Applications and Opportunities in Using Disulfides, Thiosulfinates, and Thiosulfonates as Antibacterials
Abstract
1. Introduction
2. Early Studies on Naturally-Occurring Sulfur Compounds with Antimicrobial Properties
3. Disulfides
3.1. Ajoene
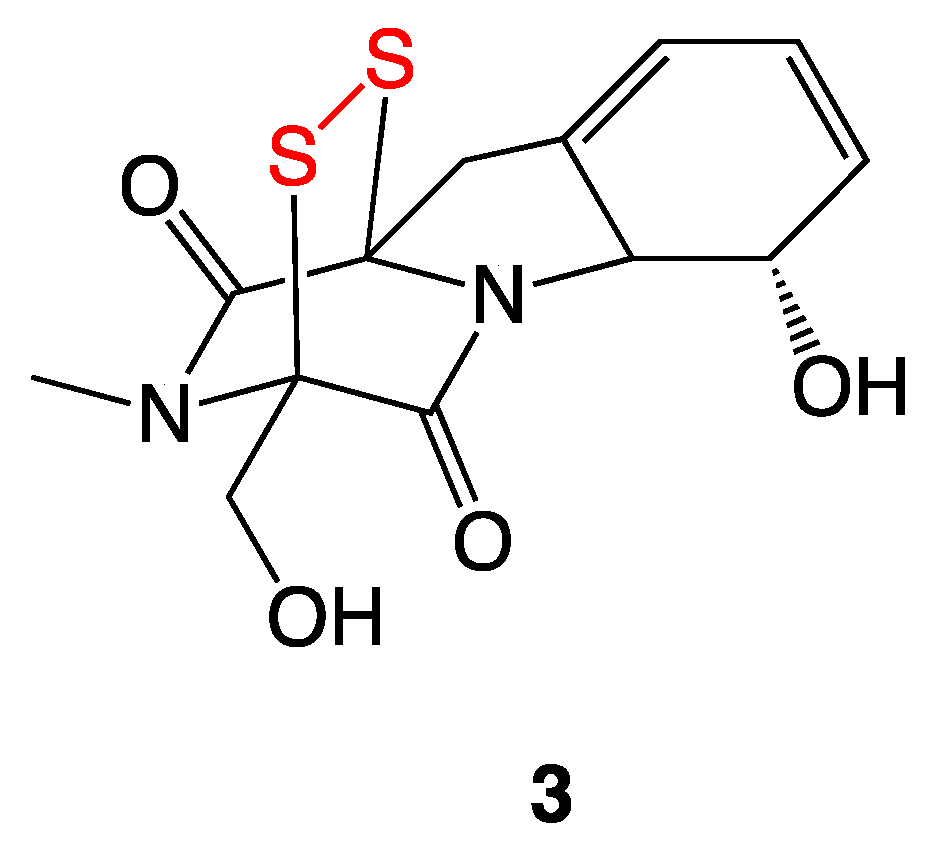
3.2. Gliotoxin
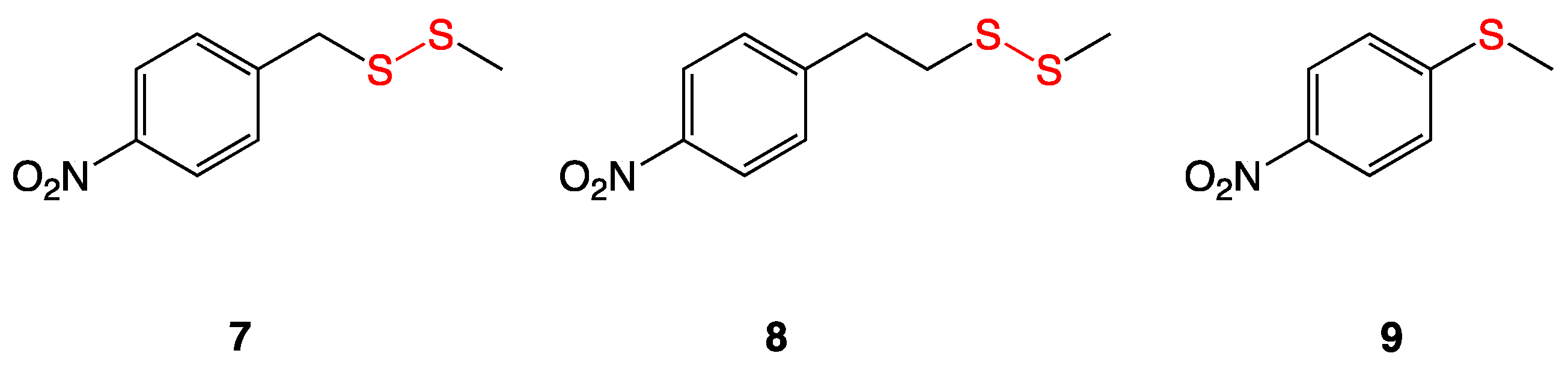
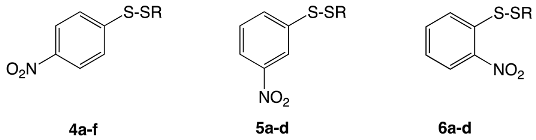
3.3. Aryl-Alkyl Disulfides
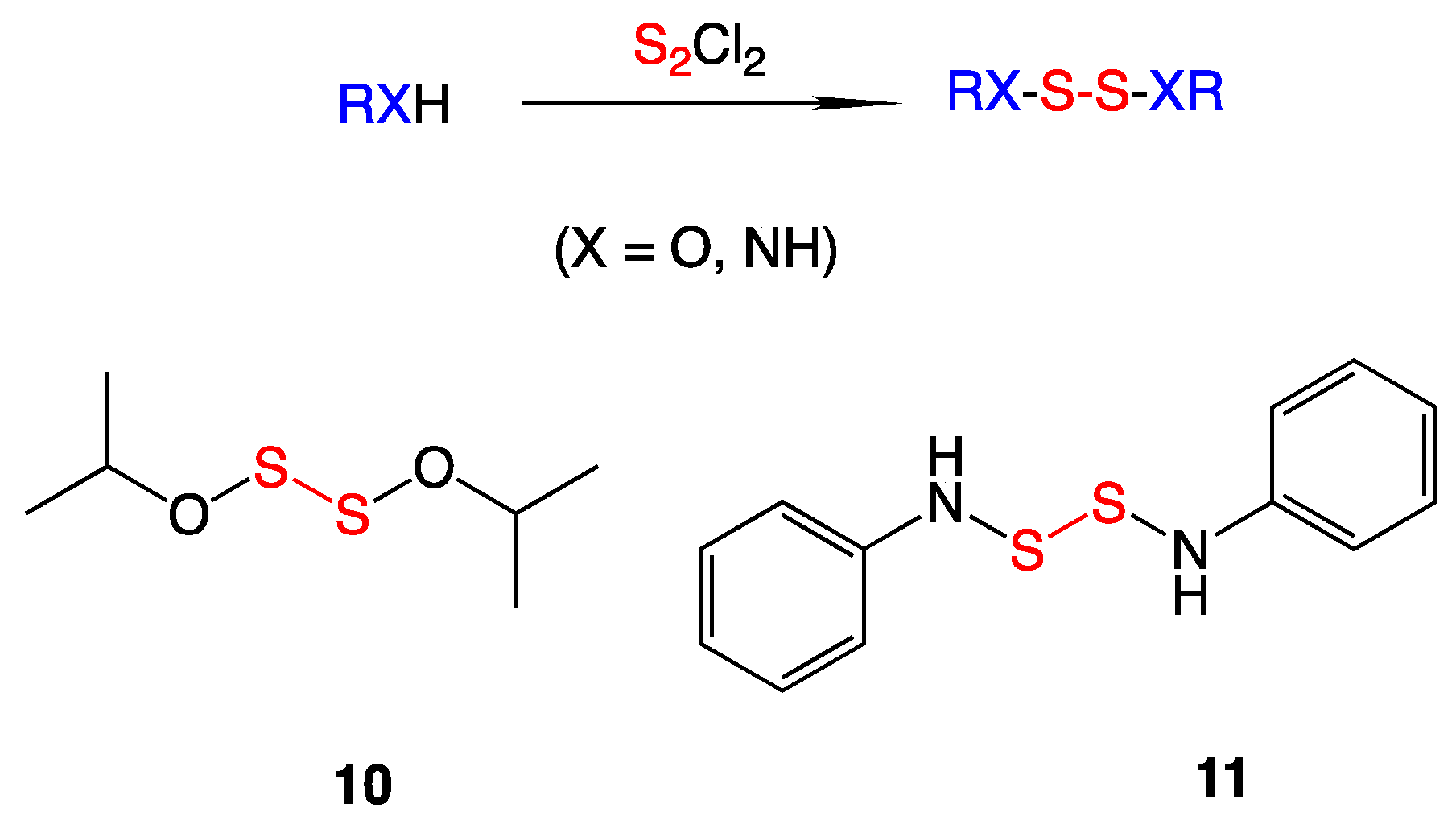
3.4. S,S′-bis(Heterosubstituted) Disulfides
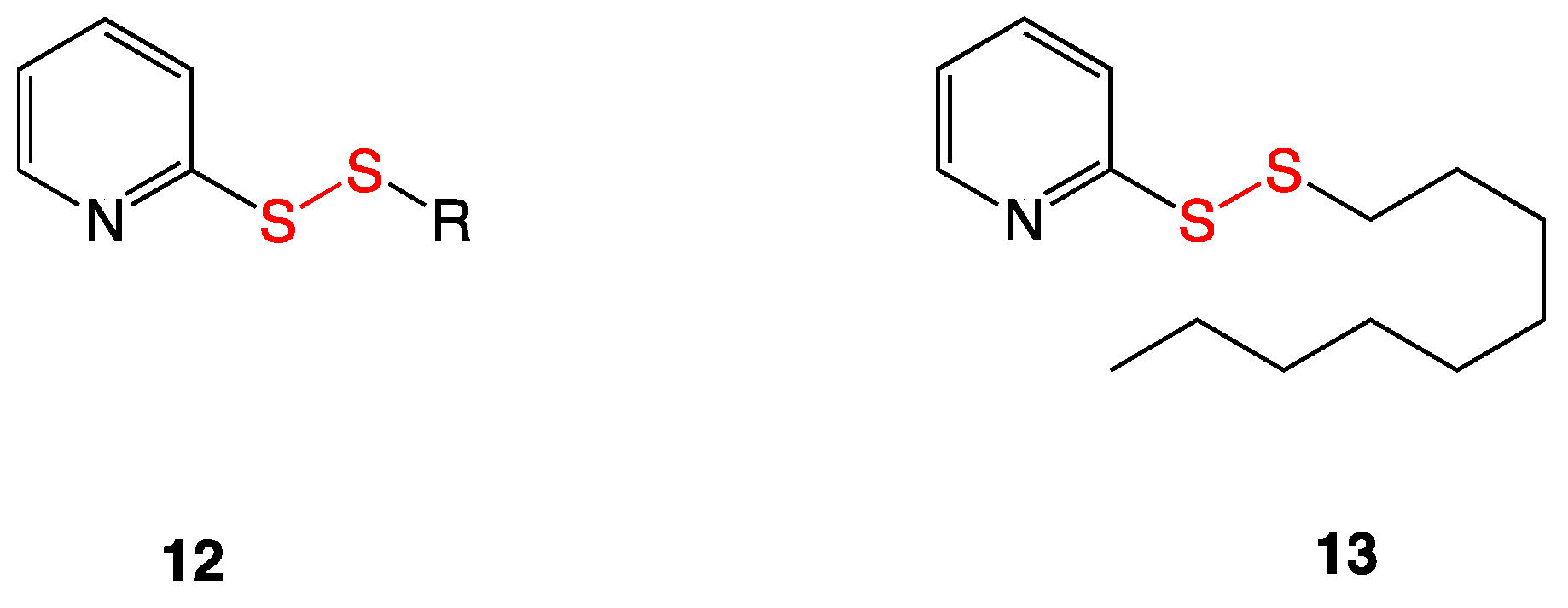
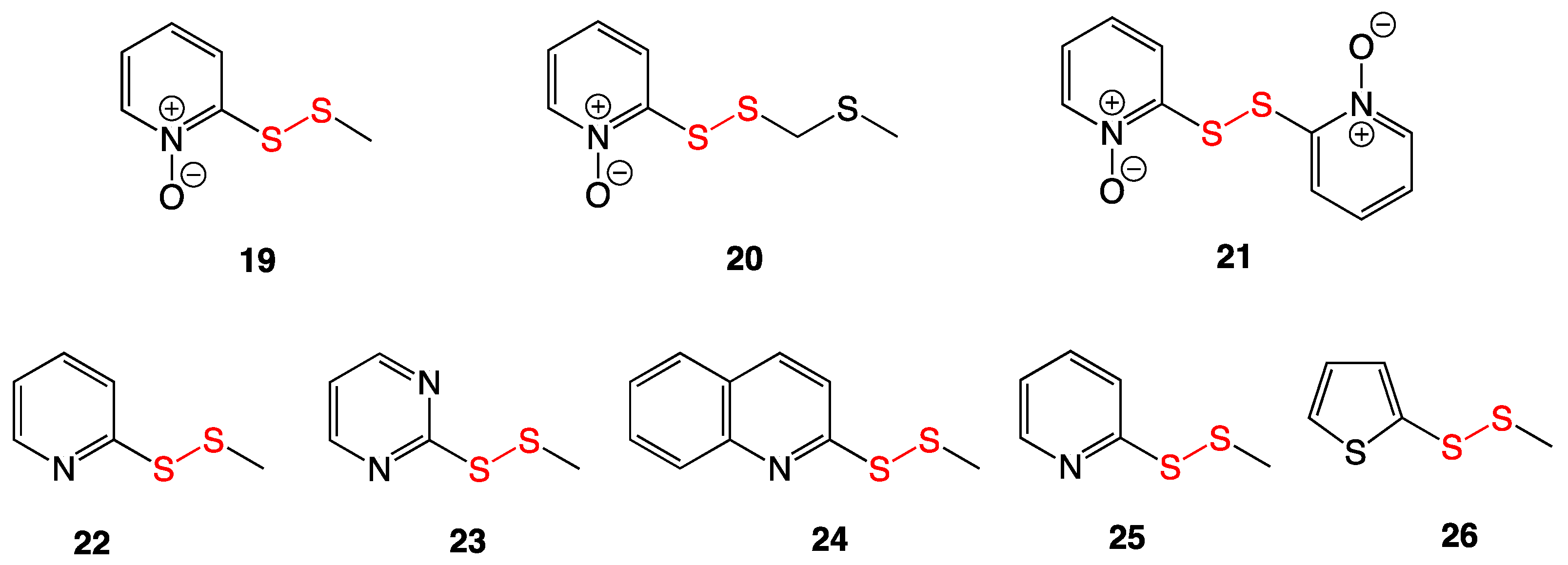
3.5. Pyridyl Disulfides
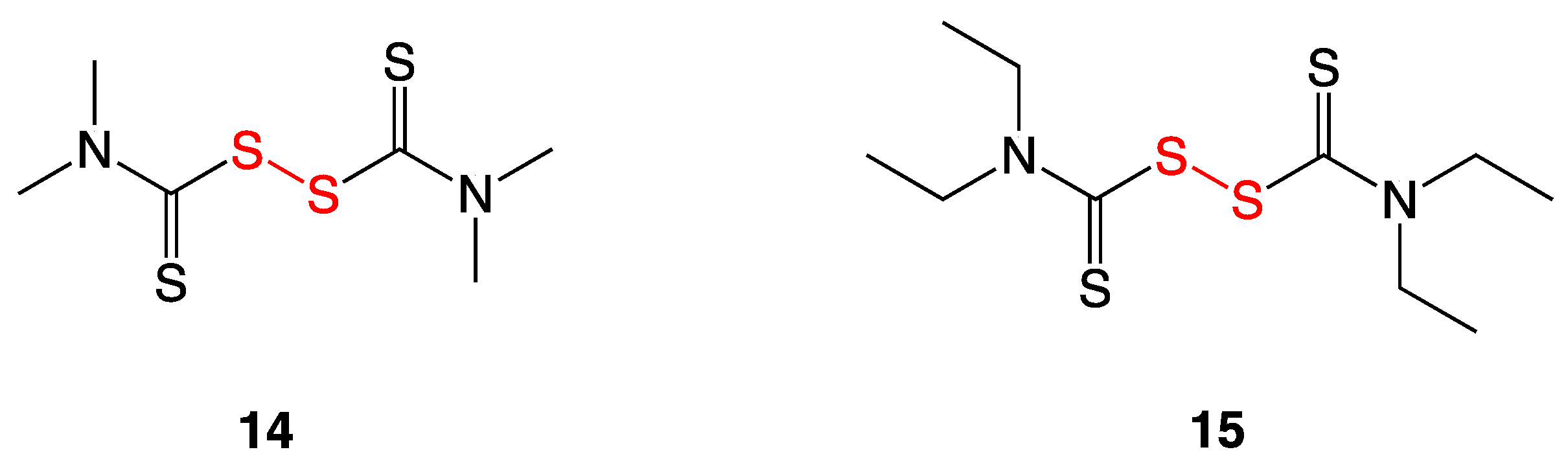
3.6. Thiuram and Disulfiram
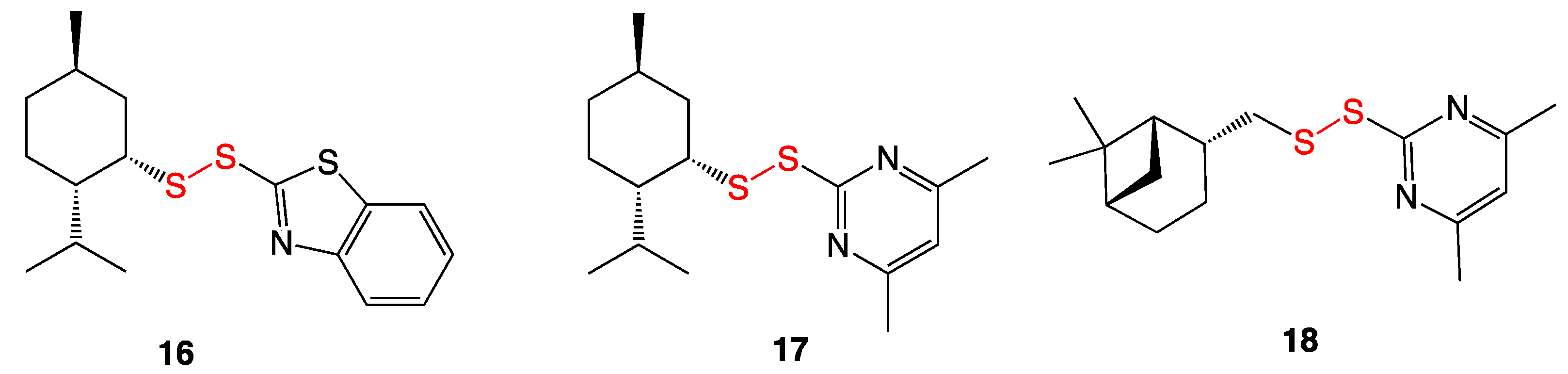
3.7. Unsymmetrical Monoterpenylheteroaryl Disulfides
3.8. Pyridine-N-oxide Disulfides
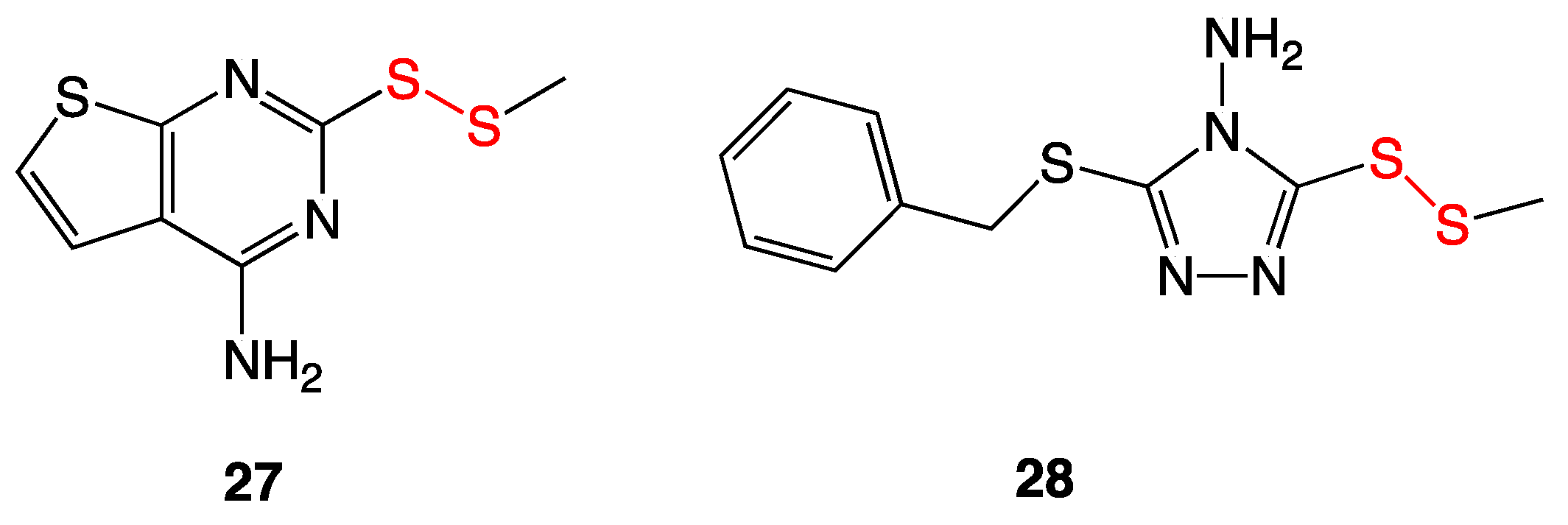
3.9. Aromatic and Heterocyclic Methyl Disulfides
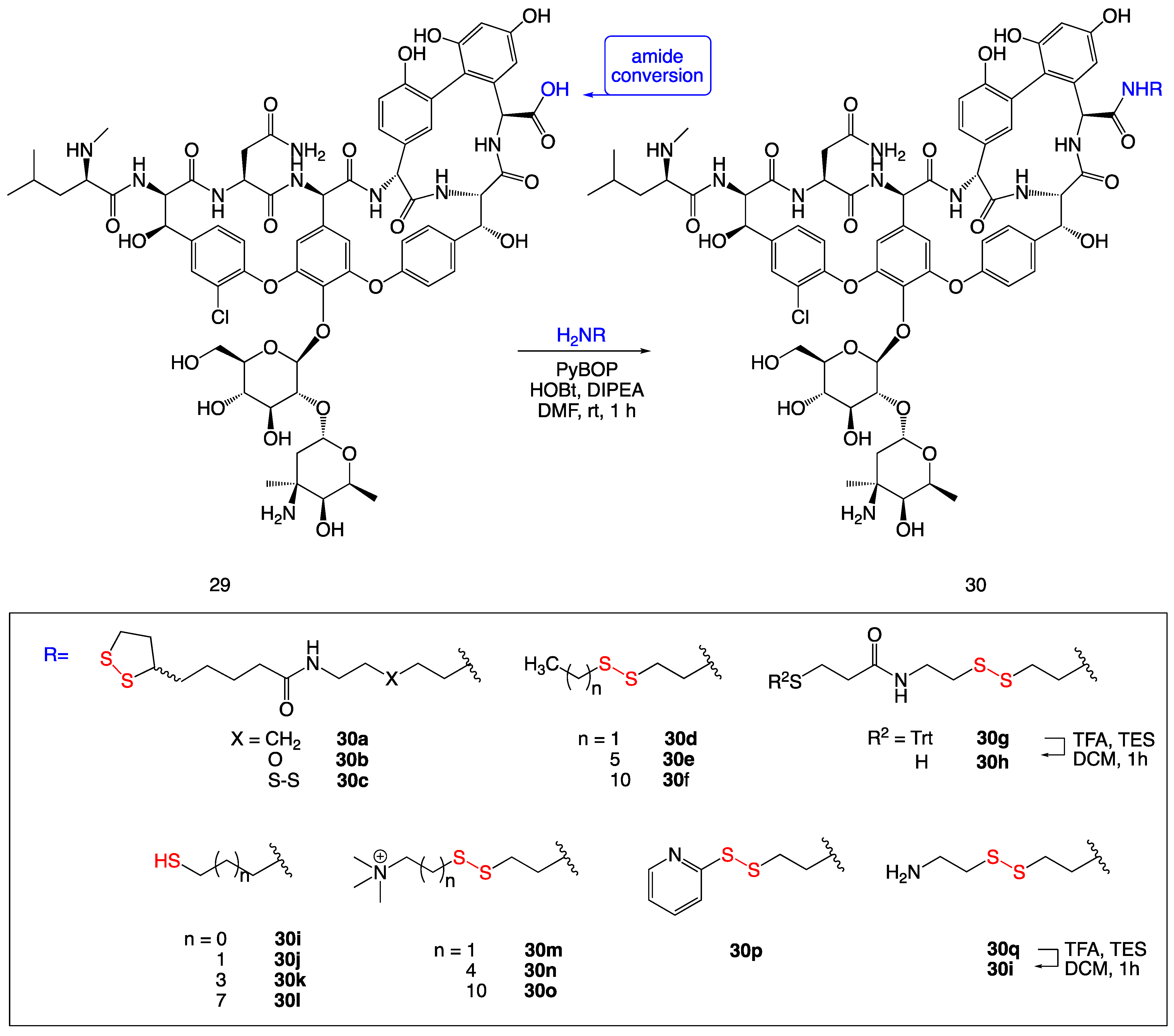
3.10. Disulfide-Containing Vancomycin Derivatives
3.11. Calicheamicins
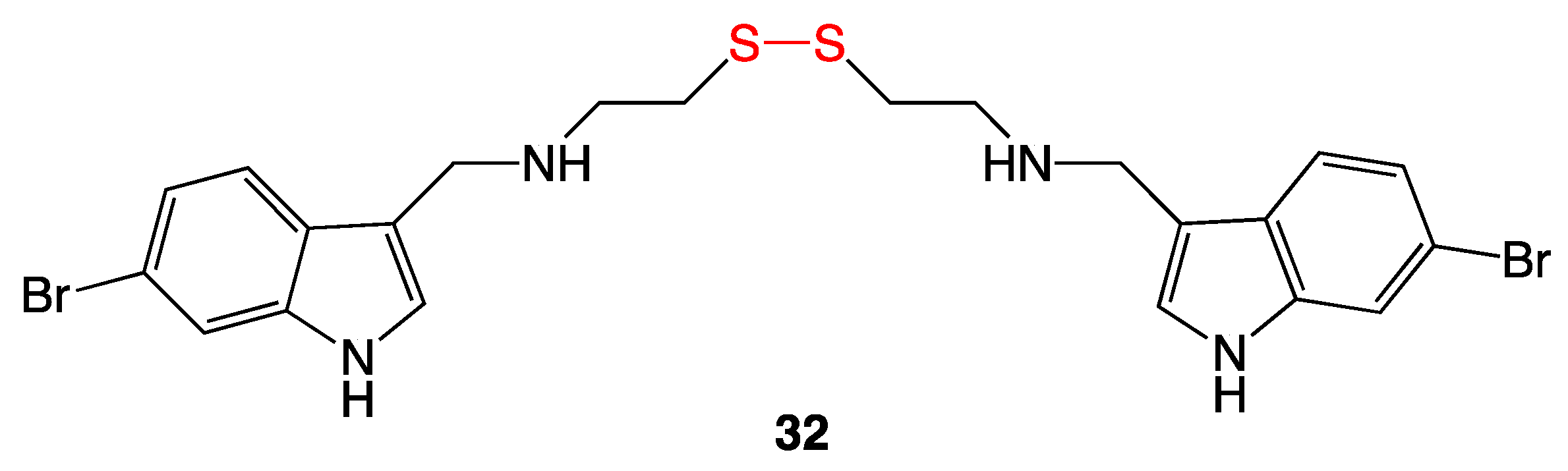
3.12. Marine Disulfide Natural Products
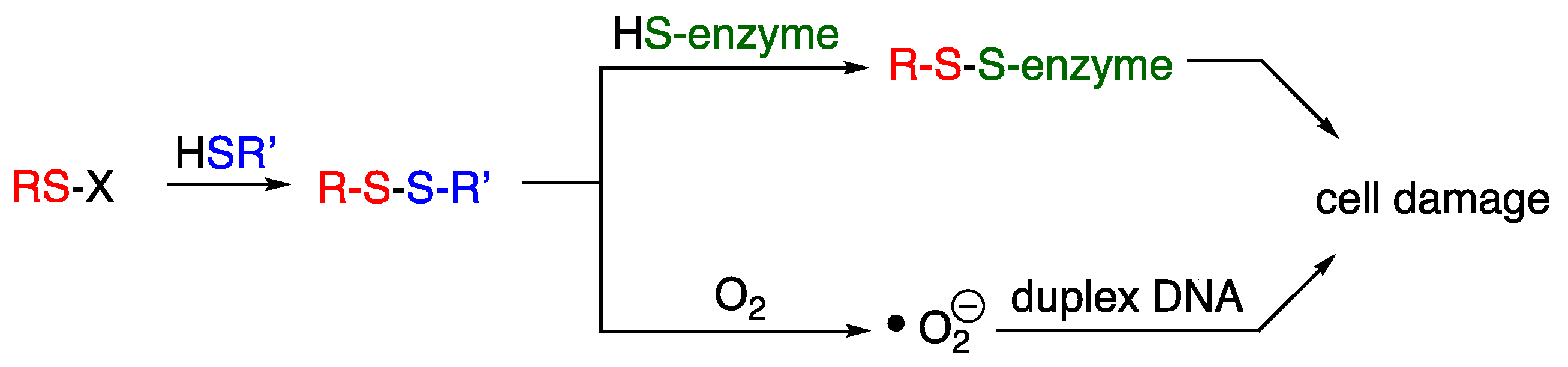
4. Thiosulfinates
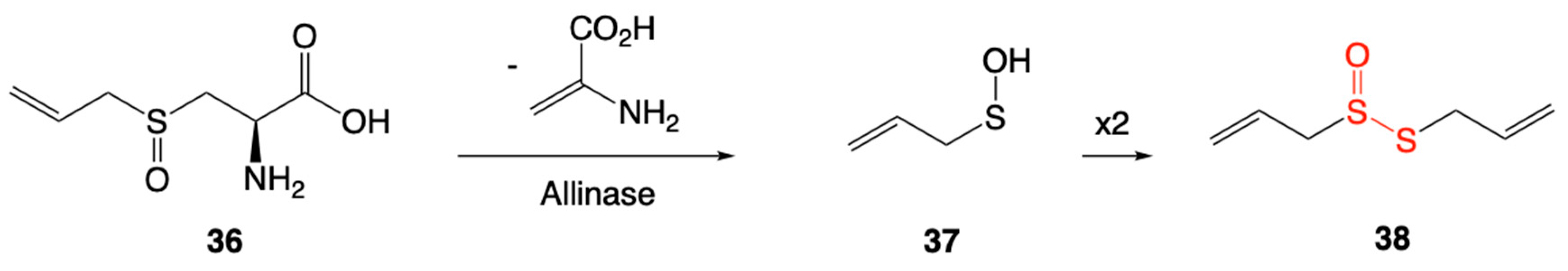
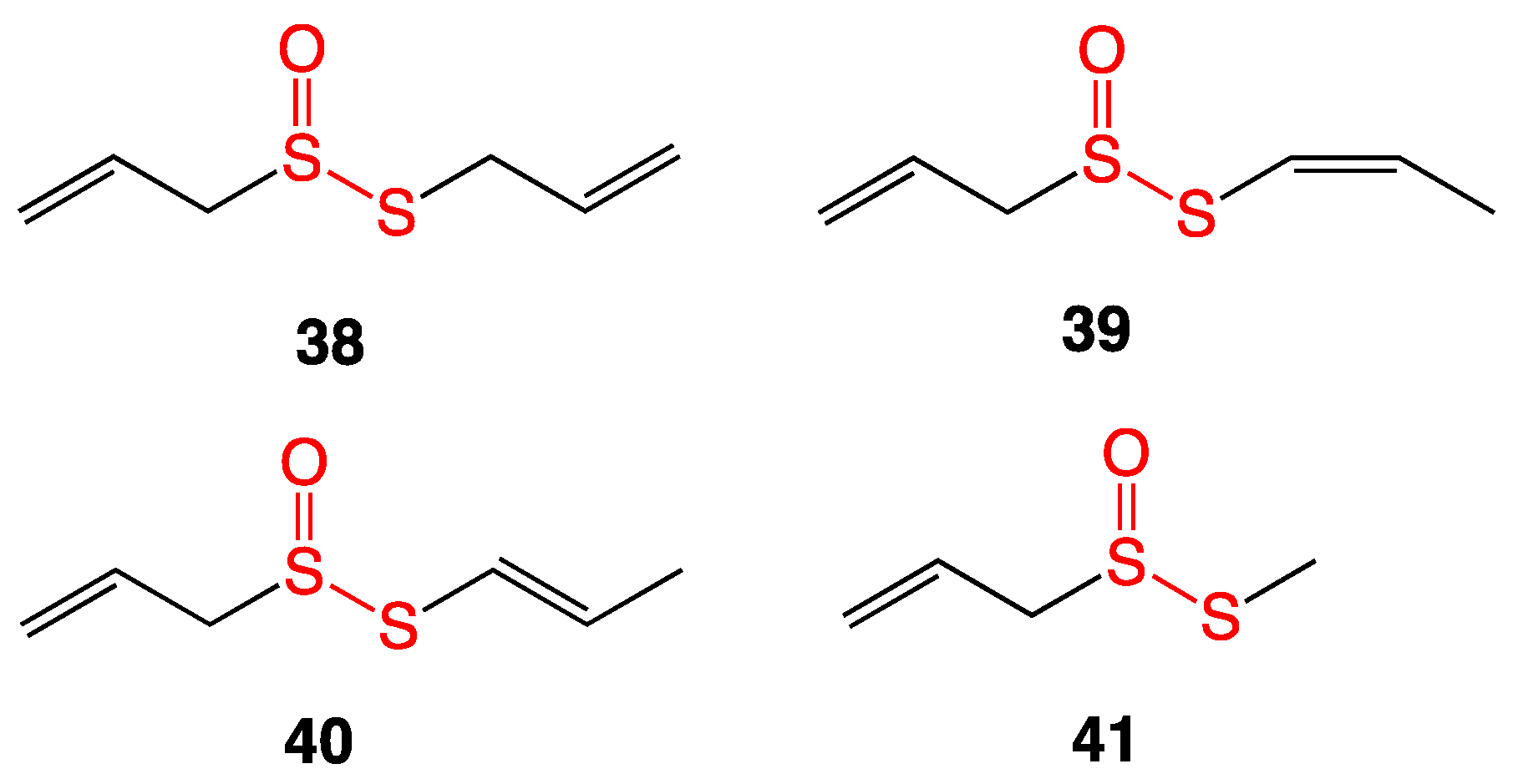
4.1. Thiosulfinates from Garlic Extract
4.2. Synthetic Allicin Derivatives
4.3. Leinamycin
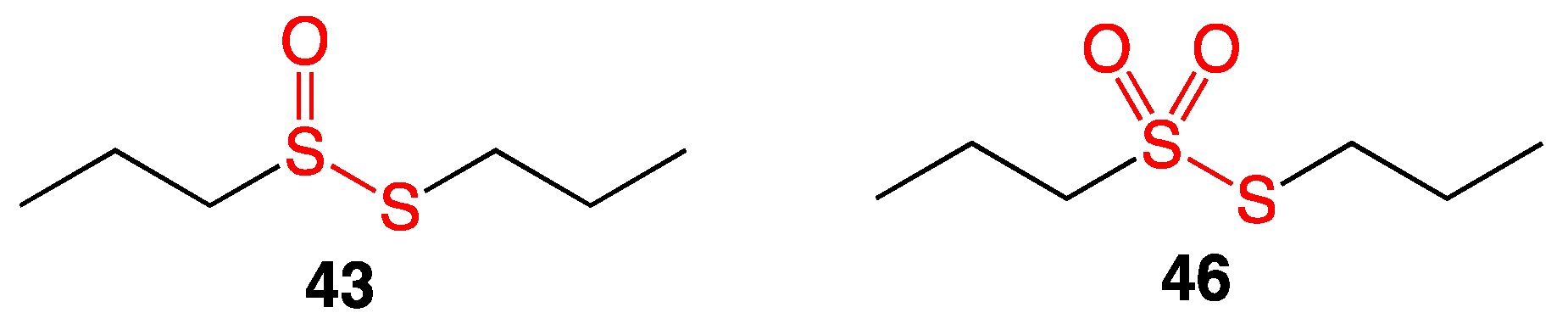
5. Thiosulfonates
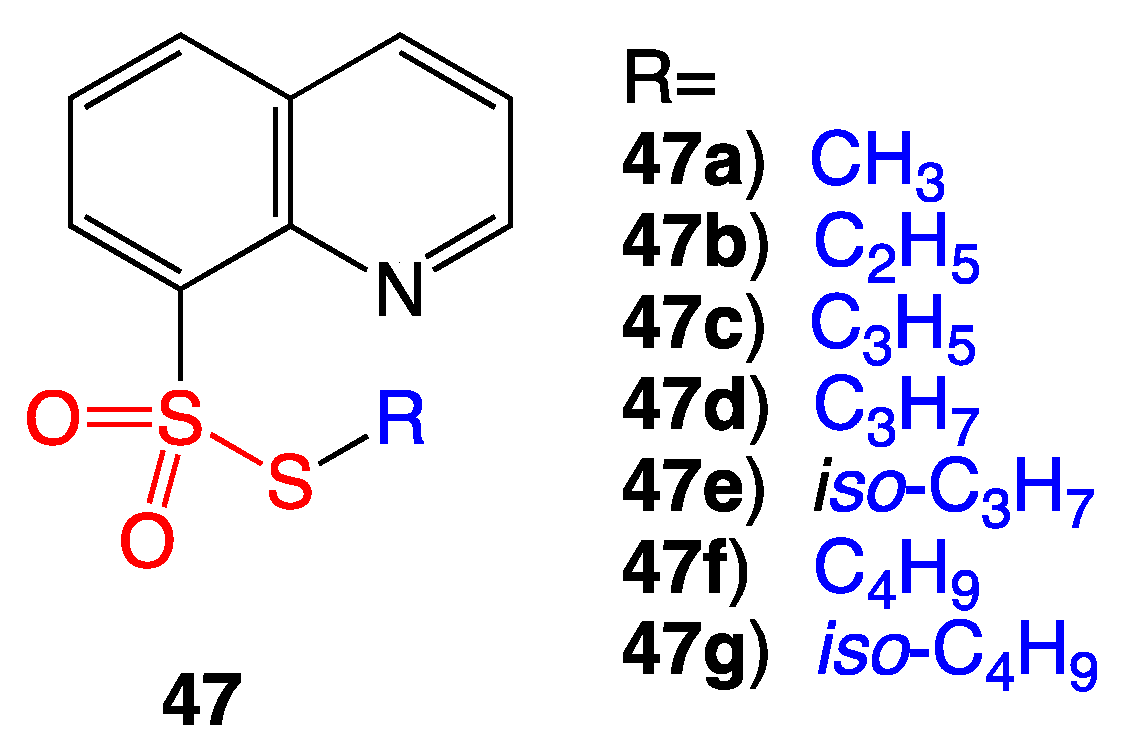
5.1. Quinoline Thiosulfonate Derivatives
5.2. Topical Thiosulfonate and Biosurfactant Combination
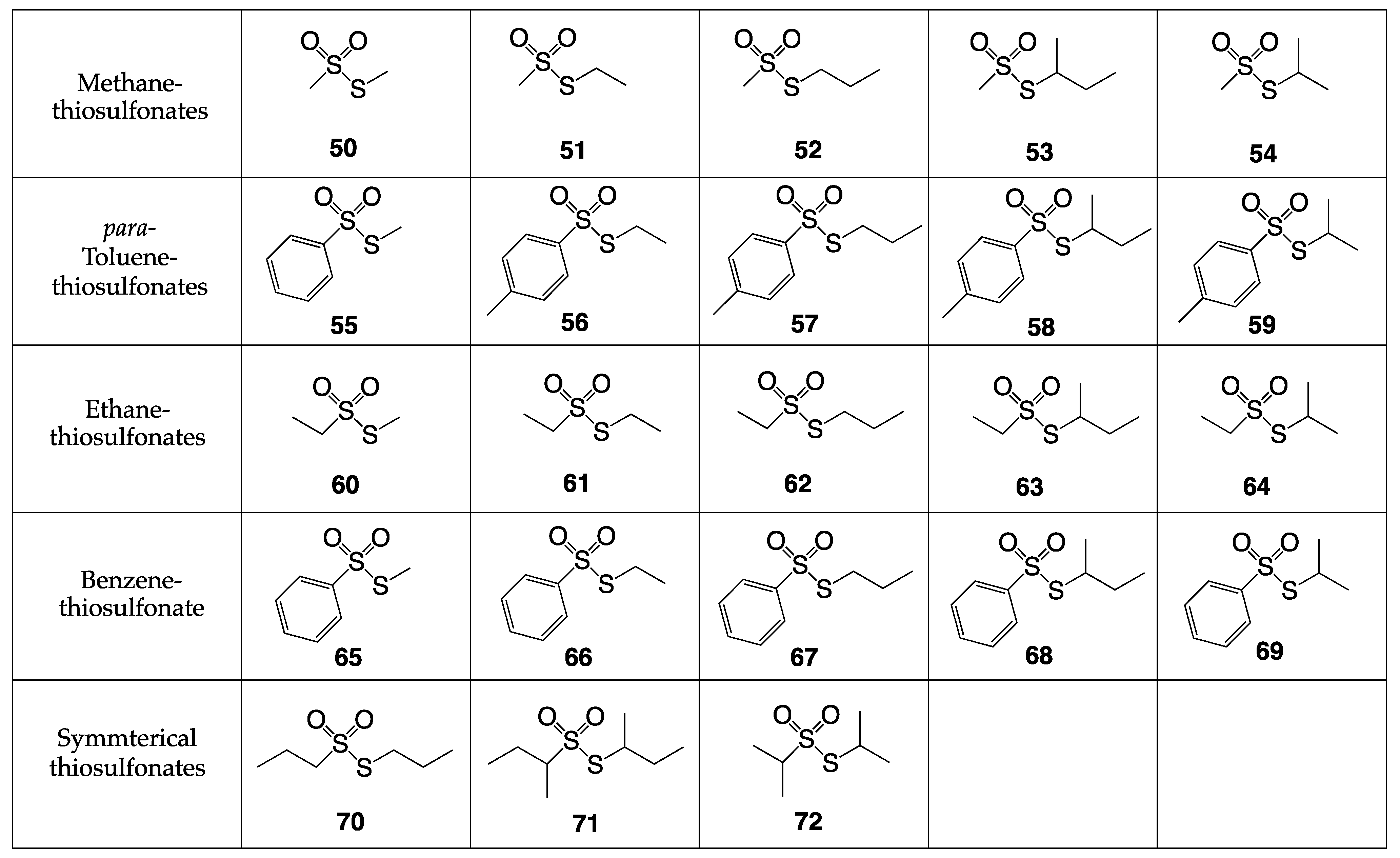
5.3. Preliminary Data for New Antibacterial Thiosulfonate Candidates
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Bcheraoui, C.; Mokdad, A.H.; Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Shirude, S.; Naghavi, M.; Murray, C.J.L. Trends and Patterns of Differences in Infectious Disease Mortality among US Counties, 1980–2014. J. Am. Med. Assoc. 2018, 319, 1248–1260. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J. The Epidemic of Antibiotic-Resistant Infections: A Call to Action for the Medical Community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- Feng, M.; Tang, B.; Liang, S.H.; Jiang, X. Sulfur Containing Scaffolds in Drugs: Synthesis and Application in Medicinal Chemistry. Curr. Top. Med. Chem. 2006, 16, 1200–1216. [Google Scholar] [CrossRef]
