Copiotrophy in a Marine-Biofilm-Derived Roseobacteraceae Bacterium Can Be Supported by Amino Acid Metabolism and Thiosulfate Oxidation
Abstract
1. Introduction
2. Results
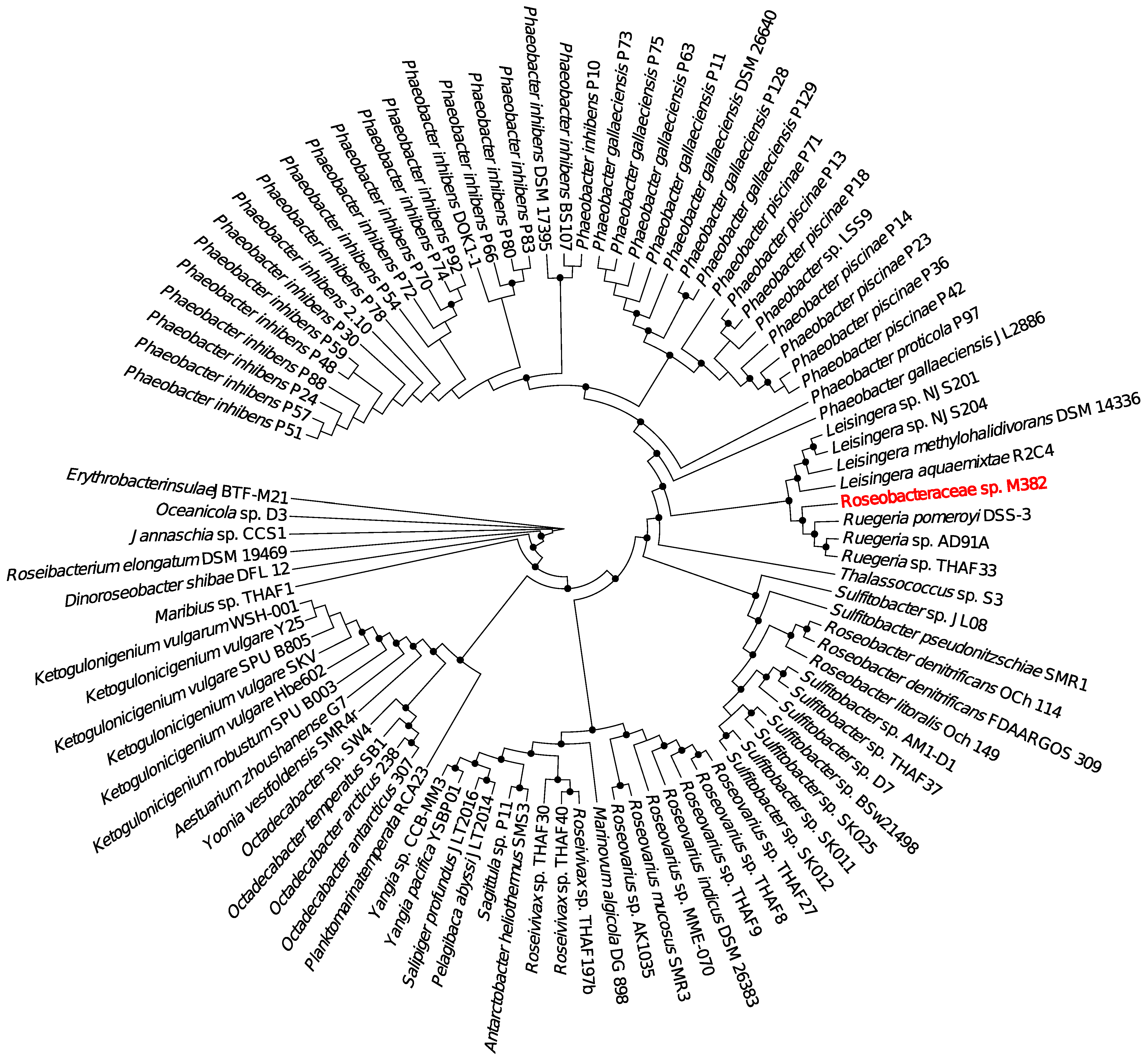
2.1. Overall Genomic Information and Phylogeny
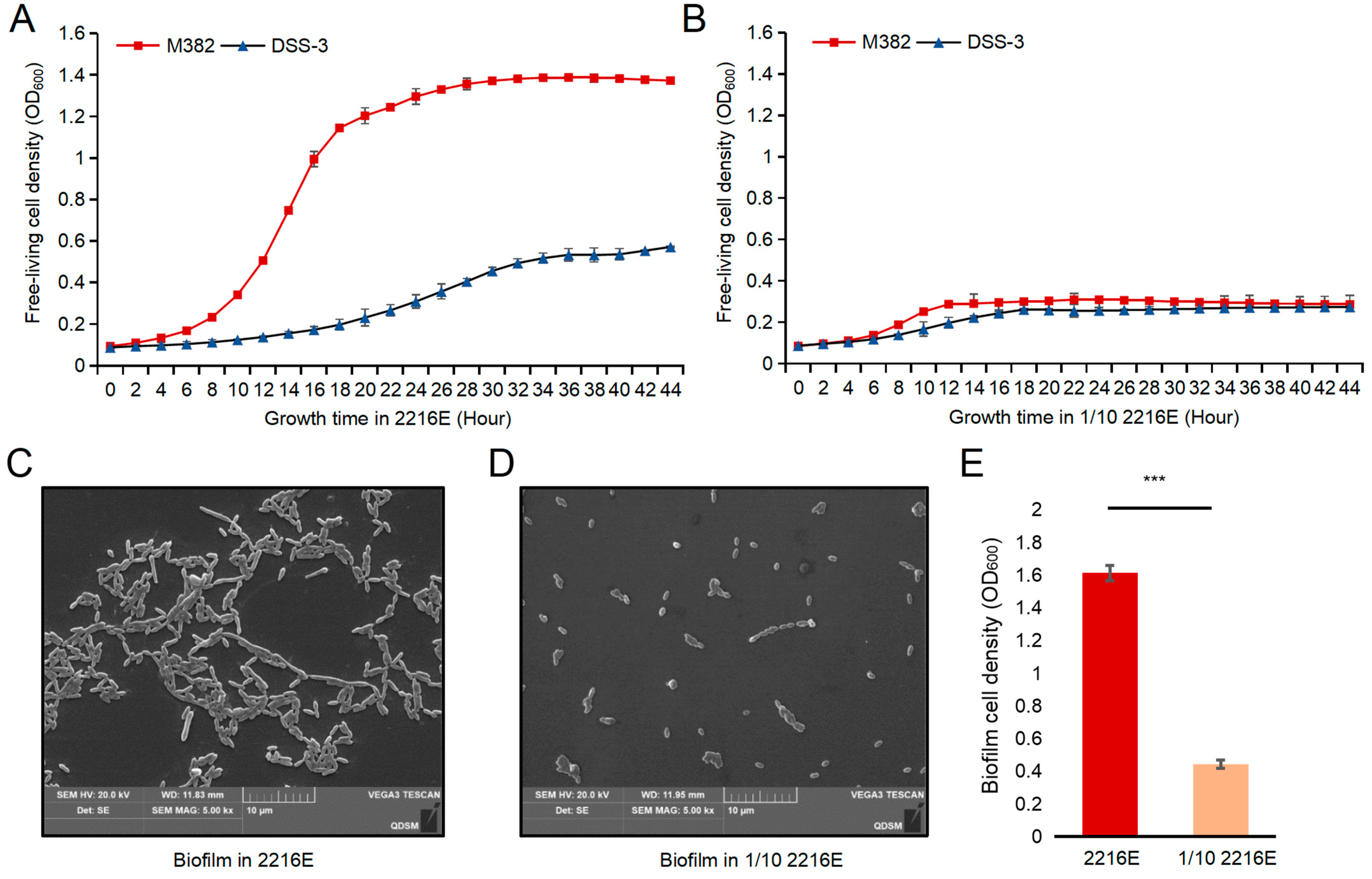
2.2. Copiotrophic Growth Features
2.3. Genomic Pathway Reconstruction
2.4. Gene Expression in Response to Carbon Source Concentration
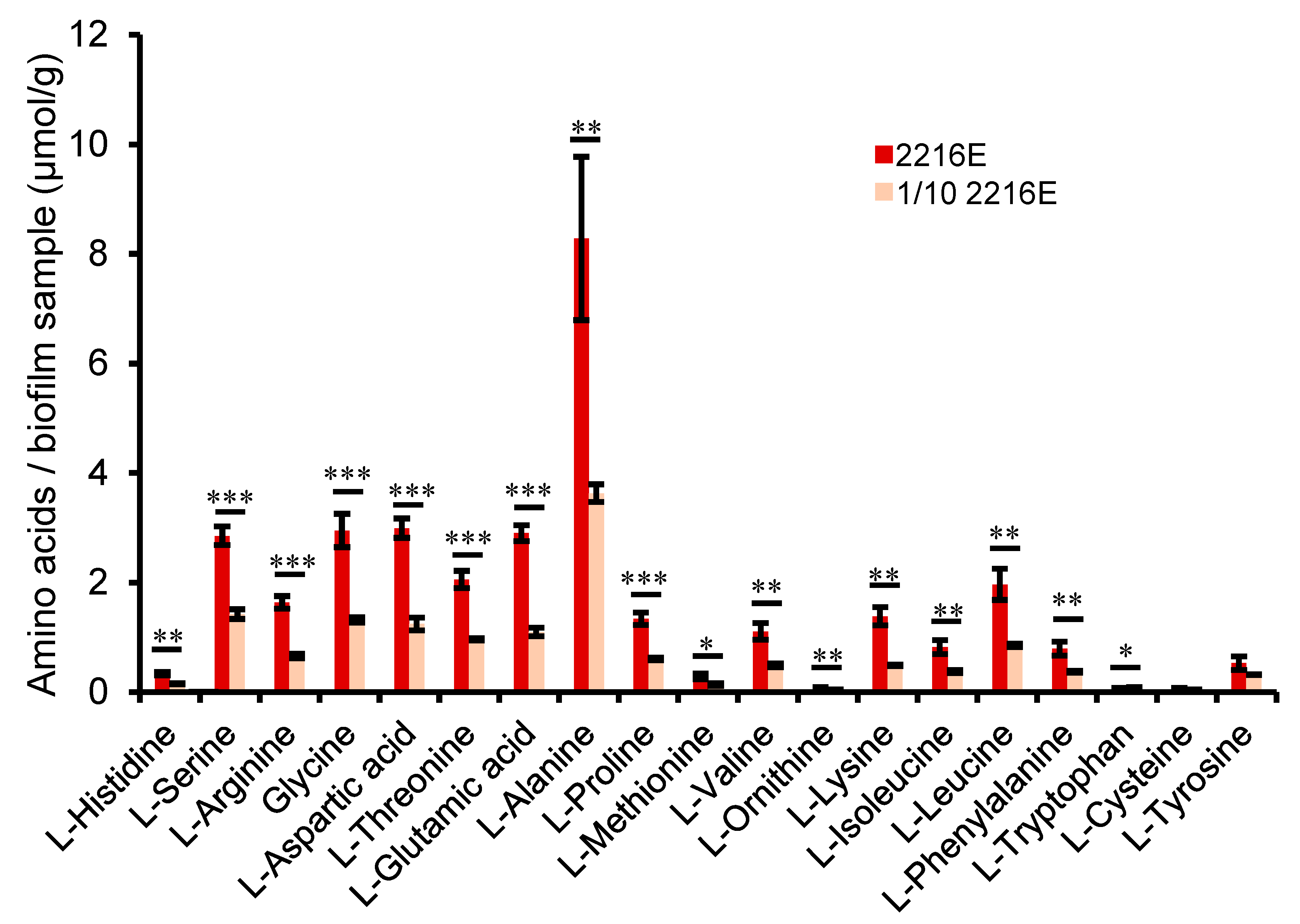
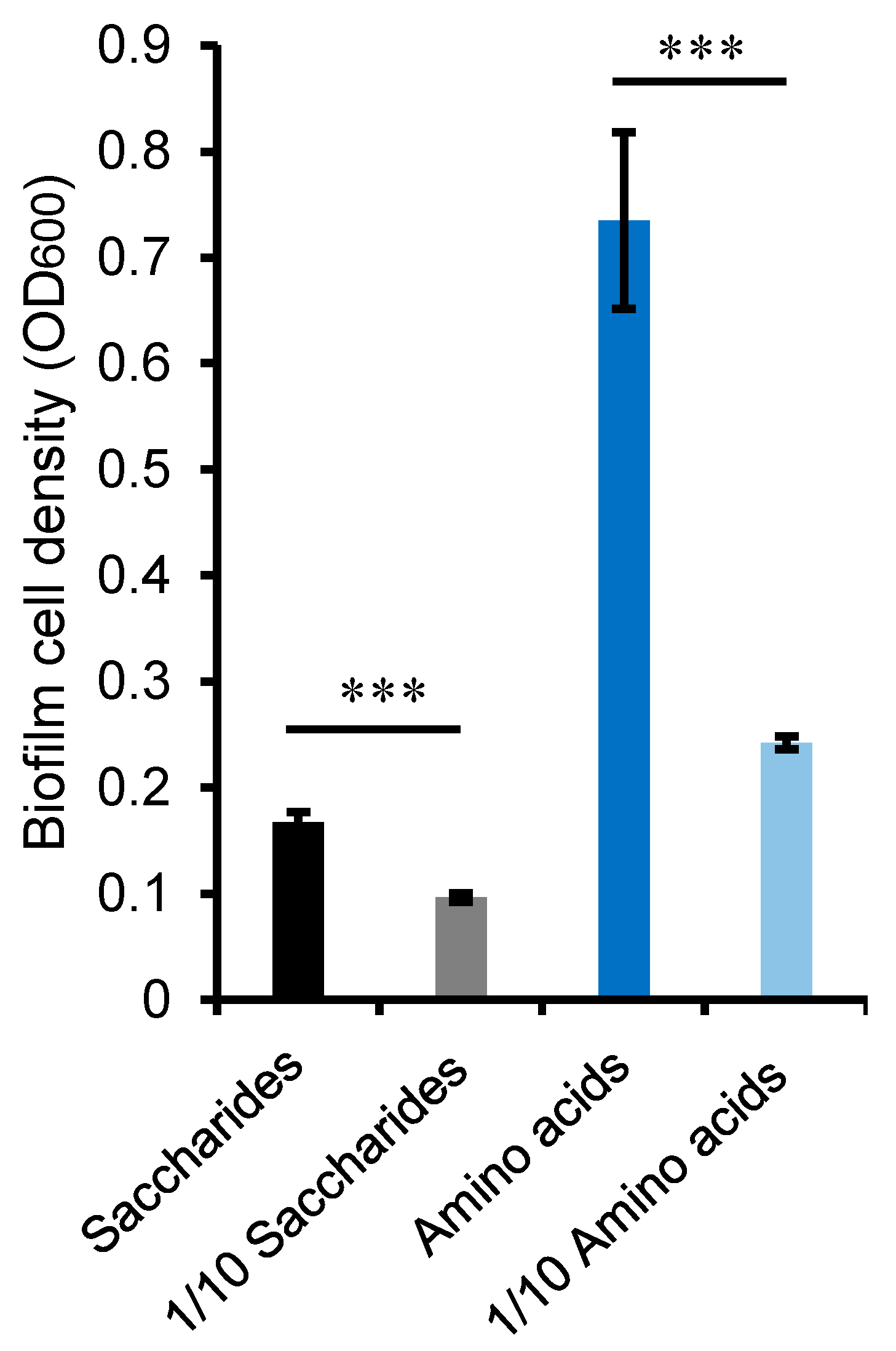
2.5. Cellular Metabolic Profiling and Preferential Utilization of Amino Acids
2.6. Association between Amino Acid Metabolism and Sulfur Oxidation
3. Discussion
4. Materials and Methods
4.1. Sampling and Bacterial Strain Isolation
4.2. Genome Sequencing and Analyses
4.3. Bacterial Growth and Biofilm Formation
4.4. Transcriptomic Sequencing and Analyses
4.5. Quantitative Determination of Cellular Amino Acids
4.6. Gene Knockout
4.7. Measurement of Membrane Potential
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lauro, F.M.; McDougald, D.; Thomas, T.; Williams, T.J.; Egan, S.; Rice, S.; DeMaere, M.Z. The genomic basis of trophic strategy in marine bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 15527–15533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.B.; Zhang, X.Y.; Dang, Y.R.; Sun, L.L.; Zhang, W.; Fu, H.H.; Yang, G.P.; Wang, M.; McMinn, A.; et al. Experimental evidence for long-term coexistence of copiotrophic and oligotrophic bacteria in pelagic surface seawater. Environ. Microbiol. 2021, 23, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Azam, F. Microbial Control of Oceanic Carbon Flux: The Plot Thickens. Science 1998, 280, 694–696. [Google Scholar] [CrossRef]
