-L1 with its receptor, PD-1, promotes T-cell tolerance and enables tumor cells to evade immune surveillance. To counteract this undesired behavior, one effective therapeutic method developed has been the anti-PD-1/PD-L1 immunotherapy. The level of PD-L1 expression in a tumor can provide information about its likelihood to respond to anti-PD-1/PD-L1 immunotherapy treatment: Typically, tumors with high PD-L1 expression are more effectively treated with anti-PD-1/PD-L1 immunotherapy, whereas tumors with low or no PD-L1 expression have a lower effectiveness of response to these treatments.
However, despite the success of anti-PD-1/PD-L1 immunotherapy in clinical, a considerable number of tumors do not respond to PD-1/PD-L1 inhibitors even though they possess high PD-L1 expression. This clinical challenge highlights the urgent need to understand the underlying mechanisms that contribute to this resistance. Several studies have shed light on the involvement of YAP in resistance to immunotherapy and its relation to PD-L1 expression. Hsu et al. find that, in H2052 and 211H human malignant pleural mesothelioma (MPM) cells, YAP binds to PD-L1 enhancer to promote expression of PD-L1. YAP expression positively regulates PD-L1 expression, examined by both overexpression and verteporfin-inhibition of YAP [98]. However, whether nuclear YAP translocation shows any causal regulation on PD-L1 expression is not investigated [67]. Additional studies contribute further evidence of YAP’s role in regulating PD-L1 expression, while also demonstrating how the YAP/PD-L1 regulatory relationship produces immunotherapy-resistant phenotypes. A study by Li L. et al. investigates the mechanisms by which Long-chain Acyl-CoA dehydrogenase (ACADL), an enzyme which has been found to act as a tumor suppressor in cancers, inhibits proliferation and enhances chemotherapeutic drug-induced apoptosis in lung adenocarcinoma [99]. The study reveals that ACADL prevents tumor immune evasion by inhibiting YAP and subsequently suppressing PD-L1 expression. Similar findings emerge in a study by Yu M. et al., in which researchers explore the mechanisms by which interferon-γ (IFN-γ) produces immunotherapy-resistant phenotypes in cancer cells. They find that IFN-γ promotes nuclear translocation and phase separation of YAP after anti-PD-1 therapy in tumor cells, indicating that YAP may be a primary mediator of the pro-tumor effect induced by IFN-γ [100].
Together, these findings suggest that YAP nuclear localization and YAP-induced increase in PD-L1 expression contribute to immune evasion, immunotherapy resistance, and drug resistance in lung adenocarcinomas.
4. Roles of ECM Played in Regulating YAP and Drug Resistance in Cancer Cells
Besides providing mechanical supports for tissue cells to reside in, extracellular matrix (ECM) functionally regulates physical-chemical interactions between cells and their surrounding microenvironments by influencing various mechanical stimulations, including substrate stiffness (both elastic and viscous properties), patterns of fluid shear stress, or active pressure [40,101,102,103,104,105]. These mechanical stimulations, along with biochemical cues, critically regulate the prognosis and behavior of cancers (Figure 3). Hence, the full investigation of the mechanistic roles that ECM has in cancers, especially in chemotherapy resistance, is critical for clinical practice.
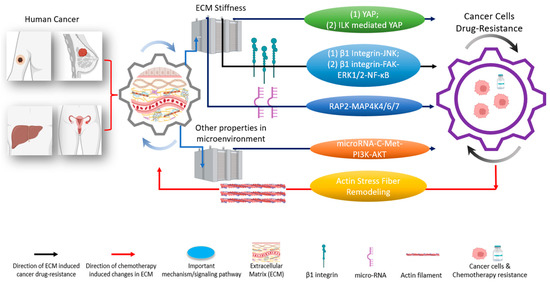
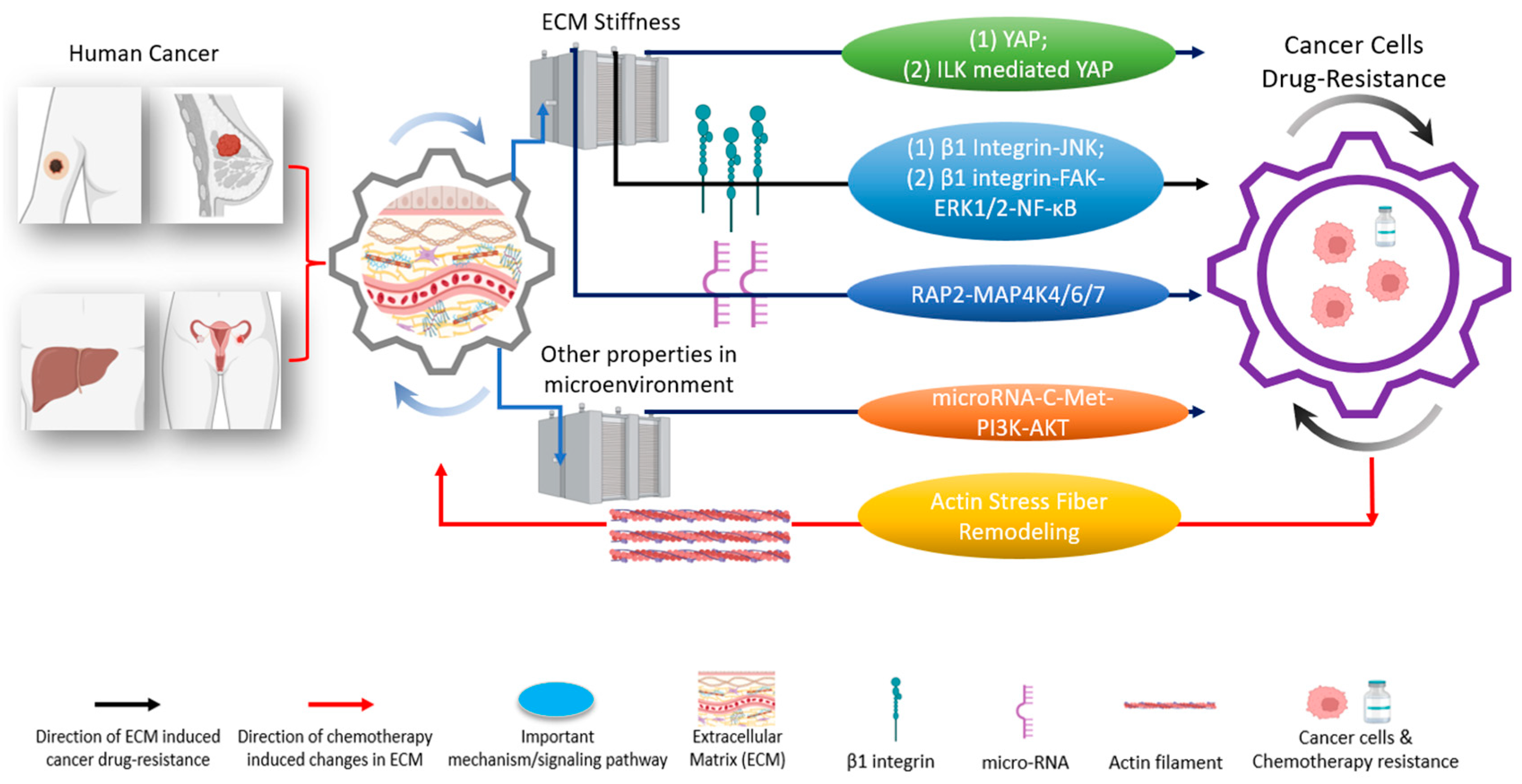
Figure 3.