- Block, E. Fifty Years of Smelling Sulfur. J. Sulfur Chem. 2013, 34, 158–207. [Google Scholar] [CrossRef]
- Revell, K.D.; Heldreth, B.; Long, T.E.; Jang, S.; Turos, E. N-Thiolated β-Lactams: Studies on the Mode of Action and Identification of a Primary Cellular Target in Staphylococcus aureus. Bioorg. Med. Chem. 2007, 15, 2453–2467. [Google Scholar] [CrossRef]
- Turos, E.; Revell, K.D.; Ramaraju, P.; Gergeres, D.A.; Greenhalgh, K.; Young, A.; Sathyanarayan, N.; Dickey, S.; Lim, D.; Alhamadsheh, M.M.; et al. Unsymmetric Aryl-Alkyl Disulfide Growth Inhibitors of Methicillin-Resistant Staphylococcus aureus and Bacillus anthracis. Bioorg. Med. Chem. 2008, 16, 6501–6508. [Google Scholar] [CrossRef]
- Yoshida, S.; Kasuga, S.; Hayashi, N.; Ushiroguchi, T.; Matsuura, H.; Nakagawa, S. Antifungal Activity of Ajoene Derived from Garlic. Appl. Environ. Microbiol. 1987, 53, 615–617. [Google Scholar] [CrossRef]
- Naganawa, R.; Iwata, N.; Ishikawa, K.; Fukuda, H.; Fujino, T.; Suzuki, A. Inhibition of Microbial Growth by Ajoene, a Sulfur-Containing Compound Derived from Garlic. Appl. Environ. Microbiol. 1996, 62, 4238–4242. [Google Scholar] [CrossRef]
- Kishi, Y. Total Synthesis of Gliotoxin, Dehydrogliotoxin, and Hyalodendrin. Tetrahedron 1981, 37, 2045–2078. [Google Scholar]
- Waring, P.; Beavert, J. Gliotoxin and Related Epipolythiodioxopiperazines. Gen. Pharmac. 1996, 27, 1311–1316. [Google Scholar] [CrossRef]
- Ramaraju, P.; Gergeres, D.; Turos, E.; Dickey, S.; Lim, D.V.; Thomas, J.; Anderson, B. Synthesis and Antimicrobial Activities of Structurally Novel S,S′-Bis(Heterosubstituted) Disulfides. Bioorg. Med. Chem. Lett. 2012, 22, 3623–3631. [Google Scholar] [CrossRef]
- Sheppard, J.G.; McAleer, J.P.; Saralkar, P.; Geldenhuys, W.J.; Long, T.E. Allicin-Inspired Pyridyl Disulfides as Antimicrobial Agents for Multidrug-Resistant Staphylococcus aureus. Eur. J. Med. Chem. 2018, 143, 1185–1195. [Google Scholar] [CrossRef]
- Custodio, M.; Sparks, J.; Long, T.E. Disulfiram: A Repurposed Drug in Preclinical and Clinical Development for the Treatment of Infectious Diseases. Anti-Infect. Agents 2022, 20, 34–45. [Google Scholar] [CrossRef]
