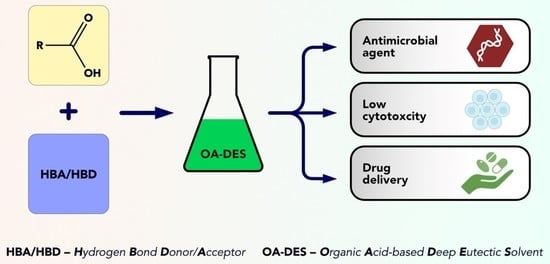
Deep Eutectic Solvents Comprising Organic Acids and Their Application in (Bio)Medicine
Abstract
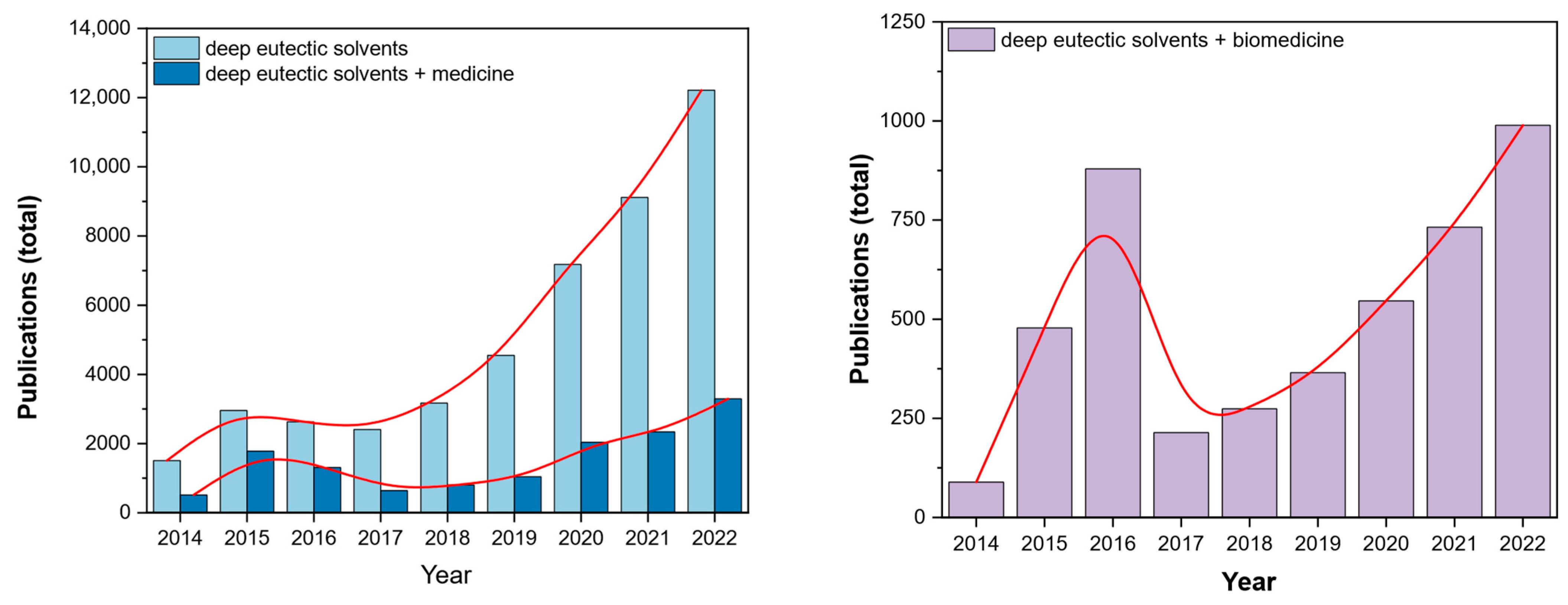
1. Introduction
1.1. Brief Background and State-of-the-Art
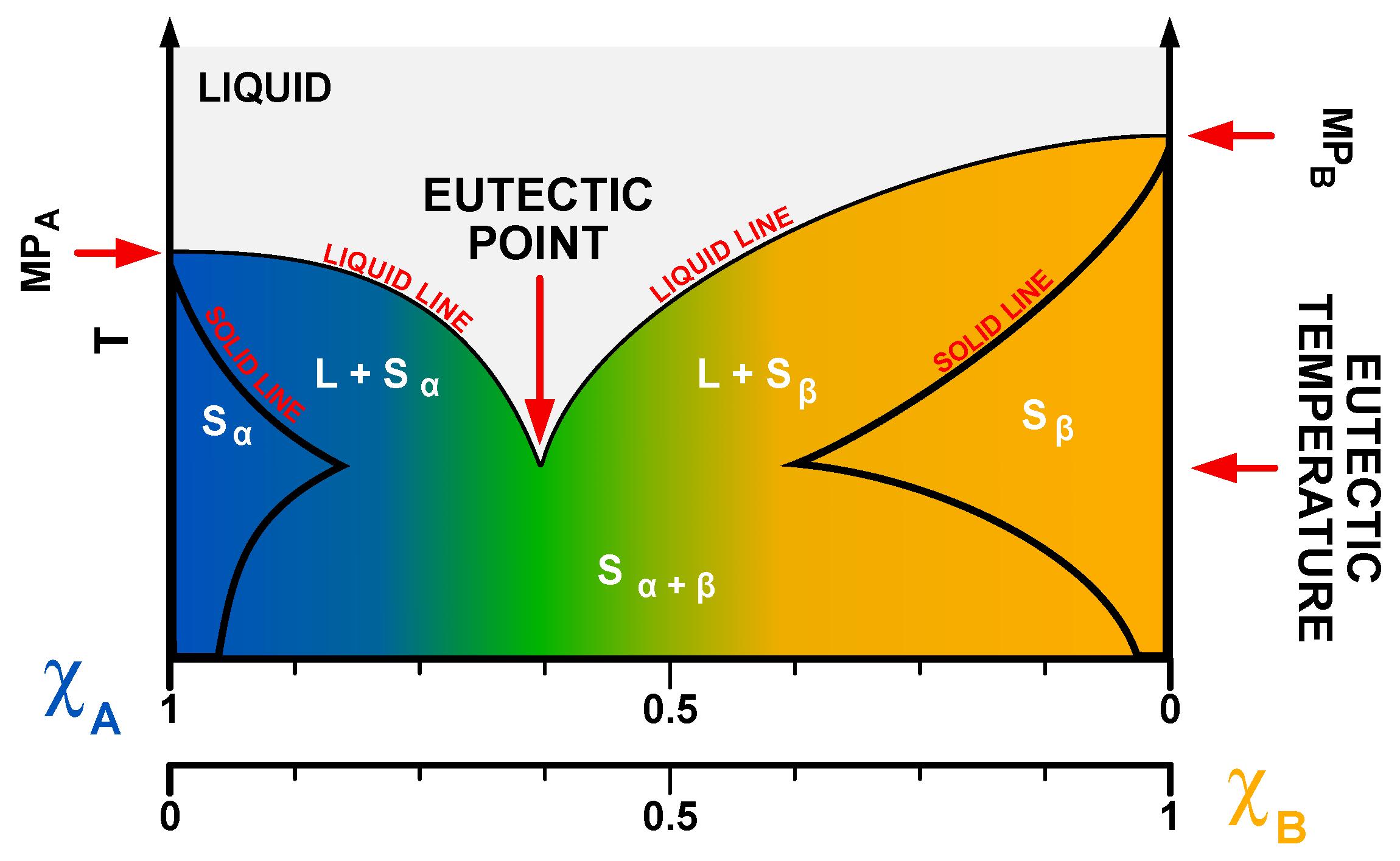
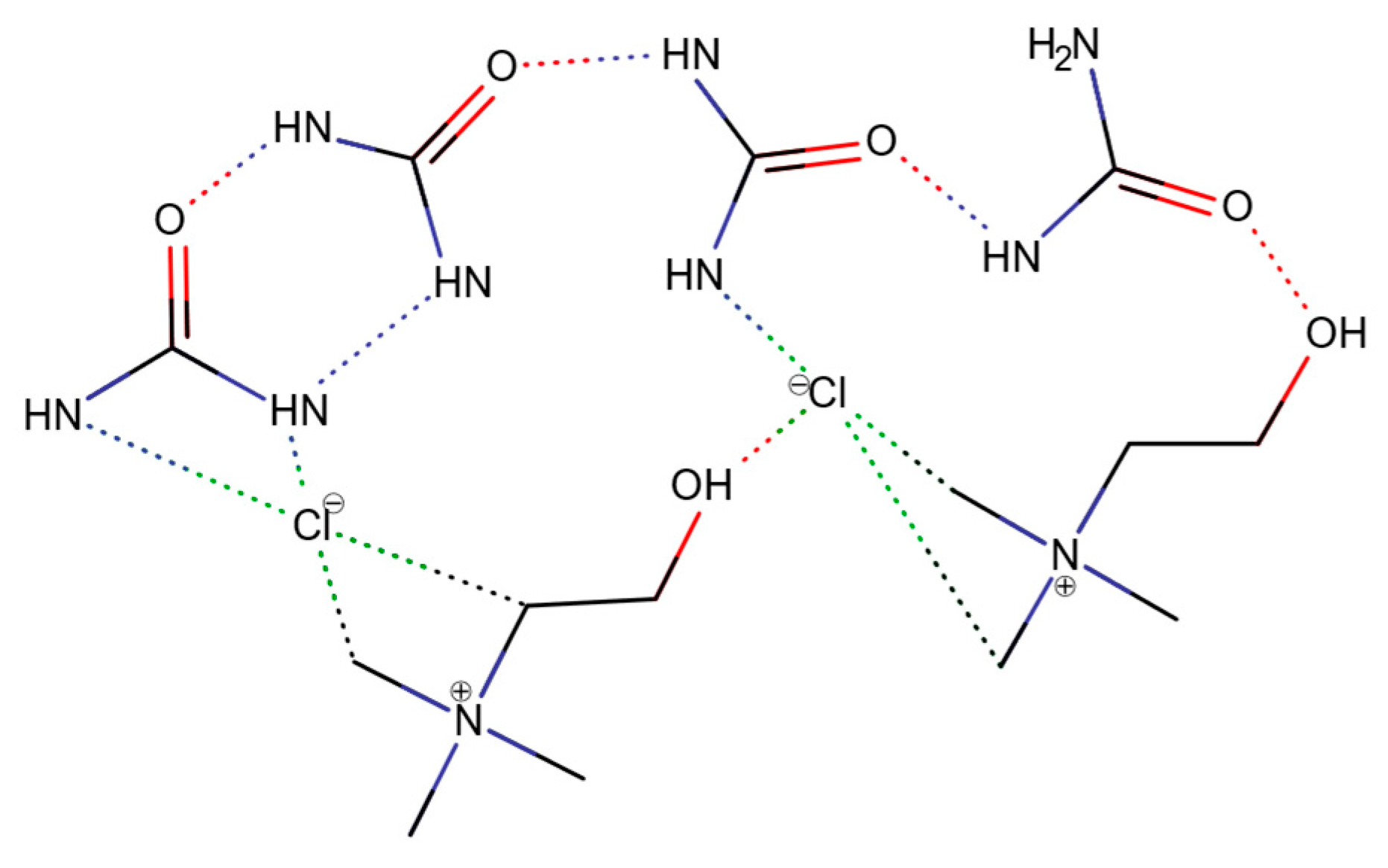
1.2. Physicochemistry of Deep Eutectic Solvents (DESs)
Current Categorization of DESs
- TYPE I: quaternary ammonium salt + metal chloride
- TYPE II: quaternary ammonium salt + metal chloride hydrate (pseudo ternary system)
- TYPE III: quaternary ammonium salt + HBD
- TYPE IV: metal chloride hydrate + HBD
1.3. Organic Acids and Their Medicinal Properties
2. OA-DESs Applications in Biomedicine
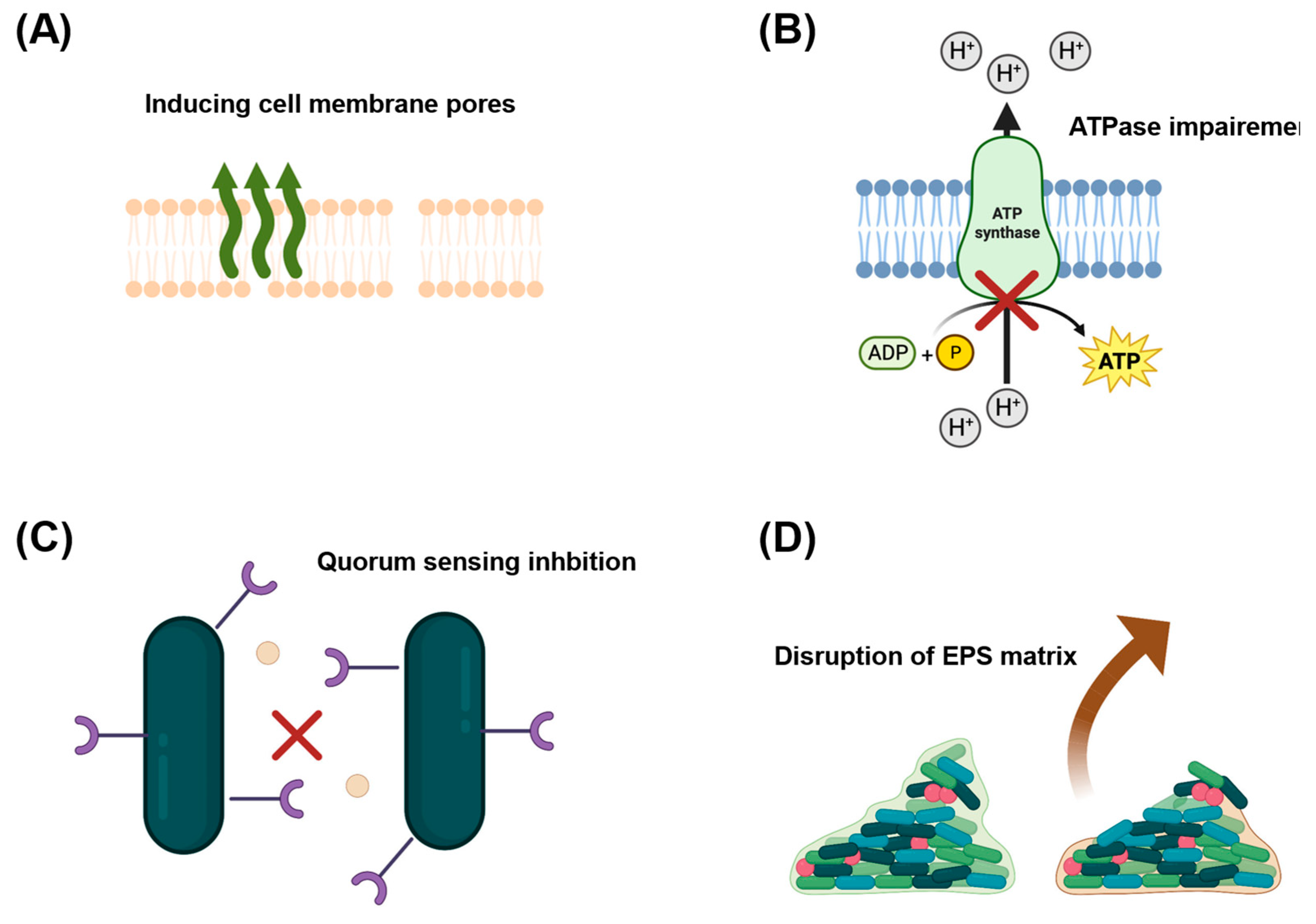
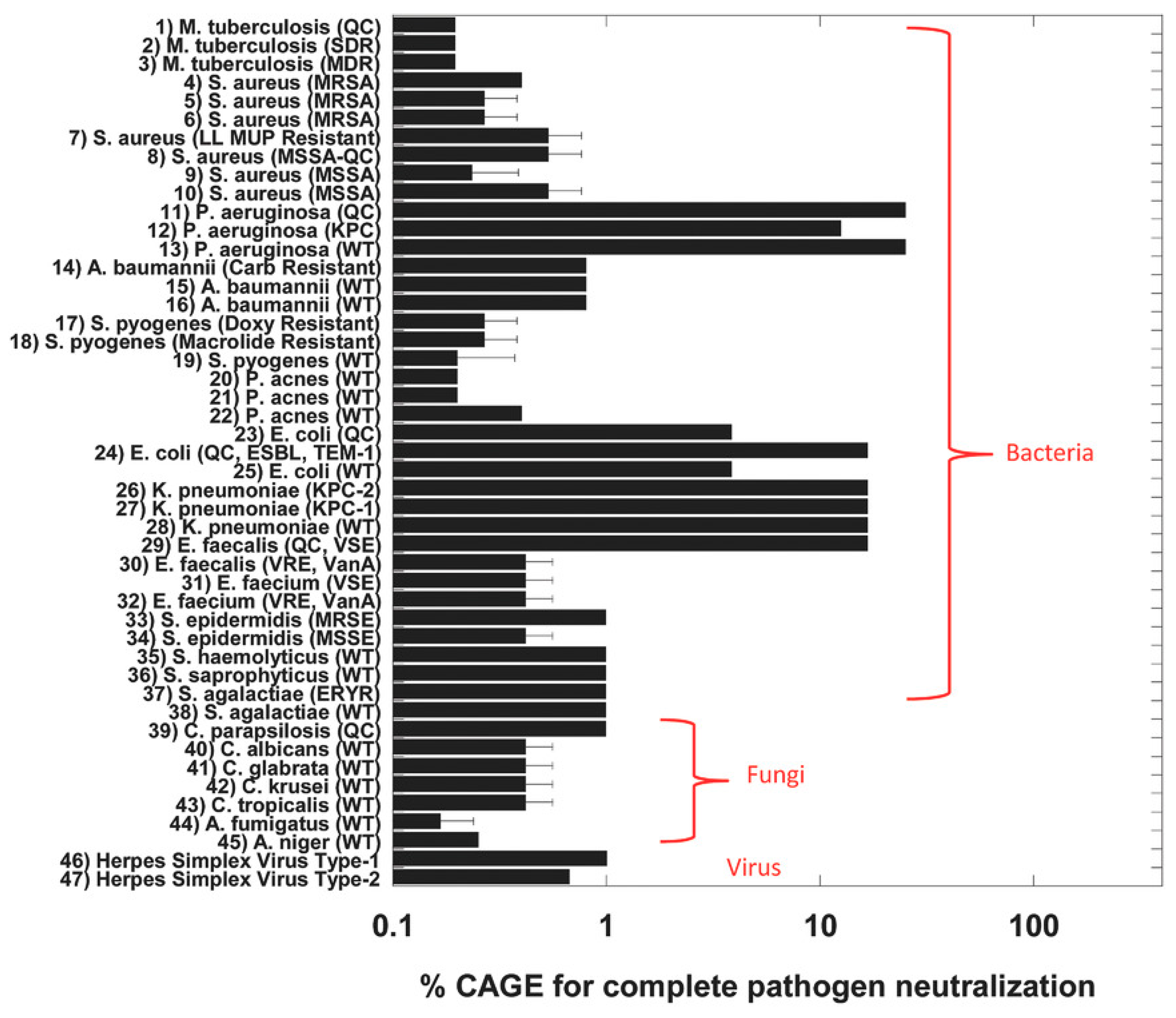
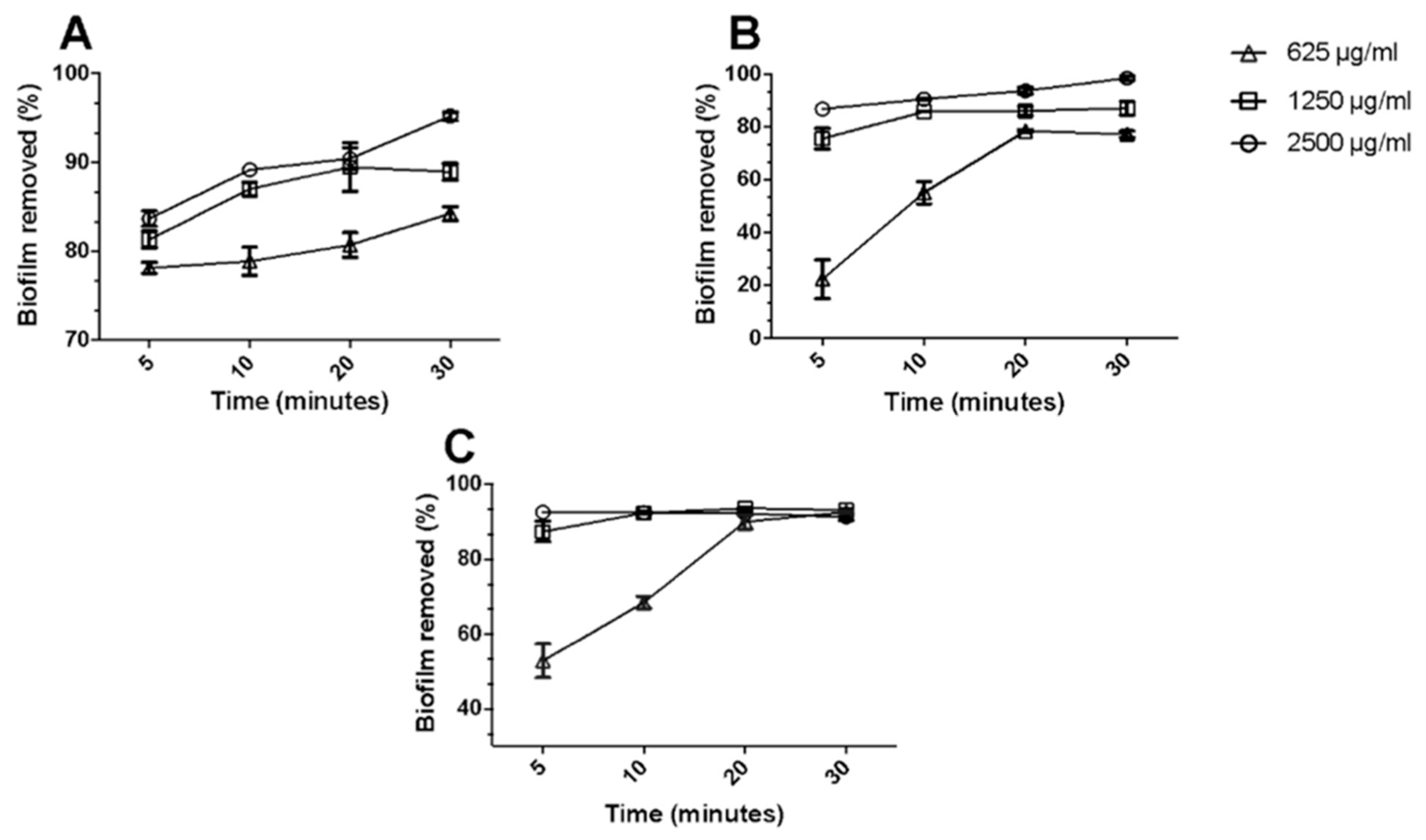
2.1. Antibacterial Properties of OA-DESs
2.2. Drug Delivery Using OA-DESs
- route of administration
- permeability
- solubility
- stability
3. Perspectives and Challenges to Overcome in the Future
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AcA | acetylsalicylic acid |
| API | active pharmaceutical ingredient |
| ATP | adenosine 5′-triphosphate |
| BenzA | benzoic acid |
| B | betaine |
| CapA | capric acid |
| CAGE | deep eutectic solvent composed of choline bicarbonate/geranic acid |
| CGLY | deep eutectic solvent composed of choline bicarbonate/glycolic acid |
| ChCl | choline chloride |
| ChBT | choline bitartate |
| CitA | citric acid |
| COX-1/2 | cyclooxygenase 1/2 |
| DESD | derived deep eutectic solvent |
| DES | deep eutectic solvent |
| DLS | dynamic light scattering |
| DMSO | dimethylsulfoxide |
| DNA | deoxyribonucleic acid |
| EACl | ethylammonium chloride |
| EC50 | half maximal effective concentration |
| EPS | extracellular polymeric substances |
| F | fructose |
| FA | mixture of nonanoic and heptanoic acid |
| FT-IR | Fourier transform infrared spectroscopy |
| G | glucose |
| GluA | glutaric acid |
| Gly | glycerol |
| GlycA | glycolic acid |
| HaCaT | immortalized human keratinocytes |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| HFA | bacteria-derived hydroxy fatty acids |
| HSV | herpes simplex virus |
| I | ibuprofen |
| IL | ionic liquid |
| LacA | lactic acid |
| LauA | lauric acid |
| LevuA | levulinic acid |
| MyrA | myristic acid |
| M | menthol |
| MalA | maleic acid |
| MaloA | malonic acid |
| MBC | minimum bactericidal concentration |
| MIC | minimum inhibitory concentration |
| MP | melting point |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MRSE | methicillin-resistant Staphylococcus epidermidis |
| NADES | natural deep eutectic solvent |
| NMR | nucleic magnetic resonance spectroscopy |
| OxA | oxalic acid |
| OA-DES | organic acid-based deep eutectic solvent |
| P | proline |
| PhaA | phenylacetic acid |
| PS | parent substance |
| QS | quorum sensing |
| SteA | stearic acid |
| T | thymol |
| TE | eutectic temperature |
| THEDES | therapeutic deep eutectic solvent |