- Liang, K.Y.H.; Orata, F.D.; Boucher, Y.F.; Case, R.J. Roseobacters in a sea of poly- and paraphyly: Whole genome-based taxonomy of the family Rhodobacteraceae and the proposal for the split of the “Roseobacter Clade” into a novel family, Roseobacteraceae fam. nov. Front. Microbiol. 2021, 12, 683109. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.; Gonzalez, J.M.; Moran, M.A. Overview of the marine Roseobacter lineage. Appl. Environ. Microbiol. 2005, 71, 5665–5677. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Dobler, I.; Biebl, H. Environmental biology of the marine Roseobacter lineage. Annu. Rev. Microbiol. 2006, 60, 255–280. [Google Scholar] [CrossRef]
- Moran, M.A.; Belas, R.; Schell, M.A.; Gonzalez, J.M.; Sun, F.; Sun, S.; Binder, B.J.; Edmonds, J.; Ye, W.; Orcutt, B.; et al. Ecological genomics of marine Roseobacters. Appl. Environ. Microbiol. 2007, 73, 4559–4569. [Google Scholar] [CrossRef]
- Luo, H.; Moran, M.A. Evolutionary ecology of the marine Roseobacter clade. Microbiol. Mol. Biol. Rev. 2014, 78, 573–587. [Google Scholar] [CrossRef]
- Yi, H.; Lim, Y.W.; Chun, J. Taxonomic evaluation of the genera Ruegeria and Silicibacter: A proposal to transfer the genus Silicibacter Petursdottir and Kristjansson 1999 to the genus Ruegeria Uchino et al. 1999. Int. J. Syst. Evol. Microbiol. 2007, 57, 815–819. [Google Scholar] [CrossRef]
- Moran, M.A.; Buchan, A.; González, J.M.; Heidelberg, J.F.; Whitman, W.B.; Kiene, R.P.; Henriksen, J.R. Genome sequence of Silicibacter pomeroyi reveals adaptations to the marine environment. Nature 2004, 432, 910–913. [Google Scholar] [CrossRef]
