Alterations in p53, Microsatellite Stability and Lack of MUC5AC Expression as Molecular Features of Colorectal Carcinoma Associated with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results
2.1. Patients and Specimens
2.2. Immunohistochemistry
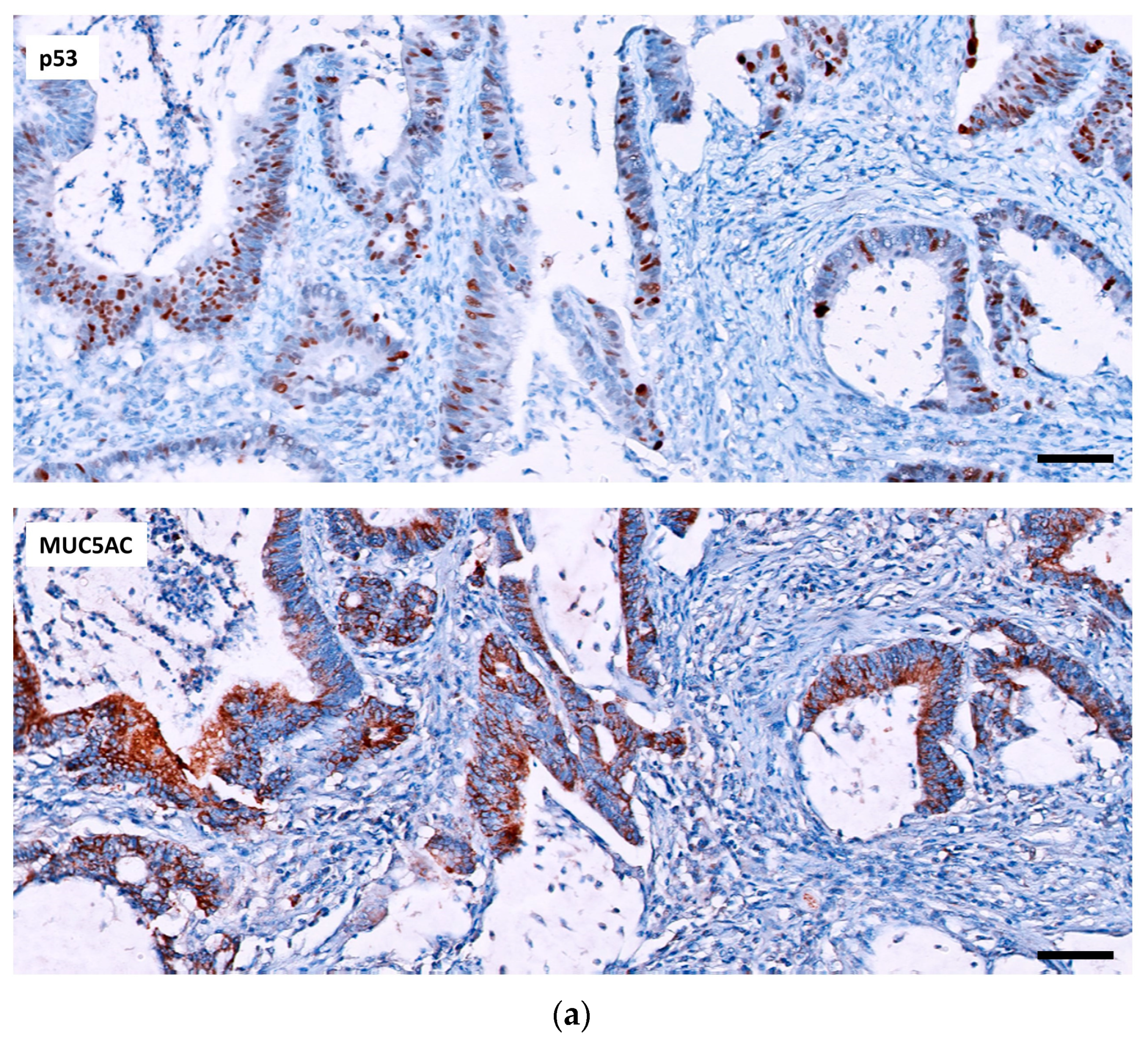
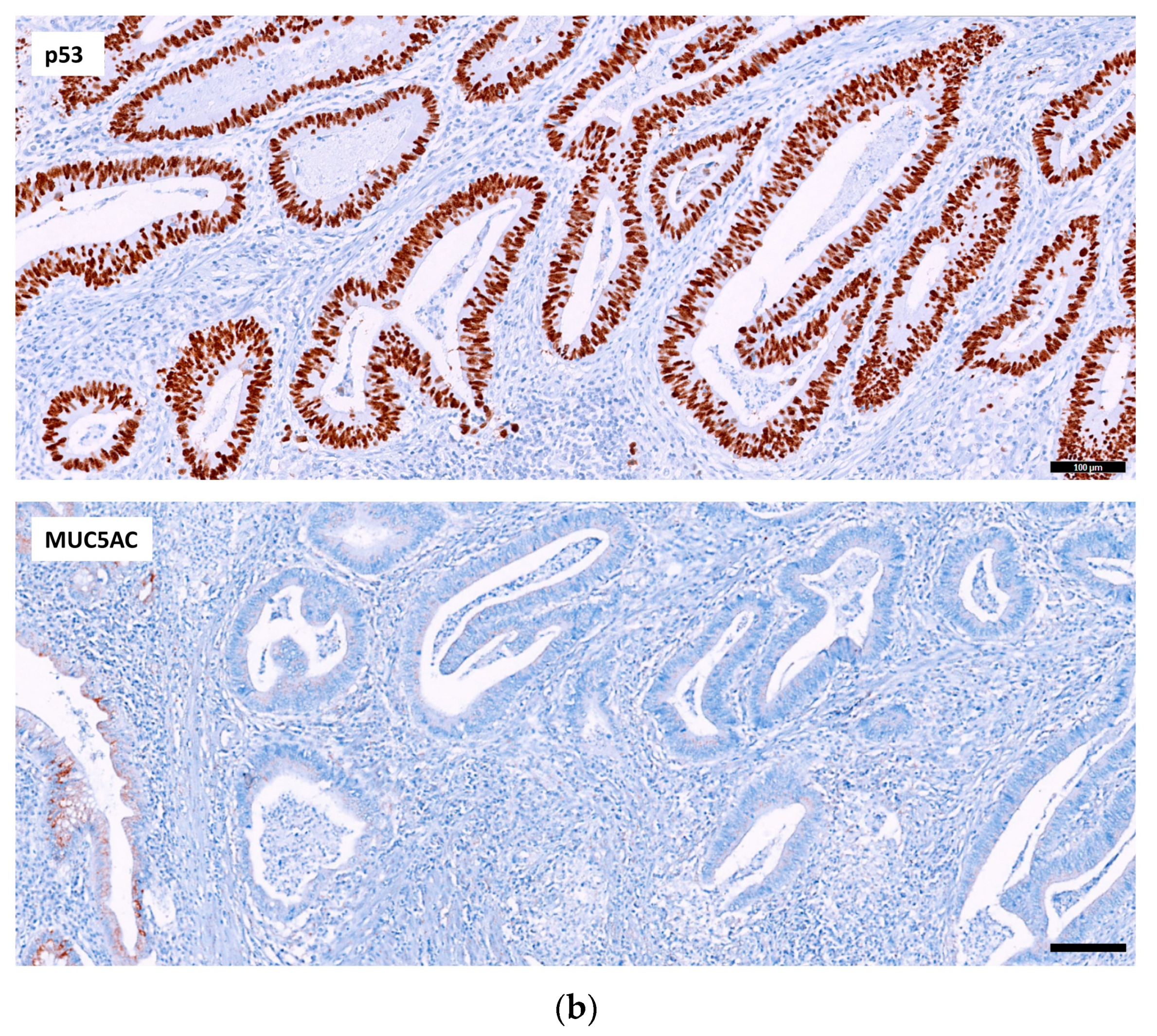
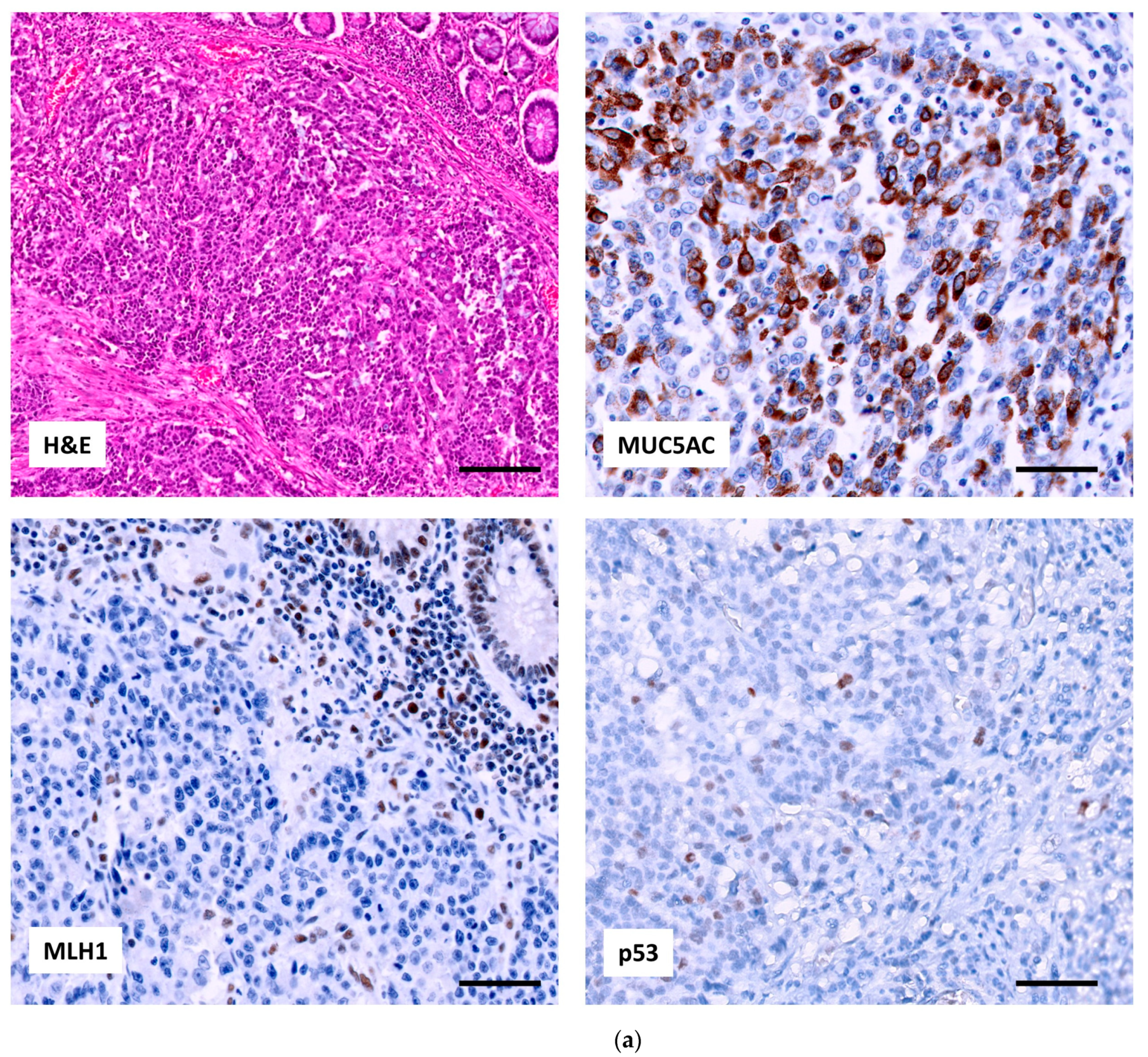
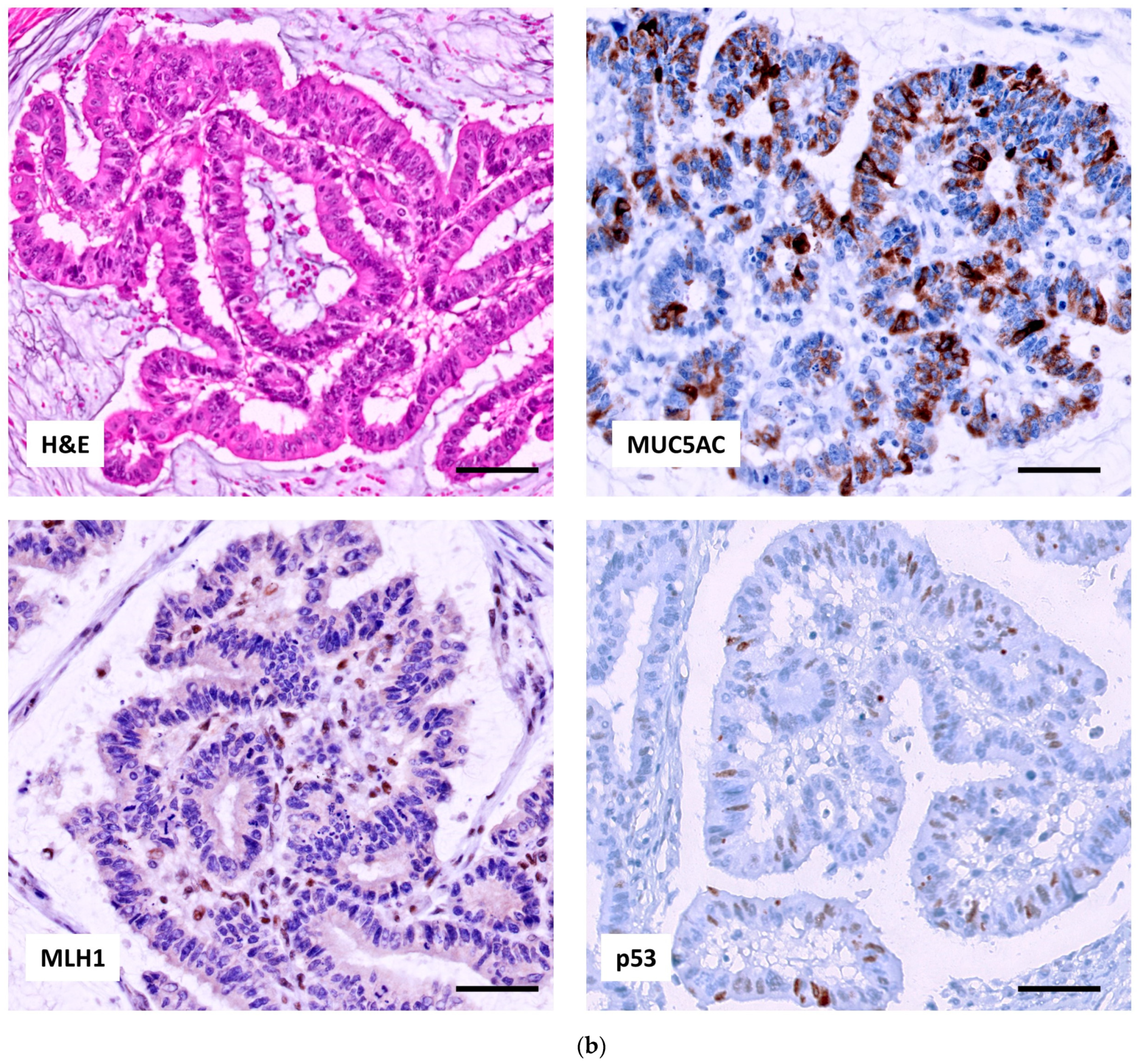
2.2.1. p53, MUC5AC and MSI in Adenocarcinoma
2.2.2. Relation of the Three IHC Markers in Adenocarcinoma
2.2.3. p53 and MUC5AC Expression in the Mucosa Adjacent to Adenocarcinomas
3. Discussion
4. Materials and Methods
4.1. Immunohistochemistry
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAC | colitis-associated colorectal carcinoma |
| IBD | inflammatory bowel disease |
| GM | gastric metaplasia |
| CRC | colorectal cancer |
| MSI | microsatellite instability |
| CD | Crohn’s disease |
| UC | ulcerative colitis |
| MSI | microsatellite instability |
| MSI-H | microsatellite instability high |
| MSS | microsatellite stability |
| LS | Lynch syndrome |
| MMR | mismatch repair |
References
- Lutgens, M.W.; van Oijen, M.G.; van der Heijden, G.J.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining risk of colorectal cancer in inflammatory bowel disease: An updated meta-analysis of population-based cohort studies. Inflamm. Bowel. Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Gamborg, M.; Matzen, P.; Munkholm, P.; Sørensen, T.I. Increased risk of intestinal cancer in Crohn’s disease: A meta-analysis of population-based cohort studies. Am. J. Gastroenterol. 2005, 100, 2724–2729. [Google Scholar] [CrossRef]
- Mattar, M.C.; Lough, D.; Pishvaian, M.J.; Charabaty, A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest. Cancer Res. 2011, 4, 53–61. [Google Scholar] [PubMed]
- Lu, C.; Schardey, J.; Zhang, T.; Crispin, A.; Wirth, U.; Karcz, K.W.; Bazhin, A.V.; Andrassy, J.; Werner, J.; Kühn, F. Survival Outcomes and Clinicopathological Features in Inflammatory Bowel Disease-associated Colorectal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. 2022, 276, e319–e330. [Google Scholar] [CrossRef] [PubMed]
- Svrcek, M.; El-Bchiri, J.; Chalastanis, A.; Capel, E.; Dumont, S.; Buhard, O.; Oliveira, C.; Seruca, R.; Bossard, C.; Mosnier, J.F.; et al. Specific clinical and biological features characterize inflammatory bowel disease associated colorectal cancers showing microsatellite instability. J. Clin. Oncol. 2007, 25, 4231–4238. [Google Scholar] [CrossRef]
