Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers
Abstract
1. Introduction
2. Evaluation of Oxidative Stress
2.1. Evaluation of Total Oxidative Status
2.2. F2-Isoprostanes (F2-IsoPs)
3. Cardiokines
3.1. GDF-8 (Myostatin)
3.2. GDF-15 (Macrophage-Inhibitory Cytokine 1)
3.3. CTRP (C1q/TNF-Related Protein) Family
3.4. Interleukine Family
3.5. Tumor Necrosis Factor (TNF) Superfamily
3.6. Transforming Growth Factor β (TGF- β) Superfamily
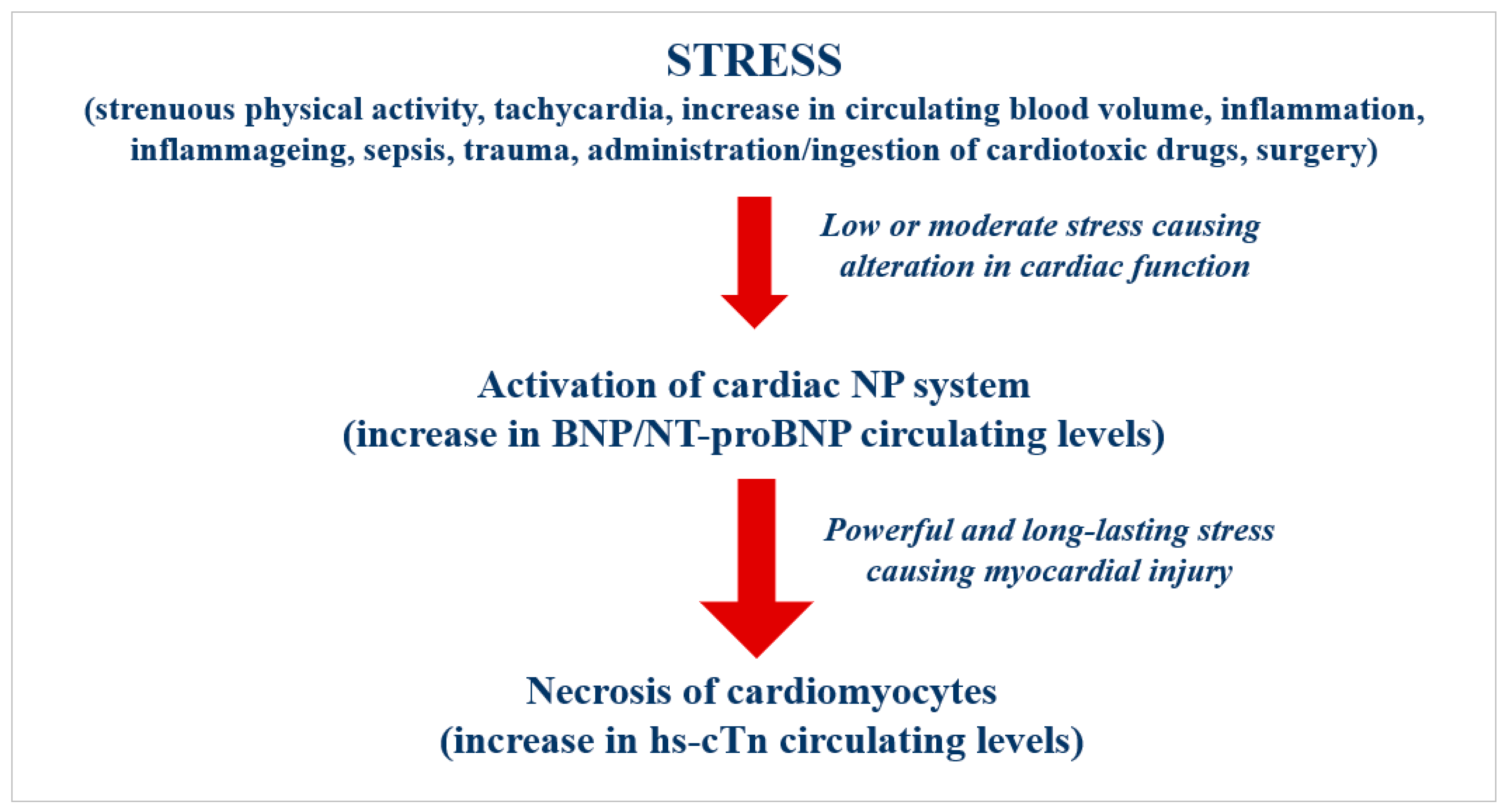
4. Cardiac-Specific Biomarkers
4.1. Cardiac Natriuretic Peptides
4.2. Cardiac Troponin I and T
4.3. Pathophysiological and Clinical Relevance of the Cardiac-Specific Biomarkers
4.4. The Potential Role of Cardiovascular Risk Screening in the General Population Using Cardio-Specific Biomarkers
5. Take-Home Messages
Funding
Conflicts of Interest
References
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflammaging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Calder, P.C.; Bosco, N.; Bourdet-Sicard, R.; Capuron, L.; Delzenne, N.; Doré, J.; Franceschi, C.; Lehtinen, M.J.; Recker, T.; Salvioli, S.; et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res. Rev. 2017, 40, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Richardson, G.; Benli, F.M.; Park, C.; de Souza, J.V.; Bronowska, A.K.; Spyridopoulos, I. Inflammageing in the cardiovascular system: Mechanisms, emerging targets, and novel therapeutic strategies. Clin. Sci. 2020, 134, 2243–2262. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Pietri, P.; Stefanidis, C. Cardiovascular Aging and Longevity: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 189–204. [Google Scholar] [CrossRef]
- Yan, M.; Sun, S.; Xu, K.; Huang, X.; Dou, L.; Pang, J.; Tang, W.; Shen, T.; Li, J. Cardiac aging: From basic research to therapeutics. Oxid. Med. Cell. Longev. 2021, 2021, 9570325. [Google Scholar] [CrossRef]
- United Nations, Department of Economic and Social Affairs Population World Population Prospects 2019: Highlights. 2019. Available online: https://www.un.org/development/desa/publications/world-population-prospects-2019-highlights.html (accessed on 1 December 2022).
- West, G. Scale. The Universal Law of Life, Growth, and Death in Organisms, Cities and Companies; Chapter 4; West, G., Ed.; Penguin Books: New York, NY, USA, 2017; p. 193. [Google Scholar]
- Hamczyk, M.R.; Nevado, R.M.; Barettino, A.; Fuster, V.; Andrés, V. Biological Versus Chronological Aging. JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Jylhävä, J.; Pedersen, N.L.; Hägg, S. Biological age predictors. EBioMedicine 2017, 21, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Vittorini, S.; Clerico, A. Cardiovascular biomarkers: Increasing impact of laboratory medicine in cardiology practice. Clin. Chem. Lab. Med. 2008, 46, 748–763. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Mueller, C.; Apple, F.S. High-sensitivity cardiac troponin assays for cardiovascular risk stratification in the general population. Eur. Heart J. 2020, 41, 4050–4056. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Zaninotto, M.; Passino, C.; Aspromonte, N.; Piepoli, M.F.; Migliardi, M.; Perrone, M.; Fortunato, A.; Padoan, A.; Testa, A.; et al. Evidence on clinical relevance of cardiovascular risk evaluation in the general population using cardio-specific biomarkers. Clin. Chem. Lab. Med. 2021, 59, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bazopoulou, D.; Knoefler, D.; Zheng, Y.; Ulrich, K.; Oleson, B.J.; Xie, L.; Kim, M.; Kaufmann, A.; Lee, Y.T.; Dou, Y.; et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature 2019, 576, 301–305. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef]
- Harrington, L.A.; Harley, C.B. Effect of vitamin E on lifespan and reproduction in Caenorhabditis elegans. Mech. Ageing Dev. 1988, 43, 71–78. [Google Scholar] [CrossRef]
- Phillips, J.P.; Campbell, S.D.; Michaud, D.; Charbonneau, M.; Hilliker, A.J. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc. Natl. Acad. Sci. USA 1989, 86, 2761–2765. [Google Scholar] [CrossRef]
- Orr, W.C.; Sohal, R.S. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 1994, 263, 1128–1130. [Google Scholar] [CrossRef]
- Parkes, T.L.; Elia, A.J.; Dickinson, D.; Hilliker, A.J.; Phillips, J.P.; Boulianne, G.L. Extension of Drosophila life span by overexpression of human SOD1 in motorneurons. Nat. Genet. 1998, 19, 171–174. [Google Scholar] [CrossRef]
- Melov, S.; Ravenscroft, J.; Malik, S.; Gill, M.S.; Walker, D.W.; Clayton, P.E.; Wallace, D.C.; Malfroy, B.; Doctrow, S.R.; Lithgow, G.J. Extension of life-span with superoxide dismutase/catalase mimetics. Science 2000, 289, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Moskovitz, J.; Bar-Noy, S.; Williams, W.M.; Requena, J.; Berlett, B.S.; Stadtman, E.R. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc. Natl. Acad. Sci. USA 2001, 98, 12920–12925. [Google Scholar] [CrossRef]
- Bakaev, V.V.; Lyudmila, M.B. Effect of ascorbic acid on longevity in the nematoda Caenorhabditis elegans. Biogerontology 2002, 3 (Suppl. 1), 12–16. [Google Scholar]
- Ruan, H.; Tang, X.D.; Chen, M.L.; Joiner, M.L.; Sun, G.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Iverson, L.; Wu, C.F.; et al. High-quality life extension by the enzyme peptide methionine sulfoxide reductase. Proc. Natl. Acad. Sci. USA 2002, 99, 2748–2753. [Google Scholar] [CrossRef]
- Ishii, N.; Senoo-Matsuda, N.; Miyake, K.; Yasuda, K.; Ishii, T.; Hartman, P.S.; Furukawa, S. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech. Ageing Dev. 2004, 125, 41–46. [Google Scholar] [CrossRef]
- Huang, T.T.; Naeemuddin, M.; Elchuri, S.; Yamaguchi, M.; Kozy, H.M.; Carlson, E.; Epstein, C.J. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum. Mol. Genet. 2006, 15, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Sinclair, J.; Wilson, M.A.; Carey, J.R.; Liedo, P.; Oropeza, A.; Kalra, A.; de Cabo, R.; Ingram, D.K.; Longo, D.L.; et al. Comparative approaches to facilitate the discovery of prolongevity interventions: Effects of tocopherols on lifespan of three invertebrate species. Mech. Ageing Dev. 2007, 128, 222–226. [Google Scholar] [CrossRef]
- Kim, J.; Takahashi, M.; Shimizu, T.; Shirasawa, T.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mech. Ageing Dev. 2008, 129, 322–331. [Google Scholar] [CrossRef]
- Quick, K.L.; Ali, S.S.; Arch, R.; Xiong, C.; Wozniak, D.; Dugan, L.L. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol. Aging 2008, 29, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.F.; Santana, L.F.; Vermulst, M.; Tomazela, D.M.; Emond, M.J.; MacCoss, M.J.; Gollahon, K.; Martin, G.M.; Loeb, L.A.; Ladiges, W.C.; et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 2009, 119, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Shibamura, A.; Ikeda, T.; Nishikawa, Y. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: Effects of oral supplementation with antioxidants on the nematode lifespan. Mech. Ageing Dev. 2009, 130, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S.; Takagi, G.; Kawabe, J.; Yang, G.; Lee, M.C.; Hong, C.; Liu, J.; Vatner, D.E.; Sadoshima, J.; Vatner, S.F.; et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc. Natl. Acad. Sci. USA 2003, 100, 9986–9990. [Google Scholar] [CrossRef] [PubMed]
- Pérez, V.I.; Van Remmen, H.; Bokov, A.; Epstein, C.J.; Vijg, J.; Richardson, A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell 2009, 8, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Tagliamonte, M.R.; Rizzo, M.R.; Manzella, D.; Gambardella, A.; Varricchio, M. Oxidative stress and advancing age: Results in healthy centenarians. J. Am. Geriatr. Soc. 1998, 46, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Mecocci, P.; Polidori, M.C.; Troiano, L.; Cherubini, A.; Cecchetti, R.; Pini, G.; Straatman, M.; Monti, D.; Stahl, W.; Sies, H.; et al. Plasma antioxidants and longevity: A study on healthy centenarians. Free Radic. Biol. Med. 2000, 28, 1243–1248. [Google Scholar] [CrossRef]
- Southam, C.M.; Ehrlich, J. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology 1943, 33, 517–524. [Google Scholar]
- Calabrese, E.J.; Baldwin, L.A. Defining hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Cypser, J.R.; Tedesco, P.; Johnson, T.E. Hormesis and aging in Caenorhabditis elegans. Exp. Gerontol. 2006, 41, 935–939. [Google Scholar] [CrossRef]
- Rattan, S.I. Hormesis in aging. Ageing Res. Rev. 2008, 7, 63–78. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Ho, E.; Karimi Galougahi, K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [PubMed]
- Marocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Fontana, J.; Zima, M.; Vetvicka, V. Biological markers of oxidative stress in cardiovascular diseases: After so many studies, what do we know? Immunol. Investig. 2018, 47, 823–843. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxid. Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Peroxidation as a biomarker of oxidative stress: Oxidative stress in dabetes, atherosclerosis, and chronic inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef]
- Tejchman, K.; Kotfis, K.; Sienko, J. Biomarkers and mechanisms of oxidative stress—Last 20 years of research with an emphasis on kidney damage and renal transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef]
- Knasmüller, S.; Nersesyan, A.; Misík, M.; Gerner, C.; Mikulits, W.; Ehrlich, V.; Hoelzl, C.; Szakmary, A.; Wagner, K.H. Use of conventional and -omics based methods for health claims of dietary antioxidants: A critical overview. Br. J. Nutr. 2008, 99 (Suppl. 1), ES3–E52. [Google Scholar] [CrossRef]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef]
- Serafini, M.; Villano, D.; Spera, G.; Pellegrini, N. Redox molecules and cancer prevention: The importance of understanding the role of the antioxidant network. Nutr. Cancer 2006, 56, 232–240. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Tomei, F.; Sancini, A.; GMorabito Serafini, M. Effect of plant foods and beverages on plasma non-enzymatic antioxidant capacity in human subjects: A meta-analysis. Br. J. Nutr. 2013, 109, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Raguzzini, A. Salivary and urinary total antioxidant capacity as biomarkers of oxidative stress in humans. Patholog. Res. Int. 2016, 2016, 5480267. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Cavaliere, A.; Palmery, M. Plasma total antioxidant capacity and peroxidation biomarkers in psoriasis. J. Biomed. Sci. 2016, 23, 52. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Bektaşoğlu, B.; Bener, M. Cupric ion reducing antioxidant capacity assay for antioxidants in human serum and for hydroxyl radical scavengers. Methods Mol. Biol. 2010, 594, 215–239. [Google Scholar] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Beretta, G.; Aldini, G.; Facino, R.M.; Russell, R.M.; Krinsky, N.I.; Yeum, K.J. Total antioxidant performance: A validated fluorescence assay for the measurement of plasma oxidizability. Anal. Biochem. 2006, 354, 290–298. [Google Scholar] [CrossRef]
- Aldini, G.; Yeum, K.J.; Russell, R.M.; Krinsky, N.I. A method to measure the oxidizability of both the aqueous and lipid compartments of plasma. Free Radic Biol. Med. 2001, 31, 1043–1050. [Google Scholar] [CrossRef]
- Takashima, M.; Horie, M.; Shichiri, M.; Hagihara, Y.; Yoshida, Y.; Niki, E. Assessment of antioxidant capacity for scavenging free radicals in vitro: A rational basis and practical application. Free Radic. Biol. Med. 2012, 52, 1242–1252. [Google Scholar] [CrossRef]
- Amorati, A.; Valmigli, L. Advantages and limitations of common testing methods for antioxidants. Free Radic. Res. 2015, 49, 633–649. [Google Scholar] [CrossRef]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Biophys. Acta 2014, 1840, 931–934. [Google Scholar] [CrossRef]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic. Res. 2010, 44, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Veglia, F.; Cighetti, G.; De Franceschi, M.; Zingaro, L.; Boccotti, L.; Tremoli, E.; Cavalca, V. Age- and gender-related oxidative status determined in healthy subjects by means of OXY-SCORE, a potential new comprehensive index. Biomarkers 2006, 11, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C. An easy and reliable automated method to estimate oxidative stress in the clinical setting. Methods Mol. Biol. 2008, 477, 31–39. [Google Scholar] [PubMed]
- Vassalle, C.; Pratali, L.; Boni, C.; Mercuri, A.; Ndreu, R. An oxidative stress score as a combined measure of the pro-oxidant and anti-oxidant counterparts in patients with coronary artery disease. Clin. Biochem. 2008, 41, 1162–1167. [Google Scholar] [CrossRef]
- Vassalle, C.; Novembrino, C.; Maffei, S.; Sciarrino, R.; De Giuseppe, R.; Vigna, L.; de Liso, F.; Mercuri, A.; Bamonti, F. Determinants of oxidative stress related to gender: Relevance of age and smoking habit. Clin. Chem. Lab. Med. 2011, 49, 1509–1513. [Google Scholar] [CrossRef]
- Vassalle, C.; Sciarrino, R.; Bianchi, S.; Battaglia, D.; Mercuri, A.; Maffei, S. Sex-related differences in association of oxidative stress status with coronary artery disease. Fertil Steril 2012, 97, 414–419. [Google Scholar] [CrossRef]
- Veglia, F.; Cavalca, V.; Tremoli, E. OXY-SCORE: A global index to improve evaluation of oxidative stress by combining pro- and antioxidant markers. Methods Mol. Biol. 2010, 594, 197–213. [Google Scholar]
- Veglia, F.; Werba, J.P.; Tremoli, E.; Squellerio, I.; Sisillo, E.; Parolari, A.; Minardi, F.; Cavalca, V. Assessment of oxidative stress in coronary artery bypass surgery: Comparison between the global index OXY-SCORE and individual biomarkers. Biomarkers 2009, 14, 465–472. [Google Scholar] [CrossRef]
- Clerico, A.; Vittorini, S.; Passino Emdin, M. New and emerging biomarkers of heart failure. Crit. Rev. Clin. Lab. Sci. 2009, 46, 107–128. [Google Scholar] [CrossRef]
- Morrow, J.D.; Awad, J.A.; Boss, H.J.; Blair, I.A.; Roberts, L.J., 2nd. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Nat. Acad. Sci. USA 1992, 89, 10721–10725. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Barnes, P.; Roberts, L.J., 2nd. Insights into oxidative stress: The isoprostanes. Curr. Med. Chem. 2007, 14, 703–717. [Google Scholar] [CrossRef]
- Milne, G.L.; Yin, H.; Hardy, K.D.; Davies, S.S.; Roberts, L.J. Isoprostane generation and function. Chem. Rev. 2011, 111, 5973–5996. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Sanchez, S.C.; Musiek, E.S.; Morrow, J.D. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat. Protoc. 2007, 2, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.S.; Roberts, L.J. F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic. Biol. Med. 2011, 50, 559–566. [Google Scholar] [CrossRef]
- Milne, G.L.; Dai, Q.; Roberts, L.J. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Il’yasova, D.; Morrow, J.D.; Ivanova, A.; Wagenknecht, L.E. Epidemiological marker for oxidant status: Comparison of the ELISA and the gas chromatography/mass spectrometry assay for urine 2,3dinor-5,6-dihydro-15-F 2t-isoprostane. Ann. Epidemiol. 2004, 14, 793–797. [Google Scholar] [CrossRef]
- Tsikas, D.; Suchy, M.T. Assessment of urinary F 2 -isoprostanes in experimental and clinical studies: Mass spectrometry versus ELISA. Hypertension 2012, 60, e14. [Google Scholar] [CrossRef]
- Klawitter, J.; Haschke, M.; Shokati, T.; Klawitter, J.; Christians, U. Quantification of 15-F 2t-isoprostane in human plasma and urine: Results from enzyme-linked immunoassay and liquid chromatography/ tandem mass spectrometry cannot be compared. Rapid Commun. Mass Spectrom. 2011, 25, 463–468. [Google Scholar] [CrossRef]
- Soffler, C.; Campbell, V.L.; Hassel, D.M. Measurement of urinary F 2 -isoprostanes as markers of in vivo lipid peroxidation: A comparison of enzyme immunoassays with gas chromatography–mass spectrometry in domestic animal species. J. Vet. Diagn. Investig. 2010, 22, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Mas, E.; Croft, K.D.; Phillips, M.; Mori, T.A. Minimizing artifactual elevation of lipid peroxidation products (F2-isoprostanes) in plasma during collection and storage. Anal. Biochem. 2014, 449, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J. Systematic review on the association between F2-isoprostanes and cardiovascular disease. Ann. Clin. Biochem. 2013, 50, 108–114. [Google Scholar] [CrossRef]
- Sakamoto, H.; Corcoran, T.B.; Laffey, J.G.; Shorten, G.D. Isoprostanes–markers of ischaemia reperfusion injury. Eur. J. Anaesthesiol. 2002, 19, 550–559. [Google Scholar] [PubMed]
- Rossi, P.; Riutta, A.; Kuukasjärvi, P.; Vehmas, T.; Mucha, I.; Salenius, J.P. Revascularization decreases 8-isoprostaglandin F2alpha excretion in chronic lower limb ischemia. Prostaglandins Leukot Essent Fat Acids 2004, 71, 97–101. [Google Scholar] [CrossRef]
