Transcriptome and Metabolome Reveal Distinct Sugar Accumulation Pattern between PCNA and PCA Mature Persimmon Fruit
Abstract
1. Introduction
2. Results
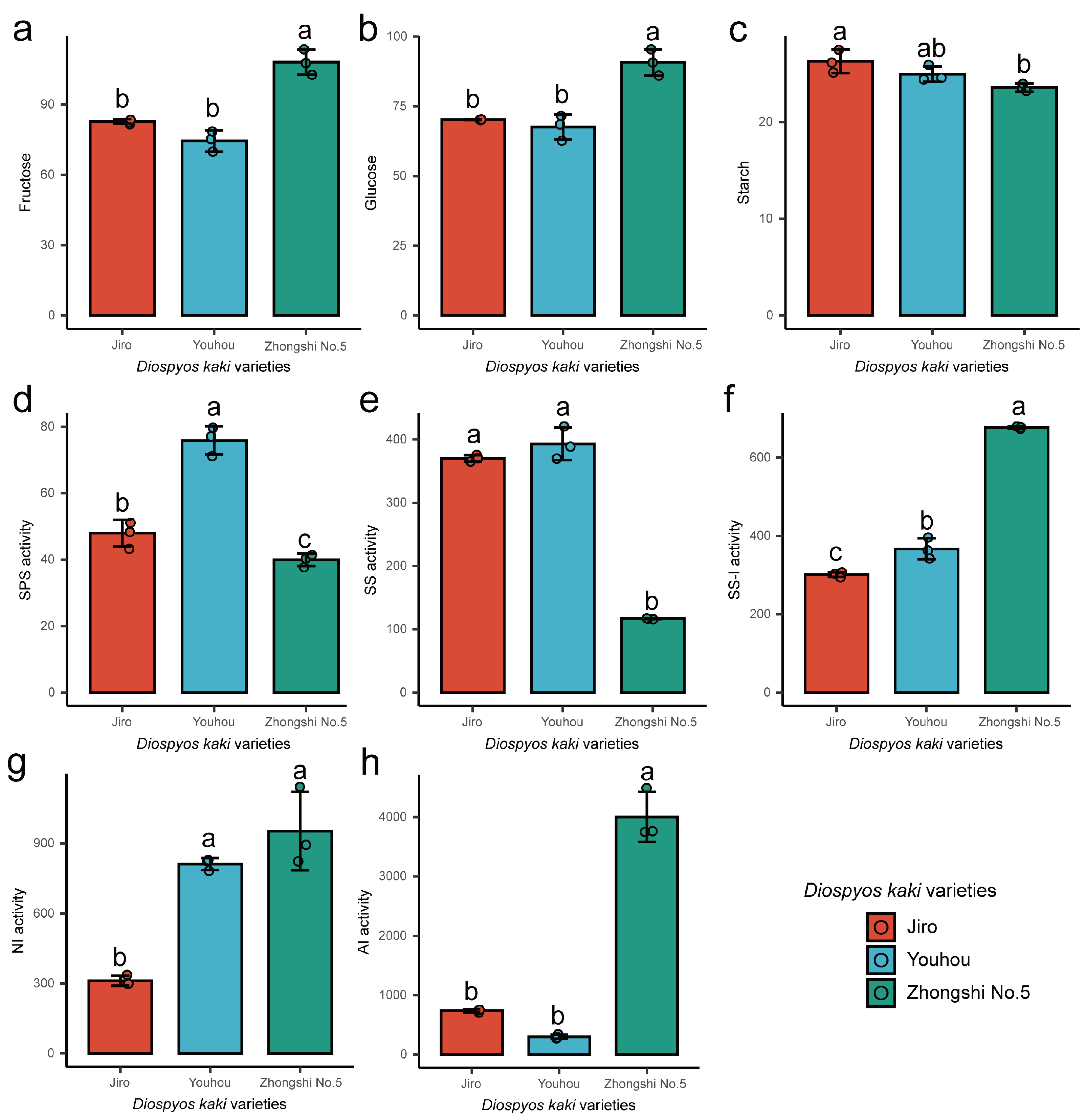
2.1. Soluble Sugar Content, Starch Content, and Sucrose Synthase and Invertase Activity
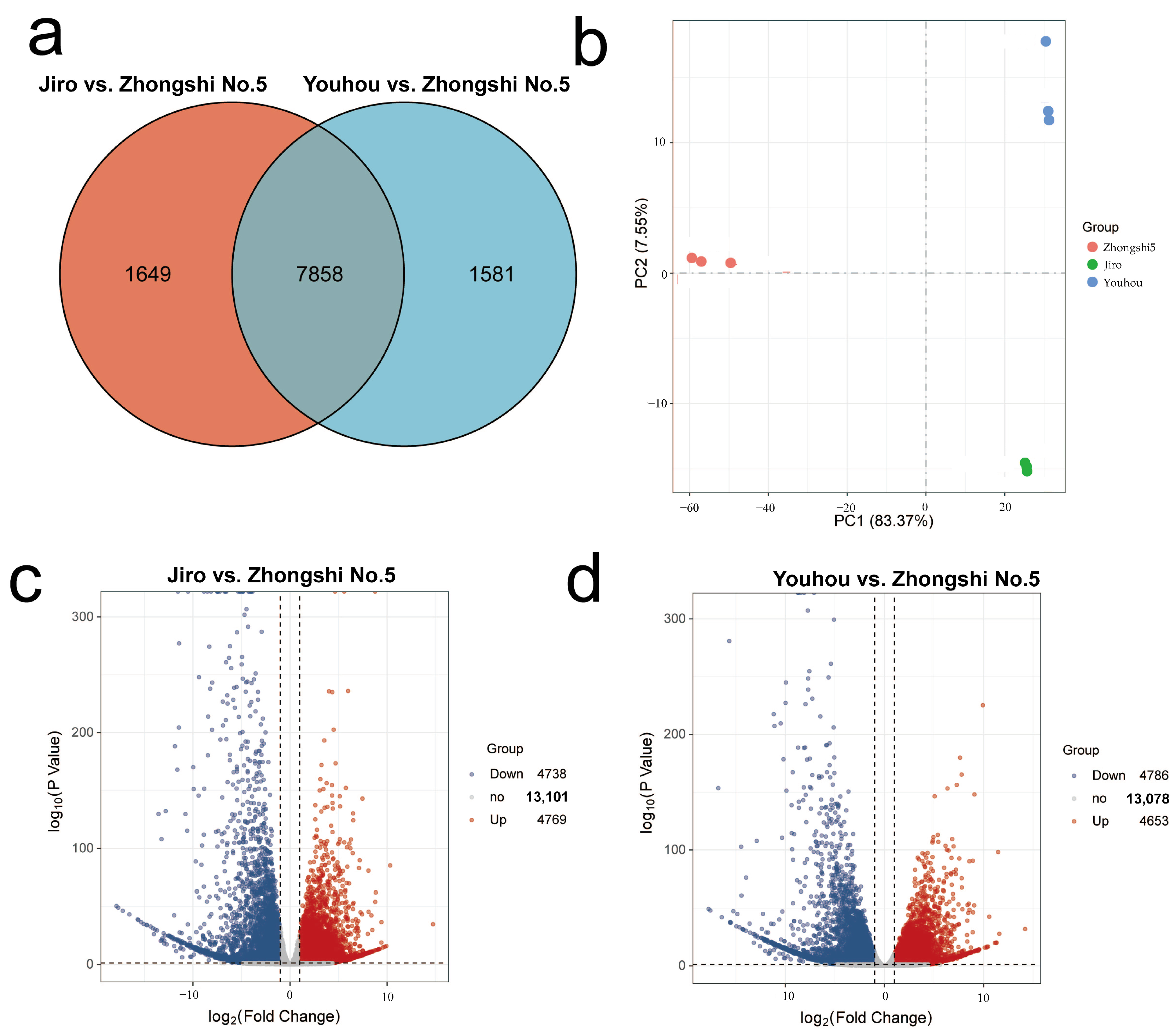
2.2. RNA-Seq of PCNA and PCA Persimmon Mature Fruit
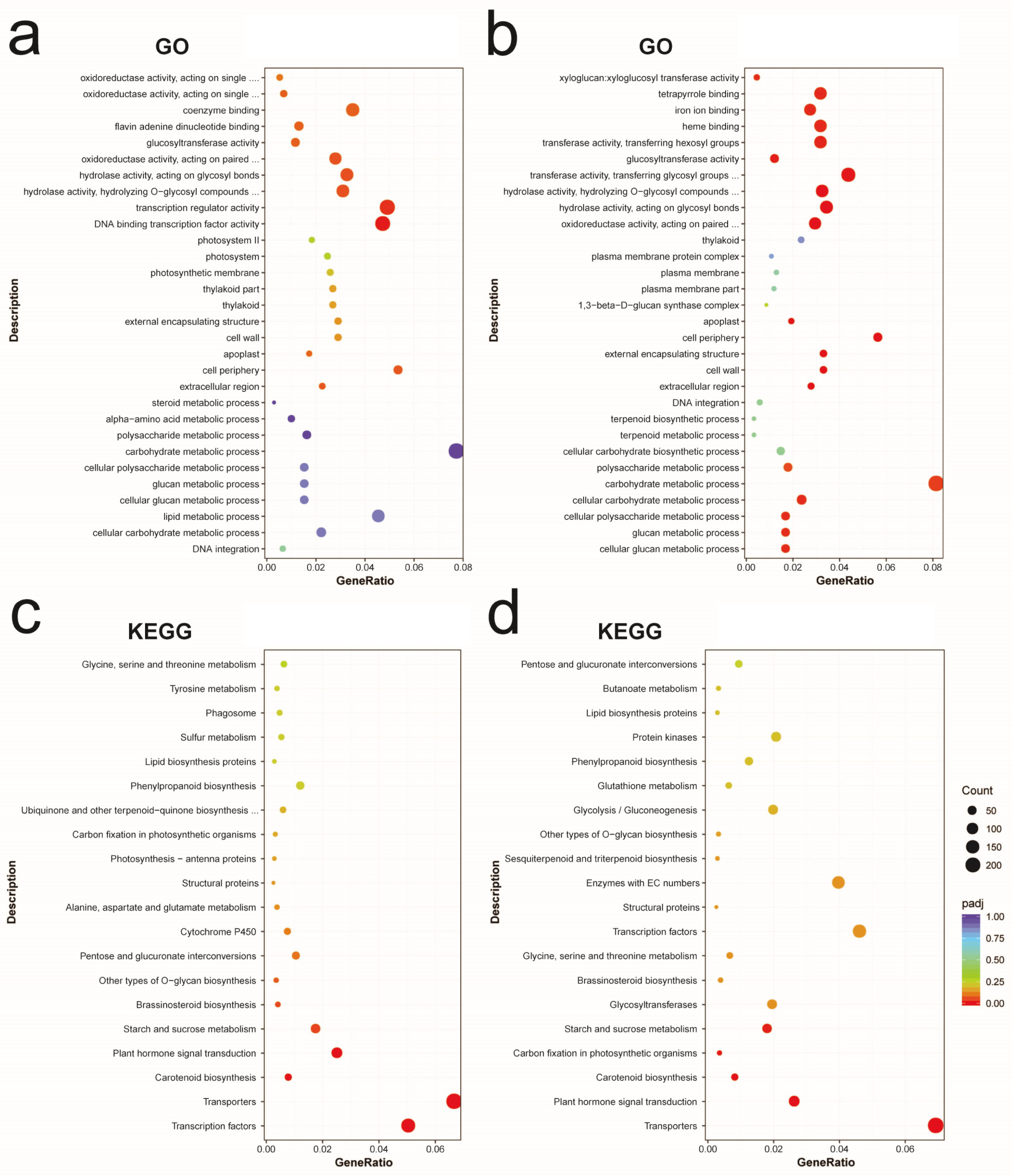
2.3. GO and KEGG Enrichment Analysis for DEGs
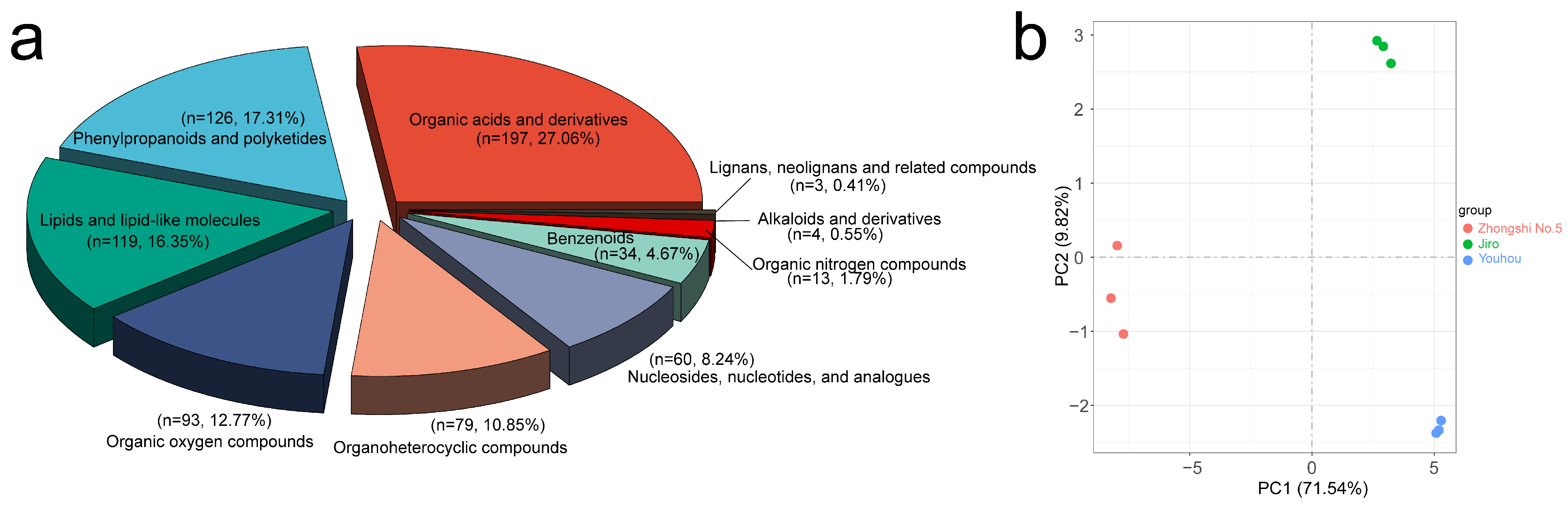
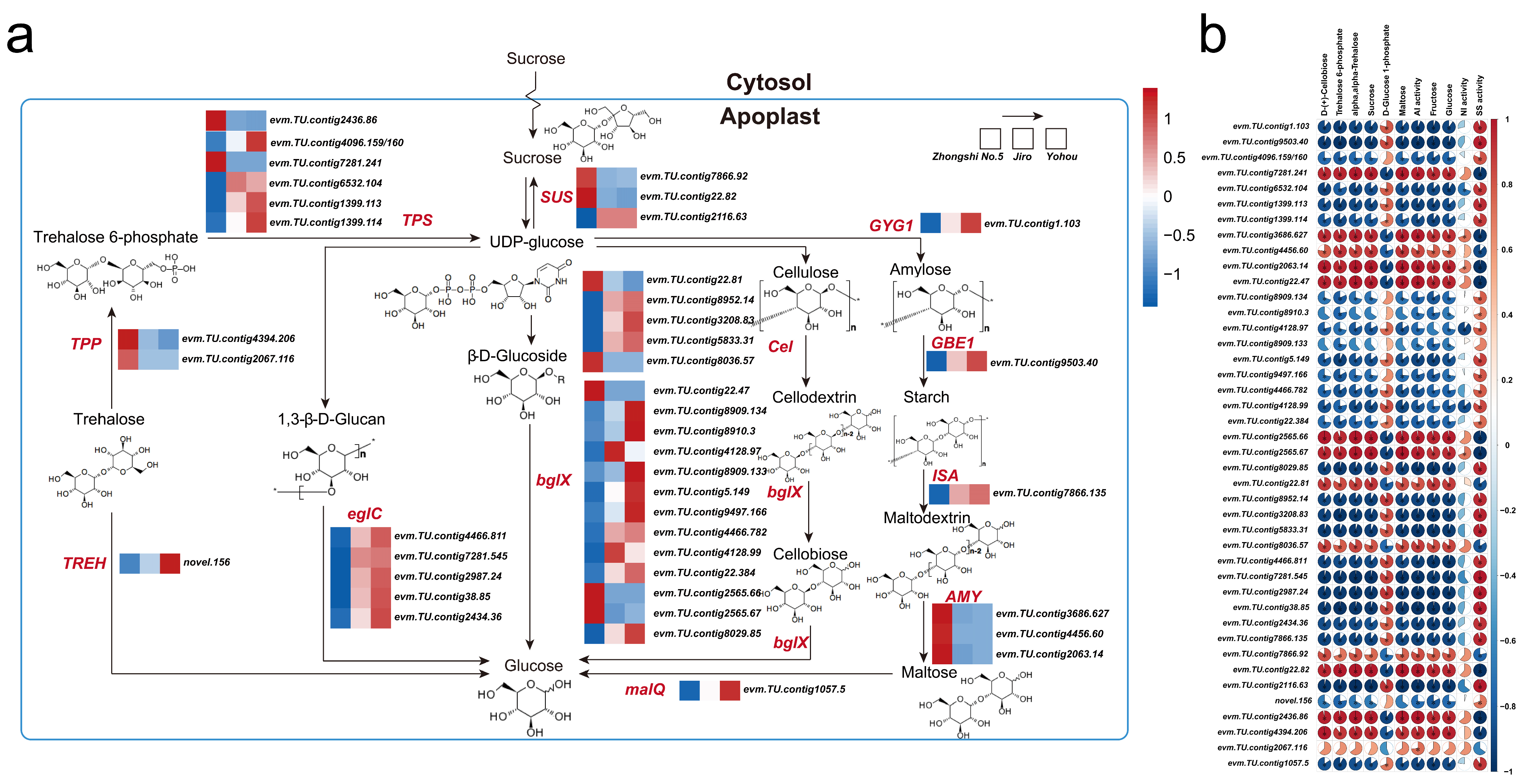
2.4. Metabolic Features of Starch and Sucrose Metabolism
2.5. Expression of Genes Implicated within Sucrose and Starch Metabolic Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Soluble Sugar, Starch, and Sucrose Synthase and Invertase Activity Measurement
4.3. Transcriptome Sequencing
4.4. Metabolite Profiling Analysis
4.5. Quantitative RT-PCR Assessments
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, C.; Zhang, P.; Hu, C.; Chachar, S.; Riaz, A.; Wang, R.; Yang, Y. Genetic diversity, germplasm identification and population structure of Diospyros kaki Thunb. from different geographic regions in China using SSR markers. Sci. Hortic. 2019, 251, 233–240. [Google Scholar] [CrossRef]
