Asymmetric Presynaptic Depletion of Dopamine Neurons in a Drosophila Model of Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. Characteristic Subsets of DANs Provide Symmetric Innervations to the Antler
2.2. Hα-SynA30P Expression Induces Age-Dependent Presynapse Depletion of ATL DANs
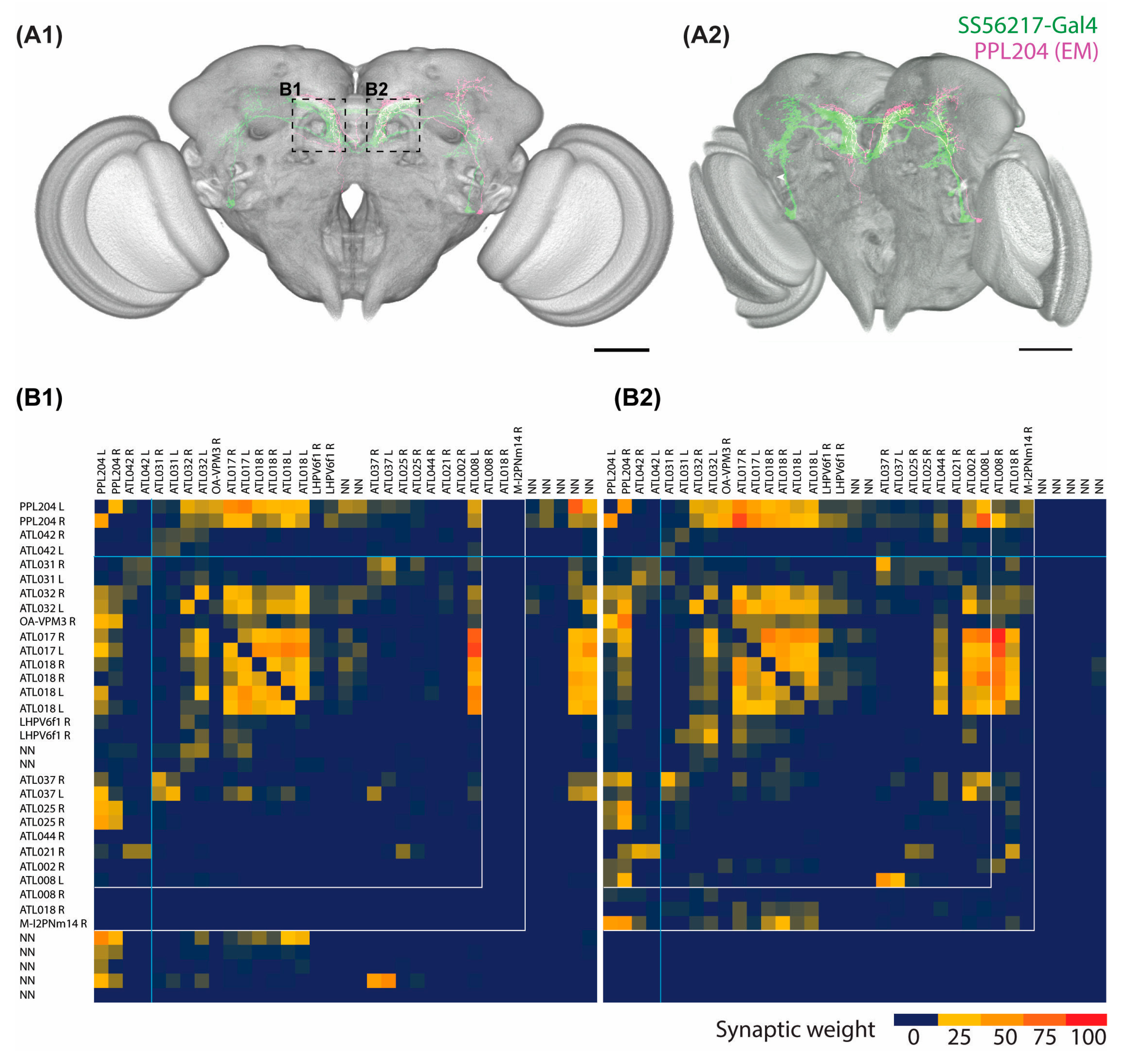
2.3. Connectome Characterization of Implicated PPL204 Neuronal Circuits
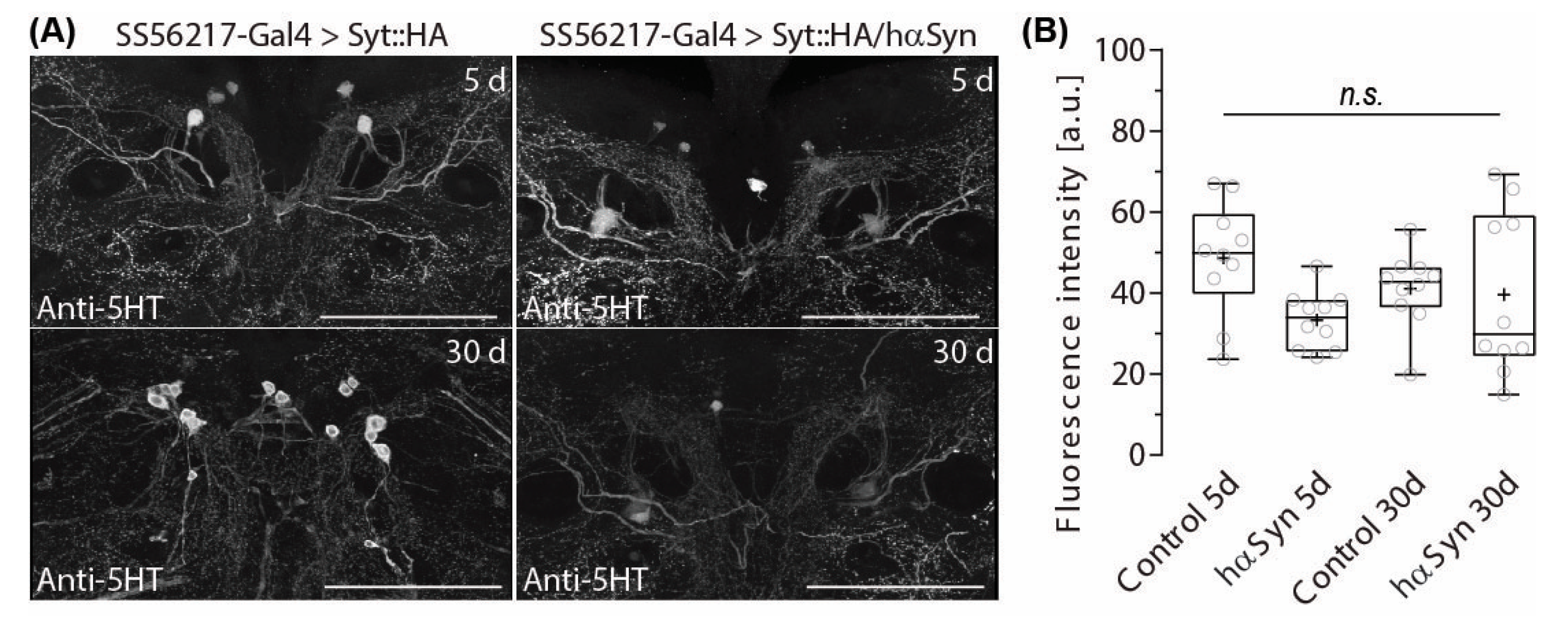
2.4. PPL204 Presynapse Depletion Does Not Affect 5-HT Immune-Reactivity in the ATL
3. Discussion
4. Materials and Methods
4.1. Drosophila Strains and Maintenance
4.2. Sample Preparation
4.3. Image Acquisition and Processing
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forno, L.S. Neuropathology of Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1996, 55, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Shulman, J.M.; De Jager, P.L.; Feany, M.B. Parkinson’s disease: Genetics and pathogenesis. Annu. Rev. Pathol. 2011, 6, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Beitz, J.M. Parkinson’s disease: A review. Front. Biosci. (Schol. Ed.) 2014, 6, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Marinus, J.; van Hilten, J.J. The significance of motor (a)symmetry in Parkinson’s disease. Mov. Disord. 2015, 30, 379–385. [Google Scholar] [CrossRef]
- Djaldetti, R.; Ziv, I.; Melamed, E. The mystery of motor asymmetry in Parkinson’s disease. Lancet Neurol. 2006, 5, 796–802. [Google Scholar] [CrossRef]
- Miller-Patterson, C.; Buesa, R.; McLaughlin, N.; Jones, R.; Akbar, U.; Friedman, J.H. Motor asymmetry over time in Parkinson’s disease. J. Neurol. Sci. 2018, 393, 14–17. [Google Scholar] [CrossRef]
- Lee, T.K.; Chau, R.; Leong, S.K. The anatomy of the basal ganglia and Parkinson’s disease: A review. Singap. Med. J 1995, 36, 74–76. [Google Scholar]
- Goelman, G.; Dan, R.; Růžička, F.; Bezdicek, O.; Jech, R. Asymmetry of the insula-sensorimotor circuit in Parkinson’s disease. Eur. J. Neurosci. 2021, 54, 6267–6280. [Google Scholar] [CrossRef]
- Antony, P.M.; Diederich, N.J.; Krüger, R.; Balling, R. The hallmarks of Parkinson’s disease. FEBS J. 2013, 280, 5981–5993. [Google Scholar] [CrossRef] [PubMed]
- Satake, W.; Nakabayashi, Y.; Mizuta, I.; Hirota, Y.; Ito, C.; Kubo, M.; Kawaguchi, T.; Tsunoda, T.; Watanabe, M.; Takeda, A.; et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 2009, 41, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H. Genetics of Parkinson’s disease. Ann. N. Y. Acad. Sci. 2000, 920, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Corti, O.; Lesage, S.; Brice, A. What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol. Rev. 2011, 91, 1161–1218. [Google Scholar] [CrossRef]