- Burgmann, H.; Howard, E.C.; Ye, W.; Sun, F.; Sun, S.; Napierala, S.; Moran, M.A. Transcriptional response of Silicibacter pomeroyi DSS-3 to dimethylsulfoniopropionate (DMSP). Environ. Microbiol. 2007, 9, 2742–2755. [Google Scholar] [CrossRef] [PubMed]
- Christie-Oleza, J.A.; Armengaud, J. In-depth analysis of exoproteomes from marine bacteria by shotgun liquid chromatography-tandem mass spectrometry: The Ruegeria pomeroyi DSS-3 case-study. Mar. Drugs. 2010, 8, 2223–2239. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, E.C.; Nielsen, K.F.; D’Alvise, P.; Porsby, C.H.; Melchiorsen, J.; Heilmann, J.; Kalatzis, P.G.; Lopez-Perez, M.; Bunk, B.; Sproer, C.; et al. Global occurrence and heterogeneity of the Roseobacter-clade species Ruegeria mobilis. ISME J. 2017, 11, 569–583. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Lee, O.O.; Tian, R.; Cao, H.; Gao, Z.; Li, Y.; Yu, L.; Xu, Y.; Qian, P.Y. Adaptation of intertidal biofilm communities is driven by metal ion and oxidative stresses. Sci. Rep. 2013, 3, 3180. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shu, Y.; Zhang, H.; Zhang, S.; Zhu, C.; Ding, W.; Zhang, W. The landscape of global ocean microbiome: From bacterioplankton to biofilms. Int. J. Mol. Sci. 2023, 24, 6491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ding, W.; Li, Y.X.; Tam, C.; Bougouffa, S.; Wang, R.; Pei, B.; Chiang, H.; Leung, P.; Lu, Y.; et al. Marine biofilms constitute a bank of hidden microbial diversity and functional potential. Nat. Commun. 2019, 10, 517. [Google Scholar] [CrossRef]
