Detection of Elevated Level of Tetrahydrobiopterin in Serum Samples of ME/CFS Patients with Orthostatic Intolerance: A Pilot Study
Abstract
1. Introduction
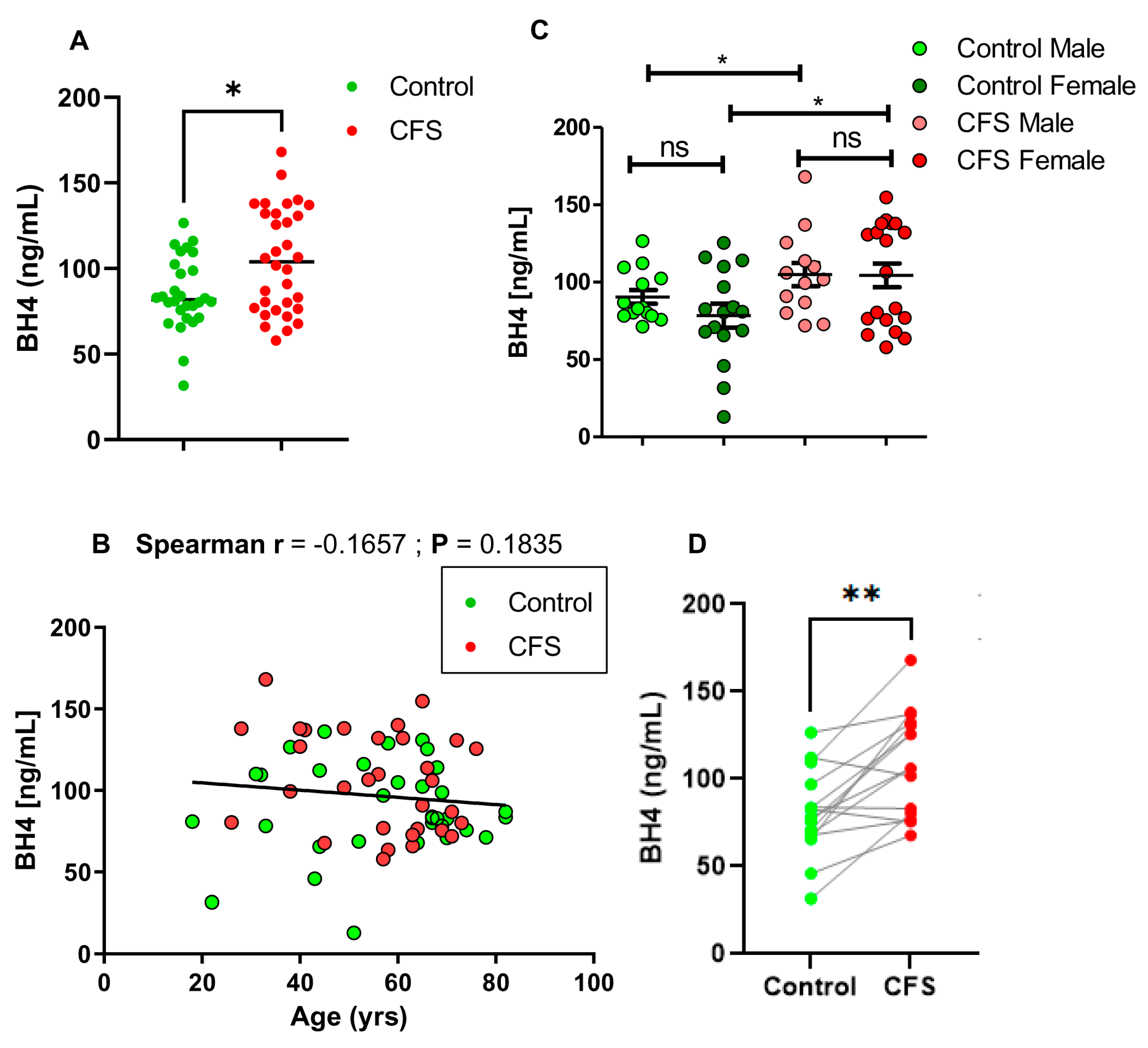
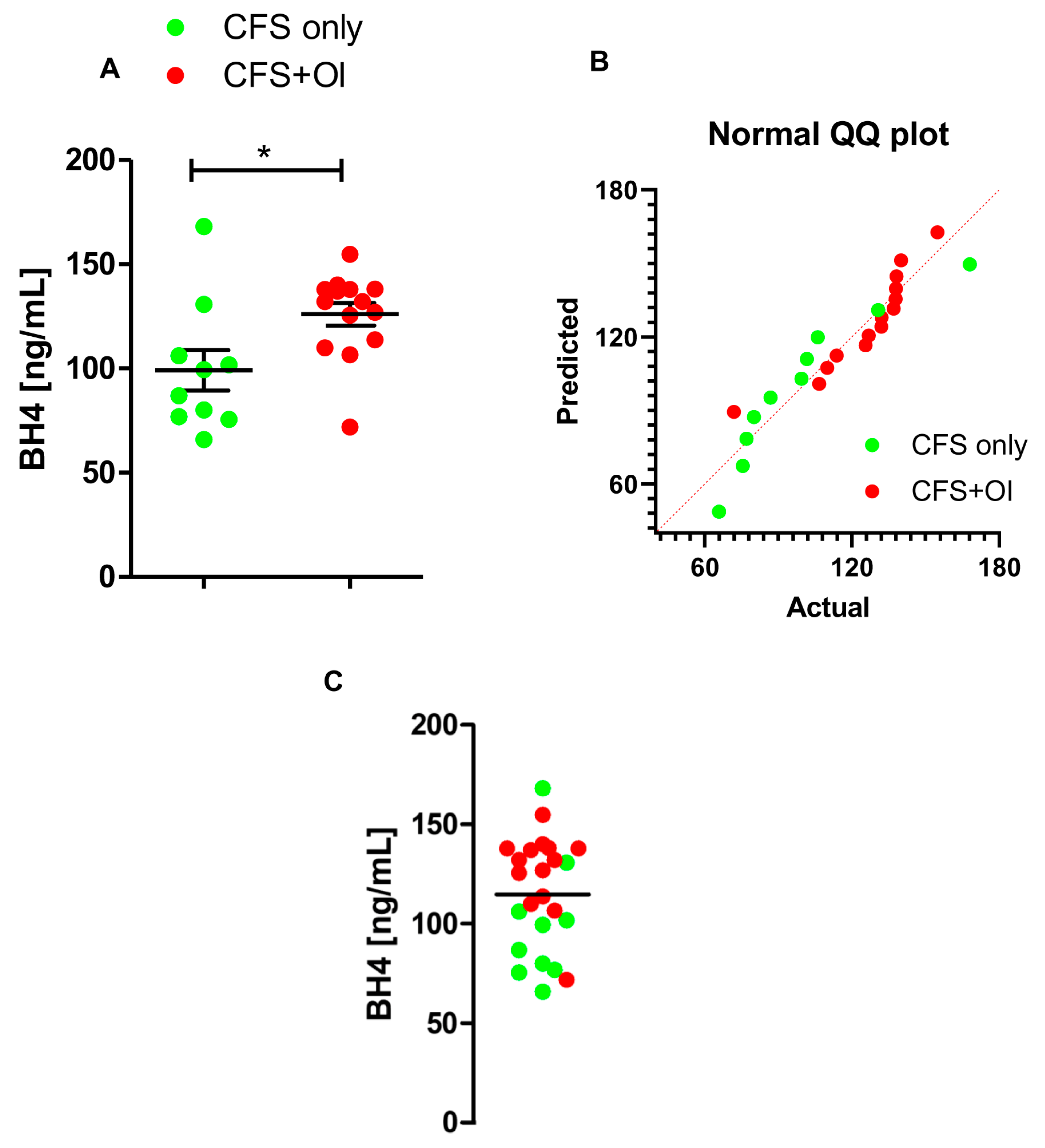
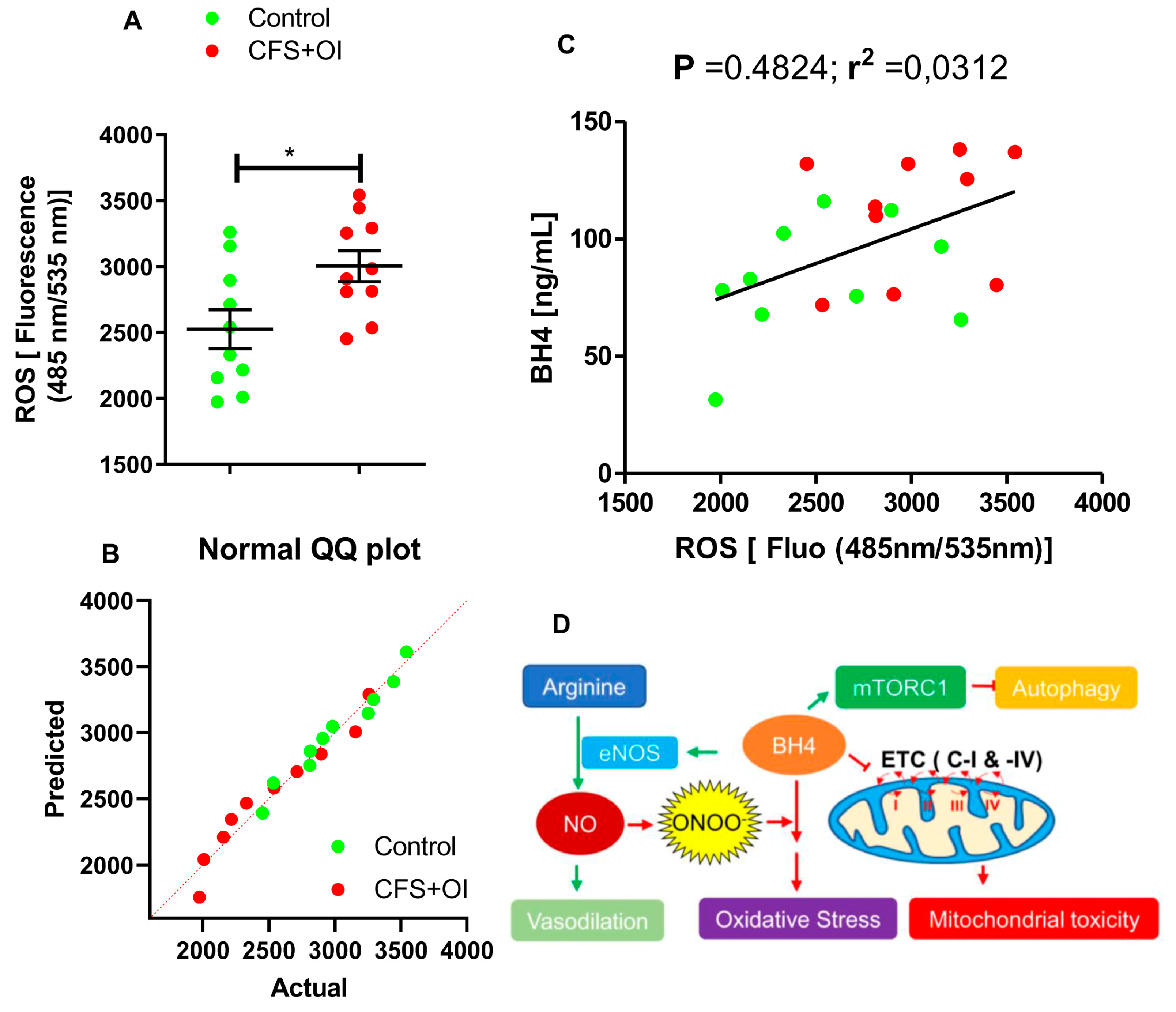
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lim, E.J.; Ahn, Y.C.; Jang, E.S.; Lee, S.W.; Lee, S.H.; Son, C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Med. 2020, 18, 100. [Google Scholar] [CrossRef]
- Son, C.-G. Review of the prevalence of chronic fatigue worldwide. J. Korean Med. 2012, 33, 25–33. [Google Scholar]
- Natelson, B.H.; Lin, J.-M.S.; Blate, M.; Khan, S.; Chen, Y.; Unger, E.R. Physiological assessment of orthostatic intolerance in chronic fatigue syndrome. J. Transl. Med. 2022, 20, 95. [Google Scholar] [CrossRef]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D. Myalgic encephalomyelitis/chronic fatigue syndrome: Essentials of diagnosis and management. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; pp. 2861–2878. [Google Scholar]
- Basantsova, N.Y.; Starshinova, A.A.; Dori, A.; Zinchenko, Y.S.; Yablonskiy, P.K.; Shoenfeld, Y. Small-fiber neuropathy definition, diagnosis, and treatment. Neurol. Sci. 2019, 40, 1343–1350. [Google Scholar] [CrossRef]
- Shoenfeld, Y.; Ryabkova, V.A.; Scheibenbogen, C.; Brinth, L.; Martinez-Lavin, M.; Ikeda, S.; Heidecke, H.; Watad, A.; Bragazzi, N.L.; Chapman, J. Complex syndromes of chronic pain, fatigue and cognitive impairment linked to autoimmune dysautonomia and small fiber neuropathy. Clin. Immunol. 2020, 214, 108384. [Google Scholar] [CrossRef]