- Meneguello, J.; Murase, L.; de Souza, J.; de Oliverira, C.; Ghiralidi-Lopes, L.; Teixeira, J.; Scodro, R.; Ferracioli, K.; Siqueira, V.L.; Campanerut-Sa, P.; et al. Systematic Review of Disulfiram as an Antibacterial Agent: What Is the Evidence? Int. J. Antimicrob. Agents 2022, 59, 106578. [Google Scholar] [CrossRef]
- Horita, Y.; Takii, T.; Yagi, T.; Ogawa, K.; Fujiwara, N.; Inagaki, E.; Kremer, L.; Sato, Y.; Kuroishi, R.; Lee, Y.; et al. Antitubercular Activity of Disulfiram, an Antialcoholism Drug, against Multidrug- and Extensively Drug-Resistant Mycobacterium tuberculosis Isolates. Antimicrob. Agents Chemother. 2012, 56, 4140–4145. [Google Scholar] [CrossRef]
- Hamblin, K.; Flick-Smith, H.; Barnes, K.; Pereira-Leal, J.; Surkont, J.; Hampson, R.; Atkins, H.; Harding, S. Disulfiram, an Alcohol Dependence Therapy, Can Inhibit the in Vitro Growth of Francisella tularensis. Int. J. Antimicrob. Agents 2019, 54, 85–88. [Google Scholar] [CrossRef]
- Alvarez-Manzo, H.; Zhang, Y.; Shi, W.; Zhang, Y. Evaluation of Disulfiram Drug Combinations and Identification of Other More Effective Combinations against Stationary Phase Borrelia burgdorferi. Antibiotics 2020, 9, 542. [Google Scholar] [CrossRef]
- Frazier, K.; Moore, J.; Long, T.E. Antibacterial Activity of Disulfiram and Its Metabolites. J. Appl. Microbiol. 2019, 126, 79–86. [Google Scholar] [CrossRef]
- Chavva, H.; Meka, Y.; Long, T.E. Antimicrobial Pharmacodynamics of Vancomycin and Disulfiram (Antabuse®) in Staphylococcus aureus. Front. Microbiol. 2023, 13, 1092257. [Google Scholar] [CrossRef]
- Sudarikov, D.V.; Gyrdymova, Y.V.; Borisov, A.V.; Lukiyanova, J.M.; Rumyantcev, R.V.; Shevchenko, O.G.; Baidamshina, D.R.; Zakarova, N.D.; Kayumov, A.R.; Sinegubova, E.O.; et al. Synthesis and Biological Activity of Unsymmetrical Monoterpenylhetaryl Disulfides. Molecules 2022, 27, 5101. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, G.; Poeschl, R.; Zimhony, O.; Gunaratnam, M.; Moreira, J.B.C.; Neidle, S.; Evangelopoulos, D.; Bhakta, S.; Malkinson, J.P.; Boshoff, H.I.; et al. Bioactive Pyridine-N-oxide Disulfides from Allium stipitatum. J. Nat. Prod. 2009, 72, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Danquah, C.A.; Kakagianni, E.; Khondkar, P.; Maitra, A.; Rahman, M.; Evangelopoulos, D.; McHugh, T.D.; Stapleton, P.; Malkinson, J.; Bhakta, S.; et al. Analogues of Disulfides from Allium stipitatum Demonstrate Potent Anti-Tubercular Activities through Drug Efflux Pump and Biofilm Inhibition. Sci. Rep. 2018, 8, 1150. [Google Scholar] [CrossRef] [PubMed]
