The Role of Cardiac Fibrosis in Diabetic Cardiomyopathy: From Pathophysiology to Clinical Diagnostic Tools
Abstract
1. Introduction
2. Pathologic Myocardial Fibrosis of Diabetic Cardiomyopathy
2.1. Hyperglycemia Induces Cardiac Fibrosis
2.2. Activating TGF-β Signaling Pathway
2.3. Activating Adipokines and ET-1 Signaling Pathway
2.4. ROS-Dependent Microvascular Inflammation
2.5. ECM Receptors Mediating DM Cardiomyopathy
3. Clinical Tools for Cardiac Functional and Anatomic Remodeling Diagnosis in DM Cardiomyopathy
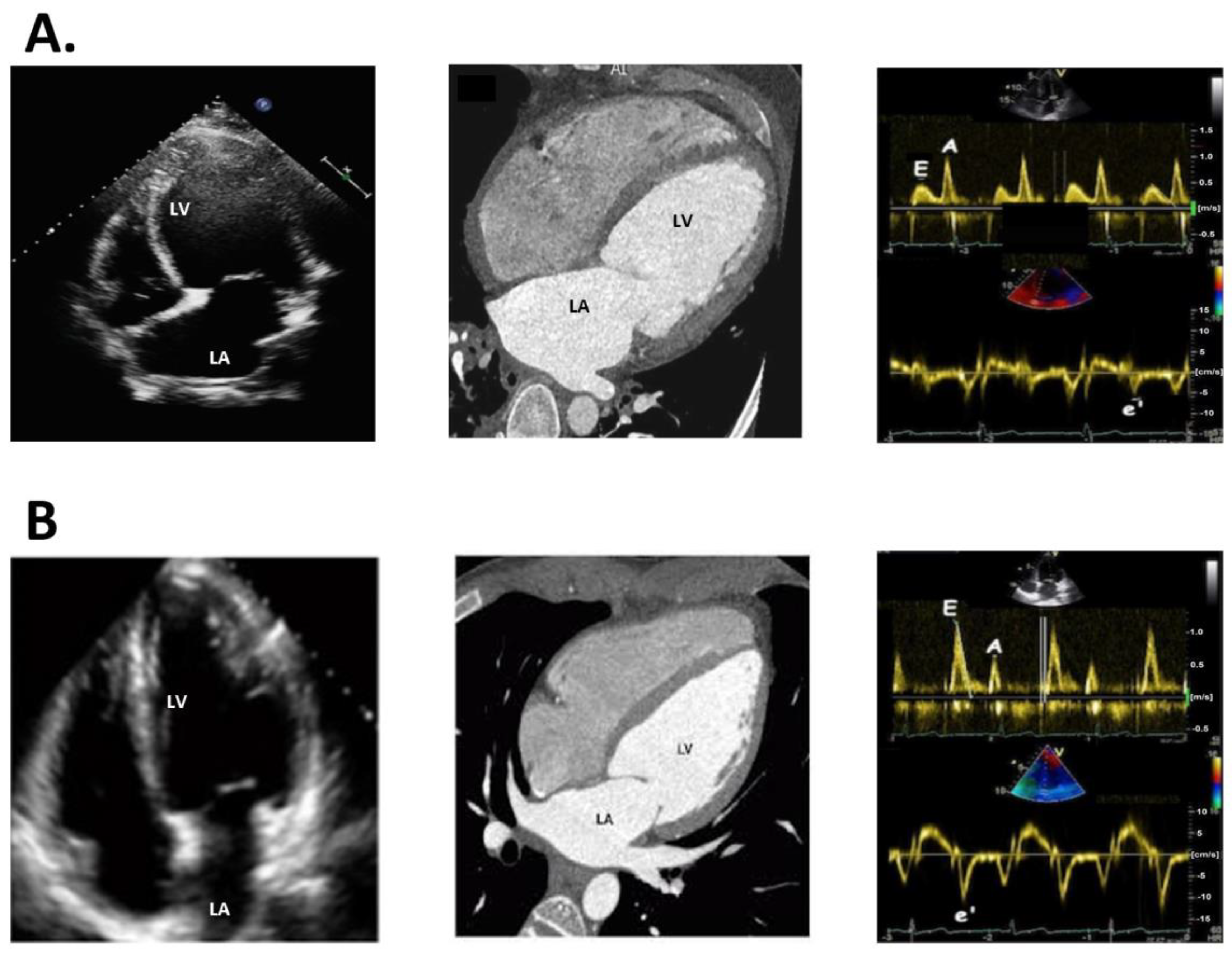
3.1. Echocardiography
3.2. Cardiac CT
3.3. Cardiac MRI
3.4. Nuclear Imaging
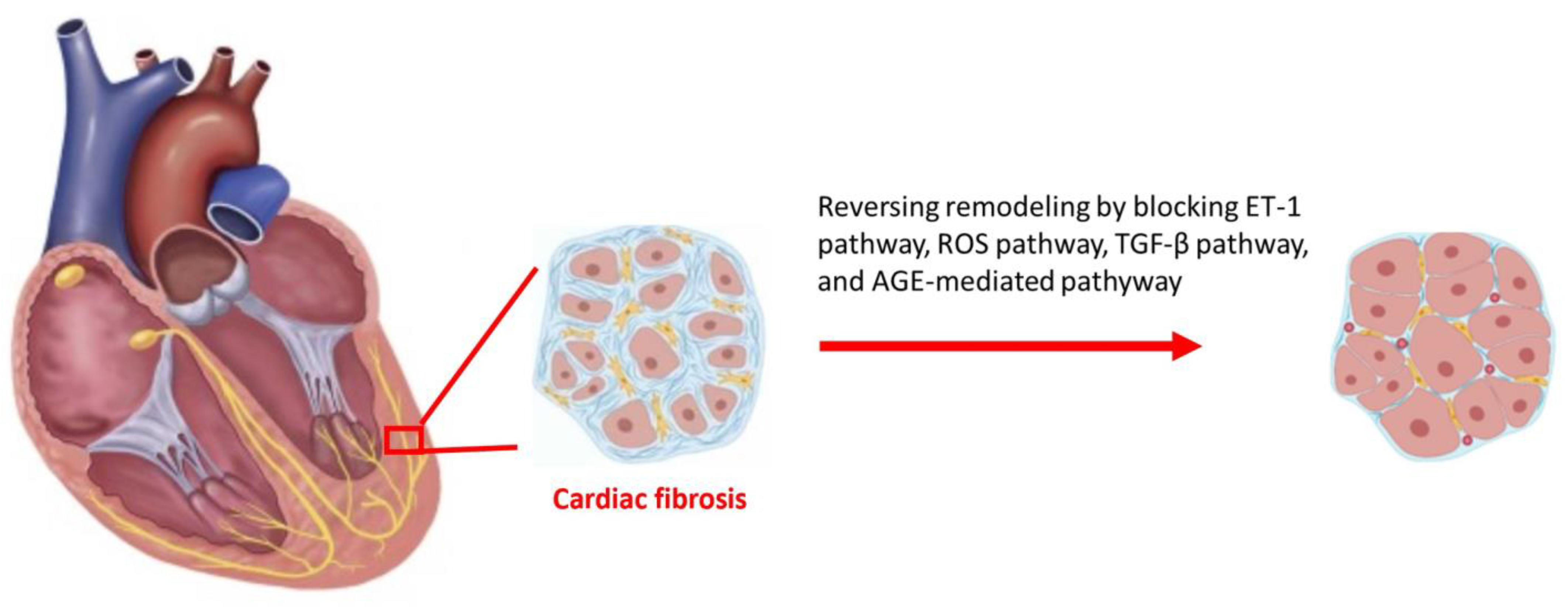
4. Current Treatment to Reverse Diabetic Cardiac Remodeling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawshani, A.; Rawshani, A.; Franzén, S.; Sattar, N.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; McGuire, D.K.; Rosengren, A.; et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018, 379, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Nichols, G.A.; Gullion, C.M.; Koro, C.E.; Ephross, S.A.; Brown, J.B. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes Care 2004, 27, 1879–1884. [Google Scholar] [CrossRef]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef]
- From, A.M.; Scott, C.G.; Chen, H.H. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J. Am. Coll. Cardiol. 2010, 55, 300–305. [Google Scholar] [CrossRef]
- Poirier, P.; Bogaty, P.; Garneau, C.; Marois, L.; Dumesnil, J.G. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: Importance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care 2001, 24, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Di Bonito, P.; Moio, N.; Cavuto, L.; Covino, G.; Murena, E.; Scilla, C.; Turco, S.; Capaldo, B.; Sibilio, G. Early detection of diabetic cardiomyopathy: Usefulness of tissue Doppler imaging. Diabet. Med. 2005, 22, 1720–1725. [Google Scholar] [CrossRef]
- Galderisi, M. Diastolic dysfunction and diabetic cardiomyopathy: Evaluation by Doppler echocardiography. J. Am. Coll. Cardiol. 2006, 48, 1548–1551. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol. Asp. Med. 2019, 65, 70–99. [Google Scholar] [CrossRef]
- Booz, G.W.; Baker, K.M. Molecular signalling mechanisms controlling growth and function of cardiac fibroblasts. Cardiovasc. Res. 1995, 30, 537–543. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef] [PubMed]