- Leonardi, L.; Adam, C.; Beaudonnet, G.; Beauvais, D.; Cauquil, C.; Not, A.; Morassi, O.; Benmalek, A.; Trassard, O.; Echaniz-Laguna, A.; et al. Skin amyloid deposits and nerve fiber loss as markers of neuropathy onset and progression in hereditary transthyretin amyloidosis. Eur. J. Neurol. 2022, 29, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Nomura, T.; Sumi-Ichinose, C. Metabolism of tetrahydrobiopterin: Its relevance in monoaminergic neurons and neurological disorders. Chem. Rec. 2008, 8, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Gray, D.W.; Shiman, R. Regulation of rat liver phenylalanine hydroxylase. III. Control of catalysis by (6R)-tetrahydrobiopterin and phenylalanine. J. Biol. Chem. 1994, 269, 24657–24665. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nagatsu, I. Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson’s disease (PD): Historical overview and future prospects. J. Neural Transm. 2016, 123, 1255–1278. [Google Scholar] [CrossRef]
- Sawada, M.; Sugimoto, T.; Matsuura, S.; Nagatsu, T. (6R)-Tetrahydrobiopterin increases the activity of tryptophan hydroxylase in rat raphe slices. J. Neurochem. 1986, 47, 1544–1547. [Google Scholar] [CrossRef]
- Chen, D.-D.; Chen, L.-Y.; Xie, J.-B.; Shu, C.; Yang, T.; Zhou, S.; Yuan, H.; Chen, A.F. Tetrahydrobiopterin regulation of eNOS redox function. Curr. Pharm. Des. 2014, 20, 3554–3562. [Google Scholar] [CrossRef] [PubMed]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Kimura, M.; Noma, K.; Hara, K.; Jitsuiki, D.; Goto, C.; Oshima, T.; Chayama, K. Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 2006, 186, 390–395. [Google Scholar] [CrossRef]
- Wang, W.Z.; Fang, X.H.; Stephenson, L.L.; Khiabani, K.T.; Zamboni, W.A. Effects of supplementation of BH4 after prolonged ischemia in skeletal muscle. Microsurgery 2007, 27, 200–205. [Google Scholar] [CrossRef]
