Enhancing the Impact of Chemotherapy on Ewing Sarcoma Cells through Combination with Cold Physical Plasma
Abstract
1. Introduction
2. Results
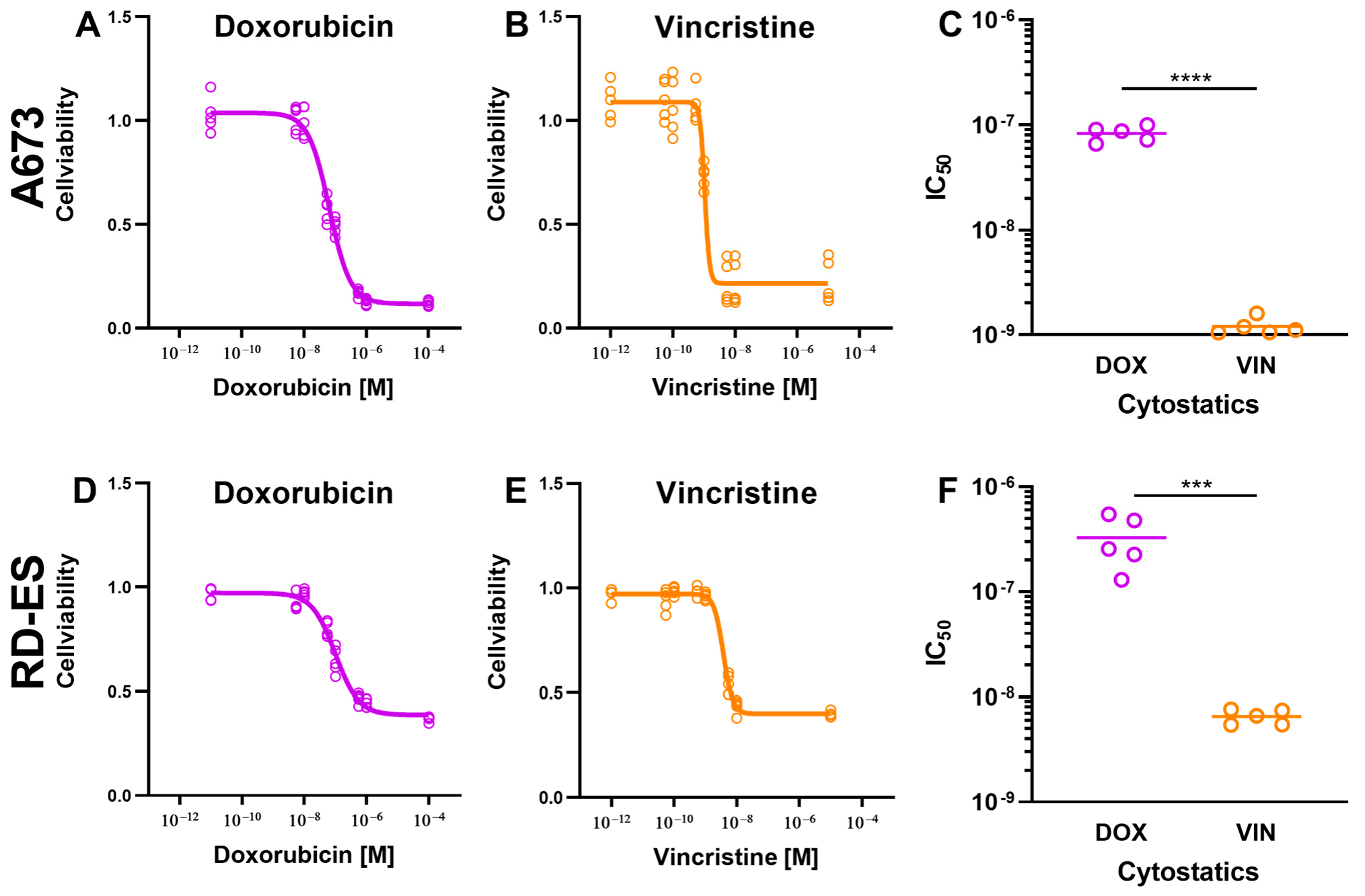
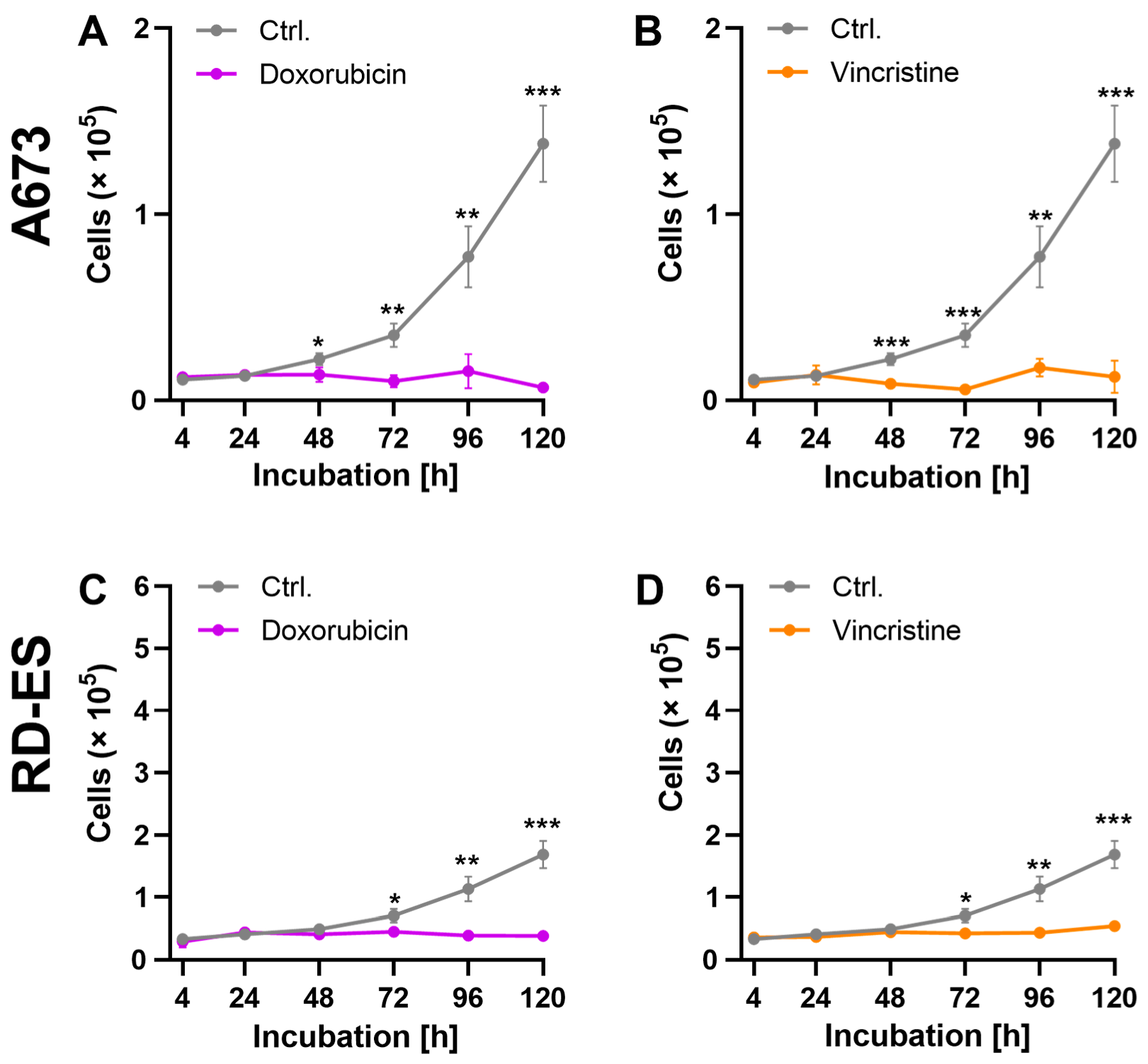
2.1. Reduction in Cell Viability and Proliferation Rate by Cytostatics
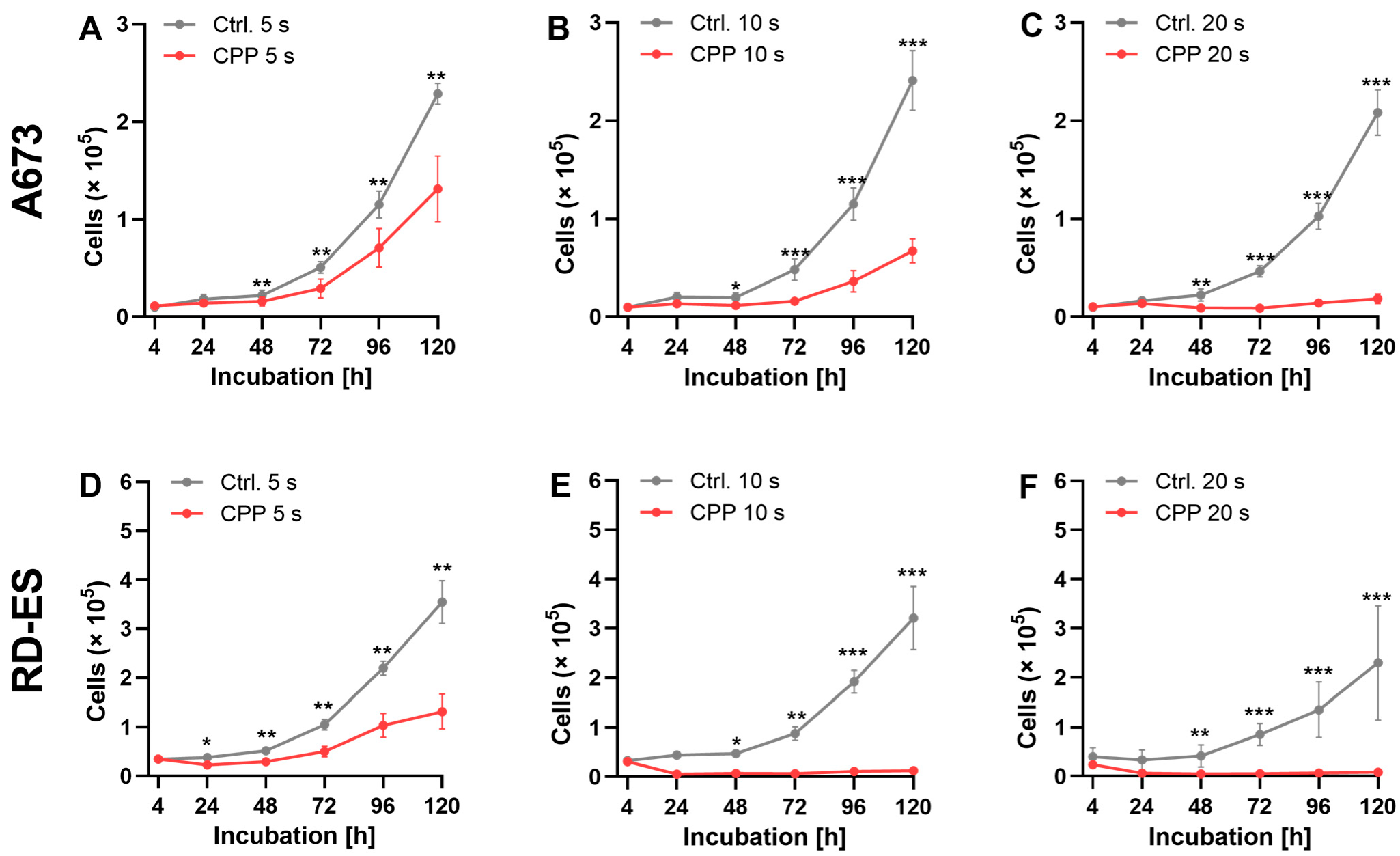
2.2. Influence of CPP on Cell Growth
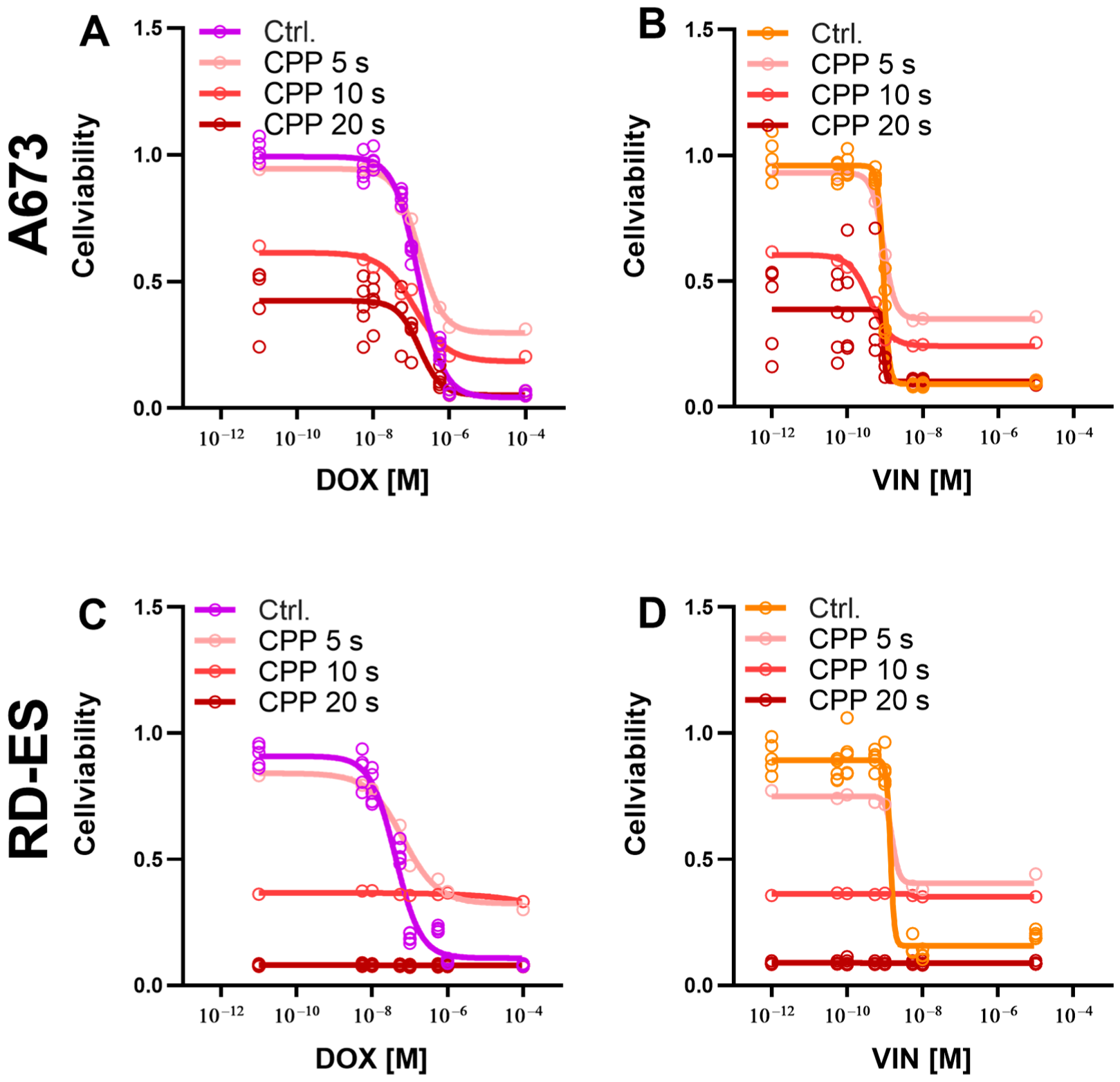
2.3. Combination of CPP and Cytostatics
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Chemotherapeutics
4.3. Proliferation Assay after CPP-Exposure
4.4. Proliferation Assay after Cytostatic Exposure
4.5. Proliferation Assay after Cytostatic and CPP Exposure
4.6. The CellTiter-Blue® Cell Viability Assay after Cytostatic Exposure
4.7. The CellTiter-Blue® Cell Viability Assay after Cytostatic and CPP Exposure
4.8. TUNEL Assay
4.9. Caspase Assay
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spector, L.G.; Hubbard, A.K.; Diessner, B.J.; Machiela, M.J.; Webber, B.R.; Schiffman, J.D. Comparative international incidence of Ewing sarcoma 1988 to 2012. Int. J. Cancer 2021, 149, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterwijk, J.G.; Anninga, J.K.; Gelderblom, H.; Cleton-Jansen, A.M.; Bovée, J.V. Update on targets and novel treatment options for high-grade osteosarcoma and chondrosarcoma. Hematol. Oncol. Clin. N. Am. 2013, 27, 1021–1048. [Google Scholar] [CrossRef] [PubMed]
- Chow, W.A. Chondrosarcoma: Biology, genetics, and epigenetics. F1000Research 2018, 7, 1826. [Google Scholar] [CrossRef] [PubMed]
- Worch, J.; Cyrus, J.; Goldsby, R.; Matthay, K.K.; Neuhaus, J.; DuBois, S.G. Racial differences in the incidence of mesenchymal tumors associated with EWSR1 translocation. Cancer Epidemiol. Biomark. Prev. 2011, 20, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Ito, Y.; Magadi, W.; Bonaventure, A.; Stiller, C.A.; Katanoda, K.; Matsuda, T.; Miyashiro, I.; Pritchard-Jones, K.; Rachet, B. Childhood cancer incidence and survival in Japan and England: A population-based study (1993–2010). Cancer Sci. 2018, 109, 422–434. [Google Scholar] [CrossRef]
- Fraumeni, J.F., Jr.; Glass, A.G. Rarity of Ewing’s sarcoma among U.S. Negro children. Lancet 1970, 1, 366–367. [Google Scholar] [CrossRef]
- Li, F.; Tu, J.-T.; Liu, F.-S.; Shiang, E. Rarity of Ewing’s Sarcoma in China. Lancet 1980, 315, 1255. [Google Scholar] [CrossRef]
- Glass, A.G.; Fraumeni, J.F., Jr. Epidemiology of bone cancer in children. J. Natl. Cancer Inst. 1970, 44, 187–199. [Google Scholar]
- Romeo, S.; Dei Tos, A.P. Soft tissue tumors associated with EWSR1 translocation. Virchows Arch. 2010, 456, 219–234. [Google Scholar] [CrossRef]
- Fisher, C. The diversity of soft tissue tumours with EWSR1 gene rearrangements: A review. Histopathology 2014, 64, 134–150. [Google Scholar] [CrossRef]