- Alex, L.; Russo, I.; Holoborodko, V.; Frangogiannis, N.G. Characterization of a mouse model of obesity-related fibrotic cardiomyopathy that recapitulates features of human heart failure with preserved ejection fraction. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H934–H949. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Huang, X.R.; Zhu, H.J.; Johnson, R.; Lan, H.Y. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003, 63, 2010–2019. [Google Scholar] [CrossRef]
- Zhao, J.; Randive, R.; Stewart, J.A. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J. Diabetes 2014, 5, 860–867. [Google Scholar] [CrossRef]
- Russo, I.; Frangogiannis, N.G. Diabetes-associated cardiac fibrosis: Cellular effectors, molecular mechanisms and therapeutic opportunities. J. Mol. Cell. Cardiol. 2016, 90, 84–93. [Google Scholar] [CrossRef]
- Cavalera, M.; Wang, J.; Frangogiannis, N.G. Obesity, metabolic dysfunction, and cardiac fibrosis: Pathophysiological pathways, molecular mechanisms, and therapeutic opportunities. Transl. Res. 2014, 164, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Derangements in adrenergic-adipokine signalling establish a neurohormonal basis for obesity-related heart failure with a preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Regan, T.J.; Lyons, M.M.; Ahmed, S.S.; Levinson, G.E.; Oldewurtel, H.A.; Ahmad, M.R.; Haider, B. Evidence for cardiomyopathy in familial diabetes mellitus. J. Clin. Investig. 1977, 60, 884–899. [Google Scholar] [CrossRef]
- Singh, V.P.; Baker, K.M.; Kumar, R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: Role in extracellular matrix production. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1675–H1684. [Google Scholar] [CrossRef]
- Muona, P.; Jaakkola, S.; Zhang, R.Z.; Pan, T.C.; Pelliniemi, L.; Risteli, L.; Chu, M.L.; Uitto, J.; Peltonen, J. Hyperglycemic glucose concentrations up-regulate the expression of type VI collagen in vitro. Relevance to alterations of peripheral nerves in diabetes mellitus. Am. J. Pathol. 1993, 142, 1586–1597. [Google Scholar]
- Muona, P.; Peltonen, J.; Jaakkola, S.; Uitto, J. Increased matrix gene expression by glucose in rat neural connective tissue cells in culture. Diabetes 1991, 40, 605–611. [Google Scholar] [CrossRef]
- Aroor, A.R.; Das, N.A.; Carpenter, A.J.; Habibi, J.; Jia, G.; Ramirez-Perez, F.I.; Martinez-Lemus, L.; Manrique-Acevedo, C.M.; Hayden, M.R.; Duta, C.; et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc. Diabetol. 2018, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, J.; Wang, P.; Wang, L.; Jia, L.; Ling, X.; Xi, W.; Min, J.; Shen, H.; Xiao, J.; et al. Glycemic control is associated with atrial structural remodeling in patients with type 2 diabetes. BMC Cardiovasc. Disord. 2019, 19, 278. [Google Scholar] [CrossRef]
