Xenon’s Sedative Effect Is Mediated by Interaction with the Cyclic Nucleotide-Binding Domain (CNBD) of HCN2 Channels Expressed by Thalamocortical Neurons of the Ventrobasal Nucleus in Mice
Abstract
1. Introduction
2. Results
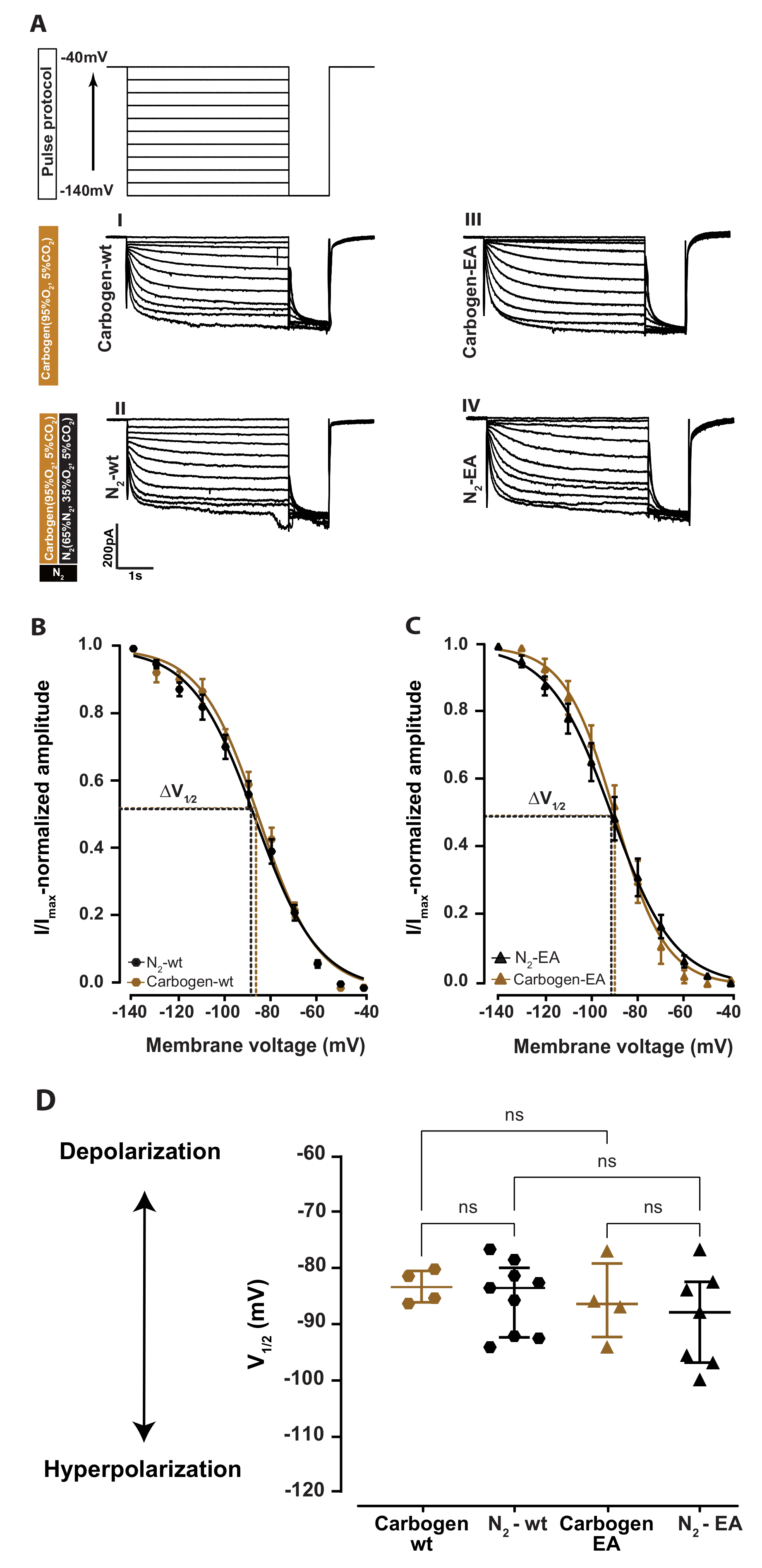
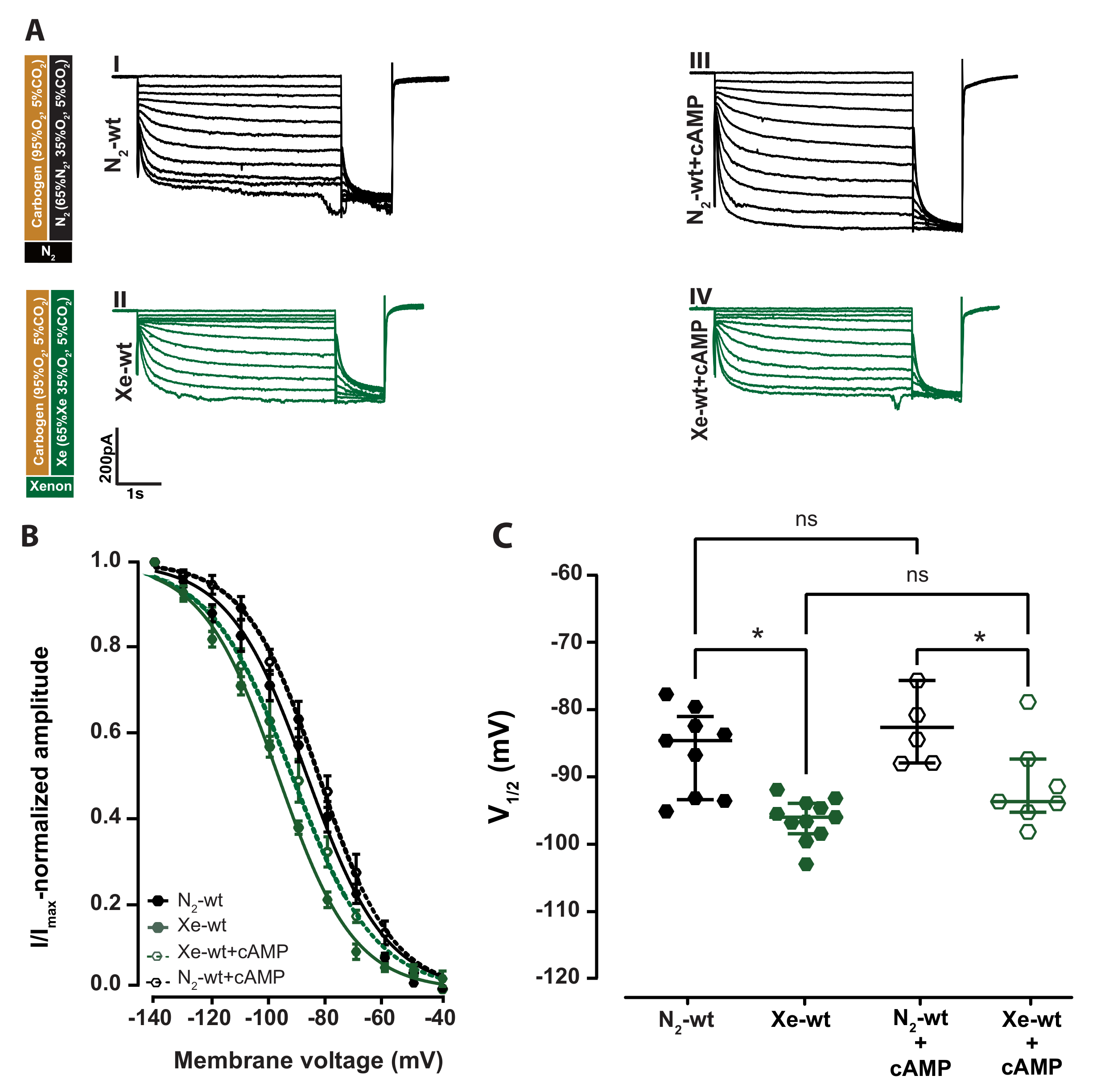
2.1. Xenon Impairs HCN2 Ih Current of VB Neurons in Wild-Type Acute Brain Slice
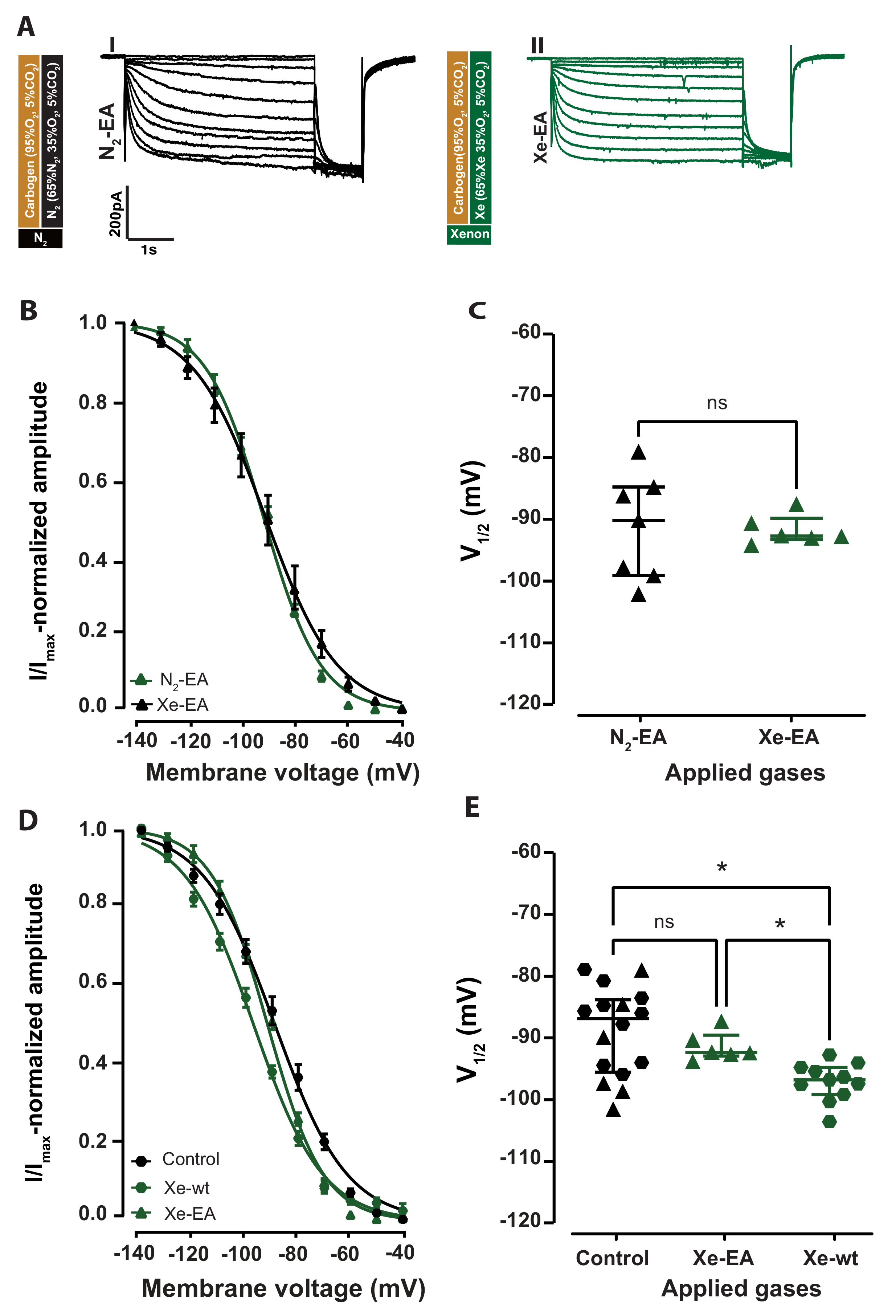
2.2. Cyclic Nucleotide Binding Domain (CNBD) Mutation Reduces the Inhibitory Effect by Xenon on Ih
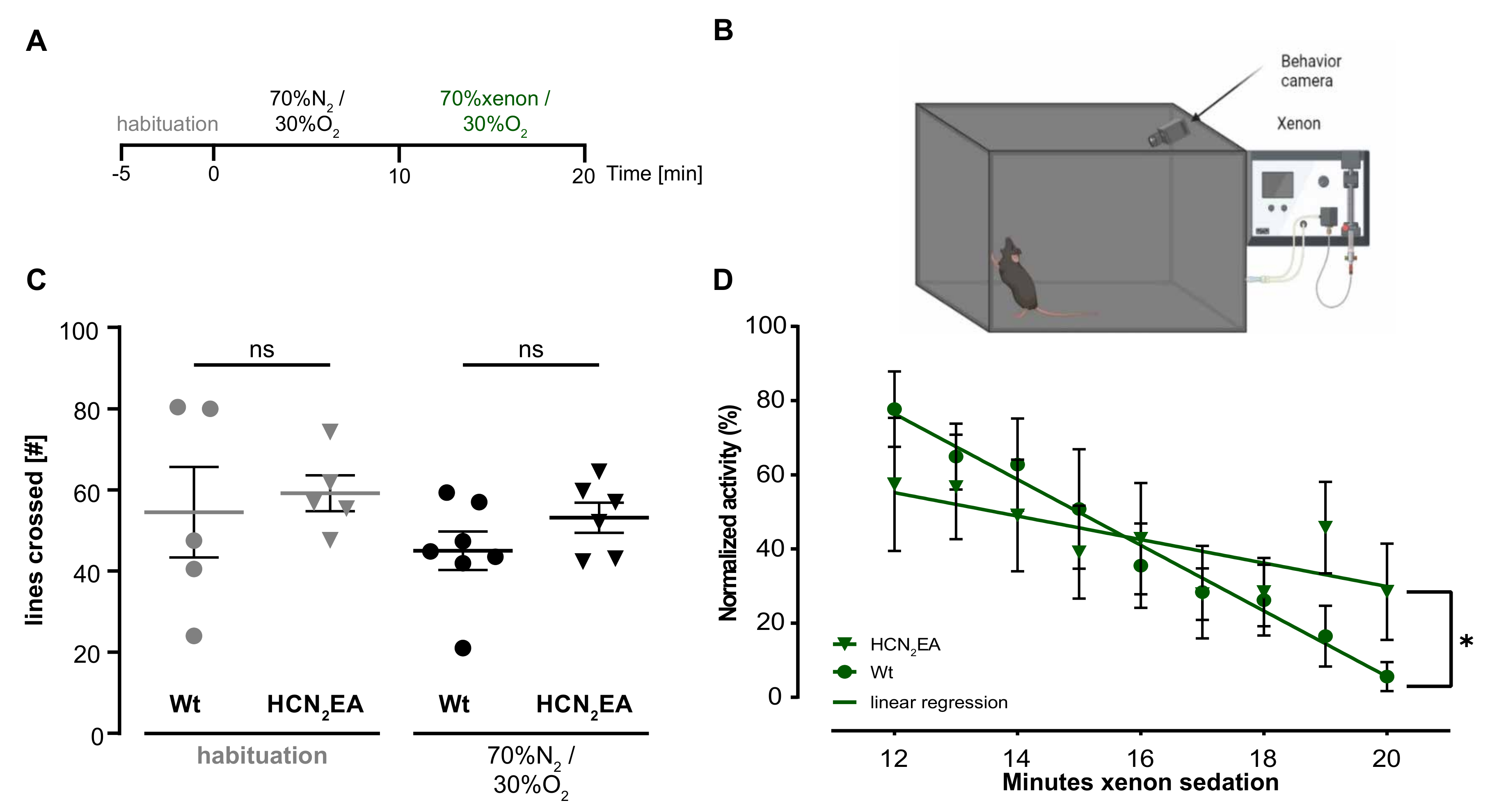
2.3. Xenon Sedation Is Absent in The HCN2 Knock-In Mouse Model (HCN2EA)
3. Discussion
4. Materials and Methods
4.1. Acute Thalamocortical Brain Slice Preparation
4.2. Whole-Cell Voltage Patch Clamp Recordings
4.3. Application of Nitrogen and Xenon Gas Mixture
4.4. Modified Open-Field Test
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCN2 | Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels channel type-2 |
| HCN2EA | Hyperpolarization-Activated Cyclic Nucleotide-Gated Channels channel type-2 |
| knocked-in channel | |
| CNBD | Cyclic nucleotide-binding domain |
| cAMP | Cyclic adenosine monophosphate |
| cGMP | Cyclic guanosine monophosphate |
| VB | Thalamus ventrobasal nucleus |
| mV | Millivolts |
| pA | PicoAmperes |
| mM | Millimolar |
| ÁM | Micromolar |
| kHz | kilohertz |
| ms | milliseconds |
| min | minutes |
| A | Amyloid beta |
| NMDA | The N-methyl-D-aspartate |
| AMPA | -amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor |
| (K2P) | two-pore domain acid-sensitive potassium channels |
References
- Hofland, J.; Ouattara, A.; Fellahi, J.L.; Gruenewald, M.; Hazebroucq, J.; Ecoffey, C.; Joseph, P.; Heringlake, M.; Steib, A.; Coburn, M.; et al. Effect of Xenon Anesthesia Compared to Sevoflurane and Total Intravenous Anesthesia for Coronary Artery Bypass Graft Surgery on Postoperative Cardiac Troponin Release: An International, Multicenter, Phase 3, Single-blinded, Randomized Noninferiority Trial. Anesthesiology 2017, 127, 918–933. [Google Scholar] [CrossRef] [PubMed]