- Agaram, N.P.; Zhang, L.; Sung, Y.S.; Singer, S.; Antonescu, C.R. Extraskeletal myxoid chondrosarcoma with non-EWSR1-NR4A3 variant fusions correlate with rhabdoid phenotype and high-grade morphology. Hum. Pathol. 2014, 45, 1084–1091. [Google Scholar] [CrossRef]
- Sankar, S.; Lessnick, S.L. Promiscuous partnerships in Ewing’s sarcoma. Cancer Genet. 2011, 204, 351–365. [Google Scholar] [CrossRef]
- Gargallo, P.; Yáñez, Y.; Juan, A.; Segura, V.; Balaguer, J.; Torres, B.; Oltra, S.; Castel, V.; Cañete, A. Review: Ewing Sarcoma Predisposition. Pathol. Oncol. Res. 2020, 26, 2057–2066. [Google Scholar] [CrossRef]
- Hancock, J.D.; Lessnick, S.L. A transcriptional profiling meta-analysis reveals a core EWS-FLI gene expression signature. Cell Cycle 2008, 7, 250–256. [Google Scholar] [CrossRef]
- Sankar, S.; Bell, R.; Stephens, B.; Zhuo, R.; Sharma, S.; Bearss, D.J.; Lessnick, S.L. Mechanism and relevance of EWS/FLI-mediated transcriptional repression in Ewing sarcoma. Oncogene 2013, 32, 5089–5100. [Google Scholar] [CrossRef]
- Takigami, I.; Ohno, T.; Kitade, Y.; Hara, A.; Nagano, A.; Kawai, G.; Saitou, M.; Matsuhashi, A.; Yamada, K.; Shimizu, K. Synthetic siRNA targeting the breakpoint of EWS/Fli-1 inhibits growth of Ewing sarcoma xenografts in a mouse model. Int. J. Cancer 2011, 128, 216–226. [Google Scholar] [CrossRef]
- Heck, R.K., Jr.; Peabody, T.D.; Simon, M.A. Staging of primary malignancies of bone. CA Cancer J. Clin. 2006, 56, 366–375. [Google Scholar] [CrossRef]
- Fisher, B.; Wolmark, N. The Current Status of Systemic Adjuvant Therapy in the Management of Primary Breast Cancer. Surg. Clin. N. Am. 1981, 61, 1347–1360. [Google Scholar] [CrossRef]
- Cripe, T.P. Ewing sarcoma: An eponym window to history. Sarcoma 2011, 2011, 457532. [Google Scholar] [CrossRef]
- Nesbit, M.E., Jr.; Gehan, E.A.; Burgert, E.O., Jr.; Vietti, T.J.; Cangir, A.; Tefft, M.; Evans, R.; Thomas, P.; Askin, F.B.; Kissane, J.M.; et al. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: A long-term follow-up of the First Intergroup study. J. Clin. Oncol. 1990, 8, 1664–1674. [Google Scholar] [CrossRef]
- Burgert, E.O., Jr.; Nesbit, M.E.; Garnsey, L.A.; Gehan, E.A.; Herrmann, J.; Vietti, T.J.; Cangir, A.; Tefft, M.; Evans, R.; Thomas, P.; et al. Multimodal therapy for the management of nonpelvic, localized Ewing’s sarcoma of bone: Intergroup study IESS-II. J. Clin. Oncol. 1990, 8, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Paulussen, M.; Ahrens, S.; Dunst, J.; Winkelmann, W.; Exner, G.U.; Kotz, R.; Amann, G.; Dockhorn-Dworniczak, B.; Harms, D.; Müller-Weihrich, S.; et al. Localized Ewing tumor of bone: Final results of the cooperative Ewing’s Sarcoma Study CESS 86. J. Clin. Oncol. 