- Cao, Y.; Zeng, W.; Cui, Y.; Kong, X.; Wang, M.; Yu, J.; Zhang, S.; Song, J.; Yan, X.; Greiser, A.; et al. Increased myocardial extracellular volume assessed by cardiovascular magnetic resonance T1 mapping and its determinants in type 2 diabetes mellitus patients with normal myocardial systolic strain. Cardiovasc. Diabetol. 2018, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.R.; Betz, B.; Sheldrake, T.A.; Manning, J.R.; Dunbar, D.R.; Dobyns, A.; Hughes, J.; Mullins, J.J. Tight blood glycaemic and blood pressure control in experimental diabetic nephropathy reduces extracellular matrix production without regression of fibrosis. Nephrology 2014, 19, 802–813. [Google Scholar] [CrossRef]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Rutschow, S.; Jäger, S.; Linderer, A.; Anker, S.; Riad, A.; Unger, T.; Schultheiss, H.P.; Pauschinger, M.; Tschöpe, C. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: The role of angiotensin type 1 receptor antagonism. Diabetes 2007, 56, 641–646. [Google Scholar] [CrossRef]
- Ziyadeh, F.N.; Sharma, K.; Ericksen, M.; Wolf, G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J. Clin. Investig. 1994, 93, 536–542. [Google Scholar] [CrossRef]
- Toblli, J.E.; Cao, G.; DeRosa, G.; Forcada, P. Reduced cardiac expression of plasminogen activator inhibitor 1 and transforming growth factor beta1 in obese Zucker rats by perindopril. Heart 2005, 91, 80–86. [Google Scholar] [CrossRef]
- Zhao, L.M.; Zhang, W.; Wang, L.P.; Li, G.R.; Deng, X.L. Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels. Pflug. Arch. 2012, 464, 613–621. [Google Scholar] [CrossRef]
- Oldfield, M.D.; Bach, L.A.; Forbes, J.M.; Nikolic-Paterson, D.; McRobert, A.; Thallas, V.; Atkins, R.C.; Osicka, T.; Jerums, G.; Cooper, M.E. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J. Clin. Investig. 2001, 108, 1853–1863. [Google Scholar] [CrossRef]
- Majumdar, P.; Chen, S.; George, B.; Sen, S.; Karmazyn, M.; Chakrabarti, S. Leptin and endothelin-1 mediated increased extracellular matrix protein production and cardiomyocyte hypertrophy in diabetic heart disease. Diabetes Metab. Res. Rev. 2009, 25, 452–463. [Google Scholar] [CrossRef]
- Hopkins, T.A.; Ouchi, N.; Shibata, R.; Walsh, K. Adiponectin actions in the cardiovascular system. Cardiovasc. Res. 2007, 74, 11–18. [Google Scholar] [CrossRef]
- Fujita, K.; Maeda, N.; Sonoda, M.; Ohashi, K.; Hibuse, T.; Nishizawa, H.; Nishida, M.; Hiuge, A.; Kurata, A.; Kihara, S.; et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 863–870. [Google Scholar] [CrossRef]