- Yonemori, K.; Sugiura, A.; Yamada, M. Persimmon genetics and breeding. Plant Breed. Rev. 2000, 19, 191–225. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Q.; Luo, Z. Comparison of four molecular markers for genetic analysis in Diospyros L. (Ebenaceae). Plant Syst. Evol. 2009, 281, 171. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Ma, F.; Dandekar, A.M.; Cheng, L. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 2018, 5, 60. [Google Scholar] [CrossRef]
- Ayre, B.G. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant 2011, 4, 377–394. [Google Scholar] [CrossRef]
- Kühn, C.; Grof, C.P.L. Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 2010, 13, 287–297. [Google Scholar] [CrossRef]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in peach fruit: A breeding perspective. Hortic. Res. 2016, 3, 15067. [Google Scholar] [CrossRef]
- Huang, Y.H.; Zeng, M. Research advances in sugar metabolism and regulatory factors in pear fruits. Plant Physiol. J. 2013, 49, 709–714. [Google Scholar]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Moing, A.; Langlois, N.; Svanella, L.; Zanetto, A.; Gaudillère, J.P. Variability in sorbitol: Sucrose ratio in mature leaves of different prunus species. J. Am. Soc. Hortic. Sci. 1997, 122, 83–90. [Google Scholar] [CrossRef]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zhang, X.; Chen, L.; Zhao, Q.; Zhang, Q.; Hua, X.; Wang, Z.; Tang, H.; Yu, Q.; Zhang, M.; et al. Comparative analysis of sucrose phosphate synthase (SPS) gene family between Saccharum officinarum and Saccharum spontaneum. BMC Plant Biol. 2020, 20, 422. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Upadhyay, S.K.; Verma, P.C.; Solomon, S.; Singh, S.B. Functional analysis of sucrose phosphate synthase (SPS) and sucrose synthase (SS) in sugarcane (Saccharum) cultivars. Plant Biol. 2011, 13, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Gayler, K.R.; Glasziou, K.T. Physiological functions of acid and neutral invertases in growth and sugar storage in sugar cane. Physiol. Plant 1972, 27, 25–31. [Google Scholar] [CrossRef]
- Tymowska-Lalanne, Z.; Kreis, M. The plant invertases: Physiology, biochemistry and molecular biology. In Advances in Botanical Research, 1st ed.; Callow, J.A., Ed.; Academic Press: San Diego, CA, USA, 1998; Volume 28, pp. 71–117. [Google Scholar]
- Novillo, P.; Salvador, A.; Crisosto, C.; Besada, C. Influence of persimmon astringency type on physico-chemical changes from the green stage to commercial harvest. Sci. Hortic. 2016, 206, 7–14. [Google Scholar] [CrossRef]
- Yildiz, E.; Kaplankiran, M. Changes in sugars content and some biochemical substances during fruit development in different persimmon cultivars. J. Agric. Fac. Mustafa Kemal Univ. 2018, 23, 12–23. [Google Scholar]
- Wang, Y.; Suo, Y.; Han, W.; Li, H.; Wang, Z.; Diao, S.; Sun, P.; Fu, J. Comparative transcriptomic and metabolomic analyses reveal differences in flavonoid biosynthesis between PCNA and PCA persimmon fruit. Front. Plant Sci. 2023, 14, 1130047. [Google Scholar] [CrossRef]