- Kelly, P.J.; Morrow, J.D.; Ning, M.; Koroshetz, W.; Lo, E.H.; Terry, E.; Milne, G.L.; Hubbard, J.; Lee, H.; Stevenson, E.; et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: The Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke 2008, 39, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; Lee, C.Y.; Chan, B.P.; Sharma, V.K.; Teoh, H.L.; Venketasubramanian, N.; Lim, E.C.; Chong, W.L.; Looi, W.F.; Huang, S.H.; et al. Oxidative damage in ischemic stroke revealed using multiple biomarkers. Stroke 2011, 42, 2326–2329. [Google Scholar] [CrossRef]
- Lindsay, T.F.; Luo, X.P.; Lehotay, D.C.; Rubin, B.B.; Anderson, M.; Walker, P.M.; Romaschin, A.D. Ruptured abdominal aortic aneurysm, a “two-hit” ischemia/reperfusion injury: Evidence from an analysis of oxidative products. J. Vasc. Surg. 1999, 30, 219–228. [Google Scholar] [CrossRef]
- Karlis, G.; Kotanidou, A.; Georgiopoulos, G.; Masi, S.; Magkas, N.; Xanthos, T. Usefulness of F2-isoprostanes in early prognostication after cardiac arrest: A topical review of the literature and meta-analysis of preclinical data. Biomarkers 2020, 25, 315–321. [Google Scholar] [CrossRef]
- Davies, S.S.; Traustadóttir, T.; Stock, A.A.; Ye, F.; Shyr, Y.; Harman, S.M.; Roberts, L.J., 2nd. Ischemia reperfusion unveils impaired capacity of older adults to restrain oxidative insult. Free Radic. Biol. Med. 2009, 47, 1014–1018. [Google Scholar] [CrossRef]
- Traustadóttir, T.; Davies, S.S.; Su, Y.; Choi, L.; Brown-Borg, H.M.; Roberts, L.J., II; Harman, S.M. Oxidative stress in older adults: Effects of physical fitness. Age 2012, 34, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Gracia, K.; Llanas-Cornejo, D.; Husi, H. CVD and oxidative stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef]
- Herder, C.; de Las Heras Gala, T.; Carstensen-Kirberg, M.; Huth, C.; Zierer, A.; Wahl, S.; Sudduth-Klinger, J.; Kuulasmaa, K.; Peretz, D.; Ligthart, S.; et al. Circulating levels of Interleukin 1-Receptor Antagonist and risk of cardiovascular disease: Meta-analysis of six population-based cohorts. Arterioscler. Thromb. Vasc. Bio. 2017, 37, 1222–1227. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Malik, R.; Björkbacka, H.; Pana, T.A.; Demissie, S.; Ayers, C.; Elhadad, M.A.; Fornage, M.; Beiser, A.S.; Benjamin, E.J.; et al. Circulating Monocyte Chemoattractant Protein-1 and risk of stroke: Meta-analysis of population-based studies involving 17 180 individuals. Circ. Res. 2019, 125, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rahimi, K.; Bautista, L.E.; Nazarzadeh, M.; Zargar, M.S.; Shab-Bidar, S. Inflammation markers and risk of developing hypertension: A meta-analysis of cohort studies. Heart 2019, 105, 686–692. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wei, J.; Du, W.; Ding, J.; Zhang, Y.; Zhang, N.; Mao, M.; Liu, P. Vitamin D for inflammation biomarkers in coronary artery disease: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e21407. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Orlenko, A.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Spires, T.E.; Yarde, M.; Wang, Z.; Seiffert, D.A.; et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2021, 75, 1281–1295. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Angelopoulos, A.; Papanikolaou, P.; Simantiris, S.; Oikonomou, E.K.; Vamvakaris, K.; Koumpoura, A.; Farmaki, A.; Trivella, M.; Vlachopoulos, C.; et al. Biomarkers of vascular inflammation for cardiovascular risk prognostication: A meta-analysis. JACC Cardiovasc. Imaging 2022, 15, 460–471. [Google Scholar] [CrossRef]
- Bergami, M.; Scarpone, M.; Bugiardini, R.; Cenko, E.; Manfrini, O. Sex beyond cardiovascular risk factors and clinical biomarkers of cardiovascular disease. Rev. Cardiovasc. Med. 2022, 23, 19. [Google Scholar] [CrossRef]
- Doroudgar, S.; Glembotski, C.C. The cardiomyokine story unfolds: Ischemic stress-induced protein secretion in the heart. Trends Mol. Med. 2011, 17, 207–214. [Google Scholar] [CrossRef]
- Dewey, C.M.; Spitler, K.M.; Ponce, J.M.; Hall, D.D.; Grueter, C.E. Cardiac-secreted factors as peripheral metabolic regulators and potential disease biomarkers. J. Am. Heart Assoc. 2016, 31, e003101. [Google Scholar] [CrossRef]
- Clerico, A.; Recchia, F.A.; Passino, C.; Emdin, M. Cardiac endocrine function is an essential component of the homeostatic regulation network: Physiological and clinical implications. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H17–H29. [Google Scholar] [CrossRef]
- Heineke, J.; Auger-Messier, M.; Xu, J.; Sargent, M.; York, A.; Welle, S.; Molkentin, J.D. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010, 121, 419–425. [Google Scholar] [CrossRef]
- Suzuki, T.; Palus, S.; Springer, J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018, 5, 1099–1107. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A.; Lichtenauer, M. Myokines and heart failure: Challenging role in adverse cardiac remodeling, myopathy, and clinical outcomes. Dis. Markers 2021, 2021, 6644631. [Google Scholar] [CrossRef]
- Oliveira, P.G.S.; Schwed, J.F.; Chiuso-Minicucci, F.; Duarte, S.R.S.; Nascimento, L.M.; Dorna, M.S.; Costa, N.A.; Okoshi, K.; Okoshi, M.P.; Azevedo, P.S.; et al. Association between serum myostatin levels, hospital mortality, and muscle mass and strength following ST-elevation myocardial infarction. Heart Lung Circ. 2022, 31, 365–371. [Google Scholar] [CrossRef]
- Wollert, K.C.; Kempf, T.; Wallentin, L. Growth Differentiation Factor 15 as a biomarker in cardiovascular disease. Clin. Chem. 2017, 63, 140–151. [Google Scholar] [CrossRef]
- Lyngbakken, M.N.; Myhre, P.L.; Røsjø, H.; Omland, T. Novel biomarkers of cardiovascular disease: Applications in clinical practice. Crit. Rev. Clin. Lab. Sci. 2019, 56, 33–60. [Google Scholar] [CrossRef]
- Zhu, L.; Li, C.; Liu, Q.; Xu, W.; Zhou, X. Molecular markers in cardiac hypertrophy. J. Cell Mol. Med. 2019, 23, 1671–1677. [Google Scholar] [CrossRef]
- Si, Y.; Fan, W.; Sun, L. A review of the relationship between CTRP family and coronary artery disease. Curr. Atheroscler. Rep. 2020, 22, 22. [Google Scholar] [CrossRef]
- Yang, C.; Xin, J.Y.; Liu, Z.L.; Fan, F.; Li, Y.M.; Jin, F.; Wang, Q.S.; Guo, F.Q.; Yu, N.W.; Le, W.D.; et al. Association between serum C1q Tumor Necrosis Factor-Related Protein 9 and the clinical characteristics and prognosis of ischemic stroke. Neurol. Ther. 2022, 11, 87–101. [Google Scholar] [CrossRef]
- Galea, J.; Armstrong, J.; Gadsdon, P.; Holden, H.; Francis, S.E.; Holt, C.M. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler. Thromb. Vasc. Biol 1996, 16, 1000–1006. [Google Scholar] [CrossRef]
- Sharma, H.S.; Das, D.K. Role of cytokines in myocardial ischemia and reperfusion. Mediat. Inflamm. 1997, 6, 175–183. [Google Scholar] [CrossRef]
- Pfeiler, S.; Winkels, H.; Kelm, M.; Gerdes, N. IL-1 family cytokines in cardiovascular disease. Cytokines 2019, 122, 154215. [Google Scholar] [CrossRef]
- Libby, P. Targeting inflammatory pathways in cardiovascular disease: The inflammasome, interleukin-1, interleukin-6 and beyond. Cells 2021, 10, 951. [Google Scholar] [CrossRef]
- Libby, P. Inflammation in atherosclerosis–No longer a theory. Clin. Chem. 2021, 67, 131–142. [Google Scholar] [CrossRef]
- Panahi, M.; Papanikolaou, A.; Torabi, A.; Zhang, J.G.; Khan, H.; Vazir, A.; Hasham, M.G.; Cleland, J.G.F.; Rosenthal, N.A.; Harding, S.E.; et al. Immunomodulatory interventions in myocardial infarction and heart failure: A systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovasc. Res. 2018, 114, 1445–1461. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; de Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; Kiernan, M.S.; et al. Role of biomarkers for the prevention, assessment, and management of heart failure: A scientific statement from the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Wu, N.; Xu, B.; Xiang, Y.; Wu, L.; Zhang, Y.; Ma, X.; Tong, S.; Shu, M.; Song, Z.; Li, Y.; et al. Association of inflammatory factors with occurrence and recurrence of atrial fibrillation: A meta-analysis. Int. J. Cardiol. 2013, 169, 62–72. [Google Scholar] [CrossRef]
- Schmitz, J.; Owyang, A.; Oldham, E.; Song, Y.; Murphy, E.; McClanahan, T.K.; Zurawski, G.; Moshrefi, M.; Qin, J.; Li, X.; et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 2005, 23, 479–490. [Google Scholar] [CrossRef]
- Demyanets, S.; Kaun, C.; Pentz, R.; Krychtiuk, K.A.; Rauscher, S.; Pfaffenberger, S.; Zuckermann, A.; Aliabadi, A.; Gröger, M.; Maurer, G.; et al. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells and in cells of the cardiac vasculature. J. Mol. Cell. Cardiol. 2013, 60, 16–26. [Google Scholar] [CrossRef]