The tumor microenvironment and extracellular matrix (ECM) induce different pathways to regulate drug resistances in various types of cancer [19,32]. These pathways are regulated through either YAP or other critical mechanisms [33]. Biophysical-chemical changes in ECM’s properties, such as cells’ substrate stiffness, could drive cancer cells’ resistance (black arrows) against chemotherapies through YAP and/or YAP-related pathways (green; two representative pathways (1) and (2) are indicated), integrin (blue; two representative pathways (1) and (2) are indicated), and other signaling pathway instead of YAP (orange) [34,35,36,37]. Besides ECM stiffness, other signals in the tumor microenvironment, such as shear stress, can induce cancer cells’ drug resistance (brown) [38]. Conversely, the application of chemotherapies in cancer cells could cause changes in the ECM’s properties (red arrow) [39]. Understanding the functional networks between ECM, ECM’s mechanical stimulations, and cancer drug resistance opens the door to designing and developing next-generation effective clinical treatments [15,40,41]. Created with Biorender.com.
4.1. ECM Induces YAP Nuclear Translocation and Influences the Resistance/Sensitivity of Cancer Cells to Chemotherapies
Increasing studies have demonstrated that YAP has mechanotransducive property which can be regulated by ECM mechanics [65]. Meanwhile, as discussed in Section 3, YAP’s expression has been proven to be closely associated with the regulation of drug resistance in multiple human cancer cell lines [12]. Therefore, a deeper understanding of how mechanical cues from the ECM induce resistance or sensitivity of cancer cells to chemotherapies via YAP’s activation and expression will offer promise for innovating new therapeutic methods from a mechanobiology perspective. In this section, we introduce and discuss the state of knowledge on the ECM-regulated chemoresistance and sensitivity of distinct cancer cell types (Figure 3).
A biphasic relationship between drug resistance and their ECM with various stiffness in breast cancer cells. Within 10 kPa (soft), intermediate stiff (38 kPa), and stiff (57 kPa) substrate, intermediate substrate rigidity (38 kPa) induces the highest level of integrin-linked kinase (ILK), nuclear YAP translocation and drug resistance [34].
Negative correlation between ECM stiffness and drug resistance is found in ovarian cancer cells (OCC). YAP has the highest nucleus/cytoplasm ratio (N/C ratio) at stiff substrate (25 kPa). However, OCC on soft substrate stiffness (0.5 kPa) shows higher resistance to cisplatin and paclitaxel, which are drugs widely used in chemotherapies of epithelial ovarian cancer. Whether the drug resistance is regulated by YAP remains to be investigated [35].
Positive correlation between ECM stiffness and drug resistance is found in two groups. First, Gao et al. found that hepatocellular carcinomas cells, which are sensitive to sorafenib (Sora-S cells), gained drug resistance when cultured on stiff substrate (4 kPa). Inversely, cells that have resistance to sorafenib (Sora-R cells) regain the sensitivity of sorafenib after adapting to the soft substrate (0.7 kPa). More importantly, the stiffness-induced YAP activation is confirmed to regulate cells’ mechano-adaption of chemotherapy. In experiments, by quantifying YAP in the nucleus and cytoplasm, researchers find that, first, YAP has higher nuclear localization in Sora-R cells than Sora-S cells; second, in Sora-R cells, the YAP nuclear localization on 4-kPa substrate is higher than that on the 0.7-kPa substrate [36]. After silencing YAP in Sora-R cells, the mechano-adaption ability of Sora-R cells disappeared, suggesting that YAP has functional roles in mechano-regulated chemoresistance of hepatocellular carcinoma cells [36]. Second, in HER2-amplified human breast cancer HCC1569 cells, both YAP nuclear translocation and drug resistance to lapatinib are higher on tissue culture plastic (2 GPa) than on Matrigel (400 Pa). In vitro knockdown of both YAP and TAZ successfully restricted the modulus-dependent resistance of lapatinib in in HCC1569 cells. Knockdown of YAP in HCC1569 cells induced a smaller tumor volume inside of mice, in contrast to that of the tumors induced by original HCC1569 cells [106].
Overall, no consistent relationship is found between (1) ECM stiffness and YAP; and (2) YAP and drug resistance. When targeting the drug resistance through mechanically regulated YAP, the strategies will be cancer-type- and drug-dependent. In addition to the observations that the changes in ECM’s properties can affect cancer cells’ chemotherapies, recent studies suggest that chemotherapies can change the ECM mechanics and contribute to drug resistance reciprocally. In BRAF-mutant melanoma, BRAF inhibitors (PLX4032) remarkably can remodel surrounding ECM by activating melanoma-associated fibroblasts (MAFs) through phosphorylation of MLC2/MYL9, which is the key regulator of actomyosin contractility. Activated MAFs are sufficient to produce denser collagen fibrils in the ECM and drive drug resistance to PLX4032 in BRAF-mutant melanoma cells. Remodeled ECM drives ERK activation and cancer cells’ resistance through integrin β1 and FAK signaling pathways [39]. Although YAP activity is not investigated in this research, the regulatory role of integrin and FAK on YAP indicates that YAP may be responsible for the integrin β1-FAK-induced drug resistance [23,24,25]. Further, in the PLX4032-resistant melanoma cancer cells, YAP activation is necessary for the maintenance of drug resistance. PLX4032-induced increasing of actin stress fibers is necessary for the YAP activation [37]. Increasing of actin stress fibers can facilitate the mechano-sensing of the cells to altered ECM stiffness [58,59,60,61] and may further activate YAP [54,55,56,57]. These results demonstrate a closed feedback loop for ECM-chemotherapy to influence each other in BRAF-mutant melanoma cancer cells mutually.
4.2. ECM Influences Resistance/Sensitivity of Cancer Cells to Chemotherapies in a YAP-Independent Manner
As discussed in Section 4.1, YAP is critical in mediating ECM-guided mechanotransduction to induce drug resistance in cancers. Importantly, besides YAP, other molecular effectors are also able to perform mechanotransduction to induce the chemotherapy resistance of carcinoma cells. Therefore, YAP likely is not the only molecular effector that links ECM mechanics to chemoresistance in cancer cells, indicating that the mechano-induced drug resistance of cancer can be YAP-independent (Figure 3).
For example, the stiffness of ECM influences cells’ sensitivity and reduces the efficiency of radiotherapies and chemotherapies by impairing DNA double-strand breaks (DSBs)’ repair [107]. This process is mediated through MAP4K4/6/7 kinases instead of LATS1/2 and YAP. The repair process of DSB has a deficiency in breast cancer cells cultured on substrates of low stiffness (0.5 and 1 kPa), which increases cellular sensitivity to genotoxic agents. The low stiffness inhibits DSB repair through modulating RNF8-mediated ubiquitin signaling. The RAP2-Hippo pathway (as an intracellular mechano-transductor) is a direct link between ECM stiffness and intracellular processes. Researchers further proved that Rap2 could be activated at low stiffness. Meanwhile, the downstream Hippo kinase MST1/2 and MAP4K4/6/7 kinases were activated at low stiffness conditions. Furthermore, researchers knocked out Rap2 and MST1/2 and MAP4K4/6/7 kinases. In experiments, cells with Rap2 knocked out become more resistant to ionizing radiation at low stiffness [107]. The knock out of MAP4K4/6/7 restores the survival rate of cells at low stiffness after applying irradiation. In conclusion, researchers showed that Rap2 and the downstream Hippo kinase MAP4K4/6/7, but not MST1/2, are required in the inhibition of DSB repair [107].