- Hecker, K.; Baumert, J.H.; Horn, N.; Rossaint, R. Xenon, a modern anaesthesia gas. Minerva Anestesiol. 2004, 70, 255–260. [Google Scholar] [PubMed]
- Thevis, M.; Piper, T.; Geyer, H.; Thomas, A.; Schaefer, M.S.; Kienbaum, P.; Schanzer, W. Measuring xenon in human plasma and blood by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2014, 28, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.S.; Christopher, A.; Pulipaka, S.K.; Suvvari, P.; Kodisharapu, P.K.; Rayani, B.K. Efficacy of xenon anesthesia in preventing postoperative cognitive dysfunction after cardiac and major non-cardiac surgeries in elderly patients: A topical review. Med. Gas Res. 2021, 5, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Burge, M.; Kratzer, S.; Mattusch, C.; Hofmann, C.; Kreuzer, M.; Parsons, C.G.; Rammes, G. The anaesthetic xenon partially restores an amyloid beta-induced impairment in murine hippocampal synaptic plasticity. Neuropharmacology 2019, 151, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, C.; Sander, A.; Wang, X.X.; Buerge, M.; Jungwirth, B.; Borgstedt, L.; Kreuzer, M.; Kopp, C.; Schorpp, K.; Hadian, K.; et al. Inhalational Anesthetics Do Not Deteriorate Amyloid-β-Derived Pathophysiology in Alzheimer’s Disease: Investigations on the Molecular, Neuronal, and Behavioral Level. J. Alzheimer’s Dis. 2021, 84, 1193–1218. [Google Scholar] [CrossRef]
- Harris, K.; Armstrong, S.P.; Campos-Pires, R.; Kiru, L.; Franks, N.P.; Dickinson, R. Neuroprotection against Traumatic Brain Injury by Xenon, but Not Argon, Is Mediated by Inhibition at the N-Methyl-d-Aspartate Receptor Glycine Site. Anesthesiology 2013, 119, 1137–1148. [Google Scholar] [CrossRef]
- Armstrong, S.P.; Banks, P.J.; McKitrick, T.J.W.; Geldart, C.H.; Edge, C.J.; Babla, R.; Simillis, C.; Franks, N.P.; Dickinson, R. Identification of Two Mutations (F758W and F758Y) in the N -methyl-D-aspartate Receptor Glycine-binding Site that Selectively Prevent Competitive Inhibition by Xenon without Affecting Glycine Binding. Anesthesiology 2012, 117, 38–47. [Google Scholar] [CrossRef]