- Liu, X.; Goldblum, J.R.; Zhao, Z.; Landau, M.; Heald, B.; Pai, R.; Lin, J. Distinct clinicohistologic features of inflammatory bowel disease-associated colorectal adenocarcinoma: In comparison with sporadic microsatellite-stable and Lynch syndrome-related colorectal adenocarcinoma. Am. J. Surg. Pathol. 2012, 36, 1228–1233. [Google Scholar] [CrossRef]
- Svrcek, M.; Fontugne, J.; Duval, A.; Fléjou, J.F. Inflammatory bowel disease-associated colorectal cancers and microsatellite instability: An original relationship. Am. J. Surg. Pathol. 2013, 37, 460–462. [Google Scholar] [CrossRef]
- Rajamäki, K.; Taira, A.; Katainen, R.; Välimäki, N.; Kuosmanen, A.; Plaketti, R.M.; Seppälä, T.T.; Ahtiainen, M.; Wirta, E.V.; Vartiainen, E.; et al. Genetic and Epigenetic Characteristics of Inflammatory Bowel Disease-Associated Colorectal Cancer. Gastroenterology 2021, 161, 592–607. [Google Scholar] [CrossRef]
- Leedham, S.J.; Graham, T.A.; Oukrif, D.; McDonald, S.A.; Rodriguez-Justo, M.; Harrison, R.F.; Shepherd, N.A.; Novelli, M.R.; Jankowski, J.A.; Wright, N.A. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology 2009, 136, 542–550.e6. [Google Scholar] [CrossRef]
- Robles, A.I.; Traverso, G.; Zhang, M.; Roberts, N.J.; Khan, M.A.; Joseph, C.; Lauwers, G.Y.; Selaru, F.M.; Popoli, M.; Pittman, M.E.; et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology 2016, 150, 931–943. [Google Scholar] [CrossRef]
- Du, L.; Kim, J.J.; Shen, J.; Chen, B.; Dai, N. KRAS and TP53 mutations in inflammatory bowel disease-associated colorectal cancer: A meta-analysis. Oncotarget 2017, 8, 22175–22186. [Google Scholar] [CrossRef] [PubMed]
- Chatila, W.K.; Walch, H.; Hechtman, J.F.; Moyer, S.M.; Sgambati, V.; Faleck, D.M.; Srivastava, A.; Tang, L.; Benhamida, J.; Ismailgeci, D.; et al. Integrated clinical and genomic analysis identifies driver events and molecular evolution of colitis-associated cancers. Nat. Commun. 2023, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Hartman, D.J.; Binion, D.G.; Regueiro, M.D.; Miller, C.; Herbst, C.; Pai, R.K. Distinct Histopathologic and Molecular Alterations in Inflammatory Bowel Disease-Associated Intestinal Adenocarcinoma: C-MYC Amplification is Common and Associated with Mucinous/Signet Ring Cell Differentiation. Inflamm. Bowel. Dis. 2018, 24, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Hardt, J.; Sauer, C.; Heselmeyer-Hadded, K.; Witt, S.H.; Kienle, P.; Ried, T.; Gaiser, T. Molecular characterization of ulcerative colitis-associated colorectal carcinomas. Mod. Pathol. 2021, 34, 1153–1166. [Google Scholar] [CrossRef]