- Vianello, E.; Dozio, E.; Tacchini, L.; Frati, L.; Corsi Romanelli, M.M. ST2/IL-33 signaling in cardiac fibrosis. Int. J. Biochem. Cell Biol. 2019, 116, 105619. [Google Scholar] [CrossRef]
- Sun, Y.; Pavey, H.; Wilkinson, I.; Fisk, M. Role of the IL-33/ST2 axis in cardiovascular disease: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0259026. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Schulz, R.; Heusch, G. TNFalpha in myocardial ischemia/ reperfusion, remodeling and heart failure. Heart Fail. Rev. 2011, 16, 49–69. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, X.; Li, G.; He, Q.; Dai, H.; Ai, C.; Shi, J. Tumor necrosis factor-alpha gene polymorphisms and susceptibility to ischemic heart disease: A systematic review and meta-analysis. Medicine 2017, 96, e6569. [Google Scholar] [CrossRef]
- Schultz Jel, J.; Witt, S.A.; Glascock, B.J.; Nieman, M.L.; Reiser, P.J.; Nix, S.L.; Kimball, T.R.; Doetschman, T. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J. Clin. Investig. 2002, 109, 787–796. [Google Scholar] [CrossRef]
- Morris, D.R.; Moxon, J.V.; Biros, E.; Krishna, S.M.; Golledge, J. Meta-analysis of the association between transforming growth factor-beta polymorphisms and complications of coronary heart disease. PLoS ONE 2012, 7, e37878. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The role of the TGF-β superfamily in myocardial infarction. Front. Cradiovasc. Med. 2019, 6, 140. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Tarighati Rasekhi, R.; Gill, D.; Alzubi, J.; Mainigi, S.K. How transforming growth factor contributes to atrial fibrillation? Life Sci. 2021, 266, 118823. [Google Scholar] [CrossRef]
- Emdin, M.; Clerico, A.; Clemenza, F.; Galvani, M.; Latini, R.; Masson, S.; Mulè, P.; Panteghini, M.; Valle, R.; Zaninotto, M.; et al. Recommendations for the clinical use of cardiac natriuretic peptides. Ital. Heart J. 2005, 6, 430–446. [Google Scholar]
- Clerico, A.; Passino, C.; Franzini, M.; Emdin, M. Natriuretic peptides as biomarkers of cardiac endocrine function in heart failure: New challenges and perspectives. Future Cardiol. 2016, 12, 573–584. [Google Scholar] [CrossRef]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological aspects of selected myokines in skeletal muscle: Focus on aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Elkasrawy, M.N.; Hamrick, M.W. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J. Musculoskelet. Neuronal Interact. 2010, 10, 56–63. [Google Scholar]
- Breitbart, A.; Auger-Messier, M.; Molkentin, J.D.; Heineke, J. Myostatin from the heart: Local and systemic actions in cardiac failure and muscle wasting. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1973–H1982. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Chen, P.; Liu, Z.; Luo, Y.; Chen, L.; Li, S.; Pan, Y.; Lei, X.; Wu, D.; Xu, D. Predictive value of serum myostatin for the severity and clinical outcome of heart failure. Eur. J. Int. Med. 2019, 64, 33–40. [Google Scholar] [CrossRef]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef]
- Moriwaki, K.; Matsumoto, H.; Tanishima, S.; Tanimura, C.; Osaki, M.; Nagashima, H. Association of serum bone- and muscle-derived factors with age, sex, body composition, and physical function in community-dwelling middle-aged and elderly adults: A cross-sectional study. BMC Musculoskelet. Disord. 2019, 20, 276. [Google Scholar] [CrossRef]
- Barrios-Silva, L.V.; Parnell, M.; Shinwari, Z.B.; Chaudhary, G.A.; Xenofontos, T.; van Bekhoven, A.; McArthur, S.; Elliott, B.T. Activin subfamily peptides predict chronological age in humans. Physiol. Rep. 2018, 6, e13823. [Google Scholar] [CrossRef]
- Hinck, A.P.; Mueller, T.D.; Springer, T.A. Structural biology and evolution of the TGF-β family. Cold Spring Harb. Perspect. Biol. 2016, 8, a022103. [Google Scholar] [CrossRef] [PubMed]
- Bergen, H.R., 3rd; Farr, J.N.; Vanderboom, P.M.; Atkinson, E.J.; White, T.A.; Singh, R.J.; Khosla, S.; LeBrasseur, N.K. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: Insights using a new mass spectrometry-based assay. Skelet. Muscle 2015, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Wischhusen, J.; Melero, I.; Fridman, W.H. Growth/Differentiation Factor-15 (GDF-15): From biomarker to novel targetable immune checkpoint. Front. Immunol. 2020, 11, 951. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Zeller, M.; Cottin, Y.; Vergely, C. GDF15 and cardiac cells: Current concepts and new insights. Int. J. Mol. Sci. 2021, 22, 8889. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, M.; de Poel, J.H.C.; de Jager, S.C.A. Growth differentiation factor 15 in adverse cardiac remodelling: From biomarker to causal player. ESC Heart Fail. 2020, 7, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Blase, P.; Adams, V.; Hildebrand, L.; Desch, S.; Schuler, G.; Thiele, H. Growth-differentiation factor 15 as predictor of mortality in acute reperfused ST-elevation myocardial infarction: Insights from cardiovascular magnetic resonance. Heart 2011, 97, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Zethelius, B.; Berglund, L.; Eggers, K.M.; Lind, L.; Lindahl, B.; Wollert, K.C.; Siegbahn, A. GDF-15 for prognostication of cardiovascular and cancer morbidity and mortality in men. PLoS ONE 2013, 8, 78797. [Google Scholar] [CrossRef] [PubMed]
- Gaggin, H.K.; Szymonifka, J.; Bhardwaj, A.; Belcher, A.; De Berardinis, B.; Motiwala, S.; Wang, T.J.; Januzzi, J.L., Jr. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JAAC Heart Fail. 2014, 2, 65–72. [Google Scholar] [CrossRef]
- Wallentin, L.; Hijazi, Z.; Andersson, U.; Alexander, J.H.; De Caterina, R.; Hanna, M.; Horowitz, J.D.; Hylek, E.M.; Lopes, R.D.; Asberg, S.; et al. Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: Insights from the Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Circulation 2014, 130, 1847–1858. [Google Scholar]
- Velders, M.A.; Wallentin, L.; Becker, R.C.; van Boven, A.J.; Himmelmann, A.; Husted, S.; Katus, H.A.; Lindholm, D.; Morais, J.; Siegbahn, A.; et al. Biomarkers for risk stratification of patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention: Insights from the Platelet Inhibition and Patient Outcomes trial. Am. Heart J. 2015, 169, 879–889.e7. [Google Scholar] [CrossRef]
- Resl, M.; Clodi, M.; Vila, G.; Luger, A.; Neuhold, S.; Wurm, R.; Adlbrecht, C.; Strunk, G.; Fritzer-Szekeres, M.; Prager, R.; et al. Targeted multiple biomarker approach in predicting cardiovascular events in patients with diabetes. Heart 2016, 102, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Demissei, B.G.; Cotter, G.; Prescott, M.F.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Pang, P.S.; Ponikowski, P.; Severin, T.M.; Wang, Y.; et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: Results from the RELAX-AHF trial. Eur. Heart Fail. 2017, 19, 1001–1010. [Google Scholar] [CrossRef]
- Bouabdallaoui, N.; Claggett, B.; Zile, M.R.; McMurray, J.J.V.; O’Meara, E.; Packer, M.; Prescott, M.F.; Swedberg, K.; Solomon, S.D.; Rouleau, J.L. Growth differentiation factor-15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: The PARADIGM-HF trial. Eur. J. Heart Fail. 2018, 20, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Frimodt-Møller, M.; von Scholten, B.J.; Reinhard, H.; Jacobsen, P.K.; Hansen, T.W.; Persson, F.I.; Parving, H.H.; Rossing, P. Growth differentiation factor-15 and fibroblast growth factor-23 are associated with mortality in type 2 diabetes—An observational follow-up study. PLoS ONE 2018, 13, e0196634. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.; Jin, X.; Gao, D.; Ma, R.; Dong, X.; Wei, J.; Wang, X. Association between growth differentiation factor 15 and left ventricular hypertrophy in hypertensive patients and healthy adults. Clin. Exp. Hypertens. 2018, 40, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Ruff, C.T.; Jarolim, P.; Giugliano, R.P.; Nordio, F.; Lanz, H.J.; Mercuri, M.F.; Antman, E.M.; Braunwald, E.; Morrow, D.A. Performance of the ABC scores for assessing the risk of stroke or systemic embolism and bleeding in patients with atrial fibrillation in ENGAGE AF-TIMI 48. Circulation 2019, 139, 760–771. [Google Scholar] [CrossRef]
- Hijazi, Z.; Verdecchia, P.; Oldgren, J.; Andersson, U.; Reboldi, G.; Di Pasquale, G.; Mazzotta, G.; Angeli, F.; Eikelboom, J.W.; Ezekowitz, M.D.; et al. Cardiac biomarkers and left ventricular hypertrophy in relation to outcomes in patients with atrial fbrillation: Experiences from the RE-LY trial. J. Am. Heart Assoc. 2019, 8, e0101107. [Google Scholar] [CrossRef]