- Gruss, M.; Bushell, T.J.; Bright, D.P.; Lieb, W.R.; Mathie, A.; Franks, N.P. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol. Pharmacol. 2004, 65, 443–452. [Google Scholar] [CrossRef]
- Robinson, R.B.; Siegelbaum, S.A. Hyperpolarization-Activated Cation Currents: From Molecules to Physiological Function. Annu. Rev. Physiol. 2003, 65, 453–480. [Google Scholar] [CrossRef]
- Mattusch, C.; Kratzer, S.; Buerge, M.; Kreuzer, M.; Engel, T.; Kopp, C.; Biel, M.; Hammelmann, V.; Ying, S.W.; Goldstein, P.A.; et al. Impact of Hyperpolarization-activated, Cyclic Nucleotide-gated Cation Channel Type 2 for the Xenon-mediated Anesthetic Effect: Evidence from In Vitro and In Vivo Experiments. Anesthesiology 2015, 122, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.M. Thalamic relays and cortical functioning. Prog. Brain Res. 2005, 149, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.M.; Guillery, R.W. Functional organization of thalamocortical relays. J. Neurophysiol. 1996, 76, 1367–1395. [Google Scholar] [CrossRef]
- McCormick, D.A.; Bal, T. Sleep and arousal: Thalamocortical mechanisms. Annu. Rev. Neurosci. 1997, 20, 185–215. [Google Scholar] [CrossRef] [PubMed]
- Biel, M.; Wahl-Schott, C.; Michalakis, S.; Zong, X. Hyperpolarization-Activated Cation Channels: From Genes to Function. Physiol. Rev. 2009, 89, 847–885. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.F.; Malleret, G.; Dudman, J.T.; Buhl, D.L.; Santoro, B.; Gibbs, E.; Vronskaya, S.; Buzsaki, G.; Siegelbaum, S.A.; Kandel, E.R.; et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell 2004, 119, 719–732. [Google Scholar] [CrossRef]
- DiFrancesco, D. The role of the funny current in pacemaker activity. Circ. Res. 2010, 106, 434–446. [Google Scholar] [CrossRef]
- Pape, H.C.; McCormick, D.A. Noradrenaline and serotonin selectively modulate thalamic burst firing by enhancing a hyperpolarization-activated cation current. Nature 1989, 340, 715–718. [Google Scholar] [CrossRef]