- Widyantoro, B.; Emoto, N.; Nakayama, K.; Anggrahini, D.W.; Adiarto, S.; Iwasa, N.; Yagi, K.; Miyagawa, K.; Rikitake, Y.; Suzuki, T.; et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 2010, 121, 2407–2418. [Google Scholar] [CrossRef]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac remodeling in obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R.; Alloatti, G.; Vercellinatto, I.; Bardini, P.; Geuna, S.; Catalano, M.G.; Danni, O.; Boccuzzi, G. Oxidative stress triggers cardiac fibrosis in the heart of diabetic rats. Endocrinology 2008, 149, 380–388. [Google Scholar] [CrossRef]
- Gui, L.; Wang, F.; Hu, X.; Liu, X.; Yang, H.; Cai, Z.; Qi, M.; Dai, C. Epigallocatechin Gallate Protects Diabetes Mellitus Rats Complicated with Cardiomyopathy through TGF-β1/JNK Signaling Pathway. Curr. Pharm. Des. 2022, 28, 2758–2770. [Google Scholar] [CrossRef]
- Yu, W.; Wu, J.; Cai, F.; Xiang, J.; Zha, W.; Fan, D.; Guo, S.; Ming, Z.; Liu, C. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS ONE 2012, 7, e52013. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lu, X.Z.; Shen, M.Z.; Xing, C.Y.; Ma, J.; Duan, Y.Y.; Yuan, L.J. N-Acetyl Cysteine improves the diabetic cardiac function: Possible role of fibrosis inhibition. BMC Cardiovasc. Disord. 2015, 15, 84. [Google Scholar] [CrossRef]
- Akgun-Unal, N.; Ozyildirim, S.; Unal, O.; Gulbahce-Mutlu, E.; Mogulkoc, R.; Baltaci, A.K. The effects of resveratrol and melatonin on biochemical and molecular parameters in diabetic old female rat hearts. Exp. Gerontol. 2023, 172, 112043. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Titus, A.S.; Ushakumary, M.G.; Venugopal, H.; Wang, M.; Lakatta, E.G.; Kailasam, S. Metformin Attenuates Hyperglycaemia-Stimulated Pro-Fibrotic Gene Expression in Adventitial Fibroblasts via Inhibition of Discoidin Domain Receptor 2. Int. J. Mol. Sci. 2022, 24, 585. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Techigawara, M.; Ishihata, T.; Asakura, T.; Saito, F.; Maehara, K.; Maruyama, Y. A comparison of ultrastructural changes on endomyocardial biopsy specimens obtained from patients with diabetes mellitus with and without hypertension. Heart Vessel. 1997, 12, 267–274. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Tajik, A.J. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J. Am. Coll. Cardiol. 1997, 30, 8–18. [Google Scholar] [CrossRef]
- Lester, S.J.; Tajik, A.J.; Nishimura, R.A.; Oh, J.K.; Khandheria, B.K.; Seward, J.B. Unlocking the mysteries of diastolic function: Deciphering the Rosetta Stone 10 years later. J. Am. Coll. Cardiol. 2008, 51, 679–689. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Kasner, M.; Westermann, D.; Steendijk, P.; Gaub, R.; Wilkenshoff, U.; Weitmann, K.; Hoffmann, W.; Poller, W.; Schultheiss, H.P.; Pauschinger, M.; et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: A comparative Doppler-conductance catheterization study. Circulation 2007, 116, 637–647. [Google Scholar] [CrossRef]