| TPAB | tetrapropylammonium bromide |
| U | urea |
References
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 9, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Vian, M.; Breil, C.; Vernes, L.; Chaabani, E.; Chemat, F. Green Solvents for Sample Preparation in Analytical Chemistry. Curr. Opin. Green Sustain. Chem. 2017, 5, 44–48. [Google Scholar] [CrossRef]
- Ünlü, A.E.; Arıkaya, A.; Takaç, S. Use of Deep Eutectic Solvents as Catalyst: A Mini-Review. Green Process. Synth. 2019, 8, 355–372. [Google Scholar] [CrossRef]
- Smith, E.L. Deep Eutectic Solvents (DESs) and the Metal Finishing Industry: Where Are They Now? Trans. Inst. Met. Finish. 2013, 91, 241–248. [Google Scholar] [CrossRef]
- Liao, H.-G.; Jiang, Y.-X.; Zhou, Z.-Y.; Chen, S.-P.; Sun, S.-G. Shape-Controlled Synthesis of Gold Nanoparticles in Deep Eutectic Solvents for Studies of Structure–Functionality Relationships in Electrocatalysis. Angew. Chem. Int. Ed. 2008, 47, 9100–9103. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Gomes, C.; Costa, R.; Martins, A.; Pereira, C.M.; Silva, F. Differential Capacity of a Deep Eutectic Solvent Based on Choline Chloride and Glycerol on Solid Electrodes. Electrochim. Acta 2009, 54, 2630–2634. [Google Scholar] [CrossRef]
- Costa, R.; Figueiredo, M.; Pereira, C.M.; Silva, F. Electrochemical Double Layer at the Interfaces of Hg/Choline Chloride Based Solvents. Electrochim. Acta 2010, 55, 8916–8920. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction Techniques with Deep Eutectic Solvents. Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Ngoh, G.C.; Wong, W.F.; Looi, C.Y. Emerging Frontiers of Deep Eutectic Solvents in Drug Discovery and Drug Delivery Systems. J. Control. Release 2019, 316, 168–195. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, X.; Wei, C.; Wang, H.; Gao, H. Deep Eutectic Solvents as Active Pharmaceutical Ingredient Delivery Systems in the Treatment of Metabolic Related Diseases. Front. Pharmacol. 2021, 12, 794939. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shi, C.; Mao, F.; Xu, Y.; Liu, J.; Wei, B.; Zhu, J.; Xiang, M.; Li, J. Discovery of New Imidazole Derivatives Containing the 2,4-Dienone Motif with Broad-Spectrum Antifungal and Antibacterial Activity. Molecules 2014, 19, 15653–15672. [Google Scholar] [CrossRef]
- Handa, M.; Almalki, W.H.; Shukla, R.; Afzal, O.; Altamimi, A.S.A.; Beg, S.; Rahman, M. Active Pharmaceutical Ingredients (APIs) in Ionic Liquids: An Effective Approach for API Physiochemical Parameter Optimization. Drug Discov. Today 2022, 27, 2415–2424. [Google Scholar] [CrossRef]
- Musiał, M.; Zorębski, E.; Malarz, K.; Kuczak, M.; Mrozek-Wilczkiewicz, A.; Jacquemin, J.; Dzida, M. Cytotoxicity of Ionic Liquids on Normal Human Dermal Fibroblasts in the Context of Their Present and Future Applications. ACS Sustain. Chem. Eng. 2021, 9, 7649–7657. [Google Scholar] [CrossRef]
- Shamsuri, A.A. Ionic Liquids: Preparations and Limitations. Makara J. Sci. 2011, 14, 101–106. [Google Scholar] [CrossRef]