- Peltomäki, P.; Nyström, M.; Mecklin, J.P.; Seppälä, T.T. Lynch Syndrome Genetics and Clinical Implications. Gastroenterology 2023, 164, 783–799. [Google Scholar] [CrossRef]
- Crockett, S.D.; Nagtegaal, I.D. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology 2019, 157, 949–966.e4. [Google Scholar] [CrossRef]
- Koukoulis, G.K.; Ke, Y.; Henley, J.D.; Cummings, O.W. Detection of pyloric metaplasia may improve the biopsy diagnosis of Crohn’s ileitis. J. Clin. Gastroenterol. 2002, 34, 141–143. [Google Scholar] [CrossRef]
- Kariv, R.; Plesec, T.P.; Gaffney, K.; Lian, L.; Fazio, V.W.; Remzi, F.H.; Lopez, R.; Goldblum, J.R.; Shen, B. Pyloric gland metaplasia and pouchitis in patients with ileal pouch-anal anastomoses. Aliment. Pharm. Ther. 2010, 31, 862–873. [Google Scholar] [CrossRef]
- Agarwal, S.; Stucchi, A.F.; Dendrinos, K.; Cerda, S.; O’Brien, M.J.; Becker, J.M.; Heeren, T.; Farraye, F.A. Is pyloric gland metaplasia in ileal pouch biopsies a marker for Crohn’s disease? Dig. Dis. Sci. 2013, 58, 2918–2925. [Google Scholar] [CrossRef]
- Tokuyama, M.; Dhingra, S.; Polydorides, A.D. Clinicopathologic Features and Diagnostic Implications of Pyloric Gland Metaplasia in Intestinal Specimens. Am. J. Surg. Pathol. 2021, 45, 365–373. [Google Scholar] [CrossRef]
- Ikezono, G.; Yao, K.; Imamura, K.; Kanemitsu, T.; Miyaoka, M.; Hirano, A.; Takeda, K.; Hisabe, T.; Ueki, T.; Tanabe, H.; et al. Gastric metaplasia of the duodenal mucosa in Crohn’s disease: Novel histological and endoscopic findings. Endosc. Int. Open 2021, 9, E181–E189. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Arslan, M.E.; Lee, E.C.; Qualia, C.M.; Mikula, M.W.; Fu, Z.; Petchers, A.; Arker, S.H.; Kmeid, M.; Boguniewicz, A.; et al. Pyloric gland metaplasia: Potential histologic predictor of severe pouch disease including Crohn’s disease of the pouch in ulcerative colitis. Pathol. Res. Pract. 2021, 220, 153389. [Google Scholar] [CrossRef]
- Chen, B.; Scurrah, C.R.; McKinley, E.T.; Simmons, A.J.; Ramirez-Solano, M.A.; Zhu, X.; Markham, N.O.; Heiser, C.N.; Vega, P.N.; Rolong, A.; et al. Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps. Cell 2021, 184, 6262–6280. [Google Scholar] [CrossRef] [PubMed]