As a mechanosensitive transcriptional coactivator [108], YAP can be activated by changes in ECM and, in turn, induces cancer cells’ chemoresistance in many cases. However, besides YAP, the change in ECM’s properties can mediate drug resistance through other molecules, such as β 1 integrin signaling pathway with its downstream effectors [109]. As the rigidity of ECM increases, triple-negative breast cancer cells show increased resistance to sorafenib. Researchers found out that breast cancer cells have significantly higher drug resistance in the stiff substrate group (400 kPa Young’s modulus gel) than in the 50 kPa group. Meanwhile, two of the downstream effectors of β1 integrin, JNK, and P38, have higher activity and phosphorylation levels in cells cultured on higher stiffness substrates. Thus, JNK and P38 become potential candidates to explain stiffness-induced drug resistance [109]. To figure out the core mechanism that drives the mechano-induced sorafenib resistance, researchers further test ERK1/2′s activity and prove the higher phosphorylation of ERK on stiff substrates at early points after sorafenib was applied. As potential molecule candidates, inhibitors of JNK, p38, and ERK are applied, respectively, combined with sorafenib. Based on these experiments, the inhibition of JNK increased the efficiency of sorafenib and restricted the impact caused by substrate stiffness [109].
More studies have been conducted on integrin and the following signaling pathway. As Wang et al. discuss, the integrin β1/FAK/ERK1/2/NF-κB signaling pathway is activated in liver cancer cells under high-rigidity substrate conditions (16 kPa) [110]. Importantly, integrin β1 was proven to drive liver cancer cells’ proliferation. In the experiments, silenced integrin β1 inhibits the proliferation effects of SMMC-7721 and HepG2 cells. Hence, researchers apply the combination of integrin inhibitor (GLPG0187) with chemotherapy agents ADM/DDP on hepatocellular cancer xenografts to investigate potential therapies. Results show that both the inhibitor-only group and the group that contains chemotherapy agents and the inhibitor have obvious effects on suppressing tumor growth and prolonging mice survival time [110]. Intriguingly, integrin signaling can be a potential therapeutic target to improve anti-cancer drugs’ efficiency. In all these studies, the roles of YAP/TAZ in mediating drug resistance remain to be investigated.
Besides the Hippo signaling pathway and YAP, the c-Met/PI3K/Akt signaling axis and a mechanosensitive microRNA, miR-199a-3p, were also proven to have critical roles in the mediation of chemoresistance in ovarian cancer cells [111]. Researchers show that miR-199a-3p’s expression is negatively correlated with the chemoresistance of ovarian cancer and the clinical treatment outcome of cisplatin, paclitaxel, and platinum [37]. In experiments, cisplatin-resistant A2780 cells show significantly lower expression of miR-1990a-3p than cisplatin-sensitive A2870 cells. More importantly, the ectopic miR-199a-3p could make cisplatin resistance cell line A2780/DDP regain the sensitivity to cisplatin and make SKOV-3 cells sensitive to paclitaxel. Hence, the medication role of miR-1990a-3p in ovarian cancer cells was proven. Furthermore, in the patient’s tumor samples, the platinum-resistance tumor shows lower expression of miR-1993-3p than the platinum-sensitive tumor, which is consistent with the results from previous experiments. In the tumor microenvironment, mechanical stimulations other than substrate stiffness play a significant role in cancer cells’ progression and potential therapies. Hassan et al.’s study [112] critically indicates that the microfluid shear stress could downregulate the expression of miR-199a-3p through the c-Met/PI3K/Akt signaling pathway. As the miR-199a-3p is downregulated by shear stress under 0.02 dynes/cm2 ascitic shear stress, tumors could become more resistant to chemotherapies. Hence, these results indicate potential therapeutic targets in ovarian cancers.
More effectors that conduct the YAP-independent mechanotransduction need to be investigated and discussed [112]. Researchers examined ductal carcinoma in situ (DCIS) from patients’ samples and created a 3D culture model to mimic the ECM stiffness. In the patients’ sample group, YAP is inactivated at the early stage of cancer. This observation is essential because the increased stiffness of ECM could drive cancer cell invasion at the early stage. Therefore, the lack of YAP activity indicates that the changes in ECM stiffness could drive cancer cells’ invasion without YAP. Furthermore, cancer cells cultured on the 3D model show no YAP activities, as stiffness increased in the in vitro group [112]. Overall, the lack of YAP’s activity was proven in the mechanotransduction of breast cancer in both 3D culture and patient sample condition, which means this mechanotransduction is independent of YAP. This research indicates the possibility that the mechanotransduction from ECM to cancer cells, which induces cancer cells’ drug resistance, can be conducted through various mechanisms or channels other than YAP.
5. Conclusions and Outlook
YAP promotes drug resistance in cancer cells and can be regulated mechanically (Figure 1). Therefore, besides using pharmacological inhibition of YAP to suppress drug resistance, the mechanical inhibition of YAP has unique potential for the development of new cancer therapies from a new perspective. We hereby propose a unified framework that elucidates how the mechanically, biochemically, and pharmacologically regulated activation of YAP contributes to drug resistance in cancer (Figure 1). Importantly, our framework, for the first time, proposes the interplay between mechanical and pharmacological regulation, which lays down a new mechanobiology foundation for the development of new combinatorial therapies.
First, besides the Hippo pathway, YAP activation can be regulated mechanically. The mechanical stimuli include: (1) extracellular mechanical signals such as ECM viscoelastic properties, solid stress generated in tissue/tumor microenvironments, and compressive/tensile force applied by body motions; and (2) intracellular mechanical signals such as endogenous cellular contractility and nuclear mechanical properties. These mechanical stimuli regulate the activation of YAP (as detailed in Section 2) and result in drug resistance in cancer cells (as detailed in Section 3). Second, drug treatment can potentially activate the upstream mechanical regulators of YAP to activate YAP and induce drug resistance, in addition to the direct regulation of YAP without any crosstalk with mechanotransduction pathways (as detailed in Section 4). This interplay can be achieved through multiple avenues: (1) drug treatment can increase extracellular ECM stiffness [95] and solid stress [113]; (2) drug treatment can induce cytoskeleton remodeling [16,37,114,115], increase cellular contractility [116], and change nuclear mechanical properties [117]; and (3) drug treatment can tune the mechano-sensitivity of cells to magnify the influences of given mechanical stimuli [117]. These intricate mechano-pharmacological interactions have not been exploited in the field. Overall, this framework suggests that, besides pharmacological treatments, mechanical treatments of cancer cells, either through inhibiting the mechanical regulators of YAP or suppressing the drug-treatment-induced activation of mechanotransduction events, can potentially reduce drug resistance.
Based on this notion, we propose three types of methods for the mechanical inhibition of YAP and drug resistance in cancer cells: (1) pharmacological modulation of abnormal ECM mechanical properties in the tumors to suppress the mechano-induced YAP activation; (2) actively applying mechanical signals (e.g., ultrasound [118,119] and physical stretching [120]) at the tumor site to inhibit YAP activity with high spatial-temporal resolution and cell-type selectivity; and (3) if specific mechano-sensing pathways in cancer cells are known, disrupting the key mediators in the pathways to suppress YAP activation or to reduce YAP’s mechano-sensitivity to minimize drug resistance. To map the real-time interplay between mechanical forces and intracellular YAP-associated signaling dynamics, the high-resolution functional and structural imaging of Ca2+ signals and CRISPR/Cas9-endogenous-tagged proteins (e.g., YAP, Piezo ½, actomyosin machinery, and nuclear constituents), accompanied by mechanobiological characterizations using molecular tension biosensors and live-cell traction force microscopy, will be instrumental.
We envision that the present unified framework (Figure 1) will help the scientific community better understand the potential of mechanobiology and biophysics for biomedical research, particularly cancer medicine. By integrating mechanobiology and biomechanics principles with oncology clinical trials, there is reason to be optimistic that more creative multidisciplinary strategies that leverage mechano-medicine will be developed to prevent, detect, treat, and ultimately cure cancer.
Author Contributions
Writing—original draft preparation, M.H., H.W., C.M., M.C.C. and X.T.; writing—review and editing, M.H., M.C.C., J.G., A.R., C.V., G.Z., M.X. and X.T. All authors have read and agreed to the published version of the manuscript.