- Yang, Y.-M.; Huang, A.; Kaley, G.; Sun, D. eNOS uncoupling and endothelial dysfunction in aged vessels. Am. J. Physiol.-Heart Circ. Physiol. 2009, 297, H1829–H1836. [Google Scholar] [CrossRef]
- Karbach, S.; Wenzel, P.; Waisman, A.; Munzel, T.; Daiber, A. eNOS uncoupling in cardiovascular diseases-the role of oxidative stress and inflammation. Curr. Pharm. Des. 2014, 20, 3579–3594. [Google Scholar] [CrossRef]
- Sindler, A.L.; Delp, M.D.; Reyes, R.; Wu, G.; Muller-Delp, J.M. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J. Physiol. 2009, 587, 3885–3897. [Google Scholar] [CrossRef]
- Gottschalk, G.; Peterson, D.; Knox, K.; Maynard, M.; Whelan, R.J.; Roy, A. Elevated ATG13 in serum of patients with ME/CFS stimulates oxidative stress response in microglial cells via activation of receptor for advanced glycation end products (RAGE). Mol. Cell. Neurosci. 2022, 120, 103731. [Google Scholar] [CrossRef] [PubMed]
- Blau, N.; Thony, B.; Spada, M.; Ponzone, A. Tetrahydrobiopterin and inherited hyperphenylalaninemias. Turk. J. Pediatr. 1996, 38, 19–35. [Google Scholar] [PubMed]
- Birnbacher, R.; Scheibenreiter, S.; Blau, N.; Bieglmayer, C.; Frisch, H.; Waldhauser, F. Hyperprolactinemia, a tool in treatment control of tetrahydrobiopterin deficiency: Endocrine studies in an affected girl. Pediatr. Res. 1998, 43, 472–477. [Google Scholar] [CrossRef]
- Hyland, K.; Kasim, S.; Egami, K.; Arnold, L.; Jinnah, H. Tetrahydrobiopterin deficiency and dopamine loss in a genetic mouse model of Lesch-Nyhan disease. J. Inherit. Metab. Dis. 2004, 27, 165–178. [Google Scholar] [CrossRef]
- Li, L.; Chen, W.; Rezvan, A.; Jo, H.; Harrison, D.G. Tetrahydrobiopterin deficiency and nitric oxide synthase uncoupling contribute to atherosclerosis induced by disturbed flow. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1547–1554. [Google Scholar] [CrossRef]