- Ding, W.; Wang, R.; Liang, Z.; Zhang, R.; Qian, P.Y.; Zhang, W. Expanding our understanding of marine viral diversity through metagenomic analyses of biofilms. Mar. Life Sci. Technol. 2021, 3, 395–404. [Google Scholar] [CrossRef]
- Ding, W.; Wang, S.; Qin, P.; Fan, S.; Su, X.; Cai, P.; Lu, J.; Cui, H.; Wang, M.; Shu, Y.; et al. Anaerobic thiosulfate oxidation by the Roseobacter group is prevalent in marine biofilms. Nat. Commun. 2023, 14, 2033. [Google Scholar] [CrossRef]
- Strokal, M.; Kroeze, C.; Wang, M.; Ma, L. Reducing future river export of nutrients to coastal waters of China in optimistic scenarios. Sci. Total Environ. 2017, 579, 517–528. [Google Scholar] [CrossRef]
- Delpech, L.M.; Vonnahme, T.R.; McGovern, M.; Gradinger, R.; Praebel, K.; Poste, A.E. Terrestrial inputs shape coastal bacterial and archaeal communities in a High Arctic Fjord (Isfjorden, Svalbard). Front. Microbiol. 2021, 12, 614634. [Google Scholar] [CrossRef]
- Chaumeil, P.A.; Mussig, A.J.; Hugenholtz, P.; Parks, D.H. GTDB-Tk: A toolkit to classify genomes with the genome taxonomy database. Bioinformatics 2019, 36, 1925–1927. [Google Scholar] [CrossRef] [PubMed]
- Michael, V.; Frank, O.; Bartling, P.; Scheuner, C.; Goker, M.; Brinkmann, H.; Petersen, J. Biofilm plasmids with a rhamnose operon are widely distributed determinants of the ‘swim-or-stick’ lifestyle in roseobacters. ISME J. 2016, 10, 2498–2513. [Google Scholar] [CrossRef] [PubMed]
- Reeves, P.J.; Whitcombe, D.; Wharam, S.; Gibson, M.; Allison, G.; Bunce, N.; Barallon, R.; Douglas, P.; Mulholland, V.; Stevens, S. Molecular cloning and characterization of 13 out genes from Erwinia carotovora subspecies carotovora genes encoding members of a general secretion pathway (GSP) widespread in gram-negative bacteria. Mol. Microbiol. 