- Hu, L.-X.; Xiong, Q.; Shi, W.-J.; Huang, G.-Y.; Liu, Y.-S.; Ying, G.-G. New Insight into the Negative Impact of Imidazolium-Based Ionic Liquid [C10mim]Cl on Hela Cells: From Membrane Damage to Biochemical Alterations. Ecotoxicol. Environ. Saf. 2021, 208, 111629. [Google Scholar] [CrossRef]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep Eutectic Solvents for the Production and Application of New Materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents–Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Yadav, A.; Trivedi, S.; Rai, R.; Pandey, S. Densities and Dynamic Viscosities of (Choline Chloride+glycerol) Deep Eutectic Solvent and Its Aqueous Mixtures in the Temperature Range (283.15–363.15)K. Fluid Phase Equilib. 2014, 367, 135–142. [Google Scholar] [CrossRef]
- van den Bruinhorst, A.; Costa Gomes, M. Is There Depth to Eutectic Solvents? Curr. Opin. Green Sustain. Chem. 2022, 37, 100659. [Google Scholar] [CrossRef]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, Hydrogen Bonding and Rheology in Choline Chloride Deep Eutectic Solvents as a Function of the Hydrogen Bond Donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef]
- Araujo, C.F.; Coutinho, J.A.P.; Nolasco, M.M.; Parker, S.F.; Ribeiro-Claro, P.J.A.; Rudić, S.; Soares, B.I.G.; Vaz, P.D. Inelastic Neutron Scattering Study of Reline: Shedding Light on the Hydrogen Bonding Network of Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2017, 19, 17998–18009. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, X.; González-Aguilera, L.; Ferrer, M.L.; Del Monte, F.; Gutiérrez, M.C. Should Deep Eutectic Solvents Be Treated as a Mixture of Two Components or as a Pseudo-Component? J. Chem. Phys. 2021, 154, 184501. [Google Scholar] [CrossRef]
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chem. Eur. J. 2007, 30, 6495–6501. [Google Scholar] [CrossRef]
- Stott, P. Transdermal Delivery from Eutectic Systems: Enhanced Permeation of a Model Drug, Ibuprofen. J. Control. Release 1998, 50, 297–308. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A Comparison between Pure Active Pharmaceutical Ingredients and Therapeutic Deep Eutectic Solvents: Solubility and Permeability Studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution Enhancement of Active Pharmaceutical Ingredients by Therapeutic Deep Eutectic Systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Banerjee, A.; Apte, S.; Kern, T.L.; Jones, M.R.; Sesto, R.E.D.; Koppisch, A.T.; Fox, D.T.; Mitragotri, S. Choline and Geranate Deep Eutectic Solvent as a Broad-Spectrum Antiseptic Agent for Preventive and Therapeutic Applications. Adv. Healthc. Mater. 2016, 5, 1282–1289. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Iwao, Y.; Zakrewsky, M.; Mitragotri, S. Transdermal Protein Delivery Using Choline and Geranate (CAGE) Deep Eutectic Solvent. Adv. Healthc. Mater. 2017, 6, 1601411. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Ibsen, K.N.; Mitragotri, S. Transdermal Insulin Delivery Using Choline-Based Ionic Liquids (CAGE). J. Control. Release 2018, 286, 137–144. [Google Scholar] [CrossRef]