- Baltacıoğlu, H.; Artık, N. Study of postharvest changes in the chemical composition of persimmon by HPLC. Turk. J. Agric. For. 2013, 37, 568–574. [Google Scholar] [CrossRef]
- Veberic, R.; Jurhar, J.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Comparative study of primary and secondary metabolites in 11 cultivars of persimmon fruit (Diospyros kaki L.). Food Chem. 2010, 119, 477–483. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D. A Study on the relationships between acid invertase, sucrose synthase and sucrose metabolism in ‘Red Fuji’ apple fruit. Acta Hortic. Sin. 2001, 28, 256–261. [Google Scholar]
- Gutiérrez-Miceli, F.A.; Rodríguez-Mendiola, M.A.; Ochoa-Alejo, N.; Méndez-Salas, R.; Dendooven, L.; Arias-Castro, C. Relationship between sucrose accumulation and activities of sucrose-phosphatase, sucrose synthase, neutral invertase and soluble acid invertase in micropropagated sugarcane plants. Acta Physiol. Plant 2002, 24, 441–446. [Google Scholar] [CrossRef]
- Wu, B.; Liu, H.; Guan, L.; Fan, P.; Li, S. Carbohydrate metabolism in grape cultivars that differ in sucrose accumulation. Vitis 2011, 50, 51–57. [Google Scholar] [CrossRef]
- Del Bubba, M.; Giordani, E.; Pippucci, L.; Cincinelli, A.; Checchini, L.; Galvan, P. Changes in tannins, ascorbic acid and sugar content in astringent persimmons during on-tree growth and ripening and in response to different postharvest treatments. J. Food Compos. 2009, 22, 668–677. [Google Scholar] [CrossRef]