- Chuaiphichai, S.; Starr, A.; Nandi, M.; Channon, K.M.; McNeill, E. Endothelial cell tetrahydrobiopterin deficiency attenuates LPS-induced vascular dysfunction and hypotension. Vasc. Pharmacol. 2016, 77, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Lee, S.Y.; Cho, Y.; No, H.; Kim, S.W.; Hwang, O. Tetrahydrobiopterin causes mitochondrial dysfunction in dopaminergic cells: Implications for Parkinson’s disease. Neurochem. Int. 2006, 48, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Moon, Y.; Hee Choi, D.; Jin Choi, H.; Hwang, O. Particular vulnerability of rat mesencephalic dopaminergic neurons to tetrahydrobiopterin: Relevance to Parkinson’s disease. Neurobiol. Dis. 2007, 25, 112–120. [Google Scholar] [CrossRef]
- Kwak, S.S.; Suk, J.; Choi, J.H.; Yang, S.; Kim, J.W.; Sohn, S.; Chung, J.H.; Hong, Y.H.; Lee, D.H.; Ahn, J.K.; et al. Autophagy induction by tetrahydrobiopterin deficiency. Autophagy 2011, 7, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Porkert, M.; Sher, S.; Reddy, U.; Cheema, F.; Niessner, C.; Kolm, P.; Jones, D.P.; Hooper, C.; Taylor, W.R.; Harrison, D.; et al. Tetrahydrobiopterin: A novel antihypertensive therapy. J. Hum. Hypertens. 2008, 22, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Channon, K. Tetrahydrobiopterin: Regulator of Endothelial Nitric Oxide Synthase in Vascular Disease. Trends Cardiovasc. Med. 2004, 14, 323–327. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Y.; Gu, L.; Liu, P.; Cao, J.; Zhang, S. The Critical Role of Tetrahydrobiopterin (BH4) Metabolism in Modulating Radiosensitivity: BH4/NOS Axis as an Angel or a Devil. Front. Oncol. 2021, 11, 720632. [Google Scholar] [CrossRef]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef] [PubMed]