- Umetani, N.; Sasaki, S.; Watanabe, T.; Shinozaki, M.; Matsuda, K.; Ishigami, H.; Ueda, E.; Muto, T. Genetic alterations in ulcerative colitis-associated neoplasia focusing on APC, K-ras gene and microsatellite instability. Jpn. J. Cancer Res. 1999, 90, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Lyda, M.H.; Noffsinger, A.; Belli, J.; Fenoglio-Preiser, C.M. Microsatellite instability and K-ras mutations in patients with ulcerative colitis. Hum. Pathol. 2000, 31, 665–671. [Google Scholar] [CrossRef]
- Fleisher, A.S.; Esteller, M.; Harpaz, N.; Leytin, A.; Rashid, A.; Xu, Y.; Liang, J.; Stine, O.C.; Yin, J.; Zou, T.T.; et al. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer Res. 2000, 60, 4864–4868. [Google Scholar] [PubMed]
- Schulmann, K.; Mori, Y.; Croog, V.; Yin, J.; Olaru, A.; Sterian, A.; Sato, F.; Wang, S.; Xu, Y.; Deacu, E.; et al. Molecular phenotype of inflammatory bowel disease-associated neoplasms with microsatellite instability. Gastroenterology 2005, 129, 74–85. [Google Scholar] [CrossRef]
- Vanoli, A.; Di Sabatino, A.; Furlan, D.; Klersy, C.; Grillo, F.; Fiocca, R.; Mescoli, C.; Rugge, M.; Nesi, G.; Fociani, P.; et al. Small Bowel Carcinomas in Coeliac or Crohn’s Disease: Clinico-pathological, Molecular, and Prognostic Features. A Study From the Small Bowel Cancer Italian Consortium. J. Crohn’s Colitis. 2017, 11, 942–953. [Google Scholar] [CrossRef]
- Giuffrida, P.; Arpa, G.; Grillo, F.; Klersy, C.; Sampietro, G.; Ardizzone, S.; Fociani, P.; Fiocca, R.; Latella, G.; Sessa, F.; et al. PD-L1 in small bowel adenocarcinoma is associated with etiology and tumor-infiltrating lymphocytes, in addition to microsatellite instability. Mod. Pathol. 2020, 33, 1398–1409, Erratum in: Mod Pathol. 2020, 33, 1453. [Google Scholar] [CrossRef]
- Mäki-Nevala, S.; Ukwattage, S.; Olkinuora, A.; Almusa, H.; Ahtiainen, M.; Ristimäki, A.; Seppälä, T.; Lepistö, A.; Mecklin, J.P.; Peltomäki, P. Somatic mutation profiles as molecular classifiers of ulcerative colitis-associated colorectal cancer. Int. J. Cancer 2021, 148, 2997–3007. [Google Scholar] [CrossRef]
- Matsuda, K.; Watanabe, T.; Shinozaki, M.; Yokoyama, T.; Sasaki, S.; Furukawa, Y.; Kanazawa, T.; Masaki, T.; Muto, T. Ulcerative colitis patients with a family history of colorectal cancer should be subjected to close and careful surveillance. Jpn. J. Clin. Oncol. 1999, 29, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Minami, N.; Yoshino, T.; Nakase, H. Unique endoscopic findings of colitisassociated colorectal cancer in a patient with ulcerative colitis and lynch syndrome. J. Crohn’s Colitis. 2014, 8, 336–337. [Google Scholar] [CrossRef] [PubMed]