2001, 19, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Le Deley, M.C.; Paulussen, M.; Lewis, I.; Brennan, B.; Ranft, A.; Whelan, J.; Le Teuff, G.; Michon, J.; Ladenstein, R.; Marec-Bérard, P.; et al. Cyclophosphamide compared with ifosfamide in consolidation treatment of standard-risk Ewing sarcoma: Results of the randomized noninferiority Euro-EWING99-R1 trial. J. Clin. Oncol. 2014, 32, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Grier, H.E.; Krailo, M.D.; Tarbell, N.J.; Link, M.P.; Fryer, C.J.; Pritchard, D.J.; Gebhardt, M.C.; Dickman, P.S.; Perlman, E.J.; Meyers, P.A.; et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N. Engl. J. Med. 2003, 348, 694–701. [Google Scholar] [CrossRef]
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Marcus, K.; Sailer, S.; Healey, J.H.; Dormans, J.P.; et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef]
- Paulussen, M.; Craft, A.W.; Lewis, I.; Hackshaw, A.; Douglas, C.; Dunst, J.; Schuck, A.; Winkelmann, W.; Köhler, G.; Poremba, C.; et al. Results of the EICESS-92 Study: Two randomized trials of Ewing’s sarcoma treatment--cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J. Clin. Oncol. 2008, 26, 4385–4393. [Google Scholar] [CrossRef]
- Obata, H.; Ueda, T.; Kawai, A.; Ishii, T.; Ozaki, T.; Abe, S.; Tanaka, K.; Tsuchiya, H.; Matsumine, A.; Yabe, H. Clinical outcome of patients with Ewing sarcoma family of tumors of bone in Japan: The Japanese Musculoskeletal Oncology Group cooperative study. Cancer 2007, 109, 767–775. [Google Scholar] [CrossRef]
- Rosen, G.; Juergens, H.; Caparros, B.; Nirenberg, A.; Huvos, A.G.; Marcove, R.C. Combination chemotherapy (T-6) in the multidisciplinary treatment of Ewing’s sarcoma. Natl. Cancer Inst. Monogr. 1981, 56, 289–299. [Google Scholar]
- Crona, D.J.; Faso, A.; Nishijima, T.F.; McGraw, K.A.; Galsky, M.D.; Milowsky, M.I. A Systematic Review of Strategies to Prevent Cisplatin-Induced Nephrotoxicity. Oncologist 2017, 22, 609–619. [Google Scholar] [CrossRef]
- Duan, Z.; Cai, G.; Li, J.; Chen, X. Cisplatin-induced renal toxicity in elderly people. Ther. Adv. Med. Oncol. 2020, 12, 1758835920923430. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Al-malky, H.S.; Al Harthi, S.E.; Osman, A.-M.M. Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance. J. Oncol. Pharm. Pract. 2020, 26, 434–444. [Google Scholar] [CrossRef]
- Ling, G.; Wang, X.; Tan, N.; Cao, J.; Li, W.; Zhang, Y.; Jiang, J.; Sun, Q.; Jiang, Y.; Wang, W.; et al. Mechanisms and Drug Intervention for Doxorubicin-Induced Cardiotoxicity Based on Mitochondrial Bioenergetics. Oxid. Med. Cell. Longev. 2022, 2022, 7176282. [Google Scholar] [CrossRef]