- Shchelik, I.S.; Gademann, K. Thiol- and Disulfide-Containing Vancomycin Derivatives against Bacterial Resistance and Biofilm Formation. ACS Med. Chem. Lett. 2021, 12, 1898–1904. [Google Scholar] [CrossRef]
- Turos, E. Biomolecules: The Organic Chemistry of Living Systems; Great River Technologies: Dubuque, IA, USA, 2021. [Google Scholar]
- Walker, S.; Landovitz, R.; Dingt, W.D.; Ellestadt, G.A.; Kahne, D. Cleavage Behavior of Calicheamicin Y1 and Calicheamicin T. Proc. Natl. Acad. Sci. USA 1992, 89, 4608–4612. [Google Scholar] [CrossRef]
- Maiese, W.M.; Lechevauert, M.P.; Lechevalier, H.A.; Korshalla, J.; Kuck, N.; Fantini, A.; Wildey, M.J.; Thomas, J.; Greenstein, M. Calicheamicins, A Novel Family of Antitumor Antibiotics: Taxonomy, Fermentation and Biological Properties. J. Antibiot. 1989, 42, 558–563. [Google Scholar] [CrossRef]
- Rol, D.M.; Is, C.; Ireland, M. Citorellamine, A New Bromoindole Derivative from Polycitorella mariae. Tetrahedron Lett. 1985, 26, 4303–4306. [Google Scholar] [CrossRef]
- Rodriguez, A.D.; Akee, R.K.; Scheuer, P.J. Two Bromotyrosine-Cysteine Derived Metabolites from a Sponge. Tetrahedron Lett. 1987, 28, 4989–4992. [Google Scholar] [CrossRef]
- Pham, N.B.; Butler, M.S.; Quinn, R.J. Isolation of Psammaplin A 11′-Sulfate and Bisaprasin 11′-Sulfate from the Marine Sponge Aplysinella rhax. J. Nat. Prod. 2000, 63, 393–395. [Google Scholar] [CrossRef]
- Wallock-Richards, D.; Doherty, C.J.; Doherty, L.; Clarke, D.J.; Place, M.; Govan, J.R.W.; Campopiano, D.J. Garlic Revisited: Antimicrobial Activity of Allicin-Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS ONE 2014, 9, e112726. [Google Scholar] [CrossRef]
- Toshiba, H.; Katsuzaki, H.; Ohta, R.; Xshikawa, K.; Fukuda, H.; Fujino, T.; Suzuki, A. Antimicrobial Activity of the Thiosulfinates Isolated from Oil-Macerated Garlic Extract. Biosci. Biotechnol. Biochem. 1999, 63, 591–594. [Google Scholar] [CrossRef]