- Wikene, K.O.; Bruzell, E.; Tønnesen, H.H. Characterization and Antimicrobial Phototoxicity of Curcumin Dissolved in Natural Deep Eutectic Solvents. Eur. J. Pharm. Sci. 2015, 80, 26–32. [Google Scholar] [CrossRef]
- Li, Z.; Lee, P.I. Investigation on Drug Solubility Enhancement Using Deep Eutectic Solvents and Their Derivatives. Int. J. Pharm. 2016, 505, 283–288. [Google Scholar] [CrossRef]
- Maag, H. Prodrugs of Carboxylic Acids. In Prodrugs; Stella, V.J., Borchardt, R.T., Hageman, M.J., Oliyai, R., Maag, H., Tilley, J.W., Eds.; Biotechnology: Pharmaceutical Aspects; Springer: New York, NY, USA, 2007; Volume V, pp. 703–729. ISBN 978-0-387-49782-2. [Google Scholar]
- Menniti, F.S.; Knoth, J.; Diliberto, E.J. Role of Ascorbic Acid in Dopamine Beta-Hydroxylation. The Endogenous Enzyme Cofactor and Putative Electron Donor for Cofactor Regeneration. J. Biol. Chem. 1986, 261, 16901–16908. [Google Scholar] [CrossRef]
- Leiros, H.-K.S.; Kozielski-Stuhrmann, S.; Kapp, U.; Terradot, L.; Leonard, G.A.; McSweeney, S.M. Structural Basis of 5-Nitroimidazole Antibiotic Resistance. J. Biol. Chem. 2004, 279, 55840–55849. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Theken, K.N.; Gong, L.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Ibuprofen Pathways. Pharmacogenet. Genomics 2015, 25, 96–106. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial Activity and Mode of Action of Ferulic and Gallic Acids Against Pathogenic Bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Aldulaimi, O. General Overview of Phenolics from Plant to Laboratory, Good Antibacterials or Not. Pharmacogn. Rev. 2017, 11, 123. [Google Scholar] [CrossRef]
- Rico-Munoz, E.; Bargiota, E.E.; Davidson, P.M. Effect of Selected Phenolic Compounds on the Membrane-Bound Adenosine Triphosphatase of Staphylococcus aureus. Food Microbiol. 1987, 4, 239–249. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.; Idzik, D.; Wąsik, T. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Rutherford, S.T.; Bassler, B.L. Bacterial Quorum Sensing: Its Role in Virulence and Possibilities for Its Control. Cold Spring Harb. Perspect. Med. 2012, 2, a012427. [Google Scholar] [CrossRef]
- Asfour, H. Anti-Quorum Sensing Natural Compounds. J. Microsc. Ultrastruct. 2018, 6, 1. [Google Scholar] [CrossRef]
- Amrutha, B.; Sundar, K.; Shetty, P.H. Effect of Organic Acids on Biofilm Formation and Quorum Signaling of Pathogens from Fresh Fruits and Vegetables. Microb. Pathog. 2017, 111, 156–162. [Google Scholar] [CrossRef]
- Silva, J.M.; Silva, E.; Reis, R.L.; Duarte, A.R.C. A Closer Look in the Antimicrobial Properties of Deep Eutectic Solvents Based on Fatty Acids. Sustain. Chem. Pharm. 2019, 14, 100192. [Google Scholar] [CrossRef]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef]