- Wahl-Schott, C.; Biel, M. HCN channels: Structure, cellular regulation and physiological function. Cell Mol. Life Sci. 2009, 66, 470–494. [Google Scholar] [CrossRef]
- Craven, K.B.; Zagotta, W.N. CNG AND HCN CHANNELS: Two Peas, One Pod. Annu. Rev. Physiol. 2006, 68, 375–401. [Google Scholar] [CrossRef]
- Baruscotti, M.; Bucchi, A.; Difrancesco, D. Physiology and pharmacology of the cardiac pacemaker (“funny”) current. Pharmacol. Ther. 2005, 107, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Pedarzani, P.; Storm, J.F. Protein kinase A-independent modulation of ion channels in the brain by cyclic AMP. Proc. Natl. Acad. Sci. USA 1995, 92, 11716–11720. [Google Scholar] [CrossRef] [PubMed]
- Hammelmann, V.; Stieglitz, M.S.; Hülle, H.; Le Meur, K.; Kass, J.; Brümmer, M.; Gruner, C.; Rötzer, R.D.; Fenske, S.; Hartmann, J.; et al. Abolishing cAMP sensitivity in HCN2 pacemaker channels induces generalized seizures. JCI Insight 2019, 4, e126418. [Google Scholar] [CrossRef] [PubMed]
- Kratzer, S.; Haseneder, R.; Goldstein, P.A.; Kochs, E.; Rammes, G. Impact of Hyperpolarization-activated, Cyclic Nucleotide-gated Cation Channel Type 2 for the Xenon-mediated Anesthetic Effect: Evidence from In Vitro and In Vivo Experiments. Anesthesiology 2017, 127, 905–910. [Google Scholar] [CrossRef]
- Lee, C.H.; MacKinnon, R. Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell 2017, 168, 111–120.e11. [Google Scholar] [CrossRef]
- Zagotta, W.N.; Olivier, N.B.; Black, K.D.; Young, E.C.; Olson, R.; Gouaux, E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature 2003, 425, 200–205. [Google Scholar] [CrossRef]
- Liu, D.T.; Tibbs, G.R.; Paoletti, P.; Siegelbaum, S.A. Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron 1998, 21, 235–248. [Google Scholar] [CrossRef]
- Patel, V.R.; Salinas, A.M.; Qi, D.; Gupta, S.; Sidote, D.J.; Goldschen-Ohm, M.P. Single-molecule imaging with cell-derived nanovesicles reveals early binding dynamics at a cyclic nucleotide-gated ion channel. Nat. Commun. 2021, 12, 6459. [Google Scholar] [CrossRef]