- Kulikova, V.V.; Chernukha, M.; Morozova, E.A.; Revtovich, S.V.; Rodionov, A.N.; Koval, V.S.; Avetisyan, L.R.; Kuliastova, D.G.; Shaginyan, I.A.; Demidkina, T.V. Antibacterial Effect of Thiosulfinates on Multiresistant Strains of Bacteria Isolated from Patients with Cystic Fibrosis. Acta Nat. 2018, 10, 77–80. [Google Scholar] [CrossRef]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef] [PubMed]
- Heldreth, B.; Turos, E. Microbiological Properties and Modes of Action of Organosulfur-Based Anti-Infectives. Curr. Med. Chem.-Anti-Infect. Agents 2005, 4, 295–315. [Google Scholar] [CrossRef]
- Mueller, L.; Oppenheim, J.J.; Kovacs, E.J.; Matsushima, K.; Durum, S.K.; Otting, G.; Wiithrich, K.; Priestle, J.P.; Schar, H.P.; Griitter, M.G.; et al. DNA Strand Scission by the Novel Antitumor Antibiotic Leinamycin. Biochemistry 1990, 29, 5676–5681. [Google Scholar]
- Pan, G.; Xu, Z.; Guo, Z.; Hindra; Ma, M.; Yang, D.; Zhou, H.; Gansemans, Y.; Zhu, X.; Huang, Y.; et al. Discovery of the Leinamycin Family of Natural Products by Mining Actinobacterial Genomes. Proc. Natl. Acad. Sci. USA 2017, 114, E11131–E11140. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Ariza-Romero, J.; Baños-Arjona, A.; Exposito-Ruiz, M.; Gutierrez-Fernandez, J. In VitroAntibacterial Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate Derived from Allium spp. against Gram-Negative and Gram-Positive Multidrug-Resistant Bacteria Isolated from Human Samples. BioMed Res. Int. 2018, 2018, 7861207. [Google Scholar] [CrossRef]
- Sorlozano-Puerto, A.; Albertuz-Crespo, M.; Lopez-Machado, I.; Gil-Martinez, L.; Ariza-Romero, J.J.; Maroto-Tello, A.; Baños-Arjona, A.; Gutierrez-Fernandez, J. Antibacterial and Antifungal Activity of Propyl-Propane-Thiosulfinate and Propyl-Propane-Thiosulfonate, Two Organosulfur Compounds from Allium cepa: In Vitro Antimicrobial Effect via the Gas Phase. Pharmaceuticals 2021, 14, 21. [Google Scholar] [CrossRef]
- Cabello-Gómez, J.F.; Aguinaga-Casañas, M.A.; Falcón-Piñeiro, A.; González-Gragera, E.; Márquez-Martín, R.; Agraso, M.d.M.; Bermúdez, L.; Baños, A.; Martínez-Bueno, M. Antibacterial and Antiparasitic Activity of Propyl-Propane-Thiosulfinate (PTS) and Propyl-Propane-Thiosulfonate (PTSO) from Allium cepa against Gilthead Sea Bream Pathogens in In Vitro and In Vivo Studies. Molecules 2022, 27, 6900. [Google Scholar] [CrossRef] [PubMed]