- Radošević, K.; Čanak, I.; Panić, M.; Markov, K.; Bubalo, M.C.; Frece, J.; Srček, V.G.; Redovniković, I.R. Antimicrobial, Cytotoxic and Antioxidative Evaluation of Natural Deep Eutectic Solvents. Environ. Sci. Pollut. Res. 2018, 25, 14188–14196. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Khalid, R.M.; Hawash, D.; Al-Kaissi, E.; Al-Adham, I.S.I.; Al-Muhtaseb, N.; Jaber, N.; Al-Remawi, M.; Collier, P.J. Antimicrobial Potential of Natural Deep Eutectic Solvents. Lett. Appl. Microbiol. 2022, 75, 607–615. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, Y.; Ding, Z.; Xia, H.; Guo, S. Physicochemical Properties and Antibacterial Activity of Hydrophobic Deep Eutectic Solvent-in-Water Nanoemulsion. J. Mol. Liq. 2021, 338, 116950. [Google Scholar] [CrossRef]
- Kitahara, T.; Koyama, N.; Matsuda, J.; Aoyama, Y.; Hirakata, Y.; Kamihira, S.; Kohno, S.; Nakashima, M.; Sasaki, H. Antimicrobial Activity of Saturated Fatty Acids and Fatty Amines against Methicillin-Resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004, 27, 1321–1326. [Google Scholar] [CrossRef]
- Desbois, A.P.; Smith, V.J. Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Nava-Ocampo, M.F.; Fuhaid, L.A.; Verpoorte, R.; Choi, Y.H.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S.; Witkamp, G.J.; Farinha, A.S.F.; Bucs, S.S. Natural Deep Eutectic Solvents as Biofilm Structural Breakers. Water Res. 2021, 201, 117323. [Google Scholar] [CrossRef]
- Park, E.-S.; Moon, W.-S.; Song, M.-J.; Kim, M.-N.; Chung, K.-H.; Yoon, J.-S. Antimicrobial Activity of Phenol and Benzoic Acid Derivatives. Int. Biodeterior. Biodegrad. 2001, 47, 209–214. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Zhou, H.-T.; Hu, Y.-H.; Tang, J.-Y.; Su, M.-X.; Guo, Y.-J.; Chen, Q.-X.; Liu, B. Antityrosinase and Antimicrobial Activities of 2-Phenylethanol, 2-Phenylacetaldehyde and 2-Phenylacetic Acid. Food Chem. 2011, 124, 298–302. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, X.; Cheng, J.-H.; Zeng, G.; Sun, D.-W. Synthesis and Antimicrobial Activities of Novel Sorbic and Benzoic Acid Amide Derivatives. Food Chem. 2018, 268, 220–232. [Google Scholar] [CrossRef]
- Carrascal, J.J.; Pinal, R.; Carvajal, T.; Pérez, L.D.; Baena, Y. Benzoic Acid Complexes with Eudragit E100®: New Alternative Antimicrobial Preservatives. Int. J. Pharm. 2021, 607, 120991. [Google Scholar] [CrossRef]
- Cook, S.D. An Historical Review of Phenylacetic Acid. Plant Cell Physiol. 2019, 60, 243–254. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, J.-Y.; Kuk, J.-H.; Moon, J.-H.; Cho, J.-I.; Kim, Y.-C.; Park, K.-H. Identification and Antimicrobial Activity of Phenylacetic Acid Produced by Bacillus Licheniformis Isolated from Fermented Soybean, Chungkook-Jang. Curr. Microbiol. 2004, 48, 312–317. [Google Scholar] [CrossRef]
- Park, H.J.; Prausnitz, M.R. Lidocaine-Ibuprofen Ionic Liquid for Dermal Anesthesia. AIChE J. 2015, 61, 2732–2738. [Google Scholar] [CrossRef]
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the Anticancer Potential of Limomene Based Therapeutic Deep Eutectic Solvents. Sci. Rep. 2019, 9, 14926. [Google Scholar] [CrossRef] [PubMed]