- Devine, M.J.; Gwinn, K.; Singleton, A.; Hardy, J. Parkinson’s disease and alpha-synuclein expression. Mov. Disord. 2011, 26, 2160–2168. [Google Scholar] [CrossRef]
- Tsai, H.C.; Zhang, F.; Adamantidis, A.; Stuber, G.D.; Bonci, A.; De Lecea, L.; Deisseroth, K. Phasic firing in dopaminergic neurons is sufficient for behavioural conditioning. Science 2009, 324, 1080–1084. [Google Scholar] [CrossRef]
- Steinberg, E.E.; Keiflin, R.; Boivin, J.; Witten, I.B.; Deisseroth, K.; Janak, P.H. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 2013, 16, 966–973. [Google Scholar] [CrossRef]
- Darvas, M.; Wunsch, A.M.; Gibbs, J.T.; Palmiter, R.D. Dopamine dependency for acquisition and performance of Pavlovian conditioned response. Proc. Natl. Acad. Sci. USA 2014, 111, 2764–2769. [Google Scholar] [CrossRef]
- Chang, C.Y.; Esber, G.; Marrero-Garcia, Y.; Yau, H.-J.; Bonci, A.; Schoenbaum, G. Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat. Neurosci. 2015, 19, 111–116. [Google Scholar] [CrossRef]
- Saunders, B.T.; Richard, J.M.; Margolis, E.B.; Janak, P.H. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 2018, 21, 1072–1083. [Google Scholar] [CrossRef]
- Fadok, J.P.; Dickerson, T.M.K.; Palmiter, R.D. Dopamine is necessary for cue-dependent fear conditioning. J. Neurosci. 2009, 29, 11089–11097. [Google Scholar] [CrossRef] [PubMed]
- Riemensperger, T.; Isabel, G.; Coulom, H.; Neuser, K.; Seugnet, L.; Kume, K.; Iché-Torres, M.; Cassar, M.; Strauss, R.; Preat, T.; et al. Behavioural consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Schwaerzel, M.; Monastirioti, M.; Scholz, H.; Friggi-Grelin, F.; Birman, S.; Heisenberg, M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 2003, 23, 10495–10502. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Griffith, L.C. The Role of Dopamine in Associative Learning in Drosophila: An Updated Unified Model. Neurosci. Bull. 2021, 37, 831–852. [Google Scholar] [CrossRef] [PubMed]
- Szczypka, M.S.; Kwok, K.; Brot, M.D.; Marck, B.T.; Matsumoto, A.M.; A Donahue, B.; Palmiter, R.D. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron 2001, 30, 819–828. [Google Scholar] [CrossRef]