- Lubenets, V.I.; Stadnitskaya, N.E.; Novikov, V.P. Synthesis of Thiosulfonates Belonging to Quinoline Derivatives. Rus. J. Org. Chem. 2000, 36, 851–853. [Google Scholar] [CrossRef]
- Lubenets, V.; Karpenko, O.; Ponomarenko, M.; Zahoriy, G.; Krychkovska, A.; Novikov, V. Development of New Antimicrobial Compositions of Thiosulfonate Structure. Chem. Chem. Technol. 2013, 7, 119–124. [Google Scholar] [CrossRef]
- Blume, L. Synthesis and Antibacterial Testing of Novel Thiosulfonate Compounds. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2022. [Google Scholar]
- Fujiki, K.; Tanifugi, N.; Sasaki, Y.; Yokoyama, T. New and Facile Synthesis of Thiosulfonates from Sulfinate/Disulfide/I2 System. Synthesis 2002, 3, 343–348. [Google Scholar] [CrossRef]
- Freeman, F.; Angeletakis, C. Formation of Elusive vic-Disulfoxides and OS-Sulfenyl Sulfinates during the m-Chloroperoxybenzoic Acid (MCPBA) Oxidation of Alkyl Aryl Disulfides and Their Regioisomeric Sulfinothioic Acid S-Esters. J. Org. Chem. 1985, 50, 793–798. [Google Scholar] [CrossRef]
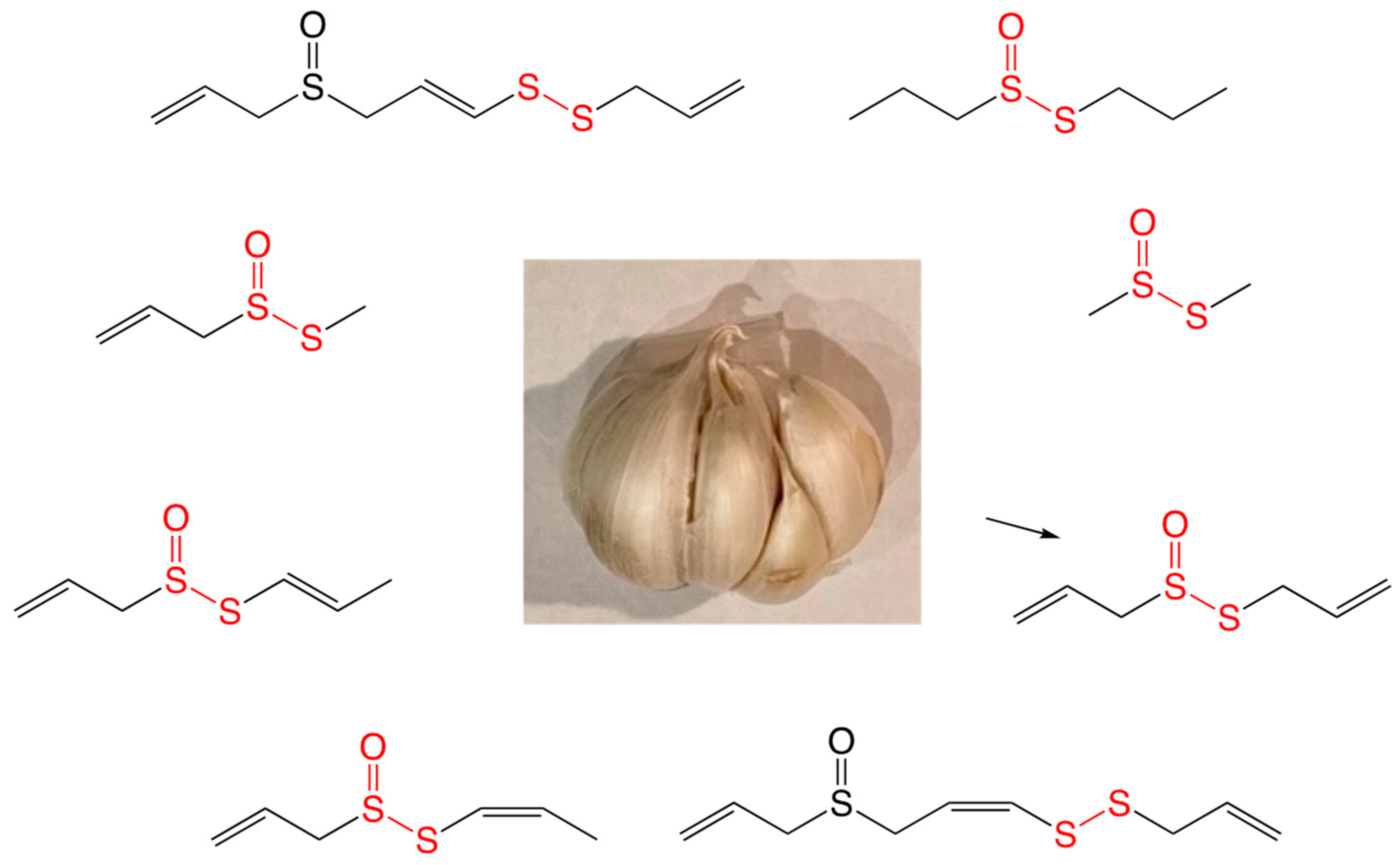
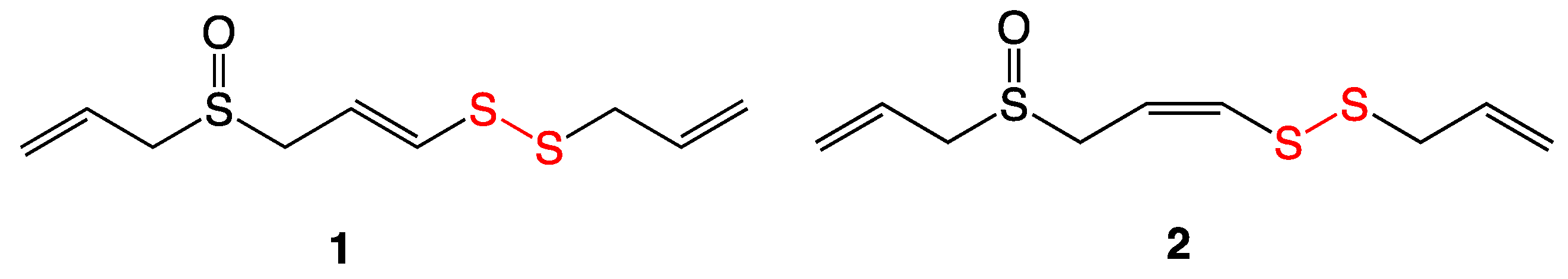




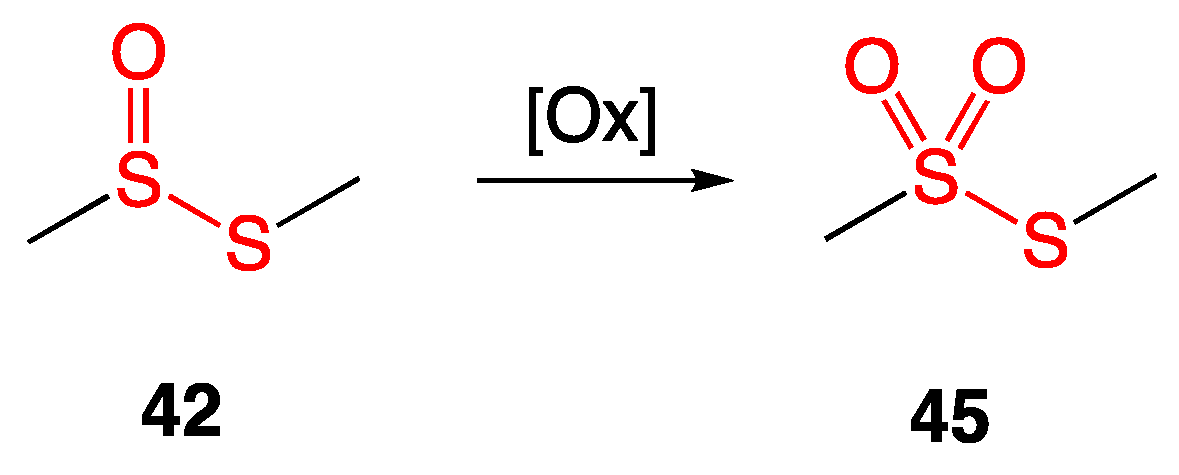

| Bacterial Species | MIC (µg/mL) |
|---|---|
| Bacillus cereus | 4 |
| Bacillus subtilis | 4 |
| Staphylococcus aureus | 16 |
| Mycobacterium smegmatis | 4 |
| Mycobacterium pheli | 14 |
| Streptomyces griseus | 4 |
 | ||||
| Alkyl | S. aureus (ATCC25923) | MRSA (ATCC 43300) | B. anthracis | |
| 4a | Methyl | 35 mm 16 µg/mL | 23 mm 32 µg/mL | 54 mm 1 µg/mL |
| 4b | Ethyl | 54 mm 1 µg/mL | 38 mm 16 µg/mL | 67 mm 0.25 µg/mL |
| 4c | Isopropyl | 85 mm <0.125 µg/mL | 75 mm <0.125 µg/mL | 71 mm 0.125 µg/mL |
| 4d | sec-Butyl | 68 mm 0.8 µg/mL | 54 mm 1.0 µg/mL | 62 mm 0.5 µg/mL |
| 4e | n-Propyl | 43 mm 16 µg/mL | 38 mm 16 µg/mL | 57 mm 1 µg/mL |
| 4f | n-Butyl | 53 mm 1 µg/mL | 28 mm 32 µg/mL | 52 mm 1 µg/mL |
| 5a | Methyl | 36 mm 16 µg/mL | 15 mm 64 µg/mL | 43 mm 8 µg/mL |
| 5b | Ethyl | 45 mm 8 µg/mL | 12 mm 64 µg/mL | 40 mm 8 µg/mL |
| 5c | Isopropyl | 85 mm <0.125 µg/mL | 38 mm 16 µg/mL | 54 mm 1 µg/mL |
| 5d | sec-Butyl | 45 mm 8 µg/mL | 22 mm 32 µg/mL | 41 mm 8 µg/mL |