- Ahmed Arafa, W.A. Deep Eutectic Solvent for an Expeditious Sono-Synthesis of Novel Series of Bis-Quinazolin-4-One Derivatives as Potential Anti-Cancer Agents. R. Soc. Open Sci. 2019, 6, 182046. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Cao, J.; Wang, N.; Su, E. Significantly Improving the Solubility of Non-Steroidal Anti-Inflammatory Drugs in Deep Eutectic Solvents for Potential Non-Aqueous Liquid Administration. MedChemComm 2016, 7, 955–959. [Google Scholar] [CrossRef]
- Hwang, B.K.; Lim, S.W.; Kim, B.S.; Lee, J.Y.; Moon, S.S. Isolation and In Vivo and In Vitro Antifungal Activity of Phenylacetic Acid and Sodium Phenylacetate from Streptomyces humidus. Appl. Environ. Microbiol. 2001, 67, 3739–3745. [Google Scholar] [CrossRef]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure-Function Relationships of the Antibacterial Activity of Phenolic Acids and Their Metabolism by Lactic Acid Bacteria: Antibacterial Phenolic Acids. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Hubbard, R.E.; Kamran Haider, M. Hydrogen Bonds in Proteins: Role and Strength. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-01590-2. [Google Scholar]
- Creighton, T.E. Disulphide Bonds and Protein Stability. BioEssays 1988, 8, 57–63. [Google Scholar] [CrossRef]
- Esquembre, R.; Sanz, J.M.; Wall, J.G.; del Monte, F.; Mateo, C.R.; Ferrer, M.L. Thermal Unfolding and Refolding of Lysozyme in Deep Eutectic Solvents and Their Aqueous Dilutions. Phys. Chem. Chem. Phys. 2013, 15, 11248. [Google Scholar] [CrossRef] [PubMed]
- Su, E.; Klibanov, A.M. Low-Transition-Temperature Mixtures (LTTMs) for Dissolving Proteins and for Drug Formulation. Appl. Biochem. Biotechnol. 2015, 177, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fernandez, A.; Edler, K.J.; Arnold, T.; Alba Venero, D.; Jackson, A.J. Protein Conformation in Pure and Hydrated Deep Eutectic Solvents. Phys. Chem. Chem. Phys. 2017, 19, 8667–8670. [Google Scholar] [CrossRef] [PubMed]
- Angsantikul, P.; Peng, K.; Curreri, A.M.; Chua, Y.; Chen, K.Z.; Ehondor, J.; Mitragotri, S. Ionic Liquids and Deep Eutectic Solvents for Enhanced Delivery of Antibodies in the Gastrointestinal Tract. Adv. Funct. Mater. 2021, 31, 2002912. [Google Scholar] [CrossRef]
- Cruz, E.; Kayser, V. Monoclonal Antibody Therapy of Solid Tumors: Clinical Limitations and Novel Strategies to Enhance Treatment Efficacy. BTT 2019, 13, 33–51. [Google Scholar] [CrossRef]
- Marhelava, K.; Pilch, Z.; Bajor, M.; Graczyk-Jarzynka, A.; Zagozdzon, R. Targeting Negative and Positive Immune Checkpoints with Monoclonal Antibodies in Therapy of Cancer. Cancers 2019, 11, 1756. [Google Scholar] [CrossRef]
- Adams, G.P.; Weiner, L.M. Monoclonal Antibody Therapy of Cancer. Nat. Biotechnol. 2005, 23, 1147–1157. [Google Scholar] [CrossRef]
- Senolt, L. Emerging Therapies in Rheumatoid Arthritis: Focus on Monoclonal Antibodies. F1000Res 2019, 8, 1549. [Google Scholar] [CrossRef]