- Harvey, W.J.; Grant, D.G.; Lammerink, J.P. Physical and sensory changes during the development and storage of buttercup squash. N. Z. J. Crop Hortic. 1997, 25, 341–351. [Google Scholar] [CrossRef]
- Irving, D.E.; Hurst, P.L.; Ragg, J.S. Changes in carbohydrates and carbohydrate metabolizing enzymes during the development, maturation, and ripening of buttercup squash (Cucurbita maxima D. ‘Delica’). J. Am. Soc. Hortic. Sci. 1997, 122, 310–314. [Google Scholar] [CrossRef]
- Phillips, T.G. Changes in the composition of squash during storage1. Plant Physiol. 1946, 21, 533–541. [Google Scholar] [CrossRef]
- Yu, J.; Xu, S.; Liu, X.; Li, T.; Zhang, D.; Teng, N.; Wu, Z. Starch degradation and sucrose accumulation of lily bulbs after cold storage. Int. J. Mol. Sci. 2022, 23, 4366. [Google Scholar] [CrossRef]
- Wingler, A. The function of trehalose biosynthesis in plants. Phytochemistry 2002, 60, 437–440. [Google Scholar] [CrossRef]
- Ponnu, J.; Wahl, V.; Schmid, M. Trehalose-6-phosphate: Connecting plant metabolism and development. Front. Plant Sci. 2011, 2, 70. [Google Scholar] [CrossRef]
- Paul, M.J.; Primavesi, L.F.; Jhurreea, D.; Zhang, Y. Trehalose Metabolism and signaling. Annu. Rev. Plant Biol. 2008, 59, 417–441. [Google Scholar] [CrossRef]