Funding
The research reported in this publication was kindly supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM150812 (X.T.), R35GM146877 (J.G.), R35GM128753 (M.X.), the National Science Foundation under grant number 2308574 (X.T.), UF Gatorade Award Start-up Package (X.T.), UFHCC Cancer Pilot Award (X.T. and Dietmar Siemann), and UF Opportunity Seed Fund (X.T., Habibeh Khoshbouei, Robert Caudle, and Christopher McCurdy).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found in the references cited.
Acknowledgments
We sincerely appreciate the invaluable scientific discussions with and technical support of Jonathan Licht (UFHCC), Dietmar Siemann (Radiation Oncology), Jianrong Lu (Biochemistry and Molecular Biology), Lizi Wu (Molecular Genetics and Microbiology), Frederic J Kaye (Hematology and Oncology), Daiqing Liao (Anatomy and Cell Biology), Greg Tyler (UFHCC), Thomas George (Hematology and Oncology), Rolf Renne (UFHCC), Hitomi Yamaguchi (Mechanical and Aerospace Engineering), Malisa Sarntinoranont (Mechanical and Aerospace Engineering), Warren Dixon (Mechanical and Aerospace Engineering), Mark Sheplak (Mechanical and Aerospace Engineering), Ting Dong (Mechanical and Aerospace Engineering), Chelsey Simmons (Mechanical and Aerospace Engineering), Blanka Sharma (Biomedical Engineering), Carlos M. Rinaldi-Ramos (Chemical Engineering), Richard B. Dickinson (Chemical Engineering), Weizhou Zhang (Pathology), Bingkan Xue (Physics), Purushottam Dixit (Physics), Shu Wang (Biostatistics), Brent P. Gila (Research Service Centers), Steven Madore (UFHCC), Alberto Riva (UFHCC), Tongjun Gu (UFHCC), Yanping Zhang (UFHCC), Franziska Haderk (UCSF), and David Hahn (University of Arizona).
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| ACADL | Long-chain Acyl-CoA dehydrogenase |
| AFM | Atomic force microscopy |
| BCL-xL | B-cell Lymphoma-Extra-Large |
| BMF | Bcl2 Modifying Factor |
| BRAF | B-raf protein (v-Raf murine sarcoma viral oncogene homolog B1) |
| C-Met | Mesenchymal-epithelial transition factor |
| CTGF | Connective tissue growth factor |
| DCIS | Ductal carcinoma in situ |
| DSBs | DNA double-strand breaks |
| ECM | Extracellular matrix |
| EGFR | Epithelial Growth Factor |
| ERK | Extracellular signal-regulated kinases |
| EMT | Epithelial-Mesenchymal Transition |
| GPCR | G-protein coupled receptors |
| Her2 | Human Epidermal Growth Factor Receptor 2 |
| ILK | Integrin-linked kinase |
| IFN-γ | Interferon-γ |
| JNK | c-Jun N-terminal kinases |
| KRAS | Kirsten Rat Sarcoma |
| LATS1 | Large Tumor Suppressor Kinase 1 |
| MAP4K4/6/7 | Mitogen-activated protein kinase-4/6/7 |
| MEK | Mitogen-Activated Protein Kinase |
| MST1/2 | Macrophage-stimulating protein |
| MPM | Malignant Pleural Mesothelioma |
| NF2 | Neurofibromatosis type 2 |
| NF-κB | Nuclear factor kappa B |
| NSCLC | Non-Small Cell Lung Cancer |
| OCC | Ovarian Cancer Cell |
| P38 | P38 mitogen-activated protein kinase |
| PD-L1 | Programmed Death-Ligand 1 |
| PI3k | Phosphatidylinositol 3-kinase |
| p-YAP | Phosphorylated Yes-Associated Protein |
| Rho-ROCK | Rho/Rho-associated coiled-coil containing protein kinase |
| ROS1 | C-ROS Oncogene 1 Receptor Tyrosine Kinase |
| siRNA | Small Interfering Ribonucleic Acid |
| SLUG | Transcription Factor Encoded by the SNAI2 Gene |
| TEAD | Transcriptional Enhanced Associate Domain |
| TCP | Tissue culture plastics |
| TKI | Tyrosine Kinase Inhibitor |
| TRP | Transient Receptor Potential |
| YAP | Yes-associated protein |
| YAP N/C ratio | YAP concentrations in nucleus vs. cytoplasm |
References
- Discher, D.E.; Janmey, P.; Wang, Y. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Vogel, V.; Sheetz, M. Local Force and Geometry Sensing Regulate Cell Functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in Development: A Growing Role for Contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a Distance: Mechanically Coupling the Extracellular Matrix with the Nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Huang, M.; Li, T.; Li, L.; Sussman, H.; Dai, Y.; Siemann, D.W.; Xie, M.; Tang, X. Towards an Integrative Understanding of Cancer Mechanobiology: Calcium, YAP, and MicroRNA under Biophysical Forces. Soft Matter 2022, 18, 1112–1148. [Google Scholar] [CrossRef]
- Carvalho, E.M.; Kumar, S. Lose the Stress: Viscoelastic Materials for Cell Engineering. Acta Biomater. 2023, 163, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.; Kumar, S. Biophysical Regulation of Tumor Cell Invasion: Moving beyond Matrix Stiffness. Integr. Biol. 2011, 3, 267. [Google Scholar] [CrossRef] [PubMed]
- Lammerding, J.; Engler, A.J.; Kamm, R. Mechanobiology of the Cell Nucleus. APL Bioeng. 2022, 6, 040401. [Google Scholar] [CrossRef]
- Tang, X.; Bajaj, P.; Bashir, R.; Saif, T.A. How Far Cardiac Cells Can See Each Other Mechanically. Soft Matter 2011, 7, 6151. [Google Scholar] [CrossRef]
- Tang, X.; Yakut Ali, M.; Saif, M.T.A. A Novel Technique for Micro-Patterning Proteins and Cells on Polyacrylamide Gels. Soft Matter 2012, 8, 7197. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, S.; Panciera, T.; Contessotto, P.; Cordenonsi, M. YAP/TAZ as Master Regulators in Cancer: Modulation, Function and Therapeutic Approaches. Nat. Cancer 2022, 4, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Sudol, M. Yes-Associated Protein (YAP65) Is a Proline-Rich Phosphoprotein That Binds to the SH3 Domain of the Yes Proto-Oncogene Product. Oncogene 1994, 9, 2145–2152. [Google Scholar]
- Hong, W.; Guan, K.-L. The YAP and TAZ Transcription Co-Activators: Key Downstream Effectors of the Mammalian Hippo Pathway. Semin. Cell Dev. Biol. 2012, 23, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.-Y.; Chinnaiyan, A.M.; et al. TEAD Mediates YAP-Dependent Gene Induction and Growth Control. Genes. Dev. 2008, 22, 1962–1971. [Google Scholar] [CrossRef]
- Haderk, F.; Fernández-Méndez, C.; Čech, L.; Yu, J.; Meraz, I.M.; Olivas, V.; Rabago, D.B.; Lucas Kerr, D.; Gomez, C.; Allegakoen, D.V.; et al. A Focal Adhesion Kinase-YAP Signaling Axis Drives Drug Tolerant Persister Cells and Residual Disease in Lung Cancer. Cancer Biol. 2021; preprint. [Google Scholar] [CrossRef]