- Helfrich-Förster, C. Light input pathways to the circadian clock of insects with an emphasis on the fruit fly Drosophila melanogaster. J. Comp. Physiol. A 2019, 206, 259–272. [Google Scholar] [CrossRef]
- Kume, K.; Kume, S.; Park, S.K.; Hirsh, J.; Jackson, F.R. Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 2005, 25, 7377–7384. [Google Scholar] [CrossRef]
- Wisor, J.P.; Nishino, S.; Sora, I.; Uhl, G.H.; Mignot, E.; Edgar, D.M. Dopaminergic role in stimulant-induced wakefulness. J. Neurosci. 2001, 21, 1787–1794. [Google Scholar] [CrossRef]
- Gjerstad, M.D.; Wentzel-Larsen, T.; Aarsland, D.; Larsen, J.P. Insomnia in Parkinson’s disease: Frequency and progression over time. J. Neurol. Neurosurg. Psychiatry 2007, 78, 476–479. [Google Scholar] [CrossRef]
- Monderer, R.; Thorpy, M. Sleep disorders and daytime sleepiness in Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2009, 9, 173–180. [Google Scholar] [CrossRef]
- Medeiros, D.D.C.; Aguiar, C.L.; Moraes, M.F.D.; Fisone, G. Sleep Disorders in Rodent Models of Parkinson’s Disease. Front. Pharmacol. 2019, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Beninger, R.J. The role of dopamine in locomotor activity and learning. Brain Res. Rev. 1983, 6, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Y.; Palmiter, R.D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 1995, 83, 1197–1209. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef]
- Yellman, C.; Tao, H.; He, B.; Hirsh, J. Conserved and sexually dimorphic behavioural responses to biogenic amines in decapitated Drosophila. Proc. Natl. Acad. Sci. USA 1997, 94, 4131–4136. [Google Scholar] [CrossRef]
- Feany, M.B.; Bender, W.W. A Drosophila model of Parkinson’s disease. Nature 2000, 404, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Auluck, A.; Pai, K.; Shetty, C.; Shenoi, S. Mandibular resorption in progressive systemic sclerosis: A report of three cases. Dentomaxillofac. Radiol. 2005, 34, 384–386. [Google Scholar] [CrossRef]
- Auluck, P.K.; Bonini, N.M. Pharmacological prevention of Parkinson disease in Drosophila. Nat. Med. 2002, 8, 1185–1186. [Google Scholar] [CrossRef]
- Cooper, A.A.; Gitler, A.D.; Cashikar, A.; Haynes, C.M.; Hill, K.J.; Bhullar, B.; Liu, K.; Xu, K.; Strathearn, K.E.; Liu, F.; et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 2006, 313, 324–328. [Google Scholar] [CrossRef]
- Trinh, K.; Moore, K.; Wes, P.D.; Muchowski, P.J.; Dey, J.; Andrews, L.; Pallanck, L.J. Induction of the phase II detoxification pathway suppresses neuron loss in Drosophila models of Parkinson’s disease. J. Neurosci. 2008, 28, 465–472. [Google Scholar] [CrossRef]