- McNamara, K.L.; Aronson, M.D.; Cohen, Z. Is there a role for prophylactic colectomy in lynch syndrome patients with inflammatory bowel disease? Int. J. Color. Dis. 2016, 31, 9–13. [Google Scholar] [CrossRef]
- Derikx, L.A.; Smits, L.J.; van Vliet, S.; Dekker, E.; Aalfs, C.M.; van Kouwen, M.C.; Nagengast, F.M.; Nagtegaal, I.D.; Hoogerbrugge, N.; Hoentjen, F. Colorectal Cancer Risk in Patients With Lynch Syndrome and Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 454–458.e1. [Google Scholar] [CrossRef] [PubMed]
- Arif, A.A.; Chahal, D.; Ladua, G.K.; Bhang, E.; Salh, B.; Rosenfeld, G.; Loree, J.M.; Donnellan, F. Hereditary and Inflammatory Bowel Disease-Related Early Onset Colorectal Cancer Have Unique Characteristics and Clinical Course Compared with Sporadic Disease. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Savarino, E.; Verstockt, B.; Fumery, M.; Pugliese, D.; Bertani, L.; Buda, A.; Dragoni, G.; Goren, I.; Laish, I.; et al. Hereditary Colorectal Cancer Syndromes and Inflammatory Bowel Diseases: An ECCO CONFER Multicentre Case Series. J. Crohn’s Colitis 2022, 16, 1845–1852. [Google Scholar] [CrossRef]
- Ayeni, A.A.; Waterland, P.; Evans, M.; Singhal, S.; Patel, R.K.; Akingboye, A. Case Report: Multiple colorectal cancers in a patient with Ulcerative colitis and Lynch syndrome: Is there a role for prophylactic colectomy? A short report and review of literature. Front. Oncol. 2022, 12, 1031606. [Google Scholar] [CrossRef]
- Rico, S.D.; Mahnken, M.; Büscheck, F.; Dum, D.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Hinsch, A.; Höflmayer, D.; Möller-Koop, C.; et al. MUC5AC Expression in various tumor types and nonneoplastic tissue: A tissue microarray study on 10,399 tissue samples. Technol. Cancer Res. Treat. 2021, 20, 15330338211043328, Erratum in: Technol Cancer Res Treat. 2022, 21, 15330338221099574. [Google Scholar] [CrossRef]
- Köbel, M.; Piskorz, A.M.; Lee, S.; Lui, S.; LePage, C.; Marass, F.; Rosenfeld, N.; Mes Masson, A.M.; Brenton, J.D. Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J. Pathol. Clin. Res. 2016, 2, 247–258. [Google Scholar] [CrossRef]