| 6a | Methyl | 37 mm 16 µg/mL | 26 mm 32 µg/mL | 31 mm 16 µg/mL |
| 6b | Ethyl | 40 mm 16 µg/mL | 28 mm 32 µg/mL | 35 mm 16 µg/mL |
| 6c | Isopropyl | 31 mm 16 µg/mL | 20 mm 32 µg/mL | 27 mm 32 µg/mL |
| 6d | sec-Butyl | 24 mm 32 µg/mL | 20 mm 32 µg/mL | 23 mm 32 µg/mL |
| Penicillin G (reference) | 33 mm 0.03 µg/mL | 14 mm 64 µg/mL | nt nt | |
| Vancomycin (reference) | 27 mm 0.25 µg/mL | 22 mm 0.5 µg/mL | nt nt | |
| Thiosulfinate | Conc. (µg/mL) | Escherichia coli K12 Ec | Pseudomonas fluorescens Pf-01 | Pseudomonas syringae pv. phaseolicola 4612 Ps4612 | Micrococcus luteus MI | Saccharomyces cerevisiae BY4742 Sc |
|---|---|---|---|---|---|---|
| Dimethyl thiosulfinate | MIC MBC | 64 64 | 16 32 | 16 16 | 64 64 | 16 MFC 16 |
| Diethyl thiosulfinate | MIC MBC | 64 64 | 128 256 | 8 16 | 32 32 | 8 MFC 8 |
| Diallyl thiosulfinate | MIC MBC | 32 32 | 128 256 | 16 16 | 32 32 | 2 MFC 4 |
| Dipropyl thiosulfinate | MIC MBC | 32 32 | 256 256 | 32 64 | 32 32 | 2 MFC 4 |
| Agent Name | Zones of Growth Inhibition (mm) | ||
|---|---|---|---|
| Candida albicans | Staphylococcus aureus | Escherichia coli | |
| Ointment of 48 and 49 | 32.2 ± 0.1 | 30.0 ± 1.0 | 23.3 ± 0.8 |
| Clotrimazole ointment | 26.5 ± 0.2 | 18.6 ± 0.4 | 20.3 ± 0.7 |
| Econazole gel | -- | 17.5 ± 0.5 | 16.4 ± 0.6 |
| Nystatin ointment | 24.2 ± 0.5 | 0 | 0 |
| Gentamicin ointment | 13.5 ± 0.2 | 26.3 ± 0.7 | 25.5 ± 0.5 |
| MRSA COL | VISA MU50 | VRSA-1 | VRE HF50104 | KP 700603 | AB5075-UW | PA 15442 | EC13047 | |
|---|---|---|---|---|---|---|---|---|
| 50 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 51 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 52 | 64 | 64 | 64 | 64 | 64 | 64 | >64 | 64 |
| 53 | 32/16 | 32 | 32 | 64 | >64 | 64 | >64 | 64 |
| 54 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| 55 | 64 | 64 | 64 | 64 | >64 | 64 | >64 | >64 |
| 56 | 64/>64 | 64 | 64 | >64 | >64 | >64 | >64 | >64 |
| 57 | 64 | 64 | 64 | 64 | >64 | >64 | >64 | >64 |
| 58 | 16/8 | 16 | 32/16 | 64 | >64 | 64 | >64 | >64 |
| 59 | 64 | 64 | 64 | >64 | >64 | >64 | >64 | >64 |
| 60 | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 |
| 61 | 64/>64 | 64 | 64 | 64 | >64 | 64 | >64 | >64 |
| 62 | 16 | 16 | 16 | 32 | 64 | 32 | >64 | 32 |