- Isaacs, J.D.; Hale, G.; Cobbold, S.P.; Waldmann, H.; Watts, R.A.; Hazleman, B.L.; Keogan, M.T. Humanised Monoclonal Antibody Therapy for Rheumatoid Arthritis. Lancet 1992, 340, 748–752. [Google Scholar] [CrossRef]
- Haraźna, K.; Walas, K.; Urbańska, P.; Witko, T.; Snoch, W.; Siemek, A.; Jachimska, B.; Krzan, M.; Napruszewska, B.D.; Witko, M.; et al. Polyhydroxyalkanoate-Derived Hydrogen-Bond Donors for the Synthesis of New Deep Eutectic Solvents. Green Chem. 2019, 21, 3116–3126. [Google Scholar] [CrossRef]

| Gram-Negative | Gram-Positive | Yeast | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. typhimurium | P. aeruginosa | P. mirabilis | E. coli | B. subtilis | S. aureus | MRSA | MRSE | P. acnes | C. albicans | ||
| CapA:LauA | N/I | − | N/I | − | N/I | + (625) | + (625) | + (625) | N/I | + (625) | [47] |
| CapA:MyrA | N/I | − | N/I | − | N/I | + (625) | + (625) | + (625) | N/I | + (625) | [47] |
| CapA:SteA | N/I | − | N/I | − | N/I | + (1250) | + (1250) | + (1250) | N/I | + (1250) | [47] |
| M:SteA | N/I | N/I | N/I | N/I | N/I | + (1018) | + (2036) | + (2036) | N/I | N/I | [48] |
| CAGE | N/I | + | + | + | N/I | N/I | + | + | + | + | [31] |
| B:MalA:P | + | + | + | + | N/I | + | N/I | N/I | N/I | N/I | [49] |
| B:MalA:G | + | + | + | + | N/I | + | N/I | N/I | N/I | N/I | [49] |
| M:BenzA | N/I | N/I | N/I | + (4000) | + (1000) | + (2000) | N/I | N/I | N/I | N/I | [30] |
| M:PhaA | N/I | N/I | N/I | + (4000) | + (2000) | + (2000) | N/I | N/I | N/I | N/I | [30] |
| M:AcA | N/I | N/I | N/I | + (4000) | + (2000) | + (2000) | N/I | N/I | N/I | N/I | [30] |
| ChCl:OxA | + | + | + | + | N/I | + | N/I | N/I | N/I | + | [30] |
| Reline | + | + | + | + | N/I | + | N/I | N/I | N/I | N/I | [30] |
| CitA:P | + | + | + | + | N/I | + | N/I | N/I | N/I | N/I | [30] |
| CitA:Gly:G | + | + | + | + | N/I | + | N/I | N/I | N/I | N/I | [30] |
| CitA:F:Gly | + | + | + | + | N/I | + | N/I | N/I | N/I | N/I | [30] |
| CapA:M:Solutol | N/I | − | N/I | + (25) | + (0.1) | + (0.1) | N/I | N/I | N/I | + | [50] |
| CapA:M | N/I | N/I | N/I | + (1120) | N/I | + (2240) | N/I | N/I | N/I | N/I | [51] |
| CapA:T | N/I | N/I | N/I | + (1140) | N/I | + (1140) | N/I | N/I | N/I | N/I | [51] |
| Aspirin | f | Acetaminophen | f | Ketoprofen | f | Naproxen | f | Ibuprofen | f | |
|---|---|---|---|---|---|---|---|---|---|---|
| WATER | 7.03 ± 0.03 | - | 19.95 ± 0.12 | - | 0.34 ± 0.00 | - | 0.06 ± 0.00 | - | 0.07 ± 0.00 | - |
| ChCl:GluA | 76.28 ± 0.61 | 10.85 | 238.10 ± 3.73 | 11.93 | 60.32 ± 0.42 | 177.41 | 21.04 ± 0.33 | 350.67 | 25.38 ± 0.20 | 362.57 |
| ChCl:GlycA | 67.43 ± 1.42 | 9.59 | 224.80 ± 3.02 | 11.27 | 22.09 ± 0.19 | 64.97 | 8.37 ± 0.09 | 139.5 | 9.49 ± 0.05 | 135.57 |
| ChCl:MaloA | 126.30 ± 2.06 | 17.96 | 266.3 ± 2.44 | 13.35 | 60.99 ± 0.31 | 179.38 | 21.98 ± 0.21 | 366.33 | 25.87 ± 0.13 | 369.57 |
| ChCl:OxA | 86.32 ± 1.16 | 12.28 | 186.70 ± 1.49 | 9.36 | 8.53 ± 0.21 | 25.09 | 13.90 ± 0.13 | 231.67 | 31.51 ± 0.20 | 450.14 |