- Li, M.; Duan, L.; Cai, Y.L.; Li, H.Y.; Hao, B.C.; Chen, J.Q.; Liu, H.B. Growth differentiation factor-15 is associated with cardiovascular outcomes in patients with coronary artery disease. Cardiovasc. Diabetol. 2020, 19, 120. [Google Scholar] [CrossRef]
- Tummalapalli, S.L.; Zelnick, L.R.; Andersen, A.H.; Christenson, R.H.; deFilippi, C.R.; Deo, R.; Go, A.S.; He, J.; Ky, B.; Lash, J.P.; et al. Association of cardiac Biomarkers with the Kansas City Cardiomyopathy Questionnaire in patients with chronic kdney disease without heart failure. J. Am. Heart Assoc. 2020, 9, e014385. [Google Scholar] [CrossRef]
- Eddy, A.C.; Trask, A.J. Growth differentiation factor-15 and its role in diabetes and cardiovascular disease. Citokine Growth Factor Rev. 2021, 57, 11–18. [Google Scholar] [CrossRef]
- Haller, P.M.; Beer, B.N.; Tonkin, A.M.; Blankenberg, S.; Neumann, J.T. Role of cardiac biomarkers in epidemiology and risk outcomes. Clin. Chem. 2021, 67, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, S.W.; Tang, J.K.K. The utility of growth differentiation factor-15, galectin-3, and sST2 as biomarkers for the diagnosis of heart failure with preserved ejection fraction and compared to heart failure with reduced ejection fraction: A systematic review. Heart Fail. Rev. 2021, 26, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Day, E.A.; Townsend, L.K.; Djordjevic, D.; Jørgensen, S.B.; Steinberg, G.R. GDF15: Emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat. Rev. Endocrinol. 2021, 17, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Klimczak-Tomaniak, D.; de Bakker, M.; Bouwens, E.; Akkerhuis, K.M.; Baart, S.; Rizopoulos, D.; Mouthaan, H.; van Ramshorst, J.; Germans, T.; Constantinescu, A.; et al. Dynamic personalized risk prediction in chronic heart failure patients: A longitudinal, clinical investigation of 92 biomarkers (Bio-SHiFT study). Sci. Rep. 2022, 12, 2795. [Google Scholar] [CrossRef] [PubMed]
- May, B.M.; Kochi, A.N.; Magalhães, A.P.A.; Scolari, F.; Zimerman, A.; Andrades, M.; Zimerman, L.I.; Rohde, L.E.; Pimentel, M. Growth/differentiation factor-15 (GDF-15) as a predictor of serious arrhythmic events in patients with nonischemic dilated cardiomyopathy. J. Electrocardiol. 2022, 70, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Kempf, T.; Giannitsis, E.; Bertsch, T.; Braun, S.L.; Maier, H.; Reim, M.; Christenson, R.H. An automated assay for Growth Differentiation Factor 15. J. App. Lab. Med. 2017, 1, 510–521. [Google Scholar] [CrossRef]
- Hamon, S.M.; Griffin, T.P.; Islam, M.N.; Wall, D.; Griffin, M.D.; O’Shea, P.M. Defining reference intervals for a serum growth differentiation factor-15 (GDF-15) assay in a Caucasian population and its potential utility in diabetic kidney disease (DKD). Clin. Chem. Lab. Med. 2019, 57, 510–520. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.; Xu, W.; Zhang, Y.; Bai, J.; Li, L.; Cui, M.; Sun, L. Association of Plasma C1q/TNF-Related Protein 3 (CTRP3) in patients with atrial fibrillation. Mediators Inflamm. 2020, 2020, 8873152. [Google Scholar] [CrossRef]
- Recinella, L.; Orlando, G.; Ferrante, C.; Chiavaroli, A.; Brunetti, L.; Leone, S. Adipokines: New potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front. Physiol. 2020, 11, 578966. [Google Scholar] [CrossRef]
- Shanaki, M.; Shabani, P.; Goudarzi, A.; Omidifar, A.; Bashash, D.; Emamgholipour, S. The C1q/TNF-related proteins (CTRPs) in pathogenesis of obesity-related metabolic disorders: Focus on type 2 diabetes and cardiovascular diseases. Life Sci. 2020, 256, 117913. [Google Scholar] [CrossRef]
- Pieri, M.; Ciotti, M.; Nuccetelli, M.; Perrone, M.A.; Caliò, M.T.; Lia, M.S.; Minieri, M.; Bernardini, S. Serum Amyloid A Protein as a useful biomarker to predict COVID-19 patients severity and prognosis. Int Immunopharmacol. 2021, 95, 107512. [Google Scholar] [CrossRef]
- Jung, H.N.; Jung, C.H. The role of anti-inflammatory adipokines in cardiometabolic disorders: Moving beyond adiponectin. Int. Mol. Sci. 2021, 22, 13529. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Meng, Z.; Gao, J.; Liu, C.; Wang, J.; Guo, R.; Zhao, J.; Lopez, B.; Christopher, T.; Lee, D.; et al. C1q Complement/Tumor Necrosis Factor-Associated proteins in cardiovascular disease and COVID-19. Proteomes 2021, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, X.; Zhou, Y.; Cai, W.; Qiu, L. C1q/TNF-related protein-9 ameliorates Ox-LDL-induced endothelial dysfunction via PGC-1α/AMPK-mediated antioxidant enzyme induction. Int. J. Mol. Sci. 2017, 18, 1097. [Google Scholar] [CrossRef]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Ge, G.; Spooner, E.; Hug, C.; Gimeno, R.; Lodish, H.F. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009, 23, 241–258. [Google Scholar] [CrossRef]
- Su, H.; Yuan, Y.; Wang, X.M.; Lau, W.B.; Wang, Y.; Wang, X.; Gao, E.; Koch, W.J.; Ma, X.L. Inhibition of CTRP9, a novel and cardiac-abundantly expressed cell survival molecule, by TNFα-initiated oxidative signaling contributes to exacerbated cardiac injury in diabetic mice. Basic Res. Cardiol. 2013, 108, 315. [Google Scholar] [CrossRef]
- Wang, J.; Hang, T.; Cheng, X.M.; Li, D.M.; Zhang, Q.G.; Wang, L.J.; Peng, Y.P.; Gong, J.B. Associations of C1q/TNF-Related Protein-9 levels in serum and epicardial adipose tissue with coronary atherosclerosis in humans. Biomed. Res. Int. 2015, 2015, 971683. [Google Scholar] [CrossRef] [PubMed]
- Moradi, N.; Fadaei, R.; Emamgholipour, S.; Kazemian, E.; Panahi, G.; Vahedi, S.; Saed, L.; Fallah, S. Association of circulating CTRP9 with soluble adhesion molecules and inflammatory markers in patients with type 2 diabetes mellitus and coronary artery disease. PLoS ONE 2018, 13, e0192159. [Google Scholar] [CrossRef]
- Gao, C.; Zhao, S.; Lian, K.; Mi, B.; Si, R.; Tan, Z.; Fu, F.; Wang, S.; Wang, R.; Ma, X.; et al. C1q/TNF-related protein 3 (CTRP3) and 9 (CTRP9) concentrations are decreased in patients with heart failure and are associated with increased morbidity and mortality. BMC Cardiovasc. Disord. 2019, 19, 139. [Google Scholar] [CrossRef]
- Pan, J.; Cui, X.; Wang, G.; Xue, K.; Hu, J.; Zhou, L. Predictive value of serum CTRP9 and STIM1 for restenosis after cerebrovascular stent implantation and its relationship with vasoactive substances and inflammatory cytokines. Exp. Ther. Med. 2020, 20, 2617–2622. [Google Scholar] [CrossRef]
- Brocker, C.; Thompson, D.; Matsumoto, A.; Nebert, D.W.; Vasiliou, V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum. Genom. 2010, 5, 30–55. [Google Scholar]
- Ancey, C.; Corbi, P.; Froger, J.; Delwail, A.; Wijdenes, J.; Gascan, H.; Potreau, D.; Lecron, J.C. Secretion of IL-6, IL-11 and LIF by human cardiomyocytes in primary culture. Cytokine 2002, 18, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Matsui, T. The cardiomyocyte as a source of cytokines in cardiac injury. J. Cell Sci. Ther. 2011, 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Van Tassell, B.W.; Toldo, S.; Mezzaroma, E.; Abbate, A. Targeting interleukin-1 in heart disease. Circulation 2013, 128, 1910–1923. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Aimo, A.; Vergaro, G.; Passino, C.; Ripoli, A.; Ky, B.; Miller, W.L.; Bayes-Genis, A.; Anand, I.; Januzzi, J.L.; Emdin, M. Prognostic value of soluble suppression of Tumorigenicity-2 in chronic heart failure: A meta-analysis. JAAC Heart Fail. 2017, 5, 280–286. [Google Scholar] [CrossRef]
- Li, H.; Liu, W.; Xie, J. Circulating interleukin-6 levels and cardiovascular and all-cause mortality in the elderly population: A meta-analysis. Arch. Gerontol. Geriatr. 2017, 73, 257–262. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, H.; Lin, Z. Association between IL-1b+3954C/T polymorphism and myocardial infarction risk. A meta-analysis. Medicine 2018, 97, e11645. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.L.; Zhao, C.R.; Pan, C.L.; Zhang, Z. Interleukin-6 as a predictor of the risk of cardiovascular disease: A meta-Analysis of prospective epidemiological studies. Immunol. Investig. 2018, 47, 689–699. [Google Scholar] [CrossRef]
- González-Castro, T.B.; Hernández-Díaz, Y.; Pérez-Hernández, N.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narvaez, M.L.; Blachman-Braun, R.; Posadas-Sánchez, R.; Vargas-Alarcón, G.; García-Flores, E.; et al. Interleukin 6 (rs1800795) gene polymorphism is associated with cardiovascular diseases: A meta-analysis of 74 studies with 86,229 subjects. EXCLI J. 2019, 18, 331–355. [Google Scholar]