| 63 | 16/8 | 8 | 8 | 32 | >64 | 64 | >64 | 64 |
| 64 | 8 | 8 | 8 | 32 | 64 | 32 | >64 | 32 |
| 65 | 32 | >64 | 64 | 32 | >64 | 64 | 64 | 64 |
| 66 | 32 | >64 | >64 | 32 | >64 | 64 | >64 | 64 |
| 67 | >64 | >64 | >64 | 16 | >64 | >64 | >64 | >64 |
| 68 | 16 | >64 | 32 | 8 | >64 | >64 | >64 | >64 |
| 69 | 8 | 16/4 | 16 | 8 | >64 | >64 | >64 | >64 |
| 70 | 8 | 4 | 2 | 32 | >64 | >64 | >64 | >64 |
| 71 | 8 | 4 | 2 | 32 | >64 | >64 | >64 | >64 |
| 72 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| COLISTIN | >64 | >64 | >64 | 16 | 1 | ≤1 | 1 | >64 |
| VANCOMYCIN | 2 | 8 | >64 | 64 | >64 | 64 | >64 | >64 |
| MRSA COL | VISA MU50 | VRSA-1 | VRE HF50104 | KP 700603 | AB5075-UW | PA 15442 | EC13047 | |
|---|---|---|---|---|---|---|---|---|
| 58 | 16/8 | 16 | 32/16 | 64 | >64 | 64 | >64 | >64 |
| 63 | 16/8 | 8 | 8 | 32 | >64 | 64 | >64 | 64 |
| 64 | 8 | 8 | 8 | 32 | 64 | 32 | >64 | 32 |
| 68 | 16 | >64 | 32 | 8 | >64 | >64 | >64 | >64 |
| 69 | 8 | 16/4 | 16 | 8 | >64 | >64 | >64 | >64 |
| 70 | 8 | 4 | 2 | 32 | >64 | >64 | >64 | >64 |
| 71 | 8 | 4 | 2 | 32 | >64 | >64 | >64 | >64 |
| Colistin | >64 | >64 | >64 | 16 | 1 | ≤1 | 1 | >64 |
| Vancomycin | 2 | 8 | >64 | 64 | >64 | 64 | >64 | >64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blume, L.; Long, T.E.; Turos, E. Applications and Opportunities in Using Disulfides, Thiosulfinates, and Thiosulfonates as Antibacterials. Int. J. Mol. Sci. 2023, 24, 8659. https://doi.org/10.3390/ijms24108659
Blume L, Long TE, Turos E. Applications and Opportunities in Using Disulfides, Thiosulfinates, and Thiosulfonates as Antibacterials. International Journal of Molecular Sciences. 2023; 24(10):8659. https://doi.org/10.3390/ijms24108659
Chicago/Turabian StyleBlume, Lindsay, Timothy E. Long, and Edward Turos. 2023. "Applications and Opportunities in Using Disulfides, Thiosulfinates, and Thiosulfonates as Antibacterials" International Journal of Molecular Sciences 24, no. 10: 8659. https://doi.org/10.3390/ijms24108659
APA StyleBlume, L., Long, T. E., & Turos, E. (2023). Applications and Opportunities in Using Disulfides, Thiosulfinates, and Thiosulfonates as Antibacterials. International Journal of Molecular Sciences, 24(10), 8659. https://doi.org/10.3390/ijms24108659