- Cullen, S.C.; Gross, E.G. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science 1951, 113, 580–582. [Google Scholar] [CrossRef]
- Campos-Pires, R.; Koziakova, M.; Yonis, A.; Pau, A.; Macdonald, W.; Harris, K.; Edge, C.J.; Franks, N.P.; Mahoney, P.F.; Dickinson, R. Xenon Protects against Blast-Induced Traumatic Brain Injury in an In Vitro Model. J. Neurotrauma 2018, 35, 1037–1044. [Google Scholar] [CrossRef]
- Neice, A.E.; Zornow, M.H. Xenon anaesthesia for all, or only a select few? Anaesthesia 2016, 71, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Devroe, S.; Devriese, L.; Debuck, F.; Fieuws, S.; Cools, B.; Gewillig, M.; Van de Velde, M.; Rex, S. Effect of xenon and dexmedetomidine as adjuncts for general anesthesia on postoperative emergence delirium after elective cardiac catheterization in children: Study protocol for a randomized, controlled, pilot trial. Trials 2020, 21, 310. [Google Scholar] [CrossRef] [PubMed]
- Rex, S.; Schaefer, W.; Meyer, P.H.; Rossaint, R.; Boy, C.; Setani, K.; Bull, U.; Baumert, J.H. Positron emission tomography study of regional cerebral metabolism during general anesthesia with xenon in humans. Anesthesiology 2006, 105, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Guillery, R.W.; Sherman, S.M. Thalamic Relay Functions and Their Role in Corticocortical Communication. Neuron 2002, 33, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Santoro, B.; Tibbs, G.R. The HCN Gene Family: Molecular Basis of the Hyperpolarization-Activated Pacemaker Channels. Ann. N. Y. Acad. Sci. 1999, 868, 741–764. [Google Scholar] [CrossRef]
- Yu, F.H.; Yarov-Yarovoy, V.; Gutman, G.A.; Catterall, W.A. Overview of Molecular Relationships in the Voltage-Gated Ion Channel Superfamily. Pharmacol. Rev. 2005, 57, 387–395. [Google Scholar] [CrossRef]