1993, 8, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Sevin, D.C.; Fuhrer, T.; Zamboni, N.; Sauer, U. Nontargeted in vitro metabolomics for high-throughput identification of novel enzymes in Escherichia coli. Nat. Methods 2017, 14, 187–194. [Google Scholar] [CrossRef]
- Bastard, K.; Perret, A.; Mariage, A.; Bessonnet, T.; Pinet-Turpault, A.; Petit, J.L.; Darii, E.; Bazire, P.; Vergne-Vaxelaire, C.; Brewee, C.; et al. Parallel evolution of non-homologous isofunctional enzymes in methionine biosynthesis. Nat. Chem. Biol. 2017, 13, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Nyyssola, A.; Kerovuo, J.; Kaukinen, P.; von Weymarn, N.; Reinikainen, T. Extreme halophiles synthesize betaine from glycine by methylation. J. Biol. Chem. 2000, 275, 22196–22201. [Google Scholar] [CrossRef]
- Miller, D.; O’Brien, K.; Xu, H.; White, R.H. Identification of a 5’-deoxyadenosine deaminase in Methanocaldococcus jannaschii and its possible role in recycling the radical S-adenosylmethionine enzyme reaction product 5’-deoxyadenosine. J. Bacteriol. 2014, 196, 1064–1072. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.H. Effects of minimal media vs. complex media on the metabolite profiles of Escherichia coli and Saccharomyces cerevisiae. Process Biochem. 2017, 57, 64–71. [Google Scholar] [CrossRef]
- Guieysse, B.; Wuertz, S. Metabolically versatile large-genome prokaryotes. Curr. Opin. Biotechnol. 2012, 23, 467–473. [Google Scholar] [CrossRef]