- Watson, C.; Gadikota, H.; Barlev, A.; Beckerman, R. A review of the risks of long-term consequences associated with components of the CHOP chemotherapy regimen. J. Drug Assess. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Kopacz-Bednarska, A.; Król, T. Cisplatin—Properties and clinical application. Oncol. Clin. Pract. 2022, 18, 166–176. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Ozaki, T. Diagnosis and treatment of Ewing sarcoma of the bone: A review article. J. Orthop. Sci. 2015, 20, 250–263. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; von Woedtke, T.; Hähnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Kong, H.; Chandel, N.S. Regulation of redox balance in cancer and T cells. J. Biol. Chem. 2018, 293, 7499–7507. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Kilmer, S.; Semchyshyn, N.; Shah, G.; Fitzpatrick, R. A pilot study on the use of a plasma skin regeneration device (Portrait PSR3) in full facial rejuvenation procedures. Lasers Med. Sci. 2007, 22, 101–109. [Google Scholar] [CrossRef] [PubMed]
- von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K. Introduction to plasma medicine. In Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–21. [Google Scholar]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef] [PubMed]
- Klebes, M.; Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Richter, H.; Bob, A.; von Hutten, J.; Krediet, J.T.; Kramer, A.; et al. Combined antibacterial effects of tissue-tolerable plasma and a modern conventional liquid antiseptic on chronic wound treatment. J. Biophotonics 2015, 8, 382–391. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients with Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Strohal, R.; Dietrich, S.; Mittlböck, M.; Hämmerle, G. Chronic wounds treated with cold atmospheric plasmajet versus best practice wound dressings: A multicenter, randomized, non-inferiority trial. Sci. Rep. 2022, 12, 3645. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Faramarzi, F.; Zafari, P.; Alimohammadi, M.; Moonesi, M.; Rafiei, A.; Bekeschus, S. Cold Physical Plasma in Cancer Therapy: Mechanisms, Signaling, and Immunity. Oxid. Med. Cell. Longev. 2021, 2021, 9916796. [Google Scholar] [CrossRef]
- Pasqual-Melo, G.; Gandhirajan, R.K.; Stoffels, I.; Bekeschus, S. Targeting malignant melanoma with physical plasmas. Clin. Plasma Med. 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Rafiei, A.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Alimohammadi, M.; Valadan, R. Inhibition of murine melanoma tumor growth in vitro and in vivo using an argon-based plasma jet. Clin. Plasma Med. 2020, 19–20, 100102. [Google Scholar] [CrossRef]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef]
- Yazdani, Z.; Mehrabanjoubani, P.; Biparva, P.; Rafiei, A. Cytotoxicity effect of cold atmospheric plasma on melanoma (B16-F10), breast (MCF-7) and lung (A549) cancer cell lines compared with normal cells. J. Maz. Univ. Med. Sci. 2020, 30, 38–48. [Google Scholar]