- Moosmang, S.; Biel, M.; Hofmann, F.; Ludwig, A. Differential distribution of four hyperpolarization-activated cation channels in mouse brain. Biol. Chem. 1999, 380, 975–980. [Google Scholar] [CrossRef]
- Ludwig, A.; Budde, T.; Stieber, J.; Moosmang, S.; Wahl, C.; Holthoff, K.; Langebartels, A.; Wotjak, C.; Munsch, T.; Zong, X.; et al. Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. Embo J. 2003, 22, 216–224. [Google Scholar] [CrossRef]
- Zobeiri, M.; Chaudhary, R.; Blaich, A.; Rottmann, M.; Herrmann, S.; Meuth, P.; Bista, P.; Kanyshkova, T.; Lüttjohann, A.; Narayanan, V.; et al. The Hyperpolarization-Activated HCN4 Channel is Important for Proper Maintenance of Oscillatory Activity in the Thalamocortical System. Cereb. Cortex 2019, 29, 2291–2304. [Google Scholar] [CrossRef]
- Wainger, B.J.; DeGennaro, M.; Santoro, B.; Siegelbaum, S.A.; Tibbs, G.R. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature 2001, 411, 805–810. [Google Scholar] [CrossRef]
- Hammelmann, V.; Zong, X.; Hofmann, F.; Michalakis, S.; Biel, M. The cGMP-Dependent Protein Kinase II Is an Inhibitory Modulator of the Hyperpolarization-Activated HCN2 Channel. PLoS ONE 2011, 6, e17078. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Pape, H.C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. 1990, 431, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Alkire, M.T. Probing the mind: Anesthesia and neuroimaging. Clin Pharmacol Ther 2008, 84, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.W.; Abbas, S.Y.; Harrison, N.L.; Goldstein, P.A. Propofol block of I(h) contributes to the suppression of neuronal excitability and rhythmic burst firing in thalamocortical neurons. Eur. J. Neurosci. 2006, 23, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Koblin, D.D.; Fang, Z.; Eger, E.I.; Laster, M.J.; Gong, D.; Ionescu, P.; Halsey, M.J.; Trudell, J.R. Minimum Alveolar Concentrations of Noble Gases, Nitrogen, and Sulfur Hexafluoride in Rats: Helium and Neon as Nonimmobilizers (Nonanesthetics). Anesth. Analg. 1998, 87, 419–424. [Google Scholar] [CrossRef]