- Tsang, T.S.; Gersh, B.J.; Appleton, C.P.; Tajik, A.J.; Barnes, M.E.; Bailey, K.R.; Oh, J.K.; Leibson, C.; Montgomery, S.C.; Seward, J.B. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J. Am. Coll. Cardiol. 2002, 40, 1636–1644. [Google Scholar] [CrossRef]
- Melenovsky, V.; Borlaug, B.A.; Rosen, B.; Hay, I.; Ferruci, L.; Morell, C.H.; Lakatta, E.G.; Najjar, S.S.; Kass, D.A. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: The role of atrial remodeling/dysfunction. J. Am. Coll. Cardiol. 2007, 49, 198–207. [Google Scholar] [CrossRef]
- Rimbas, R.C.; Visoiu, I.S.; Magda, S.L.; Mihaila-Baldea, S.; Luchian, M.L.; Chitroceanu, A.M.; Hayat, M.; Mihalcea, D.J.; Dragoi-Galrinho-Antunes-Guerra, R.; Stefan, M.; et al. New insights into the potential utility of the left atrial function analysis in heart failure with preserved ejection fraction diagnosis. PLoS ONE 2022, 17, e0267962. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Kaiume, M.; Kurokawa, R.; Maeda, E.; Daimon, M.; Abe, O. Detection of left ventricular dysfunction on axial non-contrast chest CT. Eur. J. Radiol. 2022, 150, 110274. [Google Scholar] [CrossRef]
- Lessick, J.; Mutlak, D.; Efraim, R.; Naami, R.; Mutlak, M.; Sheik-Muhamad, R.; Abadi, S.; Aronson, D. Comparison Between Echocardiography and Cardiac Computed Tomography in the Evaluation of Diastolic Dysfunction and Prediction of Heart Failure. Am. J. Cardiol. 2022, 181, 71–78. [Google Scholar] [CrossRef]
- Bottini, P.B.; Carr, A.A.; Prisant, L.M.; Flickinger, F.W.; Allison, J.D.; Gottdiener, J.S. Magnetic resonance imaging compared to echocardiography to assess left ventricular mass in the hypertensive patient. Am. J. Hypertens. 1995, 8, 221–228. [Google Scholar] [CrossRef]
- Myerson, S.G.; Bellenger, N.G.; Pennell, D.J. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension 2002, 39, 750–755. [Google Scholar] [CrossRef]
- Fyrdahl, A.; Ramos, J.G.; Eriksson, M.J.; Caidahl, K.; Ugander, M.; Sigfridsson, A. Sector-wise golden-angle phase contrast with high temporal resolution for evaluation of left ventricular diastolic dysfunction. Magn. Reason. Med. 2020, 83, 1310–1321. [Google Scholar] [CrossRef]
- Paelinck, B.P.; de Roos, A.; Bax, J.J.; Bosmans, J.M.; van Der Geest, R.J.; Dhondt, D.; Parizel, P.M.; Vrints, C.J.; Lamb, H.J. Feasibility of tissue magnetic resonance imaging: A pilot study in comparison with tissue Doppler imaging and invasive measurement. J. Am. Coll. Cardiol. 2005, 45, 1109–1116. [Google Scholar] [CrossRef]