- Chupradit, S.; Widjaja, G.; Radhi Majeed, B.; Kuznetsova, M.; Ansari, M.J.; Suksatan, W.; Turki Jalil, A.; Ghazi Esfahani, B. Recent advances in cold atmospheric plasma (CAP) for breast cancer therapy. Cell. Biol. Int. 2023, 47, 327–340. [Google Scholar] [CrossRef]
- Chen, Z.; Simonyan, H.; Cheng, X.; Gjika, E.; Lin, L.; Canady, J.; Sherman, J.H.; Young, C.; Keidar, M. A Novel Micro Cold Atmospheric Plasma Device for Glioblastoma Both In Vitro and In Vivo. Cancers 2017, 9, 61. [Google Scholar] [CrossRef]
- Adhikari, M.; Adhikari, B.; Adhikari, A.; Yan, D.; Soni, V.; Sherman, J.; Keidar, M. Cold Atmospheric Plasma as a Novel Therapeutic Tool for the Treatment of Brain Cancer. Curr. Pharm. Des. 2020, 26, 2195–2206. [Google Scholar] [CrossRef]
- Kim, C.H.; Bahn, J.H.; Lee, S.H.; Kim, G.Y.; Jun, S.I.; Lee, K.; Baek, S.J. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J. Biotechnol. 2010, 150, 530–538. [Google Scholar] [CrossRef]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.D.; Ostrikov, K.K. Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by Nox2-ASK1 apoptosis pathways and oxidative stress is mitigated by Srx-Nrf2 anti-oxidant system. Biochim. Biophys. Acta 2014, 1843, 2827–2837. [Google Scholar] [CrossRef]
- Nitsch, A.; Sander, C.; Eggers, B.; Weiss, M.; Egger, E.; Kramer, F.J.; Erb, H.H.H.; Mustea, A.; Stope, M.B. Pleiotropic Devitalization of Renal Cancer Cells by Non-Invasive Physical Plasma: Characterization of Molecular and Cellular Efficacy. Cancers 2023, 15, 481. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Tan, X.; Zhao, S.; Lei, Q.; Lu, X.; He, G.; Ostrikov, K. Single-cell-precision microplasma-induced cancer cell apoptosis. PLoS ONE 2014, 9, e101299. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, M.; Waldbeser, L.; Whitmill, A. THP-1 leukemia cancer treatment using a portable plasma device. Stud. Health Technol. Inform. 2012, 173, 515–517. [Google Scholar] [PubMed]
- Sato, Y.; Yamada, S.; Takeda, S.; Hattori, N.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Hori, M.; Kodera, Y. Effect of Plasma-Activated Lactated Ringer’s Solution on Pancreatic Cancer Cells In Vitro and In Vivo. Ann. Surg. Oncol. 2018, 25, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Yamada, S.; Torii, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fujii, T.; Nakayama, G.; et al. Effectiveness of plasma treatment on pancreatic cancer cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef]
- Tornin, J.; Mateu-Sanz, M.; Rodríguez, A.; Labay, C.; Rodríguez, R.; Canal, C. Pyruvate Plays a Main Role in the Antitumoral Selectivity of Cold Atmospheric Plasma in Osteosarcoma. Sci. Rep. 2019, 9, 10681. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Tornín, J.; Ginebra, M.P.; Canal, C. Cold Atmospheric Plasma: A New Strategy Based Primarily on Oxidative Stress for Osteosarcoma Therapy. J. Clin. Med. 2021, 10, 893. [Google Scholar] [CrossRef]