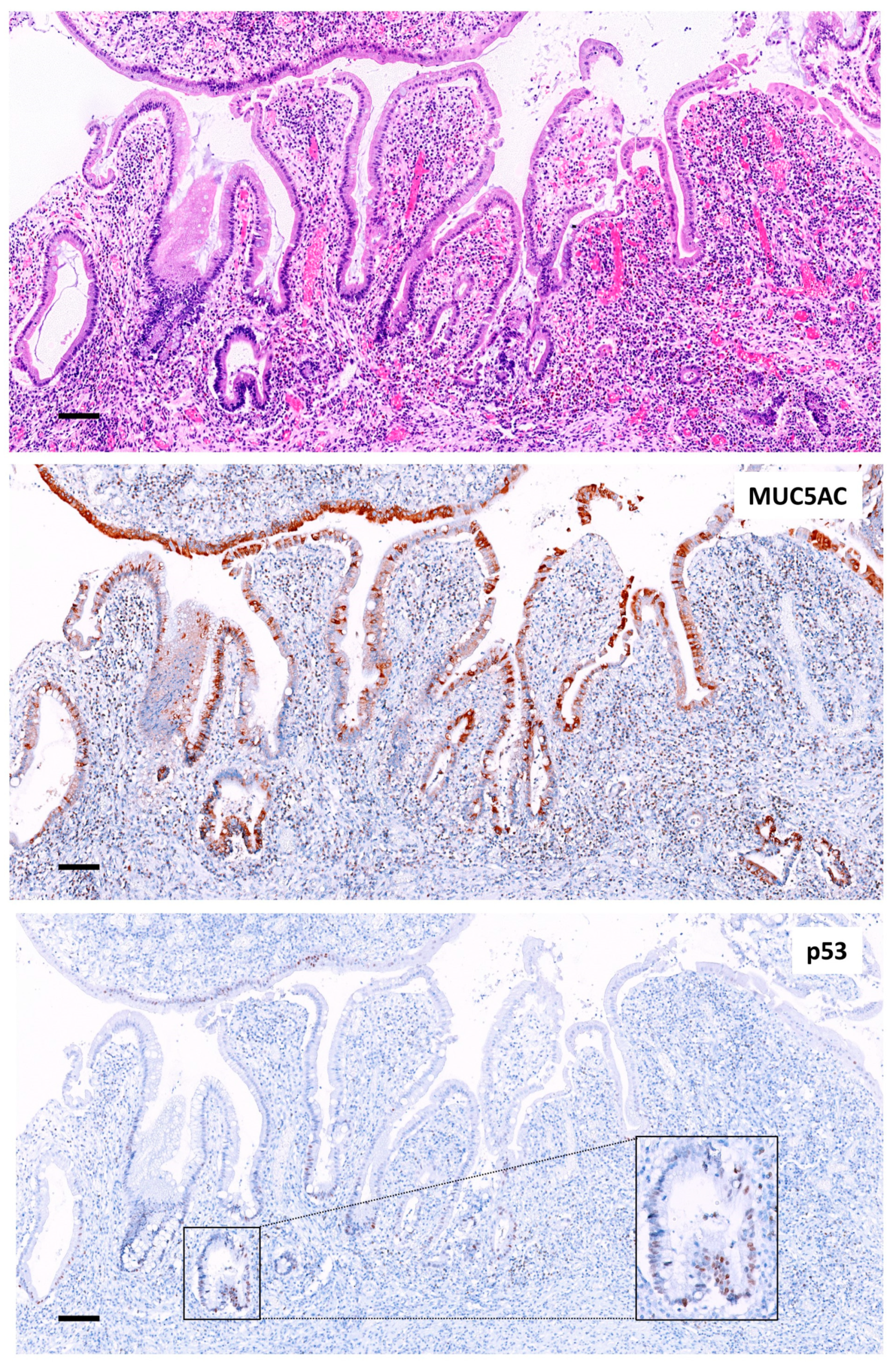
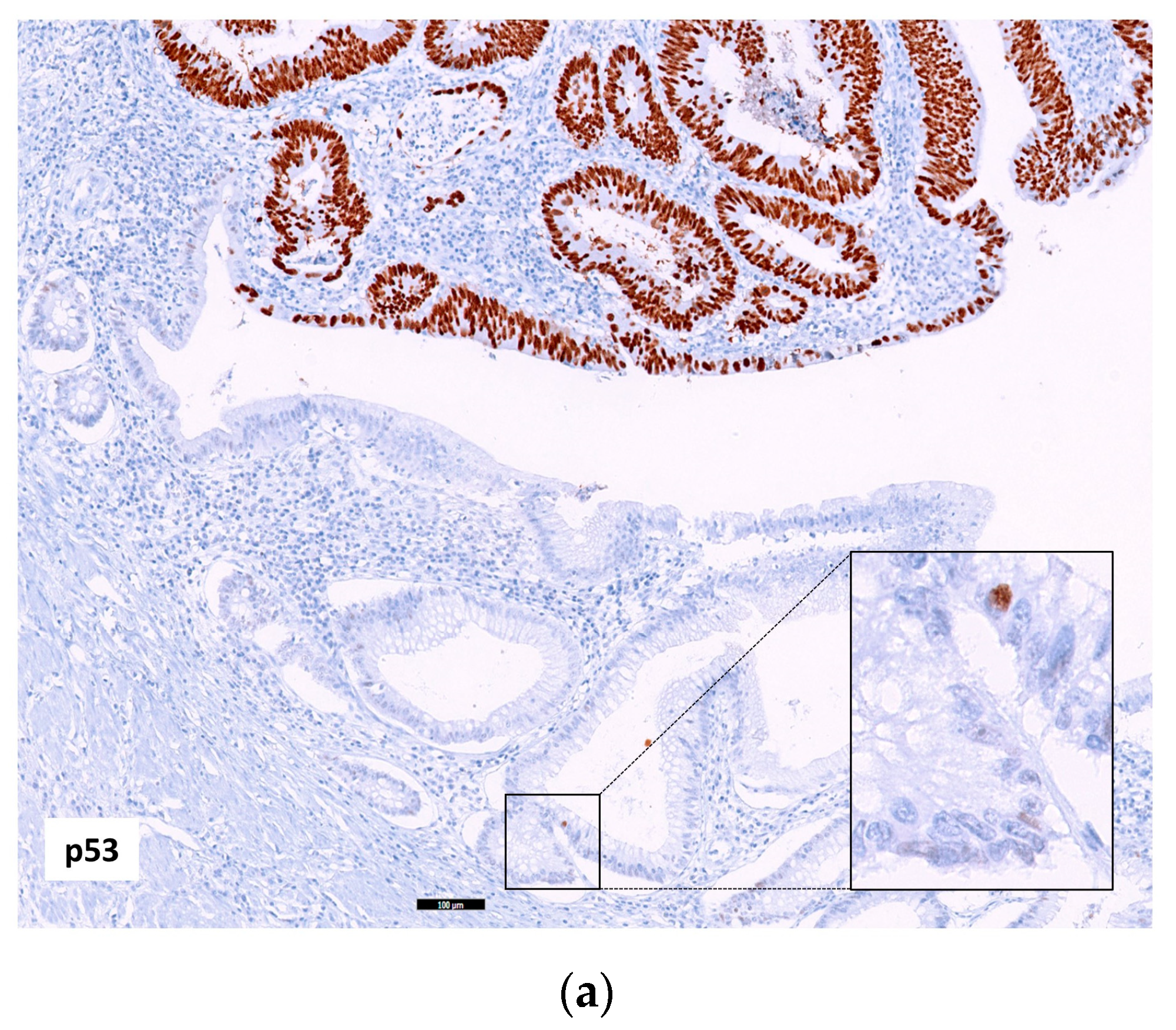
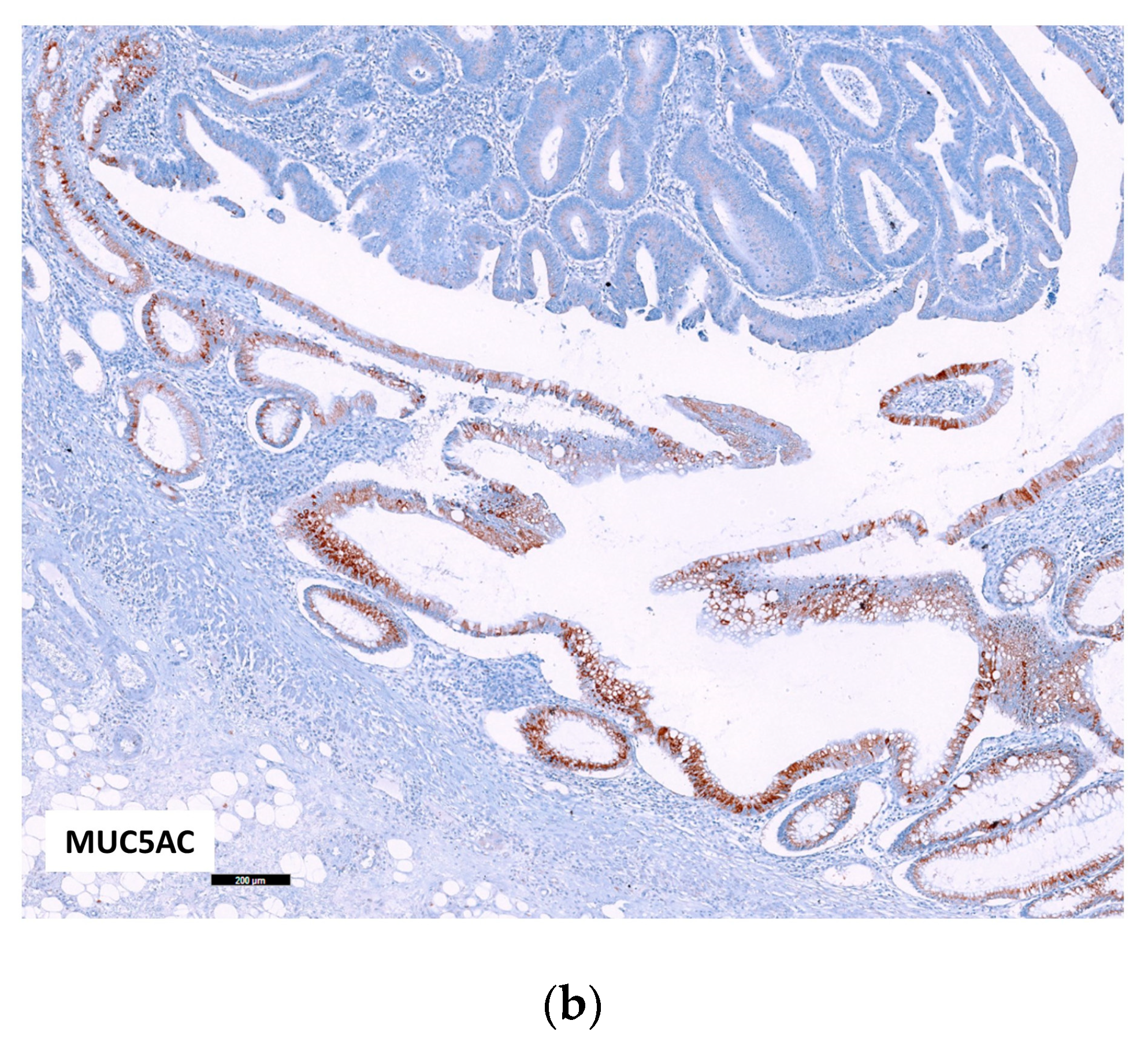

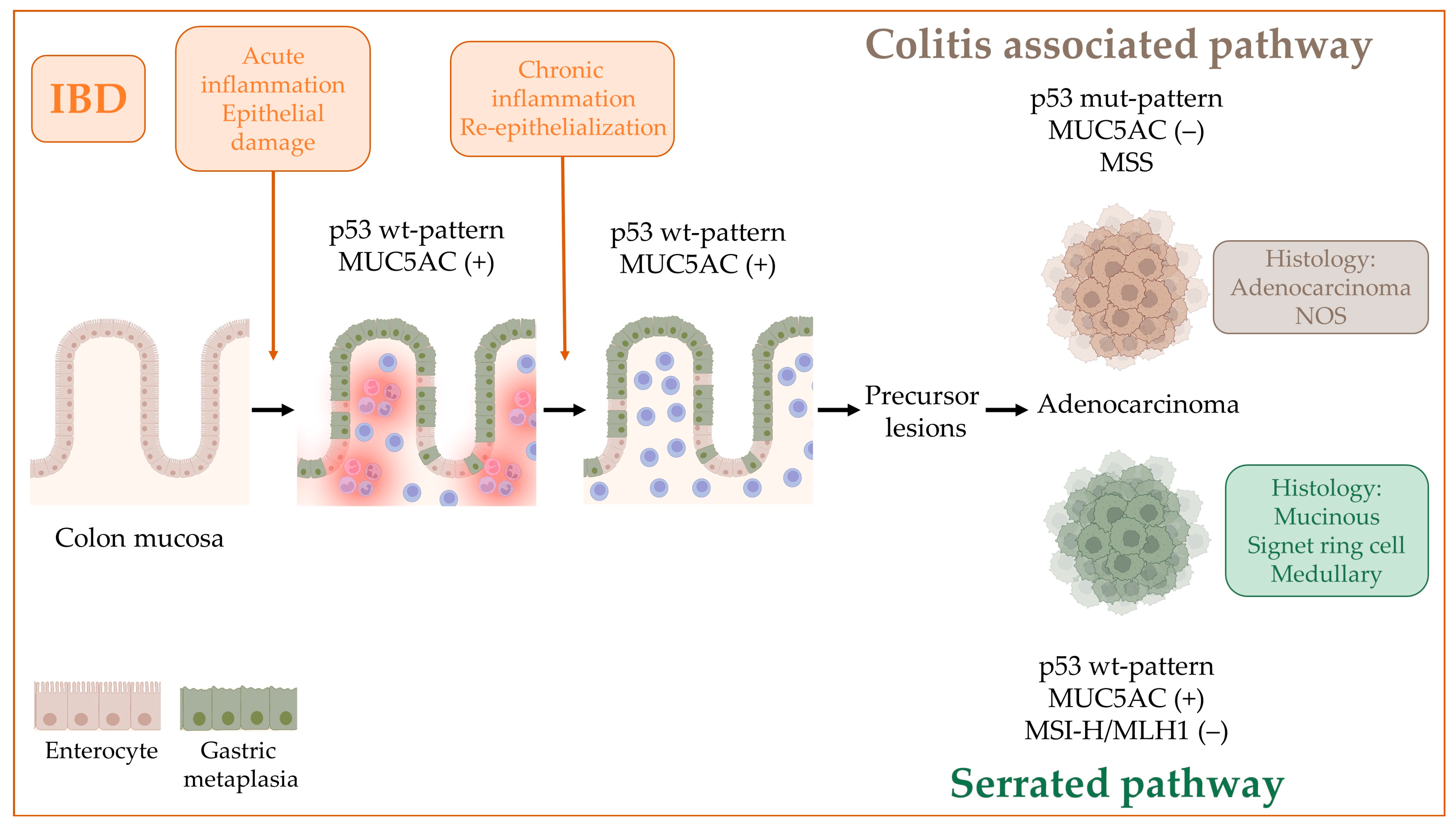
| Crohn’s Disease (%) | Ulcerative Colitis (%) | Total (%) | p Value | |
|---|---|---|---|---|
| Patients | 19 (36) | 34 (64) | 53 (100) | |
| Age Mean years [range] | 58.4 [35–88] | 64.8 [34–90] | 62.6 [34–90] | |
| Gender | 1.000 | |||
| Male | 14 (74) | 24 (71) | 38 (72) | |
| Female | 5 (26) | 10 (29) | 15 (28) | |
| IBD duration | 0.244 | |||
| Mean years [range] | 13.2 [1–43] | |||
| Concominant * | 5 (26) | 2 (6) | 7 (13.2) | |
| ≤8 years | 4 (21) | 13 (38) | 17 (32.1) | |
| 9–15 years | 3 (16) | 7 (20.6) | 10 (18.9) | |
| 16–20 years | 1 (5) | 5 (14.7) | 6 (11.3) | |
| >21 years | 5 (26) | 6 (17.6) | 11 (20.8) | |
| Unknown | 1 (5) | 1 (3) | 2 (3.8) | |
| IBD activity | 0.780 | |||
| Active | 11 (58) | 18 (53) | 29 (55) | |
| Quiescent | 8 (42) | 16 (47) | 24 (45) | |
| Synchronous CRC (n = 57) | 0.042 | |||
| Non synchronous CRC | 19 (100) | 30 (79) | 49 (86) | |
| 2 synchronous CRC | 0 | 8 (21) | 8 (14) | |
| Adenocarcinoma location (n = 57) | 0.003 | |||
| Ileum | 7 (37) | 0 | 7 (12.3) | |
| Right colon | 6 (31.5) | 11 (28.9) | 17 (29.8) | |
| Left colon | 3 (16) | 15 (39.5) | 18 (31.6) | |
| Rectum | 1 (5) | 6 (15.8) | 7 (12.3) | |
| Colon | 2 (10.5) | 5 (13.2) | 7 (12.3) | |
| Ileal reservory | 0 | 1 (2.6) | 1 (1.8) | |
| Histology (n = 57) | 0.504 | |||
| Adenocarcinoma NOS | 15 (78.9) | 32 (84.2) | 47 (82.5) | |
| Mucinous | 3 (15.8) | 4 (10.5) | 7 (12.3) | |
| Mucinous and signet ring cell | 1 (5.3) | 0 | 1 (1.8) | |
| Medullary | 0 | 1 (2.6) | 1 (1.8) | |
| Undifferentiated | 0 | 1 (2.6) | 1 (1.8) | |
| Histologic grade (n = 57) | 1.000 | |||
| Low-grade | 15 (79) | 31 (82) | 46 (81) | |
| High-grade | 4 (21) | 7 (18) | 11 (19) | |
| Adjacent mucosa to adenocarcinoma (n = 57) | 0.077 | |||
| Absent | 10 (53) | 12 (32) | 22 (39) | |
| Present | 9 (47) | 26 (68) | 35 (61) | |
| Active inflammation | 0 | 4 (15) | 4 (11.4) | |
| Chronic changes | 4 (21) | 15 (58) | 19 (54.3) | |
| Normal | 5 (26) | 7 (27) | 12 (34.3) | |
| Stage (8th ed) (n = 57) | 0.305 | |||
| I | 3 (15.8) | 9 (23.7) | 12 (21) | |
| II | 6 (31.6) | 17 (44.7) | 23 (40) | |
| III | 10 (52.6) | 12 (31.6) | 22 (39) |
| Crohn’s Disease (%) | Ulcerative Colitis (%) | Total (%) | p Value | ||
|---|---|---|---|---|---|
| p53 pattern | 0.914 | ||||