- Buss, S.J.; Krautz, B.; Schnackenburg, B.; Abdel-Aty, H.; Santos, M.F.; Andre, F.; Maertens, M.J.; Mereles, D.; Korosoglou, G.; Giannitsis, E.; et al. Classification of diastolic function with phase-contrast cardiac magnetic resonance imaging: Validation with echocardiography and age-related reference values. Clin. Res. Cardiol. 2014, 103, 441–450. [Google Scholar] [CrossRef]
- Pritchett, A.M.; Mahoney, D.W.; Jacobsen, S.J.; Rodeheffer, R.J.; Karon, B.L.; Redfield, M.M. Diastolic dysfunction and left atrial volume: A population-based study. J. Am. Coll. Cardiol. 2005, 45, 87–92. [Google Scholar] [CrossRef]
- Nguyen, J.; Weber, J.; Hsu, B.; Mulyala, R.R.; Wang, L.; Cao, J.J. Comparing left atrial indices by CMR in association with left ventricular diastolic dysfunction and adverse clinical outcomes. Sci. Rep. 2021, 11, 21331. [Google Scholar] [CrossRef]
- Jin, J.; Wang, W.; Zhu, L.; Gu, T.; Niu, Q.; Li, P.; Bi, Y.; Zhu, D. Cardiovascular Autonomic Neuropathy Is an Independent Risk Factor for Left Ventricular Diastolic Dysfunction in Patients with Type 2 Diabetes. Biomed. Res. Int. 2017, 2017, 3270617. [Google Scholar] [CrossRef]
- Didangelos, T.; Moralidis, E.; Karlafti, E.; Tziomalos, K.; Margaritidis, C.; Kontoninas, Z.; Stergiou, I.; Boulbou, M.; Papagianni, M.; Papanastasiou, E.; et al. A Comparative Assessment of Cardiovascular Autonomic Reflex Testing and Cardiac 123I-Metaiodobenzylguanidine Imaging in Patients with Type 1 Diabetes Mellitus without Complications or Cardiovascular Risk Factors. Int. J. Endocrinol. 2018, 2018, 5607208. [Google Scholar] [CrossRef]
- Wong, T.C.; Piehler, K.M.; Kang, I.A.; Kadakkal, A.; Kellman, P.; Schwartzman, D.S.; Mulukutla, S.R.; Simon, M.A.; Shroff, S.G.; Kuller, L.H.; et al. Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur. Heart J. 2014, 35, 657–664. [Google Scholar] [CrossRef]
- Bertoia, M.L.; Allison, M.A.; Manson, J.E.; Freiberg, M.S.; Kuller, L.H.; Solomon, A.J.; Limacher, M.C.; Johnson, K.C.; Curb, J.D.; Wassertheil-Smoller, S.; et al. Risk factors for sudden cardiac death in post-menopausal women. J. Am. Coll. Cardiol. 2012, 60, 2674–2682. [Google Scholar] [CrossRef]
- Iribarren, C.; Karter, A.J.; Go, A.S.; Ferrara, A.; Liu, J.Y.; Sidney, S.; Selby, J.V. Glycemic control and heart failure among adult patients with diabetes. Circulation 2001, 103, 2668–2673. [Google Scholar] [CrossRef]
- Hayward, R.A.; Reaven, P.D.; Wiitala, W.L.; Bahn, G.D.; Reda, D.J.; Ge, L.; McCarren, M.; Duckworth, W.C.; Emanuele, N.V. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2015, 372, 2197–2206. [Google Scholar] [CrossRef]