| Control (n = 34) | CFS (n = 32) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Study ID | Age | Gender | Ethnicity | Condition | Study ID | Age | Gender | Ethnicity | Condition |
| 329502 | 82 | M | white | Healthy | 211103 | 67 | M | white | CFS |
| 339105 | 70 | F | white | Healthy | 381104 | 38 | M | white | CFS |
| 939209 | 69 | M | white | Healthy | 201105 | 72 | F | white | CFS |
| 699106 | 68 | F | white | Healthy | 921106 | 69 | F | white | CFS |
| 229403 | 68 | F | white | Healthy | 991108 | 71 | M | white | CFS |
| 809208 | 67 | M | white | Healthy | 191109 | 57 | F | white | CFS |
| 499205 | 67 | F | white | Healthy | 701110 | 63 | F | white | CFS |
| 169316 | 65 | M | white | cancer | 661111 | 73 | M | white | CFS |
| 309110 | 64 | F | white | Healthy | 341112 | 33 | M | white | CFS |
| 829306 | 60 | F | white | cancer | 861113 | 49 | M | white | CFS |
| 909302 | 58 | F | white | cancer | 691114 | 49 | F | white | CFS + OI |
| 739109 | 57 | F | white | Healthy | 541201 | 41 | M | white | CFS + OI |
| 299114 | 53 | F | white | Healthy | 391203 | 61 | F | white | CFS + OI |
| 149304 | 52 | F | white | Healthy | 771204 | 56 | M | white | CFS + OI |
| 759113 | 45 | M | white | cancer | 951205 | 64 | F | white | CFS + OI |
| 109201 | 44 | M | white | Healthy | 641206 | 76 | M | white | CFS + OI |
| 639305 | 44 | F | white | Healthy | 431207 | 56 | F | white | CFS + OI |
| 219317 | 43 | F | white | Healthy | 331209 | 71 | M | white | CFS + OI |
| 129104 | 33 | M | white | Healthy | 281210 | 66 | M | white | CFS + OI |
| 369112 | 32 | M | white | Healthy | 661215 | 26 | F | white | CFS + OI |