- Budde, T.; Coulon, P.; Pawlowski, M.; Meuth, P.; Kanyshkova, T.; Japes, A.; Meuth, S.G.; Pape, H.C. Reciprocal modulation of I (h) and I (TASK) in thalamocortical relay neurons by halothane. Pflugers Arch. 2008, 456, 1061–1073. [Google Scholar] [CrossRef]
- McCormick, D.A.; Bal, T. Sensory gating mechanisms of the thalamus. Curr. Opin. Neurobiol. 1994, 4, 550–556. [Google Scholar] [CrossRef]
- Franks, N.P. General anaesthesia: From molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008, 9, 370–386. [Google Scholar] [CrossRef]
- Ávalos Prado, P.; Chassot, A.A.; Landra-Willm, A.; Sandoz, G. Regulation of two-pore-domain potassium TREK channels and their involvement in pain perception and migraine. Neurosci. Lett. 2022, 773, 136494. [Google Scholar] [CrossRef]
- Haseneder, R.; Kratzer, S.; Kochs, E.; Eckle, V.S.; Zieglgansberger, W.; Rammes, G. Xenon reduces N-methyl-D-aspartate and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor-mediated synaptic transmission in the amygdala. Anesthesiology 2008, 109, 998–1006. [Google Scholar] [CrossRef]
- Dickinson, R.; Peterson, B.K.; Banks, P.; Simillis, C.; Martin, J.C.S.; Valenzuela, C.A.; Maze, M.; Franks, N.P. Competitive Inhibition at the Glycine Site of the N -Methyl-d-aspartate Receptor by the Anesthetics Xenon and Isoflurane: Evidence from Molecular Modeling and Electrophysiology. Anesthesiology 2007, 107, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Franks, N.P.; Honoré, E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol. Sci. 2004, 25, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Bantel, C.; Maze, M.; Trapp, S. Noble gas xenon is a novel adenosine triphosphate-sensitive potassium channel opener. Anesthesiology 2010, 112, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Shigemoto, R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J. Comp. Neurol. 2004, 471, 241–276. [Google Scholar] [CrossRef] [PubMed]