- Barone, M.C.; Sykiotis, G.P.; Bohmann, D. Genetic activation of Nrf2 signaling is sufficient to ameliorate neurodegenerative phenotypes in a Drosophila model of Parkinson’s disease. Dis. Model. Mech. 2011, 4, 701–707. [Google Scholar] [CrossRef]
- Butler, E.K.; Voigt, A.; Lutz, A.K.; Toegel, J.P.; Gerhardt, E.; Karsten, P.; Falkenburger, B.; Reinartz, A.; Winklhofer, K.F.; Schulz, J.B. The mitochondrial chaperone protein TRAP1 mitigates α-synuclein toxicity. PLoS Genet. 2012, 8, e1002488. [Google Scholar] [CrossRef]
- Riemensperger, T.; Issa, A.-R.; Pech, U.; Coulom, H.; Nguyễn, M.-V.; Cassar, M.; Jacquet, M.; Fiala, A.; Birman, S. A Single dopamine pathway underlies progressive locomotor deficits in a Drosophila model of parkinson disease. Cell Rep. 2013, 5, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Coulom, H.; Birman, S. Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J. Neurosci. 2004, 24, 10993–10998. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Bowling, K.; Funderburk, C.; Lawal, H.; Inamdar, A.; Wang, Z.; O’Donnell, J.M. Interaction of genetic and environmental factors in a Drosophila parkinsonism model. J. Neurosci. 2007, 27, 2457–2467. [Google Scholar] [CrossRef]
- Hosamani, R.; Ramesh, S.R. Muralidhara attenuation of rotenone-induced mitochondrial oxidative damage and neurotoxicty in Drosophila melanogaster supplemented with creatine. Neurochem. Res. 2010, 35, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Lawal, H.O.; Chang, H.-Y.; Terrell, A.N.; Brooks, E.S.; Pulido, D.; Simon, A.F.; Krantz, D.E. The Drosophila vesicular monoamine transporter reduces pesticide-induced loss of dopaminergic neurons. Neurobiol. Dis. 2010, 40, 102–112. [Google Scholar] [CrossRef]
- Islam, R.; Yang, L.; Sah, M.; Kannan, K.; Anamani, D.; Vijayan, C.; Kwok, J.; Cantino, M.E.; Beal, M.F.; Fridell, Y.W.C. A neuroprotective role of the human uncoupling protein 2 (hUCP2) in a Drosophila Parkinson’s disease model. Neurobiol. Dis. 2012, 46, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, E. Drosophila Models of Sporadic Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3343. [Google Scholar] [CrossRef]
- Chiang, A.S.; Lin, C.Y.; Chuang, C.C.; Chang, H.M.; Hsieh, C.H.; Yeh, C.W.; Shih, C.T.; Wu, J.J.; Wang, G.T.; Chen, Y.C.; et al. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr. Biol. 2011, 21, 1–11. [Google Scholar] [CrossRef]