| 129312 | 31 | F | white | Healthy | 261302 | 58 | F | white | CFS + OI + SFN |
| 169504 | 82 | M | white | Healthy | 791304 | 54 | F | white | CFS + OI + SFN |
| 899108 | 69 | M | white | Healthy | 811305 | 45 | F | white | CFS + OI + SFN |
| 669206 | 78 | M | white | Healthy | 321306 | 57 | F | white | CFS + OI + SFN |
| 419111 | 74 | M | white | Healthy | 971309 | 60 | F | white | CFS + OI + SFN |
| 249311 | 70 | F | white | Healthy | 851310 | 65 | F | white | CFS + OI + SFN |
| 889210 | 65 | M | white | Healthy | 221311 | 67 | F | white | CFS + OI + SFN |
| 559310 | 66 | F | white | Healthy | 751312 | 28 | F | white | CFS + OI + SFN |
| 559107 | 68 | M | white | Healthy | 901313 | 65 | M | white | CFS + OI + SFN |
| 539103 | 69 | M | white | Healthy | 181316 | 63 | M | white | CFS + OI + SFN |
| 789207 | 51 | F | white | Healthy | 841317 | 40 | F | white | CFS + OI + SFN |
| 609405 | 38 | M | white | Healthy | 1004 | 40 | F | white | CFS + OI + SFN |
| 579315 | 18 | F | white | Healthy | |||||
| 431315 | 22 | F | white | Healthy | |||||
| Control | CFS + OI | ||||
|---|---|---|---|---|---|
| Age | Gender | BH4 (ng/mL) | Age | Gender | BH4 (ng/mL) |
| 44 | F | 65.63 ± 4.098 | 49 | F | 138.152 ± 24.332 |
| 44 | M | 112.296 ± 12.113 | 41 | M | 137.053 ± 13.165 |
| 57 | F | 96.841 ± 16.528 | 61 | F | 132.01 ± 18.109 |
| 65 | M | 102.42 ± 9.287 | 56 | M | 109.89 ± 11.98 |
| 64 | F | 67.852 ± 11.121 | 64 | F | 76.494 ± 23.4 |
| 74 | M | 75.669 ± 14.225 | 76 | M | 125.53 ± 4.164 |
| 53 | F | 116.068 ± 8.119 | 56 | F | 132.099± 8.119 |
| 69 | M | 78.222 ± 4.6 | 71 | M | 71.916 ± 12.01 |
| 68 | M | 82.914 ± 23.161 | 66 | M | 113.778 ± 4.33 |
| 22 | F | 31.556 ± 7.110 | 26 | F | 80.444 ± 16.12 |
| Control | CFS + OI + SFN | ||||
|---|---|---|---|---|---|