- Castagno, D.; Baird-Gunning, J.; Jhund, P.S.; Biondi-Zoccai, G.; MacDonald, M.R.; Petrie, M.C.; Gaita, F.; McMurray, J.J. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: Evidence from a 37,229 patient meta-analysis. Am. Heart J. 2011, 162, 938–948.e932. [Google Scholar] [CrossRef]
- Fatemi, O.; Yuriditsky, E.; Tsioufis, C.; Tsachris, D.; Morgan, T.; Basile, J.; Bigger, T.; Cushman, W.; Goff, D.; Soliman, E.Z.; et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am. J. Cardiol. 2014, 114, 1217–1222. [Google Scholar] [CrossRef]
- Aronson, D.; Musallam, A.; Lessick, J.; Dabbah, S.; Carasso, S.; Hammerman, H.; Reisner, S.; Agmon, Y.; Mutlak, D. Impact of diastolic dysfunction on the development of heart failure in diabetic patients after acute myocardial infarction. Circ. Heart Fail. 2010, 3, 125–131. [Google Scholar] [CrossRef]
- Pang, X.F.; Zhang, L.H.; Bai, F.; Wang, N.P.; Garner, R.E.; McKallip, R.J.; Zhao, Z.Q. Attenuation of myocardial fibrosis with curcumin is mediated by modulating expression of angiotensin II AT1/AT2 receptors and ACE2 in rats. Drug Des. Devel. Ther. 2015, 9, 6043–6054. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.; Forbes, J.M.; Thomas, M.C.; Thallas, V.; Dean, R.G.; Burns, W.C.; Tikellis, C.; Ritchie, R.H.; Twigg, S.M.; Cooper, M.E.; et al. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ. Res. 2003, 92, 785–792. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, K.-L.; Hsu, Y.-C.; Chang, S.-T.; Chung, C.-M.; Lin, C.-L. The Role of Cardiac Fibrosis in Diabetic Cardiomyopathy: From Pathophysiology to Clinical Diagnostic Tools. Int. J. Mol. Sci. 2023, 24, 8604. https://doi.org/10.3390/ijms24108604
Pan K-L, Hsu Y-C, Chang S-T, Chung C-M, Lin C-L. The Role of Cardiac Fibrosis in Diabetic Cardiomyopathy: From Pathophysiology to Clinical Diagnostic Tools. International Journal of Molecular Sciences. 2023; 24(10):8604. https://doi.org/10.3390/ijms24108604
Chicago/Turabian StylePan, Kuo-Li, Yung-Chien Hsu, Shih-Tai Chang, Chang-Min Chung, and Chun-Liang Lin. 2023. "The Role of Cardiac Fibrosis in Diabetic Cardiomyopathy: From Pathophysiology to Clinical Diagnostic Tools" International Journal of Molecular Sciences 24, no. 10: 8604. https://doi.org/10.3390/ijms24108604
APA StylePan, K.-L., Hsu, Y.-C., Chang, S.-T., Chung, C.-M., & Lin, C.-L. (2023). The Role of Cardiac Fibrosis in Diabetic Cardiomyopathy: From Pathophysiology to Clinical Diagnostic Tools. International Journal of Molecular Sciences, 24(10), 8604. https://doi.org/10.3390/ijms24108604