- Datunashvili, M.; Chaudhary, R.; Zobeiri, M.; Lüttjohann, A.; Mergia, E.; Baumann, A.; Balfanz, S.; Budde, B.; van Luijtelaar, G.; Pape, H.C.; et al. Modulation of Hyperpolarization-Activated Inward Current and Thalamic Activity Modes by Different Cyclic Nucleotides. Front. Cell. Neurosci. 2018, 12, 369. [Google Scholar] [CrossRef]
- Keine, C. Patchmaster Importer. 2023. Available online: https://github.com/ChristianKeine/HEKA_Patchmaster_Importer (accessed on 1 April 2023).
- Ghasemi, A.; Zahediasl, S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Mandrekar, J.N. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassab, N.E.D.; Mehlfeld, V.; Kass, J.; Biel, M.; Schneider, G.; Rammes, G. Xenon’s Sedative Effect Is Mediated by Interaction with the Cyclic Nucleotide-Binding Domain (CNBD) of HCN2 Channels Expressed by Thalamocortical Neurons of the Ventrobasal Nucleus in Mice. Int. J. Mol. Sci. 2023, 24, 8613. https://doi.org/10.3390/ijms24108613
Kassab NED, Mehlfeld V, Kass J, Biel M, Schneider G, Rammes G. Xenon’s Sedative Effect Is Mediated by Interaction with the Cyclic Nucleotide-Binding Domain (CNBD) of HCN2 Channels Expressed by Thalamocortical Neurons of the Ventrobasal Nucleus in Mice. International Journal of Molecular Sciences. 2023; 24(10):8613. https://doi.org/10.3390/ijms24108613
Chicago/Turabian StyleKassab, Nour El Dine, Verena Mehlfeld, Jennifer Kass, Martin Biel, Gerhard Schneider, and Gerhard Rammes. 2023. "Xenon’s Sedative Effect Is Mediated by Interaction with the Cyclic Nucleotide-Binding Domain (CNBD) of HCN2 Channels Expressed by Thalamocortical Neurons of the Ventrobasal Nucleus in Mice" International Journal of Molecular Sciences 24, no. 10: 8613. https://doi.org/10.3390/ijms24108613
APA StyleKassab, N. E. D., Mehlfeld, V., Kass, J., Biel, M., Schneider, G., & Rammes, G. (2023). Xenon’s Sedative Effect Is Mediated by Interaction with the Cyclic Nucleotide-Binding Domain (CNBD) of HCN2 Channels Expressed by Thalamocortical Neurons of the Ventrobasal Nucleus in Mice. International Journal of Molecular Sciences, 24(10), 8613. https://doi.org/10.3390/ijms24108613