- Low, B.C.; Pan, C.Q.; Shivashankar, G.V.; Bershadsky, A.; Sudol, M.; Sheetz, M. YAP/TAZ as Mechanosensors and Mechanotransducers in Regulating Organ Size and Tumor Growth. FEBS Lett. 2014, 588, 2663–2670. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP Oncoprotein by the Hippo Pathway Is Involved in Cell Contact Inhibition and Tissue Growth Control. Genes. Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, D. The Hippo Signaling Pathway in Development and Disease. Dev. Cell 2019, 50, 264–282. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, Z.; Rodriguez-Barrueco, R.; Borczuk, A.; Liu, H.; Yu, J.; Silva, J.M.; Cheng, S.K.; Perez-Soler, R.; Halmos, B. Functional Genomics Screen Identifies YAP1 as a Key Determinant to Enhance Treatment Sensitivity in Lung Cancer Cells. Oncotarget 2016, 7, 28976–28988. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.K.-H.; Du, W.; Shelton, S.J.; Oldham, M.C.; DiPersio, C.M.; Klein, O.D. An FAK-YAP-MTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice. Cell Stem Cell 2017, 21, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Arang, N.; Rigiracciolo, D.C.; Lee, J.S.; Yeerna, H.; Wang, Z.; Lubrano, S.; Kishore, A.; Pachter, J.A.; König, G.M.; et al. A Platform of Synthetic Lethal Gene Interaction Networks Reveals That the GNAQ Uveal Melanoma Oncogene Controls the Hippo Pathway through FAK. Cancer Cell 2019, 35, 457–472. [Google Scholar] [CrossRef]
- Lachowski, D.; Cortes, E.; Robinson, B.; Rice, A.; Rombouts, K.; Del Río Hernández, A.E. FAK Controls the Mechanical Activation of YAP, a Transcriptional Regulator Required for Durotaxis. FASEB J. 2018, 32, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gong, Y.; Guo, T.; Li, M.; Fang, L.; Yin, S.; Kamran, M.; Liu, Y.; Xu, J.; Xu, L.; et al. Activation of Aurora A Kinase Increases YAP Stability via Blockage of Autophagy. Cell Death Dis. 2019, 10, 432. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and Focal Adhesion Assembly: A Close Relationship Studied Using Elastic Micropatterned Substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef]
- Del Rio, A.; Perez-Jimenez, R.; Liu, R.; Roca-Cusachs, P.; Fernandez, J.M.; Sheetz, M.P. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science 2009, 323, 638–641. [Google Scholar] [CrossRef]
- Plotnikov, S.V.; Pasapera, A.M.; Sabass, B.; Waterman, C.M. Force Fluctuations within Focal Adhesions Mediate ECM-Rigidity Sensing to Guide Directed Cell Migration. Cell 2012, 151, 1513–1527. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Oria, R.; Chen, Y.; Kosmalska, A.; Pérez-González, C.; Castro, N.; Zhu, C.; Trepat, X.; Roca-Cusachs, P. Mechanical Regulation of a Molecular Clutch Defines Force Transmission and Transduction in Response to Matrix Rigidity. Nat. Cell Biol. 2016, 18, 540–548. [Google Scholar] [CrossRef]
- Zhou, D.W.; Fernández-Yagüe, M.A.; Holland, E.N.; García, A.F.; Castro, N.S.; O’Neill, E.B.; Eyckmans, J.; Chen, C.S.; Fu, J.; Schlaepfer, D.D.; et al. Force-FAK Signaling Coupling at Individual Focal Adhesions Coordinates Mechanosensing and Microtissue Repair. Nat. Commun. 2021, 12, 2359. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting Extracellular Matrix Stiffness and Mechanotransducers to Improve Cancer Therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Papavassiliou, K.A.; Papavassiliou, A.G. Mechanobiology of Solid Tumors. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2022, 1868, 166555. [Google Scholar] [CrossRef]
- Qin, X.; Lv, X.; Li, P.; Yang, R.; Xia, Q.; Chen, Y.; Peng, Y.; Li, L.; Li, S.; Li, T.; et al. Matrix Stiffness Modulates ILK-Mediated YAP Activation to Control the Drug Resistance of Breast Cancer Cells. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165625. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, Q.; Li, X.; Feng, J.; Ao, Z.; Li, X.; Wang, J. Substrate Stiffness Modulates the Growth, Phenotype, and Chemoresistance of Ovarian Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 718834. [Google Scholar] [CrossRef]
- Gao, J.; Rong, Y.; Huang, Y.; Shi, P.; Wang, X.; Meng, X.; Dong, J.; Wu, C. Cirrhotic Stiffness Affects the Migration of Hepatocellular Carcinoma Cells and Induces Sorafenib Resistance through YAP. J. Cell. Physiol. 2019, 234, 2639–2648. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.; Hong, H.; Lee, S.; Lee, J.; Jung, E.; Kim, J. Actin Remodeling Confers BRAF Inhibitor Resistance to Melanoma Cells through YAP / TAZ Activation. EMBO J. 2016, 35, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, M.; Ozasa, H.; Tsuji, T.; Funazo, T.; Yoshida, H.; Hashimoto, K.; Hosoya, K.; Ogimoto, T.; Ajimizu, H.; Yoshida, H.; et al. Yes-associated protein 1 Mediates Initial Cell Survival during Lorlatinib Treatment through AKT Signaling in ROS1 -rearranged Lung Cancer. Cancer Sci. 2023, 114, 546–560. [Google Scholar] [CrossRef]
- Hirata, E.; Girotti, M.R.; Viros, A.; Hooper, S.; Spencer-Dene, B.; Matsuda, M.; Larkin, J.; Marais, R.; Sahai, E. Intravital Imaging Reveals How BRAF Inhibition Generates Drug-Tolerant Microenvironments with High Integrin Β1/FAK Signaling. Cancer Cell 2015, 27, 574–588. [Google Scholar] [CrossRef]
- Liang, C.; Huang, M.; Tanaka, M.; Lightsey, S.; Temples, M.; Lepler, S.E.; Sheng, P.; Mann, W.P.; Widener, A.E.; Siemann, D.W.; et al. Functional Interrogation of Ca2+ Signals in Human Cancer Cells In Vitro and Ex Vivo by Fluorescent Microscopy and Molecular Tools. In Microfluidic Systems for Cancer Diagnosis; Methods in Molecular Biology; Garcia-Cordero, J.L., Revzin, A., Eds.; Springer: New York, NY, USA, 2023; Volume 2679, pp. 95–125. [Google Scholar] [CrossRef]
- Gonzalez-Molina, J.; Moyano-Galceran, L.; Single, A.; Gultekin, O.; Alsalhi, S.; Lehti, K. Chemotherapy as a Regulator of Extracellular Matrix-Cell Communication: Implications in Therapy Resistance. Semin. Cancer Biol. 2022, 86, 224–236. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Huang, B.; Shim, J.S.; Chen, Q.; Lee, S.-J.; Anders, R.A.; Liu, J.O.; Pan, D. Genetic and Pharmacological Disruption of the TEAD–YAP Complex Suppresses the Oncogenic Activity of YAP. Genes. Dev. 2012, 26, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.D.K.; Yi, C. YAP/TAZ Signaling and Resistance to Cancer Therapy. Trends Cancer 2019, 5, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, L.; Tao, Z.; Jarugumilli, G.K.; Erb, H.; Singh, A.; Li, Q.; Cotton, J.L.; Greninger, P.; Egan, R.K.; et al. Pharmacological Blockade of TEAD–YAP Reveals Its Therapeutic Limitation in Cancer Cells. Nat. Commun. 2022, 13, 6744. [Google Scholar] [CrossRef]