- Ermakov, A.M.; Ermakova, O.N.; Afanasyeva, V.A.; Popov, A.L. Dose-Dependent Effects of Cold Atmospheric Argon Plasma on the Mesenchymal Stem and Osteosarcoma Cells In Vitro. Int. J. Mol. Sci. 2021, 22, 6797. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Tornín, J.; Brulin, B.; Khlyustova, A.; Ginebra, M.P.; Layrolle, P.; Canal, C. Cold Plasma-Treated Ringer’s Saline: A Weapon to Target Osteosarcoma. Cancers 2020, 12, 227. [Google Scholar] [CrossRef]
- Jacoby, J.M.; Strakeljahn, S.; Nitsch, A.; Bekeschus, S.; Hinz, P.; Mustea, A.; Ekkernkamp, A.; Tzvetkov, M.V.; Haralambiev, L.; Stope, M.B. An Innovative Therapeutic Option for the Treatment of Skeletal Sarcomas: Elimination of Osteo- and Ewing’s Sarcoma Cells Using Physical Gas Plasma. Int. J. Mol. Sci. 2020, 21, 4460. [Google Scholar] [CrossRef]
- Haralambiev, L.; Nitsch, A.; Jacoby, J.M.; Strakeljahn, S.; Bekeschus, S.; Mustea, A.; Ekkernkamp, A.; Stope, M.B. Cold Atmospheric Plasma Treatment of Chondrosarcoma Cells Affects Proliferation and Cell Membrane Permeability. Int. J. Mol. Sci. 2020, 21, 2291. [Google Scholar] [CrossRef]
- Nitsch, A.; Strakeljahn, S.; Jacoby, J.M.; Sieb, K.F.; Mustea, A.; Bekeschus, S.; Ekkernkamp, A.; Stope, M.B.; Haralambiev, L. New Approach against Chondrosoma Cells-Cold Plasma Treatment Inhibits Cell Motility and Metabolism, and Leads to Apoptosis. Biomedicines 2022, 10, 688. [Google Scholar] [CrossRef]
- Soni, V.; Adhikari, M.; Simonyan, H.; Lin, L.; Sherman, J.H.; Young, C.N.; Keidar, M. In Vitro and In Vivo Enhancement of Temozolomide Effect in Human Glioblastoma by Non-Invasive Application of Cold Atmospheric Plasma. Cancers 2021, 13, 4485. [Google Scholar] [CrossRef]
- Yao, X.; Yan, D.; Lin, L.; Sherman, J.H.; Peters, K.B.; Keir, S.T.; Keidar, M. Cold Plasma Discharge Tube Enhances Antitumoral Efficacy of Temozolomide. ACS Appl. Bio Mater. 2022, 5, 1610–1623. [Google Scholar] [CrossRef]
- Pomeroy, A.E.; Schmidt, E.V.; Sorger, P.K.; Palmer, A.C. Drug independence and the curability of cancer by combination chemotherapy. Trends Cancer 2022, 8, 915–929. [Google Scholar] [CrossRef]
- Meads, M.B.; Hazlehurst, L.A.; Dalton, W.S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin. Cancer Res. 2008, 14, 2519–2526. [Google Scholar] [CrossRef]
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef]
- Zraik, I.M.; Heß-Busch, Y. Management von Nebenwirkungen der Chemotherapie und deren Langzeitfolgen. Urologe 2021, 60, 862–871. [Google Scholar] [CrossRef]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Calvagna, M. Chemotherapy for Cancer Treatment. Available online: https://www.wnyurology.com/content.aspx?chunkiid=32632 (accessed on 2 February 2023).