| mut-pattern | Overexpression | 8 (42) | 14 (37) | 22 (39) | |
| Null pattern | 2 (11) | 5 (13) | 7 (12) | ||
| wt-pattern | Focal nuclear staining | 9 (47) | 19 (50) | 28 (49) | |
| Microsatellite instability | 0.389 | ||||
| MSI-H | 3 (16) | 3 (8) | 6 (11) | ||
| MSS | 16 (84) | 35 (92) | 51 (89) | ||
| MUC5AC | 0.511 | ||||
| Negative | 10 (52.6) | 22 (57.9) | 32 (56.1) | ||
| Positive | Extensive | 2 (10.5) | 7 (18.4) | 9 (15.8) | |
| Focal | 7 (36.8) | 9 (23.7) | 16 (28.1) |
| Microsatellite Instability | ||||
|---|---|---|---|---|
| MSI-H (%) | MSS (%) | Total (%) | p Value | |
| p53 pattern | 0.010 | |||
| mut-pattern | 0 | 29 (57) | 29 (51) | |
| wt-pattern | 6 (100) | 22 (43) | 28 (49) | |
| MUC5AC | ||||
|---|---|---|---|---|
| Negative (%) | Positive (%) | Total (%) | p Value | |
| p53 pattern | 0.186 | |||
| mut-pattern | 19 (59) | 10 (40) | 29 (51) | |
| wt- pattern | 13 (41) | 15 (60) | 28 (49) | |
| MUC5AC | ||||
|---|---|---|---|---|
| Negative (%) | Positive (%) | Total (%) | p Value | |
| Microsatellite instability | 0.005 | |||
| MSI-H | 0 | 6 (24) | 6 (11) | |
| MSS | 32 (100) | 19 (76) | 51 (89) | |
| Patient | Age (Years) | Gender | IBD | IBD Duration (Years) | Location | Histology | p53 Pattern | MUC5AC | MLH1 | BRAF Status | MLH1 Methylation | Predisposition to Cancer |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 73 | Male | UC | 12 | Right colon | Medullary | wt | Focal positive | Negative | V600E | NP | Sporadic vs. IBD-associated |
| 8 | Right colon (transvers) | NOS with mucinous component | wt | Focal positive | Negative | V600E | NP | Sporadic vs. IBD-associated | ||||
| 14 | 52 | Male | UC | 4 | Right colon | NOS | wt | Extensive positive | Negative | wt | No methylated | Lynch syndrome |
| 20 | 50 | Male | CD | Concomitant | Ileum | NOS with mucinous component | wt | Extensive positive | Negative | wt | NP | Unknown |
| 24 | 54 | Male | CD | 1 | Ileum | NOS | wt | Focal positive | Negative | wt* | NP | Unknown |
| 53 | 52 | Female | CD | 2 | Right colon | Mucinous and signet ring cell | wt | Extensive positive | Negative | wt | NP | Unknown |
| MUC5AC | ||||
|---|---|---|---|---|
| Negative (%) | Positive (%) | Total (%) | p Value | |
| Adjacent mucosa (n = 35) | 1.000 | |||
| Location | ||||
| Colon | 8 (100) | 26 (96) | 34 (97) | |
| Small bowel | 0 | 1 (4) | 1 (3) | |
| Specific location | 0.614 | |||
| Ileum | 0 | 1 (4) | 1 (3) | |