- Scheffer, L.K.; Xu, C.S.; Januszewski, M.; Lu, Z.; Takemura, S.-Y.; Hayworth, K.J.; Huang, G.B.; Shinomiya, K.; Maitlin-Shepard, J.; Berg, S.; et al. A connectome and analysis of the adult Drosophila central brain. Elife 2020, 9, e57443. [Google Scholar] [PubMed]
- Chen, L.; Periquet, M.; Wang, X.; Negro, A.; McLean, P.J.; Hyman, B.T.; Feany, M.B. Tyrosine and serine phosphorylation of α-synuclein have opposing effects on neurotoxicity and soluble oligomer formation. J. Clin. Investig. 2009, 119, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Pech, U.; Pooryasin, A.; Birman, S.; Fiala, A. Localization of the contacts between Kenyon cells and aminergic neurons in the Drosophila melanogaster brain using SplitGFP reconstitution. J. Comp. Neurol. 2013, 521, 3992–4026. [Google Scholar] [PubMed]
- Bridi, J.C.; Hirth, F. Mechanisms of alpha-Synuclein Induced Synaptopathy in Parkinson’s Disease. Front. Neurosci. 2018, 12, 80. [Google Scholar] [CrossRef]
- Kamikouchi, A.; Shimada, T.; Ito, K. Comprehensive classification of the auditory sensory projections in the brain of the fruit fly Drosophila melanogaster. J. Comp. Neurol. 2006, 499, 317–356. [Google Scholar] [CrossRef]
- Clements, J.; Dolafi, T.; Umayam, L.; Neubarth, N.L.; Berg, S.; Scheffer, L.K.; Plaza, S.M. NeuPrint: Analysis Tools for EM Connectomics. BioRxiv 2020. [Google Scholar] [CrossRef]
- Niens, J.; Reh, F.; Çoban, B.; Cichewicz, K.; Eckardt, J.; Liu, Y.-T.; Hirsh, J.; Riemensperger, T.D. Dopamine Modulates Serotonin Innervation in the Drosophila Brain. Front. Syst. Neurosci. 2017, 11, 76. [Google Scholar] [CrossRef]
- Niederkofler, V.; Asher, T.E.; Dymecki, S.M. Functional Interplay between Dopaminergic and Serotonergic Neuronal Systems during Development and Adulthood. ACS Chem. Neurosci. 2015, 6, 1055–1070. [Google Scholar] [CrossRef]
- Linneweber, G.A.; Andriatsilavo, M.; Dutta, S.B.; Bengochea, M.; Hellbruegge, L.; Liu, G.; Ejsmont, R.K.; Straw, A.D.; Wernet, M.; Hiesinger, P.R.; et al. A neurodevelopmental origin of behavioural individuality in the Drosophila visual system. Science 2020, 367, 1112–1119. [Google Scholar] [CrossRef]
- Buchanan, S.M.; Kain, J.S.; de Bivort, B.L. Neuronal control of locomotor handedness in Drosophila. Proc. Natl. Acad. Sci. USA 2015, 112, 6700–6705. [Google Scholar]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef]