- Westoby, M.; Nielsen, D.A.; Gillings, M.R.; Litchman, E.; Madin, J.S.; Paulsen, I.T.; Tetu, S.G. Cell size, genome size, and maximum growth rate are near-independent dimensions of ecological variation across bacteria and archaea. Ecol. Evol. 2021, 11, 3956–3976. [Google Scholar] [CrossRef]
- Liao, Y.; Williams, T.J.; Ye, J.; Charlesworth, J.; Burns, B.P.; Poljak, A.; Raftery, M.J.; Cavicchioli, R. Morphological and proteomic analysis of biofilms from the Antarctic archaeon, Halorubrum lacusprofundi. Sci. Rep. 2016, 6, 37454. [Google Scholar] [CrossRef] [PubMed]
- Arias-Andres, M.; Klumper, U.; Rojas-Jimenez, K.; Grossart, H.P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Rameez, M.J.; Pyne, P.; Mandal, S.; Chatterjee, S.; Alam, M.; Bhattacharya, S.; Mondal, N.; Sarkar, J.; Ghosh, W. Two pathways for thiosulfate oxidation in the alphaproteobacterial chemolithotroph Paracoccus thiocyanatus SST. Microbiol. Res. 2020, 230, 126345. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Krasnopeeva, E.; Sinjab, F.; Pilizota, T.; Kim, M. Active Efflux Leads to Heterogeneous dissipation of proton motive force by protonophores in bacteria. mBio 2021, 12, e0067621. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Hyatt, D.; Chen, G.L.; Locascio, P.F.; Land, M.L.; Larimer, F.W.; Hauser, L.J. Prodigal: Prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 2010, 11, 119. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A toolkit for quality control of next generation sequencing data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liu, L.; Dong, Z.; Guo, S.; Gao, H. Dissociation between iron and heme biosyntheses is largely accountable for respiration defects of Shewanella oneidensis fur mutants. Appl. Environ. Microbiol. 