| Right | 3 (37.5) | 7 (26) | 10 (29) | |
| Left | 3 (37.5) | 12 (44) | 15 (43) | |
| Rectum | 0 | 4 (15) | 4 (11) | |
| Colon | 2 (25) | 3 (11) | 5 (14) | |
| Status | 0.374 | |||
| Active inflammation | 0 | 4 (14.8) | 4 (11.4) | |
| Chronic changes | 4 (50) | 15 (55.6) | 19 (54.3) | |
| Normal | 4 (50) | 8 (29.6) | 12 (34.3) | |
| MUC5AC in adenocarcinoma | 0.700 | |||
| Negative | 4 (50) | 16 (59) | 20 (57) | |
| Positive | 4 (50) | 11 (41) | 15 (43) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gené, M.; Cuatrecasas, M.; Amat, I.; Veiga, J.A.; Fernández Aceñero, M.J.; Fusté Chimisana, V.; Tarragona, J.; Jurado, I.; Fernández-Victoria, R.; Martínez Ciarpaglini, C.; et al. Alterations in p53, Microsatellite Stability and Lack of MUC5AC Expression as Molecular Features of Colorectal Carcinoma Associated with Inflammatory Bowel Disease. Int. J. Mol. Sci. 2023, 24, 8655. https://doi.org/10.3390/ijms24108655
Gené M, Cuatrecasas M, Amat I, Veiga JA, Fernández Aceñero MJ, Fusté Chimisana V, Tarragona J, Jurado I, Fernández-Victoria R, Martínez Ciarpaglini C, et al. Alterations in p53, Microsatellite Stability and Lack of MUC5AC Expression as Molecular Features of Colorectal Carcinoma Associated with Inflammatory Bowel Disease. International Journal of Molecular Sciences. 2023; 24(10):8655. https://doi.org/10.3390/ijms24108655
Chicago/Turabian StyleGené, Míriam, Míriam Cuatrecasas, Irene Amat, Jesús Alberto Veiga, María Jesús Fernández Aceñero, Victòria Fusté Chimisana, Jordi Tarragona, Ismael Jurado, Rebeca Fernández-Victoria, Carolina Martínez Ciarpaglini, and et al. 2023. "Alterations in p53, Microsatellite Stability and Lack of MUC5AC Expression as Molecular Features of Colorectal Carcinoma Associated with Inflammatory Bowel Disease" International Journal of Molecular Sciences 24, no. 10: 8655. https://doi.org/10.3390/ijms24108655
APA StyleGené, M., Cuatrecasas, M., Amat, I., Veiga, J. A., Fernández Aceñero, M. J., Fusté Chimisana, V., Tarragona, J., Jurado, I., Fernández-Victoria, R., Martínez Ciarpaglini, C., Alenda González, C., Zac, C., Ortega de la Obra, P., Fernández-Figueras, M. T., Esteller, M., & Musulen, E. (2023). Alterations in p53, Microsatellite Stability and Lack of MUC5AC Expression as Molecular Features of Colorectal Carcinoma Associated with Inflammatory Bowel Disease. International Journal of Molecular Sciences, 24(10), 8655. https://doi.org/10.3390/ijms24108655