- Lee, J.E.; Park, H.S.; Lee, D.; Yoo, G.; Kim, T.; Jeon, H.; Yeo, M.-K.; Lee, C.-S.; Moon, J.Y.; Jung, S.S.; et al. Hippo Pathway Effector YAP Inhibition Restores the Sensitivity of EGFR-TKI in Lung Adenocarcinoma Having Primary or Acquired EGFR-TKI Resistance. Biochem. Biophys. Res. Commun. 2016, 474, 154–160. [Google Scholar] [CrossRef]
- Fisher, M.L.; Grun, D.; Adhikary, G.; Xu, W.; Eckert, R.L. Inhibition of YAP Function Overcomes BRAF Inhibitor Resistance in Melanoma Cancer Stem Cells. Oncotarget 2017, 8, 110257–110272. [Google Scholar] [CrossRef]
- Haak, A.J.; Kostallari, E.; Sicard, D.; Ligresti, G.; Choi, K.M.; Caporarello, N.; Jones, D.L.; Tan, Q.; Meridew, J.; Diaz Espinosa, A.M.; et al. Selective YAP/TAZ Inhibition in Fibroblasts via Dopamine Receptor D1 Agonism Reverses Fibrosis. Sci. Transl. Med. 2019, 11, 516. [Google Scholar] [CrossRef] [PubMed]
- Fukumura, D.; Jain, R.K. Tumor Microenvironment Abnormalities: Causes, Consequences, and Strategies to Normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef]
- Pfeifer, C.R.; Alvey, C.M.; Irianto, J.; Discher, D.E. Genome Variation across Cancers Scales with Tissue Stiffness—An Invasion-Mutation Mechanism and Implications for Immune Cell Infiltration. Curr. Opin. Syst. Biol. 2017, 2, 103–114. [Google Scholar] [CrossRef]
- Chin, L.; Xia, Y.; Discher, D.E.; Janmey, P.A. Mechanotransduction in Cancer. Curr. Opin. Chem. Eng. 2016, 11, 77–84. [Google Scholar] [CrossRef]
- Poh, Y.-C.; Shevtsov, S.P.; Chowdhury, F.; Wu, D.C.; Na, S.; Dundr, M.; Wang, N. Dynamic Force-Induced Direct Dissociation of Protein Complexes in a Nuclear Body in Living Cells. Nat. Commun. 2012, 3, 866. [Google Scholar] [CrossRef]
- Poh, Y.-C.; Chen, J.; Hong, Y.; Yi, H.; Zhang, S.; Chen, J.; Wu, D.C.; Wang, L.; Jia, Q.; Singh, R.; et al. Generation of Organized Germ Layers from a Single Mouse Embryonic Stem Cell. Nat. Commun. 2014, 5, 4000. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wen, Q.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Janmey, P.A.; Saif, T.A. Attenuation of Cell Mechanosensitivity in Colon Cancer Cells during In Vitro Metastasis. PLoS ONE 2012, 7, e50443. [Google Scholar] [CrossRef] [PubMed]
- Wolfenson, H.; Yang, B.; Sheetz, M.P. Steps in Mechanotransduction Pathways That Control Cell Morphology. Annu. Rev. Physiol. 2019, 81, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in Mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; He, L.; Zhou, L.; Jing, Y.; Wang, F.; Shi, Y.; Cai, M.; Sun, J.; Xu, H.; Jiang, J.; et al. Mechanical Force Regulation of YAP by F-Actin and GPCR Revealed by Super-Resolution Imaging. Nanoscale 2020, 12, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.-L.; et al. Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Q.; Chen, X.; Liu, J.; Tanaka, M.; Wang, S.; Lepler, S.E.; Jin, Z.; Siemann, D.W.; Zeng, B.; et al. Human Cancer Cells Generate Spontaneous Calcium Transients and Intercellular Waves That Modulate Tumor Growth. Biomaterials 2022, 290, 121823. [Google Scholar] [CrossRef]
- Tang, X.; Kuhlenschmidt, T.B.; Li, Q.; Ali, S.; Lezmi, S.; Chen, H.; Pires-Alves, M.; Laegreid, W.W.; Saif, T.A.; Kuhlenschmidt, M.S. A Mechanically-Induced Colon Cancer Cell Population Shows Increased Metastatic Potential. Mol. Cancer 2014, 13, 131. [Google Scholar] [CrossRef]
- Tang, X.; Kuhlenschmidt, T.B.; Zhou, J.; Bell, P.; Wang, F.; Kuhlenschmidt, M.S.; Saif, T.A. Mechanical Force Affects Expression of an In Vitro Metastasis-Like Phenotype in HCT-8 Cells. Biophys. J. 2010, 99, 2460–2469. [Google Scholar] [CrossRef]
- Tang, X.; Tofangchi, A.; Anand, S.V.; Saif, T.A. A Novel Cell Traction Force Microscopy to Study Multi-Cellular System. PLoS Comput. Biol. 2014, 10, e1003631. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, J.; Huang, M.; Lin, G.; Tanaka, M.; Lepler, S.; Guan, J.; Siemann, D.; Tang, X. Automatic Multi-Functional Integration Program (AMFIP) towards All-Optical Mechano-Electrophysiology Interrogation. PLoS ONE 2022, 17, e0266098. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Huang, M.; Liang, C.; Zhang, J.; Lin, G.; Yu, S.; Tanaka, M.; Lepler, S.; Guan, J.; Siemann, D.; et al. All-Optical Mechanobiology Interrogation of Yes-Associated Protein in Human Cancer and Normal Cells Using a Multi-Functional System. JoVE 2021, 178, 62934. [Google Scholar] [CrossRef]
- Huang, M.; Wang, H.; Delgado, A.A.; Reid, T.A.; Long, J.; Wang, S.; Sussman, H.; Guan, J.; Yamaguchi, H.; Tang, X. Combining 3D Magnetic Force Actuator and Multi-Functional Fluorescence Imaging to Study Nucleus Mechanobiology. JoVE 2022, 185, 64098. [Google Scholar] [CrossRef]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP Regulates Cell Mechanics by Controlling Focal Adhesion Assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef]
- Elosegui-Artola, A.; Gupta, A.; Najibi, A.J.; Seo, B.R.; Garry, R.; Tringides, C.M.; De Lázaro, I.; Darnell, M.; Gu, W.; Zhou, Q.; et al. Matrix Viscoelasticity Controls Spatiotemporal Tissue Organization. Nat. Mater. 2023, 22, 117–127. [Google Scholar] [CrossRef]
- Lee, J.; Abdeen, A.A.; Tang, X.; Saif, T.A.; Kilian, K.A. Matrix Directed Adipogenesis and Neurogenesis of Mesenchymal Stem Cells Derived from Adipose Tissue and Bone Marrow. Acta Biomater. 2016, 42, 46–55. [Google Scholar] [CrossRef]
- Lee, J.; Abdeen, A.A.; Tang, X.; Saif, T.A.; Kilian, K.A. Geometric Guidance of Integrin Mediated Traction Stress during Stem Cell Differentiation. Biomaterials 2015, 69, 174–183. [Google Scholar] [CrossRef]
- Tang, X.; Saif, T.A. Adhesivity of Colon Cancer Cells During In Vitro Metastasis. Int. J. Appl. Mech. 2013, 5, 1350025. [Google Scholar] [CrossRef]
- Califano, J.P.; Reinhart-King, C.A. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cel. Mol. Bioeng. 2010, 3, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Koushki, N.; Ghagre, A.; Srivastava, L.K.; Sitaras, C.; Yoshie, H.; Molter, C.; Ehrlicher, A.J. Lamin A Redistribution Mediated by Nuclear Deformation Determines Dynamic Localization of YAP. Biophysics, 2020; preprint. [Google Scholar] [CrossRef]