- Ito, K.; Shinomiya, K.; Ito, M.; Armstrong, J.D.; Boyan, G.; Hartenstein, V.; Harzsch, S.; Heisenberg, M.; Homberg, U.; Jenett, A.; et al. A Systematic Nomenclature for the Insect Brain. Neuron 2014, 81, 755–765. [Google Scholar] [CrossRef]
- Pascual, A.; Huang, K.L.; Neveu, J.; Préat, T. Neuroanatomy: Brain asymmetry and long-term memory. Nature 2004, 427, 605–606. [Google Scholar] [CrossRef] [PubMed]
- Duistermars, B.J.; Chow, D.M.; Frye, M.A. Flies require bilateral sensory input to track odor gradients in flight. Curr. Biol. 2009, 19, 1301–1307. [Google Scholar] [CrossRef]
- Frasnelli, E.; Vallortigara, G.; Rogers, L.J. Left–right asymmetries of behaviour and nervous system in invertebrates. Neurosci. Biobehav. Rev. 2012, 36, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Scherfler, C.; Seppi, K.; Mair, K.J.; Donnemiller, E.; Virgolini, I.; Wenning, G.; Poewe, W. Left hemispheric predominance of nigrostriatal dysfunction in Parkinson’s disease. Brain 2012, 135, 3348–3354. [Google Scholar] [CrossRef]
- Versace, E.; Caffini, M.; Werkhoven, Z.; de Bivort, B.L. Individual, but not population asymmetries, are modulated by social environment and genotype in Drosophila melanogaster. Sci. Rep. 2020, 10, 4480. [Google Scholar] [CrossRef] [PubMed]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef]
- Verkhusha, V.V.; Otsuna, H.; Awasaki, T.; Oda, H.; Tsukita, S.; Ito, K. An enhanced mutant of red fluorescent protein dsred for double labeling and developmental timer of neural fiber bundle formation. J. Biol. Chem. 2001, 276, 29621–29624. [Google Scholar] [CrossRef]
- Wagh, D.A.; Rasse, T.M.; Asan, E.; Hofbauer, A.; Schwenkert, I.; Dürrbeck, H.; Buchner, S.; Dabauvalle, M.-C.; Schmidt, M.; Qin, G.; et al. Bruchpilot, a protein with homology to elks/cast, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 2006, 49, 833–844. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Preibisch, S.; Saalfeld, S.; Tomancak, P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 2009, 25, 1463–1465. [Google Scholar] [CrossRef]
- Wan, Y.; Otsuna, H.; Holman, H.A.; Bagley, B.; Ito, M.; Lewis, A.K.; Colasanto, M.; Kardon, G.; Ito, K.; Hansen, C. FluoRender: Joint freehand segmentation and visualization for many-channel fluorescence data analysis. BMC Bioinform. 2017, 18, 280. [Google Scholar] [CrossRef]
- Bogovic, J.A.; Otsuna, H.; Heinrich, L.; Ito, M.; Jeter, J.; Meissner, G.; Nern, A.; Colonell, J.; Malkesman, O.; Ito, K.; et al. An unbiased template of the Drosophila brain and ventral nerve cord. PLoS ONE 2020, 15, e0236495. [Google Scholar] [CrossRef] [PubMed]