| Age | Gender | BH4 (ng/mL) | Age | Gender | BH4 (ng/mL) |
| 57 | F | 96.84128 | 58 | F | 63.654 |
| 51 | F | 12.85 | 54 | F | 106.617 |
| 44 | F | 65.63 | 45 | F | 67.66872758 |
| 57 | F | 96.84 | 57 | F | 57.975 |
| 64 | F | 67.851 | 60 | F | 140.0630005 |
| 67 | F | 83.9012 | 65 | F | 154.8251948 |
| 68 | F | 80.614 | 67 | F | 83.041 |
| 31 | F | 110.074 | 28 | F | 137.8619147 |
| 74 | M | 75.66941 | 65 | M | 90.87 |
| 67 | M | 80.166 | 63 | M | 72.790 |
| 43 | F | 45.876 | 40 | F | 126.8371241 |
| 52 | F | 68.78 | 40 | F | 137.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gottschalk, C.G.; Whelan, R.; Peterson, D.; Roy, A. Detection of Elevated Level of Tetrahydrobiopterin in Serum Samples of ME/CFS Patients with Orthostatic Intolerance: A Pilot Study. Int. J. Mol. Sci. 2023, 24, 8713. https://doi.org/10.3390/ijms24108713
Gottschalk CG, Whelan R, Peterson D, Roy A. Detection of Elevated Level of Tetrahydrobiopterin in Serum Samples of ME/CFS Patients with Orthostatic Intolerance: A Pilot Study. International Journal of Molecular Sciences. 2023; 24(10):8713. https://doi.org/10.3390/ijms24108713
Chicago/Turabian StyleGottschalk, Carl Gunnar, Ryan Whelan, Daniel Peterson, and Avik Roy. 2023. "Detection of Elevated Level of Tetrahydrobiopterin in Serum Samples of ME/CFS Patients with Orthostatic Intolerance: A Pilot Study" International Journal of Molecular Sciences 24, no. 10: 8713. https://doi.org/10.3390/ijms24108713
APA StyleGottschalk, C. G., Whelan, R., Peterson, D., & Roy, A. (2023). Detection of Elevated Level of Tetrahydrobiopterin in Serum Samples of ME/CFS Patients with Orthostatic Intolerance: A Pilot Study. International Journal of Molecular Sciences, 24(10), 8713. https://doi.org/10.3390/ijms24108713