- Gu, L.; Li, J. Short-term and long-term prognostic value of circulating soluble suppression of tumorigenicity-2 concentration in acute coronary syndrome: A meta-analysis. Biosci. Rep. 2019, 39, BSR20182441. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Tamtaji, O.R.; Mirhosseini, N.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Borhani-Haghighi, A.; Peymani, P.; Ahmadizar, F.; Asemi, Z. The effects of statin use on inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 141, 85–103. [Google Scholar] [CrossRef] [PubMed]
- de Souza, V.H.; de Alencar, J.B.; Tiyo, B.T.; Alves, H.V.; Vendramini, E.C.L.; Sell, A.M.; Visentainer, J.E.L. Association of functional IL16 polymorphisms with cancer and cardiovascular disease: A meta-analysis. Oncotarget 2020, 11, 3405–3417. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Hang, T.; Gao, X.; Yang, W.; Kong, W.; Lou, Q.; Yang, J. The association between soluble suppression of tumorigenicity-2 and long-term prognosis in patients with coronary artery disease: A meta-analysis. PLoS ONE 2020, 15, e0238775. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Chen, H.; Zhang, H.; Gu, Y. Long-term and short-term prognostic value of crculating soluble suppression of tumorigenicity-2 concentration in chronic heart failure: A systematic review and meta-analysis. Cardiology 2021, 146, 433–440. [Google Scholar] [CrossRef]
- Li, H.; Cen, K.; Sun, W.; Feng, B. Predictive Value of blood interleukin-6 level in patients with acute coronary syndrome: A meta-analysis. Immunol. Investig. 2021, 50, 964–976. [Google Scholar] [CrossRef]
- Jia, X.; Cheng, X.; Wu, N.; Xiang, Y.; Wu, L.; Xu, B.; Li, C.; Zhang, Z.; Tong, S.; Zhong, L.; et al. Prognostic value of interleukin-6 in atrial fibrillation: A cohort study and meta-analysis. Anatol. J. Cardiol. 2021, 25, 872–879. [Google Scholar] [CrossRef]
- Perrone, M.A.; Favresse, J.; D’Alessandro, A.; Albanese, F.; De Bruyne, C.; Ceccarelli, S.; Drago, F.; Guccione, P.; Porzio, O.; Leonardi, B. Soluble Isoform of Suppression of Tumorigenicity 2 (ST2) Biomarker in a Large Cohort of Healthy Pediatric Population: Determination of Reference Intervals. J Clin Med. 2022, 11, 4693. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Z.; Li, J.; Ren, Z.; Liu, F. Meta-analysis of the relationship between interleukin-6 levels and the prognosis and severity of acute coronary syndrome. Clinics 2021, 76, e2690. [Google Scholar] [CrossRef]
- Perrone, M.A.; Pieri, M.; Marchei, M.; Sergi, D.; Bernardini, S.; Romeo, F. Serum free light chains in patients with ST elevation myocardial infarction (STEMI): A possible correlation with left ventricle dysfunction. Int. J. Cardiol. 2019, 292, 32–34. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Palaiopanos, K.; Björkbacka, H.; Peters, A.; de Lemos, J.A.; Seshadri, S.; Dichgans, M.; Georgakis, M.K. Circulating interleukin-6 levels and incident ischemic stroke: A systematic review and meta-analysis of prospective studies. Neurology 2022, 98, e1002–e1012. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Pomiato, E.; Palmieri, R.; Di Già, G.; Piemonte, F.; Porzio, O.; Gagliardi, M.G. The Effects of Exercise Training on Cardiopulmonary Exercise Testing and Cardiac Biomarkers in Adult Patients with Hypoplastic Left Heart Syndrome and Fontan Circulation. J. Cardiovasc. Dev. Dis. 2022, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Platchek, M.; Lu, Q.; Tran, H.; Xie, W. Comparative analysis of multiple immunoassays for cytokine profiling in drug discovery. SLAS Discov. 2020, 25, 1197–1213. [Google Scholar] [CrossRef]
- Pandey, S.; Malviya, G.; Chottova Dvorakova, M. Role of peptides in diagnostics. Int. J. Mol. Sci. 2021, 22, 8828. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791–796. [Google Scholar] [CrossRef]
- Lau, C.S.; Hoo, S.P.; Koh, J.M.; Phua, S.K.; Aw, T.C. Performance of the Roche IL-6 chemiluminescent immunoassay in patients with COVID-like respiratory symptoms. J. Virol. Methods 2021, 296, 114224. [Google Scholar] [CrossRef]
- Mueller, T.; Dieplinger, B. The Presage® ST2 assay: Analytical considerations and clinical applications for a high-sensitivity assay for measurement of soluble ST2. Expert Rev. Mol. Diagn. 2013, 13, 13–30. [Google Scholar] [CrossRef]
- Gao, S.; Li, J. Development of a novel homogeneous nanoparticle-based assay for rapid and high-throughput quantitation of the sST2 protein in human serum. Int. J. Nanomed. 2020, 15, 10539–10546. [Google Scholar] [CrossRef]
- Braunwald, E. Biomarkers in heart failure. N. Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef]
- Emdin, M.; Vittorini, S.; Passino, C.; Clerico, A.L. Old and new biomarkers of heart failure. Eur. J. Heart Fail. 2009, 11, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Diez-Villanueva, P.; Alfonso, F. Heart failure in the elderly. J. Geriatr. Cardiol. 2016, 13, 115–117. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Kim, J.H. Historical perspectives on tumor necrosis factor and its superfamily: 25 years later, a golden journey. Blood 2012, 119, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, J.G.; Lopus, M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020, 36, 145–164. [Google Scholar] [CrossRef]
- Dai, W.; Cheng, J.; Leng, X.; Hu, X.; Ao, Y. The potential role of necroptosis in clinical diseases (Review). Int. J. Mol. Med. 2021, 47, 89. [Google Scholar] [CrossRef]
- Linkermann, A.; Green, D.R. Necroptosis. N. Engl. J. Med. 2014, 370, 455–465. [Google Scholar] [CrossRef]
- Valaperti, A.; Li, Z.; Vonow-Eisenring, M.; Probst-Müller, E. Diagnostic methods for the measurement of human TNF-alpha in clinical laboratory. J. Pharmacol. Biomed. Anal. 2020, 179, 113010. [Google Scholar] [CrossRef]
- Gordon, K.J.; Blobe, G.C. Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochem. Biophys. Acta 2008, 1782, 197–228. [Google Scholar] [CrossRef]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M.B. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar] [CrossRef]
- Derynck, R.; Jarrett, J.A.; Chen, E.Y.; Eaton, D.H.; Bell, J.R.; Assoian, R.K.; Roberts, A.B.; Sporn, M.B.; Goeddel, D.V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature 1985, 316, 701–705. [Google Scholar] [CrossRef]
- Clerico, A.; Giannoni, A.; Vittorini, S.; Passino, C. Thirty years of the heart as an endocrine organ: Physiological role and clinical utility of cardiac natriuretic hormones. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H12–H20. [Google Scholar] [CrossRef] [PubMed]
- Del Ry, S.; Cabiati, M.; Clerico, A. Natriuretic peptide system and the heart. Front. Horm. Res. 2014, 43, 134–143. [Google Scholar] [PubMed]
- Silswal, N.; Singh, A.K.; Aruna, B.; Mukhopadhyay, S.; Ghosh, S.; Ehtesham, N.Z. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB dependent pathway. Biochem. Biophys. Res. Commun. 2005, 334, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Adya, R.; Tan, B.K.; Chen, J.; Randeva, H.S. Nuclear factor-kappaB induction by visfatin in human vascular endothelial cells: Its role in MMP-2/9 production and activation. Diabetes Care 2008, 31, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.M.; Shieh, D.C.; Chen, C.P.; Tzeng, C.Y.; Wang, S.P.; Huang, K.C.; Chiu, Y.C.; Fong, Y.C.; Tang, C.H. Leptin induces IL-8 expression via leptin receptor, IRS-1, PI3K, Akt cascade and promotion of NF-kappaB/p300 binding in human synovial fibroblasts. Cell. Signal. 2008, 20, 1478–1488. [Google Scholar] [CrossRef]
- Oikonomou, E.; Tousoulis, D.; Siasos, G.; Zaromitidou, M.; Papavassiliou, A.G.; Stefanadis, C. The role of inflammation in heart failure: New therapeutic approaches. Hellenic J. Cardiol. 2011, 52, 30–40. [Google Scholar]
- Oikonomou, E.; Zografos, T.; Papamikroulis, G.A.; Siasos, G.; Vogiatzi, G.; Theofilis, P.; Briasoulis, A.; Papaioannou, S.; Vavuranakis, M.; Gennimata, V.; et al. Biomarkers in atrial fibrillation and heart failure. Curr. Med. Chem. 2019, 26, 873–887. [Google Scholar] [CrossRef]
- Itagaki, T.; Motoki, H.; Otagiri, K.; Machida, K.; Takeuchi, T.; Kanai, M.; Kimura, K.; Higuchi, S.; Minamisawa, M.; Kitabayashi, H.; et al. Inflammation-based assessment for the risk stratification of mortality in patients with heart failure. Sci. Rep. 2021, 11, 14989. [Google Scholar] [CrossRef]
- McKechnie, D.G.; Papacosta, A.O.; Lennon, L.T.; Welsh, P.; Whincup, P.H.; Wannamethee, S.G. Inflammatory markers and incident heart failure in older men: The role of NT-proBNP. Biomark. Med. 2021, 15, 413–425. [Google Scholar] [CrossRef]