2018, 84, e00039-18. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, W.; Cheng, J.; Yang, X.; Zhu, K.; Wang, Y.; Wei, G.; Qian, P.Y.; Luo, Z.Q.; Shen, X. A Pseudomonas T6SS effector recruits PQS-containing outer membrane vesicles for iron acquisition. Nat. Commun. 2017, 8, 14888. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chan, E.W.C.; Wan, Y.; Wong, M.H.; Chen, S. Active maintenance of proton motive force mediates starvation-induced bacterial antibiotic tolerance in Escherichia coli. Commun. Biol. 2021, 4, 1068. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Cui, H.; Zhang, W. Copiotrophy in a Marine-Biofilm-Derived Roseobacteraceae Bacterium Can Be Supported by Amino Acid Metabolism and Thiosulfate Oxidation. Int. J. Mol. Sci. 2023, 24, 8617. https://doi.org/10.3390/ijms24108617
Su X, Cui H, Zhang W. Copiotrophy in a Marine-Biofilm-Derived Roseobacteraceae Bacterium Can Be Supported by Amino Acid Metabolism and Thiosulfate Oxidation. International Journal of Molecular Sciences. 2023; 24(10):8617. https://doi.org/10.3390/ijms24108617
Chicago/Turabian StyleSu, Xiaoyan, Han Cui, and Weipeng Zhang. 2023. "Copiotrophy in a Marine-Biofilm-Derived Roseobacteraceae Bacterium Can Be Supported by Amino Acid Metabolism and Thiosulfate Oxidation" International Journal of Molecular Sciences 24, no. 10: 8617. https://doi.org/10.3390/ijms24108617
APA StyleSu, X., Cui, H., & Zhang, W. (2023). Copiotrophy in a Marine-Biofilm-Derived Roseobacteraceae Bacterium Can Be Supported by Amino Acid Metabolism and Thiosulfate Oxidation. International Journal of Molecular Sciences, 24(10), 8617. https://doi.org/10.3390/ijms24108617