- Cui, Y.; Hameed, F.M.; Yang, B.; Lee, K.; Pan, C.Q.; Park, S.; Sheetz, M. Cyclic Stretching of Soft Substrates Induces Spreading and Growth. Nat. Commun. 2015, 6, 6333. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, T.P.; Cosgrove, B.D.; Heo, S.-J.; Shurden, Z.E.; Mauck, R.L. Cytoskeletal to Nuclear Strain Transfer Regulates YAP Signaling in Mesenchymal Stem Cells. Biophys. J. 2015, 108, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.W.; Meng, Z.; Yuan, H.; Plouffe, S.W.; Moon, S.; Kim, W.; Jho, E.; Guan, K. Osmotic Stress-induced Phosphorylation by NLK at Ser128 Activates YAP. EMBO Rep. 2017, 18, 72–86. [Google Scholar] [CrossRef]
- Song, X.; Xu, H.; Wang, P.; Wang, J.; Affo, S.; Wang, H.; Xu, M.; Liang, B.; Che, L.; Qiu, W.; et al. Focal Adhesion Kinase (FAK) Promotes Cholangiocarcinoma Development and Progression via YAP Activation. J. Hepatol. 2021, 75, 888–899. [Google Scholar] [CrossRef]
- Li, B.; He, J.; Lv, H.; Liu, Y.; Lv, X.; Zhang, C.; Zhu, Y.; Ai, D. C-Abl Regulates YAPY357 Phosphorylation to Activate Endothelial Atherogenic Responses to Disturbed Flow. J. Clin. Investig. 2019, 129, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Andreu, I.; Granero-Moya, I.; Chahare, N.R.; Clein, K.; Molina-Jordán, M.; Beedle, A.E.M.; Elosegui-Artola, A.; Abenza, J.F.; Rossetti, L.; Trepat, X.; et al. Mechanical Force Application to the Nucleus Regulates Nucleocytoplasmic Transport. Nat. Cell Biol. 2022, 24, 896–905. [Google Scholar] [CrossRef]
- Tyner, J.W.; Haderk, F.; Kumaraswamy, A.; Baughn, L.B.; Van Ness, B.; Liu, S.; Marathe, H.; Alumkal, J.J.; Bivona, T.G.; Chan, K.S.; et al. Understanding Drug Sensitivity and Tackling Resistance in Cancer. Cancer Res. 2022, 82, 1448–1460. [Google Scholar] [CrossRef]
- Zargar, A.; Chang, S.; Kothari, A.; Snijders, A.M.; Mao, J.-H.; Wang, J.; Hernández, A.C.; Keasling, J.D.; Bivona, T.G. Overcoming the Challenges of Cancer Drug Resistance through Bacterial-Mediated Therapy. Chronic Dis. Transl. Med. 2019, 5, 258–266. [Google Scholar] [CrossRef]
- Chatterjee, N.; Bivona, T.G. Polytherapy and Targeted Cancer Drug Resistance. Trends Cancer 2019, 5, 170–182. [Google Scholar] [CrossRef]
- Li, J.W.; Zheng, G.; Kaye, F.J.; Wu, L. PROTAC Therapy as a New Targeted Therapy for Lung Cancer. Mol. Ther. 2023, 31, 647–656. [Google Scholar] [CrossRef]
- Wei, Y.; Au, J.L.-S. Role of Tumour Microenvironment in Chemoresistance. In Integration/Interaction of Oncologic Growth; Meadows, G.G., Ed.; Cancer Growth and Progression; Springer: Berlin/Heidelberg, Germany, 2005; Volume 15, pp. 285–321. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-F.; Tseng, Y.-C.; Nguyen, P.A.; Li, Y.-C.; Ho, C.-C.; Wu, C.-W. Enhanced YAP Expression Leads to EGFR TKI Resistance in Lung Adenocarcinomas. Sci. Rep. 2018, 8, 271. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.; Kleinberg, L.; Halvorsen, A.R.; Hell, Å.; Brustugun, O.T. NSCLC Depend upon YAP Expression and Nuclear Localization after Acquiring Resistance to EGFR Inhibitors. Genes Cancer 2017, 8, 497–504. [Google Scholar] [CrossRef]
- Song, J.; Xie, L.; Zhang, X.; Hu, P.; Long, M.; Xiong, F.; Huang, J.; Ye, X. Role of YAP in Lung Cancer Resistance to Cisplatin. Oncol. Lett. 2018, 16, 3949–3954. [Google Scholar] [CrossRef]
- Lin, L.; Sabnis, A.J.; Chan, E.; Olivas, V.; Cade, L.; Pazarentzos, E.; Asthana, S.; Neel, D.; Yan, J.J.; Lu, X.; et al. The Hippo Effector YAP Promotes Resistance to RAF- and MEK-Targeted Cancer Therapies. Nat. Genet. 2015, 47, 250–256. [Google Scholar] [CrossRef]
- Kurppa, K.J.; Liu, Y.; To, C.; Zhang, T.; Fan, M.; Vajdi, A.; Knelson, E.H.; Xie, Y.; Lim, K.; Cejas, P.; et al. Treatment-Induced Tumor Dormancy through YAP-Mediated Transcriptional Reprogramming of the Apoptotic Pathway. Cancer Cell 2020, 37, 104–122.e12. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, W.C.; Varma, S.; Sousa, F.; Sunshine, M.; Abaan, O.D.; Davis, S.R.; Reinhold, S.W.; Kohn, K.W.; Morris, J.; Meltzer, P.S.; et al. NCI-60 Whole Exome Sequencing and Pharmacological CellMiner Analyses. PLoS ONE 2014, 9, e101670. [Google Scholar] [CrossRef]
- Morrison, H.; Sherman, L.S.; Legg, J.; Banine, F.; Isacke, C.; Haipek, C.A.; Gutmann, D.H.; Ponta, H.; Herrlich, P. The NF2 Tumor Suppressor Gene Product, Merlin, Mediates Contact Inhibition of Growth through Interactions with CD44. Genes Dev. 2001, 15, 968–980. [Google Scholar] [CrossRef]
- Gobbi, G.; Donati, B.; Do Valle, I.F.; Reggiani, F.; Torricelli, F.; Remondini, D.; Castellani, G.; Ambrosetti, D.C.; Ciarrocchi, A.; Sancisi, V. The Hippo Pathway Modulates Resistance to BET Proteins Inhibitors in Lung Cancer Cells. Oncogene 2019, 38, 6801–6817. [Google Scholar] [CrossRef]
- Rozengurt, E.; Eibl, G. Crosstalk between KRAS, SRC and YAP Signaling in Pancreatic Cancer: Interactions Leading to Aggressive Disease and Drug Resistance. Cancers 2021, 13, 5126. [Google Scholar] [CrossRef]
- Girotti, M.R.; Lopes, F.; Preece, N.; Niculescu-Duvaz, D.; Zambon, A.; Davies, L.; Whittaker, S.; Saturno, G.; Viros, A.; Pedersen, M.; et al. Paradox-Breaking RAF Inhibitors That Also Target SRC Are Effective in Drug-Resistant BRAF Mutant Melanoma. Cancer Cell 2015, 27, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-C.; Yang, C.-T.; Jablons, D.M.; You, L. The Crosstalk between Src and Hippo/YAP Signaling Pathways in Non-Small Cell Lung Cancer (NSCLC). Cancers 2020, 12, 1361. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Y.; Chen, P.-S.; Prakash, E.; Hsu, H.-C.; Huang, H.-Y.; Lin, M.-T.; Chang, K.-J.; Kuo, M.-L. Connective Tissue Growth Factor Confers Drug Resistance in Breast Cancer through Concomitant Up-Regulation of Bcl-XL and CIAP1. Cancer Res. 2009, 69, 3482–3491. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-C.; Huang, C.-Y.; Su, H.-L.; Tang, C.-H. CTGF Increases Drug Resistance to Paclitaxel by Upregulating Survivin Expression in Human Osteosarcoma Cells. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2014, 1843, 846–854. [Google Scholar] [CrossRef]
- Lin, M.-T.; Chang, C.-C.; Chen, S.-T.; Chang, H.-L.; Su, J.-L.; Chau, Y.-P.; Kuo, M.-L. Cyr61 Expression Confers Resistance to Apoptosis in Breast Cancer MCF-7 Cells by a Mechanism of NF-ΚB-Dependent XIAP Up-Regulation. J. Biol. Chem. 2004, 279, 24015–24023. [Google Scholar] [CrossRef]