- Greene, W.; Huffman, D.; Wiernik, P.H.; Schimpff, S.; Benjamin, R.; Bachur, N. High-dose daunorubicin therapy for acute nonlymphocytic leukemia: Correlation of response and toxicity with pharmacokinetics and intracellular daunorubicin reductase activity. Cancer 1972, 30, 1419–1427. [Google Scholar] [CrossRef]
- Holland, J.F.; Glidewell, O. Chemotherapy of acute lymphocytic leukemia of childhood. Cancer 1972, 30, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Soloway, M.S.; Hardeman, S.W.; Hickey, D.; Raymond, J.; Todd, B.; Soloway, S.; Moinuddin, M. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988, 61, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Antman, K.H.; Ryan, L.; Elias, A.; Sherman, D.; Grier, H.E. Response to ifosfamide and mesna: 124 previously treated patients with metastatic or unresectable sarcoma. J. Clin. Oncol. 1989, 7, 126–131. [Google Scholar] [CrossRef]
- Jürgens, H.; Exner, U.; Kühl, J.; Ritter, J.; Treuner, J.; Weinel, P.; Winkler, K.; Göbel, U. High-dose ifosfamide with mesna uroprotection in Ewing’s sarcoma. Cancer Chemother. Pharmacol. 1989, 24, S40–S44. [Google Scholar] [CrossRef] [PubMed]
- Miser, J.S.; Kinsella, T.; Triche, T.; Tsokos, M.; Jarosinski, P.; Forquer, R.; Wesley, R.; Magrath, I. Ifosfamide with mesna uroprotection and etoposide: An effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. J. Clin. Oncol. 1987, 5, 1191–1198. [Google Scholar] [CrossRef]
- Paladugu, R.R.; Benfield, J.R.; Pak, H.Y.; Ross, R.K.; Teplitz, R.L. Bronchopulmonary Kulchitzky cell carcinomas. A new classification scheme for typical and atypical carcinoids. Cancer 1985, 55, 1303–1311. [Google Scholar] [CrossRef]
- Gay-Mimbrera, J.; García, M.C.; Isla-Tejera, B.; Rodero-Serrano, A.; García-Nieto, A.V.; Ruano, J. Clinical and Biological Principles of Cold Atmospheric Plasma Application in Skin Cancer. Adv. Ther. 2016, 33, 894–909. [Google Scholar] [CrossRef]
- Ibañez, I.L.; Notcovich, C.; Catalano, P.N.; Bellino, M.G.; Durán, H. The redox-active nanomaterial toolbox for cancer therapy. Cancer Lett. 2015, 359, 9–19. [Google Scholar] [CrossRef]
- Murillo, D.; Huergo, C.; Gallego, B.; Rodríguez, R.; Tornín, J. Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines 2023, 11, 208. [Google Scholar] [CrossRef]
- Rasouli, M.; Mehdian, H.; Hajisharifi, K.; Amini, E.; Ostrikov, K.; Robert, E. Plasma-activated medium induces apoptosis in chemotherapy-resistant ovarian cancer cells: High selectivity and synergy with carboplatin. Plasma Process. Polym. 2021, 18, 2100074. [Google Scholar] [CrossRef]
- Brunner, T.F.; Probst, F.A.; Troeltzsch, M.; Schwenk-Zieger, S.; Zimmermann, J.L.; Morfill, G.; Becker, S.; Harréus, U.; Welz, C. Primary cold atmospheric plasma combined with low dose cisplatin as a possible adjuvant combination therapy for HNSCC cells—An in-vitro study. Head Face Med. 2022, 18, 21. [Google Scholar] [CrossRef]
- Daeschlein, G.; Hillmann, A.; Gümbel, D.; Sicher, C.; Podewils, S.v.; Stope, M.B.; Jünger, M. Enhanced Anticancer Efficacy by Drug Chemotherapy and Cold Atmospheric Plasma Against Melanoma and Glioblastoma Cell Lines In Vitro. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 153–159. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Kirschner, M.E.; Lin, L.; Sherman, J.H.; Stepp, M.A.; Keidar, M. Combination therapy of cold atmospheric plasma (CAP) with temozolomide in the treatment of U87MG glioblastoma cells. Sci. Rep. 2020, 10, 16495. [Google Scholar] [CrossRef]
- Conway, G.E.; Casey, A.; Milosavljevic, V.; Liu, Y.; Howe, O.; Cullen, P.J.; Curtin, J.F. Non-thermal atmospheric plasma induces ROS-independent cell death in U373MG glioma cells and augments the cytotoxicity of temozolomide. Br. J. Cancer 2016, 114, 435–443. [Google Scholar] [CrossRef]
- Pefani-Antimisiari, K.; Athanasopoulos, D.K.; Marazioti, A.; Sklias, K.; Rodi, M.; de Lastic, A.-L.; Mouzaki, A.; Svarnas, P.; Antimisiaris, S.G. Synergistic effect of cold atmospheric pressure plasma and free or liposomal doxorubicin on melanoma cells. Sci. Rep. 2021, 11, 14788. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Pasqual-Melo, G.; Bodnar, Y.; Gandhirajan, R.K.; Bekeschus, S. Combination of chemotherapy and physical plasma elicits melanoma cell death via upregulation of SLC22A16. Cell Death Dis. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef]