- O’Hara, L.E.; Paul, M.J.; Wingler, A. How do sugars regulate plant growth and development? New insight into the role of Trehalose-6-Phosphate. Mol. Plant 2013, 6, 261–274. [Google Scholar] [CrossRef]
- Kolbe, A.; Tiessen, A.; Schluepmann, H.; Paul, M.; Ulrich, S.; Geigenberger, P. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proc. Natl. Acad. Sci. USA 2005, 102, 11118–11123. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.P.; Ivakov, A.; Feil, R.; Duan, G.Y.; Walther, D.; Giavalisco, P.; Piques, M.; Carillo, P.; Hubberten, H.M.; Stitt, M.; et al. The sucrose–trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. J. Exp. Bot. 2014, 65, 1051–1068. [Google Scholar] [CrossRef]
- Tao, H.; Sun, H.; Wang, Y.; Song, X.; Guo, Y. New insights on ‘GALA’ apple fruit development: Sugar and acid accumulation: A transcriptomic approach. J. Plant Growth Regul. 2020, 39, 680–702. [Google Scholar] [CrossRef]
- Naraian, R.; Gautam, R.L. Penicillium enzymes for the saccharification of lignocellulosic feedstocks. In New and Future Developments in Microbial Biotechnology and Bioengineering, 2nd ed.; Gupta, V.K., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 6, pp. 121–136. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.; Ma, G. Differential expression analysis of genes related to starch and sucrose metabolism pathway in different developmental stages of dragon fruit pulp based on transcriptome. Chin. J. Trop. Crop. 2021, 42, 1520–1530. [Google Scholar] [CrossRef]