- Myhre, P.L.; Claggett, B.; Yu, B.; Skali, H.; Solomon, S.D.; Røsjø, H.; Omland, T.; Wiggins, K.L.; Psaty, B.M.; Floyd, J.S.; et al. Sex and race differences in N-terminal pro-B-type natriuretic peptide concentration and absolute risk of heart failure in the community. JAMA Cardiol. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global public health burden of heart failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation—Cause or consequence of heart failure or both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in heart failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Clerico, A.; Passino, C.; Franzini, M.; Emdin, M. Cardiac biomarker testing in the clinical laboratory: Where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin. Chim. Acta 2015, 443, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Zaninotto, M.; Passino, C.; Plebani, M. New issues on measurement of B-type natriuretic peptides. Clin. Chem. Lab. Med. 2018, 56, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.G.; Feygina, E.E. Standardization of BNP and NT-proBNP immunoassays in light of the diverse and complex nature of circulating BNP-related peptides. Adv. Clin. Chem. 2018, 85, 1–30. [Google Scholar]
- Taylor, K.S.; Verbakel, J.Y.; Feakins, B.G.; Price, C.P.; Perera, R.; Bankhead, C.; Plüddemann, A. Diagnostic accuracy of point-of-care natriuretic peptide testing for chronic heart failure in ambulatory care: Systematic review and meta-analysis. BMJ 2018, 361, k1450. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Kotani, K. Point-of-care testing of (N-terminal pro) B-type natriuretic peptide for heart disease patients in home care and ambulatory care settings. Pract. Lab. Med. 2020, 22, e00183. [Google Scholar] [CrossRef]
- Apple, F.S.; Wu, A.H.; Jaffe, A.S.; Panteghini, M.; Christenson, R.H.; NACB Committee; IFCC C-SMCD. National Academy of Clinical Biochemistry and IFCC Committee for standardization of markers of cardiac damage laboratory medicine practice guidelines: Analytical issues for biomarkers of heart failure. Clin. Biochem. 2008, 41, 222–226. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Lam, C.S.P.; Saenger, A.K.; Jaffe, A.S.; Collinson, P.; Pulkki, K.; Omland, T.; Lefèvre, G.; Body, R.; Ordonez-Llanos, J.; et al. Educational recommendations on selected analytical and clinical aspects of natriuretic peptides with a focus on heart failure: A report from the IFCC Committee on Clinical Applications of Cardiac Bio-Markers. Clin. Chem. 2019, 65, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Kavsak, P.A.; Beattie, J.; Ma, J. Effect of storage temperature for B-type natriuretic peptide concentrations for primary health care populations. Clin. Chem. 2019, 65, 811–812. [Google Scholar] [CrossRef] [PubMed]
- Prontera, C.; Zaninotto, M.; Giovannini, S.; Zucchelli, G.C.; Pilo, A.; Sciacovelli, L.; Plebani, M.; Clerico, A. Proficiency testing project for brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP (NTproBNP) immunoassays: The CardioOrmoCheck study. Clin. Chem. Lab. Med. 2009, 47, 762–768. [Google Scholar] [CrossRef]
- Clerico, A.; Zaninotto, M.; Prontera, C.; Giovannini, S.; Ndreu, R.; Franzini, M.; Zucchelli, G.C.; Plebani, M. State of the art of BNP and NT-proBNP immunoassays: The CardioOrmoCheck study. Clin. Chim. Acta 2012, 414, 112–119. [Google Scholar] [CrossRef]
- Marjot, J.; Kaier, T.E.; Martin, E.D.; Reji, S.S.; Copeland, O.; Iqbal, M.; Goodson, B.; Hamren, S.; Harding, S.E.; Marber, M.S. Quantifying the release of biomarkers of myocardial necrosis from cardiac myocytes and intact myocardium. Clin. Chem. 2017, 63, 990–996. [Google Scholar] [CrossRef]
- Mair, J.; Lindahl, B.; Hammarsten, O.; Müller, C.; Giannitsis, E.; Huber, K.; Möckel, M.; Plebani, M.; Thygesen, K.; Jaffe, A.S. How is cardiac troponin released from injured myocardium? Eur. Heart J. Acute Cardiovasc. Care 2018, 6, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Zaninotto, M.; Padoan, A.; Masotti, S.; Musetti, V.; Prontera, C.; Ndreu, R.; Zucchelli, G.; Passino, C.; Migliardi, M.; et al. Evaluation of analytical performance of immunoassay methods for cTnI and cTnT: From theory to practice. Adv. Clin. Chem. 2019, 93, 239–262. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. ESC Scientific Document Group. Fourth Universal Definition of Myocardial Infarction (2018). Eur. Heart J. 2019, 40, 237–269. [Google Scholar] [CrossRef]
- NICE. High-Sensitivity Troponin Tests for the Early Rule out of NSTEMI. Diagnostics Guidance. Published: 26 August 2020. Available online: www.nice.org.uk/guidance/dg40 (accessed on 1 December 2022).
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar]
- Apple, F.S.; Collinson, P.O.; Kavsak, P.A.; Body, R.; Ordóñez-Llanos, J.; Saenger, A.K.; Omland, T.; Hammarsten, O.; Jaffe, A.S. The IFCC Clinical Application of Cardiac Biomarkers Committee’s Appraisal of the 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Getting cardiac troponin right. Clin. Chem. 2021, 67, 730–735. [Google Scholar] [CrossRef]
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordóñez-Llanos, J.; IFCC Task Force on Clinical Application of Cardiac Bio-Markers. Cardiac troponin assays: Guide to understanding analytical characteristics and their impact on clinical care. Clin. Chem. 2017, 63, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Zaninotto, M.; Ripoli, M.; Masotti, S.; Prontera, C.; Passino, C.; Plebani, M. The 99th percentile of reference population for cTnI and cTnT assay: Methodology, pathophysiology, and clinical implications. Clin. Chem. Lab. Med. 2017, 55, 1634–1651. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Giannoni, A.; Prontera, T.; Giovannini, S. High-sensitivity troponin: A new tool for pathophysiological investigation and clinical practice. Adv. Clin. Chem. 2009, 49, 1–30. [Google Scholar] [PubMed]
- Clerico, A.; Zaninotto, M.; Passino, C.; Plebani, M. Clinical relevance of biological variation of cardiac troponins. Clin. Chem. Lab. Med. 2021, 59, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Cianflone, E.; Torella, M.; Biamonte, F.; De Angelis, A.; Urbanek, K.; Costanzo, F.S.; Rota, M.; Ellison-Hughes, G.M.; Torella, D. Targeting cardiac stem cell senescence to treat cardiac aging and disease. Cells 2020, 9, 1558. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Cellular senescence: A translational perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
- Coppé, J.P.; Kauser, K.; Campisi, J.; Beauséjour, C.M. Secretion of vascular endothelial growth factor by primary human fibroblasts at senescence. J. Biol. Chem. 2006, 281, 29568–29574. [Google Scholar] [CrossRef]
- Kuilman, T.; Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 2009, 9, 81–94. [Google Scholar] [CrossRef]
- de Boer, R.A.; De Keulenaer, G.; Bauersachs, J.; Brutsaert, D.; Cleland, J.G.; Diez, J.; Du, X.J.; Ford, P.; Heinzel, F.R.; Lipson, K.E.; et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 272–285. [Google Scholar] [CrossRef]
- Li, H.; Hastings, M.H.; Rhee, J.; Trager, L.E.; Roh, J.D.; Rosenzweig, A. Targeting age-related pathways in heart failure. Circ. Res. 2020, 126, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Alshawabkeh, L.I.; Yee, L.M.; Gardin, J.M.; Gottdiener, J.S.; Odden, M.C.; Bartz, T.M.; Arnold, A.M.; Mukamal, K.J.; Wallace, R.B. Years of able life in older persons--the role of cardiovascular imaging and biomarkers: The Cardiovascular Health Study. J. Am. Heart Assoc. 2015, 4, e001745. [Google Scholar] [CrossRef]
- Wijsman, L.W.; de Craen, A.J.; Trompet, S.; Sabayan, B.; Muller, M.; Stott, D.J.; Ford, I.; Welsh, P.; Westendorp, R.G.; Jukema, J.W.; et al. High-sensitivity cardiac troponin T is associated with cognitive decline in older adults at high cardiovascular risk. Eur. J. Prev. Cardiol. 2016, 23, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Veugen, M.G.J.; Henry, R.M.A.; Brunner-La Rocca, H.P.; Dagnelie, P.C.; Schram, M.T.; van Agtmaal, M.J.M.; van der Kallen, C.J.H.; Sep, S.J.S.; van Boxtel, M.P.J.; Bekers, O.; et al. Cross-sectional associations between cardiac biomarkers, cognitive performance, and structural brain changes are modified by age. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1948–1958. [Google Scholar] [CrossRef]