- Sarangapani, C.; Ziuzina, D.; Behan, P.; Boehm, D.; Gilmore, B.F.; Cullen, P.; Bourke, P. Degradation kinetics of cold plasma-treated antibiotics and their antimicrobial activity. Sci. Rep. 2019, 9, 3955. [Google Scholar] [CrossRef]
- Liedtke, K.-R.; Freund, E.; Hermes, M.; Oswald, S.; Heidecke, C.-D.; Partecke, L.-I.; Bekeschus, S. Gas Plasma-Conditioned Ringer’s Lactate Enhances the Cytotoxic Activity of Cisplatin and Gemcitabine in Pancreatic Cancer In Vitro and In Ovo. Cancers 2020, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Mateu-Sanz, M.; Ginebra, M.-P.; Tornín, J.; Canal, C. Cold atmospheric plasma enhances doxorubicin selectivity in metastasic bone cancer. Free. Radic. Biol. Med. 2022, 189, 32–41. [Google Scholar] [CrossRef]
- Haralambiev, L.; Bandyophadyay, A.; Suchy, B.; Weiss, M.; Kramer, A.; Bekeschus, S.; Ekkernkamp, A.; Mustea, A.; Kaderali, L.; Stope, M.B. Determination of Immediate vs. Kinetic Growth Retardation in Physically Plasma-treated Cells by Experimental and Modelling Data. Anticancer Res. 2020, 40, 3743–3749. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Cai, D.; Ning, M.; Yu, L.; Zhang, Z.; Han, P.; Dai, X. Cold atmospheric plasma selectively induces G0/G1 cell cycle arrest and apoptosis in AR-independent prostate cancer cells. J. Cancer 2021, 12, 5977–5986. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mang, X.; Li, X.; Cai, Z.; Tan, F. Cold atmospheric plasma induces apoptosis in human colon and lung cancer cells through modulating mitochondrial pathway. Front. Cell Dev. Biol. 2022, 10, 915785. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zeng, W.; Xia, Y.; Wang, B.; Xu, D.; Liu, D.; Kong, M.G.; Dong, Y. Cold atmospheric plasma induces apoptosis of melanoma cells via Sestrin2-mediated nitric oxide synthase signaling. J. Biophotonics 2019, 12, e201800046. [Google Scholar] [CrossRef]
- Haralambiev, L.; Wien, L.; Gelbrich, N.; Lange, J.; Bakir, S.; Kramer, A.; Burchardt, M.; Ekkernkamp, A.; Gümbel, D.; Stope, M.B. Cold atmospheric plasma inhibits the growth of osteosarcoma cells by inducing apoptosis, independent of the device used. Oncol. Lett. 2020, 19, 283–290. [Google Scholar] [CrossRef]
- Xu, S.; Wang, Y.; Que, Y.; Ma, C.; Cai, S.; Wang, H.; Yang, X.; Yang, C.; Cheng, C.; Zhao, G.; et al. Cold atmospheric plasma-activated Ringer’s solution inhibits the proliferation of osteosarcoma cells through the mitochondrial apoptosis pathway. Oncol. Rep. 2020, 43, 1683–1691. [Google Scholar] [CrossRef]
- Limanowski, R.; Yan, D.; Li, L.; Keidar, M. Preclinical Cold Atmospheric Plasma Cancer Treatment. Cancers 2022, 14, 3461. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.-D. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Cranio-Maxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Friedman, P.C.; Miller, V.; Fridman, G.; Lin, A.; Fridman, A. Successful treatment of actinic keratoses using nonthermal atmospheric pressure plasma: A case series. J. Am. Acad. Dermatol. 2017, 76, 349–350. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitsch, A.; Qarqash, S.; Römer, S.; Schoon, J.; Ekkernkamp, A.; Niethard, M.; Reichert, J.C.; Wassilew, G.I.; Tzvetkov, M.V.; Haralambiev, L. Enhancing the Impact of Chemotherapy on Ewing Sarcoma Cells through Combination with Cold Physical Plasma. Int. J. Mol. Sci. 2023, 24, 8669. https://doi.org/10.3390/ijms24108669
Nitsch A, Qarqash S, Römer S, Schoon J, Ekkernkamp A, Niethard M, Reichert JC, Wassilew GI, Tzvetkov MV, Haralambiev L. Enhancing the Impact of Chemotherapy on Ewing Sarcoma Cells through Combination with Cold Physical Plasma. International Journal of Molecular Sciences. 2023; 24(10):8669. https://doi.org/10.3390/ijms24108669
Chicago/Turabian StyleNitsch, Andreas, Sara Qarqash, Sarah Römer, Janosch Schoon, Axel Ekkernkamp, Maya Niethard, Johannes C. Reichert, Georgi I. Wassilew, Mladen V. Tzvetkov, and Lyubomir Haralambiev. 2023. "Enhancing the Impact of Chemotherapy on Ewing Sarcoma Cells through Combination with Cold Physical Plasma" International Journal of Molecular Sciences 24, no. 10: 8669. https://doi.org/10.3390/ijms24108669
APA StyleNitsch, A., Qarqash, S., Römer, S., Schoon, J., Ekkernkamp, A., Niethard, M., Reichert, J. C., Wassilew, G. I., Tzvetkov, M. V., & Haralambiev, L. (2023). Enhancing the Impact of Chemotherapy on Ewing Sarcoma Cells through Combination with Cold Physical Plasma. International Journal of Molecular Sciences, 24(10), 8669. https://doi.org/10.3390/ijms24108669