- Otsuna, H.; Ito, M.; Kawase, T. Color depth MIP mask search: A new tool to expedite Split-GAL4 creation. BioRxiv 2018. [CrossRef]
- Bates, A.S.; Manton, J.D.; Jagannathan, S.R.; Costa, M.; Schlegel, P.; Rohlfing, T.; Jefferis, G.S. The natverse, a versatile toolbox for combining and analysing neuroanatomical data. Elife 2020, 9, e53350. [Google Scholar] [CrossRef]
- Chockley, A.S.; Dinges, G.F.; Di Cristina, G.; Ratican, S.; Bockemühl, T.; Büschges, A. Subsets of leg proprioceptors influence leg kinematics but not interleg coordination in Drosophila melanogaster walking. J. Exp. Biol. 2022, 225, jeb244245. [Google Scholar] [CrossRef]
- Lim, R.S.; Eyjolfsdottir, E.; Shin, E.; Perona, P.; Anderson, D.J. How food controls aggression in Drosophila. PLoS ONE 2014, 9, e105626. [Google Scholar] [CrossRef]
- DeAngelis, B.D.; Zavatone-Veth, J.A.; Gonzalez-Suarez, A.D.; Clark, D.A. Spatiotemporally precise optogenetic activation of sensory neurons in freely walking Drosophila. Elife 2020, 9, e54183. [Google Scholar] [CrossRef] [PubMed]



| Light Microscopy (Bouton) | Electron Microscopy (T-Bar) | |||
|---|---|---|---|---|
| Mean ± SD of 10 Samples | PPL204_R | PPL204_L | Total | |
| ATL (left) | 435.2 ± 57.7 | 201 | 379 | 580 |
| ATL (right) | 374.6 ± 41.4 | 462 | 295 | 757 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Lentz, L.; Goldammer, J.; Iliescu, J.; Tanimura, J.; Riemensperger, T.D. Asymmetric Presynaptic Depletion of Dopamine Neurons in a Drosophila Model of Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 8585. https://doi.org/10.3390/ijms24108585
Zhang J, Lentz L, Goldammer J, Iliescu J, Tanimura J, Riemensperger TD. Asymmetric Presynaptic Depletion of Dopamine Neurons in a Drosophila Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(10):8585. https://doi.org/10.3390/ijms24108585
Chicago/Turabian StyleZhang, Jiajun, Lucie Lentz, Jens Goldammer, Jessica Iliescu, Jun Tanimura, and Thomas Dieter Riemensperger. 2023. "Asymmetric Presynaptic Depletion of Dopamine Neurons in a Drosophila Model of Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 10: 8585. https://doi.org/10.3390/ijms24108585
APA StyleZhang, J., Lentz, L., Goldammer, J., Iliescu, J., Tanimura, J., & Riemensperger, T. D. (2023). Asymmetric Presynaptic Depletion of Dopamine Neurons in a Drosophila Model of Parkinson’s Disease. International Journal of Molecular Sciences, 24(10), 8585. https://doi.org/10.3390/ijms24108585