- Kaura, A.; Panoulas, V.; Glampson, B.; Davies, J.; Mulla, A.; Woods, K.; Omigie, J.; Shah, A.D.; Channon, K.M.; Weber, J.N.; et al. Association of troponin level and age with mortality in 250 000 patients: Cohort study across five UK acute care centres. BMJ 2019, 367, l6055. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Ripoli, A.; Zaninotto, M.; Masotti, S.; Musetti, V.; Ciaccio, M.; Aloe, R.; Rizzardi, S.; Dittadi, R.; Carrozza, C.; et al. Head-to-head comparison of plasma cTnI concentration values measured with three high-sensitivity methods in a large Italian population of healthy volunteers and patients admitted to emergency department with acute coronary syndrome: A multi-center study. Clin. Chim. Acta 2019, 496, 25–34. [Google Scholar] [CrossRef]
- Franzini, M.; Lorenzoni, V.; Masotti, S.; Prontera, C.; Chiappino, D.; Della Latta, D.; Daves, M.; Deluggi, I.; Zuin, M.; Ferrigno, L.; et al. The calculation of the cardiac troponin T 99th percentile of the reference population is affected by age, gender, and population selection: A multicenter study in Italy. Clin. Chim. Acta 2015, 438, 376–381. [Google Scholar] [CrossRef]
- Ishii, J.; Nomura, M.; Nakamura, Y.; Naruse, H.; Mori, Y.; Ishikawa, T.; Ando, T.; Kurokawa, H.; Kondo, T.; Nagamura, Y.; et al. Risk stratification using a combination of cardiac troponin T and brain natriuretic peptide in patients hospitalized for worsening chronic heart failure. Am. J. Cardiol. 2002, 89, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T.; Kitaoka, H.; Okawa, M.; Yamanaka, S.; Hirota, T.; Baba, Y.; Hayato, K.; Yamasaki, N.; Matsumura, Y.; Yasuda, N.; et al. Combined measurements of cardiac troponin I and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circ. J. 2011, 75, 919–926. [Google Scholar] [CrossRef]
- du Fay de Lavallaz, J.; Badertscher, P.; Nestelberger, T.; Zimmermann, T.; Miró, Ò.; Salgado, E.; Christ, M.; Geigy, N.; Cullen, L.; Than, M.; et al. B-type natriuretic peptides and cardiac troponins for diagnosis and risk-stratification of syncope. Circulation 2019, 139, 2403–2418. [Google Scholar] [CrossRef]
- Perrone, M.A.; Zaninotto, M.; Masotti, S.; Musetti, V.; Padoan, A.; Prontera, C.; Plebani, M.; Passino, C.; Romeo, F.; Bernardini, S.; et al. The combined measurement of high-sensitivity cardiac troponins and natriuretic peptides: A useful tool for clinicians? J. Cardiovasc. Med. 2020, 21, 953–963. [Google Scholar] [CrossRef]
- Iorio, A.; Lombardi, C.M.; Specchia, C.; Merlo, M.; Nuzzi, V.; Ferraro, I.; Peveri, G.; Oriecuia, C.; Pozzi, A.; Inciardi, R.M.; et al. Combined role of troponin and natriuretic peptides measurements in patients with Covid-19 (from the Cardio-COVID-Italy Multicenter Study). Am. J. Cardiol. 2022, 167, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, F. The shift of the paradigm between ageing and disease. Clin. Lab. Med. 2020, 58, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Sze, J.; Mooney, J.; Barzi, F.; Hillis, G.S.; Chow, C.K. Cardiac troponin and its relationship to cardiovascular outcomes in community populations—A systematic review and meta-analysis. Heart Lung Circ. 2016, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Van der Lindel Klinkenberg, L.J.J.; Bekers, O.; Loon, L.J.C.V.; Dieijen-Visser, M.P.V.; Zeegers, M.P.; Meex, S.J.R. Prognostic value of basal high-sensitive cardiac troponin levels on mortality in the general population: A meta-analysis. Medicine 2016, 95, e5703. [Google Scholar] [CrossRef]
- Willeit, P.; Welsh, P.; Evans, J.D.W.; Tschiderer, L.; Boachie, C.; Jukema, J.W.; Ford, I.; Trompet, S.; Stott, D.J.; Kearney, P.M.; et al. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J. Am. Coll. Cardiol. 2017, 70, 558–568. [Google Scholar] [CrossRef]
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Predicting mortality with cardiac troponins: Recent insights from meta-analyses. Diagnosis 2019, 8, 37–49. [Google Scholar] [CrossRef]
- Aimo, A.; Georgiopoulos, G.; Panichella, G.; Vergaro, G.; Passino, C.; Emdin, M.; Clerico, A. High-sensitivity troponins for outcome prediction in the general population: A systematic review and meta-analysis. Eur. J. Intern. Med. 2022, 98, 61–68. [Google Scholar] [CrossRef]
- Hughes, M.F.; Ojeda, F.; Saarela, O.; Jørgensen, T.; Zeller, T.; Palosaari, T.; O’Doherty, M.G.; Borglykke, A.; Kuulasmaa, K.; Blankenberg, S.; et al. Association of repeatedly measured high-sensitivity-assayed troponin I with cardiovascular disease events in a general population from the MORGAM/BiomarCaRE Study. Clin. Chem. 2017, 63, 334–342. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.L.; Gruson, D.; Bernardini, S.; Clerico, A.; Perrone, M. The underestimated issue of non-reproducible cardiac troponin I and T results: Case series and systematic review of the literature. Clin. Chem. Lab. Med. 2021, 59, 1201–1211. [Google Scholar] [CrossRef]
- Perrone, M.A.; Aimo, A.; Bernardini, S.; Clerico, A. Natriuretic Peptides and Troponins to Predict Cardiovascular Events in Patients Undergoing Major Non-Cardiac Surgery. Int. J. Environ. Res. Public Health 2022, 19, 5182. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Storti, S.; Salvadori, S.; Pecori, A.; Bernardini, S.; Romeo, F.; Guccione, P.; Clerico, A. Cardiac troponins: Are there any differences between T and I? J. Cardiovasc. Med. 2021, 22, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.A.; Spolaore, F.; Ammirabile, M.; Romeo, F.; Caciagli, P.; Ceriotti, F.; Bernardini, S. The assessment of high sensitivity cardiac troponin in patients with COVID-19: A multicenter study. Int J Cardiol Heart Vasc. 2021, 32, 100715. [Google Scholar] [CrossRef] [PubMed]
| Oxidative stress |
| Inflammatory activation |
| Metabolic disorders: hyperglycemia, hyperinsulinemia, insulin resistance, dyslipidemia |
| Vascular disorders |
| Endothelial dysfunction, arterial hypertension, arterial stiffness |
| Genetic-epigenetic mechanisms |
| Telomere length DNA methylation |
| Acceptable to patient |
| Stability in vivo and in vitro |
| Adequate analytical sensitivity (functional sensitivity) |
| Good degree in reproducibility and accuracy |
| Easy to perform |
| Complete automation of assay |
| International standardization |
| Low cost |
| Low biological variation |
| Reference range and cut-off values tested for gender, age, and ethnicity dependence |
| Good diagnostic and prognostic accuracy |
| Cost-benefit ratio favorable |
| Cardiokines | Related Conditions | References |
|---|---|---|
| Natriuretic Peptides (ANP, BNP, CNP and related peptides) | Cardiac stress, activation of neuro-immune-inflammatory systems, stretching of right atrium, hypoxia | [12,16,94,104] |
| GDF-8 (myostatin) | In heart failure, increased levels of cardiac derived GDF-8 act in an endocrine fashion on skeletal muscle to reduce muscle mass. | [94,103,105,106,107,108] |
| GDF-15 (macrophage-inhibitory cytokine 1) | Cardiac hypertrophy and chronic heart failure, ischemia/reperfusion injury, myocardial infarction | [94,103,109,110,111] |
| CTRP (C1q/TNF-related protein) family | Diabetes mellitus, coronary artery disease, ischemia/reperfusion injury, myocardial infarction, ischemic stroke | [94,103,112,113] |
| IL-1 family | Atherosclerosis, myocardial infarction | [103,114,115,116,117,118,119] |
| IL-6 | Atherothrombosis, heart failure, atrial fibrillation | [99,103,115,117,118,120,121,122] |
| IL-33/ST2 pathway | Heart failure, inflammation, cardiac fibrosis | [99,103,123,124,125,126] |
| TNF-a | Coronary artery disease, Ischemia/reperfusion injury, heart failure | [99,103,115,127,128] |
| TGF-b1 | Coronary artery disease, cardiac hypertrophy, myocardial infarction, atrial fibrillation | [103,129,130,131,132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrone, M.A.; Aimo, A.; Bernardini, S.; Clerico, A. Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers. Int. J. Mol. Sci. 2023, 24, 844. https://doi.org/10.3390/ijms24010844
Perrone MA, Aimo A, Bernardini S, Clerico A. Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers. International Journal of Molecular Sciences. 2023; 24(1):844. https://doi.org/10.3390/ijms24010844
Chicago/Turabian StylePerrone, Marco Alfonso, Alberto Aimo, Sergio Bernardini, and Aldo Clerico. 2023. "Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers" International Journal of Molecular Sciences 24, no. 1: 844. https://doi.org/10.3390/ijms24010844
APA StylePerrone, M. A., Aimo, A., Bernardini, S., & Clerico, A. (2023). Inflammageing and Cardiovascular System: Focus on Cardiokines and Cardiac-Specific Biomarkers. International Journal of Molecular Sciences, 24(1), 844. https://doi.org/10.3390/ijms24010844