- Hsu, P.; Miao, J.; Wang, Y.; Zhang, W.; Yang, Y.; Wang, C.; Yang, C.; Huang, Z.; You, J.; Xu, Z.; et al. Inhibition of Yes-associated Protein Down-regulates PD-L1 (CD274) Expression in Human Malignant Pleural Mesothelioma. J. Cell. Mol. Med. 2018, 22, 3139–3148. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.-L.; Wang, T.-L.; Zheng, F.-M. ACADL Suppresses PD-L1 Expression to Prevent Cancer Immune Evasion by Targeting Hippo/YAP Signaling in Lung Adenocarcinoma. Med. Oncol. 2023, 40, 118. [Google Scholar] [CrossRef]
- Yu, M.; Peng, Z.; Qin, M.; Liu, Y.; Wang, J.; Zhang, C.; Lin, J.; Dong, T.; Wang, L.; Li, S.; et al. Interferon-γ Induces Tumor Resistance to Anti-PD-1 Immunotherapy by Promoting YAP Phase Separation. Mol. Cell 2021, 81, 1216–1230.e9. [Google Scholar] [CrossRef]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular Matrix: A Dynamic Microenvironment for Stem Cell Niche. Biochim. Biophys. Acta (BBA)—General. Subj. 2014, 1840, 2506–2519. [Google Scholar] [CrossRef]
- Yue, B. Biology of the Extracellular Matrix: An Overview. J. Glaucoma 2014, 23, S20–S23. [Google Scholar] [CrossRef]
- Yahyazadeh Shourabi, A.; Kashaninejad, N.; Saidi, M.S. An Integrated Microfluidic Concentration Gradient Generator for Mechanical Stimulation and Drug Delivery. J. Sci. Adv. Mater. Devices 2021, 6, 280–290. [Google Scholar] [CrossRef]
- Li, J.; Hou, B.; Tumova, S.; Muraki, K.; Bruns, A.; Ludlow, M.J.; Sedo, A.; Hyman, A.J.; McKeown, L.; Young, R.S.; et al. Piezo1 Integration of Vascular Architecture with Physiological Force. Nature 2014, 515, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Li, K.; Yang, M.; Tan, Y. Fluid Shear Stress Induces EMT of Circulating Tumor Cells via JNK Signaling in Favor of Their Survival during Hematogenous Dissemination. Int. J. Mol. Sci. 2020, 21, 8115. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Pelissier, F.A.; Zhang, H.; Lakins, J.; Weaver, V.M.; Park, C.; LaBarge, M.A. Microenvironment Rigidity Modulates Responses to the HER2 Receptor Tyrosine Kinase Inhibitor Lapatinib via YAP and TAZ Transcription Factors. MBoC 2015, 26, 3946–3953. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Lin, J.; Nowsheen, S.; Liu, T.; Zhao, Y.; Villalta, P.W.; Sicard, D.; Tschumperlin, D.J.; Lee, S.; Kim, J.; et al. Extracellular Matrix Stiffness Determines DNA Repair Efficiency and Cellular Sensitivity to Genotoxic Agents. Sci. Adv. 2020, 6, eabb2630. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Irnaten, M.; Hopkins, A.; O’Callaghan, J.; Stamer, W.D.; Clark, A.F.; Wallace, D.; O’Brien, C.J. Matrix Mechanotransduction via Yes-Associated Protein in Human Lamina Cribrosa Cells in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2022, 63, 16. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Sleiman, M.; Moriarty, T.; Herrick, W.G.; Peyton, S.R. Sorafenib Resistance and JNK Signaling in Carcinoma during Extracellular Matrix Stiffening. Biomaterials 2014, 35, 5749–5759. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, X.; Huang, B.; Zhou, W.; Cui, X.; Zheng, C.; Liu, F.; Bi, J.; Zhang, Y.; Luo, H.; et al. Inhibition of Matrix Stiffness Relating Integrin Β1 Signaling Pathway Inhibits Tumor Growth in Vitro and in Hepatocellular Cancer Xenografts. BMC Cancer 2021, 21, 1276. [Google Scholar] [CrossRef]
- Hassan, A.A.; Artemenko, M.; Tang, M.K.S.; Shi, Z.; Chen, L.-Y.; Lai, H.-C.; Yang, Z.; Shum, H.-C.; Wong, A.S.T. Ascitic Fluid Shear Stress in Concert with Hepatocyte Growth Factor Drive Stemness and Chemoresistance of Ovarian Cancer Cells via the C-Met-PI3K/Akt-MiR-199a-3p Signaling Pathway. Cell Death Dis. 2022, 13, 537. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chang, J.K.; Dominguez, A.A.; Lee, H.; Nam, S.; Chang, J.; Varma, S.; Qi, L.S.; West, R.B.; Chaudhuri, O. YAP-Independent Mechanotransduction Drives Breast Cancer Progression. Nat. Commun. 2019, 10, 1848. [Google Scholar] [CrossRef]
- Cao, S.; Tang, J.; Huang, Y.; Li, G.; Li, Z.; Cai, W.; Yuan, Y.; Liu, J.; Huang, X.; Zhang, H. The Road of Solid Tumor Survival: From Drug-Induced Endoplasmic Reticulum Stress to Drug Resistance. Front. Mol. Biosci. 2021, 8, 620514. [Google Scholar] [CrossRef]
- Sharma, S.; Santiskulvong, C.; Bentolila, L.A.; Rao, J.; Dorigo, O.; Gimzewski, J.K. Correlative Nanomechanical Profiling with Super-Resolution F-Actin Imaging Reveals Novel Insights into Mechanisms of Cisplatin Resistance in Ovarian Cancer Cells. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 757–766. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Kieu, Q.M.N.; Dawson, M.R. The Malignancy of Metastatic Ovarian Cancer Cells Is Increased on Soft Matrices Through a Mechanosensitive Rho–ROCK Pathway. J. Cell Sci. 2014, 127, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Barai, A.; Thakur, B.; Das, A.; Patwardhan, S.R.; Monteiro, M.; Gaikwad, S.; Bukhari, A.B.; Mogha, P.; Majumder, A.; et al. Soft Drug-Resistant Ovarian Cancer Cells Migrate via Two Distinct Mechanisms Utilizing Myosin II-Based Contractility. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2018, 1865, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, Á.; Cook, A.W.; Gough, R.E.; Schilling, M.; Olszok, N.A.; Brown, I.; Wang, L.; Aaron, J.; Martin-Fernandez, M.L.; Rehfeldt, F.; et al. DNA Damage Alters Nuclear Mechanics through Chromatin Reorganization. Nucleic Acids Res. 2021, 49, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.S.; Yoon, C.W.; Wang, Q.; Moon, S.; Koo, K.M.; Jung, H.; Chen, R.; Jiang, L.; Lu, G.; Fernandez, A.; et al. Focused Ultrasound Stimulates ER Localized Mechanosensitive PANNEXIN-1 to Mediate Intracellular Calcium Release in Invasive Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 504. [Google Scholar] [CrossRef]
- Huo, S.; Liao, Z.; Zhao, P.; Zhou, Y.; Göstl, R.; Herrmann, A. Mechano-Nanoswitches for Ultrasound-Controlled Drug Activation. Adv. Sci. 2022, 9, 2104696. [Google Scholar] [CrossRef]
- Tijore, A.; Yao, M.; Wang, Y.-H.; Hariharan, A.; Nematbakhsh, Y.; Lee Doss, B.; Lim, C.T.; Sheetz, M. Selective Killing of Transformed Cells by Mechanical Stretch. Biomaterials 2021, 275, 120866. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).