- Bhatia, Y.; Mishra, S.; Bisaria, V.S. Microbial β-glucosidases: Cloning, properties, and applications. Crit. Rev. Biotechnol. 2002, 22, 375–407. [Google Scholar] [CrossRef]
- Tomasik, P.; Horton, D. Enzymatic conversions of starch. In Advances in Carbohydrate Chemistry and Biochemistry, 2nd ed.; Horton, D., Ed.; Academic Press: San Diego, CA, USA, 2012; Volume 2, pp. 59–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Cao, J.; Jiang, W.; Zhao, Y. Experiment Guidance of Postharvest Physiology and Biochemistry of Fruits and Vegetables, 1st ed.; China Light Industry Press Ltd.: Beijing, China, 2007; pp. 76–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, C.; Bennett, C.; Thornton, M.; Kim, D. Rapid and accurate alignment of nucleotide conversion sequencing reads with HISAT-3N. Genome Res. 2021, 31, 1290–1295. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2022, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H.; Murphy, R.C., Jr.; Raetz, C.R.H.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Du, G.; Wang, L.; Li, H.; Sun, P.; Fu, J.; Suo, Y.; Han, W.; Diao, S.; Mai, Y.; Li, F. Selection and validation of reference genes for quantitative gene expression analyses in persimmon (Diospyros kaki Thunb.) using real-time quantitative PCR. Biol. Futur. 2019, 70, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Miao, B.; Wang, S.; Dong, W.; Xu, H.; Si, C.; Wang, W.; Duan, S.; Lou, J.; Bao, Z.; et al. Hiplot: A comprehensive and easy-to-use web service for boosting publication-ready biomedical data visualization. Brief. Bioinform. 