| ChCl:LevuA | 135.00 ± 0.19 | 19.2 | 279.00 ± 3.20 | 13.98 | 192.90 ± 3.62 | 567.35 | 31.67 ± 0.40 | 527.83 | 78.69 ± 0.93 | 1124.14 |
| ChCl:LacA | 91.30 ± 1.83 | 12.99 | 254.00 ± 1.84 | 12.73 | 38.88 ± 0.53 | 114.35 | 12.95 ± 0.07 | 215.83 | 17.95 ± 0.25 | 256.43 |
| EACl:LacA | 44.57 ± 0.71 | 6.34 | 141.20 ± 0.73 | 7.08 | 32.24 ± 0.18 | 94.82 | 3.58 ± 0.04 | 59.67 | 20.67 ± 0.47 | 295.29 |
| TPAB:LevuA | 163.70 ± 2.30 | 23.29 | 316.50 ± 1.94 | 15.86 | 299.80 ± 3.33 | 881.76 | 129.10 ± 0.83 | 2151.67 | 194.80 ± 1.57 | 2782.86 |
| B:LevuA | 149.80 ± 0.96 | 21.31 | 212.10 ± 1.92 | 10.63 | 329.10 ± 4.42 | 967.94 | 76.64 ± 0.72 | 1277.33 | 280.20 ± 2.68 | 4002.86 |
| ChBT:LevuA | 37.06 ± 0.51 | 5.27 | 89.07 ± 1.04 | 4.46 | 51.02 ± 0.64 | 150.06 | 9.45 ± 0.11 | 157.5 | 35.05 ± 0.42 | 500.71 |
| PS/OA-DES | Dissolution Efficiency (%) | Permeability (105 cm s−1) | Diffusion Coefficient (106 cm2 s−1) | Time * (h) |
|---|---|---|---|---|
| AcA | 11 | N/I | N/I | N/I |
| M:AcA (3:1) | 72 | N/I | N/I | N/I |
| BenzA | 70 | 0.9 ± 0.01 | 0.73 ± 0.09 | 3.5 |
| M:BenzA (3:1) | 87 | 6.8 ± 0.63 | 6.43 ± 1.42 | 0.5 |
| PhaA | 37 | 16 ± 2.3 | 3.14 ± 0.18 | 2 |
| M:PhaA (3:1) | 81 | 18 ± 0.38 | 4.66 ± 0.13 | 3 |
| M:PhaA (2:1) | 78 | 13 ± 0.59 | 5.73 ± 0.43 | 2 |
| I | N/I | 4.6 ± 0.14 | 2.39 ± 0.43 | >8 |
| M:I (3:1) | N/I | 14 ± 1.53 | 4.32 ± 0.34 | 3 |
| OA-DES | Protein | Reference |
|---|---|---|
| CAGE | BVA | [32] |
| CAGE | OVA | [32] |
| CGLY | TNFα | [32] |
| CAGE | INS | [32,33] |
| CAGE | GLP-1 | [76] |
| OA-DES/PS | Lignin Solubility (mg/mL) |
|---|---|
| HFA:TBMACl (1:1) | 0.6 |
| HFA:TBMACl (2:1) | 0.9 |
| HFA:EMlmCl (1:1) | 0.8 |
| HFA:EMlmCl (2:1) | 26.9 |
| HFA:ChCl (1:1) | - |
| HFA:ChCl (2:1) | 25.6 |
| FA:TBMACl (1:1) | 7.2 |
| FA:TBMACl (2:1) | 15.6 |
| FA:EMlmCl (1:1) | 29.8 |
| FA:EMlmCl (2:1) | 30.7 |
| FA:ChCl (1:1) | - |
| FA:ChCl (2:1) | - |
| HFA | 0.3 |
| FA | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swebocki, T.; Barras, A.; Abderrahmani, A.; Haddadi, K.; Boukherroub, R. Deep Eutectic Solvents Comprising Organic Acids and Their Application in (Bio)Medicine. Int. J. Mol. Sci. 2023, 24, 8492. https://doi.org/10.3390/ijms24108492
Swebocki T, Barras A, Abderrahmani A, Haddadi K, Boukherroub R. Deep Eutectic Solvents Comprising Organic Acids and Their Application in (Bio)Medicine. International Journal of Molecular Sciences. 2023; 24(10):8492. https://doi.org/10.3390/ijms24108492
Chicago/Turabian StyleSwebocki, Tomasz, Alexandre Barras, Amar Abderrahmani, Kamel Haddadi, and Rabah Boukherroub. 2023. "Deep Eutectic Solvents Comprising Organic Acids and Their Application in (Bio)Medicine" International Journal of Molecular Sciences 24, no. 10: 8492. https://doi.org/10.3390/ijms24108492
APA StyleSwebocki, T., Barras, A., Abderrahmani, A., Haddadi, K., & Boukherroub, R. (2023). Deep Eutectic Solvents Comprising Organic Acids and Their Application in (Bio)Medicine. International Journal of Molecular Sciences, 24(10), 8492. https://doi.org/10.3390/ijms24108492