2022, 23, bbac261. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, W.; Wang, Y.; Li, H.; Diao, S.; Suo, Y.; Li, T.; Sun, P.; Li, F.; Fu, J. Transcriptome and Metabolome Reveal Distinct Sugar Accumulation Pattern between PCNA and PCA Mature Persimmon Fruit. Int. J. Mol. Sci. 2023, 24, 8599. https://doi.org/10.3390/ijms24108599
Han W, Wang Y, Li H, Diao S, Suo Y, Li T, Sun P, Li F, Fu J. Transcriptome and Metabolome Reveal Distinct Sugar Accumulation Pattern between PCNA and PCA Mature Persimmon Fruit. International Journal of Molecular Sciences. 2023; 24(10):8599. https://doi.org/10.3390/ijms24108599
Chicago/Turabian StyleHan, Weijuan, Yiru Wang, Huawei Li, Songfeng Diao, Yujing Suo, Taishan Li, Peng Sun, Fangdong Li, and Jianmin Fu. 2023. "Transcriptome and Metabolome Reveal Distinct Sugar Accumulation Pattern between PCNA and PCA Mature Persimmon Fruit" International Journal of Molecular Sciences 24, no. 10: 8599. https://doi.org/10.3390/ijms24108599
APA StyleHan, W., Wang, Y., Li, H., Diao, S., Suo, Y., Li, T., Sun, P., Li, F., & Fu, J. (2023). Transcriptome and Metabolome Reveal Distinct Sugar Accumulation Pattern between PCNA and PCA Mature Persimmon Fruit. International Journal of Molecular Sciences, 24(10), 8599. https://doi.org/10.3390/ijms24108599






